Abstract
Laryngeal squamous cell cancer (LSCC) is a common carcinoma with high morbidity and mortality. Metastasis constitutes the major cause of death and poor prognosis among patients with LSCC. Recent evidence confirms critical function of Wnt1-inducible signaling protein 1 (WISP1) in several cancers. However, its contribution in LSCC metastasis remains unclear. Specimens of tumor tissues and adjacent normal mucosa were collected from patients with LSCC. The mRNA and protein levels were determined using quantitative real-time PCR and Western blot, respectively. RNA interference was applied to silence the expression of WISP1 and TGF-β, and recombinant adenovirus was used to overexpress WISP1 in human LSCC cell line TU212 cells. Cell invasion and migration were determined by transwell assay. High expression of WISP1 was observed in LSCC tissues, especially in those from metastatic groups. Ectopic expression of WISP1 enhanced invasion and migration of TU212 cells. On the contrary, WISP1 knockdown reduced numbers of invasive and migrated cells. Additionally, elevation of WISP1 depressed the expression of epithelial marker E-cadherin and increased levels of mesenchymal marker vimentin in TU212 cells, whereas WISP suppression yielded the opposite effects. Further analysis corroborated that WISP1 overexpression enhanced activation of TGF-β-Smad signaling by increasing expression of TGF-β1, p-Smad2, and p-Smad3, which was abrogated following WISP1 down-regulation. Moreover, TGF-β1 exposure facilitated LSCC cell invasion and migration. Notably, blockage of the TGF-β-Smad pathway by si-TGF-β overturned WISP-1-evoked epithelial-to-mesenchymal transition (EMT), and subsequent cell invasion and migration. These findings highlight the pro-metastatic function of WISP1 in LSCC by regulating cell invasion and migration via TGF-β-Smad-mediated EMT, supporting a promising invention target for LSCC therapy.
Keywords: Laryngeal squamous cell cancer, Wnt1-inducible signaling protein 1, invasion, migration, transforming growth factor-beta, epithelial-to-mesenchymal transition
Impact statement
This work expanded the knowledge of the molecular mechanisms underlying metastasis of LSCC by exploring the role of WISP1 LSCC cells. It was showed that WISP1 was highly expressed in metastatic LSCC tissues. Overexpression of WISP1 enhanced invasion and migration, and enhanced EMT of LSCC cells. TGF-β-Smad2/3 signaling pathway was involved in the function of WISP1 in LSCC cells. These findings highlight the pro-metastatic function of WISP1 in LSCC by regulating cell invasion and migration via TGF-β-Smad-mediated EMT, supporting a promising invention target for LSCC therapy.
Introduction
Head and neck cancer is an anatomically diverse and molecularly heterogeneous malignancy that poses a major health risk, especially for elderly people.1 Laryngeal squamous cell cancer (LSCC) ranks as the most prevalent head and neck cancer and approximately 40% of patients with LSCC usually are diagnosed at clinical stage III–IV. Currently, despite the advance in available and standard treatments including surgery and chemotherapy, therapeutic efficacy remains poor in advanced stages due to the uncontrolled invasion and metastasis.2 Therefore, better understanding the molecular mechanism orchestrating the progression of LSCC is an urgent and pivotal goal in developing more effective strategies for LSCC therapy.
Metastasis usually results in poor prognosis and is a major obstacle for the treatment of cancer.3 During metastasis, cells transform to a fibroblastic phenotype that exerts the indispensable role in local invasion and further metastasis. Convincing evidence has identified epithelial to mesenchymal transition (EMT) as a major driver in cancer cell transformation and metastatic progression.4,5 EMT is a proverbial process, whereby epithelial cells can reprogram to a mesenchymal-like phenotype that will facilitate cancer cells escape from the primary site and invade distant tissue.6 Additionally, EMT phenotype is typically characterized by a decrease in epithelial marker E-cadherin and an increase in mesenchymal markers, such as vimentin. Canonical transforming growth factor-beta (TGF-β) signaling can act as a driver to induce EMT, and thus has a critical role in cancer progression by regulating various cellular processes of cell growth, invasion, and mobility.7,8 Intriguingly, recent evidence supports a new approach for cancer treatment that targets EMT.9
Wnt1-inducible signaling protein 1 (WISP1), a cysteine-rich protein located on chromosome 8q24.1–q24.3, belongs to CCN (CyR61, CTGF, NOV) family. Analogous with other CCNs, WISP1 possess multiple functions in various physiological and pathogenic processes, including tissue repair.10,11 WISP1 is originally implicated as a downstream target of Wnt/β-catenin, and its aberrant expression has been recently observed in several cancers, including LSCC.12–14 Currently, WISP1 has moved into the limelight within cancer research where it exerts the prominent roles in cancer cell proliferation, invasion, chemoresistance, and radioresistance. Notably, high expression of WISP1 can serve as a potential marker for poor prognosis of patients with colon cancer.12 In glioblastoma, WISP1 acts as an oncogenic gene to control cell proliferation, apoptosis, and invasion, supporting a promising therapeutic target for glioblastoma.13 Our previous study confirmed high levels of WISP1 in LSCC patients and its positive roles in LSCC cell growth.14 Up to now, the role and underlying mechanism of WISP1 in LSCC metastasis remain poorly understand.
In the present study, the expression of WISP1 in LSCC patients was investigated. Furthermore, we conducted the gain- and loss-of-function tests of WISP1 in cell invasion, migration, and EMT in LSCC cells. The mechanisms lying beneath these processes were also elucidated.
Materials and methods
Specimen collection and ethics statement
Pair specimens of tumor tissues and adjacent normal mucosa (ANM) were collected from 35 patients with LSCC (20 cases without metastasis, 15 cases with metastasis). All patients were diagnosed and underwent partial or total laryngectomy from 2012 to 2017 at the Department of Otolaryngology–Head and Neck Surgery, the First Affiliated Hospital of Zhengzhou University. Patients involved in this research did not receive any systemic anti-carcinoma therapy before sample collection. The clinical characteristics of patients are shown in Table 1. Samples isolated from surgical excision were preserved in liquid nitrogen and stored at −80°C until processed. All experimental protocols were conducted according to the Declaration of Helsinki and approved by the Research Ethics Committee of the First Affiliated Hospital of Zhengzhou University. All participants gave written informed consent before specimen collection.
Table 1.
Clinical characteristics of patients with LSCC (n = 35).
| Clinical parameter | Number (%) |
|---|---|
| Gender | |
| Male | 22 (62.8) |
| Female | 13 (37.2) |
| Age | |
| ≤60 | 19 (54.3) |
| >60 | 16 (45.7) |
| Cancer differentiation | |
| Well | 17 (48.6) |
| Moderate or poor | 18 (51.3) |
| Stage | |
| I–II | 21 (60.0) |
| III–IV | 14 (40.4) |
| LSCC metastasis | |
| Without metastasis | 20 (57.1) |
| With metastasis | 15 (42.9) |
Antibodies
Rabbit polyclonal antibodies against human WISP1 (ab155654), E-cadherin (ab15148), TGF-β (ab92486), rabbit monoclonal antibody against human vimentin (ab92547), and mouse monoclonal antibody against β-actin (ab8226) were obtained from Abcam (Cambridge, MA, USA). Rabbit monoclonal antibodies against human Smad2 (#5339), phospho-Smad2 (p-Smad2, #18338), Smad3 (#9523), p-Smad3 (#9520) were purchased from Cell Signaling (Danvers, MA, USA). HRP-conjugated goat anti-rabbit IgG (ab6721) and HRP-conjugated rabbit anti-mouse IgG (ab97046) were purchased from Abcam.
Cell culture and treatment
Human LSCC cell line TU212 was obtained from ATCC (Manassas, VA, USA). For culture, cells were grown in Dulbecco’s Modified Eagle medium (DMEM) containing 10% fetal bovine serum (Hyclone, Loan, UT, USA), 100 U/mL of penicillin, and 100 μg/mL of streptomycin. Cells were stimulated with recombinant human TGF-β1 (5 ng/mL; Abcam) for 24 h. All cells were maintained in a humidified incubator with 5% CO2 at 37°C.
Knockdown of WISP1 and TGF-β expression by siRNA transfection
RNA interference was applied to silence the expression of WISP1 and TGF-β in TU212 cells. The specific siRNA targeting WISP1 (sc-39335), TGF-β (sc-270322) and the control scramble siRNA (si-NC) were obtained from Santa Cruz Biotechnology, Inc. (Santa Cruz, CA, USA). To perform the siRNA transfection, cells were seeded into 6-well plates and grown to 60%–80% confluence. Then, cells were transfected with 100 nmol/L si-WISP1 or si-TGF-β by Lipofectamine 2000 (Invitrogen, Carlsbad, CA, USA) according to the recommended protocols of manufacture. Approximately 48 h later, Western blotting was carried out to evaluate transfection efficiency.
Recombinant adenovirus vector construction and transduction
To construct WISP1-overexpressed cells, the full-length of WISP1 cDNA was inserted into an adenoviral shuttle plasmid of pAdTrack-CMV (Agilent, Santa Clara, CA, USA) containing green fluorescent protein (GFP). Subsequently, the recombinant Ad-WISP1 vectors were homologously recombinated in E. coli strain BJ5183, followed by the transfection into 293 T cells to generate recombinant adenovirus. After propagation into 293 T cells, the virus was purified via CsCl2 gradient centrifugation, and the titer of virus was identified by the Adeno-X Rapid Titer kit (BD Biosciences, San Jose, CA, USA). Then, the culture medium containing virus of Ad-WISP1 (1 × 109 TU/mL) was supplemented into TU212 cells for transduction. At 48 h after adenovirus infection, the effects on WISP1 expression were measured.
RNA extraction and quantitative real-time PCR
Cells were transfected with si-WISP1 or Ad-WISP1 plasmids, and the total RNA was extracted using RNAiso Plus (TaKaRa Bio Inc., Dalian, China). Afterwards, RNA was reverse transcribed to synthesize cDNA according to the directions provided by the PrimeScript RT Reagent Kit (TaKaRa). Real-time PCR was then carried out in a 20 µL reaction system to detect the transcript of WISP1 using the SYBR Premix Ex TaqTM II Kit (Takara). The specific primer sequences for WISP1 were 5′-CTACAACAACGGCCAGTCCT-3′, reverse 5′-ACATACCCACTGCTCACAGC-3′, and synthesized by Sangon Biotech (Shanghai, China). For reactions, all specimens were subjected to the ABI PRISM 7000 system (Applied Biosystems; Foster City, CA, USA), and data were analyzed by the 2−ΔΔCt equation. β-actin was applied as an endogenous control to normalize the transcriptional levels of WISP1.
Western blotting
Cells were rinsed with PBS before treatment with RIPA lysis buffer (Beyotime, Nantong, China), and the protein concentrations extracted from cells were measured using a BCA protein assay kit (Beyotime). Equal amounts of protein were loaded in each lane and separated by 12% SDS–PAGE, followed by the transfer to PVDF membrane. To interdict the non-specific binding, the membrane was incubated with 5% nonfat milk. Then, the primary antibodies against WISP1 (1:1500), E-cadherin (1:500), vimentin (1:700), TGF-β (1:1000), Smad2 (1:1000), p-Smad2 (1:1000), Smad3 (1:1000), p-Smad3 (1:1000) were added for further incubation at 4°C overnight. After treatment with HRP-conjugated secondary antibody (1:4000) for 2 h at room temperature, the binding signal was revealed by an ECL reagent (Beyotime). The intensities of the immunoreactive bands were analyzed by Image J software (National Institutes of Health, Bethesda, MD, USA).
Transwell assay for cell invasion and migration
Cell invasion ability was evaluated using a Transwell system (Corning, Corning, NY, USA) in accordance with the manufacturer’s instructions. Briefly, cells were treated with si-WISP1, Ad-WISP1, or TGF-β (10 ng/mL). Then, cells resuspended in serum-free medium were seeded into upper chamber where the membrane had been coated with 0.5-mm thickness of Matrigel (50 µg/chamber, BD Biosciences). Then, the lower chamber was filled with 500 µL of culture medium containing 10% fetal bovine serum as a chemoattractant. Following incubation for 48 h, the upper surface of chamber was scrapped to remove the residual cells. After fixating with 4% paraformaldehyde, 0.1% crystal violet was used to stain cells that passed through the upper chamber to the lower surface. Cell migration assay was also conducted as the above protocols except for the absence of Matrigel in upper chamber membrane. The invasive and migrated cells were photographed under a light microscope (×200), and the number of cells was counted on each membrane in five random microscopic fields.
Statistical analysis
All data from at least three independent experiments were analyzed by SPSS 16.0 software (SPSS Inc., Chicago, IL, USA), and are presented as the mean ± SD. Comparisons between groups were evaluated using the Student’s t-test (two groups) or one-way ANOVA (three or more groups), followed by the Student–Newman–Keuls (SNK) post hoc tests. A value of P <0.05 was considered statistically significant.
Results
High expression of WISP1 observes in metastatic LSCC tissues
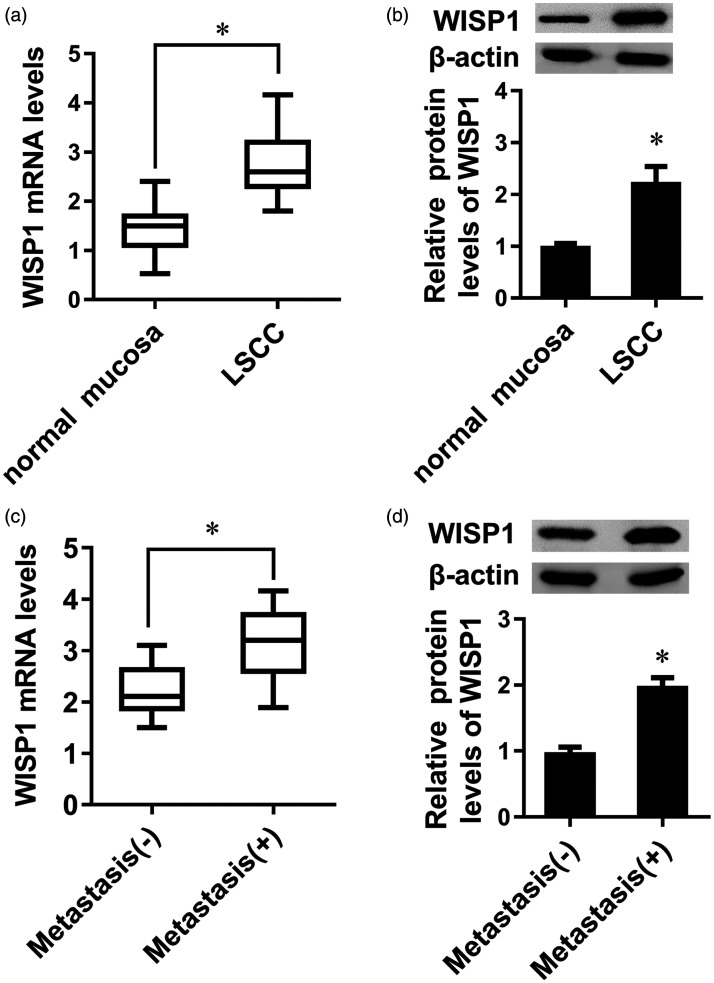
To investigate the function of WISP1 in LSCC progression, we analyzed the expression of WISP1 in clinical specimens from patients with LSCC. As shown in Figure 1(a), higher transcriptional levels of WISP1 were observed in LSCC tissues relative to adjacent normal mucosa tissues. Simultaneously, WISP1 protein expression was higher in tissues from LSCC groups than those from the control groups (Figure 1(b)). More importantly, higher expression of WISP1 mRNA (Figure 1(c)) and protein (Figure 1(d)) was validated in LSCC tissues with metastasis than in LSCC tissues without metastasis.
Figure 1.
Expression of WISP1 in LSCC tissues. The mRNA (a) and protein (b) levels of WISP1 in normal mucosa and LSCC tissues were determined by qRT-PCR and Western blotting. The transcription (c) and protein (d) expression of WISP1 in LSCC with metastasis and without metastasis groups. *P < 0.05.
Elevation of WISP1 expression facilitates cell metastatic potential of LSCC cells in vitro
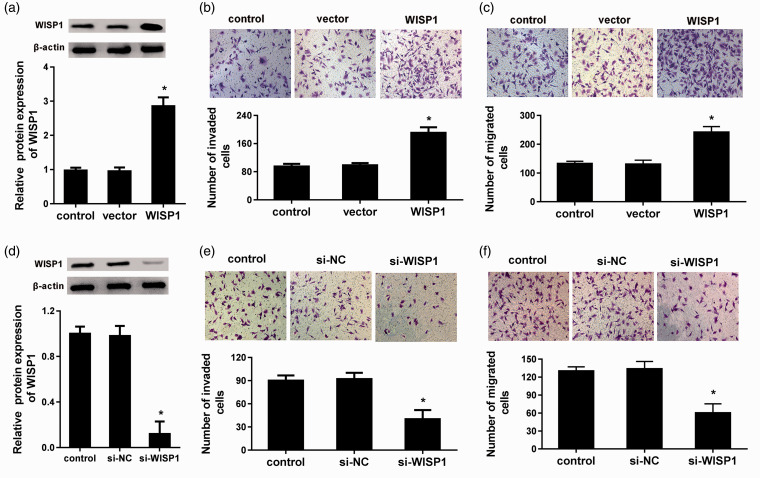
To further elucidate a potential role of WISP1 in the progression of LSCC metastasis in vitro, the expression of WISP1 protein was enhanced in LSCC cell line TU212 by infection with Ad-WISP (Figure 2(a)). Transwell assay substantiated that overexpression of WISP1 increased the invasiveness of TU212 cells (Figure 2(b)). Moreover, cells with elevated WISP1 expression exhibited stronger ability to facilitate cell migration relative to control groups (Figure 2(c)). These findings suggest that WISP1 may exert the positive role in LSCC metastasis.
Figure 2.
WISP1 regulated cell invasion and migration in LSCC cells. (a) The LSCC cell line TU212 was infected with Ad-WISP1, and the protein levels of WISP1 were measured. (b) After overexpression of WISP1 in LSCC cells, the effects on cell invasion were evaluated by transwell assay. (c) Effects of WISP1 elevation on cell migration. (d) Cells were transfected with si-WISP1, then the expression of WISP1 was assessed. (e) Effects of WISP1 knockdown on cell invasion. (f) Transwell assay was performed to detect cell migration. *P < 0.05. (A color version of this figure is available in the online journal.)
Knockdown of WISP1 reversely restrains the metastatic potential of LSCC cells
We next clarified the effects of WISP1 inhibition on LSCC cell invasion and migration. After transfection with siRNA targeting WISP1, the protein levels of WISP1 were notably suppressed (Figure 2(d)). Furthermore, compared with control groups, the number of invasive cells was decreased to 40 ± 11 after WISP1 knockdown in TU212 cells (Figure 2(e)). In striking contrast, cessation of WISP1 also restrained the ability of migration in LSCC cells (Figure 2(f)).
WISP1 regulates EMT in LSCC cells
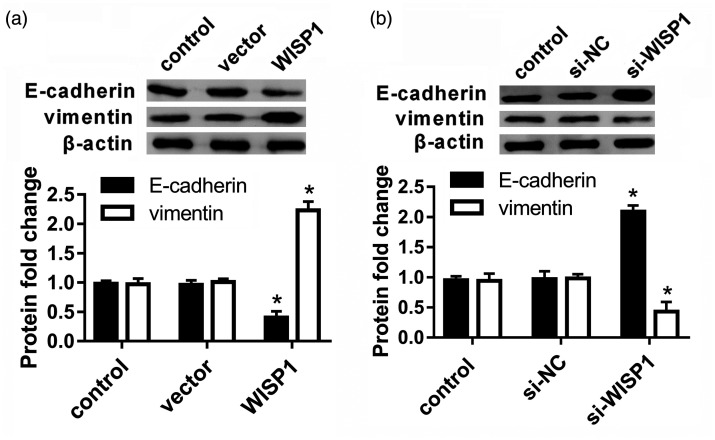
Accumulating evidence has emphasized the critical role of EMT in metastatic propensity of cancers including LSCC. Accordingly, we explored the effects of WISP1 on EMT of LSCC cells. As present in Figure 3(a), overexpression of WISP1 inhibited the protein expression of mesenchymal marker E-cadherin in LSCC cells. Concomitantly, the expression of epithelial marker vimentin was elevated following WISP1 up-regulation (Figure 3(a)). Additionally, further Western blotting analysis corroborated that depression of WISP1 induced E-cadherin expression and decreased vimentin protein levels. Thus, these data indicate that WISP1 may facilitate EMT in LSCC cells (Figure 3(b)).
Figure 3.
Elevation of WISP1 facilitated EMT, whereas WISP1 depression yielded the opposite effects. (a) LSCC cells were infected with Ad-WISP1, and the expression of EMT markers E-cadherin and vimentin was analyzed. (b) Following knockdown of WISP1 by si-WISP1 transfection, the expression of E-cadherin and vimentin was measured. *P < 0.05.
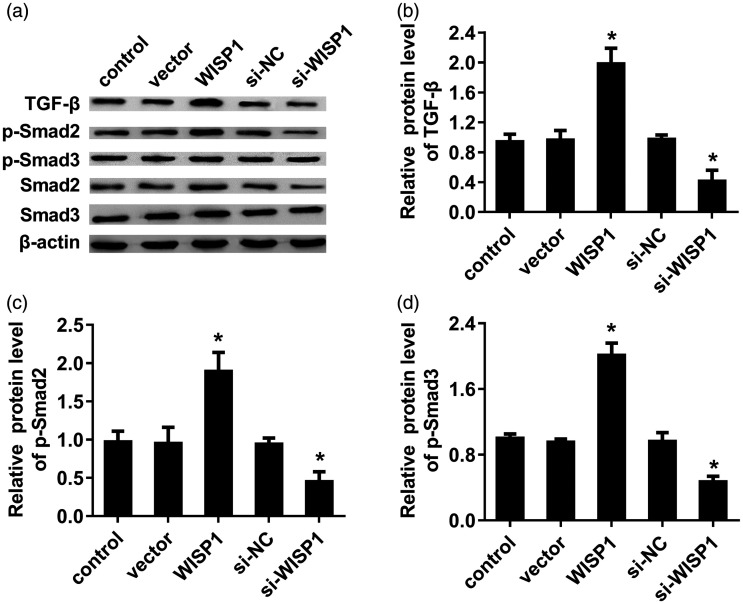
Enhancement of WISP1 activates the TGF-β-Smad2/3 signaling
TGF-β/Smad pathway has been identified as an oncogenic signaling pathway to drive tumor cell invasion and metastasis.15,16 Mechanism analysis confirmed that elevation of WISP1 enhanced the protein levels of TGF-β1 in LSCC cells (Figure 4(a) and (b)). Simultaneously, expression of downstream p-Smad2 (Figure 4(c)) and p-Smad3 (Figure 4(d)) was also activated following WISP1 overexpression, indicating that WISP1 enhances the activation of TGF-β-Smad2/3 signaling. Conversely, knockdown of WISP1 suppressed the activation of TGF-β-Smad2/3 signaling by inhibiting TGF-β1, p-Smad2 and p-Smad3 expression (Figure 4(a) to (d)).
Figure 4.
Effects of WISP1 expression on TGF-β-Smad signaling in LSCC cells. (a) TU212 cells were transfected with WISP1 vectors or si-WISP1, then the protein levels of TGF-β, p-Smad2, Smad2, p-Smad3, and Smad3 were analyzed by Western blotting. (b–d) The quantified analysis on binding bands was evaluated by Image J software. *P < 0.05.
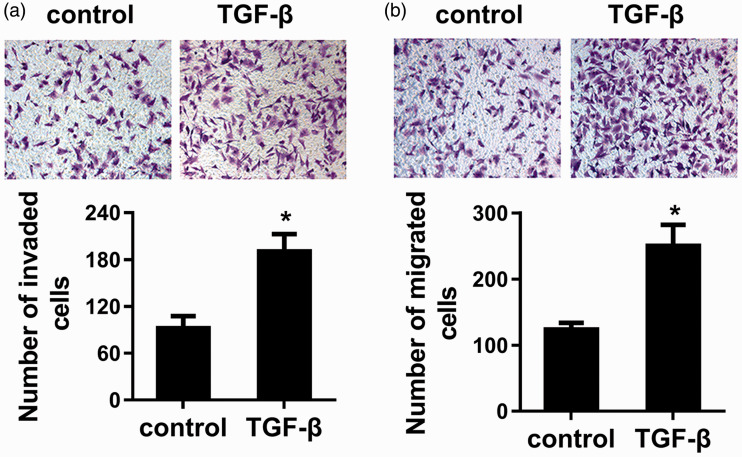
TGF-β acts as a potent factor to promote cell invasion and migration in LSCC cells
Next, we corroborated the function of TGF-β in metastatic potential of LSCC cells. As shown in Figure 5(a), exposure to TGF-β1 dramatically increased cell invasion, in contrast to control groups. Moreover, TGF-β1 stimulation also enhanced the ability of cell migration in LSCC cells relative to control groups (Figure 5(b)). These data suggest the oncogenic potential of TGF-β in cell invasion and migration in LSCC cells.
Figure 5.
TGF-β enhanced LSCC cell invasion and migration. Cells were exposed to TGF-β for 48 h. Then, the effects on cell invasion (a) and migration (b) were assessed by Transwell assay. *P < 0.05. (A color version of this figure is available in the online journal.)
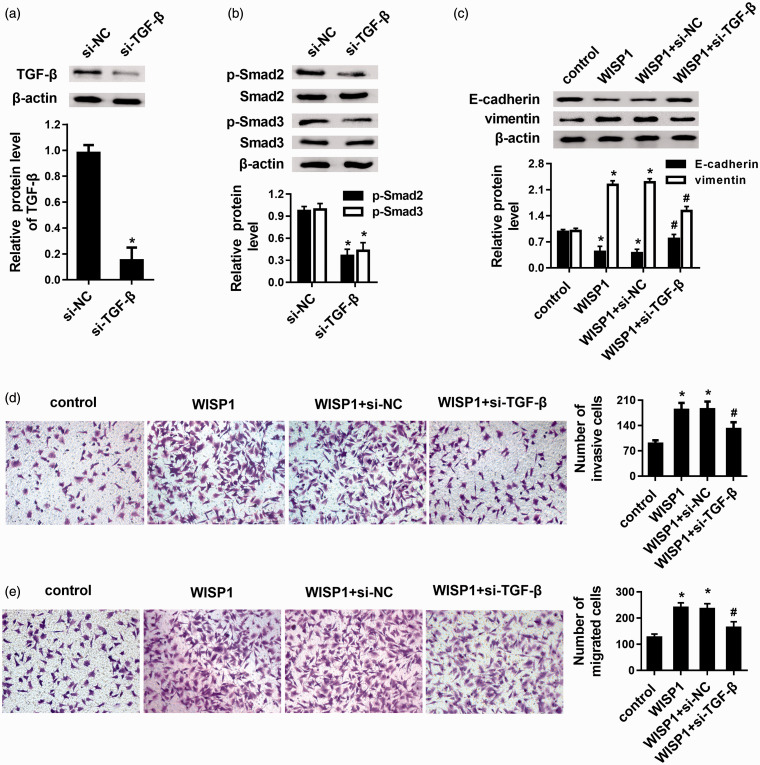
Blocking the TGF-β-Smad pathway overturns the EMT and pro-metastatic effects of WISP1 on LSCC cells
To further elucidate the relationship between TGF-β-Smad pathway and WISP1 in the progression of LSCC metastatic potential, cells were transfected with si-TGF-β. As expected, treatment with si-TGF-β1 engendered not only the decrease in the expression of TGF-β (Figure 6(a)) but also abrogated the expression of p-Smad2 and p-Smad3 (Figure 6(b)), implying the inhibition of TGF-β knockdown on the activation of TGF-β-Smad signaling. Intriguingly, blockage of TGF-β-Smad pathway by si-TGF-β reversed the suppressive effects of WISP1 on E-cadherin expression, and antagonized WISP1-induced vimentin expression (Figure 6(c)). Additionally, abrogating this pathway also afforded the effective intervention in response to WISP1 elevation-induced cancer cell invasion (Figure 6(d)) and migration (Figure 6(e)).
Figure 6.
Abrogating the TGF-β-Smad pathway reversed the effects of WISP1 on EMT and pro-metastatic potential of LSCC cells. (a) TU212 cells were transfected with si-TGF-β, then the expression of TGF-β protein was detected. The corresponding quantified analysis was carried out based on Western blot. (b) The expression of p-Smad2, Smad2, p-Smad3, Smad3 was determined after TGF-β knockdown by Western blotting. The corresponding quantified analysis was carried out based on Western blot. (c) After blockage of TGF-β signaling by si-TGF-β transfection, the effects on EMT markers E-cadherin and vimentin were measured using Western blot. The consequents on cell invasion (d) and migration (e) were analyzed. *P < 0.05 vs. control groups; #P < 0.05 vs. WISP1-overexpressed groups. (A color version of this figure is available in the online journal.)
Discussion
WISP1, also known as CCN4, is a cysteine-rich protein with pleiotropic functions that has been implicated in tissue repair, inflammation, and insulin resistance.10,17 Numerous studies corroborate the aberrant expression of WISP1 in various cancers that has been identified as a clinical marker for poor prognosis in patients with oral squamous cell carcinoma and colon cancer.12,18 Until now, the function of WISP1 in LSCC remains exclusive. Intriguingly, the current study confirmed the high expression of WISP1 in LSCC tissues. Notably, LSCC tissues from patients with metastasis revealed higher expression of WISP1 than those from non-metastatic groups. Analogously, previous research also verified a positive correlation between WISP1 high expression and lymph node metastasis in patients with esophageal squamous cell carcinoma.19 Contradictorily, lower levels of WISP1 were observed in metastatic breast cancer in contrast to non-metastatic groups.20 Undoubtedly, an in-depth investigation is essential to elucidate the roles of WISP1 in metastatic progression of LSCC.
LSCC constitutes a major health threaten due to its high metastasis potential that involves multiple procedures, including cell invasion and migration. Increasing evidence supports the critical roles of WISP1 in tumorigenesis, radioresistance, and chemoresistance of several carcinomas.12,21 For instance, WISP1 may act as a oncoprotein in colon cancer as the evidence that WISP1 overexpression confers cancer cell growth and invasion, whereas its inhibition slows the progression of tumor formation and metastasis.12 Our previous finding corroborated that targeting WISP1 by miR-384 suppressed laryngeal cancer cell proliferation.14 The present research emphasized the potential roles of WISP1 in LSCC metastasis, and confirmed that elevation of WISP1 enhanced cancer cell invasion and migration, whereas knockdown of WISP yielded the opposite effects. These data indicate that WISP1 present within tumor microenvironment may facilitate metastasis of LSCC. Similarly, WISP1 enhancement by PTEN knockdown increased breast cancer cell metastasis.22 Intriguingly, there is a contradictory finding that low levels of WISP1 were validated in breast cancer patients with metastasis and poor prognosis, implying that WISP1 may act as a possible anti-oncogene in breast cancer progression.20,23
EMT is a critical step for the process of orchestrating cancer metastasis.4,24 Cancer cells undergoing EMT switch from the epithelial phenotype into an invasive mesenchymal phenotype, contributing to the migration from primary site and disseminate to distant sites. Here, we observed that WISP1 elevation suppressed the expression of epithelial marker E-cadherin and increased the levels of mesenchymal marker vimentin. Analogous with observations for glioma, knockdown of WISP1 enhanced E-cadherin expression and reduced vimentin levels, suggesting that WISP1 may function as an EMT induced in glioblastoma.13 EMT that can endow cancer cells with increased aggressiveness, is recently proved to be involved in therapy resistance and recurrence of carcinomas.24 Increasing evidence has support a potential of targeting EMT for cancer therapy.4 Intriguingly, up-regulation of WISP1 also induces the occurrence of EMT in alveolar type II cells, supporting a new target for pulmonary fibrosis treatment.25
Further mechanism analysis elucidated that WISP1 elevation evoked the activation of TGF-β-Smad signaling. In LSCC, expression of TGF-β is validated to be associated with clinical stages and lymph node metastasis.26 TGF-β can regulate the expression of matrix metalloproteinases (MMPs) and downstream Smad signaling to degrade the extracellular matrix, an essential step for EMT occurrence.15,27 Currently, TGF-β is widely accepted as a key inducer of EMT. Initiation of TGF-β pathway will facilitate cell invasion and migration by inducing EMT, thus aggravating cancer metastasis.15,28 To further decipher the correlation between TGF-β-Smad signal activation and WISP1-mediated metastatic potential in LSCC cells, we firstly corroborated that TGF-β exposure promoted cell invasion and migration in LSCC. More importantly, blocking the TGF-β pathway by employing si-TGF-β antagonized WISP1-enhanced EMT, invasion, and migration. Intriguingly, targeting TGF-β signaling has been identified as a novel strategy to fight carcinoma based on its ability to mediate EMT and metastasis.29,30
Conclusions
This work identified high expression of WISP1 in LSCC tissues, especially metastatic tissues. Notably, gain and loss of WISP1 confirmed that WISP1 may afford a pro-metastatic potential in LSCC by enhancing cell invasion and migration through activating TGF-β-Smad signaling-mediated EMT. Together, these findings may highlight a new opinion about how WISP1 aggravates the progression of LSCC. Accordingly, this research may offer a new and promising therapeutic strategy against LSCC. Of note, the further study involving the function of WISP1 in LSCC metastasis in vivo will be investigated in our next plan.
Footnotes
AUTHORS’ CONTRIBUTIONS: LW and DS conceived the study. LW, KS, HW, and JL analyzed and interpreted the data. HW provided samples. LW, KS, HW, JL, and DS performed experiments and statistical analysis. LW, KS, and DS confirmed statistical analysis. DS was a major contributor in writing the manuscript. All authors read and approved the final manuscript.
DECLARATION OF CONFLICTING INTERESTS: The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
ETHICAL APPROVAL: This research involved in human experiment was performed according to the Declaration of Helsinki, and approved by the Research Ethics Committee of the First Affiliated Hospital of Zhengzhou University (approval number: 2018–172).
FUNDING: This study was supported by the National Natural Science Foundation of China (No. U2004109).
ORCID iD: Liang Wang https://orcid.org/0000-0003-1555-7277
References
- 1.Torre LA, Bray F, Siegel RL, Ferlay J, Lortet-Tieulent J, Jemal A.Global cancer statistics, 2012. CA Cancer J Clin 2015; 65:87–108 [DOI] [PubMed] [Google Scholar]
- 2.Gourin CG, Conger BT, Sheils WC, Bilodeau PA, Coleman TA, Porubsky ES.The effect of treatment on survival in patients with advanced laryngeal carcinoma. Laryngoscope 2009; 119:1312–7 [DOI] [PubMed] [Google Scholar]
- 3.Furtado de Araujo Neto VJ, Cernea CR, Aparecido Dedivitis R, Furtado de Araujo Filho VJ, Fabiano Palazzo J, Garcia Brandao L.Cervical metastasis on level IV in laryngeal cancer. Acta Otorhinolaryngol Ital 2014; 34:15–8 [PMC free article] [PubMed] [Google Scholar]
- 4.Singh M, Yelle N, Venugopal C, Singh SK.EMT: mechanisms and therapeutic implications. Pharmacol Ther 2018; 182:80–94 [DOI] [PubMed] [Google Scholar]
- 5.Chaffer CL, San Juan BP, Lim E, Weinberg RA.EMT, cell plasticity and metastasis. Cancer Metastasis Rev 2016; 35:645–54 [DOI] [PubMed] [Google Scholar]
- 6.Pastushenko I, Blanpain C.EMT transition states during tumor progression and metastasis. Trends Cell Biol 2019; 29:212–26 [DOI] [PubMed] [Google Scholar]
- 7.Jia-Ray Y, Yilin T, Ying J, Hammell MC, J, Erby W, Jae-Seok R, Vakoc CR, Linda VA.TGF-β/smad signaling through DOCK4 facilitates lung adenocarcinoma metastasis. Genes Dev 2015; 29:250–61 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Syed V.TGF-beta signaling in cancer. J Cell Biochem 2016; 117:1279–87 [DOI] [PubMed] [Google Scholar]
- 9.Cho ES, Kang HE, Kim NH, Yook JI.Therapeutic implications of cancer epithelial-mesenchymal transition (EMT). Arch Pharm Res 2019; 42:14–24 [DOI] [PubMed] [Google Scholar]
- 10.Jung TW, Kang C, Goh J, Chae SI, Kim HC, Lee TJ, Abd El-Aty AM, Jeong JH.WISP1 promotes non-alcoholic fatty liver disease and skeletal muscle insulin resistance via TLR4/JNK signaling. J Cell Physiol 2018; 233:6077–87 [DOI] [PubMed] [Google Scholar]
- 11.Yu Z, Wang T, Zhang L, Yang X, Li Q, Ding X.WISP1 and TLR4 on macrophages contribute to ventilator-induced lung injury. Inflammation 2020; 43:425–32 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Wu J, Long Z, Cai H, Du C, Liu X, Yu S, Wang Y.High expression of WISP1 in Colon cancer is associated with apoptosis, invasion and poor prognosis. Oncotarget 2016; 7:49834–47 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Jing D, Zhang Q, Yu H, Zhao Y, Shen L.Identification of WISP1 as a novel oncogene in glioblastoma. Int J Oncol 2017; 51:1261–70 [DOI] [PubMed] [Google Scholar]
- 14.Wang L, Sun J, Cao H.MicroRNA-384 regulates cell proliferation and apoptosis through directly targeting WISP1 in laryngeal cancer. J Cell Biochem 2019; 120:3018–26 [DOI] [PubMed] [Google Scholar]
- 15.Zhang X, Zhang P, Shao M, Zang X, Zhang J, Mao F, Qian H, Xu W.SALL4 activates TGF-beta/SMAD signaling pathway to induce EMT and promote gastric cancer metastasis. Cancer Manag Res 2018; 10:4459–70 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Drabsch Y, ten Dijke P.TGF-beta signalling and its role in cancer progression and metastasis. Cancer Metastasis Rev 2012; 31:553–68 [DOI] [PubMed] [Google Scholar]
- 17.Ding X, Tong Y, Jin S, Chen Z, Li T, Billiar TR, Pitt BR, Li Q, Zhang LM.Mechanical ventilation enhances extrapulmonary sepsis-induced lung injury: role of WISP1-alphavbeta5 integrin pathway in TLR4-mediated inflammation and injury. Crit Care 2018; 22:302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Clausen MJ, Melchers LJ, Mastik MF, Slagter-Menkema L, Groen HJ, van der Laan BF, van Criekinge W, de Meyer T, Denil S, Wisman GB, Roodenburg JL, Schuuring E.Identification and validation of WISP1 as an epigenetic regulator of metastasis in oral squamous cell carcinoma. Genes Chromosomes Cancer 2016; 55:45–59 [DOI] [PubMed] [Google Scholar]
- 19.Nagai Y, Watanabe M, Ishikawa S, Karashima R, Kurashige J, Iwagami S, Iwatsuki M, Baba Y, Imamura Y, Hayashi N, Baba H.Clinical significance of Wnt-induced secreted protein-1 (WISP-1/CCN4) in esophageal squamous cell carcinoma. Anticancer Res 2011; 31:991–7 [PubMed] [Google Scholar]
- 20.Taghavi A, Akbari ME, Hashemi-Bahremani M, Nafissi N, Khalilnezhad A, Poorhosseini SM, Hashemi-Gorji F, Yassaee VR.Gene expression profiling of the 8q22-24 position in human breast cancer: TSPYL5, MTDH, ATAD2 and CCNE2 genes are implicated in oncogenesis, while WISP1 and EXT1 genes may predict a risk of metastasis. Oncol Lett 2016; 12:3845–55 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Chen J, Yin J, Li X, Wang Y, Zheng Y, Qian C, Xiao L, Zou T, Wang Z, Liu J, Zhang W, Zhou H, Liu Z.WISP1 polymorphisms contribute to platinum-based chemotherapy toxicity in lung cancer patients. Int J Mol Sci 2014; 15:21011–27 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Chiang KC, Hsu SY, Lin SJ, Yeh CN, Pang JH, Wang SY, Hsu JT, Yeh TS, Chen LW, Kuo SF, Cheng YC, Juang HH.PTEN insufficiency increases breast cancer cell metastasis in vitro and in vivo in a xenograft zebrafish model. Anticancer Res 2016; 36:3997–4005 [PubMed] [Google Scholar]
- 23.Davies SR, Watkins G, Mansel RE, Jiang WG.Differential expression and prognostic implications of the CCN family members WISP-1, WISP-2, and WISP-3 in human breast cancer. Ann Surg Oncol 2007; 14:1909–18 [DOI] [PubMed] [Google Scholar]
- 24.Smith BN, Bhowmick NA.Role of EMT in metastasis and therapy resistance. J Clin Med 2016; 5:17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Heise RL, Stober V, Cheluvaraju C, Hollingsworth JW, Garantziotis S.Mechanical stretch induces epithelial-mesenchymal transition in alveolar epithelia via hyaluronan activation of innate immunity. J Biol Chem 2011; 286:17435–44 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Cui W, Meng W, Zhao L, Cao H, Wang CW.B. TGF-beta-induced long non-coding RNA MIR155HG promotes the progression and EMT of laryngeal squamous cell carcinoma by regulating the miR-155-5p/SOX10 axis. Int J Oncol 2019; 54:2005–18 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Yeh HW, Hsu EC, Lee SS, Lang YD, Lin YC, Chang CY, Lee SY, Gu DL, Shih JH, Ho CM, Chen CF, Chen CT, Tu PH, Cheng CF, Chen RH, Yang RB, Jou YS.PSPC1 mediates TGF-beta1 autocrine signalling and Smad2/3 target switching to promote EMT, stemness and metastasis. Nat Cell Biol 2018; 20:479–91 [DOI] [PubMed] [Google Scholar]
- 28.Zhou Q, Zheng X, Chen L, Xu B, Yang X, Jiang J, Wu C.Smad2/3/4 pathway contributes to TGF-beta-Induced MiRNA-181b expression to promote gastric cancer metastasis by targeting Timp3. Cell Physiol Biochem 2016; 39:453–66 [DOI] [PubMed] [Google Scholar]
- 29.Xu M, He J, Li J, Feng W, Zhou H, Wei H, Zhou M, Lu Y, Zeng J, Peng W, Du F, Gong A.Methyl-CpG-binding domain 3 inhibits epithelial-mesenchymal transition in pancreatic cancer cells via TGF-beta/smad signalling. Br J Cancer 2017; 116:91–9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Yu Y, Luo W, Yang ZJ, Chi JR, Li YR, Ding Y, Ge J, Wang X, Cao XC.miR-190 suppresses breast cancer metastasis by regulation of TGF-beta-induced epithelial-mesenchymal transition. Mol Cancer 2018; 17:70. [DOI] [PMC free article] [PubMed] [Google Scholar]