Abstract
This experimental research aimed to investigate the effects of non-thermal plasma on nerve regeneration after transected nerve damage using the sciatic nerve in Wistar albino (A) rats. The experiments were performed on 27 Wistar A rats. The rats underwent surgery for right sciatic nerve exposure and were divided into three groups (each group, n = 9) according to sciatic nerve transected injury (SNTI) and non-thermal plasma application: a non-nerve damage (non-ND) group, a only nerve damage without non-thermal plasma application (ND) group, and a nerve damage with non-thermal plasma application (ND + NTP) group. Subsequent to SNTI and immediate suture, non-thermal plasma was administered three times per week for eight weeks. Evaluation for functional recovery was performed using the static sciatic index measured over the full treatment period of eight weeks. The sciatic nerve specimens were obtained after euthanasia and third day from the last non-thermal plasma application. The sciatic nerve tissues were subjected to histological analysis. Behavior analysis presented that the ND + NTP group showed improved static sciatic index compared with the nerve damage group. Histopathological findings demonstrated that the ND + NTP group had more dense Schwann cells and well-established continuity of nerve fibers, greater than the nerve damage group. Immunohistochemistry showed that the ND + NTP group had increased levels of markers for microtubule-associated protein 2 (MAP2), tau, S100 calcium-binding protein B, and neurofilament-200 and regulated the overexpression of CD68 and MAP2. These results indicated that non-thermal plasma enhanced the motor function and restored the neuronal structure by accelerating myelination and axonal regeneration. Additionally, non-thermal plasma was confirmed to have a positive effect on the recovery of SNTI in rats.
Keywords: Nerve regeneration, nerve repair, non-thermal atmospheric-pressure plasma, cold plasma, plasma medicine, sciatic nerve injury of rat
Impact statement
The regeneration of injured peripheral nerve is rare, which thereby impairs the associated motor, sensory, and autonomic functions of the nerve. To date, several treatment modalities have been introduced, but none have shown promising results. The standard treatment of injured peripheral nerve is via a direct suture and/or an autologous nerve graft. However, in addition to the limitations of functional recovery, nerve harvesting complications can also occur. This is the first study to confirm the effect of NTP on transected injured nerve. The novel findings of this study are that NTP application has the ability to enhance the recovery of the injured nerve and minimize the complications of donor site due to autologous nerve graft. Thus, NTP could have a positive impact on clinical practice for better treatment outcome of peripheral nerve injury in the future. Further research in this field would be of great help for patients suffering from peripheral nerve damage.
Introduction
Peripheral nerve injury (PNI) usually results from trauma, iatrogenic surgical injury, or diseases such as tumors, cystic lesions, or inflammation. PNI can lead to demyelination and axonal degeneration of the peripheral nerves. A complete regeneration of a damaged peripheral nerve is rare.1,2 Hence, there is impairment of the associated motor, sensory, and autonomic functions of affected nerve. Moreover, the resultant pain, altered sensations, and muscle paralysis can compromise the patient’s quality of life.3,4 Currently, researches are being conducted to develop treatment methods to aid recovery of posttraumatic nerve damage and reduce iatrogenic surgical nerve damage, but there are few satisfactory results thus far. The effectiveness of different treatment modalities using laser, electricity, ultrasound, and various pharmacological agents, such as steroids, vitamin B, alpha lipoic acid, nimodipine, to provide functional recovery from PNI before or after surgery has been studied, but have failed to provide promising results.5–7 Thus, direct surgical suture, autologous nerve graft, and nerve bridging remain the clinical standards of treatment for addressing nerve damage. However, these are microsurgical procedures requiring expert microsurgeons and dedicated operating rooms, and hence cannot be performed in general clinics. Moreover, surgical outcomes from the above-mentioned procedures are poor and include the risks of painful complications, such as loss of function, sensory disturbances, neuralgia, or neuroma formation, at the site of nerve harvest.8,9 Therefore, there is an urgent need for a treatment that would enable functional and histological recovery of the damaged nerve without donor site complications.
Plasma is defined as “the fourth phase of matter” along with solids, liquids, and gases. In the 1850s, plasma was first used to make ozone and has been used in biomedical, environmental, agricultural, and other domains since the mid-1990s.10–13 The application of low-temperature atmospheric-pressure plasma has been used as bactericidal. Medically used plasma is referred as cold plasma or non-thermal atmospheric-pressure plasma (NTP) because it stays at room temperature, as compared to other plasma which requires temperatures of 3000°C or higher. The mechanism of action of NTP can be described by the biological effect of reactive oxygen and nitrogen species on the cells and tissues produced by NTP.14,15 Recently, there have been wide applications of NTP, including sterilization, disinfection, wound healing, oncology, pharmacology, and enhanced biocompatibility of implants.16–18 The beneficial effects of NTP on cutaneous wounds and muscle regeneration have been reported; however, its effects on the physically injured nerves are yet to be studied. As NTP has been demonstrated to affect not only the surface but also the deep tissues,19,20 it would be reasonable to assume that NTP could also affect the thinly distributed peripheral branches of nerves.
In a previous study, which was the first experiment to investigate the therapeutic effects of NTP on peripheral nerve injuries, axonotmesis was reproduced by administering a crush injury to the sciatic nerve (SN) of rats, yielding a physiological response similar to that in human peripheral nerves following this type of injury.21 The study revealed that plasma treatment enhances functional recovery and neuronal regeneration. In addition, treatment with NTP also accelerates muscle healing.
This experimental study aims to investigate the effects of NTP on nerve recovery after neurotmesis in a rat model with sciatic nerve transected injury (SNTI). We explored the application of NTP to treat nerve damage as a possible therapeutic method using the static sciatic index (SSI), histological analysis, and immunofluorescence (IF) analysis. This is only the second study to investigate the effect of NTP on regeneration of injured peripheral nerve, and the first to do so using a rat model of SNTI.
Materials and methods
NTP generating device
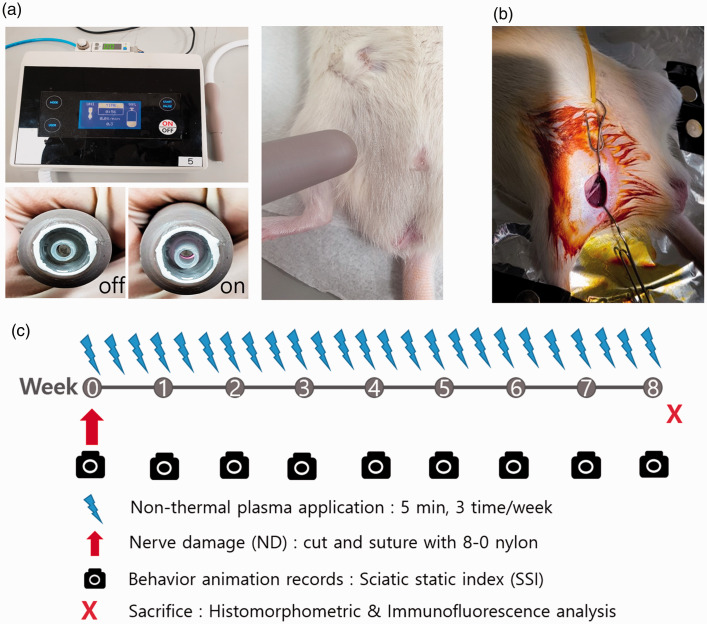
NTP was generated using equipment produced by the Feagle Corporation (Yangsan-si, Gyeonsangnam-do, Republic of Korea) for the treatment of nerve injury (Figure 1(a)). A plasma jet generating module in this equipment was a coaxial dielectric barrier discharge (DBD)-type, and had two electrodes and one dielectric. A single, medium gas-based NTP ejecting module generates a plasma flow within the electrodes. The target temperature of the flow at the end of the electrodes was 35°C for 10 min, and ultraviolet emissions were not detectable.
Figure 1.
(a) Shows the NTP (argon-based coaxial-DBD plasma)-generating device used in this study. NTP application was done for 5 min and 5 mm away from the surgical wound. (b) Surgical field for anastomosis of the transected sciatic nerve (right hind limb). The surgical procedure was performed through the ventral approach according to the preference of a single operator, considering the operability of the instrument and the accessibility of the surgery during anastomosis. The technique was as follows: Transection of sciatic nerve with metzenbaum scissor, followed by two epineural sutures with 8–0 nylon. (c) Experiment schedule of NTP application, nerve damage, and behavior animation records for the static sciatic index (SSI), sacrifice for histological and immunological evaluations. (A color version of this figure is available in the online journal.)
Type of medium gas and the time of application
In order to select the plasma-generating medium gas to be used in this experiment, optical emission spectroscopy (OES) to find out the gas composition and an in vitro experiment to compare the differentiation ability of the SH-SY5Y neuroblastoma cell line into neurons were performed using helium and argon gas. OES was performed under the condition of the same gas pressure and voltage. The majority of the beneficial active species in the argon-based NTP were generated much more than the helium-based NTP. In an in vitro experiment in which NTP was applied to SH-SY5Y cells at various times, it was confirmed that while helium-based NTP further inhibited the growth of normal cells, the cell length promoting effect that appeared during cell differentiation was remarkably effective in argon-based NTP.
Based on these results, it was set as the basic condition to treat the argon-based NTP for 5 min. This condition was also applied to the previous study with sciatic nerve crush injury (SNCI) model and had good recovery effect.21 Additional information regarding the NTP-generating device and details of the NTP application protocol have been provided in previous studies.22–24
Animal model setup and NTP treatment
Twenty-seven male Wistar A rats (eight weeks old; 250–300 g weight roughly) were obtained from Samtako Bio (Osan-si, Gyeonggi-do, Republic of Korea). Wistar A rats are ideal for behavioral analyses as they are more active and less aggressive than other breeds, and they have large feet and well-defined toes.24 The distance between the nerves and skin is less in the rat, so NTP can be applied without re-incision. We used young male rats to investigate the effects of NTP in order to minimize the effects of sex-related hormone differences on peripheral nerve regeneration.25 All experiments adhered to the ethical guidelines of the Pusan National University Institutional Animal Care and Use Committee (South Korea) and regulations set out by the International Association for Study of Pain in Animals (ED-PNU2017-0183).
An intraperitoneal injection of a cocktail of 100 mg/kg of ketamine (Yuhan, Seoul, South Korea) and 10 mg/kg of xylazine (Rompun; Bayer Korea Ltd, Seoul, South Korea) was administered to anesthetize the animals. Local anesthesia in the surgical field was provided via a subcutaneous injection of 0.3 mL of a solution containing 1:100,000 epinephrine and 2% lidocaine hydrochloride (Huons, Seoul, South Korea). Cefazolin (50 mg/kg intramuscular; Yuhan, Seoul, South Korea) was injected prior to surgery as a preventive antibiotic. After confirmation of complete anesthesia, the limbs of the rats were tied up in a prone position on a sterilized surgical plate. The surgical field on the right limb of the rat was shaved with electrical clipper and scrubbed with povidone-iodine solution for disinfection.
Experiments were carried out in three groups (each group, n = 9): non-nerve damage (non-ND), nerve damage (ND), and nerve damage with NTP treatment (ND + NTP). The non-ND group served as a placebo group and received a sham operation in which a fake surgical procedure was performed conducted with the same steps as the real procedure but without ND. This group served as a means to evaluate the extent to which skin and muscle damage can affect functional impairment and recovery. In the ND group, an identical surgical procedure was performed along with the SN transection and immediate anastomosis with 8–0 nylon 2-point epineural suture. The NTP treatment was not given to this group. The ND + NTP group underwent the same surgical procedure as the ND group, but additionally received NTP to the skin overlying the damaged SN (Table 1). With the rats anesthetized, NTP was irradiated 0.5 cm away from the skin overlying the SN for 5 min. Considering the general anesthesia of experimental animal for NTP application, NTP was irradiated three times a week for eight weeks (Figure 1(b) and (c)).
Table 1.
Experimental and control groups, the criteria that determine them.
| Exposure of sciatic nerve | Transected injury of sciatic nerve | NTP Applying | Number of rats | |
|---|---|---|---|---|
| Non-ND | O | X | X | 9 |
| ND | O | O | X | 9 |
| ND-NTP | O | O | O | 9 |
Non-ND: non-nerve damage with sham operation; ND: transected injury and 2point epineural suture without NTP treatment; ND+NTP: transected injury and 2point epineural suture with NTP treatment.
Functional analysis and SSI scoring
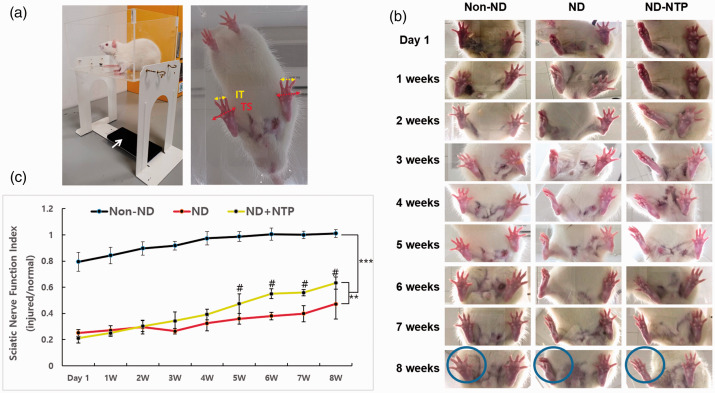
Functional data were recorded before surgery to check rat activity and to acclimatize them to the customized transparent box. Functional analysis was conducted once a week for eight weeks. The customized box composed of a transparent acrylic plate 25 × 16 × 12 cm in size (Figure 2(a)). A cellular phone camera (i-Phone 6S Plus; Apple Inc., Cupertino, CA) was placed 25 cm from the bottom of the box. Video recordings were made three days after the SNTI operation (Friday) in the first week and then every Wednesday for eight weeks. Rats were placed into the box gently and free to move independently in the clear acrylic box. Video clips were recorded for 1 min to obtain natural foot shape after more than 5 min to get used to a new circumstance before recording. It was recorded only during daytime in a quiet and independent room with not too bright light.
Figure 2.
The effect of NTP on sciatic nerve transected injury recovery. (a) Experimental setup of animal, smart phone camera (white arrow), and transparent box for the animation video recording. Captured image for SSI calculation; IT (intermediary toe spread): 2nd-4th toe spread distance, yellow and solid line, TS (total toe spread): 1st–5th toe spread distance, red and dotted line. (b) Captured images using smartphone video recording of animal free movement for SSI analysis after sciatic nerve transected injury procedure. Note the circle of injured right hind limb. (c) Statistically significant improvement of injured-to-normal ratio to date (SSI results) was observed in NTP-treated groups at the last day of the study period. Data shown are representative of each group (n = 9), **P < 0.01, ***P < 0.001 (two-way ANOVA for repeated measures followed by Bonferroni post hoc test), #P < 0.05 when compare with ND group. (A color version of this figure is available in the online journal.)
The SSI with a static video record analysis proposed by Bervar was used as a for functional recovery analysis.26 Measurements for SSI were taken from prints of captured images (Figure 1(d)).
SSI formula = 108.44 × TSxF + 31.85 × ITxF − 5.49
TS: Total toe spread, 1st–5th toe spread distance (mm)
IT: Intermediary toe spread, 2nd–4th toe spread distance (mm)
F = 563.357 is a constant value
The relative SSI values were estimated with the SSI values of both sides (no surgical and surgical side) of hind limbs to evaluate the degree of recovery of the nerve function after the SNTI procedure.
Histopathological findings
All rats were sacrificed one day after their last NTP application. Specimens from the SN of the experimental surgical field were extracted. All specimens were treated with 4% paraformaldehyde for one day to fix and then paraffin-embedding and transected into 5 μm slices followed by hematoxylin and eosin (H&E) stain. Histopathological evaluation of nerve tissues was conducted by examining pictures taken with an iCM 9.0 digital camera system (IMT i-Solution Inc., Rochester, NY) under light microscopy (CX31; Olympus, Tokyo, Japan).
IF analysis
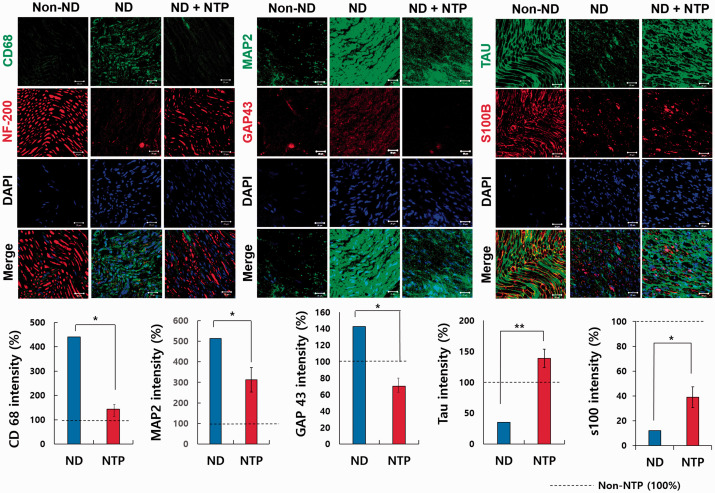
IF staining was conducted using antibodies of various markers to evaluate the recovery of transected SN after SNTI. The 5-μm nerve tissue sections were dealt with antibodies against myelin basic protein (MBP) (1:100; Santa Cruz Biotechnology/sc-271524, Santa Cruz, CA) and neurofilament 200 (NF-200) (1:100; Abcam/ab8135, Cambridge, MA) for 2 h at 37°C. Samples were then washed four times with phosphate-buffered saline and dealt with anti-mouse Alexa Fluor-488 and anti-rabbit Alexa Fluor-594 dyes (1:100; Thermo Fisher Scientific, Rockford, IL) for 1 h at 37°C. Then, 4′,6-diamidino-2-phenylindole (DAPI) counterstaining to visualize cell nuclei was followed. MBP as a myelin sheath marker, NF-200 as a marker for large myelinated A-fiber neurons, and DAPI as a marker for the number of nuclei were observed in the captured images. All tissue sections were also treated with antibodies against CD68 (1:100; Santa Cruz Biotechnology/sc-20060, Santa Cruz, CA), growth-associated protein-43 (GAP-43) (1:500; Abcam/ab75810, Cambridge, United Kingdom), microtubule-associated protein 2 (MAP2) (1:100, Santa Cruz Biotechnology/sc-74421, Santa Cruz, CA), S100 calcium-binding protein B (S100B) (1:100, Abcam/ab868, Cambridge, United Kingdom), tau protein (tau) (2 ug/mL; Abcam/ab80579, Cambridge, United Kingdom). CD68 was used as a phagocytic marker for monocytes and macrophage. GAP43 was used as a marker for growth and regenerating neurons and was associated with the regulation of axonal growth and plasticity. MAP2 was used as a marker on neuronal cells, their perikaryal, and neuronal dendrites. Tau was used as a marker on neuronal axons. S100B was used as a marker for assessing the proliferation of Schwann cells.
The fluorescence image of the tissue section in each group (n = 9) was obtained under a Carl Zeiss LSM 780 confocal laser microscope. Acquired image was analyzed using ImageJ program (NIH- https://rsb.info.nih.gov/ij/). The basic functions of ImageJ software were used, and no additional plug-in was used. The intensity of fluorescence in the suture area was measured, and the non-ND group was set as 100%, and a relative value was determined as the fluorescence intensity of ND and ND + NTP group.
Data analysis and statistics
To evaluate the reproducibility of the SSI, the measurements of two observers who were not previously trained were compared with the measurement taken by the principal observer. The evaluation was repeated with collected measurements from five footprints within a two-week interval.
Experimental results are recorded as the mean ± SEM for every group. Data analysis was accessed with SPSS software (version 12.0; SPSS Inc., Chicago, IL). Functional analyses were conducted with two-way analysis of variance (ANOVA) for repeated measures followed by post hoc test with Bonferroni method for pairwise comparisons to find out the influence of time and treatments, and time point comparisons were carried out by the least significant difference. Immunofluorescence staining measurement of the ND and ND + NTP groups was analyzed statistically with Mann–Whitney U test. A P value under 0.05(*) was decided to have a statistically significant difference.
Results
NTP promoted the functional improvement of the injured nerve after SNTI
Foot print analysis was evaluated to address functional recovery after SNTI (Table 2). In the non-ND group, the right hind limb function (SNTI-operated side) was virtually fully recovered within one week. However, only little difference appeared until two weeks after the operation in the ND and ND + NTP groups (Figure 2(b)). SSI differences between the ND and ND + NTP groups were observed since week 4. After eight weeks, the ND + NTP group was able to spread the toes more clearly compared with the ND group. And, the function of ND + NTP group was restored to almost 60%, whereas that of ND group was only at 47% based on SSI scores (Figure 2(c)). Although the SN function of the ND + NTP group was not showed to be restored completely until eight weeks, the overall recovery tendency of the sciatic function was statistically different with the ND group (Figure 2(c)). Application of NTP promoted functional recovery over time.
Table 2.
RM ANOVA for effect of NTP treatment and time on SFI.
| Type III SS | Df | MS | F | sig. | |
|---|---|---|---|---|---|
| NTP treatment | 0.881 | 2 | 0.19 | 404.523 | 0.000 |
| Time | 0.971 | 4.114 | 0.236 | 78.736 | 0.000 |
| Interaction | 0.211 | 8.228 | 0.026 | 8.544 | 0.000 |
RM ANOVA: repeated measures analysis of variance; SS: sum of squares; df: degrees of freedom; MS: mean squares, based on Greenhouse–Geisser correction.
NTP was helpful in the recovery of damaged SN tissue after SNTI and the restoration of the continuity of the transected SN
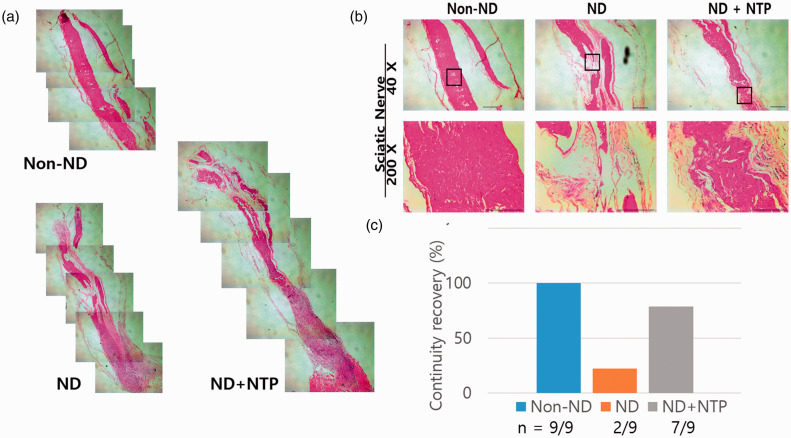
The difference in recovery from SNTI between the three groups after eight weeks is shown in Figure 3(a). After SNTI, the anastomosis site was observed under a light microscope using 40× magnification image of the stained samples. SN tissues that appeared eosinophilic in H&E stain represented an axon and myelin sheath.27 In the non-ND group, which underwent only skin and muscle incision and nerve exposure without SNTI, no specific damage to SN tissues was observed, and the arrangement and continuity of SN fiber was well maintained. When observed at low magnification (40×), there was a slight defect in the anastomosis site after SNTI in the ND + NTP group, but a continuity of SN tissue was restored, as in the non-ND group. A continuity of SN tissue was not observed in the ND group (Figure 3(b)). The number and density of SN fibers in the anastomosis site after SNTI were presented similarly in the ND + NTP group and non-ND group. When observed under high magnification (200×), the SN cell nuclei were presented between the nerve fibers. The nerve fiber in the ND + NTP group was restored with intact continuity, and arranged densely. However, the nuclei of SN cell in ND group were not well observed. The nerve fiber of the ND group was relatively short, and arranged loosely. The differences in arrangement of nerve fibers were also clearly visible. In the ND + NTP group, the arrangement of neurons was more regular and well-structured, whereas in the ND group, it was irregular and edematous. The whole tissue (n = 9) was examined, and histological continuity was confirmed in seven of the ND + NTP group, and continuity was confirmed in two of the ND group.
Figure 3.
(a) SN tissues with hematoxylin and eosin (H&E) staining were presented. (b) NTP effectively recovered the tissue density. The photographs were taken using optical microscopy at 40× and 200× magnification. Data shown are representative of each group (n = 9). (c) The continuity of the transected sciatic nerve was restored in ND + NTP group more than ND group. (A color version of this figure is available in the online journal.)
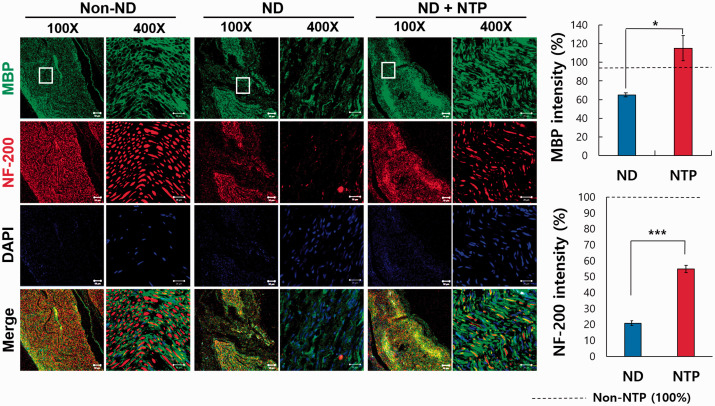
In the merged images taken after the last NTP treatment, the nerves in the ND + NTP group that had been transected were restored structurally, appeared similar to the non-injured nerves in the non-ND group, and were significantly more restored than in the ND group (Figures 4 and 5). When observed at a low magnification, the transected area in the ND + NTP group was continuously immune-stained. In the ND group, however, no conspicuous continuity was detected, as in ND + NTP group.
Figure 4.
Immunofluorescence in the neural tissue in three groups: MBP, NF-200, and DAPI. IF assay of SN tissue with anti- NF-200 and MBP antibodies was done to identify the recovery of neuronal axon and myelin sheath. The white box indicates the transected site. The representative photographs of each group (n = 9) were taken using confocal microscopy. Scale bars at the bottom of each figure indicate 50 μm (white solid line), 20 μm (white line) (*, P < 0.05; ***, P < 0.001, Mann–Whitney U test, n = 9 in each groups). (A color version of this figure is available in the online journal.)
Figure 5.
Immunofluorescence in the neural tissue in three groups (×400): CD68, MAP2, GAP43, S100B, and tau. The representative photographs of each group (n = 9) were taken using confocal microscopy. Scale bars (white line) at the bottom of each figure indicate 20 μm (*, P < 0.05; **, P < 0.01, Mann–Whitney U test, n = 9 in each groups). (A color version of this figure is available in the online journal.)
NTP can enhance the axonal and myelin sheath regeneration of damaged SN by regulating the expression of factors related to nerve regeneration
To investigate the functional restoration of the SNs after SNTI at the molecular level, the SN tissues were observed after IF staining against MBP,28,29 NF-200,29,30 S100B31 as markers of axon filament and myelination of Schwann cells, and tau protein which maintains the stability of the microtubule and modulates Schwann cell proliferation, migration, differentiation, and axonal regeneration after PNI.32,33
The MBP expression of the ND + NTP group was observed to be similar to that in the non-ND group (Figure 4), but was not well recognized in the ND group. The expression of NF-200 was also manifested more distinctly in the ND + NTP group and was similar to the expression of NF-200 in the no-injury non-ND group. NF-200 expression was difficult to observe in the ND group. MAP and NF-200 were expressed significantly more in the ND + NTP group than the ND group. S100B was expressed less in the ND group than both the non-ND and ND + NTP groups. However, S100B was expressed more significantly in the ND + NTP group than the ND group. Tau protein was expressed at a comparable level with the non-ND group in the ND + NTP group and significantly more than the ND group (Figure 5). This suggests that NTP promotes the recovery of structure and function of injured SN.
NTP regulated the structural overgrowth of the somatodendritic and axonal regions
The overexpression of CD68 and MAP2 might be implicated in the formation of neuroma,34–36 and GAP43 might lead to aberrant axonal sprouting. CD68 and MAP2 were significantly overexpressed in the ND group in comparison with the non-ND group, but were expressed in the ND + NTP group at a level similar to the non-ND group. Although IF staining of GAP43 was not noticeable in all three groups, quantitative analysis confirmed that it was significantly reduced in the ND + NTP group in comparison with the ND group (Figure 4). This suggests that NTP prevents the formation of neuroma after SNTI.
NTP application prevented the accumulation of CD68 positive macrophage
To investigate the effect of NTP on inflammatory macrophages after the SNTI, the SN tissues were observed under IF staining with antibodies against CD68. Although macrophages play a pivotal role in neural recovery, overexpression slows the recovery of nerves, causes chronic nerve inflammation, and is associated with fibroblast formation.32,37 CD68-positive macrophages increased at the SNTI site of the ND group (Figure 5), which indicate that NTP reduced the macrophage-mediated destruction of the damaged SN and the scar formation.
Discussion
Plasma occurs in nature and can be created in the laboratory by applying high voltage to gas. Artificial plasma is further divided into two types, thermal and non-thermal. Although thermal plasma can damage tissue owing to its high temperature, NTP can maintain the gas close to room temperature and prevent adverse effects on adjacent tissue, rendering it suitable for medical use.24,38,39 Several studies have shown that NTP can interact with biological cells and induce biological outcomes, leading to an increase in the study of biomedical applications of NTP in recent years.40–42 Furthermore, plasma therapy has been used clinically in various medical departments.43–45
The mechanism of action of NTP is explained by the effect of reactive oxygen and nitrogen species generated by NTP on cells and tissues,14,15 and it has been shown to affect not only the surface but also the deep tissues such as muscle and nerve tissue.19,20 It is thus reasonable to assume that NTP can also affect the thinly distributed peripheral branches of nerves. Therefore, we studied the effect of NTP application after SNCI in rats by reproducing PNI in a previous study, which was the first study that used NTP in PNI.21 A microvascular clamp was compressed for 60 s to inflict crush injury, and NTP was applied similarly as in the current study. The results showed that nerve function was completely restored in the ND + NTP group after three weeks, whereas only 60% of the nerve function was restored in the ND group without NTP application. In the ND + NTP group, reduced macrophage accumulation was observed, and the damaged axon fibers and myelin sheaths were regenerated overall. These results showed that NTP application could be helpful in the restoration of damaged peripheral nerves. PNI is classified as neuropraxia, axonotmesis, or neurotmesis depending on the extent of injury. In the current study, we administered SNTI to reproduce neurotmesis, which is the total transection of the entire nerve structure.46,47 This experimental report is the first to investigate the therapeutic effects of NTP on the regeneration of SNTI in rats, and it investigated the recovery of function, as well as histological and immunological features.
Meanwhile, in our previous study, a histological analysis was also conducted on the effect of NTP on the skin and muscle tissue located above the SNCI site where the same application was applied.21,22 Histological examination demonstrated the possibility of promoting wound healing. The muscle tissue of the non-ND and ND groups had a significant decrease in density of muscle tissue and accumulation of mononuclear cells around the nerve repair site. On the contrary, the ND + NTP group had an increase in the diameter of the muscle fibers and a significant decrease in the number of mononuclear cells around the muscle fibers. Choi et al. revealed that nitrogen gas-based NTP treatment promotes damaged muscle healing by accelerating proliferation and differentiation of satellite cell simultaneously with skin wound regeneration in rat model.20 The result of the previous study might also be due to the NTP-mediated satellite cell activation. Furthermore, the result of IF staining for CD68 and type I collagen in our previous study showed significantly increased type I collagen and reduced macrophages in the muscle tissue around the sutured site.21,22 Therefore, NTP might prevent a chronic inflammatory reaction on the damaged muscle tissue.
As our previous study had confirmed the effect of NTP on the muscle, this study did not perform histological examination of the injured muscle above the SNTI site, but instead focused on the analyzing effect of NTP application on the SNTI-treated nerve tissue.
The SSI, introduced by Bervar in 2000, was used to investigate the functional motor recovery of the SN26. The SSI method is convenient for footprint analysis after peripheral ND in rats. In the past, complex equipment and video cameras were required for the analysis; however, this experiment was conducted using the video function of a cellular phone owing to the improved technological functions, such as storage, capture, and image transfer, among others. Using a cellular phone rendered the process easier and more convenient in terms of data acquisition for SSI.48,49
The result of our functional analysis reveals that SNTI leads to about an 80% decrease in motor function one and two weeks after SNTI. The non-ND group, which received a sham operation, had reduced SN function only to 20% in one week and almost recovered at the end of week 2. This 20% loss is likely the result of damage to the skin and muscle tissues. Interestingly, there was approximately 20% to 30% restoration of movement function after transection by anastomosis of the SN alone. In the ND group, the SN function was reduced for the first three weeks after injury, with full function restored after three weeks. The ND + NTP group recovered with the improved rate of functional recovery higher than that of the ND group after the application of NTP.
According to the results of histopathological and immunofluorescence analysis, the application of NTP had a positive effect in promoting the recovery of the structure of SNTI. The application of NTP leads to an increase in MBP, NF-200, S100B, and tau, which are involved in axonal regeneration. NTP also leads to a decrease of CD68, MAP2, and GAP43, which have negative effects on regeneration. In the functional analysis, the SSI of the NTP-treated rats is recovered to 60% of normal by eight weeks, and NTP is found to promote the regeneration of SNTI.
However, there are some limitations in this study. Additional study is needed to observe inflammatory indicator such as TNFα, IL-1α, IL-1β, etc. in order to confirm the capability of NTP to reduce chronic inflammation. Because the number of experimental subjects was relatively small, future experiments should be conducted on more individuals. Studies on larger animals in stages should also be conducted. In addition, because the human peripheral nerve has a more complicated histological structure than rodents and recovers more slowly after surgical treatment, this should also be considered. Additionally, hematoma and scar formation after surgery should also be considered. Moreover, the current study was conducted in the situation of direct anastomosis. However, in accidents resulting in human ND, segmental loss may occur, and treatment in this situation would be performed with artificial nerve growth conduit or nerve graft. Therefore, in the future further studies to investigate the influence of NTP application on the functional and structural restoration of nerves in these various treatment situations is warranted. Thus, patients with peripheral ND can be treated more conservatively if further studies on PNI are performed with due consideration given to the above-mentioned limitations. Further, we can hope to apply NTP treatment in the era of invasive treatments, such as nerve transplantation, with reduced donor site complications and better functional outcomes.
Finally, the present study revealed that the use of NTP can have a significant positive effect on nerve regeneration after PNI. The current results combined with the results of our previous study for an SNCI model show that NTP application can be an alternative in the treatment of nerve injury.
Footnotes
AUTHORS’ CONTRIBUTIONS: All authors participated in the design and interpretation of the studies, analysis of the data, and review of the manuscript. STL and YSJ designed the study, and DSH supervised the study. Most of the experiments and data analysis and interpretation were performed by YSJ, STL, and DSH. The NTP device for this study was presented by GCK, who also advised on the device operation setting. STL and DSH wrote most of the manuscript HJK, MHR, GCK, UKK, and DSH advised it.
DECLARATION OF CONFLICTING INTERESTS: The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
FUNDING: The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: This research was supported by the Basic Science Research Program through the National Research Foundation of Korea funded by the Ministry of Education (No. 2017R1D1A1B03033708).
ORCID iD: Sung-Tak Lee https://orcid.org/0000-0001-6651-8046
References
- 1.Ichihara S, Inada Y, Nakamura T.Artificial nerve tubes and their application for repair of peripheral nerve injury: update of current concepts. Injury 2008; 39:29–39 [DOI] [PubMed] [Google Scholar]
- 2.Reid AJ, de Luca AC, Faroni A, Downes S, Sun M, Terenghi G, Kingham PJ.Long term peripheral nerve regeneration using a novel PCL nerve conduit. Neurosci Lett 2013; 544:125–30 [DOI] [PubMed] [Google Scholar]
- 3.Korompilias AV, Payatakes AH, Beris AE, Vekris MD, Afendras GD, Soucacos PN.Sciatic and peroneal nerve injuries. Microsurgery 2006; 26:288–94 [DOI] [PubMed] [Google Scholar]
- 4.Shahid KR, Dellon AL, Amrami KK, Spinner RJ.Sciatic and peroneal nerve injuries after endovascular ablation of lower extremity varicosities: case reports and review of the literature. Ann Plast Surg 2015; 74:64–8 [DOI] [PubMed] [Google Scholar]
- 5.Terzis JK, Sun DD, Thanos PK.Historical and basic science review: past, present, and future of nerve repair. J Reconstr Microsurg 1997; 13:215–25 [DOI] [PubMed] [Google Scholar]
- 6.Tsuang YH, Liao LW, Chao YH, Sun JS, Cheng CK, Chen MH, Weng PW.Effects of low intensity pulsed ultrasound on rat Schwann cells metabolism. Artif Organs 2011; 35:373–83 [DOI] [PubMed] [Google Scholar]
- 7.Horasanli B, Hasturk AE, Arikan M, Togral G, Helvacioglu F, Dagdeviren A, Mut S, Harman F, Argun G.Comparative evaluation of the electrophysiological, functional and ultrastructural effects of alpha lipoic acid and cyanocobalamin administration in a rat model of sciatic nerve injury. J Back Musculoskelet Rehabil 2017; 30:967–74 [DOI] [PubMed] [Google Scholar]
- 8.Ray WZ, Mackinnon SE.Management of nerve gaps: autografts, allografts, nerve transfers, and end-to-side neurorrhaphy. Exp Neurol 2010; 223:77–85 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Bozkurt A, Lassner F, O'Dey D, Deumens R, Böcker A, Schwendt T, Janzen C, Suschek CV, Tolba R, Kobayashi E, Sellhaus B, Tholl S, Eummelen L, Schügner F, Damink LO, Weis J, Brook GA, Pallua N.The role of microstructured and interconnected pore channels in a collagen-based nerve guide on axonal regeneration in peripheral nerves. Biomaterials 2012; 33:1363–75 [DOI] [PubMed] [Google Scholar]
- 10.Arndt S, Unger P, Wacker E, Shimizu T, Heinlin J, Li YF, Thomas HM, Morfill GE, Zimmermann JL, Bosserhoff AK, Karrer S.Cold atmospheric plasma (CAP) changes gene expression of key molecules of the wound healing machinery and improves wound healing in vitro and in vivo. PLoS One 2013; 8:e79325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Fathollah S, Mirpour S, Mansouri P, Dehpour AR, Ghoranneviss M, Rahimi N, Safaie Naraghi Z, Chalangari R, Chalangari KM.Investigation on the effects of the atmospheric pressure plasma on wound healing in diabetic rats. Sci Rep 2016; 6:19144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Laroussi M.Plasma medicine: a brief introduction. Plasma 2018; 1:47–60 [Google Scholar]
- 13.Babington P, Rajjoub K, Canady J, Siu A, Keidar M, Sherman JH.Use of cold atmospheric plasma in the treatment of cancer. Biointerphases 2015; 10:029403. [DOI] [PubMed] [Google Scholar]
- 14.Tanaka H, Mizuno M, Ishikawa K, Nakamura K, Utsumi F, Kajiyama H, Kano H, Maruyama S, Kikkawa F, Hori M.Cell survival and proliferation signaling pathways are downregulated by plasma activated medium in glioblastoma brain tumor cells. Plasma Med 2012; 2:207–20 [Google Scholar]
- 15.Woedtkea TV, Reuterab S, Masurab K, Weltmann KD.Plasma for medicine. Phys Rep 2013; 530:291–20 [Google Scholar]
- 16.Gazeli K, Noël C, Clément F, Daugé C, Svarnas P, Belmonte T.A study of helium atmospheric-pressure guided streamers for potential biological applications. Plasma Sources Sci Technol 2013; 22:025020 [Google Scholar]
- 17.Kumar N, Attri P, Yadav DK, Choi J, Choi EH, Uhm HS.Induced apoptosis in melanocytes cancer cell and oxidation in biomolecules through deuterium oxide generated from atmospheric pressure non-thermal plasma jet. Sci Rep 2014; 4:7589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kubinova S, Zaviskova K, Uherkova L, Zablotskii V, Churpita O, Lunov O, Dejneka A.Non-thermal air plasma promotes the healing of acute skin wounds in rats. Sci Rep 2017; 24:45183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Heinlin J, Isbary G, Stolz W, Morfill G, Landthaler M, Shimizu T, Steffes B, Nosenko T, Zimmermann J, Karrer S.Plasma applications in medicine with a special focus on dermatology. J Eur Acad Dermatol Venereol 2011; 25:1–11 [DOI] [PubMed] [Google Scholar]
- 20.Choi JW, Kang SU, Kim YE, Park JK, Yang SS, Kim YS, Lee YS, Lee Y, Kim CH.Novel therapeutic effects of monthermal atmospheric pressure plasma for muscle regeneration and differentiation. Sci Rep 2016; 6:28829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Lee HG, Choi JH, Jang YS, Kim UK, Kim GC, Hwang DS.Non-thermal plasma accelerates the healing process of peripheral nerve crush injury in rats. Int J Med Sci 2020; 17:1112–20 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Choi JH, Song YS, Lee HJ, Hong JW, Kim GC.Inhibition of inflammatory reactions in 2,4-Dinitrochlorobenzene induced Nc/nga atopic dermatitis mice by non-thermal plasma. Sci Rep 2016; 6:27376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Choi JH, Lee HW, Lee JK, Hong JW, Kim GC.Low-temperature atmospheric plasma increases the expression of anti-aging genes of skin cells without causing cellular damages. Arch Dermatol Res 2013; 305:133–40 [DOI] [PubMed] [Google Scholar]
- 24.Setsuhara Y.Low-temperature atmospheric-pressure plasma sources for plasma medicine. Arch Biochem Biophys 2016; 605:3–10 [DOI] [PubMed] [Google Scholar]
- 25.Kovacic U, Zele T, Osredkar J, Sketelj J, Bajrović FF.Sex-related differences in the regeneration of sensory axons and recovery of nociception after peripheral nerve crush in the rat. Exp Neurol 2004; 189:94–104 [DOI] [PubMed] [Google Scholar]
- 26.Bervar M.Video analysis of standing–an alternative footprint analysis to assess functional loss following injury to the rat sciatic nerve. J Neurosci Methods 2000; 102:109–16 [DOI] [PubMed] [Google Scholar]
- 27.Ayan H, Yildirim ED, Pappas DD, Sun W.Development of a cold atmospheric pressure microplasma jet for freeform cell printing. Appl Phys Lett 2011; 99:111502 [Google Scholar]
- 28.Li J, Parker B, Martyn C, Natarajan C, Guo J.The PMP22 gene and its related diseases. Mol Neurobiol 2013; 47:673–98 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Amici SA, Dunn WA, Jr, Murphy AJ, Adams NC, Gale NW, Valenzuela DM, Yancopoulos GD, Notterpek L.Peripheral myelin protein 22 is in complex with alpha6beta4 integrin, and its absence alters the Schwann cell basal lamina. J Neurosci 2006; 26:1179–89 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Ruscheweyh R, Forsthuber L, Schoffnegger D, Sandkühler J.Modification of classical neurochemical markers in identified primary afferent neurons with abeta-, adelta-, and C-fibers after chronic constriction injury in mice. J Comp Neurol 2007; 502:325–36 [DOI] [PubMed] [Google Scholar]
- 31.Schmalbruch H.Fiber composition of the rat sciatic nerve. Anat Rec 1986; 215:71–81 [DOI] [PubMed] [Google Scholar]
- 32.Fujiwara S, Hoshikawa S, Ueno T, Hirata M, Saito T, Ikeda T, Kawaguchi H, Nakamura K, Tanaka S, Ogata T.SOX10 transactivates S100B to suppress Schwann cell proliferation and to promote myelination. PLoS One 2014; 9:e115400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Chen P, Piao X, Bonaldo P.Role of macrophages in wallerian degeneration and axonal regeneration after peripheral nerve injury. Acta Neuropathol 2015; 130:605–18 [DOI] [PubMed] [Google Scholar]
- 34.Kiguchi N, Kobayashi D, Saika F, Matsuzaki S, Kishioka S.Inhibition of peripheral macrophages by nicotinic acetylcholine receptor agonists suppresses spinal microglial activation and neuropathic pain in mice with peripheral nerve injury. J Neuroinflammation 2018; 15:96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Shapiro LA, Whitaker-Azmitia PM.Expression levels of cytoskeletal proteins indicate pathological aging of S100B transgenic mice: an immunohistochemical study of MAP-2, drebrin and GAP-43. Brain Res 2004; 1019:39–46 [DOI] [PubMed] [Google Scholar]
- 36.T, Tang L, Lu Y, Zheng W, Li Y.Overexpression of MAP-2 via formation of microtubules plays an important role in the sprouting of mossy fibers in epileptic rats. J Mol Neurosci 2014; 53:103–8 [DOI] [PubMed] [Google Scholar]
- 37.Yi S, Liu Q, Wang X, Qian T, Wang H, Zha G, Yu J, Wang P, Gu X, Chu D, Li S.Tau modulates Schwann cell proliferation, migration and differentiation following peripheral nerve injury. J Cell Sci 2019; 132:jcs222059. [DOI] [PubMed] [Google Scholar]
- 38.Alekseev O, Donovan K, Limonnik V, Azizkhan-Clifford J.Nonthermal dielectric barrier discharge (DBD) plasma suppresses herpes simplex virus type 1 (HSV-1) replication in corneal epithelium. Transl Vis Sci Technol 2014; 3:2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Wagner HE, Brandenburg R, Kozlov KV, Sonnenfeld A, Michel P, Behnke JF.The barrier discharge: basic properties and applications to surface treatment. Vacuum 2003; 71:417–36 [Google Scholar]
- 40.Stoffels E, Flikweert AJ, Stoffels WW, Kroesen GMWl.Plasma needle: a non-destructive atmospheric plasma source for fine surface treatment of (bio)materials. Plasma Sources Sci Technol 2012; 11:383–8 [Google Scholar]
- 41.Fridman G, Friedman G, Gutsol A, Shekhter AB, Vasilets VN, Fridman A.Applied plasma medicine. Plasma Process Polym 2008; 5:503–33 [Google Scholar]
- 42.Laroussi M, Richardson JP, Dobbs FC.Effects of non-equilibrium atmospheric pressure plasmas on the heterotrophic pathways of bacteria and their cell morphology. Appl Phys Lett 2002; 81:772–4 [Google Scholar]
- 43.Laroussi M, Mohades S, Barekzi N.Killing of adherent and non-adherent cancer cells by the plasma pencil. Biointerphases 2015; 10:029410 [Google Scholar]
- 44.Babaeva N, Kushner MJ.Reactive fluxes delivered by dielectric barrier discharge filaments to slightly wounded skin. J Phys D 2013; 46:025401 [Google Scholar]
- 45.Kim JY, Ballato J, Foy P, Hawkins T, Wei Y, Li J, Kim SO.Apoptosis of lung carcinoma cells induced by a flexible optical fiber-based cold microplasma. Biosens Bioelectron 2011; 28:333–8 [DOI] [PubMed] [Google Scholar]
- 46.Sunderland S.A classification of peripheral nerve injuries producing loss of function. Brain 1951; 74:491–516 [DOI] [PubMed] [Google Scholar]
- 47.Zuo J, Neubauer D, Graham J, Krekoski CA, Ferguson TA, Muir D.Regeneration of axons after nerve transection repair is enhanced by degradation of chondroitin sulfate proteoglycan. Exp Neurol 2002; 176:221–8 [DOI] [PubMed] [Google Scholar]
- 48.Baptista AF, Gomes JR, Oliveira JT, Santos SM, Vannier-Santos MA, Martinez AA.New approach to assess function after sciatic nerve lesion in the mouse – adaptation of the sciatic static index. J Neurosci Methods 2007; 161:259–64 [DOI] [PubMed] [Google Scholar]
- 49.Bozkurt A, Tholl S, Wehner S, Tank J, Cortese M, O'Dey Dm Deumens R, Lassner F, Schügner F, Gröger A, Smeets R, Brook G, Pallua N.Evaluation of functional nerve recovery with visual-SSI – a novel computerized approach for the assessment of the static sciatic index SSI. J Neurosci Methods 2008; 170:117–22 [DOI] [PubMed] [Google Scholar]