Abstract
Bacteriophages are present in fluids from cirrhosis patients. However, their effect on the immune response is unknown. In this work, we explore the role of phages in the phenotype, function, and cytokine production of monocytes. We stimulated healthy monocytes with five different butanol-purified phage suspensions infective for Gram-negative and Gram-positive bacteria. We studied the expression of the monocyte markers involved in lipopolysaccharide recognition (LPS; CD14), antigen presentation (HLA-DR) and co-stimulation (CD86), and the concentration of induced cytokines (TNF-α, IFN-α, and IL-10) by phages. To confirm the direct role of phages without the interference of contaminating soluble LPS in phage suspensions, polymyxin B was added to the cell cultures. Phagocytosis experiments were assessed by flow cytometry using labeled phage suspensions. We observed that butanol-purified phages reduced the surface levels of CD14 and CD86 in monocytes and increased the secreted levels of TNF-α and IL-10 compared with the control sample containing only butanol buffer. All phage suspensions showed downregulation of HLA-DR expression but only Staphylococcus aureus phage contaminated with Escherichia coli reached statistical significance. The addition of polymyxin B did not restore the monocytic response induced by phages, suggesting that the effect was not caused by the presence of LPS. Monocytes were able to phagocyte phages in a dose- and time-dependent manner. To conclude, the phagocytosis of butanol-purified phages altered the phenotype and cytokine production of monocytes suggesting they become tolerogenic.
Keywords: Bacteriophages, immunity, cirrhosis
Impact statement
It is known that patients with liver cirrhosis have phages in their ascitic fluids. Our work demonstrates that phages induce an immune response in healthy monocytes, in terms of phenotype, production of soluble factors, and phagocytosis function. Furthermore, our work validates the usefulness of polymyxin B and 1-butanol protocol as easy strategies to discard the interference of bacterial products in the phage suspensions.
Introduction
Bacteriophages, also called phages, are viruses present in 45% of the ascitic fluid (AF) samples from patients with spontaneous bacterial peritonitis (SBP).1,2 SBP, which is the most severe complication in patients with liver cirrhosis, is generated by the translocation of bacteria from the gut to the peritoneal cavity caused by an increased intestinal permeability.3 The diagnosis of SBP is based on a positive bacteriological culture result and a count of polymorphonuclear cells above 250 cells/mm3 in the AF. However, bacteria are not detected in a half of the patients with >250 cells/mm3 in AF. Interestingly, these patients have a similar severity and prognosis than those patients with positive bacteriological result,4 suggesting that not only the presence of bacteria in AF induces an immune response.
Bacterial cells are infected by specific phages through lysogenic cycles and then, bacteria are lysed through lytic cycles.5 Although phages do not infect eukaryotic cells, immune cells can be stimulated directly by phages or indirectly by the massive pathogen-associated molecular patterns (PAMPs), released after the lysis of phage-induced or phage-infected bacteria.6 Phagocytes (monocytes and neutrophils) are the main cell populations found in AF from patients with cirrhosis. We have previously reported that ascitic monocytes from SBP expressed low levels of antigen presentation (HLA-DR) and activation (CD86) markers. Furthermore, they produced high levels of TNF-α and IL-10 and they have a reduced ability to phagocytose Escherichia coli.7 Surprisingly, ascitic monocytes from SBP patients with either positive or negative bacteriological culture show a similar immune response.7
In the present study, we hypothesized that the immune response displayed by ascitic monocytes in cirrhosis is not only due to the presence of bacteria but also to the presence of phages in the AF. To confirm our hypothesis, first, healthy monocytes were stimulated with butanol-purified phage suspensions and we studied the markers related with LPS receptor (CD14), antigen presentation (HLA-DR), and co-stimulation (CD86), and the soluble factors (TNF-α, IFN-α, and IL-10) observed in the ascitic monocytes. Second, it was analyzed whether the immune response observed was phage-host specific. Third, the interference of soluble LPS in the phage suspensions was discarded through two strategies: by the addition of polymyxin B and using phage suspensions infecting Gram-positive Staphylococcus aureus, which do not contain LPS in their bacterial cell wall. Finally, the phagocytosis was studied by flow cytometry using labeled phages in dose- and time-dependent assays.
Materials and methods
Bacteriophages and bacterial strains
Five different phage suspensions were studied. They included three virulent phages infecting E. coli WG5 (ATCC 700078) (phages SOM1, SOM3 and SOM4)8 and one phage infecting S. aureus strain RN4220 (phage ɸ11)8 (Table 1). The fifth phage suspension contained the same S. aureus phage but propagated in a S. aureus RN4220 culture contaminated with a sonicated culture of E. coli strain WG5 (prepared as described in the following section). This phage suspension was included to confirm that the butanol protocol removes the contaminating soluble lipopolysaccharide (LPS) from E. coli. This is in contrast with the non-contaminated ɸ11 phage suspension where LPS could not be present as S. aureus lacks LPS in its cell wall.
Table 1.
Characteristics of the different phages used in the cell stimulations.
| Bacteriophage | Phage family | Tail type | Source | Bacterial host strain | Titer after purification (PFU/mL) | References |
|---|---|---|---|---|---|---|
| SOM1 | Siphoviridae | Curly | Sewage | E. coli WG5 ATCC 700078 | 8.0 ×107 | Muniesa et al.9 |
| SOM3 | Myoviridae | Contractile | Sewage | E. coli WG5 ATCC 700078 | 7.0 × 108 | Muniesa et al.9 |
| SOM4 | Siphoviridae | Flexible | Sewage | E. coli WG5 ATCC 700078 | 4.2 × 108 | Muniesa et al.9 |
| ɸ11 | Siphoviridae | Flexible | S. aureus RN451 | S. aureus RN4220 | 3.2 × 108 | Ubeda et al.10 |
PFU: plaque-forming unit.
Butanol-purification of phage suspensions
Phage preparations were purified following the butanol protocol described by Szermer-Olearnik B et al.11 For it, bacteria culture in Luria broth was carried at 37°C for 8–16 h, until the optical density (OD, 600 nm) reached 0.3, which corresponded to about 108 bacterial cells/mL.9 At this point, the culture was infected with phage in a proportion of 0.1 PFU/bacterial cells, and incubated at 37°C for 8 h. Crude bacterial lysates (5 mL–20 mL) were filtered through 0.22 μm low protein binding polyethersulfone (PES) membranes (Millex-GP, Merck). 1-butanol was added (about 40% v/v) to the bacterial lysate and shaken for 1–3 h at room temperature. Then, the two-phase mixture was cooled to 4°C for 1–3 h and separated by centrifugation at 4000 ×g for 10 min. The collected aqueous phases were dialyzed in a buffer containing NaCl 0.15 M and concentrated with Amicon Ultra-15 Centrifugal Filters 50 K (Millipore) to a final volume of 5 mL in NaCl 0.15 M. A buffer control using the same bacterial cultures in the absence of phages was processed in parallel and included in the analysis. In parallel, one phage ɸ11 suspension was contaminated with E. coli to include LPS in the suspensions and monitor its removal by the butanol protocol. An overnight culture of E. coli strain WG5 containing 109 bacterial cells/mL was sonicated for 30 s and placed on dry ice for 30 s in four consecutive steps to disrupt the cells; 1 mL of this culture was added to 200 mL culture of S. aureus RN4220 containing phage ɸ11 culture in a proportion of 0.1 plaque-forming unit (PFU)/bacterial cells, and incubated at 37°C for 8 h. Purification of phages was performed after incubation as described above. After purification and concentration of the phage suspensions, phage titer was determined using the double layer agar technique.12
SYBR-Gold staining of phage suspensions
The phage SOM1 and SOM3 suspensions were stained with SYBR-Gold (Molecular probes, Thermofisher) as previously reported.13 Briefly, 20 µL of SYBR-Gold 100× was added per mL of phage suspension (109 PFU/mL). Suspensions were gently mixed and incubated 1 h in the dark. Suspensions were washed four times with NaCl 0.15 M using Amicon Ultra-15 Centrifugal Filters 50 K (Millipore) to remove the excess of SYBR-Gold and the suspension was obtained in a final volume of 1 mL using NaCl 0.15 M. After purification and concentration of the phage suspensions, the total number of labeled phages was counted by flow cytometry and adjusted to ca 107 phage particles/mL using buffer NaCl 0.15 M.
A buffer control using culture media in the absence of phages was processed in parallel for SYBR Gold staining and purification and included in the analysis.
PBMCs isolation and stimulation with phage suspensions
PBMCs were isolated from 10 mL of peripheral blood of healthy donors using a Lymphoprep gradient (AXIS-SHIELD, PoCAs, Oslo, Norway). The total number of cells was counted by flow cytometry and adjusted to 2 × 105 monocytes/mL with RPMI medium supplemented with 25 mM HEPES buffer (hereafter referred as RPMI-HEPES medium; Sigma-Aldrich, St.Louis, MO). Subsequently, 100,000 PBMCs were stimulated with 1/100 of phage suspensions diluted in RPMI-HEPES medium in a total volume of 200 µL in 96-well culture plates. As a negative control, PBMCs were also stimulated with 1/100 of the butanol buffer. Furthermore, to discard the possibility that soluble LPS molecules remained in the purifications of phage infecting E. coli, PBMCs were pre-cultured with 10 µg/mL of polymyxin B (Sigma-Aldrich) for 30 min at 37°C before the stimulation with phage suspensions. Then, PBMCs were incubated for 24 h at 37°C. After incubation, cells were harvested from wells, stained for 15 min at room temperature and darkness with anti-CD14 PECy7 (BioLegend), anti-CD86 PE (BioLegend), and anti-HLA-DR APC (Immunotools), and washed with phosphate-buffered saline (PBS 1×) before the acquisition by flow cytometry (MACSQuant Analyzer; Miltenyi, Germany).
Soluble factors measured in supernatants of phage stimulated PBMCs
Supernatants of PBMCs stimulated with phage suspensions for 24 h were collected and the levels of TNF-α, IFN-α (BD Biosciences, San Diego, CA), and IL-10 (ImmunoTools, Friesoythe, Germany) were measured by ELISA. The limit of detection was 30 pg/mL for TNF-α, 7 pg/mL for IFN-α, and 9.4 pg/mL for IL-10. Supernatants were diluted 1/2 for TNF-α, 1/20 for IFN-α, and 1/10 for IL-10.
Phagocytosis assay with labeled phage
Phage suspensions containing 107 PFU/mL labeled with SYBR-Gold as described above were used for the phagocytosis experiments; 2 mL of whole blood were lysed using red blood cell lysis (BioLegend, San Diego, CA). Phagocytes (monocytes and neutrophils) were counted by flow cytometry and adjusted to 200,000 cells with RPMI-HEPES medium. Phagocytes were incubated in 96-well culture plates with 1/5, 1/10, and 1/20 labeled phage SOM1 and SOM3 for periods of 20, 40, and 120 min at 37°C and at 4°C to inhibit the phagocytosis process. After the incubation, cells were harvested from wells, stained for 15 min at room temperature and darkness with anti-CD14-PECy7 (BioLegend) and washed with PBS 1× before the acquisition by flow cytometry. Phagocytic monocytes were defined as CD14 positive, low granularity, and SYBR-Gold positive.
Statistical analysis
Experimental groups were compared by t-test for paired data with the respective negative controls. Correlations were analyzed by Spearman test. Significance was established at P < 0.05. Values were expressed as mean ± SD.
Results
Effect of phages on the expression of monocyte markers
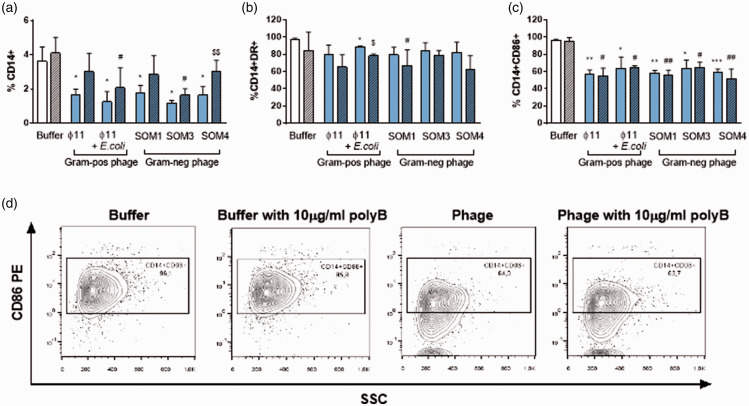
It was first confirmed that either the butanol buffer used for the phage purification or the phage suspensions did not affect the viability, size, and granularity of CD14+ cells (monocytes) (Figure S1). Upon stimulation, we found that all phage suspensions significantly reduced the percentage and the expression of CD14 and CD86 compared with monocytes stimulated with buffer in the absence of phages. Only ɸ11 phage contaminated with E. coli significantly down-regulated HLA-DR expression compared with buffer in absence of phages (Figure 1).
Figure 1.
Changes in monocyte markers expression induced by butanol-purified Gram-negative and Gram-positive phages. (a) Percentage of CD14+, (b) CD14+HLA-DR+ , and (c) CD14+CD86+ cells from PBMCs stimulated with 1/100 butanol buffer and 1/100 butanol-purified phages without adding polymixin B (polyB; solid bar) or after adding polyB (stripe pattern). (d) Representative flow cytometry image of CD14+CD86+ cells from PBMCs stimulated with 1/100 butanol buffer or 1/100 SOM1 phage with or without polyB. *<0.05; **<0.01; ***<0.001; *Buffer vs Phage; #Buffer with polyB vs. Phage with polyB; $Phage vs. Phage with polyB. (A color version of this figure is available in the online journal.)
To validate that the observed changes in monocytes were not due to contaminating LPS from Gram-negative bacteria in the phage suspensions, we performed the monocyte stimulation in the presence of polymyxin B (polyB). PolyB neutralizes the effect of LPS but at higher concentrations can also down-regulate CD14.14 Therefore, we first tested the concentration of polyB to counteract the effect of LPS without down-regulating CD14. At 10 µg/mL, polyB maintained the expression of CD14 and reverted the downregulation of HLA-DR and CD86 produced by LPS (Figure S2). However, polyB did not revert the reduction of CD14, HLA-DR, and CD86 expression on monocytes after phage stimulus, confirming that the specificity of immune response in monocytes was induced by the phages. Furthermore, we did not observe significant differences between the S. aureus ɸ11 phage suspension and S. aureus ɸ11 phage contaminated with E. coli (Figure 1). This observation validates that butanol protocol removed efficiently soluble LPS from the phage suspensions, either those propagated in E. coli or those in S. aureus that were contaminated with E. coli. Only the phage suspension SOM4 cultured in presence of polyB increased the levels of CD14 in monocytes compared with the culture without polyB, but without reaching the CD14 levels observed under buffer conditions. However, the effect of polyB in this phage suspension was not observed neither in HLA-DR nor in CD86 levels, suggesting that only a minimal source of residual LPS could exist in this phage suspension.
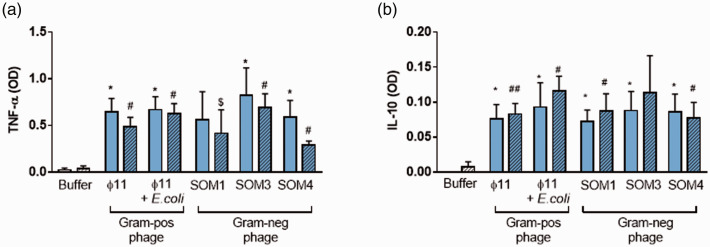
Effect of phages on the production of soluble factors by PBMCs
The concentration of IFN-α, TNF-α, and IL-10 produced by PBMCs stimulated with the five phage suspensions was measured in the supernatants. IFN-α levels were undetectable in all the conditions in PBMCs cultured neither with buffer alone nor upon stimulation with phage suspensions. All phage suspensions increased the TNF-α and IL-10 production by PBMCs compared with buffer alone (Figure 2(a) and (b)). We have also demonstrated using butanol-purified phages that the presence of polyB during the stimulation with phage suspensions did not revert the TNF-α and IL-10 levels.
Figure 2.
Inflammation induced by butanol-purified Gram-negative and Gram-positive phages. (a) TNF-α and (b) IL-10 levels measured by ELISA assays in the supernatants of PBMCs stimulated with 1/100 butanol-purified phages without (solid bar) or after adding polyB (stripe pattern). *<0.05; **<0.01; ***<0.001; *Buffer vs Phage; #Buffer with polyB vs. Phage with polyB; $Phage vs. Phage with polyB. (A color version of this figure is available in the online journal.)
Taking together the results about phenotype and soluble factors induced by phages, a negative correlation between the expression of CD86 on monocytes stimulated with phage suspensions and the TNF-α levels in their supernatants was observed (rho=−0.66, P = 0.007) (Figure S3).
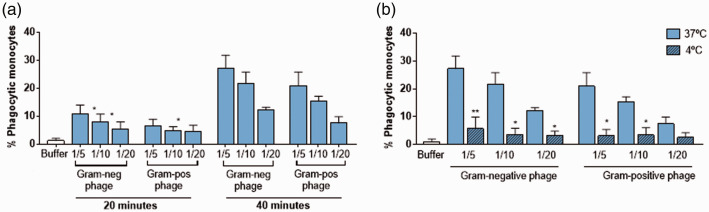
Ability of monocytes to phagocytose phages
The phage-monocyte interaction using labeled phages infecting Gram-negative (SOM4) and Gram-positive (ɸ11) bacteria was assessed by flow cytometry. We observed an increased phagocytosis at the highest phage concentration used (1/5) compared with the lowest phage concentration (1/20) and at 40 min of incubation (Figure 3(a)). Since phagocytosis and unspecific binding of phages to the cell surface cannot be distinguished by flow cytometry, we repeated the experiment at 4°C, a temperature at which phagocytosis activity does not take place, to confirm that monocytes efficiently phagocyte phages in an early and dose-dependent process (Figure 3(b)).
Figure 3.
Phagocytosis of Gram-negative and Gram-positive phages by monocytes. (a) Kinetic assay of phagocytosis: Percentage of phagocytic monocytes cultured with 1/5, 1/10, and 1/20 of SYBR-Gold-labeled Gram-negative (SOM4) and Gram-positive (ɸ11) phage for 20 and 40 min at 37°C. Phagocytosis was determined using flow cytometry and was expressed as the percentage of phagocytic monocytes stained with anti-CD14. *<0.05, Phagocytosis 1/5 vs. 1/10 or 1/20. (b) Temperature assay to determine the non-specific phagocytosis of phages: monocytes were cultured with 1/5, 1/10, and 1/20 of SYBR-Gold-labeled Gram-negative (SOM4) and Gram-positive (ɸ11) phage for 40 min at 4°C to inhibit the mechanism of phagocytosis. *<0.05, **<0.01, Phagocytosis at 37°C vs. phagocytosis at 4°C. (A color version of this figure is available in the online journal.)
Discussion
In the present study, by using butanol-purified phages, we have demonstrated that phages are able to induce in monocytes a tolerant immune response after being phagocytosed. This response is similar to that observed in our previous paper about ascitic monocytes from cirrhotic patients. It is mainly characterized by a reduction in the expression of CD14 and CD86 and an increase in the soluble TNF-α and IL-10 levels. Furthermore, we have validated the butanol purification as a useful protocol to be used in cell stimulation assays without any interference of free endotoxin in phage suspensions.
The reduction in CD14 and CD86 observed on monocytes stimulated by phages suggest a strategy of phages to avoid being removed by the immune system through turning cells into tolerogenic state. HLA-DR expression was also down-regulated by ɸ11 phage contaminated with E. coli. During an infection, it is likely that the tolerogenic monocytes induced by phages favor the infection progression. Our previous results in ascitic monocytes from infected SBP patients showed a similarly reduced expression of CD14, HLA-DR, and CD86 7 than the response observed in healthy monocytes stimulated by phages. Particularly, low HLA-DR levels in the ascitic monocytes from SBP with the negative bacteriological result were associated with a high bacterial DNA burden.15 However, studies with isolated phages from infected fluids are needed to better understand their role during the infection. It is also well known that soluble LPS from Gram-negative bacteria is a potent inductor of the immune response. Our results demonstrate that polyB did not revert the changes induced by phage suspensions in monocytes. Therefore, our work highlight the use of polyB as an easy strategy to validate that the changes observed in immune cells after phage stimulations are not affected by contaminating soluble LPS in phage suspensions.
We did not observe significant differences in the immune response induced in monocytes by the different phages infecting the different bacterial hosts. Van Belleghem et al. have also observed comparable induced immune responses by Gram-negative and Gram-positive phages.16 One possibility is that the changes in CD14, HLA-DR, and CD86 expression are induced by phage proteins common in all these phages. In any case, differences attributable to remaining LPS fragments from the host bacteria in the phage suspensions can be ruled out.
In our assays, we were not able to detect IFN-α levels. This fact could be surprising since monocytes and dendritic cells are able to produce type I IFN during viral infections. However, it could be explained by the fact that IFN-α needs shorter stimulation times to be detected.17 We found that phage suspensions increased the TNF-α and IL-10 levels. IL-10 results are in line with the findings in cirrhosis, since SBP patients had also an increased ascitic IL-10 levels compared with patients without ascitic infection. However, patients with SBP did not show any difference in TNF-α levels compared with patients without ascitic infection.7 This finding can be explained by the elevated production of inflammatory mediators that patients with cirrhosis display regardless of any ascitic infection.18 According to our results, both pro- and anti-inflammatory gene expression profiles of PBMCs stimulated with CsCl-purified phages are reported,16 supporting the results about the immunogenicity of phages. Taking together the phenotypic changes and the soluble factors induced, we found a negative correlation between CD86 and TNF-α levels. This finding is consistent with the downregulation of CD86 on monocytes by TNF-α described in patients infected by human immunodeficiency virus (HIV). This phenotypic change induces an altered production of IL-2 and, consequently, results in a deficient proliferative response of lymphocytes.19 It is likely that phages in SBP induce soluble factors that contribute to the tolerant state of ascitic monocytes and, consequently, favor the infection.
We have validated a flow cytometry assay to study the phage-monocyte interaction through phagocytosis assays with labeled phage suspensions. We have observed the phagocytosis of phages by monocytes at short times. According to other reports using microscopic analysis, phagocytosis of phages is an early process since, at longer times of incubation, the intracellular destruction of phages begins.20 In line with these results, we did not detect phagocytosis of phages at longer times of incubation (120 min). In cirrhosis, we have previously shown that ascitic monocytes from patients with SBP had impaired phagocytosis of E. coli.7 It is possible that the tolerant state induced by phages also contributes to the impaired phagocytosis of monocytes against bacterial infection. The outcome for the phages is to reduce their own elimination by monocytes while promoting the survival of their bacterial host.
Finally, our work suggests that phages infecting Gram-negative and Gram-positive bacteria turn healthy monocytes into a tolerogenic state. We can speculate that in certain infection-associated pathologies, such as cirrhosis, not only bacteria but also phages could induce an immune response in monocytes to avoid the clearance of the infection. However, further studies using isolated phages from infected fluids are needed to support our hypothesis.
Supplemental Material
Supplemental material, sj-pdf-1-ebm-10.1177_1535370221995154 for Bacteriophages immunomodulate the response of monocytes by Lídia Perea, Lorena Rodríguez-Rubio, Juan C Nieto, Carlos Zamora, Elisabet Cantó, German Soriano, Maria Poca, Pedro Blanco-Picazo, Ferran Navarro, Maite Muniesa and Silvia Vidal in Experimental Biology and Medicine
Footnotes
AUTHORS’ CONTRIBUTIONS: Conceptualization, LP, LR-R, FN, MM and SV; methodology, PB-P, LR-R, LP, JCN, CZ, EC, GS, MP; software analysis, LP, LR-R; validation, LP, LR-R; formal analysis, LP, LR-R, PB-P, JCN, CZ, EC, GS, MP; investigation, LP, LR-R, PB-P, JCN, CZ, EC, GS, MP; resources, SV, FN, MM; data curation, LP, LR-R, PB-P, JCN, CZ, EC, GS, MP; writing—original draft preparation, LP, LR-R, MM, SV; writing—review and editing, LP, L R-R, MM, FN, SV; visualization, LP, LR-R, PB-P, JCN, CZ, EC, GS, MP; supervision, MM, SV, FN; project administration, SV; funding acquisition, SV, MM All authors have read and agreed to the published version of the manuscript.
DECLARATION OF CONFLICTING INTERESTS: The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
FUNDING: The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: This research was supported by the Spanish Ministerio de Innovación y Ciencia [AGL2016-75536-P]; the Agencia Estatal de Investigación (AEI); the European regional fund (ERF); and the Generalitat de Catalunya [2017SGR170]. P.B.-P. has a grant from the Spanish Ministry of Economy, Industry and Competitiveness [BES-2017–081296]. L.R-R. is a Serra Húnter Fellow.
ORCID iDs: Lídia Perea https://orcid.org/0000-0002-1624-0012
Ferran Navarro https://orcid.org/0000-0002-4302-2838
References
- 1.Blanco-Picazo P, Fernández-Orth D, Brown-Jaque M, Miró E, Espinal P, Rodríguez-Rubio L, Muniesa M, Navarro F.Unravelling the consequences of the bacteriophages in human samples. Sci Rep 2020; 10:6737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Brown-Jaque M, Muniesa M, Navarro F.Bacteriophages in clinical samples can interfere with microbiological diagnostic tools. Sci Rep 2016; 6:33000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Guarner C, Runyon BA, Young S, Heck M, Sheikh MY.Intestinal bacterial overgrowth and bacterial translocation in cirrhotic rats with ascites. J Hepatol 1997; 26:1372–8 [DOI] [PubMed] [Google Scholar]
- 4.Căruntu FA, Benea L.Spontaneous bacterial peritonitis: pathogenesis, diagnosis, treatment. J Gastrointestin Liver Dis 2006; 15:51–6 [PubMed] [Google Scholar]
- 5.Campbell A.The future of bacteriophage biology. Nat Rev Genet 2003; 4:1–7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.D Browska K, Miernikiewicz P, Piotrowicz A, Hodyra K, Owczarek B, Lecion D, Ka Mierczak Z, Letarov A, Gorski A.Immunogenicity studies of proteins forming the T4 phage head surface. J Virol 2014; 88:12551–7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Nieto JC, Sanchez E, Romero C, Roman E, Poca M, Guarner C, Juarez C, Soriano G, Vidal S.Impaired innate immune response of leukocytes from ascitic fluid of patients with spontaneous bacterial peritonitis. J Leukoc Biol 2015; 98:819–25 [DOI] [PubMed] [Google Scholar]
- 8.Kreiswirth BN, Löfdahl S, Betley MJ, O’reilly M, Schlievert PM, Bergdoll MS, Novick RP.The toxic shock syndrome exotoxin structural gene is not detectably transmitted by a prophage. Nature 1983; 305:709–12 [DOI] [PubMed] [Google Scholar]
- 9.Muniesa M, Mocé-Llivina L, Katayama H, Jofre J.Bacterial host strains that support replication of somatic coliphages. Antonie Van Leeuwenhoek 2003; 83:305–15 [DOI] [PubMed] [Google Scholar]
- 10.Ubeda C, Maiques E, Knecht E, Lasa I, Novick RP, Penadés JR.Antibiotic-induced SOS response promotes horizontal dissemination of pathogenicity island-encoded virulence factors in staphylococci. Mol Microbiol 2005; 56:836–44 [DOI] [PubMed] [Google Scholar]
- 11.Szermer-Olearnik B, Boratyński J.Removal of endotoxins from bacteriophage preparations by extraction with organic solvents. PLoS One 2015; 10:e0122672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Adams M.Bacteriophages. New York: Interscience Publishers, Inc., 1959 [Google Scholar]
- 13.Mosier-Boss PA, Lieberman SH, Andrews JM, Rohwer FL, Wegley LE, Breitbart M.Use of fluorescently labeled phage in the detection and identification of bacterial species. Appl Spectrosc 2003; 57:1138–44 [DOI] [PubMed] [Google Scholar]
- 14.Lin S-M, Frevert CW, Kajikawa O, Wurfel MM, Ballman K, Mongovin S, Wong VA, Selk A, Martin TR.Differential regulation of membrane CD14 expression and endotoxin-tolerance in alveolar macrophages. Am J Respir Cell Mol Biol 2014; 31:162–70 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Fagan KJ, Rogers GB, Melino M, Arthur DM, Costello ME, Morrison M, Powell EE, Irvine KM.Ascites bacterial burden and immune cell profile are associated with poor clinical outcomes in the absence of overt infection. PLoS One 2015; 10:e0120642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Van Belleghem JD, Clement F, Merabishvili M, Lavigne R, Vaneechoutte M.Pro- and anti-inflammatory responses of peripheral blood mononuclear cells induced by Staphylococcus aureus and Pseudomonas aeruginosa phages. Sci Rep 2017; 7:8004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ali S, Mann-Nüttel R, Schulze A, Richter L, Alferink J, Scheu S.Sources of type I interferons in infectious immunity: plasmacytoid dendritic cells not always in the driver’s seat. Front Immunol 2019; 10:778. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Giròn-González JA, Rodríguez-Ramos C, Elvira J, Galán F, Del Álamo CFG, D́az F, Martín-Herrera L.Serial analysis of serum and ascitic fluid levels of soluble adhesion molecules and chemokines in patients with spontaneous bacterial peritonitis. Clin Exp Immunol 2001; 123:56–61 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kumar A, Angels JB, Aucoin S, Creery WD, Daftarian MP, Cameron DW, Filion I, Diaz-Mitoma F.Dysregulation of B7.2 (CD86) expression on monocytes of HIV-infected individuals is associated with altered production of IL-2. Clin Exp Immunol 1999; 117:84–91 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Aronow R, Danon D, Shahar A, Aronson M.Electron microscopy of in vitro endocytosis of T2 phage by cells from rabbit peritoneal exudate. J Exp Med 1964; 120:943–54 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental material, sj-pdf-1-ebm-10.1177_1535370221995154 for Bacteriophages immunomodulate the response of monocytes by Lídia Perea, Lorena Rodríguez-Rubio, Juan C Nieto, Carlos Zamora, Elisabet Cantó, German Soriano, Maria Poca, Pedro Blanco-Picazo, Ferran Navarro, Maite Muniesa and Silvia Vidal in Experimental Biology and Medicine