Abstract
Sarcopenia is becoming a well-established player in evaluating patients with chronic liver disease. Data regarding its clinical significance and consequences in the course of liver disease have been growing; many of the data support the idea that it impacts decompensation event frequency, prolonged hospitalization, and mortality, as well as providing the possibility to better prioritize patients on lists awaiting liver transplantation. When assessing the whole clinical scope of the field, which includes malnutrition and frailty, as well as the complete spectrum of muscle mass, strength, and function, it becomes clear that a well-founded approach in everyday clinical practice is essential. In this respect, this article attempts to unveil the most recently published data regarding possible methods and modalities that could be used to diagnose sarcopenia as early as possible, along with the required accuracy and reliability. From the most important field discoveries to data that need further clarification, the merits and weaknesses of the very diverse existing evaluation methods are presented. Finally, a critical overview is given, in an attempt to discern study lines of importance from those that could pose further ambiguity for the theme. The author also poses relevant questions that remain unanswered but are of clinical importance in the field.
Keywords: Sarcopenia, Liver cirrhosis, Frailty, Muscle strength, Nutrition indices, Risk factors
Core Tip: Knowledge regarding the influence of sarcopenia in the course of chronic liver disease has greatly expanded in the past ten years, especially with respect to cirrhosis. Data show that it has a great influence on disease decompensation and patient mortality, providing clues for the development of newer evaluation modalities and sarcopenia indices. Nonetheless, data regarding the therapeutic consequences and interventions remain scarce. This article attempts to summarize the current state of knowledge of this important clinical topic with a critical evaluation of some related groundbreaking studies.
INTRODUCTION
Sarcopenia is an important part of the medical evaluation and treatment for chronic liver disease, especially in the advanced stage of the disease (i.e., cirrhosis), not only due to the very frequent coexistence of both medical entities but also because of its impact on the related clinical outcomes. This article focuses on the clinical aspects and consequences of sarcopenia in liver cirrhosis, common diagnostic procedures, and suggestions for clinical therapy. A review of the presented field in the scientific literature is provided, with the intentional omission of pathophysiology, for better transparency, clinical applicability, and an attempt to critically evaluate the most recent study findings.
To facilitate the understanding of common medical terms, a short paragraph summarizing their definitions is presented (see also Figure 1), followed by a brief description of the pathophysiological processes involved. For a more detailed review of the pathophysiology of sarcopenia in liver cirrhosis-especially regarding the possible influence of the gastrointestinal microbiota on the occurrence and course of muscle dysfunction-the author recommends some excellent reviews in this field[1-4].
Figure 1.
Main definitions of the presented topic.
DEFINITIONS
Malnutrition is defined as a measurable change in physical and mental functions secondary to altered body composition and cell mass, resulting in an impaired quality of life and poor clinical outcomes. It is the consequence of insufficient protein and energy supplies.
It is well admitted that malnutrition participates in the onset of sarcopenia but the link between these two nutritional concepts remains confusing[5].
Sarcopenia is defined as the loss of muscle mass, muscle strength, and reduced physical function.
Frailty is a condition of increased vulnerability to endogenous and/or exogenous stressors associated with physiological decline.
Myosteatosis is pathological fat accumulation in muscles.
PATHOPHYSIOLOGY
Sarcopenia is often equated as a state of advanced malnutrition. The condition is associated with complications such as poor mobility and quality of life and increased mortality. Muscle loss in sarcopenia is not solely at the expense of muscle atrophy, it also involves replacing muscle cells with fat and connective tissue. Protein supply in a sarcopenic patient is low due to chronic muscle degradation which is most clearly manifested during metabolic stress when muscle proteins should be mobilized very quickly to provide the body with amino acids for the liver, gut and immune system function. Sarcopenia in patients with liver cirrhosis is connected to age-related decline in muscle mass and to malnutrition. Studies show that malnutrition develops due to negative energy balance, loss of appetite, rapid satiety and poor food absorption. Concurrent illnesses or addictions (bacterial growth in the gut, pancreatic exocrine insufficiency, alcoholism) exacerbate the condition. Iatrogenic losses include ascites paracentesis and use of laxatives and diuretics. Frequent endoscopic imaging and laboratory examinations also affect diet and weight loss as well as sarcopenia. Therefore, liver cirrhosis is a state of accelerated starvation. In liver cirrhosis, cytokines that stimulate catabolic processes in the body are released. Due to the low glycogen stores, energy production is directed towards the breakdown of fatty acids. Gluconeogenesis is mainly pursued through amino acids breakdown, which worsens protein loss. Advancement of the state is also exacerbated by growth hormone and testosterone deficiency, decreased physical activity, and more frequent occurrence of hepatocellular carcinoma (HCC). Patients with liver cirrhosis are prone to replacing muscle fibers with fat cells (myosteatosis), which may further accelerate loss of muscle function and decline in muscle strength. Connection between hepatic encephalopathy (HE) and sarcopenia is in a form of a vicious cycle where ammonia is likely to be directly responsible for mitochondrial oxidative damage and autophagy of skeletal muscle cells; it also inhibits protein synthesis in skeletal muscle through stimulation of myostatin[1-4].
CLINICAL SIGNIFICANCE AND CONSEQUENCES OF SARCOPENIA
To facilitate the clinical application and evaluation of sarcopenia tests, The European Working Group on Sarcopenia and Older People provided consensus criteria for the diagnosis of sarcopenia using muscle mass, muscle strength, and muscle function as a practical clinical definition. In 2019, a revised definition was published. Pre-sarcopenia is defined as the presence of low muscle strength without its impact on muscle mass/quality or muscle function. Sarcopenia is defined as a low muscle strength with additional low muscle mass or quality. For severe sarcopenia, muscle mass, muscle strength, and muscle function needed to be impaired. As can be seen from the definition, muscle mass does not condition muscle function or strength. The end result of the sarcopenia process is a decline in muscle functional abilities assessed by frailty[6].
To assess frailty, the 5 Fried’s phenotypes of frailty as cited by Sinclair[7] are used. While patients with 3 or more phenotypes are defined as frail, patients with 1 or 2 phenotypes are defined as prefrail, and those with no phenotype as robust[7]. The concomitant presence of sarcopenia and obesity is significantly associated with prefrailty and frailty, especially in women with cirrhosis[8]. According to a well-defined description by Buchard et al[5], “sarcopenia [is] not identical to frailty but there is a major overlap between definitions and diagnosis criteria of the two phenotypes. Frailty is a more multidimensional concept encompassing not only muscle conditions but also exhaustion, well-being, disability, dependency, and cognitive state. Loss of skeletal muscle mass and function remains a strong substratum of frailty, as in malnutrition. The absence of sarcopenia certainly does not rule out frailty but clues of frailty must lead to a complete body composition evaluation”.
In a well-designed study, a group of authors led by Traub et al[8] evaluated the applicability of the latest definition of sarcopenia in patients with cirrhosis. Computed tomography (CT) examination was used to assess muscle mass, hand grip test to assess muscle strength, and 4 m gait speed test to assess muscle function. Compared to the 2019 definition, they observed that the 2010 definition identified more sarcopenia cases in male patients. In patients with cirrhosis, muscle strength seems to be preserved longer, while muscle mass is already reduced, leading to a significant difference in sarcopenia diagnosis rates when using the 2019 definition. However, the gender imbalance seen in the 2010 definition seemed to be less pronounced with the 2019 definition. They objected to the lack of data whether the 2010 or 2019 criteria is better to predict clinical complications, poorer prognoses, and the effect of specific interventions[8].
Sarcopenia is present in 30% to 70% of patients with cirrhosis, probably less frequently in the population of patients with metabolic liver disease. The prevalence increases with the disease stage and increases sharply before liver transplantation[7,9]. Based on the Child–Pugh score, the annual rate of decrease in skeletal muscle mass is 1.3% in Child–Pugh A patients, 3.5% in Child–Pugh B patients, and 6.1% in Child–Pugh C patients[10]. The risk of developing sarcopenia is associated with male gender, ascites, and the degree of renal and hepatic dysfunction[9]. Overweight and obesity are as frequent as in general population, ranging from 20% to 40% and aggravating prognosis both in compensated and in decompensated cirrhosis[11]. Sarcopenic obesity (coexistence of sarcopenia and obesity) is present in one-fifth to one-third of patients with cirrhosis. It is determined by using an assessment of sarcopenia to which an assessment of obesity using a body mass index (BMI ≥ 25 or ≥30 kg/m2) is added. BMI corrected for ascites is probably the most practical to use (see below under the Anthropometry section), although it may shift patients to a lower BMI grade[12]. Myosteatosis occurs in up to 50% of patients with cirrhosis and can be identified in both sarcopenic and non-sarcopenic patients with or without obesity. It meditates inflammatory responses and has been associated with lower muscle function and strength, muscle atrophy, and physical disabilities[12]. It affects mortality, more through loss of muscle function than through liver cirrhosis determinants. It probably plays an important role in the occurrence of HE[13]. Microscopic analysis of skeletal muscle fibers references special attention to atrophy of fast-twitch fibers and reduction in muscle fiber size. Reduced skeletal muscle fiber size and shift toward wider fast-twitch fibers (increased proportion of type IIA/IIX fibers concurrent with lower proportions of type IIA fibers) were visible in the muscle of a sarcopenic patient[3].
The main clinical consequences of sarcopenia are increased mortality per se, increased mortality from systemic bacterial infection, the occurrence of HE after transjugular intrahepatic portosystemic shunt (TIPSS) insertion, and the more frequent occurrence of acute on chronic liver failure. Regardless of the underlying disease, it has a significant effect on mortality in patients with HCC[9]. Ebadi et al[3] point out that it would be important to define strategies maintaining muscle mass in candidates prior to the TIPSS insertion. A study by Al-Azzawi et al[14] confirmed that the existence of sarcopenia in alcoholic hepatitis prolongs hospital treatment. In the case of sarcopenia, patients had a more severe course of the disease. To date, there is no solid evidence linking portal hypertension to known sarcopenia indices. In a well-defined study, hepatic venous pressure gradient (HVPG) was shown to inversely correlate with adipose tissue indices, while CT sarcopenia markers were unrelated to the degree of portal hypertension[15]. On the other hand, the Austrian study group demonstrated poorer survival and an increased risk of further cirrhosis decompensation in the group of patients with HVPG > 20 mmHg by comparing CT sarcopenia indices and invasively assessed clinically significant portal hypertension (CSPH)[16].
In the group of patients awaiting liver transplantation, sarcopenia can affect relegation from the waiting list due to excessive perioperative risk, increased need for hospitalization, and prolonged hospitalization with multiple complications after organ transplantation[9]. Decline of muscle function has a significant impact on mortality and the occurrence of complications requiring hospital treatment[17]. The presence of sarcopenic obesity in this group of patients is relatively common and is associated with higher mortality than if the entities were present separately[11]. After organ transplantation, there is a lack of clinical data regarding the impact of sarcopenia on long-term clinical outcomes[13].
Given the above-mentioned situation, it is not surprising that there are calls for the inclusion of sarcopenia among the factors of transplantation priority [Model for End-Stage Liver Disease (MELD) – score vs sarcopenia-MELD score]. A thorough study in this area evaluated patients on a transplant waiting list. To define sarcopenia, CT measurements with proposed cut-off values were used (see below). In this cohort of European patients, they demonstrated the sarcopenia impact on the increased mortality. When comparing MELD to sarcopenia-MELD score, the authors also confirmed a much lower priority of patients with MELD < 15. It has been observed that a better scoring option would include one encompassing the patient’s age and previous occurrence of HE[18]. In the literature, other authors support the idea for better transplant priority of patients with sarcopenia and low MELD score to reduce the risk of waiting list mortality and to improve the overall outcome[19]. They also point to the strong impact of sarcopenia reflected in the mere fact that it is equivalent to adding 10 points to the MELD score if presented[20].
From a clinical point of view, it is important whether sarcopenia is worth assessing in the group of patients with cirrhosis and HCC. Sarcopenia probably affects the recurrence or progression of HCC in patients who are candidates for liver transplantation. Presumably, sarcopenia in this group of patients affects long-term mortality after surgical treatment of a single lesion with partial hepatectomy. While sarcopenic patients have a smaller volume of preserved liver tissue, there is no data connecting sarcopenia and postoperative decompensation in this group. There is also almost no data relating the effect of sarcopenia in HCC treatment with radiofrequency ablation. In the group of patients receiving transarterial chemoembolization, sarcopenia affected survival and response to treatment. In the group of patients receiving chemotherapy with sorafenib, sarcopenia affected disease progression. Numerous data suggest that sorafenib itself has a significant effect on reducing muscle mass. The condition is codependent in both directions as sarcopenia has an impact on the systemic treatment side effects severity (e.g., enteritis), and thus on the sorafenib dosage reduction[21].
In recent years, it has been pointed out that sarcopenia is a systemic disease. Cardiac sarcopenia likely manifests as a heart failure with preserved ejection fraction[1]. Relationships between heart failure and depleted lean muscle mass are indisputable and go both ways. Increased mortality in sarcopenic patients could be partly explained by this bilateral effect, especially in the posttransplant period. Cardiac ultrasound (US) is suggested to assess cardiac function in a sarcopenic patient with cirrhosis[22]. Involvement of the diaphragm leads to reduced peak cough flow in the elderly, increased rate of respiratory infections through impaired airway clearance and difficulties when weaning sarcopenic patients with cirrhosis from mechanical ventilation[5]. Impaired function of the cardiac and respiratory muscles likely contributes to clinical manifestations of dyspnea, weakness, fatigue, reduced exercise tolerance, and loss of appetite, all of which can further contribute to the propagation of sarcopenia and frailty[1].
DIAGNOSTIC METHODS IN THE FIELD OF SARCOPENIA
Questionnaires
Questionnaires take precedence over other tests because of their relatively short evaluation time and their possibility to monitor the condition in a dynamic timely manner[7]. Across the investigative options to assess malnutrition, Subjective Global Assessment (SGA) is the most frequently mentioned. Features of the SGA include a physical exam component that evaluates the loss of subcutaneous fat, peripheral or sacral edema, and muscle wasting. The quantity of muscle and subcutaneous tissue is graded subjectively by the examiner who then categorizes it as normal, mildly, moderately, or severely decreased. Multiple components on patient history are also evaluated. The first component is the amount of weight loss in the previous 6 mo. Supplementary historical features of the SGA include patient’s dietary intake and the presence of gastrointestinal symptoms experienced daily for at least 2 wk. Once the history and physical examination sections are completed, patients are classified as well nourished (SGA grade A), moderately malnourished or suspected of being malnourished (SGA grade B), or severely malnourished (SGA grade C). SGA is a partially subjective method constituted by quantitative and qualitative variables, subject to varied interpretations, and reported as having low sensitivity in cirrhotic patients, as it can underestimate the nutritional state in their population[22].
Nutritional Risk Screening 2002 (NRS-2002), Liver Disease Undernutrition Screening Tool (LDUST), Royal Free Hospital-Nutritional Prioritizing Tool (RFH-NPT), and Malnutrition Universal Screening Tool (MUST) can be also used to assess malnutrition in hospitalized patients.
The NRS-2002 is a nutrition screening tool recommended by the ESPEN guidelines[17]. It includes three components―the nutritional score (BMI, weight loss, and dietary intake included), the disease severity score, and the age score (age > 70 years)[17,23]. Patients are classified as having no or low risk.
The LDUST assesses 6 factors that were identified as having the strongest associations with malnutrition in patients with chronic liver disease (nutrient intake, weight loss, loss of subcutaneous fat, loss of muscle mass, fluid accumulation, and a decline in functional status). The three potential patient responses are labeled and indicated as no signs of malnutrition, “mild to moderate” malnutrition, and “moderate to severe” malnutrition[24].
The RFH-NPT is a nutrition screening tool developed in the United Kingdom. It includes three major steps: (1) Patients who have alcoholic hepatitis or are undergoing tube feeding are immediately evaluated as high risk without proceeding to the next step; (2) Patients who do not have alcoholic hepatitis and are not undergoing tube feeding are assessed for fluid overload and its impact on food intake and weight loss; and (3) Patients who do not have fluid overload are assessed for nutritional status (BMI, unplanned weight loss, and daily dietary intake). Patients are stratified as being at low, moderate, or high risk[2,25].
The MUST includes three categories: Current BMI, unintentional weight loss, and the presence of any acute disease that could compromise nutritional intake for more than 5 d[25].
A large study assessed the importance of RFH-NPT in the Asian population with predominantly viral liver cirrhosis. The questionnaire proved to be useful in the group of patients with a low MELD score and for assessing the prognosis of the disease. The disadvantage of the study is that different questionnaires are only compared with each other, and with only basic laboratory and anthropometric parameters[25]. In a recent publication, Buchard et al[5] noted that RFH-NPT and LDUST, despite recommendations for their use in patients with liver cirrhosis, have not yet been associated with clinical issues such as survival and complications appearance. Moreover, LDUST is based on the patient’s statement and lacks objective data.
Laboratory tests
Myostatin is a natural muscle growth inhibitor. As a marker, it depends on gender and inflammatory processes in the body. In men with liver cirrhosis, it can be used to assess muscle mass, prognosis of decompensation events, and fit for surgery status. In contrast to Oshida et al[27] who came to different conclusions in the group of patients with compensated disease, the cited study used CT sarcopenia indices in a large group of patients with decompensated advanced chronic liver disease (dACLD). The limitation of the test is the decrease in the level of serum myostatin at a very low muscle mass stage which presents a major problem when defining possible cut-off values[26].
Another potential biomarker is irisin, a myokine, mainly expressed and secreted by skeletal muscles as well as functioning as an adipokine. A significant lower irisin level is proved to be a marker for muscle weakness and atrophy. In the group of patients with dACLD, the cited study demonstrated higher irisin levels in women without evidence of an association with irisin levels and the degree of hepatic impairment according to CHILD/MELD score, or the presence of ascites. Sarcopenia was assessed using a hand grip test and CT assessment of muscle mass[2]. In a related publication, a group led by Zhao et al[10] studied irisin levels in a group of patients with liver cirrhosis where a hand grip test and CT-assessed muscle mass were also used for the evaluation of sarcopenia. The difference between the studies was in the CT index used, as the latter used the proposed gold standard (see below). Lower irisin levels were demonstrated in the group of patients with a higher CHILD score. They also defined that it is not entirely clear whether this is the cause or the consequence of sarcopenia. Deficiency of the article are poorly defined criteria for the diagnosis of liver cirrhosis[10].
If muscles are damaged by diseases or vigorous exercise, titin is decomposed by proteolytic enzymes, and various titin fragments are detected in serum and urine. One of the isolated fragments is titin-N. The cited study defined its urinary excretion in a group of patients with metabolic-induced fatty liver disease. The study had a well-defined control group, but poorly defined comparative indices (CT indices? muscle and liver elastography? US parameters of skeletal muscle assessment?) in the article itself. According to their observations, titin-N was negatively associated with the amount of muscle mass (higher level in urine correlated with lower skeletal muscle mass) and positively associated with the occurrence of muscle myosteatosis (higher level in urine correlated with higher levels of US-assessed skeletal muscle fat). They also identified an association of the biomarker with the degree of muscle fibrosis progression which may be associated with functional muscle decline. For the latter, a knee extension test and a hand grip test were used as a comparative test[27].
Anthropometry
According to EASL guidelines, two simple criteria can be used in everyday clinical practice to stratify patients at high risk of malnutrition: being underweight (BMI < 18.5 kg/m2) and having advanced decompensated cirrhosis (Child C patients)[28]. In patients with ascites and peripheral edema, dry weight assessment is proposed, either estimated by the post paracentesis body weight, the weight recorded before fluid retention if available, or by subtracting a percentage of weight based upon the severity of ascites (mild 5%; moderate 10%; severe 15%), with an additional 5% subtracted if bilateral peripheral edema is present[25,28].
Of the measurements that can be performed with an ordinary tape measure, the most common are mid-arm muscle circumference (MAMC), mid-arm muscle area, and triceps skinfold (TSF), all of which are simple and rapid to perform low-cost tests that are not affected by the presence of fluid retention[7,28]. Some Asian studies mention calf circumference which showed a good association to the frailty[29] in their study population and for the assessment of sarcopenia in the group of patients with compensated ACLD (cACLD), or in patients without ascites[30]. The disadvantage of both studies is the assessment of muscle mass with tests that are less useful in the overweight or hypervolemic patients’ population.
Simple tests of muscle function
This group of tests can be performed at the bedside or during outpatient consultations.
The Short Physical Performance Battery consisting of three methods - balance test, gait speed test, and five chair stand test - can be used to assess the patient’s functional ability[2,7].
Handgrip strength test (HS) is the most commonly studied test in this group using a calibrated dynamometer. The test depends on the patient’s age and BMI. An interesting study has shown that HS in combination with the MELD score in men awaiting liver transplantation can be superior to CT modality if mortality was observed as a clinical outcome. Study results could be explained due to the early decline in muscle function even before the decline in muscle composition and mass occurs[31].
To assess muscle function, a test cited by Buchard et al[5] is commonly mentioned - the Liver Frailty Index (LFI). Its role is to evaluate frailty by combining HS, chair stands, and balance tests. Using a provided cut-off, LFI was associated with mortality independently of the presence of HE and ascites[5,7].
The next frequently used test is a six-minute walk test which has a sensitivity of 90% for identifying patients with increased risk for pre-LT mortality when performed at less than 250 m[1,7].
A walking speed/gait test is offered as a third option[2,32]. In a study by Nishikawa et al[32], a 6 m walking test was performed to measure muscle function in a group of patients with liver cirrhosis, with walking speed (WS) and gait speed (GS) defined, respectively. As a reference to define sarcopenia, bioelectrical impedance analysis (BIA) was used and therefore only patients without ascites were included. They observed that improvement in WS requires quick movement, whereas the improvement in the GS does not. WS requires maintained cognitive function, and muscle strength may be necessary for its improvement, while the GS value will not decrease as long as muscle strength is maintained even if cognitive function is reduced. They confirmed that hypervolemia or tissue edema and the presence of various orthopedic diseases have a significant effect on both tests. It is likely that WS could have a greater impact on the assessment of frailty than on the assessment of sarcopenia[32].
Body composition measurements
These diagnostic methods represent the foundation of modern clinical body composition analysis but with several important limitations when evaluating patients with liver cirrhosis.
BIA: A fixed, low-voltage, high-frequency alternating current is introduced into the human body to assess body electrical conductivity together with resistance (impedance). Subsequently, capacitance is the parameter that makes the current lag behind the voltage, which results in a phase shift. This shift is measured geometrically as the angular transformation of the capacitance to resistance ratio, or the phase angle (PA). BIA measures belong to safe, rapid, easy-to-perform, and quite accurate methods of estimating fat mass and fat-free mass. The main limitation of its use in the group of patients with liver cirrhosis is the influence of hypervolemia, physical activity, use of diuretic therapy, BMI, and liquid or food intake before the test[13,24,33].
There are several parameters that can be measured by means of BIA: Body cell mass, total body water, extracellular water, extracellular mass, and body fat. Multifrequency BIA analysis has been proposed lately because it is less influenced by overhydration. It measures the above-mentioned parameters by passing a series of different electrical currents and electrical frequencies through the body. Segmental BIA can also be used to overcome the fluid retention bias[33].
PA was observed to be less affected by overhydration while being a reliable indicator of clinical outcome. As such, a PA result less or equal to 5.4 degrees was a predictor of reduced survival in one of the conducted surveys. The data obtained from another investigation indicated PA cut-off less than or equal to 4.9 as a predictor of disease progression and mortality in cirrhotic patients[17,33]. A Japanese study comparing the applicability of BIA to equivalent anthropometries (MAMC, TSF) found that BIA measures comparably 6%-16% higher values of muscle mass estimates. Nevertheless, the results coincided with the prognosis of mortality in the cohort where cirrhosis was proven histologically in all patients. The disadvantage of the study is the inclusion of mostly compensated patients, and that no comparison with other tests was performed[18].
Dual energy x-ray absorptiometry: Dual energy x-ray absorptiometry (DEXA) uses low-dose x-rays to provide a comprehensive 3-dimensional analysis of the entire body, thus automatically breaking down each body compartment into bone mass, fat mass, and fat-free (or lean) mass. It is safe, inexpensive, readily available, and reproducible. In comparison to the CT scan, it uses less radiation. As with BIA, the problem of analysis may be water accumulation in muscle and fibrous tissue, especially in the elderly. Also, it is difficult to access in some medical centers[13,31,33].
In the group of patients with liver cirrhosis, it shows an association between muscle mass assessment and mortality[35]. Appendicular lean mass (APLM) has been proposed as the most appropriate method in cirrhosis to minimize confounding by ascites[31]. A well-designed Greek study that excluded the population of patients with active alcohol consumption compared the proposed APLM index (ALMI - APMI balanced to the patient’s height; unit kg/m2) to CT indices of muscle mass, together with multivariate analyses of patient’s age and gender. They compared the success of detecting sarcopenia by DEXA and by the CT cut-off values proposed by several study groups (Carey, Montano-Loza, Mourtzakis, ESPEN, and indices proposed from oncological populations - Martin, Prado). They found that the proposed cut-off values in the DEXA-assisted analysis matched the detection of sarcopenia very well when comparing the CT cut-off values proposed by the various research groups[36]. An important finding of the study was also that the prevalence of low muscle mass did not reveal any statistically significant difference in relation to disease etiology in general. However, it should be noted that a significantly higher percentage of patients with an alcoholic etiology was found in the low muscle mass subgroup compared to the normal muscle mass group. As a weak point of the DEXA analysis, they mention the accessibility of the investigation in smaller hospital centers[36].
On the other hand, some studies report only a weak concordance between DEXA and CT when identifying sarcopenia in cirrhosis[24]. The rest state that DEXA indices show a sex-related distribution of body compartments. In cirrhotic women, more reduction in fat stores is observed with the maintenance of lean tissue. In men, the loss of lean tissue is the most featured early phenomenon. A described pattern is reflected by a weak association between muscle strength and muscle mass in cirrhotic women[33].
The following study in this area states the possibility of using proposed DEXA limb muscle mass indices which showed a good correlation compared to CT indices. The study was conducted in a small cohort of patients awaiting liver transplantation. They observed that fewer women were identified sarcopenic with DEXA than expected. They also cite the well-known DEXA deficiency as not being able to offer muscle structure quality determination compared to the CT examination[37].
US: US can be used to evaluate echogenicity, diameter, cross-sectional area, and muscle volume. Performing three consecutive measurements and using their mean value as a final result is proposed. The patient should rest for at least 5 min before the measurement and not exercise less than 30 min before the examination. To assess the muscle contraction potential, muscle thickness can be measured before and after the movement, especially in muscles that have a significant change in diameter during contraction. The possibility of assessing microvascularization with contrast enhanced US and the assessment of pennation angle are mentioned. A special expert group for this field lists 39 muscle groups that can be evaluated using US examination. They propose standardizations of the measurement site and explore main problems of the investigative methodology that should be solved. The inability of assessing some muscle components with US is the most common problem. Other problems mentioned are visibility, dependency of the operator’s experience as well as the patient’s general condition impact on the measurement value, and its dependency on the equipment quality[38].
In this area, the quadriceps muscle evaluation, especially its thickness and quality, is most often cited in the literature. Pita et al[39] estimated a daily decline in muscle mass in patients with cirrhosis who were waiting for a liver transplant in the intensive care unit. In a relatively small cohort, CT-estimated muscle mass was used for comparison demonstrating the ability of the US to monitor muscle mass decline with daily measurements of rectus femoris muscle diameter, as well as the association between this result and mortality in the studied patients. Another frequently cited study from this area performed quadriceps diameter measurement and proposed a model that included BMI. It states that the US of quadriceps muscle is a low-cost, reliable, reproducible, and accurate estimate of muscle mass that can be completed at the bedside or in an outpatient clinical setting as well as repeated without concern of radiation exposure. Gender-specific nomograms that correlated well with CT control were suggested. The cohort of patients was small, but the comparability between the two operators was good[40].
Measurements of the tongue muscle thickness in the group of patients with liver cirrhosis showed an association with the CHILD and MELD score, and a distinctive difference between the group of healthy control group, but with no proven CT control correlation. The definition of liver cirrhosis in the article was relatively loose[41].
In a population of Japanese cirrhosis patients, Kobayashi et al[42] measured the area of the psoas muscle in the right groin area and balanced it by the square of body height. The examination was quick and performed at bedside in a large cohort of patients. The study showed a significant correlation between US and CT measurements, suggesting the reliability of the US measurement. In addition, the proposed US index was significantly associated with CT obtained measurements. There was also a good comparison between the two investigators and 100% applicability of the method in the investigated cohort. A small number of patients with dACLD, especially patients with ascites, can be mentioned as a study deficit[42]. Our group also used the psoas muscle measurement in a small group of patients with dACLD. The infero-lateral area in respect to the right kidney was used as the measurement site and the estimated psoas muscle diameter was balanced to the patient’s height. The proposed index showed clinical significance when predicting any subsequent decompensation and patient mortality. The measurement showed an important limitation in terms of practical use, as it was not feasible in about a quarter of patients due to technical limitations (ascites, obesity, visibility). Also, no comparison with any of the standard investigatory methods was made[43].
In recent years, the use of elastography has been suggested to assess muscle stiffness which could indirectly point to the loss of muscle function. A well-structured study in a small cohort of patients showed an association between femoral muscle elastography results and frailty[44].
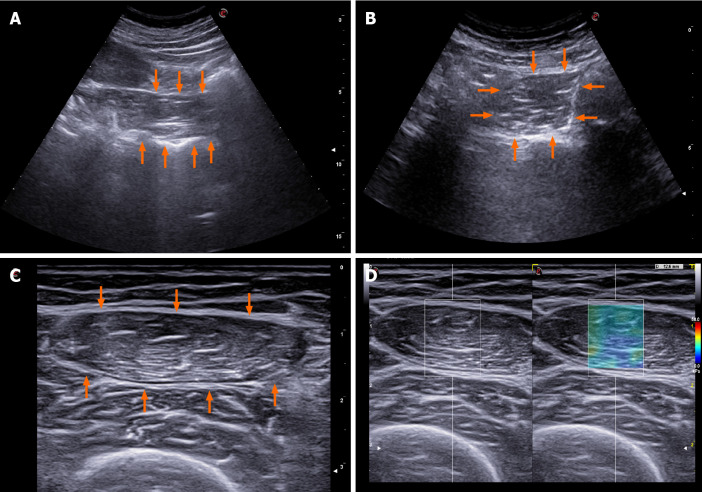
The US imaging of some of the above mentioned methods is showed in Figure 2.
Figure 2.
Reported options for ultrasound muscle mass assessment in cirrhosis. A: Psoas muscle evaluation as proposed by Hari et al[43]; B: Psoas muscle evaluation as proposed by Kobayashi et al[42]; C: Femoral muscle evaluation as proposed by Tandon et al[40]; D: 2D-shearwave elastography of the femoral muscle.
Cross-sectional imaging: CT: CT allows an accurate cross section assessment of muscle mass area and an estimate of tissue density using Hounsfield units (HU)[13]. It is recommended to use tissue that shows HU estimated density from -29 to +150 on two consecutive CT slices at L3 level. To make it more clinically applicable, it is suggested to use various computer programs which automatically and very reliably evaluate the muscle area (e.g., SliceOmatic, ImageJ, FatSeg, OsiriX)[7]. All these programs require manual image analysis by a radiologist. In this regard, some studies have tested the concept of a methodology to measure psoas muscle features on incidental CT scans using an automated, deep convolutional neural network model, a technology routinely used for facial recognition software. Automated measurement in the cirrhosis patient population proved to be comparable to that where the measurement was performed with standardized programs. It was significantly faster and independent of human error[45]. An alternative option is open-source software for image processing which requires manual tracing of various abdominal regions to obtain body composition information. Using these results and a preprogrammed template, the operator can easily generate various muscle indices. Alternatively, the use of the method first published by Durand et al[35] (see below) is suggested[45].
The most common shortcomings of CT sarcopenia analysis are unclear possibility of recurrent/dynamic evaluation due to radiation exposure and the impact of the result on clinical decisions; unclear protocol of the examination method; radiation impact; examination costs; and the need for additional software to analyze images. In addition to the ability to differentiate three main body compartments, i.e., muscle, visceral, and subcutaneous adipose tissue, the ability to identify muscle radiodensity to determine ectopic fat accumulation in muscles, and the fact that it is probably not affected by the presence of ascites or edema, the pros are the relative ease, speed, accuracy, and accessibility of the examination in a hospital setting. There is a strong possibility of price and radiation exposure reduction to only 2.6 mSv by a single slice CT, and CT shows a very good reproducibility between different performers[7,19,31].
The test of choice is the L3 skeletal muscle index (SMI), a muscle area on a CT scan at the level of the L3 vertebrae corrected for height (Figure 3)[7]. Carey et al[24] recently defined sarcopenia as SMI < 50 cm2/m2 in males and 39 cm2/m2 in females in a cohort of cirrhotic patients in the North American region awaiting liver transplantation[1,13,24]. In this cohort, a CT measured SMI was a strong surrogate of whole-body muscle mass and can be applied as a reliable marker of the whole-body muscularity. Defined in this way, sarcopenia was mainly delineated as sex-specific SMI values associated with mortality that were independent of age and MELD score[13,24]. When assessing sarcopenia by SMI on an abdominal CT scan, there does not appear to be a large difference between measurements at L3 vs L4 vertebrae[10]. There is also an excellent agreement between various software programs with respect to measurements of abdominal skeletal muscle area[46].
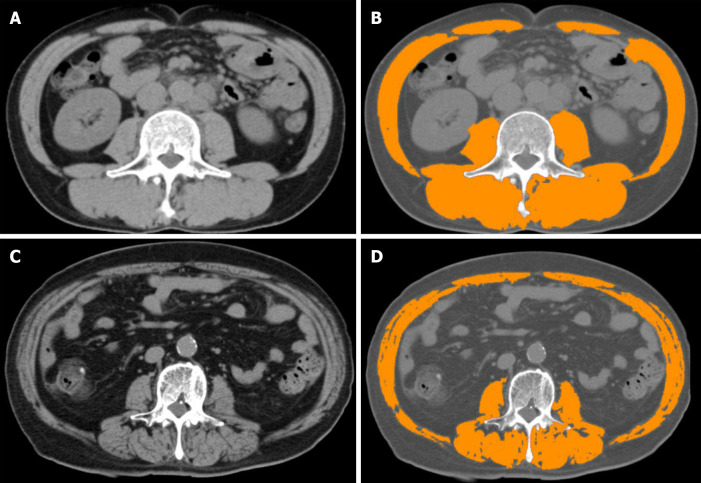
Figure 3.
Computed tomography modality and skeletal muscle index. A and C: Analysing computed tomography slices obtained at the third lumbar vertebra level; B: Patient with normal skeletal muscle index (SMI) values; D: Patient with reduced SMI values. After using medical imaging software and analysing areas of predefined Hounsfield units, SMI values are calculated.
SMI seems to be a more complete and robust measurement than individual measurement of the psoas muscle or the psoas muscle index (PMI), especially in men with cirrhosis. In addition, low PMI identifies an incomplete subset of patients at increased risk of mortality indicated by low SMI[13,47,48]. The degree of sarcopenia can also be defined by SMI as severe sarcopenia [SMI two SD below the sex-specific mean value of a young donor (18-40 years old), sarcopenia (SMI between -1 and -2 SD of sex-specific young) donor mean values] and as a non-sarcopenic group (SMI higher than one SD below the sex-specific mean for young donors)[49]. SMI study validations include numerous publications. Important study of this area published by Ebadi et al[49] defined cut-off values compared to healthy controls. In their cohort, cut-off values for SMI were < 42 cm2/m2 in men and < 30 cm2/m2 in women (for severe sarcopenia) and < 50 cm2/m2 and < 37 cm2/m2 for sarcopenia. Mortality was considered as a clinical outcome. The cohort showed the presence of severe sarcopenia in 8% of patients, the majority of which had alcohol-related cirrhosis[49].
The second common cited choice is the index first proposed by Durand et al[35].They demonstrated that the ratio between the transverse diameter of the psoas muscle measured at the L3 level (umbilicus is suggested as a reference point) and balanced for the patient’s height [psoas to height ratio (PTHR); unit mm/m] is an objective indicator of muscle loss and a predictor of mortality in patients with cirrhosis, independent of MELD or Na-MELD score. In their retrospective study, they also demonstrated the effect of the PTHR on mortality in patients with refractory ascites[35]. A study by Paternostro et al[47] tested the value of a similar index. They demonstrated the clinical applicability and calculative ease of PTHR to define sarcopenia with similar cut-off values as proposed by Durand et al[35]. A weak point of this methodology is a variable landmark (the umbilicus) to measure muscle thickness; thus, CT-derived index from single muscle measurement can still not be recommended[46].
To determine myosteatosis, CT should be performed without the use of a contrast agent. Muscle density is assessed by HU values[13]. To define myosteatosis, the proposed cut-off values are > 41 HU in patients with BMI < 24.9, and > 33 HU in those with a BMI > 25. In a group of patients with liver cirrhosis, Bhanji et al[50] demonstrated the effect of CT-assessed myosteatosis on the occurrence of HE within one year of the measurement. It is probably a risk independent of sarcopenia.
An interesting option offered by CT is the already mentioned possibility to assess the concomitant presence of sarcopenia and myosteatosis. In a small cohort of patients with cirrhosis, Nardelli et al[51] assessed the presence of sarcopenia and myosteatosis with suggested CT SMI and HU values. Minimal HE defined by psychomotor tests was more common in the group of patients with both factors, as was elevated blood ammonia.
Magnetic resonance imaging: Magnetic resonance imaging (MRI) is considered as an appealing test for the diagnosis of muscle wasting due to the lack of radiation exposure and high-quality images, including information on muscle quality as evident by fat infiltration[7]. When comparing results from CT and MRI images in Traub et al[8] study cohort, there was no difference in the detection rate of reduced muscle mass. However, the study did not compare CT and MRI images in the same patients since they used only imaging studies that were routinely performed. The study by Beer et al[52] included patients with cirrhosis who had clinical or imaging cirrhosis parameters. Additionally, the FIB-4 score was used to assess the degree of liver fibrosis. Portal hypertension was defined invasively, or its signs were evaluated by gastroscopy. MRI-assessed sarcopenia using PTHR index demonstrated an association between sarcopenia rates and mortality in patients with cACLD and the influence of sarcopenia on infection or mortality due to infections. Mortality was particularly high in the PTHR < 8 mm/m (female) and < 12 mm/m (male) group. They also pointed out the advantage of the MRI for its possibility to provide sarcopenia data without the use of a contrast agent and without radiation exposure and reported good comparability with CT-PTHR measurements as well as good agreement between the two readers[52].
MRI offers another imaging technique, namely diffusion weighted imaging (DWI). DWI offers additional information regarding the composition and architecture of investigated tissue. DWI quantified by apparent diffusion coefficient (ADC) can reflect different pathological changes, such as cell density, extracellular matrix, nucleic areas, and membrane permeability, and may have a role in the diagnosis of different muscle disorders. In the cited study, ADC maps were thus created by the implemented software and manually drawn regions of interest on the ADC maps along the contours of the iliopsoas and paravertebral muscles to avoid fat areas and vessels. They confirmed that ADC can reflect pathological muscle changes since myositis and myopathy had statistically significantly higher ADC values in comparison to unaffected muscles. The study does not offer a direct proposal for the clinical use of the proposed indices in the assessment of sarcopenia. In the multivariate analysis, the authors also used only the MELD score[53].
CLINICAL TREATMENT OPTIONS
The treatment of sarcopenia is focused on drug treatment possibilities, exercise and dietary measures, and the treatment of decompensated liver cirrhosis. There are currently no studies that prove the benefit of one or the other in terms of reducing mortality. There is a general benefit of the interventions in terms of reducing the feeling of fatigue; increasing vital capacity; improving muscle mass and ability to exercise; and last but not least, improving quality of life[7].
The general objectives of drug treatment are to focus on lowering blood ammonia and improving the action of growth hormone and testosterone in certain parts of the population with liver cirrhosis. Special emphasis should be placed on the elimination of poor appetite and complete absence of physical activity[1,2,7]. Studies suggest a possible beneficial effect of testosterone on improving muscle mass and function without significant side effects of treatment, but the evidence is yet not enough to recommend replacement therapy[2,7]. Extensive study data on the use of growth hormone in this area are not available. The importance of regulating thyroid hormone and blood sugar levels is also mentioned[24].
General nutrition advice concerns sufficient energy and adequate protein intake while avoiding prolonged fasting periods (> 6 h). The patient should eat three to five meals a day while the target caloric intake varies according to the patient’s BMI. As per EASL guidelines, at least 35 kcal/kg of actual body weight per day is recommended in non-obese individuals (BMI < 30). Second, all patients should be provided with a target protein intake - guidelines have been consistent in the recommendation of 1.2 g/kg to 1.5 g/kg per day. This can be achieved by using multiple sources including meat, dairy, and vegetable proteins, with some support from the literature stating that the latter two sources may have additional benefits against HE. In obese patients with cirrhosis, a moderate decrease in caloric intake (500 kcal to 800 kcal daily and not less than 70% of previous caloric intake) with a tailored, individualized dietary plan is recommended. Best results on liver histology and on the HVPG were observed in patients achieving a weight loss of 10%, and this should probably be considered the target of lifestyle interventions. A late evening snack before night sleep with 50 g of complex carbohydrates and approximately 15 g of protein content is acceptable and significantly increases muscle mass and may also have beneficial effects on HE reccurence. Patients should be counseled on the need to eat breakfast[11,13]. Evidence is mounting regarding the benefit of Mediterranean diet -rich in vegetables, fruit, and olive oil - in the cirrhotic population. In this fashion, patients are advised to avoid processed foods and use fresh ingredients[11,17]. Among the dietary supplements, the daily use of branched chain amino acids and aromatic amino acids which are probably associated with event-free life and a quality of life is much mentioned. Special attention is also paid to the effect of L-carnitine, vitamin D, omega-3 fatty acids, and leucine[1,7,13]. Vitamin and microelement deficiency should be excluded, and in case of a deficit, vitamin D and zinc should be supplemented. Branched chain amino acid supplements and leucine-enriched amino acid supplements (6-8 g per day) should be considered, particularly in decompensated patients[11]. Ghrelin, a peptide produced in the stomach, has anabolic and anti-inflammatory properties that make it a promising agent[10]. A small study assessing ghrelin infusion in patients with heart failure showed improvement in LV function, muscle strength, lean body mass, and exercise capacity[1].
In the field of exercise, moderate intensity exercise is recommended for at least 30 min per day, 3-5 times per week. Physical exercise should start with a short-term warm-up and end with a stretching/cool-down phase. Although a combination of resistance and aerobic exercise is recommended, resistance exercise is more effective in reversing sarcopenia[9,13]. Avoidance of sedentary behavior should be recommended even in patients not willing to undergo a formal exercising program to increase daily physical activity within the context of NEAT (nonexercise activity thermogenesis). As opposed to scheduled exercise, NEAT encourages patients to take opportunities to increase their activity within their day-to-day activities[11]. Exercise brings improvement in insulin sensitivity, an increase in muscle protein synthesis, and the use of muscle as an alternative route for ammonia detoxification. According to EASL guidelines, the target for moderate aerobic activity is to eventually reach up to 150 min per week, incorporating resistance activities on two or more days per week, except for abdominal workouts which are not recommended as they might abruptly increase abdominal pressure[11].
In the general complications of liver cirrhosis treatment area, there is evidence of the beneficial effect of TIPSS insertion on sarcopenia[1,7]. Thus, an uncontrolled study found that the insertion led to an increase in muscle mass and an improvement in overall prognosis. It also showed an improvement in psoas muscle area in 70% of patients with an increase in the mean muscle area after TIPSS insertion[2]. As previously stated, an already present sarcopenia could be considered a risk factor for mortality in patients who undergo TIPS placement[3], data, confirmed with another retrospective study in patients with cirrhosis who undergo TIPS placement for refractory ascites[54]. The therapeutic consequences of this findings are yet to be provided. The final treatment option for many patients with advanced liver cirrhosis is, of course, liver transplantation, which has been shown to have a beneficial effect on reversibility of muscle function after liver transplantation[9].
Dilemmas remain regarding the timeline of the proposed measures. It is hypothesized that an earlier intervention at a time when anabolic potential exists may be more effective than an intervention at a refractory stage of muscle wasting. Studies in this area are rare and have mostly been performed in very small populations in the field of cirrhosis[3]. To date, there is no evidence that exercise or dietary supplements affect sarcopenia through dysbiosis[4]. There is also no clear guidance on the group of patients with HCC and liver cirrhosis where interventions regarding exercise and dietary substitutions before and after treatment would probably be beneficial[21].
CONCLUSION
From their repeatability, comparability, accessibility, and realistic range for the correct clinical assessment of an individual patient, the main qualitative problem of sarcopenia assessment in the population of patients with liver cirrhosis is the great heterogeneity of the proposed clinical tests (Figure 4). Control tests in clinical trials that are not generally proposed to assess sarcopenia in patients with liver cirrhosis are also common. From the patient’s point of view, correct assessment of the sarcopenia degree in the female population as well as in the population of overweight patients further exists. The unanswered question is the possibility of transferring study-confirmed findings between different races. When analysing different published studies, it is common that they address study issues within a small cohort that is occasionally incoherent or too isolative. Many studies are retrospective and represent an analysis of a difficult to verify or an incomplete mass of data stored in hospital computer systems rather than in study-specific storage programs. A common problem is the definition of the liver cirrhosis diagnosis which should in modern cACLD treatment be mostly elastographic, but certainly not a result of the ICD coding nomenclature. All with the aim to cover enough patients in the early disease stages and to discern patients who have sarcopenia at the expense of actual chronically impaired liver function. On the other side of the spectrum, many studies with sarcopenia do not pay enough attention to the population of patients with permanent decompensated disease, especially to those with refractory ascites. Many authors also point out that sarcopenia studies rarely include tests to define muscle function and focus solely on static information regarding total muscle mass which is easier to perform from the study complexity point of view. In this area, the question remains unanswered as to what is the long-term relationship between muscle mass and function, which is probably not linear throughout, and whether this relationship has a significant impact on the risks associated with the presence of sarcopenia. There is considerable incoherence in the studies regarding the use and comparison of different (especially CT-defined) indices of muscle mass or function as well as the inclusion of own study-defined claims in sequential studies (possibility of continuing primary study error).
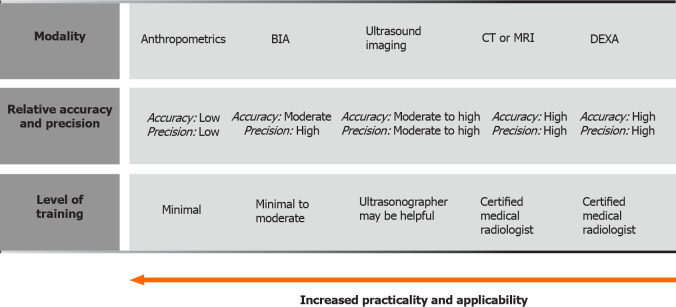
Figure 4.
Sarcopenia evaluating methods. Distinctions regarding required training, applicability, practicality, accuracy and precision are presented. Adapted from Tandon et al[55]. BIA: Bioelectrical impedance analysis; CT: Computed tomography; MRI: Magnetic resonance imaging; DEXA: Dual energy x-ray absorptiometry.
The definition of CT-SMI as the gold standard for muscle mass assessment and the efforts of several study groups to define valid cirrhosis related sarcopenia thresholds are an important foundation for the future study and clinical applicability of this field. Indeed, some data suggest that even small changes in the cut-off values could mean very relevant shifts in the detection and study evaluation of sarcopenia in this population[8].
Study-malnourished areas in the field of sarcopenia and liver cirrhosis are the area of sarcopenia-CSPH interdependence, the area of vulnerable groups (HCC patients, alcoholism, morbid obesity), and the influence of sarcopenia on clinical outcomes in addition to mortality, especially on various forms of disease decompensation. A small number of studies pays attention to the field of sarcopenia treatment through the therapeutic interventions. Such studies are, of course, necessary for the long-term clinical applicability of the field but difficult to perform in larger cohorts.
According to certain clues, sarcopenia could have an important decision regarding the clinical choice for liver transplantation, both in terms of the patient’s higher priority and in terms of the patient’s ability to be fit for a major surgery. In countries that allow such graft allocation, this could lead to the selection of poorer transplants or living donor transplants, or to the rejection of the transplant process in the event of an estimated poor yield.
At the very end, an issue remains that we as clinical professionals find difficult to face. Does the current definition of sarcopenia by the above-mentioned tests have any relevant clinical implications regarding patients’ survival? Although most of the answers point to the affirmative, we are still quite a long way from the objective long-term goals of successful treatment in this area.
Nevertheless, we can certainly be justifiably pleased and proud to look at the huge leap of the last decade in terms of the knowledge gained in this clinically most relevant field. New possibilities for the use of existing and more modern indices of declining muscle mass and function in a patient with cirrhosis are coming to the fore. The tests mentioned in the article are increasingly striving for repeatability and simplicity, both in enabling dynamic sarcopenia monitoring and in reducing investigator dependent errors. A realistic desire for a study breakthrough in the upcoming years remains to define sarcopenia tests and indices that would ensure further ease of affordability and mass clinical applicability for a patient with liver cirrhosis.
Footnotes
Conflict-of-interest statement: The author declare that he has no conflict of interest.
Manuscript source: Invited manuscript
Peer-review started: March 7, 2021
First decision: April 17, 2021
Article in press: July 19, 2021
Specialty type: Gastroenterology and hepatology
Country/Territory of origin: Slovenia
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): 0
Grade C (Good): C
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Huang HC S-Editor: Liu M L-Editor: A P-Editor: Yuan YY
References
- 1.Bhanji RA, Montano-Loza AJ, Watt KD. Sarcopenia in Cirrhosis: Looking Beyond the Skeletal Muscle Loss to See the Systemic Disease. Hepatology. 2019;70:2193–2203. doi: 10.1002/hep.30686. [DOI] [PubMed] [Google Scholar]
- 2.Kukla M, Skladany L, Menżyk T, Derra A, Stygar D, Skonieczna M, Hudy D, Nabrdalik K, Gumprecht J, Marlicz W, Koulaouzidis A, Koller T. Irisin in Liver Cirrhosis. J Clin Med. 2020;9:3158. doi: 10.3390/jcm9103158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ebadi M, Bhanji RA, Mazurak VC, Montano-Loza AJ. Sarcopenia in cirrhosis: from pathogenesis to interventions. J Gastroenterol. 2019;54:845–859. doi: 10.1007/s00535-019-01605-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Nishikawa H, Enomoto H, Nishiguchi S, Iijima H. Liver Cirrhosis and Sarcopenia from the Viewpoint of Dysbiosis. Int J Mol Sci. 2020;21:5254. doi: 10.3390/ijms21155254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Buchard B, Boirie Y, Cassagnes L, Lamblin G, Coilly A, Abergel A. Assessment of Malnutrition, Sarcopenia and Frailty in Patients with Cirrhosis: Which Tools Should We Use in Clinical Practice? Nutrients. 2020;12:186. doi: 10.3390/nu12010186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Cruz-Jentoft AJ, Bahat G, Bauer J, Boirie Y, Bruyère O, Cederholm T, Cooper C, Landi F, Rolland Y, Sayer AA, Schneider SM, Sieber CC, Topinkova E, Vandewoude M, Visser M, Zamboni M Writing Group for the European Working Group on Sarcopenia in Older People 2 (EWGSOP2); and the Extended Group for EWGSOP2. Sarcopenia: revised European consensus on definition and diagnosis. Age Ageing. 2019;48:601. doi: 10.1093/ageing/afz046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Sinclair M. Controversies in Diagnosing Sarcopenia in Cirrhosis-Moving from Research to Clinical Practice. Nutrients. 2019;11:2454. doi: 10.3390/nu11102454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Traub J, Bergheim I, Eibisberger M, Stadlbauer V. Sarcopenia and Liver Cirrhosis-Comparison of the European Working Group on Sarcopenia Criteria 2010 and 2019. Nutrients. 2020;12:547. doi: 10.3390/nu12020547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hsu CS, Kao JH. Sarcopenia and chronic liver diseases. Expert Rev Gastroenterol Hepatol. 2018;12:1229–1244. doi: 10.1080/17474124.2018.1534586. [DOI] [PubMed] [Google Scholar]
- 10.Zhao M, Zhou X, Yuan C, Li R, Ma Y, Tang X. Association between serum irisin concentrations and sarcopenia in patients with liver cirrhosis: a cross-sectional study. Sci Rep. 2020;10:16093. doi: 10.1038/s41598-020-73176-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Tandon P, Berzigotti A. Management of Lifestyle Factors in Individuals with Cirrhosis: A Pragmatic Review. Semin Liver Dis. 2020;40:20–28. doi: 10.1055/s-0039-1696639. [DOI] [PubMed] [Google Scholar]
- 12.Eslamparast T, Montano-Loza AJ, Raman M, Tandon P. Sarcopenic obesity in cirrhosis-The confluence of 2 prognostic titans. Liver Int. 2018;38:1706–1717. doi: 10.1111/liv.13876. [DOI] [PubMed] [Google Scholar]
- 13.Ebadi M, Montano-Loza AJ. Clinical relevance of skeletal muscle abnormalities in patients with cirrhosis. Dig Liver Dis. 2019;51:1493–1499. doi: 10.1016/j.dld.2019.05.034. [DOI] [PubMed] [Google Scholar]
- 14.Al-Azzawi Y, Albo B, Fasullo M, Coukos J, Watts GJ, Tai R, Radcliffe D, Kroll-Desrosiers A, Devuni D, Szabo G. Sarcopenia is associated with longer hospital stay and multiorgan dysfunction in alcoholic hepatitis. Eur J Gastroenterol Hepatol. 2020;32:733–738. doi: 10.1097/MEG.0000000000001583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Rodrigues SG, Brabandt B, Stirnimann G, Maurer MH, Berzigotti A. Adipopenia correlates with higher portal pressure in patients with cirrhosis. Liver Int. 2019;39:1672–1681. doi: 10.1111/liv.14175. [DOI] [PubMed] [Google Scholar]
- 16.Paternostro R, Bardach C, Hofer BS, Scheiner B, Schwabl P, Asenbaum U, Ba-Ssalamah A, Scharitzer M, Bucscis T, Simbrunner B, Bauer D, Trauner M, Mandorfer M, Reiberger T, Lampichler K. Prognostic impact of sarcopenia in cirrhotic patients stratified by different severity of portal hypertension. Liver Int. 2021;41:799–809. doi: 10.1111/liv.14758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Plauth M, Bernal W, Dasarathy S, Merli M, Plank LD, Schütz T, Bischoff SC. ESPEN guideline on clinical nutrition in liver disease. Clin Nutr. 2019;38:485–521. doi: 10.1016/j.clnu.2018.12.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.van Vugt JLA, Alferink LJM, Buettner S, Gaspersz MP, Bot D, Darwish Murad S, Feshtali S, van Ooijen PMA, Polak WG, Porte RJ, van Hoek B, van den Berg AP, Metselaar HJ, IJzermans JNM. A model including sarcopenia surpasses the MELD score in predicting waiting list mortality in cirrhotic liver transplant candidates: A competing risk analysis in a national cohort. J Hepatol. 2018;68:707–714. doi: 10.1016/j.jhep.2017.11.030. [DOI] [PubMed] [Google Scholar]
- 19.Stirnimann G, Ebadi M, Tandon P, Montano-Loza AJ. Should Sarcopenia Increase Priority for Transplant or Is It a Contraindication? Curr Gastroenterol Rep. 2018;20:50. doi: 10.1007/s11894-018-0656-3. [DOI] [PubMed] [Google Scholar]
- 20.Montano-Loza AJ, Duarte-Rojo A, Meza-Junco J, Baracos VE, Sawyer MB, Pang JX, Beaumont C, Esfandiari N, Myers RP. Inclusion of Sarcopenia Within MELD (MELD-Sarcopenia) and the Prediction of Mortality in Patients With Cirrhosis. Clin Transl Gastroenterol. 2015;6:e102. doi: 10.1038/ctg.2015.31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Marasco G, Serenari M, Renzulli M, Alemanni LV, Rossini B, Pettinari I, Dajti E, Ravaioli F, Golfieri R, Cescon M, Festi D, Colecchia A. Clinical impact of sarcopenia assessment in patients with hepatocellular carcinoma undergoing treatments. J Gastroenterol. 2020;55:927–943. doi: 10.1007/s00535-020-01711-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Montano-Loza AJ. Clinical relevance of sarcopenia in patients with cirrhosis. World J Gastroenterol. 2014;20:8061–8071. doi: 10.3748/wjg.v20.i25.8061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Anand AC. Nutrition and Muscle in Cirrhosis. J Clin Exp Hepatol. 2017;7:340–357. doi: 10.1016/j.jceh.2017.11.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Carey EJ, Lai JC, Sonnenday C, Tapper EB, Tandon P, Duarte-Rojo A, Dunn MA, Tsien C, Kallwitz ER, Ng V, Dasarathy S, Kappus M, Bashir MR, Montano-Loza AJ. A North American Expert Opinion Statement on Sarcopenia in Liver Transplantation. Hepatology. 2019;70:1816–1829. doi: 10.1002/hep.30828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Wu Y, Zhu Y, Feng Y, Wang R, Yao N, Zhang M, Liu X, Liu H, Shi L, Zhu L, Yang N, Chen H, Liu J, Zhao Y, Yang Y. Royal Free Hospital-Nutritional Prioritizing Tool improves the prediction of malnutrition risk outcomes in liver cirrhosis patients compared with Nutritional Risk Screening 2002. Br J Nutr. 2020;124:1293–1302. doi: 10.1017/S0007114520002366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Skladany L, Koller T, Molcan P, Vnencakova J, Zilincan M, Jancekova D, Kukla M. Prognostic usefulness of serum myostatin in advanced chronic liver disease: its relation to gender and correlation with inflammatory status. J Physiol Pharmacol. 2019;70 doi: 10.26402/jpp.2019.3.03. [DOI] [PubMed] [Google Scholar]
- 27.Oshida N, Shida T, Oh S, Kim T, Isobe T, Okamoto Y, Kamimaki T, Okada K, Suzuki H, Ariizumi SI, Yamamoto M, Shoda J. Urinary Levels of Titin-N Fragment, a Skeletal Muscle Damage Marker, are Increased in Subjects with Nonalcoholic Fatty Liver Disease. Sci Rep. 2019;9:19498. doi: 10.1038/s41598-019-56121-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.European Association for the Study of the Liver. European Association for the Study of the Liver. EASL Clinical Practice Guidelines on nutrition in chronic liver disease. J Hepatol. 2019;70:172–193. doi: 10.1016/j.jhep.2018.06.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Nishikawa H, Yoh K, Enomoto H, Ikeda N, Aizawa N, Koriyama T, Nishimura T, Nishiguchi S, Iijima H. Anthropometric Measurements and Frailty in Patients with Liver Diseases. Diagnostics (Basel) 2020;10:433. doi: 10.3390/diagnostics10060433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Nishikawa H, Yoh K, Enomoto H, Iwata Y, Sakai Y, Kishino K, Shimono Y, Ikeda N, Takashima T, Aizawa N, Takata R, Hasegawa K, Koriyama T, Yuri Y, Nishimura T, Nishiguchi S, Iijima H. Calf Circumference as a Useful Predictor of Sarcopenia in Patients With Liver Diseases. In Vivo. 2020;34:2561–2569. doi: 10.21873/invivo.12073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Sinclair M, Chapman B, Hoermann R, Angus PW, Testro A, Scodellaro T, Gow PJ. Handgrip Strength Adds More Prognostic Value to the Model for End-Stage Liver Disease Score Than Imaging-Based Measures of Muscle Mass in Men With Cirrhosis. Liver Transpl. 2019;25:1480–1487. doi: 10.1002/lt.25598. [DOI] [PubMed] [Google Scholar]
- 32.Nishikawa H, Enomoto H, Yoh K, Iwata Y, Sakai Y, Kishino K, Ikeda N, Takashima T, Aizawa N, Takata R, Hasegawa K, Ishii N, Yuri Y, Nishimura T, Iijima H, Nishiguchi S. Walking Speed: Japanese Data in Chronic Liver Diseases. J Clin Med. 2020;9:166. doi: 10.3390/jcm9010166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Cichoż-Lach H, Michalak A. A Comprehensive Review of Bioelectrical Impedance Analysis and Other Methods in the Assessment of Nutritional Status in Patients with Liver Cirrhosis. Gastroenterol Res Pract. 2017;2017:6765856. doi: 10.1155/2017/6765856. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Moriwaki EI, Enomoto H, Saito M, Hara N, Nishikawa H, Nishimura T, Iwata Y, Iijima H, Nishiguchi S. The Anthropometric Assessment With the Bioimpedance Method Is Associated With the Prognosis of Cirrhotic Patients. In Vivo. 2020;34:687–693. doi: 10.21873/invivo.11825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Durand F, Buyse S, Francoz C, Laouénan C, Bruno O, Belghiti J, Moreau R, Vilgrain V, Valla D. Prognostic value of muscle atrophy in cirrhosis using psoas muscle thickness on computed tomography. J Hepatol. 2014;60:1151–1157. doi: 10.1016/j.jhep.2014.02.026. [DOI] [PubMed] [Google Scholar]
- 36.Georgiou A, Papatheodoridis GV, Alexopoulou A, Deutsch M, Vlachogiannakos I, Ioannidou P, Papageorgiou MV, Papadopoulos N, Yannakoulia M, Kontogianni MD. Validation of cutoffs for skeletal muscle mass index based on computed tomography analysis against dual energy X-ray absorptiometry in patients with cirrhosis: the KIRRHOS study. Ann Gastroenterol. 2020;33:80–86. doi: 10.20524/aog.2019.0432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Lindqvist C, Brismar TB, Majeed A, Wahlin S. Assessment of muscle mass depletion in chronic liver disease: Dual-energy x-ray absorptiometry compared with computed tomography. Nutrition. 2019;61:93–98. doi: 10.1016/j.nut.2018.10.031. [DOI] [PubMed] [Google Scholar]
- 38.Perkisas S, Bastijns S, Baudry S, Bauer J, Beaudart C, Beckwée D, Cruz-Jentoft A, Gasowski J, Hobbelen H, Jager-Wittenaar H, Kasiukiewicz A, Landi F, Małek M, Marco E, Martone AM, de Miguel AM, Piotrowicz K, Sanchez E, Sanchez-Rodriguez D, Scafoglieri A, Vandewoude M, Verhoeven V, Wojszel ZB, De Cock AM. Application of ultrasound for muscle assessment in sarcopenia: 2020 SARCUS update. Eur Geriatr Med. 2021;12:45–59. doi: 10.1007/s41999-020-00433-9. [DOI] [PubMed] [Google Scholar]
- 39.Pita A, Ziogas IA, Ye F, Chen Y, Rauf MA, Matsuoka LK, Kaur N, Whang G, Zielsdorf SM, Bastas G, Izzy M, Alexopoulos SP. Feasibility of Serial Ultrasound Measurements of the Rectus Femoris Muscle Area to Assess Muscle Loss in Patients Awaiting Liver Transplantation in the Intensive Care Unit. Transplant Direct. 2020;6:e618. doi: 10.1097/TXD.0000000000001067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Tandon P, Low G, Mourtzakis M, Zenith L, Myers RP, Abraldes JG, Shaheen AA, Qamar H, Mansoor N, Carbonneau M, Ismond K, Mann S, Alaboudy A, Ma M. A Model to Identify Sarcopenia in Patients With Cirrhosis. Clin Gastroenterol Hepatol. 2016;14:1473–1480.e3. doi: 10.1016/j.cgh.2016.04.040. [DOI] [PubMed] [Google Scholar]
- 41.Tandon M, Singh H, Singla N, Jain P, Pandey CK. Tongue thickness in health vs cirrhosis of the liver: Prospective observational study. World J Gastrointest Pharmacol Ther. 2020;11:59–68. doi: 10.4292/wjgpt.v11.i3.59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Kobayashi K, Maruyama H, Kiyono S, Ogasawara S, Suzuki E, Ooka Y, Chiba T, Kato N, Yamaguchi T. Application of transcutaneous ultrasonography for the diagnosis of muscle mass loss in patients with liver cirrhosis. J Gastroenterol. 2018;53:652–659. doi: 10.1007/s00535-017-1378-2. [DOI] [PubMed] [Google Scholar]
- 43.Hari A, Berzigotti A, Štabuc B, Caglevič N. Muscle psoas indices measured by ultrasound in cirrhosis - Preliminary evaluation of sarcopenia assessment and prediction of liver decompensation and mortality. Dig Liver Dis. 2019;51:1502–1507. doi: 10.1016/j.dld.2019.08.017. [DOI] [PubMed] [Google Scholar]
- 44.Becchetti C, Germani G, Burra P, Dufour JF, Berzigotti A. Bidimensional shear wave elastography of the rectus femoris muscle in patients with cirrhosis. J Hepatol. 2020;73:S696. doi: 10.1016/j.clinre.2023.102080. [DOI] [PubMed] [Google Scholar]
- 45.Wang NC, Zhang P, Tapper EB, Saini S, Wang SC, Su GL. Automated Measurements of Muscle Mass Using Deep Learning Can Predict Clinical Outcomes in Patients With Liver Disease. Am J Gastroenterol. 2020;115:1210–1216. doi: 10.14309/ajg.0000000000000662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Marasco G, Sadalla S, Vara G, Golfieri R, Festi D, Colecchia A, Renzulli M. Imaging Software-Based Sarcopenia Assessment in Gastroenterology: Evolution and Clinical Meaning. Can J Gastroenterol Hepatol. 2021;2021:6669480. doi: 10.1155/2021/6669480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Paternostro R, Lampichler K, Bardach C, Asenbaum U, Landler C, Bauer D, Mandorfer M, Schwarzer R, Trauner M, Reiberger T, Ferlitsch A. The value of different CT-based methods for diagnosing low muscle mass and predicting mortality in patients with cirrhosis. Liver Int. 2019;39:2374–2385. doi: 10.1111/liv.14217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Ebadi M, Wang CW, Lai JC, Dasarathy S, Kappus MR, Dunn MA, Carey EJ, Montano-Loza AJ From the Fitness; Life Enhancement and Exercise in Liver Transplantation (FLEXIT) Consortium. Poor performance of psoas muscle index for identification of patients with higher waitlist mortality risk in cirrhosis. J Cachexia Sarcopenia Muscle. 2018;9:1053–1062. doi: 10.1002/jcsm.12349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Ebadi M, Bhanji RA, Dunichand-Hoedl AR, Mazurak VC, Baracos VE, Montano-Loza AJ. Sarcopenia Severity Based on Computed Tomography Image Analysis in Patients with Cirrhosis. Nutrients. 2020;12:3463. doi: 10.3390/nu12113463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Bhanji RA, Moctezuma-Velazquez C, Duarte-Rojo A, Ebadi M, Ghosh S, Rose C, Montano-Loza AJ. Myosteatosis and sarcopenia are associated with hepatic encephalopathy in patients with cirrhosis. Hepatol Int. 2018;12:377–386. doi: 10.1007/s12072-018-9875-9. [DOI] [PubMed] [Google Scholar]
- 51.Nardelli S, Gioia S, Faccioli J, Riggio O, Ridola L. Sarcopenia and cognitive impairment in liver cirrhosis: A viewpoint on the clinical impact of minimal hepatic encephalopathy. World J Gastroenterol. 2019;25:5257–5265. doi: 10.3748/wjg.v25.i35.5257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Beer L, Bastati N, Ba-Ssalamah A, Pötter-Lang S, Lampichler K, Bican Y, Lauber D, Hodge J, Binter T, Pomej K, Simbrunner B, Semmler G, Trauner M, Mandorfer M, Reiberger T. MRI-defined sarcopenia predicts mortality in patients with chronic liver disease. Liver Int. 2020;40:2797–2807. doi: 10.1111/liv.14648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Surov A, Paul L, Meyer HJ, Schob S, Engelmann C, Wienke A. Apparent Diffusion Coefficient Is a Novel Imaging Biomarker of Myopathic Changes in Liver Cirrhosis. J Clin Med. 2018;7:359. doi: 10.3390/jcm7100359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Petridis I, Miraglia R, Maruzzelli L, Wan T, Berzigotti A, Bosch J, Volpes R. Sarcopenia predicts mortality after transjugular intrahepatic portosystemic shunt creation in patients with refractory ascites. J Hepatology. 2020;73:S717–S718. [Google Scholar]
- 55.Tandon P, Raman M, Mourtzakis M, Merli M. A practical approach to nutritional screening and assessment in cirrhosis. Hepatology. 2017;65:1044–1057. doi: 10.1002/hep.29003. [DOI] [PubMed] [Google Scholar]