Abstract
Background
Marzeptacog alfa (activated) (MarzAA), a novel recombinant activated human factor VII (FVIIa) variant, was developed to provide increased procoagulant activity, subcutaneous (SC) administration, and longer duration of action in people with hemophilia.
Objectives
To investigate if daily SC administration of MarzAA in subjects with inhibitors can provide effective prophylaxis.
Methods
This multicenter, open‐label phase 2 trial (NCT03407651) enrolled men with severe congenital hemophilia with an inhibitor. All subjects had a baseline annualized bleeding rate (ABR) of ≥12 events/year. Subjects received a single 18 μg/kg intravenous dose of MarzAA to measure 24‐hour pharmacokinetics (PK) and pharmacodynamics (PD), single 30 μg/kg SC dose to measure 48‐hour PK/PD, then daily SC 30 μg/kg MarzAA for 50 days. If spontaneous bleeding occurred, the dose was sequentially escalated to 60, 90, or 120 μg/kg, with 50 days at the final effective dose without spontaneous bleeding to proceed to a 30‐day follow‐up. The primary end point was reduction in ABR. Secondary end points were safety, tolerability, and antidrug antibody (ADA) formation.
Results
In the 11 subjects, the mean ABR significantly reduced from 19.8 to 1.6, and the mean proportion of days with bleeding significantly reduced from 12.3% to 0.8%. Of a total of 517 SC doses, six injection site reactions in two subjects were reported. No ADAs were detected. One fatal unrelated serious adverse event occurred: intracerebral hemorrhage due to untreated hypertension.
Conclusions
The data demonstrated that MarzAA was highly efficacious for prophylactic treatment in patients with inhibitors by significantly decreasing bleed frequency and duration of bleeding episodes.
Keywords: hemophilia, marzeptacog alfa (activated), prophylaxis, recombinant FVIIa, subcutaneous injection
Essentials.
Marzeptacog alfa (activated; MarzAA) is a novel variant of activated human factor VII.
MarzAA efficacy, safety, pharmacokinetics, and pharmacodynamics were evaluated in people with hemophilia with an inhibitor.
MarzAA greatly reduced annualized bleeding rates and average proportion of days with bleeding.
This data supports additional clinical trials for people with hemophilia with inhibitors.
1. INTRODUCTION
Hemophilia A and B are X‐linked, recessive, hereditary bleeding disorders caused by a deficiency of coagulation factor VIII (FVIII) or factor IX (FIX), respectively.1 Treatment of hemophilia A or B typically involves factor replacement therapy by intravenous (IV) injection of FVIII or FIX, respectively, to treat episodic bleeding or provide prophylaxis against bleeding episodes.1, 2, 3 Neutralizing antibodies (inhibitors) to the injected FVIII or FIX can be a complication of factor replacement therapy.4, 5, 6, 7 Inhibitors can occur at high or low titers (quantitated in Bethesda Units), can neutralize the activity of the replacement therapy, and thus render treatment of bleeding episodes unsuccessful, resulting in potentially catastrophic clinical consequences for the patient, including an increased morbidity and mortality risk.1, 4 Prophylaxis treatment options have considerably improved for people with hemophilia A with inhibitors with the use of subcutaneous (SC) emicizumab (Hemlibra, Genentech, South San Francisco, CA, USA), a bispecific antibody that is an activated factor VIII mimetic.8 Unfortunately, not all people with hemophilia are appropriate candidates for emicizumab prophylaxis. People who develop anti‐drug antibodies (ADAs) to emicizumab, those who have inadequate bleeding prevention, those with adverse effects precluding use, or those who have hemophilia B with inhibitors, require treatment with other agents, namely wild‐type recombinant FVIIa (wt‐rFVIIa) or activated prothrombin complex concentrates (aPCCs). 9, 10, 11, 12, 13, 14 Due to the short half‐life, prophylaxis with wt‐rFVIIa or aPCCs require frequent IV dosing.9, 12, 13 Intravenous dosing often requires a medical professional or family member to perform the venipuncture, making home IV prophylaxis cumbersome.3, 15 Treatment of people who have inhibitors with wt‐rFVIIa or aPCCs have limitations with regard to efficacy, safety, and convenience.16, 17, 18 Subcutaneous administration presents a major advantage over IV administration because it reduces the treatment burden, improves quality of life (QoL), and reduces health care costs.19, 20
Marzeptacog alfa (activated) (MarzAA), a novel rFVIIa variant, was developed using a structure‐based rational protein design approach intended to enhance the biological properties of wt‐rFVIIa via four amino acid substitutions. Two substitutions in the heavy‐chain protease domain (Q286R and M298Q) increase catalytic activity for factor X activation in both a tissue factor–dependent and tissue factor–independent manner.21 The two other substitutions are in the light chain (T128N and P129A) to yield an additional N‐linked glycosylation site to provide an extended duration of effect.21, 22, 23, 24 These qualities of increased potency and resistance to protease destruction allow low‐volume SC injection of MarzAA for bleeding prophylaxis (Figure 1).
FIGURE 1.
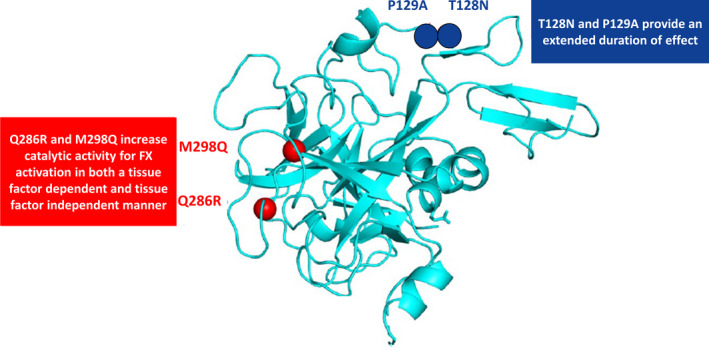
Marzeptacog alfa (activated) has four amino acid substitutions: Q286R, M298Q, T128N, and P129A. FX, factor X
Herein, we describe the phase 2 trial in people with hemophilia with inhibitors receiving MarzAA prophylaxis.
2. MATERIALS AND METHODS
This open‐label, multicenter, dose‐escalation trial, was designed to investigate the pharmacokinetics (PK), bioavailability, pharmacodynamics (PD), efficacy, and safety of a daily SC injection of MarzAA for bleeding prophylaxis in adult subjects with hemophilia A or B with an inhibitor. The trial was registered at ClinicalTrials.gov (Registration Number: NCT03407651).
This trial was conducted in accordance with the US Code of Federal Regulations, International Conference on Harmonisation Guidelines on Good Clinical Practices, and national and local laws and regulations. The trial protocol and all amendments were reviewed and approved by the institutional review board or independent ethics committees at each participating trial site. All subjects gave written informed consent before participation in the trial. Emicizumab was not approved in any participating country at the time this trial was conducted.
2.1. Study population
Men aged ≥18 years with a confirmed diagnosis of severe congenital hemophilia with an inhibitor and a history of frequent spontaneous bleeding episodes (historical annualized bleeding rate [ABR] ≥12, as per the subject’s bleeding and treatment records), were eligible for enrollment. Individuals with a known hypersensitivity to the trial product or related products, known positive antibody to FVII or FVIIa detected by the central laboratory, and concomitant therapy with immunomodulating drugs were excluded. A complete list of inclusion and exclusion criteria can be found in Table S1. At screening, subjects received training and a diary to enter all investigational drug administration, injection site assessments, bleeding episodes, bleeding treatment, adverse events (AE) and patient‐reported outcome (PRO) scales. During Part 1b, subjects received SC injection training.
2.2. Study design
The trial included the following three parts, which occurred consecutively (Figure 2):
FIGURE 2.
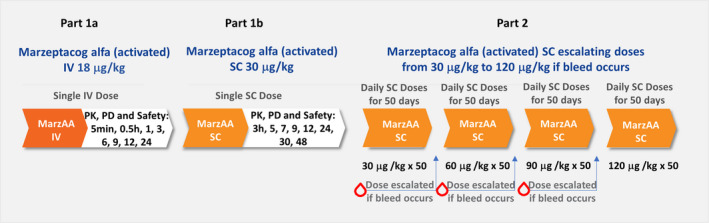
Phase 2, multicenter, open‐label trial design. IV, intravenous; MarzAA: marzeptacog alfa (activated); SC, subcutaneous
Part 1a (24 hours) measured PK, PD, and safety parameters after IV administration of a single 18 μg/kg dose of MarzAA. Assessments were done before dosing and after dosing at 5 and 30 minutes and at 1, 3, 6, 9, 12, and 24 hours.
Part 1b (48 h) measured PK, PD, and safety parameters after SC administration of a single 30 μg/kg dose of MarzAA. Assessments were performed before dosing and repeated at 3, 5, 7, 9, 12, 24, 30, and 48 hours after dosing.
Part 2 aimed to (i) find the optimal dose of MarzAA for each subject (ie, dose that prevented spontaneous bleeding); and (ii) determine the peak and trough MarzAA concentrations, PD, and safety of daily SC administration of MarzAA for each subject for an extended duration, reflecting the intended SC prophylactic use of MarzAA in subjects with hemophilia. At day 1 of daily SC dosing, subjects began their daily self‐administered dosing regimen (at approximately the same time each day), starting with a SC dose of 30 μg/kg MarzAA for 50 consecutive days. At each dose, subjects recorded the day and time of the SC injections in their diary. If a spontaneous bleeding episode occurred before the fifth daily dose, subjects continued at the current dosing level. If a spontaneous bleeding episode occurred after the fifth daily dose, the MarzAA dose was escalated to the next dose level. Three dose escalations of 60, 90, and 120 μg/kg (maximum dose) were allowed. The dosing regimen in this trial was selected on the basis of previous preclinical and clinical studies. At each dose escalation level, safety, MarzAA concentration, and PD were monitored to ensure that dose escalation to a higher dose level was appropriate. If a subject required a dose escalation, they continued treatment with that dose for an additional 50 days to complete the trial. Subjects were allowed to use their current bypass regimen for any spontaneous or traumatic bleed that occurred while on the study drug. Subjects were required to contact the clinical investigative team immediately to report the event and have a treatment dose authorized. Follow‐up plans for that event were made, including whether to arrange for a blood specimen to be drawn (if feasible) before further administration of either study drug or the bypassing agent used for treatment. The decision of whether to continue daily study drug administration was determined by the clinical trial team after discussion with the sponsor. This information was also recorded in the subject’s diary. Daily SC MarzAA injections could be interrupted if there was a need for a surgical procedure, an event requiring extended hospitalization (>48 hours), a thrombotic event, clinical evidence of inhibitor formation, or laboratory results suggesting an antibody may be developing.
2.3. Investigation product
MarzAA was provided as a powder for injection, at a 4.62 mg/vial dosage strength, supplied in a 5‐mL vial.
2.4. Outcome measures
The primary objective was to evaluate the efficacy and safety of MarzAA for bleeding prophylaxis in adult subjects with hemophilia with an inhibitor, by (i) evaluating ABR (spontaneous and total) during Part 2 when on the final MarzAA dose level versus recorded ABR; and (ii) evaluating spontaneous bleeding, which required escalation to a higher dose level. A spontaneous bleeding episode was defined as one that was precipitated by normal activities of daily living. The secondary objectives were to (i) determine the PK and PD of SC administration of MarzAA in subjects with hemophilia with inhibitors and compare IV versus SC administration of MarzAA with regards to PK, PD, and safety parameters; (ii) find the optimal dose of MarzAA for each subject, that is, the dose that prevented spontaneous bleeding, and to determine the peak and trough concentrations, PD, and safety of daily SC administration of MarzAA at the optimal dose for each subject for an extended duration; (iii) determine the occurrence of and categorize breakthrough bleeds requiring escalation to a higher dose level as follows: (a) number of bleeds that were life threatening, (b) number of bleeds that required hospitalization and/or blood transfusion, (c) number of muscle bleeds, and (d) median time (and interquartile range) to resolution of bleeds. A breakthrough bleed was defined as any spontaneous or traumatic bleed. Exploratory objectives were to (i) identify potential biomarkers and determine the effect of SC administration of MarzAA on the QoL of subjects with hemophilia with inhibitors; (ii) identify biomarker(s) such as antigen levels, activity levels, or global thrombosis assay evaluation that could be used to predict or correlate with a subject’s lack of spontaneous bleeding; (iii) identify biomarker(s) such as D‐dimer, or prothrombin fragment 1+2 (F1+2), or a functional assay of the FVIIa activity that would identify or predict clinical thrombogenicity of daily SC administration of MarzAA when use of rescue medication was required, and (iv) record PRO measurements using the European QoL‐5 Dimensions (EQ‐5D), Visual Analogue Scale (VAS), Haemophilia A Quality of Life Questionnaire (Haem‐A‐QoL), and Haemophilia Activities List (HAL).
2.5. Laboratory analysis
MarzAA antigen and coagulation parameters were measured at day 1 (before dosing and 7 hours after dosing), day 3 (before dosing and postdose hour 7), day 5 (before dosing and postdose hour 7), and day 7 (before dosing and postdose hour 7). Fibrinogen was tested using HemosIL Fibrinogen‐C; D‐Dimer with HemosIL D‐Dimer HS; thrombin‐antithrombin (TAT) with Siemens Enzygnost TAT kit and F1+2 with Seimens Enzygnost F1+2 kit (Siemens Healthcare Diagnostics Products GmbH, Marburg, Germany). The MarzAA activity assays were performed using STACLOT VIIa‐rTF kits manufactured by Diagnostica Stago (Parsippany, NJ, USA) on the Stago ST4/Start4 coagulation analyzer, and the test procedure was adapted from the STACLOT kit instructions. If spontaneous bleeding occurred after the fifth daily dose, MarzAA antigen levels were measured within 6 hours of the spontaneous bleed (if feasible). Specimens for coagulation and immunogenicity testing were also drawn. If it was determined to escalate to the next dose level because of spontaneous bleeding, a before dose and postdose 7‐hour specimen was drawn for PK, coagulation, and thrombogenicity markers on day 7 after the escalation to estimate the new trough and peak concentrations and PD. MarzAA antigen and coagulation parameters were then measured before and after dosing at days 14, 21, and 28, and before dosing on day 50 after escalation.
Specimens for ELISA‐based immunogenicity testing (antibody to MarzAA, neutralizing activity, cross reactivity with wt‐rFVIIa) were drawn at screening, Part 1a before dose, Part 1b before dose, and during Part 2 before dose on days 1, 7, 14, 21, 28, and 42, and then every 2 weeks until the end‐of‐trial visit at 30 days after the last dose. Immunogenicity assessments followed Food and Drug Administration guidance25 and included assay for any occurrence of antibody formation resulting in a decreased endogenous level of FVII or FVIIa, occurrence of antibody response to MarzAA, and the number and percentage of antibody formation resulting in a decreased endogenous level of FVII or FVIIa. Coagulation assays, thrombogenicity markers, and immunogenicity marker evaluations were performed at a central laboratory.
2.6. PK and PD analysis
Standard PK parameters included terminal phase elimination half‐life (t1/2), total plasma clearance, volume of distribution at steady state, area under the FVIIa activity time curve (AUC) from time zero to a definite time t and AUC from time zero to infinity, mean residence time (MRT), and bioavailability of the SC administration. The PK sampling schedule was specific to each part and dose administration. Pharmacokinetic analysis was performed using a noncompartmental analysis in professional PK software Phoenix WinNonLin version 6.4 or higher (Certara, Princeton, NJ, USA). A semiparametric model described by Lee et al26, 27 was used to calculate the t1/2. A noncompartmental model using the trapezoidal rule was used to compute the various AUCs and the parameters derived from them. Descriptive statistics were reported for each parameter including PK. Tables, listings, and figures were produced using SAS statistical package version 9.3 or higher (SAS Institute, Cary, NC, USA).
Pharmacodynamic assessments including coagulation assays (activated partial thromboplastin time [aPTT], prothrombin time [PT], thrombin generation time, and fibrinogen); and thrombogenicity biomarkers (D‐dimer, F1+2, and TAT complexes), were derived by noncompartmental analysis using intravascular or extravascular trapezoidal log‐linear rule using Phoenix WinNonLin or SAS software packages.
2.7. Statistical analysis
The null hypothesis (H0) was that the ABR (for MarzAA) equaled 12 versus the alternative hypothesis (H1) ABR (for MarzAA) <12. With a total of 12 subjects, if the true ABR for MarzAA is ≤6, with a one‐tailed 2.5% significance level, there was near 100% power to demonstrate this using a one‐sample Poisson test of the H0. Even if only six subjects were available for the analysis, the power would be in excess of 99%. Thus, a sample of 12 subjects was chosen and expected to provide sufficient power for the primary end point analysis in this trial. If a subject did not complete the trial as defined in the protocol, that is, before receipt of the study drug for 50 days at the same dose level (maximum, 120 μg/kg), a replacement subject could be enrolled. Missing data were not imputed in this trial. All analyses were based on available data. Baseline is defined as the last assessment before the first administration of study drug in Part 1a. This baseline was used for both Part 1a and Part 1b and was used in all efficacy and safety analyses.
The analysis of the primary end point (ABR) of the final dose used to treat a subject was based on the evaluation of the ABR of MarzAA compared to a rate seen with prior 6‐month episodic therapy. The latter was assumed to be 12 (or one bleed per month) as the null hypothesis. The comparison of the actual ABR for MarzAA, either pooled across all doses used or evaluated at the highest dose for an individual, was compared to this null hypothesized value using the one‐sample test for a Poisson rate (using an exact calculation of the P value based on the program StatXact 11, Cytel, Inc., 2015). Because this was a one‐tailed test (the alternative hypothesis: ABR for MarzAA will be <12), a one‐tailed 2.5% significance level was used. All other statistical tests were performed at the 0.05 significance level using two‐sided tests.
3. RESULTS
3.1. Subjects included
A total of 17 subjects were screened, and 11 subjects received the study drug and were included in the Safety Analysis Set. Three subjects did not complete the trial. The efficacy/PD modified intent‐to‐treat population, defined as any patient who received at least seven SC doses of MarzAA at 30 μg/kg (Table S2 and Figure 3), was used to present all primary and secondary efficacy results in the trial. Demographic and baseline characteristics are shown in Table 1. All subjects in the trial were White, with an average age of 31 years (18‐47 years), and average BMI of 23.025 kg/m² (14.62‐32.89 kg/m²). Ten (90.9%) subjects had hemophilia A, and one (9.1%) subject had hemophilia B. The average age of hemophilia diagnosis was at 2 years (1‐7 years). Overall, the median number of bleeds in the prior 6 months and prior 50 days upon entering the trial was 8.0 (4‐12) and 2.0 (1‐5), respectively. A list of episodic treatments before MarzAA treatment can be found in Table S3.
FIGURE 3.
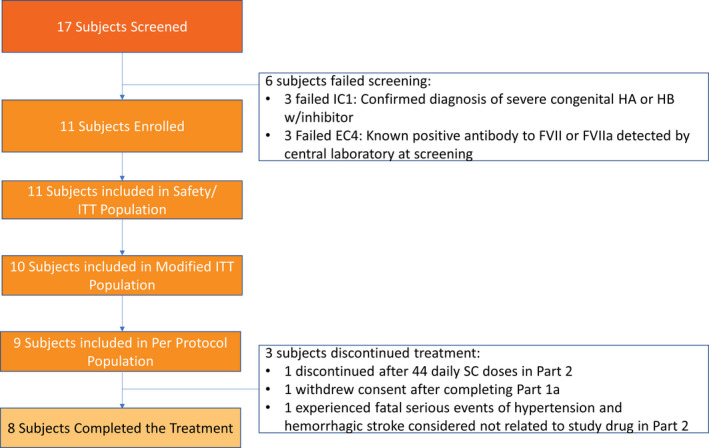
Patient enrollment flowchart. EC, exclusion criterion; FVII, factor VII; HA, hemophilia A; HB, hemophilia B; IC, inclusion criterion; ITT, intent‐to‐treat; SC, subcutaneous
TABLE 1.
Subject demographics and characteristics at screening
| Subject | Median Age (y) | Height (cm) | Weight (kg) | BMI (kg/m2) | Hemophilia A or B | Highest inhibitor level (BU) | Age when inhibitor diagnosed (years) | ABR |
PDB (%) |
Bleeds in the past 6 months | Bleeds in the past 50 days |
|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | 43 | 175 | 75 | 24.5 | A | 5.5 | 39 | 22.2 | 22 | 6 | 2 |
| 2 | 31 | 160 | 61 | 23.8 | B | 1.73 | 31 | 21.2 | 18 | 9 | 2 |
| 3 | 47 | 150 | 44 | 18.6 | A | 1.07 | 40 | 20.5 | 8 | 9 | 4 |
| 4a | 36 | 170 | 75 | 26.0 | A | 40 | 15 | 12.2 | 6 | 4 | 1 |
| 5 | 18 | 185 | 62 | 18.7 | A | 5 | 14 | 26.7 | 18 | 12 | 4 |
| 6b | 30 | 180 | 72 | 22.2 | A | 2.7 | 26 | 18.3 | 11 | 9 | 2 |
| 7 | 31 | 184 | 111 | 32.9 | A | 27.5 | 10 | 24.3 | 9 | 12 | 5 |
| 8c | 29 | 167 | 58 | 20.8 | A | 4.2 | 27 | 15.9 | 12 | 7 | 1 |
| 9 | 35 | 166 | 40 | 14.6 | A | 4.7 | 35 | 16.6 | 11 | 8 | 1 |
| 10 | 23 | 182 | 92 | 27.8 | A | 4.5 | 21 | 15.2 | 4 | 7 | 1 |
| 11 | 18 | A | 56 | 6 | 15.9 | 9 | 8 | 2 | |||
| All | 31 | 172.5 | 67 | 23.0 | 4.7 | 26 | 18.3 | 11 | 8 | 2 |
Patients can have a very different proportion of days with bleeding despite similar ABR.
Abbreviations: ABR, annualized bleeding rate; BMI, body mass index; BU, Bethesda Units; PDB, proportion of days with bleeding.
Withdrew consent after completing Part 1a of the trial, skipping Part 1b, and receiving a single SC 30 μg/kg dose in Part 2 and had no adverse events.
Discontinued after experiencing serious events of hypertension and hemorrhagic stroke considered not related to study drug in Part 2, which resulted in the subject's death (day 11).
Discontinued after 44 daily SC doses in Part 2 because of relocation.
3.2. Efficacy
The mean baseline ABR was 19.8 (12.2‐26.7), and the mean proportion of days with bleeding (days when bleeding was recorded divided by the days of observation) was 12.3% (4%‐22%). All subjects who completed the trial were adherent with all doses of SC MarzAA; the median exposure days was 50 in Part 2. Three dose escalations of 60, 90, and 120 μg/kg (maximum dose) were allowed during Part 2. Nine subjects did not require a dose escalation and remained on 30 μg/kg throughout Part 2. Four subjects experienced bleeds in Part 2 of the trial. One subject experienced a spontaneous bleed (hematoma to the abdominal wall) before 5 days of dosing of Part 2 and did not dose escalate. One subject experienced a traumatic bleed, which did not warrant dose escalation, caused by a contact injury to his right thumb muscle after 36 days of treatment with 30 μg/kg SC MarzAA in Part 2. Two subjects experienced spontaneous bleeds (hematuria and right knee joint injury, respectively) that required dose escalation to 60 μg/kg. Dose escalation beyond 60 μg/kg was not required. Subjects experienced >90% reduction in all bleeding, and seven of nine subjects had no bleeding (spontaneous or traumatic) at the final dose level (Figure 4). The mean ABR significantly reduced from 19.8 to 1.6 (95% confidence interval [CI], 15.2‐21.1; P = .009), while the mean proportion of days with bleeding significantly declined from 12.3% to 0.8% (95% CI, 7.5%‐15.6%; P = .009). The median pretreatment bleeding interval was 19.5 days (range, 13.9‐25.0 days) and was prolonged to a median of 50 days (range, 5‐50 days) while receiving MarzAA. No subjects required a blood transfusion. None of the enrolled subjects had been on a prophylactic treatment. In Part 2, the injection volume ranged from 0.6 to 1.3 mL for the 30 μg/kg SC dose, and for the 2 subjects who required 60 μg/kg the volumes were 1.7 and 1.75 mL, respectively; all doses were given as a single injection.
FIGURE 4.
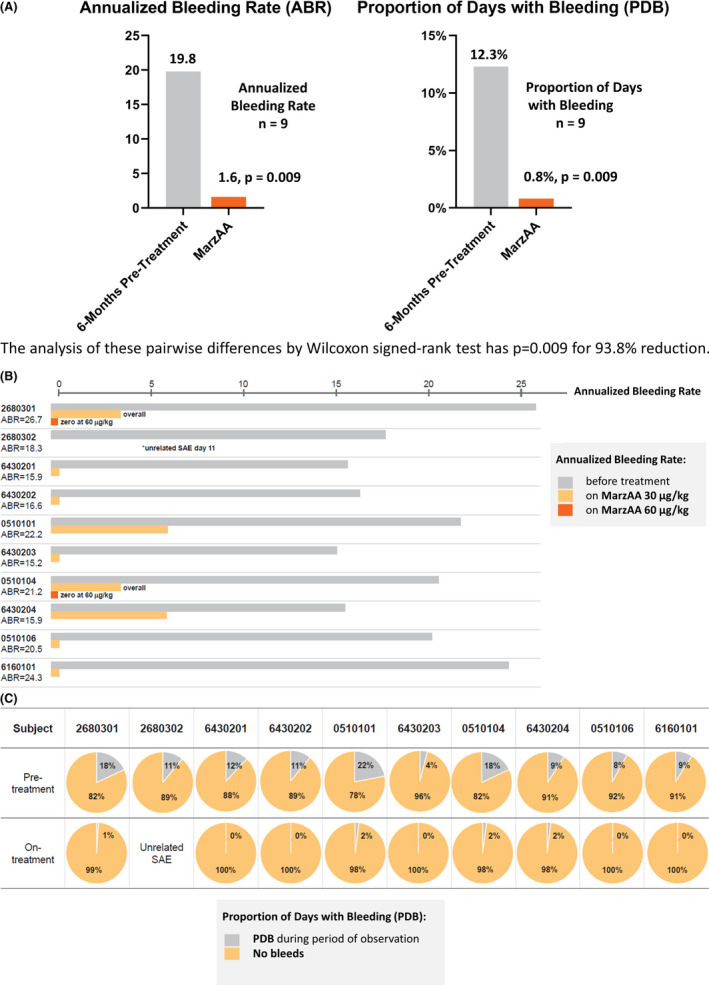
Marzeptacog alfa (activated) demonstrated statistically significant reduction in all bleeding. (A) Overall; (B) ABR; (C) PDB. ABR, annualized bleeding rate; PDB, proportion of days with bleeding
3.3. PK and PD
PK parameters of SC versus IV MarzAA are summarized in Table 2. Of note, levels of MarzAA at the time taken to reach the maximum or peak drug concentration (tmax) for SC administration (SC tmax=7 hours) were comparable to the declining levels of MarzAA at 12 hours after IV infusion (SC = 18.1 ng/mL, IV = 16.0 ng/mL). The mean SC bioavailability was 27% (95% CI, 16%‐37%). The t1/2β was prolonged when MarzAA was administered via SC administration compared with IV (SC t1/2β=17.0 hours, IV t1/2β=3.65 hours); the MRT for MarzAA was also prolonged when administered SC compared with IV dosing (SC MRT=25.8 hours, IV MRT=4.05 hours). These data document a more prolonged effect when MarzAA is administered SC.
TABLE 2.
Summary of pharmacokinetic antigen parameters for IV and SC administration of marzeptacog alfa (activated)
| PK parameter | IV | SC | ||||
|---|---|---|---|---|---|---|
| Mean | SEM | CI | Mean | SEM | CI | |
| t1/2α (h) | 1.47 | 0.29 | 1.08‐2.12 | NP | NP | NP |
| t1/2β(h) | 3.65 | 0.23 | 3.23‐4.13 | 17.0 | 3.1 | 10.9‐23.0 |
| MRT (h) | 4.05 | 0.39 | 3.30‐4.80 | 25.8 | 4.5 | 17.0‐34.6 |
| Cmax (ng/mL) | 375 | 54 | 257‐467 | 24.0 | 4.5 | 15.7‐32.8 |
| tmax (h) | 0.5 | 0.4 | 0‐0.9 | 7.0 | 0.8 | 5.4‐8.6 |
| AUC0‐t (h • ng/mL) | 1076 | 97 | 866, 1252 | 473 | 132 | 188‐688 |
| AUC0‐inf (h • ng/mL) | 1102 | 101 | 902‐1295 | 609 | 190 | 186‐891 |
| Bioavailability (%) | NP | NP | NP | 27 | 6 | 16‐37 |
| Trough (ng/mL) during 30 μg/kg dosing | NP | NP | NP | 6.3 | 1.15 | 3.69‐8.02 |
| Peak (ng/mL) during 30 μg/kg dosing | NP | NP | NP | 18.7 | 1.59 | 15.7‐22.1 |
| Trough (ng/mL) during 60 μg/kg dosing | NP | NP | NP | 16.1 | 4.08 | 7.99‐23.8 |
| Peak (ng/mL) during 60 μg/kg dosing | NP | NP | NP | 51.5 | 9.79 | 30.0‐69.1 |
Abbreviations: AUC0‐inf, area under the curve from time zero to infinity; AUC0‐t, area under the curve at the last measurable concentration; CI, confidence interval; Cmax, maximum or peak drug concentration; IV, intravenous; MRT, mean residence time; NP, not performed; SC, subcutaneous; SEM, standard error of mean; t1/2α, half‐life due to redistribution; t1/2β, half‐life due to drug elimination/metabolism; tmax, time taken to reach Cmax.
For MarzAA treatment in the PD population, the change from baseline for the following parameters were analyzed: aPTT, D‐dimer, fibrinogen, PT, F1+2, and TAT values (Table 3). Prothrombin time in Part 1a and Part 1b decreased and returned to baseline within 24 and 48 hours, respectively; in Part 2, minor fluctuations were noted throughout the 50‐day period, with a return to baseline at the end of the trial. In Parts 1a and 1b, aPTT decreased from baseline and returned to baseline within 24 or 48 hours, respectively. Fibrinogen in Parts 1a and 1b decreased slightly from baseline with a return to baseline levels within 24 and 48 hours, respectively; in Part 2, fibrinogen decreased through day 5 and returned to baseline values for the remainder of the 50‐day period.
TABLE 3.
Summary of change from baseline for specific pharmacodynamic parameters
| Parameter (unit) | Statistic | Part 1a and 1b | Part 2 | ||
|---|---|---|---|---|---|
|
MarzAA IV 18 μg/kg (N=10) |
MarzAA SC 30 μg/kg (N=9) |
MarzAA SC 30 μg/kg (N=10) |
MarzAA SC 60 μg/kg (N=2) |
||
| PT (sec) | Median | −3.7 | −2.8 | −3.0 | −4.8 |
| Min, Max | −5.3, 1.5 | −4.8, −0.3 | −6.5, 1.2 | −4.8, 3.8 | |
| aPTT (sec) | Median | −10.6 | 1.3 | −8.3 | −15 |
| Min, Max | −57.4, 140.8 | −30.7, 50.1 | −47.3, 30.3 | −20.15, 2.0 | |
| Fibrinogen (mg/dL) | Median | −10 | −15 | −4 | −4 |
| Min, Max | −173, 51 | −133, 108 | −112, 146 | −84, 116 | |
| D‐dimer (μg/L) | Median | 0a | 0a | 17 | 112.5 |
| Min, Max | −73, 176 | −47, 266 | −47, 778 | 0, 125 | |
| F1+2 (pmol/L) | Median | 156.5 | 57 | 76.5 | 93 |
| Min, Max | −136, 967 | −42, 1093 | −49, 1090 | 34, 1058 | |
| TAT complexes (μg/L) | Median | −6.9 | 3.7 | −0.05 | 1.3 |
| Min, Max | −3.0, 497.6 | −4.8, 370.4 | −6.7, 379.9 | −3.7, 37 | |
Modified intent‐to‐treat population.
Abbreviations: aPTT, activated partial thromboplastin time; F1+2, prothrombin fragments 1+2; IV, intravenous; MarzAA, marzeptacog alfa (activated); PT, prothrombin time; SC, subcutaneous; TAT, thrombin‐antithrombin.
The median change of zero reflect that the majority of values were set to 200 (μg/L) because D‐dimer was below the level of quantification at all time points.
A more complete PK/PD profile for MarzAA will be reported separately; however, the data in this trial are conformant with those from the phase 1 trial (NCT04072237).
3.4. Safety
3.4.1. Adverse events
An overall summary of treatment‐emergent adverse events (TEAEs) in the Safety Population in Parts 1 and 2 of the trial, is presented in Table 4. In Part 1, no related TEAE or serious adverse events (SAEs, AE leading to discontinuation, or death) were reported. In Part 2 (MarzAA 30 μg/kg), eight (80.0%) subjects reported a TEAE, and three (30.0%) subjects had TEAE considered related to the study drug (injection site reaction, anemia, and hematoma). There was one fatal SAE: intracerebral hemorrhage (ICH) and hypertension, which resulted in the subject’s death on day 11. This patient had a history of episodes of hypertension for which he was prescribed captopril but was not taking it at the time of trial enrollment. In the 4 weeks before the ICH, the subject had normal measurements of his blood pressure (highest systolic level was 125 mm Hg with the highest diastolic 75 mm Hg). Blood pressure on admission with ICH was 195/95 mm Hg. Computed tomography scan showed 120 mL left temporal and frontal lobe ICH with spread to ventricles and displacement of midline structures. One subject experienced a TEAE of blepharitis and dyspepsia, considered not related to the study drug. A total of 517 SC doses were administered with six injection site reactions in two subjects reported (one subject reported moderate swelling on day 21; one had mild redness on days 15 and 18; moderate redness on days 19, 20, and 21 and all resolved without sequelae); 1 was reported as an AE and was treated with topical hydrocortisone. No adverse or abnormal findings were shown for clinical laboratory, chemistry, vital signs, or other safety measurements. No subjects experienced a thrombotic event.
TABLE 4.
Treatment‐emergent adverse events by system organ class and preferred term
| System organ classa n (%)b | MarzAA IV 18 μg/kg (N = 10) | MarzAA SC 30 μg/kg (N = 9) |
|---|---|---|
| Part 1 | ||
| Any TEAE | 1 (10.0) | 1 (11.1) |
| Gastrointestinal disorders | 0 | 1 (11.1) |
| Vomiting | 0 | 1 (11.1) |
| Metabolism and nutrition disorders | 1 (10.0) | 0 |
| Hyperglycemia | 1 (10.0) | 0 |
| Nervous system disorders | 0 | 1 (11.1) |
| Headache | 0 | 1 (11.1) |
| Vascular disorders | 1 (10.0) | 0 |
| Hypertension | 1 (10.0) | 0 |
| System organ classa | MarzAA 30 μg/kg (N = 10) | MarzAA 60 μg/kg (N = 2) |
|---|---|---|
| Part 2 | ||
| Any TEAE | 8 (80.0) | 1 (50.0) |
| Blood and lymphatic system disorders | 2 (20.0) | 0 |
| Anemia | 2 (20.0) | 0 |
| Eye disorders | 0 | 1 (50.0) |
| Blepharitis | 0 | 1 (50.0) |
| Gastrointestinal disorders | 0 | 1 (50.0) |
| Dyspepsia | 0 | 1 (50.0) |
| General disorders and administration site conditions | 1 (10.0) | 0 |
| Injection site reaction | 1 (10.0) | 0 |
| Infections and infestations | 1 (10.0) | 0 |
| Respiratory tract infection | 1 (10.0) | 0 |
| Injury, poisoning, and procedural complications | 1 (10.0) | 0 |
| Contusion | 1 (10.0) | 0 |
| Musculoskeletal and connective tissue disorders | 2 (20.0) | 0 |
| Arthralgia | 1 (10.0) | 0 |
| Hemarthrosis | 1 (10.0) | 0 |
| Nervous system disorders | 1 (10.0) | 0 |
| Hemorrhagic stroke | 1 (10.0) | 0 |
| Psychiatric disorders | 1 (10.0) | 0 |
| Panic attack | 1 (10.0) | 0 |
| Vascular disorders | 3 (30.0) | 0 |
| Hematoma | 1 (10.0) | 0 |
| Hemorrhagic vasculitis | 1 (10.0) | 0 |
| Hypertension | 1 (10.0) | 0 |
A subject with multiple events in a given system organ class or preferred term was counted only once per system organ class or preferred term. TEAEs are defined as AEs that occurred on or after the first dose of study medication. TEAEs were assigned to a treatment based on the time of occurrence in relation to the last treatment administered prior to the onset of the TEAE.
Abbreviations: AE, adverse event; IV, intravenous; MarzAA, marzeptacog alfa (activated); SC, subcutaneous; TEAE, treatment‐emergent adverse event.
Adverse events preferred term were coded using the Medical Dictionary for Regulatory Activities Version 20.1. Percentages are based on the number of subjects in the safety population in each treatment group.
Number of patients with events and percentage of patients with event in the defined group.
3.4.2. Immunogenicity
Immunogenicity assessments were negative or normal for any occurrence of antibody formation resulting in a decreased endogenous level of FVII or FVIIa, occurrence of antibody response to MarzAA, and the number and the percentage of antibody formation resulting in a decreased endogenous level of FVII or FVIIa was zero. Therefore, no ADAs to MarzAA or FVIIa were detected at baseline or throughout the trial or safety follow‐up period in treated subjects.
3.5. Exploratory outcomes
For exploratory parameters in Parts 1 and 2 of the trial, change from baseline in QoL measurements for EQ‐5D‐5L and EQ VAS, Haem‐A‐QoL, and HAL showed no clinically meaningful findings.
4. DISCUSSION
A total of 17 subjects were screened, and 11 subjects received the study drug. The primary efficacy end point was met: The mean ABR and proportion of days with bleeding had a 90% reduction. Seven of 9 participants had no bleeding at their final dose level for 50 days. Results suggest that SC MarzAA achieves therapeutic levels rapidly and significantly decreases frequency of bleeding. Furthermore, the SC t1/2β was extended (17 hours compared with an IV t1/2β of 3.65 hours). MRT for MarzAA was also prolonged when administered SC compared with IV dosing (SC MRT = 25.8 hours, IV MRT = 4.05 hours). Pharmcodynamic results showed appropriate reduction in PT and aPTT with SC administration; D‐dimer, F1+2, TAT complex, and fibrinogen changes did not show thrombogenic concerns, and SC changes were similar to those after IV administration.
A fatal SAE of ICH occurred in a subject participating in Part 2. This was ascribed by the principal investigator as due to untreated episodic hypertension. The subject reported concomitant medication of nimesulide (a nonsteroidal anti‐inflammatory drug), which he took intermittently. While reactive hypertension can be present after ICH, the principal investigator assessed the hypertension as related to his prior history of isolated episodes of hypertension, and causative of the ICH that was not related to use of MarzAA. The external safety monitors concurred with this decision. ICH is associated with high mortality accounting for 10% to 20% of all stroke cases and is mostly induced by hypertension. There was no evidence of any thrombotic event. The dose of MarzAA (30 μg/kg) was not protective, and it is unknown whether any dose level could have prevented ICH.
Overall, MarzAA demonstrated an acceptable safety profile when dosed daily for 50 days, and up to 97 days in one subject. No ADAs were detected during treatment and in the posttreatment follow‐up period. More than 500 SC injections were administered, with six injection site reactions reported for two subjects; all resolved without sequelae. MarzAA was well tolerated and without any treatment‐related SAE.
Several emerging nonfactor therapies are in clinical trials for bleed prevention in people with hemophilia with inhibitors, including fitusiran (siRNA‐AT3),28 anti–tissue factor pathway inhibitor29 and anti–activated protein C.30 A prophylaxis trial of daily IV dosing of rVIIa showed bleeding frequency was reduced by 45% and 59% during prophylaxis with 90 and 270 μg/kg, respectively (P < .0001), with no significant difference between the two dose levels31; however, a mean of eight and five joint bleeds per month, respectively, occurred during the treatment period, considerably greater than in our trial. Intravenous infusion of three times per week of aPCC was associated with a 62% reduction in all bleeding episodes (P < .001) and a 61% reduction in hemarthroses (P < .001), but participants had a mean of five bleeds during the 6‐month treatment period compared with episodic treatment, thus higher than in our trial.32
The addition of MarzAA, an improved rFVIIa variant with a unique PD profile allowing SC use, may provide a new treatment option to prevent and treat bleeding events in patients and may potentially help address existing unmet medical need in patients with FVII deficiency or hemophilia B with inhibitors, in whom the bispecific antibody emicizumab cannot work, and in people with hemophilia A with inhibitors who may experience loss of efficacy with emicizumab. The objective of this trial was to evaluate the use of SC MarzAA as a prophylactic agent; however, MarzAA may also be useful to treat breakthrough bleeds by means of SC injections based on the PK data in this trial and other work. MarzAA has at least nine times the specific activity of wt‐FVIIa as measured by IV comparative PD in dogs. This increased specific activity allows for SC dosing for prophylaxis and treatment of bleeding. A pivotal registrational phase 3 trial is currently under way to determine whether treatment of bleeding with SC MarzAA is noninferior to IV standard‐of‐care treatment (NCT04489537). Intravenous MarzAA remains an option for treatment of bleeding should SC prove not be efficacious. One of the main arguments against nonfactor replacement therapies (NFTs) such as emicizumab is the need to use different hemostatic drugs in addition to NFTs for breakthrough bleeds with some concerns around thrombotic potential of such combinations.8 In normal physiology, the endogenous procoagulant and anticoagulant pathways are interwoven with multiple regulatory interactions to provide hemostasis while minimizing thrombosis.33 The hemostatic effects of NFTs circumvent these regulatory interactions to therapeutically “rebalance” the coagulation cascade to address the underlying bleeding disorder. However, the new NFT‐induced hemostatic balance is likely not as stable as that of normal physiology or with targeted coagulation factor replacement. Thrombotic complications are rare but are a well‐recognized risk, especially when combined with other hemostatic therapies.8
It is important to highlight that any molecular modifications of therapeutic proteins, such as FVIIa, may create a non–self‐epitope that can stimulate innate immune responses. Consequently, such alterations could trigger the development of ADAs, with or without neutralizing activity, hypersensitivity reactions, or breakdown of immune tolerance to the endogenous protein. Neutralizing antibodies may, therefore, be reported following administration of novel therapies. Clinical development trials for other bioengineered extended t1/2 rFVIIa products, including glycopegylated rFVIIa (N7‐GP),34 vatreptacog alfa (activated),35 and BAY 86‐615036, 37 were prematurely stopped due to a lack of a dose‐response or development of neutralizing ADAs. However, antibodies to a therapeutic protein may not have clinical effects, as shown by Whelan et al., who found that ≈30% of people with hemophilia A have antibodies binding to FVIII without any effect on treatment outcome.36, 37, 38, 39, 40 Although there were no ADAs to MarzAA or FVIIa detected at baseline or throughout the trial or safety follow‐up period in treated subjects, and >500 exposure days were recorded for 46 to 99 exposure days by participants, immunogenicity should continue to be closely monitored in future studies with MarzAA. A total of 46 individuals have been exposed to MarzAA without detection of ADAs. The phase 3 trial (NCT04489537) currently recruiting patients will provide further evidence of the safety evaluation of MarzAA in a larger cohort of people with hemophilia A and B treated with inhibitors for multiple bleeding episodes over a longer period.
This phase 2, open‐label, SC prophylaxis trial met all primary and secondary end points. The results of this trial must be interpreted in light of certain limitations. First, there is no single established and validated means for assessment of efficacy. Thus, efficacy in this trial was defined as ABR (spontaneous and total) during Part 2 when on final MarzAA dose level versus recorded ABR. Part 2 relied on patient diaries to assess treatment in the home setting. There are some limitations of patient diaries, mainly due to the reliance on patient adherence and self‐reported measures.
5. CONCLUSION
This multicenter, open‐label, phase 2 trial with MarzAA (NCT03407651) enrolled adults with severe congenital hemophilia A or B with an inhibitor. The data demonstrated that an individualized dose of daily SC MarzAA can significantly decrease the frequency of bleeding and provide effective prophylaxis in people with hemophilia with inhibitors.
RELATIONSHIP DISCLOSURE
JM reports receiving research trial grants from BioMarin, Catalyst Biosciences, CSL Behring, Freeline Therapeutics, Novo Nordisk, Novartis, Pfizer, Sanofi, Roche, Spark; Takeda; advisory board/consultation fees from CSL Behring, Catalyst Biosciences, Freeline Therapeutics, Novo Nordisk, Roche, Sanofi, Spark, Takeda, and speaker fees from CSL Behring, Catalyst Biosciences, Freeline Therapeutics, Novo Nordisk, Roche, Sanofi, Spark, and Takeda. GI, BK, KH, LM, and MK have nothing to disclose. ML, HL, and FDG report personal fees from Catalyst Bioscience, during the conduct of the trial.
AUTHOR CONTRIBUTIONS
JM was involved in conceptualization, methodology, data collection, and report critical analysis and approval. HL was involved in conceptualization, methodology, interpretation of data, and report critical analysis and approval. ML was involved in the formal analysis and interpretation of data. MVK was involved in the data collection and report critical analysis and approval. HK was involved in the data collection and report critical analysis and approval. BK was involved in the data collection and report critical analysis and approval. LM was involved in the data collection and report critical analysis and approval. GI was involved in the data collection and report critical analysis and approval. FDG was involved in conceptualization, methodology, and report critical analysis and approval.
Supporting information
Table S1‐S3
ACKNOWLEDGMENTS
This trial was supported by Catalyst Biosciences, Inc. Medical writing support was provided by Bronwyn Boyes and sponsored by Catalyst Biosciences.
Mahlangu J, Levy H, Kosinova MV, et al. Subcutaneous engineered factor VIIa marzeptacog alfa (activated) in hemophilia with inhibitors: Phase 2 trial of pharmacokinetics, pharmacodynamics, efficacy, and safety. Res Pract Thromb Haemost. 2021;5:e12576. 10.1002/rth2.12576
Handling Editor: Pantep Angchaisuksiri.
Contributor Information
Johnny Mahlangu, @johnnynmahlangu.
Howard Levy, Email: hlevy@catbio.com, @catalystbio.
REFERENCES
- 1.Mannucci PM, Tuddenham EG. The hemophilias‐from royal genes to gene therapy. N Engl J Med. 2001;344(23):1773‐1779. [DOI] [PubMed] [Google Scholar]
- 2.Nienhuis AW, Nathwani AC, Davidoff AM. Gene therapy for hemophilia. Mol Ther. 2017;25(5):1163‐1167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Peyvandi F, Garagiola I, Young G. The past and future of haemophilia: diagnosis, treatments, and its complications. Lancet. 2016;388(10040):187‐197. [DOI] [PubMed] [Google Scholar]
- 4.Astermark J, Santagostino E, Keith HW. Clinical issues in inhibitors. Haemophilia. 2010;16(suppl 5):54‐60. [DOI] [PubMed] [Google Scholar]
- 5.Franchini M, Mannucci PM. Inhibitors of propagation of coagulation (factors VIII, IX and XI): a review of current therapeutic practice. Br J Clin Pharmacol. 2011;72(4):553‐562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Sultan Y. Prevalence of inhibitors in a population of 3435 hemophilia patients in France. French Hemophilia Study Group. Thromb Haemost. 1992;67(6):600‐602. [PubMed] [Google Scholar]
- 7.Wight J, Paisley S. The epidemiology of inhibitors in haemophilia A: a systematic review. Haemophilia. 2003;9(4):418‐435. [DOI] [PubMed] [Google Scholar]
- 8.Oldenburg J, Mahlangu JN, Kim B, et al. Emicizumab prophylaxis in hemophilia A with inhibitors. N Engl J Med. 2017;377(9):809‐818. [DOI] [PubMed] [Google Scholar]
- 9.FEIBA US Package Insert. (2020). FEIBA Prescribing Insert. Retrieved from https://www.shirecontent.com/PI/PDFs/FEIBA_USA_ENG.pdf. Accessed 22 October 2020.
- 10.Kempton CL, Meeks SL. Toward optimal therapy for inhibitors in hemophilia. Blood. 2014;124(23):3365‐3372. [DOI] [PubMed] [Google Scholar]
- 11.Kempton CL, White GC 2nd. How we treat a hemophilia A patient with a factor VIII inhibitor. Blood. 2009;113(1):11‐17. [DOI] [PubMed] [Google Scholar]
- 12.NovoSeven RT US Package Insert. (2020). NovoSeven RT Prescribing Information. Retrieved from https://www.novo‐pi.com/novosevenrt.pdf. Accessed 22 October 2020.
- 13.SevenFact US Package Insert. (2020). SevenFact Prescribing Insert. Retrieved from https://sevenfact.com/Content/PDF/Sevenfact_PI.pdf. Accessed 22 October 2020.
- 14.Carcao M, Goudemand J. Inhibitors in Hemophilia: A Primer. World Federation of Hemophilia (WFH). Nov 2018. Retrieved from www1.wfh.org/publication/files/pdf‐1122.pdf#:~:text=Inhibitors%20in%20Hemophilia%3A%20A%20Primer%205%20Of%20the,and%20immune%20regulatory%20gene%20polymorphisms%20are%20fairly%20weak. Accessed 22 October 2020.
- 15.Hartmann J, Croteau SE. 2017 Clinical trials update: innovations in hemophilia therapy. Am J Hematol. 2016;91(12):1252‐1260. [DOI] [PubMed] [Google Scholar]
- 16.Abshire T, Kenet G. Recombinant factor VIIa: review of efficacy, dosing regimens and safety in patients with congenital and acquired factor VIII or IX inhibitors. J Thromb Haemost. 2004;2(6):899‐909. [DOI] [PubMed] [Google Scholar]
- 17.Lusher JM. Early treatment with recombinant factor VIIa results in greater efficacy with less product. Eur J Haematol. 1998;61(S63):7‐10. [DOI] [PubMed] [Google Scholar]
- 18.Pipe SW. The promise and challenges of bioengineered recombinant clotting factors. J Thromb Haemost. 2005;3(8):1692‐1701. [DOI] [PubMed] [Google Scholar]
- 19.Stoner KL, Harder H, Fallowfield LJ, Jenkins VA. Intravenous versus subcutaneous drug administration. Which do patients prefer? A systematic review. Patient. 2015;8(2):145‐153. 10.1007/s40271-014-0075-y. [DOI] [PubMed] [Google Scholar]
- 20.Srivastava A, Santagostino E, Dougall A, et al. WFH Guidelines for the Management of Hemophilia, 3rd edition. Haemophilia. 2020;26(S6):1‐158. 10.1111/hae.14046. [DOI] [PubMed] [Google Scholar]
- 21.Pittman D, Weston S, Shields K, et al. A novel FVIIa variant with increased potency and duration of effect compared to wildtype FVIIa. A study in a dog model of hemophilia A. Blood. 2011;118(21):2252. [Google Scholar]
- 22.Patel‐Hett S, Rakhe S, Zhao ML, Shields K, Madison EL, Arkin S, Murphy JE, Pittman DD. PF‐05280602 (CB813d), a factor VIIa variant with enhanced in vitro potency compared to wildtype factor VIIa in hemophilic hemostasis assays [poster]. Presented at: Annual Meeting of the International Society on Thrombosis and Haemostasis, Toronto, Ontario, Canada, 20–25 June 2015.
- 23.Patel‐Hett S, Rakhe S, Zhao ML, et al. PF‐05280602, a factor VIIA variant with enhanced in vitro potency compared to wild‐type factor VIIA in hemophilic hemostasis assays [abstract PO597‐WED]. J Thromb Haemost. 2015;13(suppl 1):968. [Google Scholar]
- 24.Varki A. Biological roles of oligosaccharides: all of the theories are correct. Glycobiology. 1993;3:97‐130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Food and Drug Administration (FDA) . Immunogenicity Testing of Therapeutic Protein Products —Developing and Validating Assays for Anti‐Drug Antibody Detection. Guidance for Industry. January 2019. Retrieved from https://www.fda.gov/media/119788/download. Accessed on April 20, 2021.
- 26.Lee ML, Poon W‐Y, Kingdon HS. A two‐phase linear regression model for biological half‐life data. J Lab Clin Med. 1990;115:745‐748. [PubMed] [Google Scholar]
- 27.Lee ML, Schroth P, Bray G, Gomperts ED. The use of robust regression techniques to obtain improved coagulation factor half‐life estimates. XVIth Congress of the International Society for Thrombosis and Hemostasis, Florence, Italy, 1997.
- 28.Pasi KJ, Rangarajan S, Georgiev P, et al. Targeting of antithrombin in hemophilia A or B with RNAi therapy. N Engl J Med. 2017;377(9):819‐828. [DOI] [PubMed] [Google Scholar]
- 29.Eichler H, Angchaisuksiri P, Kavakli K, et al. A randomized trial of safety, pharmacokinetics and pharmacodynamics of concizumab in people with hemophilia A. J Thromb Haemost. 2018;16(11):2184‐2195. [DOI] [PubMed] [Google Scholar]
- 30.Polderdijk SG, Adams TE, Ivanciu L, Camire RM, Baglin TP, Huntington JA. Design and characterization of an APC‐specific serpin for the treatment of hemophilia. Blood. 2017;129(1):105‐113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Konkle BA, Ebbesen LS, Erhardtsen E, et al. Randomized, prospective clinical trial of recombinant factor VIIa for secondary prophylaxis in hemophilia patients with inhibitors. J Thromb Haemost. 2007;5(9):1904‐1913. [DOI] [PubMed] [Google Scholar]
- 32.Leissinger C, Gringeri A, Antmen B, et al. Anti‐inhibitor coagulant complex prophylaxis in hemophilia with inhibitors. N Engl J Med. 2011;365(18):1684‐1692. [DOI] [PubMed] [Google Scholar]
- 33.Willyard C. Thrombosis: balancing act. Nature. 2014;515(7528):S168‐S169. [DOI] [PubMed] [Google Scholar]
- 34.Ljung R, Karim FA, Saxena K, et al. Pioneer™1 Investigators. 40K glycoPEGylated, recombinant FVIIa: 3‐month, double‐blind, randomized trial of safety, pharmacokinetics and preliminary efficacy in hemophilia patients with inhibitors. J Thromb Haemost. 2013;11(7):1260‐1268. [DOI] [PubMed] [Google Scholar]
- 35.Mahlangu JN, Weldingh KN, Lentz SR, et al. adept™2 Investigators. Changes in the amino acid sequence of the recombinant human factor VIIa analog, vatreptacog alfa, are associated with clinical immunogenicity. J Thromb Haemost. 2015;13(11):1989‐1998. [DOI] [PubMed] [Google Scholar]
- 36.Mahlangu J, Paz P, Hardtke M, Aswad F, Schroeder J. TRUST trial: BAY 86–6150 use in haemophilia with inhibitors and assessment for immunogenicity. Haemophilia. 2016;22(6):873‐879. [DOI] [PubMed] [Google Scholar]
- 37.Koh PL, Ng HJ, Lissitchkov T, Hardtke M, Schroeder J. The TRUST trial: anti‐drug antibody formation in a patient with hemophilia with inhibitors after receiving the activated factor VII product Bay 86–6150. Blood. 2013;122(21):573. [Google Scholar]
- 38.Shapiro AD, Mitchell IS, Nasr S. The future of bypassing agents for hemophilia with inhibitors in the era of novel agents. J Thromb Haemost. 2018;16:2362‐2374. [DOI] [PubMed] [Google Scholar]
- 39.de van Weert M, Horn Møller E, eds. Immunogenicity of Biopharmaceuticals. Springer; 2008. [Google Scholar]
- 40.Shankar G, Pendley C, Stein KE. A risk‐based bioanalytical strategy for the assessment of antibody immune responses against biological drugs. Nat Biotechnol. 2007;25:555‐561. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Table S1‐S3


