Abstract
Hepatitis C virus (HCV) is a global health concern associated with significant morbidity and mortality. Before the approval of second-generation direct-acting antiviral agents (DAAs), interferon therapy and liver transplantation constituted the mainstay of treatment. The introduction of well-tolerated oral DAAs in late 2013 has revolutionized HCV management with over 95% cure rates. The predominance of HCV-related liver transplantations has declined following the widespread approval of DAAs. Despite the unparallel efficacy observed among these novel therapies, pharmaceutical costs continue to limit equitable access to healthcare and likely contribute to the differential HCV infection rates observed globally. To reduce the burden of disease worldwide, essential agenda items for all countries must include the prioritization of integrated care models and access to DAAs therapies. Through transparent negotiations with the pharmaceutical industry, the consideration for compassionate release of medications to promote equitable division of care is paramount. Here we provide a literature review of HCV, changes in epidemiologic trends, access issues for current therapies, and global inequities in disease burden.
Keywords: Hepatitis C virus, Direct-acting antivirals, Global health, Liver transplantation, People who inject drugs, Liver cirrhosis
Core Tip: Hepatitis C virus (HCV) is a global health concern associated with significant morbidity and mortality. The introduction of well-tolerated oral direct-acting antiviral agents in late 2013 has revolutionized HCV management with over 90% cure rates. Equitable access to these agents remains an important issue, given that countries with the greatest burden of disease have the least access to curative therapies. Here we provide a literature review of HCV, changes in epidemiologic trends, current therapies, and highlight inequities in the burden of disease and access to treatment.
INTRODUCTION
Hepatitis C virus (HCV) is a blood-borne RNA virus first described in the 1980s. During the acute phase of the infection, a large proportion of individuals are asymptomatic, contributing to increased spread and transmission. Thirty percent of individuals clear the infection spontaneously within the acute phase or the first six months[1]. According to 2017 World Health Organization (WHO) estimates, less than 20% of individuals infected with HCV are aware of their diagnosis[1].
HCV infection is a global health concern leading to significant morbidity and mortality worldwide[1]. According to the most recent global statistics, approximately 71 million persons are chronically infected with HCV, comprising 1% of the population[1]. Chronic HCV infection is associated with significant downstream complications, including liver cirrhosis, hepatocellular carcinoma (HCC), and end-stage liver disease[2]. In 2016, the WHO developed a global strategy with the aim of reducing the incidence of HCV by 90% and associated deaths by 65% by 2030[3]. This strategy entails raising awareness, preventing transmission, increasing detection, as well as optimizing access to pharmacotherapies to ultimately ensure equitable division of resources globally[3]. Here we provide a literature review of HCV, changes in epidemiologic trends, access issues for current therapies, and global inequities in disease burden.
GLOBAL EPIDEMIOLOGICAL TRENDS
Globally, the seroprevalence of HCV is highly variable and likely underestimated in low- to middle-income countries due to inadequate infrastructure for population-wide testing. The Global Burden of Diseases, Injuries, and Risk Factors 2010 (GBD 2010) Study estimated > 184 million have been infected with HCV[4]. This study identified Central Asia, East Asia, and North Africa–Middle East as the most endemic regions, with > 3.5% of their populations affected by HCV[4]. Of these regions, Asia carries the highest burden of disease, with more than 100 million HCV-affected individuals across South and East Asia[4]. Evaluation of global epidemiologic trends with a focus on regional prevalence identifies Asian and African countries to have some of the highest reported cases, with Egypt (18%-22%) and Pakistan (4.9%) exhibiting the highest rate of chronic infections. Among high-income countries, Italy (2.5%-10%) remains the most impacted, with as high as 10% of its population reporting chronic HCV infection.
Contaminated needles are considered to be the primary cause of spread in low-to middle-income countries[5]. The population-level spread is seen in countries where infection control practices were historically insufficient. For example, treatment of schistosomiasis, an endemic parasitic infection in Egypt, between the late 1950s to 1980s with intravenous tartar emetic unintentionally contributed to HCV transmission[6,7]. Contaminated needles also account for the majority of new HCV cases in high-income countries. In fact, the highest rates of HCV transmission in the United States are currently seen among people who inject drugs, comprising 23% of new infections (1.75 million new infections in 2015) and 33% of deaths from HCV[8]. Approximately 67% of individuals who endorse a history of intravenous drug use test positive for anti-HCV, indicating current or past infection[8].
In the United States, the estimated prevalence of HCV infection in the non-institutionalized civilian population is 1.0%, corresponding to 2.7 million in 2010[9]. HCV increases the risk of HCC by up to 20-folds, making it the leading cause of HCC worldwide and accounting for approximately 34% of cases in the United States[10]. Globally, HCV-related cirrhosis and HCC together contributed to 399000 deaths in 2016[11]. For decades, HCC had been the fastest-rising cause of cancer-related death in the United States, likely attributed to the HCV epidemic, obesity, and increased prevalence of non-alcoholic steatohepatitis[12]. In 2016 we observed the first decline in the rates of HCC (Relative Rate 0.96; P = 0.007), resulting from improved treatments for HCC and increased availability of direct-acting antiviral agents (DAAs)[12]. In terms of HCV-related mortality, since the introduction of DAAs in late 2013, there has been a significant decrease in United States nationwide HCV-related mortality rates compared with the pre-DAAs era. Although cirrhosis and HCC-related mortality continues to increase in recent decades, HCV cirrhosis-related mortality rates markedly declined from 2014 to 2016[13].
In the advent of DAAs, global declines in HCV viremia are anticipated, and over the past decade epidemiologic surveys have demonstrated such a trend[6]. Unfortunately, few direct data exist to establish global trends of HCV burden in the post DAA era[14]. Employing previously validated simulation models, HCV is projected to become a rare disease by 2036 in the United States[15]. Population-based modeling studies employing European data predict that the number of cured HCV patients will exceed the number of viraemic patients[16]. Irrespective of cure, the same researchers foresee a large number of patients remaining untreated, undiagnosed, and developing advanced sequelae of liver disease[16]. Ultimately, it is expected the burden of the disease will shift from patients with viremia to those with virologically cured HCV and expected to reach older age[17].
HCV VIROLOGY AND GENOMIC SEQUENCING
HCV is a single-stranded RNA virus which is easily transmissible via microscopic amounts of as little as 0.6 microlitres of blood[18]. It is primarily transmitted through contaminated needles, inadequate sterilization of medical equipment, transfusion of unscreened blood, as well as high-risk sexual practices. We see higher rates of infection among people from low and middle-income countries, in populations who inject drugs, and in men who have sex with men[5,19].
Following exposure, the virus incubates for 2 wk to 24 wk, though only 20% of patients actually develop symptoms[11]. While a minority of patients spontaneously clear the infection within six months, 70% develop chronic HCV. These individuals are at an elevated risk for significant morbidity and mortality, whereby approximately 20-30% will subsequently develop cirrhosis at 20-30 years[5].
This virus was first described in 1989 as a non-A non-B hepatitis. This was discovered after a significant number of post-transfusion hepatitis cases were identified despite what was felt to be adequate testing for the previously identified hepatitis A and B viruses[20]. It was not until Dr. Michael Houghton’s team isolated complementary DNA from a “non-A non-B” infected patient, cultivating the isolation of viral RNA, which was ultimately termed ‘HCV’[21]. Following the isolation of this strain, Houghton’s team were able to develop accurate serological diagnostic tests, including Elisa immunoassays[21]. Unfortunately, the establishment of HCV through antibody testing alone would under detect a quarter of chronically infected patients. These misclassifications resulted from the seroconversion processes, which occur 4-10 wk after transmission, can be circumvented by HCV RNA reverse transcription-polymerase chain reaction testing[22]. Identification of active infection is the primary utility of the HCV RNA testing modality, which is now universally employed. Genomic sequencing of the HCV was completed by Choo et al[23] in 1991. This remarkable achievement led to the distinct categorization of HCV by varying genotypes, now numbered from 1-7, with further subcategorizations of related HCV genotypic strains known as subtypes (labelled alphabetically)[22,24]. Despite the description of seven known genotypes, the first six remain the most commonly studied and reported[25]. Genomic isolates of HCV have been well characterized by their geographic variation, which is partly explained by human migration, environmental epidemiologic changes (attempts at eradication of schistosomiasis), and viral evolutions observed across continents[25]. A summary of the HCV genotypes, subtypes, and geographic variation can be found in Figure 1. Understanding the genotypes, in addition to their geographic variation, is crucial, as interventions are organized for distinct genotype targets. This significant discovery and contribution to science ultimately garnered international recognition, winning Dr. Houghton’s team the 2020 Nobel Prize in Physiology or Medicine[26].
Figure 1.
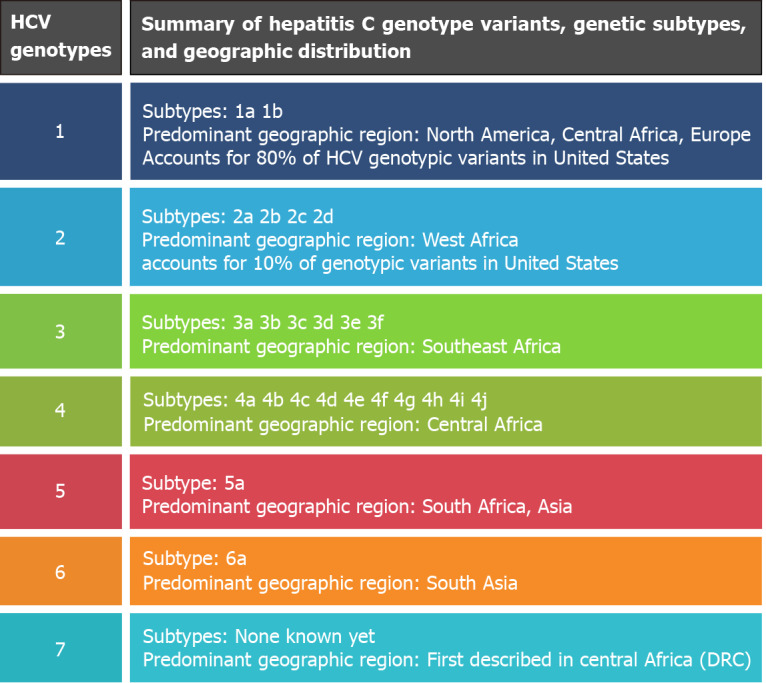
Summary of hepatitis C virus genotypes. HCV: Hepatitis C virus.
OUT WITH THE OLD, IN WITH NEW–NOVEL THERAPEUTICS IN THE TREATMENT OF HCV
The goal of HCV treatment is to cure patients through the eradication of HCV RNA as measured by the sustained virologic response (SVR) at 12 wk after treatment completion[5]. Evaluation of SVR at 12 wk is still considered controversial, where historically, assessment of SVR at 24 wk following treatment completion was considered the gold standard. While high concordance between SVR measured at 12 and 24 wk has been previously demonstrated in trials of pegylated interferon and ribavirin[27], there is less evidence demonstrating the appropriateness of a 12-wk SVR target in the literature for DAAs. A recent systematic review from 2016 did evaluate the concordance of SVR at 12 and 24 wk across two studies of DAAs, demonstrating a reliable correlation of SVR at 12 and 24 wk among multiple populations infected with HCV, including treatment naïve, varying viral genotype, and prior null responders[27]. This evidence was ultimately used to inform future clinical trial design and current recommendations.
Approximately 99% of patients who attain SVR will remain infection-free 5 years later, in the absence of re-infection[28]. Treatment is associated with reduced rates of HCC, liver-related morbidity, as well as liver- and all-cause mortality[5,29,30]. Unless contraindicated, all patients with chronic HCV should be offered treatment. Between 1991 and 2011, combination treatment with pegylated interferon and ribavirin was the mainstay of therapy, achieving SVR rates of up to 40%-50%[5].
DAAs function by targeting proteins involved in HCV replication[29]. The first-generation DAAs for HCV treatments (boceprevir and telaprevir) were approved for use in combination with pegylated interferon and ribavirin in 2011, but have been largely replaced by second-generation DAAs since[29]. The introduction of second-generation DAAs from late 2013 has revolutionized HCV care, achieving cure rates of over 95% among treatment-naïve patients. These interferon-free oral regimens have allowed for major advancements in HCV management, given their ease of administration, much more tolerable side effects profile, and superior efficacy[5,29]. The choice of regimen is guided by HCV genotype, as well as other factors, including previous treatments received and liver cirrhosis[5,29]. Genotypic viral variations add to the complexity of DAA selection. The most desirable therapy would be a pangenotypic option that can be utilized across viral genotypes and subtypes[31]. This was the motive behind the novel NS5A inhibitor, velpatasvir, demonstrated to have 90% SVR at 12 wk across patients with genotypes 1 through 6[32]. Other combination therapies, including glecaprevir/pibrentasvir have also been demonstrated to show pangenoytpic effectiveness[33-35].
Drug-drug interactions (DDIs) are concerning for patients with HCV, especially in the era of DAAs where complex treatment regimens require multiple medications, in conjunction with the other therapies utilized for optimal control of comorbid disease and the side effects of hepatitis treatment itself[31]. The majority of DDIs involve interaction between drugs using the cytochrome P450 enzymes[36]. Careful observation must be given prior to any drug treatment initiation within this population. Often managing teams will include specialist pharmacists responsible for determining the risk for DDIs, approving patients already existing medications, or adding new ones.
Among pregnant women, HCV prevalence estimates range between 0.1% to 8%, depending on the setting and country[37]. However, despite the prevalence of HCV in this population, until recently, the mainstay treatment of HCV, including ribavirin, was considered contraindicated due to the teratogenic and embryotoxic properties of this therapy[37-39]. The introduction of DAAs has opened up questions around the role of DAAs in pregnancy. While there are investigations underway, overall safety data in this population is lacking, and, therefore, treatment with DAAS in pregnancy is currently not recommended in most guidelines[37-39]. For similar reasons, treatment of HCV is also not recommended in breastfeeding women or during lactation[37-39]. Where possible, women of reproductive age should be screened for HCV infection, and if found to have HCV infection, recommendations suggest offering treatment prior to conception and pregnancy, or following the completion of pregnancy and lactation in the postpartum period, and screening[38,39].
Compared to existing guidelines for the treatment of HCV or HBV mono-infection are well delineated in both national and international settings, treatment guidelines for HCV/HBV co-infection are less established and remain an area requiring further data[40]. Early reports on the use of DAAs and interferon-based intervention have suggested an increased risk for HBV reactivation with treatment of HCV without concomitant suppression of HBV[41]. However, the clinical significance of this is inconclusive.
While there has been evidence for lower response rates to DAA treatment in HCV populations with parallel injectable recreational drug use, a 2020 study led by Koustenis et al[42] offers real-world data that sustained virological response to DAAs is possible in high-risk patients who inject drugs[42]. This is in keeping with established trends revealing similar treatment responses in these higher-risk populations to that of the general population[42]. However, adherence and loss to follow-up remain ongoing concerns among high-risk populations, and during the study, over 10% of patients who inject drugs were lost to follow-up during or after treatment[42]. The risk of being lost to follow-up was correlated with several factors, including younger age and parallel recreational drug use[42].
There have been some controversies as to the safety and efficacy of DAAs for reducing HCC[43,44]. Recent studies have reported that DAAs-based SVR resulted in approximately 50%-80% risk reduction of HCC in patients with cirrhosis[45,46]. Based on the recent expert review, successfully eradicating HCV infection by DAAs confers a protective benefit with reduced incidence of HCC[47]. In terms of HCC recurrence, DAAs therapy was not associated with an overall or early increase in HCC recurrence after complete response to HCC treatment[48].
The availability and access to DAAs worldwide differ by national income and setting[49]. For example, Gilead Sciences has issued licenses that would enable over 100 countries to access generic DAA regimens, and some countries, such as Pakistan and Egypt, have enabled other companies to manufacture unlicensed DAAs in generic format. However, there are disparities among low-income countries, and several countries have not been registered for access to DAAs[49]. Lack of global funding and license provision may present potential barriers[49]. Among higher-income countries, such as countries in Europe, Japan, and the United States, overall generic drug competition among manufacturers has significantly lowered drugs prices, which has improved accessibility[49].
In the table below, we summarize drugs within each category based on their target site of action, and the HCV genotype for which they are employed[29] (Table 1).
Table 1.
Classification of direct-acting antiviral agents[29]
|
Class
|
Drug name
|
HCV genotype
|
| NS3/4A inhibitor | Boceprevir1 | Genotypes 1a and 1b |
| Telaprevir1 | ||
| Simeprevir | ||
| Asunaprevir | ||
| Paritaprevir | ||
| Grazoprevir | ||
| Glecaprevir | ||
| Voxilaprevir | ||
| NS5A inhibitor | Daclatasvir | All HCV genotypes |
| Ledipasvir | ||
| Ombitasvir | ||
| Elbasvir | ||
| Velpatasvir | ||
| Pibrentasvir | ||
| NS5B polymerase inhibitor: Nucleoside analogs | Sofosbuvir | All HCV genotypes |
| NS5B polymerase inhibitor: Non-nucleoside analogs | Dasabuvir | Genotypes 1a and 1b |
First generation direct-acting antiviral agents.
HCV: Hepatitis C virus.
Considerable efforts have focused on the design and development of a HCV vaccine. Challenges resulting from differential rates of transmission among distinct subgroups, in conjunction with the notable variability in treatment access, acceptance, and compliance, could be prevented the development of an effective vaccine. Unfortunately, many barriers have prevented its development. Challenges to the generation of live-attenuated inactivated whole virus for HCV stems largely from our inability to culture the virus effectively[50]. In the rare instances this has been accomplished, the cultured strains exhibited adaptive mutations, which increased replication in vitro[50]. These processes become further complicated by the enormous genetic diversity observed with HCV, with 7 known genotypes and more than 80 subtypes. Ultimately, major barriers to vaccine development include the significant genetic variability between HCV strains, challenges in vaccine production and testing models, as well as our incomplete understanding of the innate protective immune responses to HCV[50].
LIVER TRANSPLANTATION IN THE CONTEXT OF HCV
Prior to the approval of second-generation DAAs, interferon treatment and liver transplantation constituted the mainstay treatment options for liver disease attributed to HCV infection. HCV was previously identified as the leading cause of liver transplantation waitlist additions and surgeries over the last two decades in the United States[51-53]. However, newer data suggest that the predominance of HCV-related liver disease-related transplantations has declined following the widespread use and approval of second-generation DAAs[54]. More recently, HCV-related liver disease has been replaced by alcoholic liver disease as the leading cause of liver transplantation in the United States[51]. Between 2012 and 2015, HCV accounted for 28% of liver transplantations on average[51], by 2016, following the use of DAAs, 18% of liver transplantations were attributed to HCV-related liver disease[51].
Without curative therapies including liver transplantation, re-infection with HCV occurs in high frequency and commonly results in significant liver graft destruction, resulting in poor patient survival[55]. Approximately 80% of patients will develop allograft hepatitis within two years of liver transplantation without eradication, and 20% of those patients progress to advanced fibrosis or cirrhosis within five years of transplantation[56]. Of note, cholestatic hepatitis C, which can occur in up to 5% to 10% of patients within one year of liver transplantation, can be rapidly progressive and life-threatening[55]. For this reason, many eligible patients will undergo treatment with DAAs prior to or following liver transplantation, ideally before recurrence[55].
Although the optimal timing of DAAs in the context of liver transplantation is still to be clarified, many eligible patients receive treatment prior to transplantation. The overall low burden of DDIs with widely used immunosuppressants poses no contraindication for use in the post-transplantation period[55]. The advantages of early or pre-transplantation viral eradication can include improved overall liver function, quality of life and liver-related complications, potential avoidance of the need for liver transplantation, and lower rate of post-transplantation infection recurrence[56]. However, pre-transplantation eradication can increase wait times for transplantation by limiting access to HCV-positive donor livers and through the improvement of liver function to the degree that de-prioritizes patients on organ transplantation wait lists[56]. This concept has been referred to as ‘Model for End-stage Liver Disease (MELD) purgatory’ or ‘MELD limbo’[55].
The financial burden of liver transplantation remains considerable and is almost exclusive to high-income countries. In Canada, the average cost of liver transplantation in Ontario is $121732 CAN, and in the United States, the average cost is estimated to be around $163438 USD, including follow-up care and medication-related costs. This is compared to other Organization for Economic Cooperation and Development countries, where costs have been estimated at a mean of $103548 USD[57,58]. On average, the out-of-pocket expense for liver transplantation in the United States was noted to be $476.60 USD[59]. Approximately half (53.9%) patients required personal savings and credit (25%) to offset the costs. When compared to patients undergoing kidney transplantation, liver recipients were more likely to rely on alternative sources of funds, including personal loans from friends or family members, retirement funds, and community-based fundraising campaigns[59]. It is anticipated that the burden of financial costs will proportionally reduce with the lower rates of required transplantation with novel therapeutics such as DAAs.
GLOBAL INEQUITIES IN ACCESS TO CARE: THE PHARMACEUTICAL DILEMMA
The global burden of HCV is disproportionately higher among low and middle-income countries[60]. Previous epidemiologic studies from 2013 estimated 12% of globally prevalent cases lived in countries with reliable access to HCV-targeted therapies[4]. Estimates of global disease burden are directly impacted by disease screening practices. Limitations in public health funding among low- and middle-income countries is a barrier to screening and ultimately prevent widescale testing. This leads to the underestimation of HCV prevalence. It is currently estimated that 41% of the world’s population lives in countries with no public health funding for the screening and management for viral hepatitis B and C[60]. Overall, limitations in public health funding and infrastructure, organization and costs of screening, expensive of therapeutics, shortages of highly specialized clinicians, and political prioritization of other infectious diseases such as HIV and tuberculosis, all contribute to the barriers inequitable access to care[61].
When distinguishing the Millennium Development Goals, prioritization of infectious diseases excluded viral hepatitis as a ‘top 10’ contributor to global morbidity and mortality[60,62]. The prioritization of other infectious diseases above HCV leads to considerable diversion of resources away from targeting the important health outcomes resulting from hepatitis infection, including cirrhosis and HCC[60,62]. Provided the global estimates of morbidity and mortality of both cirrhosis and HCC resulting from viral hepatitis were combined, viral hepatitis would securely rank among the top 10 infectious diseases, redefining its importance on the global arena and focus of resources[63].
The prohibitive costs of treatment for HCV infection have been well reported. Many countries will prioritize curative treatment for HCV with DAAs to mitigate downstream costs associated with end-stage liver disease[64,65]. Irrespective of savings, the total cost of DAAs are substantial. Current estimates place DAAs in the highest cost category, with wholesale acquisition prices listed between $417 USD (glecaprevir-pibrentasvir) to as high as $1125 USD (ledpasvir-sofosbuvir)[66]. Discrepancies between production and wholesale acquisition exist and contribute to substantial grievances with the pharmaceutical industry. For instance, Daclatasvir production cost for a 12-wk course is estimated to be between $10-30 USD, yet wholesale acquisition cost is marked up to $ 63000 USD for one 12-wk treatment course[66].
DAA drug costs continue to fluctuate as a result of generic drug competition, local manufacturing, and geography[49]. In Iran, reducing pricing on generic DAAs, such as sofosbuvir/daclatasvir, has been made possible by local production and manufacturing of generic formulations, together with resultant market competition[67]. This described process has been documented in other countries, such as India, Pakistan, Bangladesh, China, Brazil, and Morocco[67]. These market factors have allowed for generic DAAs to be priced at approximately $60 USD, compared to a previously quoted price of approximately $10000 USD a decade prior[67]. Concerns, however, have been highlighted regarding the degree of oversight over generic manufacturing in these settings, which may be balanced against quality assurances based mainly on clinical observations of the safety and effectiveness of these drugs in the community[67].
Globally, the United States pays disproportionately higher costs for DAAs, even when compared to other high-income countries. For instance, the cost of sofosbuvir for 12-wk of treatment is $84000 USD, which is disproportionately higher than European countries such as Spain where the cost is estimated to be $25000 USD for the same treatment course[68]. Such discrepancies reflect the purchasing power single-payer healthcare systems hold when negotiating with the pharmaceutical industry[68].
Despite the recent United States Food and Drug Administration approval of the more affordable pan-genotypic glecaprevir-pibrentasvir through federally funded programs[66], there remains considerable inequity in access to DAAs and treatment with curative intent. This is partially driven by the lack of standardized policies within federally funded insurance such as Medicare or Medicaid to define eligibility for treatment[66]. Some insurers have gone so far as prioritizing patients with advanced liver fibrosis or those with clinical features of severe disease[66]. Variability in access to treatment is a serious problem within the United States, driven largely by limitations within the health infrastructure. Disproportionate allocation of resources has led insurers to enact restrictions on the delivery of HCV medications[68]. Regardless of the power publicly funded healthcare systems may hold, such costs are prohibitive to equitable treatment distribution worldwide.
INNOVATIVE EXAMPLES OF HCV ELIMINATION EFFORTS IN RESOURCE-LIMITED SETTINGS
Despite the advent of safely targeted pharmacotherapies, treatment access, as well as the rates of infection and re-infection, continue to soar in specific subgroups, particularly those with substance use disorders[69]. Novel approaches to reducing HCV transmission and improving access to therapeutics include integrative HCV care models, whereby delivery of care through addiction services can serve as an effective approach to HCV prevention. In fact, the scale-up of both opioid substitution therapy as well as needle exchange programs can contribute to substantial reductions in HCV transmission and prevalence[70].
Within addiction services, a variety of integrated models for improving HCV treatment have been proposed and evaluated. These models include referrals to primary care or harm reduction settings, developing specialty clinics with inter-disciplinary teams, organization of hepatology and addiction providers within primary care settings, utilizing technology to improve access with telemedicine, as well as onsite delivery of DAAs within opioid substitution clinics[71]. Prison-based HCV programs have also been proposed as a method to improve the delivery of HCV treatment among specific vulnerable subgroups. Targeting penitentiary populations provides an opportunity for micro-elimination, given the controlled setting and ease at which delivery of care can be executed, as well as organized upon transfer or release[72]. In the largest study of European inmates with hepatitis, HCV care was arranged for more than 80% of inmates exhibiting antibody positivity, with similar rates of treatment acceptance and compliance regardless of high-risk practices (e.g., injecting drug)[73]. Ultimately this model of care proved effective for treatment delivery and completion[73].
Comprehensive community-based HCV care models (“educate, test, and treat” schemas) have been trialed with success in Egypt, a country that experiences one of the highest global burdens of HCV infection[74]. A study by Shiha et al[74] tested the real-world applicability of such models[74]. First, eligible villagers who met inclusion criteria for age were identified and screened for HCV infection using rapid diagnostic tests[74]. Patients who screened possessive would provide an immediate sample to be tested for HCV RNA, and those who had a positive result would be scheduled for a clinic visit for education and initiation of baseline testing and, later, treatment using DAAs[74]. Patients with cirrhosis were followed up clinically at ELRIAH or satellite facilities during treatment, and among these patients, 5.2% (166 patients) were diagnosed with HCC[9]. As a result, treatment cure was established for 84.6% of the 17137 persons identified across 70 villages, and established this community-based care model as feasible and effective in this setting[74]. The data may suggest that community-based HCV care models can be a feasible model to consider in treating HCV patients with similar characteristics as those in the study. Despite their novelty and demonstrated impact within specific settings, few of these models have been tested in a clinical trial, and none have been directly compared.
FUTURE DIRECTIONS
Despite the unparallel efficacy observed among second-generation DAAs, with SVR of over 95%[75-78], the cost of such treatments continues to limit equitable access to care and likely contributes to differential rates of HCV infection globally. To reduce the burden of disease worldwide, essential agenda items for low-, middle-, and high-income countries must prioritize access to DAAs therapies. We must acknowledge the complexity of the proposed task beyond negotiations for lower prices from the pharmaceutical industry.
Ancillary costs contributing to the safe and effective delivery of HCV care are undoubtedly expensive. These expenses are largely driven by the costs associated with healthcare personnel, as well as diagnostic and laboratory testing. Provider experience (specialized training in hepatology, infectious disease, general internal medicine), technology for fibrotic staging, as well as baseline laboratory measures including transaminase levels, HCV RNA level, HCV genotype, measures of synthetic liver function (glucose, platelets, albumin), and current renal function account for the bulk of health delivery expenses. Within the United States alone, accomplishing this task may require federal level funding to negotiate in a single-payer fashion for the United States population in one pool. Given the United States is a multi-payer system, there no incentive for pharmaceutical companies to provide the bulk prices single-payer systems negotiate such as the United Kingdom.
CONCLUSION
On a global scale, continued commitment to the prioritization of HCV is required to increase its importance on the international agenda, specifically the WHO. Additionally, pharmaceutical companies need to consider providing compassionate release of medications to promote equitable healthcare worldwide, especially given that the highest burden of disease rests within low- and middle-income countries.
Footnotes
Conflict-of-interest statement: All authors have no conflict of interest to declare. We report no competing interests for this investigation.
Manuscript source: Invited manuscript
Peer-review started: January 28, 2021
First decision: March 6, 2021
Article in press: July 13, 2021
Specialty type: Gastroenterology and hepatology
Country/Territory of origin: United States
Peer-review report’s scientific quality classification
Grade A (Excellent): A, A
Grade B (Very good): B, B
Grade C (Good): C
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Poustchi H, Tavabie OD, Zhao J S-Editor: Fan JR L-Editor: A P-Editor: Yuan YY
Contributor Information
Brittany B Dennis, Department of Medicine, McMaster University, Hamilton L8S 4L8, ON, Canada; Division of Gastroenterology and Hepatology, Stanford University School of Medicine, Stanford, CA 94304, United States.
Leen Naji, Department of Family Medicine, McMaster University, Hamilton L8P 1H6, ON, Canada; Department of Health Research Methods, Evidence and Impact, McMaster University, Hamilton L8S 4K1, ON, Canada.
Yasmin Jajarmi, Department of Medicine, McMaster University, Hamilton L8S 4L8, ON, Canada.
Aijaz Ahmed, Division of Gastroenterology and Hepatology, Stanford University School of Medicine, Stanford, CA 94304, United States.
Donghee Kim, Division of Gastroenterology and Hepatology, Stanford University School of Medicine, Stanford, CA 94304, United States. dhkimmd@stanford.edu.
References
- 1.World Health Organization. Global hepatitis report 2017. [cited 10 January 2021]. Available from: https://www.who.int/hepatitis/publications/global-hepatitis-report2017/en/
- 2.Perz JF, Armstrong GL, Farrington LA, Hutin YJ, Bell BP. The contributions of hepatitis B virus and hepatitis C virus infections to cirrhosis and primary liver cancer worldwide. J Hepatol. 2006;45:529–538. doi: 10.1016/j.jhep.2006.05.013. [DOI] [PubMed] [Google Scholar]
- 3.EMCDDA Global health sector strategy on viral hepatitis 2016-2021. Towards ending viral hepatitis (No. WHO/HIV/2016.06). [cited 10 January 2021]. Available from: https://www.emcdda.europa.eu/drugs-library/who-global-health-sector-strategy-viral-hepatitis-2016-2021_en .
- 4.Mohd Hanafiah K, Groeger J, Flaxman AD, Wiersma ST. Global epidemiology of hepatitis C virus infection: new estimates of age-specific antibody to HCV seroprevalence. Hepatology. 2013;57:1333–1342. doi: 10.1002/hep.26141. [DOI] [PubMed] [Google Scholar]
- 5.Mohamed AA, Elbedewy TA, El-Serafy M, El-Toukhy N, Ahmed W, Ali El Din Z. Hepatitis C virus: A global view. World J Hepatol. 2015;7:2676–2680. doi: 10.4254/wjh.v7.i26.2676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Elgharably A, Gomaa AI, Crossey MM, Norsworthy PJ, Waked I, Taylor-Robinson SD. Hepatitis C in Egypt - past, present, and future. Int J Gen Med. 2017;10:1–6. doi: 10.2147/IJGM.S119301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Rao MR, Naficy AB, Darwish MA, Darwish NM, Schisterman E, Clemens JD, Edelman R. Further evidence for association of hepatitis C infection with parenteral schistosomiasis treatment in Egypt. BMC Infect Dis. 2002;2:29. doi: 10.1186/1471-2334-2-29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.World Health Organization. Guidelines for the Screening Care and Treatment of Persons with Chronic Hepatitis C Infection Updated Version April 2016: Guidelines. [cited 10 January 2021]. Available from: www.who.int/iris/bitstream/10665/204452/1/WHO_HIV_2016.01_eng.pdf . [PubMed]
- 9.Denniston MM, Jiles RB, Drobeniuc J, Klevens RM, Ward JW, McQuillan GM, Holmberg SD. Chronic hepatitis C virus infection in the United States, National Health and Nutrition Examination Survey 2003 to 2010. Ann Intern Med. 2014;160:293–300. doi: 10.7326/M13-1133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.El-Serag HB. Epidemiology of viral hepatitis and hepatocellular carcinoma. Gastroenterology. 2012;142:1264–1273.e1. doi: 10.1053/j.gastro.2011.12.061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.World Health Organization. Hepatitis C World Report. World Health Organization. [cited 10 January 2021]. Available from: https://www.who.int/news-room/fact-sheets/detail/hepatitis-c .
- 12.Shiels MS, O'Brien TR. Recent Decline in Hepatocellular Carcinoma Rates in the United States. Gastroenterology. 2020;158:1503–1505.e2. doi: 10.1053/j.gastro.2019.12.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kim D, Li AA, Gadiparthi C, Khan MA, Cholankeril G, Glenn JS, Ahmed A. Changing Trends in Etiology-Based Annual Mortality From Chronic Liver Disease, From 2007 Through 2016. Gastroenterology. 2018;155:1154–1163.e3. doi: 10.1053/j.gastro.2018.07.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Thrift AP, El-Serag HB, Kanwal F. Global epidemiology and burden of HCV infection and HCV-related disease. Nat Rev Gastroenterol Hepatol. 2017;14:122–132. doi: 10.1038/nrgastro.2016.176. [DOI] [PubMed] [Google Scholar]
- 15.Kabiri M, Jazwinski AB, Roberts MS, Schaefer AJ, Chhatwal J. The changing burden of hepatitis C virus infection in the United States: model-based predictions. Ann Intern Med. 2014;161:170–180. doi: 10.7326/M14-0095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Chen Q, Ayer T, Bethea E, Kanwal F, Wang X, Roberts M, Zhuo Y, Fagiuoli S, Petersen J, Chhatwal J. Changes in hepatitis C burden and treatment trends in Europe during the era of direct-acting antivirals: a modelling study. BMJ Open. 2019;9:e026726. doi: 10.1136/bmjopen-2018-026726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Chen Q, Ayer T, Adee MG, Wang X, Kanwal F, Chhatwal J. Assessment of Incidence of and Surveillance Burden for Hepatocellular Carcinoma Among Patients With Hepatitis C in the Era of Direct-Acting Antiviral Agents. JAMA Netw Open. 2020;3:e2021173. doi: 10.1001/jamanetworkopen.2020.21173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Shteyer E, Shekhtman L, Zinger T, Harari S, Gafanovich I, Wolf D, Ivgi H, Barsuk R, Dery I, Armoni D, Rivkin M, Pipalia R, Cohen Eliav M, Skorochod Y, Breuer GS, Tur-Kaspa R, Weil Wiener Y, Stern A, Cotler SJ, Dahari H, Lurie Y. Modeling suggests that microliter volumes of contaminated blood caused an outbreak of hepatitis C during computerized tomography. PLoS One. 2019;14:e0210173. doi: 10.1371/journal.pone.0210173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Rauch A, Wandeler G. Hepatitis C among men who have sex with men: knowing your epidemic. Lancet Gastroenterol Hepatol. 2021;6:5–6. doi: 10.1016/S2468-1253(20)30358-7. [DOI] [PubMed] [Google Scholar]
- 20.National Institutes of Health. Story of Discovery: Hepatitis C: From Non-A, Non-B Hepatitis to a Cure. 2016. [cited 10 January 2021]. Available from: https://www.niddk.nih.gov/news/archive/2016/story-discovery-hepatitis-c-from-non-a-non-b-hepatitis-cure .
- 21.Choo QL, Kuo G, Weiner AJ, Overby LR, Bradley DW, Houghton M. Isolation of a cDNA clone derived from a blood-borne non-A, non-B viral hepatitis genome. Science. 1989;244:359–362. doi: 10.1126/science.2523562. [DOI] [PubMed] [Google Scholar]
- 22.Pol S, Lagaye S. The remarkable history of the hepatitis C virus. Genes Immun. 2019;20:436–446. doi: 10.1038/s41435-019-0066-z. [DOI] [PubMed] [Google Scholar]
- 23.Choo QL, Richman KH, Han JH, Berger K, Lee C, Dong C, Gallegos C, Coit D, Medina-Selby R, Barr PJ. Genetic organization and diversity of the hepatitis C virus. Proc Natl Acad Sci USA. 1991;88:2451–2455. doi: 10.1073/pnas.88.6.2451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Zein NN. Clinical significance of hepatitis C virus genotypes. Clin Microbiol Rev. 2000;13:223–235. doi: 10.1128/cmr.13.2.223-235.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Wang LS, D'Souza LS, Jacobson IM. Hepatitis C-A clinical review. J Med Virol. 2016;88:1844–1855. doi: 10.1002/jmv.24554. [DOI] [PubMed] [Google Scholar]
- 26.The Nobel Prize. The Nobel Prize in Physiology or Medicine 2020. [cited 14 March 2021]. Available from: https://www.nobelprize.org/prizes/medicine/2020/houghton/Lecture/
- 27.Burgess SV, Hussaini T, Yoshida EM. Concordance of sustained virologic response at weeks 4, 12 and 24 post-treatment of hepatitis c in the era of new oral direct-acting antivirals: A concise review. Ann Hepatol. 2016;15:154–159. doi: 10.5604/16652681.1193693. [DOI] [PubMed] [Google Scholar]
- 28.Simmons B, Saleem J, Hill A, Riley RD, Cooke GS. Risk of Late Relapse or Reinfection With Hepatitis C Virus After Achieving a Sustained Virological Response: A Systematic Review and Meta-analysis. Clin Infect Dis. 2016;62:683–694. doi: 10.1093/cid/civ948. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kohli A, Shaffer A, Sherman A, Kottilil S. Treatment of hepatitis C: a systematic review. JAMA. 2014;312:631–640. doi: 10.1001/jama.2014.7085. [DOI] [PubMed] [Google Scholar]
- 30.Russo MW. Antiviral therapy for hepatitis C is associated with improved clinical outcomes in patients with advanced fibrosis. Expert Rev Gastroenterol Hepatol. 2010;4:535–539. doi: 10.1586/egh.10.60. [DOI] [PubMed] [Google Scholar]
- 31.Bidell MR, McLaughlin M, Faragon J, Morse C, Patel N. Desirable Characteristics of Hepatitis C Treatment Regimens: A Review of What We Have and What We Need. Infect Dis Ther. 2016;5:299–312. doi: 10.1007/s40121-016-0118-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Everson GT, Towner WJ, Davis MN, Wyles DL, Nahass RG, Thuluvath PJ, Etzkorn K, Hinestrosa F, Tong M, Rabinovitz M, McNally J, Brainard DM, Han L, Doehle B, McHutchison JG, Morgan T, Chung RT, Tran TT. Sofosbuvir With Velpatasvir in Treatment-Naive Noncirrhotic Patients With Genotype 1 to 6 Hepatitis C Virus Infection: A Randomized Trial. Ann Intern Med. 2015;163:818–826. doi: 10.7326/M15-1000. [DOI] [PubMed] [Google Scholar]
- 33.Asselah T, Kowdley KV, Zadeikis N, Wang S, Hassanein T, Horsmans Y, Colombo M, Calinas F, Aguilar H, de Ledinghen V, Mantry PS, Hezode C, Marinho RT, Agarwal K, Nevens F, Elkhashab M, Kort J, Liu R, Ng TI, Krishnan P, Lin CW, Mensa FJ. Efficacy of Glecaprevir/Pibrentasvir for 8 or 12 Weeks in Patients With Hepatitis C Virus Genotype 2, 4, 5, or 6 Infection Without Cirrhosis. Clin Gastroenterol Hepatol. 2018;16:417–426. doi: 10.1016/j.cgh.2017.09.027. [DOI] [PubMed] [Google Scholar]
- 34.Gane E, Lawitz E, Pugatch D, Papatheodoridis G, Bräu N, Brown A, Pol S, Leroy V, Persico M, Moreno C, Colombo M, Yoshida EM, Nelson DR, Collins C, Lei Y, Kosloski M, Mensa FJ. Glecaprevir and Pibrentasvir in Patients with HCV and Severe Renal Impairment. N Engl J Med. 2017;377:1448–1455. doi: 10.1056/NEJMoa1704053. [DOI] [PubMed] [Google Scholar]
- 35.Kwo PY, Poordad F, Asatryan A, Wang S, Wyles DL, Hassanein T, Felizarta F, Sulkowski MS, Gane E, Maliakkal B, Overcash JS, Gordon SC, Muir AJ, Aguilar H, Agarwal K, Dore GJ, Lin CW, Liu R, Lovell SS, Ng TI, Kort J, Mensa FJ. Glecaprevir and pibrentasvir yield high response rates in patients with HCV genotype 1-6 without cirrhosis. J Hepatol. 2017;67:263–271. doi: 10.1016/j.jhep.2017.03.039. [DOI] [PubMed] [Google Scholar]
- 36.Di L. The role of drug metabolizing enzymes in clearance. Expert Opin Drug Metab Toxicol. 2014;10:379–393. doi: 10.1517/17425255.2014.876006. [DOI] [PubMed] [Google Scholar]
- 37.Freriksen JJM, van Seyen M, Judd A, Gibb DM, Collins IJ, Greupink R, Russel FGM, Drenth JPH, Colbers A, Burger DM. Review article: direct-acting antivirals for the treatment of HCV during pregnancy and lactation - implications for maternal dosing, foetal exposure, and safety for mother and child. Aliment Pharmacol Ther. 2019;50:738–750. doi: 10.1111/apt.15476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Ghany MG, Morgan TR AASLD-IDSA Hepatitis C Guidance Panel. Hepatitis C Guidance 2019 Update: American Association for the Study of Liver Diseases-Infectious Diseases Society of America Recommendations for Testing, Managing, and Treating Hepatitis C Virus Infection. Hepatology. 2020;71:686–721. doi: 10.1002/hep.31060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Society for Maternal-Fetal Medicine (SMFM), Hughes BL, Page CM, Kuller JA. Hepatitis C in pregnancy: screening, treatment, and management. Am J Obstet Gynecol. 2017;217:B2–B12. doi: 10.1016/j.ajog.2017.07.039. [DOI] [PubMed] [Google Scholar]
- 40.Jamma S, Hussain G, Lau DT. Current Concepts of HBV/HCV Coinfection: Coexistence, but Not Necessarily in Harmony. Curr Hepat Rep. 2010;9:260–269. doi: 10.1007/s11901-010-0060-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Mavilia MG, Wu GY. HBV-HCV Coinfection: Viral Interactions, Management, and Viral Reactivation. J Clin Transl Hepatol. 2018;6:296–305. doi: 10.14218/JCTH.2018.00016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Koustenis KR, Anagnostou O, Kranidioti H, Vasileiadi S, Antonakaki P, Koutli E, Pantsas P, Deutsch M, Manolakopoulos S. Direct-acting antiviral treatment for chronic hepatitis C in people who use drugs in a real-world setting. Ann Gastroenterol. 2020;33:195–201. doi: 10.20524/aog.2020.0449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Guarino M, Viganò L, Ponziani FR, Giannini EG, Lai Q, Morisco F Special Interest Group on Hepatocellular carcinoma and new anti-HCV therapies” of the Italian Association for the Study of the Liver. Recurrence of hepatocellular carcinoma after direct acting antiviral treatment for hepatitis C virus infection: Literature review and risk analysis. Dig Liver Dis. 2018;50:1105–1114. doi: 10.1016/j.dld.2018.08.001. [DOI] [PubMed] [Google Scholar]
- 44.Piscaglia F, Granito A, Bolondi L. DAAs for HCV and risk of hepatocellular carcinoma: current standpoint. Lancet Gastroenterol Hepatol. 2018;3:736–738. doi: 10.1016/S2468-1253(18)30238-3. [DOI] [PubMed] [Google Scholar]
- 45.Romano A, Angeli P, Piovesan S, Noventa F, Anastassopoulos G, Chemello L, Cavalletto L, Gambato M, Russo FP, Burra P, Vincenzi V, Scotton PG, Panese S, Tempesta D, Bertin T, Carrara M, Carlotto A, Capra F, Carolo G, Scroccaro G, Alberti A. Newly diagnosed hepatocellular carcinoma in patients with advanced hepatitis C treated with DAAs: A prospective population study. J Hepatol. 2018;69:345–352. doi: 10.1016/j.jhep.2018.03.009. [DOI] [PubMed] [Google Scholar]
- 46.Calvaruso V, Cabibbo G, Cacciola I, Petta S, Madonia S, Bellia A, Tinè F, Distefano M, Licata A, Giannitrapani L, Prestileo T, Mazzola G, Di Rosolini MA, Larocca L, Bertino G, Digiacomo A, Benanti F, Guarneri L, Averna A, Iacobello C, Magro A, Scalisi I, Cartabellotta F, Savalli F, Barbara M, Davì A, Russello M, Scifo G, Squadrito G, Cammà C, Raimondo G, Craxì A, Di Marco V Rete Sicilia Selezione Terapia–HCV (RESIST-HCV) Incidence of Hepatocellular Carcinoma in Patients With HCV-Associated Cirrhosis Treated With Direct-Acting Antiviral Agents. Gastroenterology. 2018;155:411–421.e4. doi: 10.1053/j.gastro.2018.04.008. [DOI] [PubMed] [Google Scholar]
- 47.Singal AG, Lim JK, Kanwal F. AGA Clinical Practice Update on Interaction Between Oral Direct-Acting Antivirals for Chronic Hepatitis C Infection and Hepatocellular Carcinoma: Expert Review. Gastroenterology. 2019;156:2149–2157. doi: 10.1053/j.gastro.2019.02.046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Singal AG, Rich NE, Mehta N, Branch A, Pillai A, Hoteit M, Volk M, Odewole M, Scaglione S, Guy J, Said A, Feld JJ, John BV, Frenette C, Mantry P, Rangnekar AS, Oloruntoba O, Leise M, Jou JH, Bhamidimarri KR, Kulik L, Tran T, Samant H, Dhanasekaran R, Duarte-Rojo A, Salgia R, Eswaran S, Jalal P, Flores A, Satapathy SK, Wong R, Huang A, Misra S, Schwartz M, Mitrani R, Nakka S, Noureddine W, Ho C, Konjeti VR, Dao A, Nelson K, Delarosa K, Rahim U, Mavuram M, Xie JJ, Murphy CC, Parikh ND. Direct-Acting Antiviral Therapy Not Associated With Recurrence of Hepatocellular Carcinoma in a Multicenter North American Cohort Study. Gastroenterology. 2019;156:1683–1692.e1. doi: 10.1053/j.gastro.2019.01.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Graham CS. The Current Status of US and Global Access to Direct-Acting Antiviral Regimens for Hepatitis C Virus Infection. Clin Liver Dis (Hoboken) 2020;16:16–19. doi: 10.1002/cld.925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Bailey JR, Barnes E, Cox AL. Approaches, Progress, and Challenges to Hepatitis C Vaccine Development. Gastroenterology. 2019;156:418–430. doi: 10.1053/j.gastro.2018.08.060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Cholankeril G, Ahmed A. Alcoholic Liver Disease Replaces Hepatitis C Virus Infection as the Leading Indication for Liver Transplantation in the United States. Clin Gastroenterol Hepatol. 2018;16:1356–1358. doi: 10.1016/j.cgh.2017.11.045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Goldberg D, Ditah IC, Saeian K, Lalehzari M, Aronsohn A, Gorospe EC, Charlton M. Changes in the Prevalence of Hepatitis C Virus Infection, Nonalcoholic Steatohepatitis, and Alcoholic Liver Disease Among Patients With Cirrhosis or Liver Failure on the Waitlist for Liver Transplantation. Gastroenterology. 2017;152:1090–1099.e1. doi: 10.1053/j.gastro.2017.01.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Flemming JA, Kim WR, Brosgart CL, Terrault NA. Reduction in liver transplant waitlisting in the era of direct-acting antiviral therapy. Hepatology. 2017;65:804–812. doi: 10.1002/hep.28923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Cholankeril G, Li AA, March KL, Yoo ER, Kim D, Snyder H, Gonzalez SA, Younossi ZM, Ahmed A. Improved Outcomes in HCV Patients Following Liver Transplantation During the Era of Direct-Acting Antiviral Agents. Clin Gastroenterol Hepatol. 2018;16:452–453. doi: 10.1016/j.cgh.2017.08.020. [DOI] [PubMed] [Google Scholar]
- 55.Sugawara Y, Hibi T. Direct-acting agents for hepatitis C virus before and after liver transplantation. Biosci Trends. 2018;11:606–611. doi: 10.5582/bst.2017.01293. [DOI] [PubMed] [Google Scholar]
- 56.Daniel KE, Said A. Considerations When Treating Hepatitis C in a Cirrhotic Transplant Candidate. Curr Gastroenterol Rep. 2018;20:20. doi: 10.1007/s11894-018-0626-9. [DOI] [PubMed] [Google Scholar]
- 57.Sullivan P. New price tag put on liver transplants. CMAJ. 2003;168:206–206. [Google Scholar]
- 58.van der Hilst CS, Ijtsma AJ, Slooff MJ, Tenvergert EM. Cost of liver transplantation: a systematic review and meta-analysis comparing the United States with other OECD countries. Med Care Res Rev. 2009;66:3–22. doi: 10.1177/1077558708324299. [DOI] [PubMed] [Google Scholar]
- 59.Rodrigue JR, Reed AI, Nelson DR, Jamieson I, Kaplan B, Howard RJ. The financial burden of transplantation: a single-center survey of liver and kidney transplant recipients. Transplantation. 2007;84:295–300. doi: 10.1097/01.tp.0000269797.41202.79. [DOI] [PubMed] [Google Scholar]
- 60.Lemoine M, Thursz M. Hepatitis C, a global issue: access to care and new therapeutic and preventive approaches in resource-constrained areas. Semin Liver Dis. 2014;34:89–97. doi: 10.1055/s-0034-1371082. [DOI] [PubMed] [Google Scholar]
- 61.Lemoine M, Nayagam S, Thursz M. Viral hepatitis in resource-limited countries and access to antiviral therapies: current and future challenges. Future Virol. 2013;8:371–380. doi: 10.2217/fvl.13.11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Cooke GS, Lemoine M, Thursz M, Gore C, Swan T, Kamarulzaman A, DuCros P, Ford N. Viral hepatitis and the Global Burden of Disease: a need to regroup. J Viral Hepat. 2013;20:600–601. doi: 10.1111/jvh.12123. [DOI] [PubMed] [Google Scholar]
- 63.Lozano R, Naghavi M, Foreman K, Lim S, Shibuya K, Aboyans V, Abraham J, Adair T, Aggarwal R, Ahn SY, Alvarado M, Anderson HR, Anderson LM, Andrews KG, Atkinson C, Baddour LM, Barker-Collo S, Bartels DH, Bell ML, Benjamin EJ, Bennett D, Bhalla K, Bikbov B, Bin Abdulhak A, Birbeck G, Blyth F, Bolliger I, Boufous S, Bucello C, Burch M, Burney P, Carapetis J, Chen H, Chou D, Chugh SS, Coffeng LE, Colan SD, Colquhoun S, Colson KE, Condon J, Connor MD, Cooper LT, Corriere M, Cortinovis M, de Vaccaro KC, Couser W, Cowie BC, Criqui MH, Cross M, Dabhadkar KC, Dahodwala N, De Leo D, Degenhardt L, Delossantos A, Denenberg J, Des Jarlais DC, Dharmaratne SD, Dorsey ER, Driscoll T, Duber H, Ebel B, Erwin PJ, Espindola P, Ezzati M, Feigin V, Flaxman AD, Forouzanfar MH, Fowkes FG, Franklin R, Fransen M, Freeman MK, Gabriel SE, Gakidou E, Gaspari F, Gillum RF, Gonzalez-Medina D, Halasa YA, Haring D, Harrison JE, Havmoeller R, Hay RJ, Hoen B, Hotez PJ, Hoy D, Jacobsen KH, James SL, Jasrasaria R, Jayaraman S, Johns N, Karthikeyan G, Kassebaum N, Keren A, Khoo JP, Knowlton LM, Kobusingye O, Koranteng A, Krishnamurthi R, Lipnick M, Lipshultz SE, Ohno SL, Mabweijano J, MacIntyre MF, Mallinger L, March L, Marks GB, Marks R, Matsumori A, Matzopoulos R, Mayosi BM, McAnulty JH, McDermott MM, McGrath J, Mensah GA, Merriman TR, Michaud C, Miller M, Miller TR, Mock C, Mocumbi AO, Mokdad AA, Moran A, Mulholland K, Nair MN, Naldi L, Narayan KM, Nasseri K, Norman P, O'Donnell M, Omer SB, Ortblad K, Osborne R, Ozgediz D, Pahari B, Pandian JD, Rivero AP, Padilla RP, Perez-Ruiz F, Perico N, Phillips D, Pierce K, Pope CA 3rd, Porrini E, Pourmalek F, Raju M, Ranganathan D, Rehm JT, Rein DB, Remuzzi G, Rivara FP, Roberts T, De León FR, Rosenfeld LC, Rushton L, Sacco RL, Salomon JA, Sampson U, Sanman E, Schwebel DC, Segui-Gomez M, Shepard DS, Singh D, Singleton J, Sliwa K, Smith E, Steer A, Taylor JA, Thomas B, Tleyjeh IM, Towbin JA, Truelsen T, Undurraga EA, Venketasubramanian N, Vijayakumar L, Vos T, Wagner GR, Wang M, Wang W, Watt K, Weinstock MA, Weintraub R, Wilkinson JD, Woolf AD, Wulf S, Yeh PH, Yip P, Zabetian A, Zheng ZJ, Lopez AD, Murray CJ, AlMazroa MA, Memish ZA. Global and regional mortality from 235 causes of death for 20 age groups in 1990 and 2010: a systematic analysis for the Global Burden of Disease Study 2010. Lancet. 2012;380:2095–2128. doi: 10.1016/S0140-6736(12)61728-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.NICE National Institute for Health and Care Excellence: Single technology appraisal - Daclatasvir for treating chronic hepatitis C: final scope. [cited 10 January 2021]. Available from: http://www.nice.org.uk/guidance/gid-tag487/documents/hepatitis-c-chronic-daclatasvir-final-scope2 .
- 65.NICE National Institute for Health and Care Excellence: Single technology appraisal. Ledipasvir–sofosbuvir for treating chronic hepatitis C: final scope. 2014. [cited 10 January 2021]. Available from: http://www.nice.org.uk/guidance/gid-tag484/documents/hepatitis-c-chronic-ledipasvirsofosbuvir-final-scope2 .
- 66.Woolston S, Kim N. Cost and Access to Direct-Acting Antiviral Agents [Internet]. 2018. [cited 10 January 2021]. Available from: https://www.hepatitisc.uw.edu/go/evaluation-treatment/cost-access-medications/core-concept/all .
- 67.Hajarizadeh B. Generic direct acting antiviral treatment: The first step towards elimination of hepatitis C in Iran. Hepat Mon. 2017 [Google Scholar]
- 68.Rosenthal ES, Graham CS. Price and affordability of direct-acting antiviral regimens for hepatitis C virus in the United States. Infect Agent Cancer. 2016;11:24. doi: 10.1186/s13027-016-0071-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Backmund M, Meyer K, Von Zielonka M, Eichenlaub D. Treatment of hepatitis C infection in injection drug users. Hepatology. 2001;34:188–193. doi: 10.1053/jhep.2001.25882. [DOI] [PubMed] [Google Scholar]
- 70.Vickerman P, Martin N, Turner K, Hickman M. Can needle and syringe programmes and opiate substitution therapy achieve substantial reductions in hepatitis C virus prevalence? Addiction. 2012;107:1984–1995. doi: 10.1111/j.1360-0443.2012.03932.x. [DOI] [PubMed] [Google Scholar]
- 71.Perlman DC, Jordan AE, Uuskula A, Huong DT, Masson CL, Schackman BR, Des Jarlais DC. An international perspective on using opioid substitution treatment to improve hepatitis C prevention and care for people who inject drugs: Structural barriers and public health potential. Int J Drug Policy. 2015;26:1056–1063. doi: 10.1016/j.drugpo.2015.04.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Lazarus JV, Safreed-Harmon K, Thursz MR, Dillon JF, El-Sayed MH, Elsharkawy AM, Hatzakis A, Jadoul M, Prestileo T, Razavi H, Rockstroh JK, Wiktor SZ, Colombo M. The Micro-Elimination Approach to Eliminating Hepatitis C: Strategic and Operational Considerations. Semin Liver Dis. 2018;38:181–192. doi: 10.1055/s-0038-1666841. [DOI] [PubMed] [Google Scholar]
- 73.Fiore V, De Matteis G, Ranieri R, Saderi L, Pontali E, Muredda A, Ialungo AM, Caruso R, Madeddu G, Sotgiu G, Babudieri S. HCV testing and treatment initiation in an Italian prison setting: A step-by-step model to micro-eliminate hepatitis C. Int J Drug Policy. 2021;90:103055. doi: 10.1016/j.drugpo.2020.103055. [DOI] [PubMed] [Google Scholar]
- 74.Shiha G, Soliman R, Mikhail NNH, Easterbrook P. An educate, test and treat model towards elimination of hepatitis C infection in Egypt: Feasibility and effectiveness in 73 villages. J Hepatol. 2020;72:658–669. doi: 10.1016/j.jhep.2019.11.004. [DOI] [PubMed] [Google Scholar]
- 75.Sulkowski MS, Gardiner DF, Rodriguez-Torres M, Reddy KR, Hassanein T, Jacobson I, Lawitz E, Lok AS, Hinestrosa F, Thuluvath PJ, Schwartz H, Nelson DR, Everson GT, Eley T, Wind-Rotolo M, Huang SP, Gao M, Hernandez D, McPhee F, Sherman D, Hindes R, Symonds W, Pasquinelli C, Grasela DM AI444040 Study Group. Daclatasvir plus sofosbuvir for previously treated or untreated chronic HCV infection. N Engl J Med. 2014;370:211–221. doi: 10.1056/NEJMoa1306218. [DOI] [PubMed] [Google Scholar]
- 76.Lawitz E, Mangia A, Wyles D, Rodriguez-Torres M, Hassanein T, Gordon SC, Schultz M, Davis MN, Kayali Z, Reddy KR, Jacobson IM, Kowdley KV, Nyberg L, Subramanian GM, Hyland RH, Arterburn S, Jiang D, McNally J, Brainard D, Symonds WT, McHutchison JG, Sheikh AM, Younossi Z, Gane EJ. Sofosbuvir for previously untreated chronic hepatitis C infection. N Engl J Med. 2013;368:1878–1887. doi: 10.1056/NEJMoa1214853. [DOI] [PubMed] [Google Scholar]
- 77.Ferenci P, Bernstein D, Lalezari J, Cohen D, Luo Y, Cooper C, Tam E, Marinho RT, Tsai N, Nyberg A, Box TD, Younes Z, Enayati P, Green S, Baruch Y, Bhandari BR, Caruntu FA, Sepe T, Chulanov V, Janczewska E, Rizzardini G, Gervain J, Planas R, Moreno C, Hassanein T, Xie W, King M, Podsadecki T, Reddy KR PEARL-III Study; PEARL-IV Study. ABT-450/r-ombitasvir and dasabuvir with or without ribavirin for HCV. N Engl J Med. 2014;370:1983–1992. doi: 10.1056/NEJMoa1402338. [DOI] [PubMed] [Google Scholar]
- 78.Nelson DR, Cooper JN, Lalezari JP, Lawitz E, Pockros PJ, Gitlin N, Freilich BF, Younes ZH, Harlan W, Ghalib R, Oguchi G, Thuluvath PJ, Ortiz-Lasanta G, Rabinovitz M, Bernstein D, Bennett M, Hawkins T, Ravendhran N, Sheikh AM, Varunok P, Kowdley KV, Hennicken D, McPhee F, Rana K, Hughes EA ALLY-3 Study Team. All-oral 12-week treatment with daclatasvir plus sofosbuvir in patients with hepatitis C virus genotype 3 infection: ALLY-3 phase III study. Hepatology. 2015;61:1127–1135. doi: 10.1002/hep.27726. [DOI] [PMC free article] [PubMed] [Google Scholar]


