Abstract
Background
Clostridioides difficile (C. difficile) is one of the primary pathogens responsible for infectious diarrhea. Antibiotic treatment failure, occurring in about 30% of patients, and elevated rates of antibiotic resistance pose a major challenge for therapy. Reinfection often occurs by isolates that produce biofilm, a protective barrier impermeable to antibiotics. We explored the association between antibiotic resistance (in planktonic form) and biofilm-production in 123 C. difficile clinical isolates.
Results
Overall, 66 (53.6%) out of 123 isolates produced a biofilm, with most of them being either a strong (44%) or moderate (34.8%) biofilm producers. When compared to susceptible isolates, a statistically higher percentage of isolates with reduced susceptibility to metronidazole or vancomycin were biofilm producers (p < 0.0001, for both antibiotics). Biofilm production intensity was higher among tolerant isolates; 53.1% of the metronidazole-susceptible isolates were not able to produce biofilms, and only 12.5% were strong biofilm-producers. In contrast, 63% of the isolates with reduced susceptibility had a strong biofilm-production capability, while 22.2% were non-producers. Among the vancomycin-susceptible isolates, 51% were unable to produce biofilms, while all the isolates with reduced vancomycin susceptibility were biofilm-producers. Additionally, strong biofilm production capacity was more common among the isolates with reduced vancomycin susceptibility, compared to susceptible isolates (72.7% vs. 18.8%, respectively). The distribution of biofilm capacity groups was statistically different between different Sequence-types (ST) strains (p =0.001). For example, while most of ST2 (66.7%), ST13 (60%), ST42 (80%) isolates were non-producers, most (75%) ST6 isolates were moderate producers and most of ST104 (57.1%) were strong producers.
Conclusions
Our results suggest an association between reduced antibiotic susceptibility and biofilm production capacity. This finding reinforces the importance of antibiotic susceptibility testing, mainly in recurrence infections that may be induced by a strain that is both antibiotic tolerant and biofilm producer. Better adjustment of treatment in such cases may reduce recurrences rates and complications. The link of biofilm production and ST should be further validated; if ST can indicate on isolate virulence, then in the future, when strain typing methods will be more available to laboratories, ST determination may aid in indecision between supportive vs. aggressive treatment.
Keywords: C. difficile, reduced antibiotic susceptibility, recurrence, metronidazole, vancomycin, biofilm production
Introduction
Clostridioides difficile (C. difficile) is a Gram-positive, spore-forming, anaerobic bacteria, that constitutes one of the primary pathogens responsible for nosocomial diarrhea (Johnson and Gerding, 1998; De Roo and Regenbogen, 2020). C. difficile infection (CDI) is primarily induced by antibiotic therapy, which alters the gut microbiome (Khurana et al., 2020). Along with elevated CDI incidence, the evolution of CDI has changed over the past few decades, due to emergence of hypervirulent strains associated with increased disease severity and high complication and morbidity rates (Goudarzi et al., 2014). The major virulence factors contributing to CDI are toxin production and spore formation (Voth and Ballard, 2005; Rupnik et al., 2009; Semenyuk et al., 2014; Janoir, 2016). Ingestion of the highly resistant spores is the first step toward CDI onset, which is followed by spore germination in the gut and bacterial colonization (Semenyuk et al., 2014). Toxins A and B secreted by the vegetative cells, mediate CDI pathogenesis, which manifests as damage to intestinal cells and by a proinflammatory host response (Voth and Ballard, 2005; Goudarzi et al., 2014; Janoir, 2016).
The accepted treatments had long been vancomycin and metronidazole, with the last being the first line treatment (Vuotto et al., 2016). However, due to higher recurrences rates and lower clinical cure rates, compared to vancomycin, metronidazole was replaced in 2018 with oral vancomycin or fidaxomicin (McDonald et al., 2018). Paradoxically, antibiotics may induce further changes in the gut microbiome, resulting in recurrence of CDI, which occurs in approximately 25% of the patients following treatment completion (Johnson and Gerding, 1998; Johnson, 2009; Goudarzi et al., 2014). Patients with disease recurrence, are prone to episodic reappearances, with risks of 45% and 64% for second and third episodes, respectively (Surawicz and Alexander, 2011). Mostly, recurrence is associated with the original strain (Figueroa et al., 2012), assigned to either resistance to the administered antibiotic or to a protective, antibiotic-impermeable barrier it forms (Dawson et al., 2021). This barrier is composed of sessile, surface-associated microbial communities, known as a biofilm (Dawson et al., 2012). Previous studies have demonstrated in vitro and in vivo formation of biofilms by C. difficile (Lawley et al., 2009; Buckley et al., 2011; Dawson et al., 2012; Donelli et al., 2012; Dapa and Unnikrishnan, 2013; Ethapa et al., 2013; Semenyuk et al., 2014; Semenyuk et al., 2015; Crowther et al., 2016; Vuotto et al., 2016; Jain et al., 2017; Soavelomandroso et al., 2017; James et al., 2018; Poquet et al., 2018; Dawson et al., 2021; Normington et al., 2021), which have been shown to include polysaccharides, proteins and extracellular DNA (Dawson et al., 2021), as well as toxins and spores (Dapa and Unnikrishnan, 2013; Semenyuk et al., 2014) [Reviewed at (Frost et al., 2021)].
The biofilm has been suggested to play several roles, including evasion of the host immune system and protection from antimicrobials (Semenyuk et al., 2014; Hall and Mah, 2017). While various studies have reported on an association between biofilm and C. difficile antibiotic resistance in the biofilm form, there is scant evidence whether the antibiotic susceptibility of the planktonic cells is associated with their ability to produce a biofilm. This prompted us to investigate whether resistant isolates are more likely to form biofilms as compared to sensitive isolates.
Materials and Methods
Bacterial Isolates
This study was performed at the clinical microbiology laboratory of the Baruch Padeh Medical Center, Israel. One-hundred and twenty-three clinical C. difficile isolates were randomly chosen for this study. These isolates were previously isolated from stool samples of patients aged ≥18 years, who were diagnosed with CDI during hospitalization at the center, between January 2018 and April 2020. Poriya Baruch Padeh Medical Center Helsinki Committee, POR-0085-15, approved the study. A signed informed consent for participation was obtained from all patients.
Stool samples were inoculated on chromID™ C. difficile (bioMérieux, Durham, NC). Following incubation of the agar plates at 37°C under anaerobic conditions for 48 h, C. difficile colonies were identified using the Bruker Biotyper system (Bruker Daltonics, Bremen, Germany), which is based on the matrix-assisted laser desorption ionization-time of flight (MALDI TOF) technique (Singhal et al., 2015).
Antibiotic Susceptibility Testing
Antimicrobial susceptibility testing (AST) was performed using the Etest technique, which determines the minimum inhibitory concentration (MIC), i.e., the minimal antibiotic concentration that inhibits the growth of bacteria under specific conditions. To this end, C. difficile colonies were suspended in Thioglycolate broth medium (Becton Dickinson, Heidelberg, Germany) until 1.0 McFarland turbidity. The inoculum was then seeded on Brucella blood agar growth medium (Hy Laboratories, Rehovot, Israel). Gradient Etest strips (bioMérieux, Durham, NC) of vancomycin and metronidazole were added to the plates. Following incubation under anaerobic conditions, at 37°C, for 24 h, the MIC was determined for each antibiotic. Test results (susceptible/with reduced susceptibility) were interpreted according to the European Committee on Antimicrobial Susceptibility Testing (EUCAST) recommendations (EUCAST, 2021), that determined the following epidemiological cut-offs- reduced susceptibility for vancomycin is defined with an MIC above 2 µg/mL; reduced susceptibility to metronidazole is defined with an MIC above 2 µg/mL.
Table S1 presents all the collected data regarding the study’s isolates (antibiotic susceptibility, ST strain and biofilm production capacity). Table S2 presents a comparison of MIC between Etest and micro broth dilution, for the validation of Etest.
Microtiter Plate Assay for the Assessment of Biofilm Production
Biofilm production by the different strains was assessed using a microtiter plate, as previously described (Hammond et al., 2014). Each C. difficile strain was cultured in brain heart infusion agar supplemented with yeast extract + 0.1% L-cysteine (BHIS), at 37°C, for 24 h. A sample was taken from the BHIS broth and normalized to an OD600nm of 0.8. Then, the inoculum was diluted 1:100 in BHIS broth and a 200 μl aliquot of each diluted inoculum was dispensed into a 96-well Nunc flat-bottom microtiter plate.
BHIS was used as a negative control and the biofilm-forming ATCC® BAA-1382 C. difficile (strain 630) was used as a positive control. The plate was incubated at 37°C, for 24 h, in an anaerobic cabinet. Following incubation, spent medium was removed and wells were washed twice with phosphate buffered saline (PBS) (Oxoid, Cambridge, UK). PBS was discarded and 200 μl of 0.25% (w/v) aqueous crystal violet were added to each well. After 5 min, the wells were washed with PBS eight times and air-dried. Then, 200 μl ethanol: acetone (1:1) solution was added to each well in order to solubilize the dye from adherent cells. Absorbance was measured within 5 min, at 570 nm, using an ELISA reader (Multiskan Go, Fisher-Scientific Ltd., Vantaa, Finland). The cut-off OD570 (ODc) was determined as three standard deviations above the mean OD of the negative control.
The isolates were classified as: non-producers - isolates with OD<ODC, weak producers - isolates with ODc < OD ≤ 2× ODc, moderate producers - 2× ODc < OD ≤ 4× ODc and strong producers - OD> 4× ODc (Stepanovic et al., 2000). According to this calculation, the following ranges of OD570 were used: Non producers- OD < 0.35, Weak producers- 0.36< OD< 0.7, Moderate producers- 0.71< OD< 1.4, Strong producers- OD> 1.41. The OD570 range of the positive control strain 630 was 2.1-2.4.
The experiment was repeated 3 times, with triplicates of each strain tested in each experiment.
Multi-Locus Sequence Typing (MLST) of Bacterial Isolates
Total genomic DNA was extracted from bacterial isolates using the QIAamp DNA Mini Kit (QIAGEN, Hilden, Germany), according to the manufacturer’s instructions. MLST was performed as previously described (Griffiths et al., 2010):
A. Amplification: The DNA of 7 housekeeping genes of each isolate was amplified in a polymerase chain reaction (PCR) using the qPCRBIO SyGreen Blue Mix Hi-ROX kit (PCR Biosystems Inc., Wayne, Pennsylvania, USA), on a real-time PCR device (BioRad CFX96 Real-Time Detection System, Hercules, CA, USA). Amplification conditions were: 95°C for 2 min, followed by 40 cycles of (95°C for 5 s, and 60–65°C for 20–30 s).
B. Sequencing and Sequence Type (ST) Determination: the 7 PCR amplicons were purified and their nucleotide sequences were determined using amplification primers, as previously described (Griffiths et al., 2010). The capillary electrophoresis method was used for genotyping on an ABI PRISM® 310 Genetic Analyzer (Applied Biosystems, Foster City, CA, USA). Data analysis was performed using ChromasLite v2.01 (Technelysium DNA Sequencing Software, South Brisbane, Australia) and Sequencher v5.1 (Gene Codes Corporation, Ann Arbor, USA).
Following sequencing, the allelic numbers of each gene and the STs were determined using the PubMLST C. difficile database (http://pubmlst.org/cdifficile/). Each ST number was determined for each specific combination of alleles.
Statistical Analysis
Chi-square test was applied for analyzing differences in the proportions of the different categories of biofilm production capacity, between susceptible isolates and isolates with reduced susceptibility to metronidazole and vancomycin. Tables S3 and S4 presents the expected and observed values for each Chi square analysis.
Fisher’s Exact Test was performed to determine if there were ST specific differences in the biofilm production capacity (p<0.05 indicates there were ST specific differences). Fisher’s exact test was performed to determine if there were ST specific differences in antibiotic susceptibility (p<0.05 indicates there were ST specific differences).
The Analysis of Variance (ANOVA) test was applied for analyzing differences in MIC values between the different isolates’ groups according to the biofilm producing capacity.
All assays were repeated 3 times (n=3). All tests applied were two-tailed, and a p value of 5% or less was considered statistically significant. The data was analyzed using the SAS® version 9.3 (SAS Institute, Cary, North Carolina). Additional data can be found in the Supplementary File.
Results
Sequence Types of Study’s Isolates
Following MLST analysis, we categorized the isolates into eight major ST groups (each group containing at least 5 isolates) and additional group called “others”, which combined several different STs (with less than 5 isolates per ST). The most common ST among our isolates was ST4 (24/119, 20.2%), followed by ST42 (10/119, 8.4%), ST13 (10/119, 8.4%), ST37 (9/119, 7.6%), ST6 (8/119, 6.7%), ST1 (7/119, 5.9%), ST104 (7/119, 5.9%) and ST2 (6/119, 5%).
Antibiotic Susceptibility Profiles
Of the 123 tested clinical C. difficile isolates, 27 (22%) showed reduced susceptibility to metronidazole (Table 1). Average and median MIC of the metronidazole-susceptible isolates were 0.4 μg/mL and 0.25 μg/mL, respectively. In contrast, isolates with reduced susceptibility to metronidazole had an average MIC of 246.7 μg/mL and a median MIC of 256 μg/mL.
Table 1.
Antibiotic susceptibility profiles of study isolates.
| Antibiotic/Characteristics | Metronidazole | Vancomycin | ||
|---|---|---|---|---|
| Susceptible Isolates (MIC≤ 2) n=96 | Isolates with Reduced Susceptibility (MIC>2) n=27 | Susceptible Isolates (MIC≤ 2) n=112 | Isolates with Reduced Susceptibility (MIC>2) n=11 | |
| Average MIC, (μg/mL) |
0.4 | 246.7 | 0.6 | 118.7 |
| Median MIC (Q1, Q4*), (μg/mL) |
0.25 (0.125, 1.5) | 256 (256, 256) | 0.38 (0.25, 2) | 8 (4, 256) |
*Q1, Q4 indicate quartiles 1 and 4, respectively.
Reduced susceptibility to vancomycin was found in 11 (9%) isolates. Average and median MIC of the vancomycin-susceptible isolates were 0.6 μg/mL and 0.38 μg/mL, respectively. The isolates with reduced susceptibility to vancomycin had an average and a median MIC of 118.7 μg/mL and 8 μg/mL, respectively (Table 1). Figures S1 and S2 present the distribution of metronidazole and vancomycin MICs for all isolates, respectively. MIC of the control strain 630 was 0.094 μg/mL and 1 μg/mL for metronidazole and vancomycin, respectively.
Next, we tested whether there were ST specific differences in antibiotic susceptibility. We found that metronidazole susceptibility pattern was diverse between different ST (p= 0.015) (Table S5). Specifically, ST104 accounted for most of the differences. For vancomycin, the differences in susceptibility pattern between STs were not statistically significant.
Biofilm Production Capacity
Overall, 53.6% (66/123) isolates were able to produce a biofilm (Table 2). Most of the biofilm-producing isolates were either strong (29/66, 44%) or moderate (23/66, 34.8%) biofilm producers.
Table 2.
Biofilm production capacity of study isolates.
| Non-biofilm producing isolates | Biofilm-producing isolates | |||
|---|---|---|---|---|
| Weak producers | Moderate producers | Strong producers | ||
| N (%) | 57 (46.3) | 14 (11.4) | 23 (18.7) | 29 (23.6) |
Association Between ST and Biofilm Production Capacity
We compared the biofilm production capacity of isolates from different STs and found that the distribution of biofilm capacity groups (non-producers, weak-, moderate- and strong producers) was statistically different between the different STs (p =0.001) (Table 3). For example, while most isolates of ST13 (60%), ST42 (80%) and ST2 (66.7%) were non-producers, most (75%) isolates of ST6 were moderate producers and most of ST104 (57.1%) isolates were strong producers. One isolate which belong to ST43 (mean OD570 = 2.22) produced a biofilm as strong as the control 630 strain which belong to ST54 (Saito et al., 2019). Two other isolates that belong to ST8 and ST239 had a stronger biofilm production capacity, compared to the control strain (mean OD570 = 3.93 and 3.34, respectively). All three strains, as well as the control strain 630, belong to clade 1.
Table 3.
Biofilm production capacity among the different STs.
| ST*(%) | Non-biofilm producing isolates N(%) | Biofilm-producing isolates | Total N (%) | p value** | ||
|---|---|---|---|---|---|---|
| Weak producers N(%) | Moderate producers N(%) | Strong producers N(%) | ||||
| 1 (5.9) | 1 (14.3) | 3 (42.9) | 3 (42.9) | 0 | 7 (5.9) | 0.001 |
| 2 (5) | 4 (66.7) | 1 (16.7) | 0 | 1 (16.7) | 6 (5) | |
| 4 (20.2) | 13 (54.2) | 2 (8.3) | 2 (8.3) | 7 (29.2) | 24 (20.2) | |
| 6 (6.7) | 2 (25) | 0 | 6 (75) | 0 | 8 (6.7) | |
| 13 (8.4) | 6 (60) | 1 (10) | 3 (30) | 0 | 10 (8.4) | |
| 37 (7.6) | 4 (44.4) | 1 (11.1) | 0 | 4 (44.4) | 9 (7.6) | |
| 42 (8.4) | 8 (80) | 0 | 0 | 2 (20) | 10 (8.4) | |
| 104 (5.9) | 3 (42.9) | 0 | 0 | 4 (57.1) | 7 (5.9) | |
| Others*** (31.9) | 15 (39.5) | 3 (7.9) | 9 (23.7) | 11 (28.9) | 38 (31.9) | |
*The MLST analysis of 4 isolates is missing.
** Fisher’s Exact Test was performed to determine if there were ST specific differences in the biofilm production capacity; p value < 0.05.
*** Others= ST3, ST8, ST10, ST12, ST17, ST35, ST43, ST54, ST55, ST59, ST60, ST103, ST153, ST239, ST421 and ST439.
Association Between Antibiotic Resistance and Biofilm Production Capacity
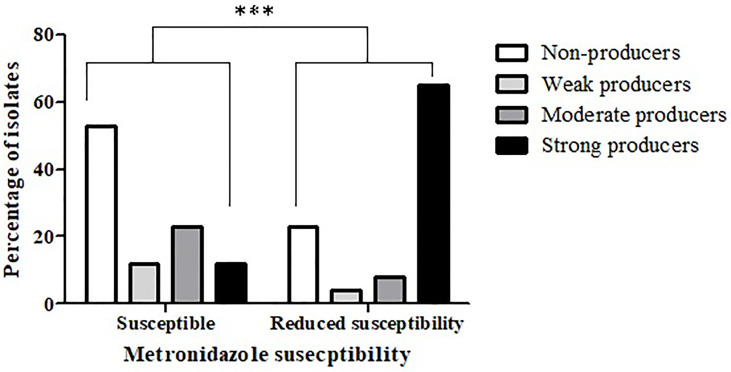
Comparison of biofilm production capacity between isolates that were metronidazole-susceptible versus isolates with reduced susceptibility to metronidazole, revealed a significantly higher rate of biofilm producers among isolates with reduced susceptibility (p < 0.0001) (Figure 1). Specifically, 53.1% (51/96) of the metronidazole-susceptible isolates were not able to produce biofilms, and only 12.5% (12/96) were strong biofilm-producers. In contrast, most (63%, 17/27) of the isolates with reduced susceptibility had a strong biofilm-producing capacity, while 22.2% (6/27) isolates were non-producers.
Figure 1.
Biofilm production capacity among metronidazole-susceptible isolates and isolates with reduced susceptibility. The biofilm production capacity of C. difficile isolates was compared between metronidazole-susceptible (n = 96) and reduced metronidazole susceptibility isolates (n = 27) (***p < 0.0001). The p value represents the comparison of the biofilm production capacity between susceptible isolates and isolates with reduced susceptibility (Chi square analysis).
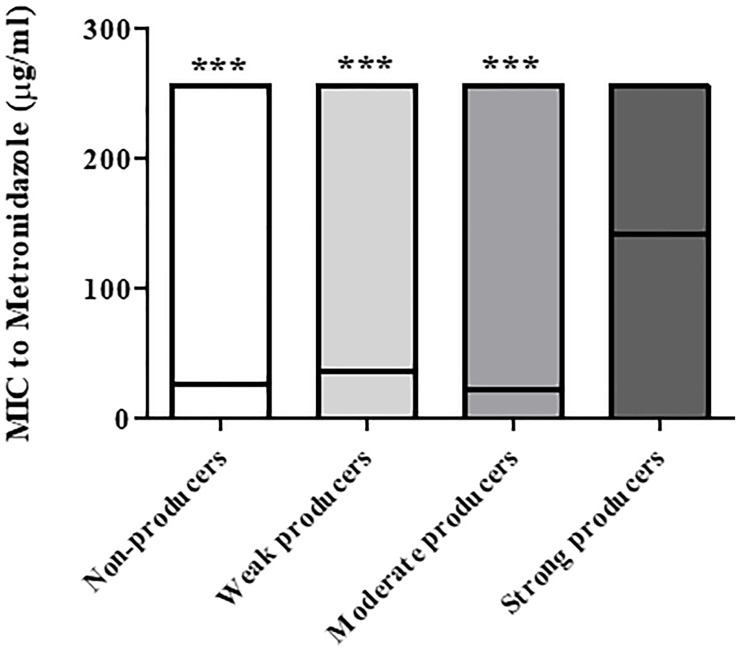
Figure 2 presents the distribution of metronidazole MIC values among the different isolates in relation to their biofilm production capacity. Strong biofilm producers had statistically higher mean MIC (141.6 μg/mL) compared to all other biofilm production categories (27.3, 36.8, and 22.6 μg/mL for non-producers, weak producers and moderate producers, respectively; p < 0.0001).
Figure 2.
Distribution of Metronidazole MIC values of non-biofilm producers, weak-, moderate- and strong biofilm-producing C. difficile isolates. The minimal, maximal and average MIC values are shown (the average is indicated by the line within the bar) for each group of isolates (non-producers, weak-, moderate- and strong producers’ groups). The mean metronidazole MIC of strong biofilm-producing isolates (n = 29) were significantly higher compared to MIC values of isolates that were non-producers (n = 57), weak producers (n = 14) and moderate producers (n = 23), ***p < 0.001. The p value represents the comparison of the mean MIC between the four categories of biofilm production capacity (ANOVA analysis).
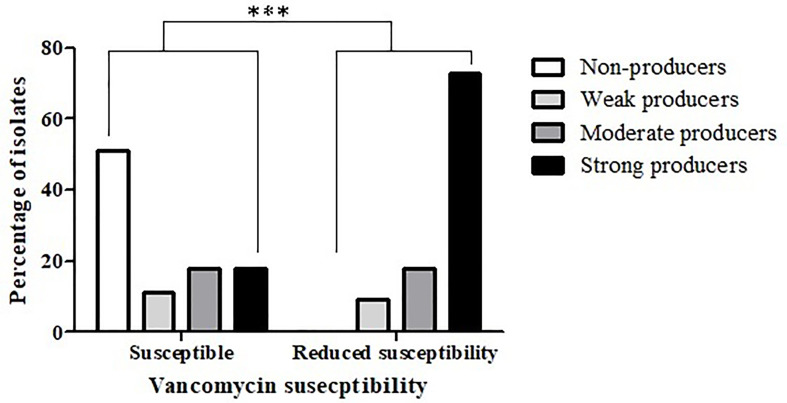
An association between reduced antibiotic susceptibility and biofilm production capacity was also noted in relation to vancomycin (p < 0.0004) (Figure 3); while 51% (57/112) of the vancomycin-susceptible isolates were not able to produce biofilms, all the isolates with reduced vancomycin susceptibility were biofilm-producers. Additionally, strong biofilm production capacity was more common among the isolates with reduced vancomycin susceptibility, compared to the susceptible isolates (72.7% vs. 18.8%, respectively).
Figure 3.
Biofilm production capacity among isolates that are vancomycin-susceptible and isolates with reduced vancomycin- susceptibility. The biofilm-production capacity of C. difficile isolates was compared between isolates that are vancomycin-susceptible (n = 112) and isolates with reduced vancomycin susceptibility (n = 11) isolates (***p < 0.0001). The p value represents the comparison of the biofilm production capacity between susceptible isolates and isolates with reduced susceptibility (Chi square analysis).
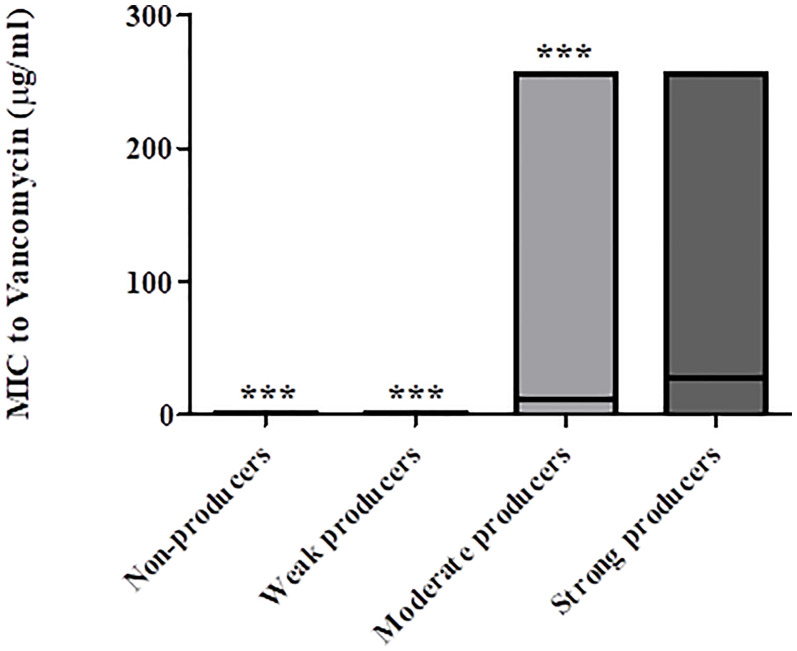
The distribution of vancomycin MIC values also indicated an association between reduced vancomycin susceptibility and biofilm-producing capacity (Figure 4); strong biofilm-producing isolates had higher mean vancomycin MIC (28.9 μg/mL) than non-producers, weak- and moderate- biofilm producers (0.5 μg/mL, 20.3 μg/mL, and 12.2 μg/mL, respectively; p < 0.0001).
Figure 4.
Distribution of vancomycin MIC values of non-biofilm producers, weak-, moderate- and strong biofilm producers. The minimal, maximal and average MIC values are shown (the average is indicated by the line within the bar) for each group of isolates (non-producers, weak-, moderate- and strong producers’ groups). The mean vancomycin MIC of strong biofilm producers (n = 29) were significantly higher as compared to MIC values of isolates that were non-producers (n = 57), weak producers (n = 14) and moderate producers (n = 23), ***p < 0.001. The p value represents the comparison of the mean MIC between the four categories of biofilm production capacity (ANOVA analysis).
Discussion
C. difficile biofilm production has been associated with disease recurrence, which poses a major challenge for CDI treatment (Dapa and Unnikrishnan, 2013). Although C. difficile biofilms have been studied both in vitro and in vivo, most of them explored only a limited number of strains (Lawley et al., 2009; Buckley et al., 2011; Dawson et al., 2012; Dapa and Unnikrishnan, 2013; Ethapa et al., 2013; Semenyuk et al., 2014; Semenyuk et al., 2015; Crowther et al., 2016; Vuotto et al., 2016; Soavelomandroso et al., 2017; James et al., 2018; Poquet et al., 2018; Dubois et al., 2019; Dawson et al., 2021; Normington et al., 2021). Additionally, previous studies have not compared the biofilm production capacity of antibiotic-susceptible versus antibiotic-tolerant isolates.
In the current study, we reported on the antibiotic susceptibility and biofilm-production capacity of 123 C. difficile isolates. This information is novel, considering the fact that most in vitro studies of C. difficile biofilms focused on 3-7 different well-characterized strains (Dapa and Unnikrishnan, 2013; Ethapa et al., 2013; Semenyuk et al., 2014; Vuotto et al., 2016; James et al., 2018). We found that 53.6% of the tested isolates were able to produce a biofilm. This finding is interesting in light of the fact that only one previous study has found non-biofilm producers among C. difficile isolates and their percentage was very low (2/102, 1.9%) (Tijerina-Rodriguez et al., 2019). However, 81.4% of the isolates in this study belonged to ribotype 027 (RT027, ST1), which is known to produce biofilm (Semenyuk et al., 2014). Another previously reported study that profiled 37 isolates, applied different biofilm production classification system, and had no category of non-producers (Pantaleon et al., 2018). Regarding the other categories of biofilm production, Tijerina-Rodriguez et al., 2019 found that 87.25% of the study’s isolates were strong producers (Tijerina-Rodriguez et al., 2019). As mentioned earlier, most of these isolates were RT027. Thus, is it possible that these isolates originated from the same strain. In the study of Pantaleon et al., 2018, the distribution of biofilm production capacity was: 50%- low, 17.6%-moderate and 32.4%-strong capacity (Pantaleon et al., 2018). Although the distribution differs from our results, it is difficult to compare the isolates’ biofilm production capacity, since we used different classification system.
Interestingly, we found an association between the biofilm capacity to the ST. However, this association should further be explored due to the low number of isolates in each ST group. A former study with 37 isolates, has not found an association between biofilm production and ST, notwithstanding biofilm production capacity was variable among the different strains (Pantaleon et al., 2018). In the same study, strong biofilm- producing isolates belonged to ST3 (RT027), ST19 (RT05), ST21 (RT03), ST26 (RT156), ST33 (RT095), ST34 (RT014/020) and ST36 (RT165) (Pantaleon et al., 2018). In contrast, our strong biofilm- producing isolates belonged to other STs including ST2, ST4, ST8, ST37, ST42, ST43, ST55, ST59, ST60, ST104, ST239, ST421, and ST439. In another study, RT014 (ST 49/13/2) and RT017 (ST37/45) were found to be strong biofilm producers (Kullin et al., 2016). It is important to note that most of these ST are genetically different and even strains that belong to the same phylogenetic clade are genetically divergent due to frequent horizontal gene transfer and homologous recombination (Didelot et al., 2012; Stabler et al., 2012). On the other hand, we noticed that most strong producers in our study and in a previous study (Pantaleon et al., 2018) belong to clade 1. In another study which investigated biofilm production and structure in five strains representative of the five phylogenetic lineages, the strongest biofilm producer, strain RT012 (our control strain 630), also belong to clade 1 (Dawson et al., 2021). Similar biofilm formation capabilities were seen in RT023 and RT078, that belong to clade 3 and to the most divergent clade 5, respectively (Dawson et al., 2021). In the current study, ST37 which belong to clade 4 also presented high biofilm production capacity. Thus, further studies should be performed in order to confirm whether there is an association between ST and biofilm production or alternatively between clade and biofilm production. If such an association truly exists, ST determination at the beginning of CDI may aid in identification of hypervirulent isolates and rapid adjustment of specific treatment.
The main aim of the current work was to test whether isolates with reduced susceptibility to antibiotics have a greater biofilm-producing capacity than susceptible isolates. We found a higher percentage of biofilm producers among isolates with reduced metronidazole- or vancomycin- susceptibility as compared to the susceptible isolates. Furthermore, isolates with reduced metronidazole or vancomycin isolates were characterized with a stronger biofilm-producing capacity as compared to susceptible isolates. To the best of our knowledge, this is the first time that biofilm production capacity is associated with reduced antibiotic susceptibility in C. difficile planktonic cells. Most studies have determined the antibiotic susceptibility of C. difficile biofilms, showing that biofilm cells are more tolerant of several antibiotics, compared to their planktonic counterparts (Dawson et al., 2012; Dapa and Unnikrishnan, 2013; Crowther et al., 2014; Semenyuk et al., 2014; Dawson et al., 2021). In contrast, we defined the antibiotic susceptibility of the different isolates before biofilm production, and associated the susceptibility profile with the biofilm-forming capacity. Similar findings were reported in other bacterial species (Arciola et al., 2005; Klingenberg et al., 2005; de Araujo et al., 2006; Agarwal and Jain, 2012; Yekani et al., 2017; Ratajczak et al., 2021). For example, Staphylococcus epidermidis strains that produced exopolysaccharide had a significantly higher prevalence of resistance to aminoglycosides, sulfamethoxazole and ciprofloxacin, compared to non-producing isolates. Additionally, multiple resistance to antibiotics was more common among biofilm-producing isolates (Arciola et al., 2005). In a previous study with Pseudomonas aeruginosa, biofilm-producing isolates showed higher resistance rate to β-lactams and aminoglycosides antibiotics than non-biofilm-producing isolates. Additionally, biofilm production capacity and multi drug resistance (MDR) were significantly associated (Yekani et al., 2017).
Our findings arouse a question whether reduced antibiotic susceptibility and biofilm production are regulated by the same pathways. Alternatively, it is possible that genes conferring reduced antibiotic susceptibility and genes that are associated with biofilm production pathway are co-located on the same genetic element. Although there are no supportive evidences in C. difficle to our hypothesis, several reports in other species may reinforce our assumptions; for example, genes encoding the flagella and fimbriae proteins in Escherichia coli (E. coli) O157:H7 were found to be located on plasmids (Karch et al., 1987). Since some antibiotic resistance genes are also located in plasmids, it is possibly that the same plasmid constitute genes of both traits. Another possible explanation which also concern with plasmids is the promotion of biofilm formation by conjugative plasmids [reviewed at (Madsen et al., 2012)]; it was shown that factors expressed by conjugative plasmid induced biofilm in E. coli and removal of this plasmid resulted in inability of biofilm formation (Ghigo, 2001). These results were later supported by another study in which co-culture of plasmid-bearing and non-bearing E. coli strains resulted in gaining of the biofilm production capacity among the non-bearing isolates. The authors suggested that this capacity was acquired by conjugative plasmid transfer (Reisner et al., 2006).
Interestingly, biofilm development was shown to be influenced by efflux pumps presence. A previous study in Salmonella enterica isolates induced mutations in the efflux pumps that contribute to tetracycline resistance. As a result of the pumps’ loss or inhibition, mutants were unable to produce curli, one of the biofilm’s component in Salmonella. Further experiments revealed that mutants had a transcriptional repression of the two curli biosynthetic operons. The authors proposed there was a link between the regulation of multidrug efflux and biofilm formation via global regulators of efflux (Baugh et al., 2012; Baugh et al., 2014).
Since mechanisms of C. difficile antibiotic resistance to both metronidazole and vancomycin are not clearly understood, it is possible that efflux pumps or plasmids that harbor antibiotic resistance genes are part of these mechanisms. As mentioned above, the presence of either efflux pumps or plasmids may contribute to biofilm production. Therefore, the association of antibiotic resistance and biofilm production in C. difficle should be further investigated in order to find the exact mechanism that is responsible for this association. Nevertheless, this association between reduced susceptibility to antibiotics and biofilm production reinforces the importance of performing antibiotic susceptibility testing. This is mainly necessary in recurrence infections that may be induced by a strain that has both reduced susceptibilities to antibiotics and biofilm production capacity, thus has multiple pathogenicity traits. Better adjustment of antimicrobial treatment in such cases may reduce recurrences rates and complications.
It is known that biofilm production, as well as alterations in antibiotic targets and/or in metabolic routes/paths that directly confer antibiotic resistance can be induced by in vivo exposure to antibiotics (Peng et al., 2017). Sub-inhibitory concentrations of metronidazole (Semenyuk et al., 2014; Vuotto et al., 2016) and vancomycin (Ethapa et al., 2013) were shown to induce biofilm production in several C. difficile strains. Thus, a future study should investigate whether isolates that presented reduced susceptibility to metronidazole/vancomycin and biofilm-producing capacity, were isolated from patients that were previously treated with these antibiotics. Additionally, mutations in genes that confer resistance to antibiotics should be investigated.
The study has some limitations; first, it should be noted that a previous study reported on a heme-dependent increase in the metronidazole-MIC of C. difficile isolates for metronidazole, which in some cases led to interpretation of reduced susceptibility (MIC > 2 mg/L) (Boekhoud et al., 2020). Therefore, it is necessary to validate the MIC values of all the isolates with metronidazole- reduced susceptibility. Second, we have not performed sequencing thus we do not have information regarding the presence of specific resistance genes or plasmids that are known to be associated with antibiotic resistance.
In summary, this study correlated between antibiotic susceptibility and biofilm production, two main pathogenicity traits of C. difficile. Additionally, we found a link between ST to antibiotic susceptibility and to biofilm production. Further studies should be performed to confirm these finding and to reveal the mechanisms responsible for these associations. If ST can indicate on isolate virulence, then in the future, when strain typing methods will be more available to laboratories, ST determination may aid in indecision between supportive vs. aggressive treatment.
Data Availability Statement
The original contributions presented in the study are included in the article/Supplementary Material. Further inquiries can be directed to the corresponding author.
Ethics Statement
The studies involving human participants were reviewed and approved by Poriya Baruch Padeh Medical Center Helsinki Committee, POR-0085-15. The patients/participants provided their written informed consent to participate in this study.
Author Contributions
Conceptualization, LA, MA, and AP. Data curation, LA and MA. Formal analysis, LA and MA. Methodology, LA, MA, and AP. Project administration, MA, and AP. Supervision, MA, and AP. Validation, LA, and MA. Visualization, LA, MA, and AP. Writing—original draft preparation, LA, MA, and AP. Writing—review and editing, LA, MA, and AP. All authors contributed to the article and approved the submitted version.
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s Note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Acknowledgments
We thank Mr. Wadie Abu Dahoud for the statistical analysis.
Supplementary Material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fcimb.2021.683464/full#supplementary-material
References
- Agarwal A., Jain A. (2012). Association Between Drug Resistance & Production of Biofilm in Staphylococci. Indian J. Med. Res. 135, 562–564. [PMC free article] [PubMed] [Google Scholar]
- Arciola C. R., Campoccia D., Gamberini S., Donati M. E., Pirini V., Visai L., et al. (2005). Antibiotic Resistance in Exopolysaccharide-Forming Staphylococcus Epidermidis Clinical Isolates From Orthopaedic Implant Infections. Biomaterials 26, 6530–6535. 10.1016/j.biomaterials.2005.04.031 [DOI] [PubMed] [Google Scholar]
- Baugh S., Ekanayaka A. S., Piddock L. J., Webber M. A. (2012). Loss of or Inhibition of All Multidrug Resistance Efflux Pumps of Salmonella Enterica Serovar Typhimurium Results in Impaired Ability to Form a Biofilm. J. Antimicrob. Chemother. 67, 2409–2417. 10.1093/jac/dks228 [DOI] [PubMed] [Google Scholar]
- Baugh S., Phillips C. R., Ekanayaka A. S., Piddock L. J., Webber M. A. (2014). Inhibition of Multidrug Efflux as a Strategy to Prevent Biofilm Formation. J. Antimicrob. Chemother. 69, 673–681. 10.1093/jac/dkt420 [DOI] [PubMed] [Google Scholar]
- Boekhoud I. M., Sidorov I., Nooij S., Harmanus C., Bos-Sanders I. M. J. G., Viprey V., et al. (2020). Heme Is Crucial for Medium-Dependent Metronidazole Resistance in Clinical Isolates of C. Difficile. bioRxiv. 2020.2011.2018.388959. 10.1101/2020.11.18.388959 [DOI] [PMC free article] [PubMed]
- Buckley A. M., Spencer J., Candlish D., Irvine J. J., Douce G. R. (2011). Infection of Hamsters With the UK Clostridium Difficile Ribotype 027 Outbreak Strain R20291. J. Med. Microbiol. 60, 1174–1180. 10.1099/jmm.0.028514-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crowther G. S., Chilton C. H., Todhunter S. L., Nicholson S., Freeman J., Baines S. D., et al. (2014). Development and Validation of a Chemostat Gut Model to Study Both Planktonic and Biofilm Modes of Growth of Clostridium Difficile and Human Microbiota. PloS One 9, e88396. 10.1371/journal.pone.0088396 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crowther G. S., Wilcox M. H., Chilton C. H. (2016). An In Vitro Model of the Human Colon: Studies of Intestinal Biofilms and Clostridium Difficile Infection. Methods Mol. Biol. 1476, 223–234. 10.1007/978-1-4939-6361-4_17 [DOI] [PubMed] [Google Scholar]
- Dapa T., Unnikrishnan M. (2013). Biofilm Formation by Clostridium Difficile. Gut Microbes 4, 397–402. 10.4161/gmic.25862 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dawson L. F., Peltier J., Hall C. L., Harrison M. A., Derakhshan M., Shaw H. A., et al. (2021). Extracellular DNA, Cell Surface Proteins and C-Di-GMP Promote Biofilm Formation in Clostridioides Difficile. Sci. Rep. 11, 3244. 10.1038/s41598-020-78437-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dawson L. F., Valiente E., Faulds-Pain A., Donahue E. H., Wren B. W. (2012). Characterisation of Clostridium Difficile Biofilm Formation, a Role for Spo0A. PloS One 7, e50527. 10.1371/journal.pone.0050527 [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Araujo G. L., Coelho L. R., De Carvalho C. B., Maciel R. M., Coronado A. Z., Rozenbaum R., et al. (2006). Commensal Isolates of Methicillin-Resistant Staphylococcus Epidermidis Are Also Well Equipped to Produce Biofilm on Polystyrene Surfaces. J. Antimicrob. Chemother. 57, 855–864. 10.1093/jac/dkl071 [DOI] [PubMed] [Google Scholar]
- De Roo A. C., Regenbogen S. E. (2020). Clostridium Difficile Infection: An Epidemiology Update. Clin. Colon Rectal Surg. 33, 49–57. 10.1055/s-0040-1701229 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Didelot X., Eyre D. W., Cule M., Ip C. L., Ansari M. A., Griffiths D., et al. (2012). Microevolutionary Analysis of Clostridium Difficile Genomes to Investigate Transmission. Genome Biol. 13, R118. 10.1186/gb-2012-13-12-r118 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Donelli G., Vuotto C., Cardines R., Mastrantonio P. (2012). Biofilm-Growing Intestinal Anaerobic Bacteria. FEMS Immunol. Med. Microbiol. 65, 318–325. 10.1111/j.1574-695X.2012.00962.x [DOI] [PubMed] [Google Scholar]
- Dubois T., Reynaert C., Jacques D., Lepiece B., Zdanowicz N. (2019). Role of Gut Microbiota in the Interaction Between Immunity and Psychiatry: A Literature Review. Psychiatr. Danub 31, 381–385. [PubMed] [Google Scholar]
- Ethapa T., Leuzzi R., Ng Y. K., Baban S. T., Adamo R., Kuehne S. A., et al. (2013). Multiple Factors Modulate Biofilm Formation by the Anaerobic Pathogen Clostridium Difficile. J. Bacteriol. 195, 545–555. 10.1128/JB.01980-12 [DOI] [PMC free article] [PubMed] [Google Scholar]
- EUCAST . (2021). Available at: https://www.eucast.org/fileadmin/src/media/PDFs/EUCAST_files/Breakpoint_tables/v_11.0_Breakpoint_Tables.pdf.
- Figueroa I., Johnson S., Sambol S. P., Goldstein E. J., Citron D. M., Gerding D. N. (2012). Relapse Versus Reinfection: Recurrent Clostridium Difficile Infection Following Treatment With Fidaxomicin or Vancomycin. Clin. Infect. Dis. 55 (Suppl 2), S104–S109. 10.1093/cid/cis357 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frost L. R., Cheng J. K. J., Unnikrishnan M. (2021). Clostridioides Difficile Biofilms: A Mechanism of Persistence in the Gut? PloS Pathog. 17, e1009348. 10.1371/journal.ppat.1009348 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ghigo J. M. (2001). Natural Conjugative Plasmids Induce Bacterial Biofilm Development. Nature 412, 442–445. 10.1038/35086581 [DOI] [PubMed] [Google Scholar]
- Goudarzi M., Seyedjavadi S. S., Goudarzi H., Mehdizadeh Aghdam E., Nazeri S. (2014). Clostridium Difficile Infection: Epidemiology, Pathogenesis, Risk Factors, and Therapeutic Options. Scientifica (Cairo) 2014, 916826. 10.1155/2014/916826 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Griffiths D., Fawley W., Kachrimanidou M., Bowden R., Crook D. W., Fung R., et al. (2010). Multilocus Sequence Typing of Clostridium Difficile. J. Clin. Microbiol. 48, 770–778. 10.1128/JCM.01796-09 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hall C. W., Mah T. F. (2017). Molecular Mechanisms of Biofilm-Based Antibiotic Resistance and Tolerance in Pathogenic Bacteria. FEMS Microbiol. Rev. 41, 276–301. 10.1093/femsre/fux010 [DOI] [PubMed] [Google Scholar]
- Hammond E. N., Donkor E. S., Brown C. A. (2014). Biofilm Formation of Clostridium Difficile and Susceptibility to Manuka Honey. BMC Complement Altern. Med. 14, 329. 10.1186/1472-6882-14-329 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jain S., Smyth D., O’hagan B. M. G., Heap J. T., Mcmullan G., Minton N. P., et al. (2017). Inactivation of the dnaK Gene in Clostridium Difficile 630 Deltaerm Yields a Temperature-Sensitive Phenotype and Increases Biofilm-Forming Ability. Sci. Rep. 7, 17522. 10.1038/s41598-017-17583-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- James G. A., Chesnel L., Boegli L., Delancey Pulcini E., Fisher S., Stewart P. S. (2018). Analysis of Clostridium Difficile Biofilms: Imaging and Antimicrobial Treatment. J. Antimicrob. Chemother. 73, 102–108. 10.1093/jac/dkx353 [DOI] [PubMed] [Google Scholar]
- Janoir C. (2016). Virulence Factors of Clostridium Difficile and Their Role During Infection. Anaerobe 37, 13–24. 10.1016/j.anaerobe.2015.10.009 [DOI] [PubMed] [Google Scholar]
- Johnson S. (2009). Recurrent Clostridium Difficile Infection: Causality and Therapeutic Approaches. Int. J. Antimicrob. Agents 33 Suppl 1, S33–S36. 10.1016/S0924-8579(09)70014-7 [DOI] [PubMed] [Google Scholar]
- Johnson S., Gerding D. N. (1998). Clostridium Difficile–Associated Diarrhea. Clin. Infect. Dis. 26, 1027–1034; quiz 1035-1026. 10.1086/520276 [DOI] [PubMed] [Google Scholar]
- Karch H., Heesemann J., Laufs R., O’brien A. D., Tacket C. O., Levine M. M. (1987). A Plasmid of Enterohemorrhagic Escherichia Coli O157:H7 Is Required for Expression of a New Fimbrial Antigen and for Adhesion to Epithelial Cells. Infect. Immun. 55, 455–461. 10.1128/iai.55.2.455-461.1987 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khurana S., Kahl A., Yu K., Dupont A. W. (2020). Recent Advances in the Treatment of Clostridioides Difficile Infection: The Ever-Changing Guidelines. Fac Rev. 9, 13. 10.12703/b/9-13 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klingenberg C., Aarag E., Ronnestad A., Sollid J. E., Abrahamsen T. G., Kjeldsen G., et al. (2005). Coagulase-Negative Staphylococcal Sepsis in Neonates. Association Between Antibiotic Resistance, Biofilm Formation and the Host Inflammatory Response. Pediatr. Infect. Dis. J. 24, 817–822. 10.1097/01.inf.0000176735.20008.cd [DOI] [PubMed] [Google Scholar]
- Kullin B., Brock T., Rajabally N., Anwar F., Vedantam G., Reid S., et al. (2016). Characterisation of Clostridium Difficile Strains Isolated From Groote Schuur Hospital, Cape Town, South Africa. Eur. J. Clin. Microbiol. Infect. Dis. 35, 1709–1718. 10.1007/s10096-016-2717-6 [DOI] [PubMed] [Google Scholar]
- Lawley T. D., Clare S., Walker A. W., Goulding D., Stabler R. A., Croucher N., et al. (2009). Antibiotic Treatment of Clostridium Difficile Carrier Mice Triggers a Supershedder State, Spore-Mediated Transmission, and Severe Disease in Immunocompromised Hosts. Infect. Immun. 77, 3661–3669. 10.1128/IAI.00558-09 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Madsen J. S., Burmolle M., Hansen L. H., Sorensen S. J. (2012). The Interconnection Between Biofilm Formation and Horizontal Gene Transfer. FEMS Immunol. Med. Microbiol. 65, 183–195. 10.1111/j.1574-695X.2012.00960.x [DOI] [PubMed] [Google Scholar]
- Mcdonald L. C., Gerding D. N., Johnson S., Bakken J. S., Carroll K. C., Coffin S. E., et al. (2018). Clinical Practice Guidelines for Clostridium Difficile Infection in Adults and Children: 2017 Update by the Infectious Diseases Society of America (IDSA) and Society for Healthcare Epidemiology of America (SHEA). Clin. Infect. Dis. 66, 987–994. 10.1093/cid/ciy149 [DOI] [PubMed] [Google Scholar]
- Normington C., Moura I. B., Bryant J. A., Ewin D. J., Clark E. V., Kettle M. J., et al. (2021). Biofilms Harbour Clostridioides Difficile, Serving as a Reservoir for Recurrent Infection. NPJ Biofilms Microbiomes 7, 16. 10.1038/s41522-021-00184-w [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pantaleon V., Monot M., Eckert C., Hoys S., Collignon A., Janoir C., et al. (2018). Clostridium Difficile Forms Variable Biofilms on Abiotic Surface. Anaerobe 53, 34–37. 10.1016/j.anaerobe.2018.05.006 [DOI] [PubMed] [Google Scholar]
- Peng Z., Addisu A., Alrabaa S., Sun X. (2017). Antibiotic Resistance and Toxin Production of Clostridium Difficile Isolates From the Hospitalized Patients in a Large Hospital in Florida. Front. Microbiol. 8, 2584. 10.3389/fmicb.2017.02584 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Poquet I., Saujet L., Canette A., Monot M., Mihajlovic J., Ghigo J. M., et al. (2018). Clostridium Difficile Biofilm: Remodeling Metabolism and Cell Surface to Build a Sparse and Heterogeneously Aggregated Architecture. Front. Microbiol. 9, 2084. 10.3389/fmicb.2018.02084 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ratajczak M., Kaminska D., Nowak-Malczewska D. M., Schneider A., Dlugaszewska J. (2021). Relationship Between Antibiotic Resistance, Biofilm Formation, Genes Coding Virulence Factors and Source of Origin of Pseudomonas Aeruginosa Clinical Strains. Ann. Agric. Environ. Med. 28, 306–313. 10.26444/aaem/122682 [DOI] [PubMed] [Google Scholar]
- Reisner A., Holler B. M., Molin S., Zechner E. L. (2006). Synergistic Effects in Mixed Escherichia Coli Biofilms: Conjugative Plasmid Transfer Drives Biofilm Expansion. J. Bacteriol 188, 3582–3588. 10.1128/JB.188.10.3582-3588.2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rupnik M., Wilcox M. H., Gerding D. N. (2009). Clostridium Difficile Infection: New Developments in Epidemiology and Pathogenesis. Nat. Rev. Microbiol. 7, 526–536. 10.1038/nrmicro2164 [DOI] [PubMed] [Google Scholar]
- Saito R., Usui Y., Ayibieke A., Nakajima J., Prah I., Sonobe K., et al. (2019). Hypervirulent Clade 2, Ribotype 019/Sequence Type 67 Clostridioides Difficile Strain From Japan. Gut Pathog. 11, 54. 10.1186/s13099-019-0336-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Semenyuk E. G., Laning M. L., Foley J., Johnston P. F., Knight K. L., Gerding D. N., et al. (2014). Spore Formation and Toxin Production in Clostridium Difficile Biofilms. PloS One 9, e87757. 10.1371/journal.pone.0087757 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Semenyuk E. G., Poroyko V. A., Johnston P. F., Jones S. E., Knight K. L., Gerding D. N., et al. (2015). Analysis of Bacterial Communities During Clostridium Difficile Infection in the Mouse. Infect. Immun. 83, 4383–4391. 10.1128/IAI.00145-15 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singhal N., Kumar M., Kanaujia P. K., Virdi J. S. (2015). MALDI-TOF Mass Spectrometry: An Emerging Technology for Microbial Identification and Diagnosis. Front. Microbiol. 6, 791. 10.3389/fmicb.2015.00791 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Soavelomandroso A. P., Gaudin F., Hoys S., Nicolas V., Vedantam G., Janoir C., et al. (2017). Biofilm Structures in a Mono-Associated Mouse Model of Clostridium Difficile Infection. Front. Microbiol. 8, 2086. 10.3389/fmicb.2017.02086 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stabler R. A., Dawson L. F., Valiente E., Cairns M. D., Martin M. J., Donahue E. H., et al. (2012). Macro and Micro Diversity of Clostridium Difficile Isolates From Diverse Sources and Geographical Locations. PloS One 7, e31559. 10.1371/journal.pone.0031559 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stepanovic S., Vukovic D., Dakic I., Savic B., Svabic-Vlahovic M. (2000). A Modified Microtiter-Plate Test for Quantification of Staphylococcal Biofilm Formation. J. Microbiol. Methods 40, 175–179. 10.1016/S0167-7012(00)00122-6 [DOI] [PubMed] [Google Scholar]
- Surawicz C. M., Alexander J. (2011). Treatment of Refractory and Recurrent Clostridium Difficile Infection. Nat. Rev. Gastroenterol. Hepatol 8, 330–339. 10.1038/nrgastro.2011.59 [DOI] [PubMed] [Google Scholar]
- Tijerina-Rodriguez L., Villarreal-Trevino L., Baines S. D., Morfin-Otero R., Camacho-Ortiz A., Flores-Trevino S., et al. (2019). High Sporulation and Overexpression of Virulence Factors in Biofilms and Reduced Susceptibility to Vancomycin and Linezolid in Recurrent Clostridium [Clostridioides] Difficile Infection Isolates. PloS One 14, e0220671. 10.1371/journal.pone.0220671 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Voth D. E., Ballard J. D. (2005). Clostridium Difficile Toxins: Mechanism of Action and Role in Disease. Clin. Microbiol. Rev. 18, 247–263. 10.1128/CMR.18.2.247-263.2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vuotto C., Moura I., Barbanti F., Donelli G., Spigaglia P. (2016). Subinhibitory Concentrations of Metronidazole Increase Biofilm Formation in Clostridium Difficile Strains. Pathog. Dis. 74, 1–7. 10.1093/femspd/ftv114 [DOI] [PubMed] [Google Scholar]
- Yekani M., Memar M. Y., Alizadeh N., Safaei N., Ghotaslou R. (2017). Antibiotic Resistance Patterns of Biofilm-Forming Pseudomonas Aeruginosa Isolates From Mechanically Ventilated Patients. Int. J. Sci. Study. 5, 1–5. 10.17354/ijss/2017/1 [DOI] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The original contributions presented in the study are included in the article/Supplementary Material. Further inquiries can be directed to the corresponding author.