Abstract
BACKGROUND
A decline in serum carbohydrate antigen 19-9 (CA19-9) levels during systemic chemotherapy is considered as a prognostic marker for patients with advanced pancreatic cancer. Neutrophil-to-lymphocyte ratio (NLR) has been extensively studied as a simple and useful indicator of prognosis in various cancers including pancreatic cancer.
AIM
To assess the prognostic significance of NLR and CA19-9 in patients with advanced pancreatic adenocarcinoma received first-line chemotherapy according to CA19-9 positivity.
METHODS
We retrospectively analyzed patients diagnosed with advanced pancreatic cancer who received first-line chemotherapy between January 2010 and July 2017 at the Catholic University of Seoul St. Mary’s Hospital. Patients were divided according to CA19-9 positivity (CA19-9-positive vs -negative groups) and pre-and post-treatment NLR levels. To determine cut-off value of NLR and CA19-9 reduction, time-dependent receiver operating characteristic curve was applied. We evaluated overall survival (OS) and progression-free survival (PFS) for each group using Kaplan-Meier method, and we performed multivariate analyses on the entire cohort.
RESULTS
We included 271 patients in this study. Cut-off value of NLR and CA19-9 reduction was determined as 2.62 and 18%. Multivariate analysis showed that post-treatment NLR < 2.62 and reduction of ≥ 18% of baseline CA19-9 were significantly associated with OS and PFS. Post-treatment NLR ≥ 2.62 showed hazard ratio (HR) of 2.47 [95% confidence interval (CI): 1.84-3.32, P < 0.001] and CA19-9 decline (≥ 18%) showed HR of 0.51 (95%CI: 0.39-0.67, P < 0.001) for OS. When CA19-9-positive patients were divided into groups according to CA19-9 response (responder vs non-responder) and post-treatment NLR (< 2.62 vs ≥ 2.62), CA19-9 responder and post-treatment NLR < 2.62 group showed better survival than CA19-9 non-responder and post-treatment NLR ≥ 2.62 group (OS 11.0 mo vs 3.9 mo, P < 0.001; PFS 6.3 mo vs 2.0 mo, P < 0.001). The combination of CA19-9 decline and post-treatment NLR showed a significant correlation with clinical response in CA 19-9 positive group. Within the CA19-9-negative group, the post-treatment NLR < 2.62 group showed better survival than the post-treatment NLR ≥ 2.62 group (OS 12.7 mo vs 7.7 mo, P < 0.001; PFS 6.7 mo vs 2.1 mo, P < 0.001), and post-treatment NLR showed correlation with clinical response.
CONCLUSION
In advanced pancreatic cancer patients positive for CA19-9 and treated with systemic chemotherapy, the combination of post-treatment NLR < 2.62 and 18% decline of CA19-9 at the first response evaluation is a good prognostic marker. Post-treatment NLR < 2.62 alone could be used as a prognostic marker and an adjunctive tool for response evaluation in CA19-9-negative patients.
Keywords: Pancreatic adenocarcinoma, Serum carbohydrate antigen 19-9, Neutrophil-to-lymphocyte ratio, Multivariate analysis, Prognosis, Chemotherapy
Core Tip: In pancreatic cancer patients treated with first-line chemotherapy, carbohydrate antigen 19-9 (CA19-9) decline is considered as a prognostic marker. However, there has been no consensus regarding the degree of CA19-9 decline, and certain populations show false negativity. We evaluated the cut-off value of decline of CA19-9 and post-treatment neutrophil-to-lymphocyte ratio (NLR) as prognostic makers. Combination of post-treatment NLR (< 2.62) and decline of CA19-9 (≥ 18%) for CA19-9 positive group or post-treatment NLR alone for CA19-9 negative group could be used as a prognostic marker and an adjuvant tool for response evaluation.
INTRODUCTION
Pancreatic cancer is the seventh leading cause of cancer-related death worldwide and the fourth leading cause of cancer-related death in western countries[1,2]. Prognosis remains dismal with a 5-year survival rate of only 8%[1]. At the time of diagnosis, less than 20% of patients are eligible for curative surgery[3]. For patients with advanced pancreatic cancer (either locally advanced or metastatic disease), the mainstay of treatment is systemic chemotherapy. Gemcitabine base regimen and 5-FU based regimen displayed survival benefit and have been recommended as fist-line therapies[4-6].
Radiologic evaluation based on the response evaluation criteria in solid tumors (RECIST) can be used to assess the response to systemic chemotherapy, although radiologic evaluation is considered unreliable because the desmoplastic reaction in the tumor microenvironment makes it difficult to differentiate between normal pancreatic tissue, malignant tissue, inflammation, and fibrosis[7]. This necessitates biomarkers to support imaging assessments.
Carbohydrate antigen 19-9 (CA19-9) is a sialylated Lewis blood group antigen and is the most widely investigated tumor marker for pancreatic cancer. CA19-9 has proven useful in the diagnosis of pancreatic cancer in symptomatic patients with a sensitivity and specificity of 79%-81% and 82%-90%, respectively[8]. The pre-operative CA19-9 Level is associated with prognosis and resectability in resectable diseases. Post-operative CA19-9 Levels are associated with prognosis and recurrence. In advanced diseases, pre-treatment CA19-9 Level is prognostic, and CA19-9 decline during chemotherapy seems to be an indicator of treatment response[9].
Roughly, 5%-10% of the population is considered to be of the Lea-b- phenotype and cannot synthesize CA19-9. Evaluating serum CA19-9 levels is not useful in a CA19-9-negative population, and false positivity can be detected by the presence of biliary obstruction, infection, or inflammation. Therefore, the value of CA19-9 as a tumor marker is limited in certain populations[10,11].
Inflammatory responses in the tumor microenvironment play vital roles in tumor initiation and promotion (survival, proliferation, growth, angiogenesis, and invasion)[12]. Blood neutrophil-to-lymphocyte ratio (NLR), calculated using the absolute lymphocyte and neutrophil count of peripheral blood, is one of the most studied indicators of the systemic inflammatory response. Neutrophils contribute to the inflammatory response by infiltrating tumors and the surrounding microenvironment and by secreting various cytokines and vascular endothelial growth factor (VEGF). Lymphocytes contribute to the immune surveillance of tumors by inducing cytotoxic cell death and by inhibiting tumor cell proliferation and migration[13,14].
Many recent studies have shown that NLR is associated with prognosis in various cancers including pancreatic cancer. Low NLR is associated with a better prognosis than high NLR, and the link between response to chemotherapy and NLR has been studied[15-18]. Several studies also have shown that combination of NLR and CA 19-9 could be a prognostic marker in patients with pancreatic cancer, but they did not focus on the changes in NLR and CA 19-9 between pre- and post-treatment[19-21].
In the present study, we sought to assess the prognostic significance of NLR and CA19-9 in patients with advanced pancreatic adenocarcinoma who receive first-line chemotherapy. We also aimed to investigate the potential association of changes in NLR and CA19-9 from baseline to first response evaluation with the prognosis and chemotherapy response according to CA19-9 positivity.
MATERIALS AND METHODS
Patients
We retrospectively investigated the data of patients diagnosed with pancreatic cancer who received first-line chemotherapy between January 2010 and July 2017 at the Catholic University of Seoul St. Mary’s Hospital.
Eligible patients were aged 18 years or older, had histologically confirmed pancreatic adenocarcinoma, metastatic disease, or locally advanced disease, had at least one cycle of the first line systemic chemotherapy, had a CA19-9 levels and complete blood count (CBC) at the baseline and when the first response evaluation was performed.
Patients with unresolved biliary obstruction or biliary tract infection and active malignancies other than pancreatic cancer were excluded.
NLR was defined as the absolute neutrophil count divided by the absolute lymphocyte count in peripheral blood. NLR and CA19-9 data were collected within one week before treatment and within one week after response evaluation was performed.
Other clinico-pathological variables included age, sex, Eastern Cooperative Oncology Group performance status (ECOG PS), stage (locally advanced or metastatic), number of metastatic sites, liver metastasis, pathologic differentiation, biliary drainage, chemotherapy regimen, and carcinoembryonic antigen (CEA).
True CA19-9 negativity was defined as having a serum CA19-9 concentration within the normal range (< 37 U/mL) from baseline to the follow-up period, including when disease status progressed. CA19-9 false-negativity was defined as having a serum CA19-9 concentration within the normal range (< 37 U/mL) from baseline, but elevation was confirmed during the follow-up period.
The Institutional Review Board of the Catholic University of Seoul Saint Mary’s Hospital approved the study (KC20RASI0321). Requirement for informed consent was waived because the study was based on retrospective analyses of existing administrative and clinical data.
Follow-up evaluation
Response evaluation was performed with abdominal computed tomography or magnetic resonance imaging every two cycles of treatment with tumor markers CA19-9 and NLR. When signs or symptoms indicated a possible disease progression, response evaluation was performed with CA19-9 and NLR.
Statistical analysis
Progression-free survival (PFS) was defined as the time from the start of treatment to disease progression or death from any cause. Overall survival (OS) was measured as the time from the start of treatment until death from any cause or until the last follow-up date. Treatment response was evaluated using Response Evaluation Criteria in Solid Tumors version 1.1.
Chi-squared and Fisher’s test for categorical variables were used to compare the demographics between groups regarding baseline characteristics. To determine the optimal cut-off value of NLR and CA19-9 reduction, time-dependent receiver operating characteristic (ROC) curve was applied. The Kaplan-Meier method and log-rank test were used to estimate cumulative survival and for comparison between the groups.
Univariate and multivariate analysis models of patient and tumor characteristics in association with PFS and OS were based on Cox-proportional hazards regression analyses. Significance was considered as P < 0.05. All statistical analyses were conducted using R version 3.5.3 (http://www.r-project.org).
RESULTS
Clinical characteristics
A total of 271 patients between January 2010 and July 2017 were eligible for analysis. The median follow-up duration was 7.9 mo. The baseline characteristics of the population are summarized in Table 1. The median age was 63 years [interquartile range (IQR), 57-70 years] and the male to female ratio was 178 (65.7%) to 93 (34.3%). Patients with ECOG PS 0-1 and in the metastatic stage were 79.7% and 91.9%, respectively. Majority of patients received gemcitabine based chemotherapy (86.7%) and 80.8% of the patients had elevated CA19-9 (> 37 U/mL) at diagnosis; median CA19-9 was 362.80 U/mL (IQR, 64.98-1585.90).
Table 1.
Baseline characteristics
|
Variables
|
Number of patients, n = 271
|
%
|
Median (interquartile range)
|
CA19-9 positive, n = 219
|
%
|
CA19-9 negative, n = 52
|
%
|
P
value
1
|
| Age (yr) | 63 (57-70) | |||||||
| < 65 | 167 | 61.6 | 133 | 60.7 | 34 | 65.4 | 0.535 | |
| ≥ 65 | 104 | 38.4 | 86 | 39.3 | 18 | 34.6 | ||
| Sex | ||||||||
| Male | 178 | 65.7 | 147 | 67.1 | 31 | 59.6 | 0.305 | |
| Female | 93 | 34.3 | 72 | 32.9 | 21 | 40.4 | ||
| ECOG | ||||||||
| 0-1 | 216 | 79.7 | 171 | 78.1 | 45 | 86.5 | 0.173 | |
| ≥ 2 | 55 | 20.3 | 48 | 21.9 | 7 | 13.5 | ||
| Stage | ||||||||
| Locally advanced | 22 | 8.1 | 15 | 6.8 | 7 | 13.5 | 0.117 | |
| Metastatic | 249 | 91.9 | 204 | 93.2 | 45 | 86.5 | ||
| Differentiation | ||||||||
| Well to moderately | 177 | 65.3 | 142 | 64.8 | 35 | 67.3 | 0.262 | |
| Poorly | 94 | 34.7 | 77 | 35.2 | 17 | 32.7 | ||
| Biliary stent | ||||||||
| Yes | 30 | 11.1 | 28 | 12.8 | 2 | 3.8 | 0.084 | |
| No | 241 | 88.9 | 191 | 87.2 | 50 | 96.2 | ||
| Chemotherapy | ||||||||
| Gemcitabine | 86 | 31.7 | 72 | 32.9 | 14 | 26.9 | 0.967 | |
| Gemcitabine/Elrotinib | 110 | 40.6 | 87 | 39.7 | 23 | 44.2 | ||
| Gemcitabine/Nab-paclitaxel | 39 | 14.4 | 31 | 14.2 | 8 | 15.4 | ||
| Gemcitabine/Fluoropyrimidine | 30 | 11.1 | 24 | 11.0 | 6 | 11.5 | ||
| FOLFIRINOX | 6 | 2.2 | 5 | 2.3 | 1 | 1.9 | ||
| No. of metastatic sites | ||||||||
| 0-2 | 229 | 84.5 | 180 | 82.2 | 49 | 94.2 | 0.033 | |
| 3- | 42 | 15.5 | 39 | 17.8 | 3 | 5.8 | ||
| Liver metastasis | ||||||||
| Yes | 122 | 45.0 | 106 | 48.4 | 16 | 30.8 | 0.022 | |
| No | 149 | 55.0 | 113 | 51.6 | 36 | 69.2 | ||
| Pretreatment NLR | 2.66 (1.78-3.85) | |||||||
| < 2.60 | 130 | 48.0 | 97 | 44.3 | 33 | 63.5 | 0.013 | |
| ≥ 2.60 | 141 | 52.0 | 122 | 55.7 | 19 | 36.5 | ||
| Post-treatment NLR | 2.53 (1.63-4.03) | |||||||
| < 2.62 | 143 | 52.8 | 106 | 48.4 | 37 | 71.2 | 0.003 | |
| ≥ 2.62 | 128 | 47.2 | 113 | 51.6 | 15 | 28.8 | ||
| CA19-9 (U/mL) | 362.80 (64.98-1585.90) | |||||||
| ≤ 37 | 52 | 19.2 | 0 | 0.0 | 52 | 100.0 | ||
| > 37 | 219 | 80.8 | 219 | 100.0 | 0 | 0.0 | ||
| CEA (ng/mL) | 3.26 (2.00-7.95) | |||||||
| ≤ 5 | 175 | 64.6 | 133 | 60.7 | 42 | 80.8 | 0.003 | |
| > 5 | 96 | 35.4 | 86 | 39.3 | 10 | 19.2 |
P value from Chi-square test or Fisher's exact test for categorical variables. ECOG PS: Eastern Cooperative Oncology Group Performance Status; NLR: Neutrophil-to-lymphocyte ratio; CA19-9: Carbohydrate antigen 19-9; CEA: Carcinoembryonic antigen; FOLFIRINOX: Folinic acid, 5-Fluorouracil, Irinotecan and Oxaliplatin.
Median pre-treatment NLR was 2.66 (IQR, 1.78-3.85) and median post-treatment NLR was 2.53 (IQR, 1.63-4.03).
Among 52 patients who initially had a normal range of CA19-9, 35 patients presented with elevated values of CA19-9 during follow-up. They were categorized as the CA19-9 false-negative group. The remaining 17 patients maintained normal CA19-9 levels (< 37 U/mL) during the follow-up, regardless of disease status; thus, they were categorized as the CA19-9 true-negative group.
Compared to CA19-9 negative group, factors found to be significantly associated with CA19-9 positive group included number of metastatic sites (≥ 3), liver metastasis, higher number of pretreatment and post-treatment NLR, and elevated CEA. The baseline characteristics of the cohort according to CA19-9 positivity are summarized in Table 1.
In addition, compared to CA 19-9 false-negative group, factors found to be significantly associated with CA19-9 positive group included number of metastatic sites (≥ 3), higher number of pretreatment and post-treatment NLR, and elevated CEA. CA19-9 positive group showed tendency to be positively associated with liver metastasis. There were no significant differences between the CA19-9-positive and CA19-9 true-negative groups (Supplementary Table 1).
Determination of optimal cut-off value of Pre- and post-treatment NLR, decline of CA19-9
Time-dependent ROC curve was generated with a 1-year survival time point, and the cut-off value was determined by the value that maximized the Youden index. Pre-treatment NLR 2.60, post-treatment NLR 2.62, and 18% decline of CA19-9 were selected as cut-off values (Supplementary Figure 1).
Association of the pre-treatment NLR and CA19-9 with baseline clinical characteristics
The clinical characteristics of the cohort according to pre-treatment NLR (< 2.60 vs ≥ 2.60) are summarized in Supplementary Table 2. The pre-treatment NLR ≥ 2.60 group was associated with poor differentiation and elevated CA19-9 (P = 0.039 and P < 0.001, respectively). Pre-treatment NLR was positively correlated with post-treatment NLR (P < 0.001).
CA19-9 response and survival
The cohort was divided into three groups: Group A, comprising the CA19-9 responders, including patients with 18% decline or normalization of CA19-9 from baseline to the first response evaluation; Group B, comprising the CA19-9 non-responders, including patients with less than 18% decline or increased CA19-9 at the first response evaluation; Group C, comprising patients with negative CA19-9. The survival curves are summarized in Figure 1.
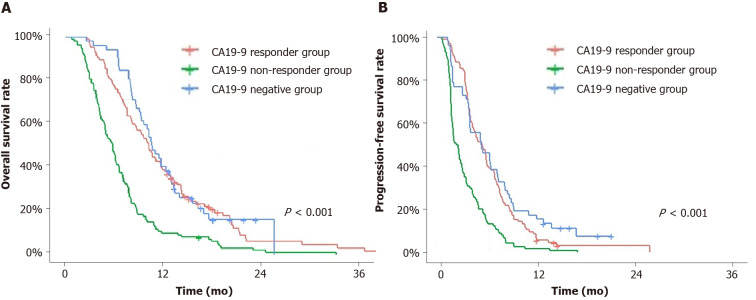
Figure 1.
Overall survival and progression-free survival. A and B: Overall survival (A) and progression-free survival (B) of the following groups: Carbohydrate antigen 19-9 (CA19-9) responder group, CA19-9 non-responder group and CA19-9-negative group. CA19-9 responder group and CA19-9 negative group showed significantly longer overall survival and progression-free survival than CA19-9 non-responder group. CA19-9: Carbohydrate antigen 19-9.
Group A showed longer OS and PFS than Group B (P < 0.001 and P < 0.001, respectively), with a median OS of 10.2 mo [95% confidence interval (CI): 8.6-11.8] and a median PFS of 5.7 mo (95%CI: 4.4-6.5). Group C showed longer OS and PFS than Group B (P < 0.001 and P < 0.001, respectively), with median OS of 10.5 mo (95%CI: 9.3-13.0) and a median PFS of 5.6 mo (95%CI: 4.1-7.6). Group B showed a worse prognosis with a median OS of 5.8 mo (95%CI: 4.9-6.6) and a median PFS of 2.5 mo (2.0-3.1) than Group A or C. Group A and Group C showed no difference in both OS and PFS (P = 0.620 and P = 0.310, respectively).
When group C was subdivided by CA 19-9 false-negative and CA 19-9 true negative group, false-negative group showed statistically better OS and PFS (OS 12.3 mo, 95%CI: 10.5-15.5 vs 8.1 mo, 95%CI: 7.7-11.0, P = 0.040; PFS 6.7 mo, 95%CI; 4.1-9.5 vs 4.0 mo, 95%CI: 1.9-6.7, P = 0.020).
Pre- and post-treatment NLR and survival
When the cohort was divided according to pre-treatment NLR (< 2.60 vs ≥ 2.60), groups were different in PFS and OS by log-rank test. Pre-treatment NLR < 2.60 group showed better OS and PFS (OS 10.2 mo, 95%CI: 8.8-11.7 vs 6.5 mo, 95%CI: 5.8-7.7, P < 0.001; PFS 5.63 mo, 95%CI: 4.40-6.53 vs 3.43 mo, 95%CI: 2.73-3.97, P < 0.001).
When the cohort was divided by post-treatment NLR (< 2.62 vs ≥ 2.62), groups were different in PFS and OS by log-rank test. Post-treatment NLR < 2.62 group showed better OS and PFS (OS 10.8 mo, 95%CI: 9.6-12.3 vs 5.8 mo, 95%CI: 5.0-6.6, P < 0.001; PFS 6.2 mo, 95%CI: 4.9-7.2 vs 2.7 mo, 95%CI: 2.1-3.5, P < 0.001).
Univariate and multivariate analyses of PFS and OS
Univariate analysis showed that OS, ECOG PS (0-1 vs ≥ 2), stage (locally advanced vs metastatic), differentiation, chemotherapy regimen (Gemcitabine single vs other regimens), pre-treatment NLR (< 2.60 vs ≥ 2.60), post-treatment NLR (< 2.62 vs ≥ 2.62), CA19-9 18% reduction, and CEA were prognostic factors associated with survival. After adjusting for covariates, multivariate analysis showed that ECOG, stage, differentiation, chemotherapy regimen, post-treatment NLR, and CA19-9 reduction were independent prognostic factors of OS.
Univariate and multivariate analysis of PFS showed that stage, liver metastasis, post-treatment NLR, and CA19-9 reduction were independent prognostic factors of PFS (Table 2).
Table 2.
Univariate and multivariate analysis of overall survival, progression-free survival
|
Overall survival
|
Progression-free survival
|
|||||||||||
|
Univariate
|
Multivariate
|
Univariate
|
Multivariate
|
|||||||||
|
HR
|
95%CI
|
P
value
|
HR
|
95%CI
|
P
value
|
HR
|
95%CI
|
P
value
|
HR
|
95%CI
|
P
value
|
|
| Age (yr) | ||||||||||||
| < 65 | 1 | 1 | ||||||||||
| ≥ 65 | 1.08 | 0.84-1.39 | 0.557 | 0.97 | 0.75-1.24 | 0.786 | ||||||
| Sex | ||||||||||||
| Female | 1 | 1 | ||||||||||
| Male | 1.12 | 0.86-1.46 | 0.406 | 1.08 | 0.83-1.39 | 0.573 | ||||||
| ECOG | ||||||||||||
| 0-1 | 1 | 1 | 1 | |||||||||
| ≥ 2 | 1.5 | 1.10-2.03 | 0.009 | 1.40 | 1.00-1.97 | 0.050 | 1.23 | 0.81-0.91 | 0.173 | |||
| Stage | ||||||||||||
| Locally advanced | 1 | 1 | 1 | 1 | ||||||||
| Metastatic | 1.84 | 1.13-2.98 | 0.014 | 2.17 | 1.28-3.69 | 0.004 | 1.95 | 1.23-3.09 | 0.005 | 1.91 | 1.16-3.15 | 0.011 |
| Differentiation | ||||||||||||
| Well to moderately | 1 | 1 | 1 | |||||||||
| Poorly | 1.35 | 1.04-1.74 | 0.026 | 1.48 | 1.11-1.96 | 0.007 | 1.06 | 0.82-1.36 | 0.662 | |||
| Biliary stent | ||||||||||||
| No | 1 | 1 | ||||||||||
| Yes | 0.98 | 0.66-1.46 | 0.939 | 0.81 | 0.55-1.20 | 0.297 | ||||||
| Liver metastasis | ||||||||||||
| No | 1 | 1 | 1 | 1 | ||||||||
| Yes | 1.25 | 0.98-1.61 | 0.077 | 1.22 | 0.93-1.60 | 0.135 | 1.51 | 1.19-1.93 | 0.001 | 1.54 | 1.19-2.01 | 0.001 |
| Chemotherapy | ||||||||||||
| Gemcitabine single | 1 | 1 | 1 | 1 | ||||||||
| Other regimens | 0.68 | 0.52-0.89 | 0.004 | 0.70 | 0.53-0.93 | 0.015 | 0.78 | 0.60-1.01 | 0.062 | 0.86 | 0.65-1.14 | 0.303 |
| Pretreatment NLR | ||||||||||||
| < 2.60 | 1 | 1 | 1 | 1 | ||||||||
| ≥ 2.60 | 1.77 | 1.37-2.29 | < 0.001 | 1.09 | 0.81-1.48 | 0.545 | 1.61 | 1.26-2.07 | < 0.001 | 1.03 | 0.78-1.36 | 0.816 |
| Post-treatment NLR | ||||||||||||
| < 2.62 | 1 | 1 | 1 | 1 | ||||||||
| ≥ 2.62 | 2.42 | 1.87-3.14 | < 0.001 | 2.47 | 1.84-3.32 | < 0.001 | 2.51 | 1.93-3.26 | < 0.001 | 2.59 | 1.94-3.47 | < 0.001 |
| CA19-9 reduction | ||||||||||||
| < 18% | 1 | 1 | 1 | 1 | ||||||||
| ≥ 18% | 0.53 | 0.41-0.69 | < 0.001 | 0.51 | 0.39-0.67 | < 0.001 | 0.54 | 0.46-0.75 | < 0.001 | 0.6 | 0.47-0.78 | < 0.001 |
| CEA (ng/mL) | ||||||||||||
| ≤ 5 | 1 | 1 | 1 | 1 | ||||||||
| > 5 | 1.27 | 0.98-1.65 | 0.066 | 1.20 | 0.91-1.57 | 0.187 | 1.26 | 0.98-1.62 | 0.074 | 1.17 | 0.90-1.52 | 0.231 |
Univariate analysis and multivariate survival analysis were performed using Cox proportional hazard model, and P values < 0.05 were considered to indicate statistical significance. Significant values are in boldface type. CI: Confidence interval; HR: Hazard ratio; ECOG: Eastern Cooperative Oncology Group; NLR: Neutrophil-to-lymphocyte ratio; CA19-9: Carbohydrate antigen 19-9; CEA: Carcinoembryonic antigen.
Multivariate analysis of both OS and PFS showed that post-treatment NLR and CA19-9 decline were the most significant prognostic factors. Post-treatment NLR ≥ 2.62 showed hazard ratio (HR) of 2.47 (95%CI: 1.84-3.32, P < 0.001) and CA 19-9 decline (≥ 18%) showed HR of 0.51 (95%CI: 0.39-0.67, P < 0.001) for OS.
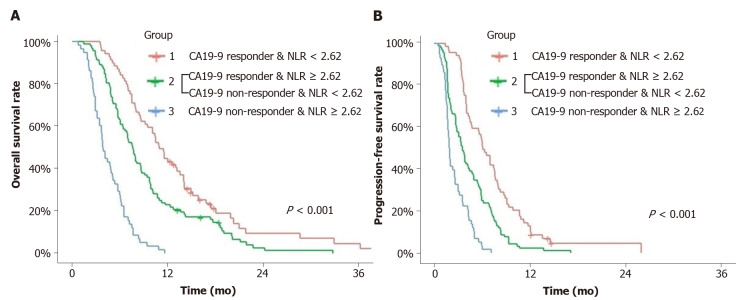
Combination of CA19-9 decline and post-treatment NLR as prognostic markers in CA19-9-positive group
CA19-9-positive patients (n = 219) were divided into three groups according to CA19-9 reduction and post-treatment NLR. Group 1 comprised CA19-9 responders and post-chemotherapy NLR < 2.62, Group 2 comprised CA19-9 responders or post-chemotherapy NLR < 2.62, and Group 3 comprised CA19-9 non-responders and post-chemotherapy NLR ≥ 2.62. OS and PFS were compared among the three groups using Kaplan-Meier survival curve with log-rank test.
All groups were significantly different from each other in the OS and PFS curves using the log-rank test. The median OS of Group 1 was 11.0 mo (95%CI: 9.6-14.2), 7.7 mo (95%CI: 6.5-8.8) for Group 2 and 3.9 mo (95%CI: 3.5-5.2) for Group 3. The median PFS of Group 1 was 6.3 mo (95%CI: 4.9-7.8), 3.8 mo (95%CI: 2.9-4.9) for Group 2, 2.0 mo (95%CI: 1.8-2.7) for Group 3 (Figure 2).
Figure 2.
Overall survival and progression-free survival. A and B: Overall survival (A) and progression-free survival (B) of the groups divided by carbohydrate antigen 19-9 response and post-treatment neutrophil-to-lymphocyte ratio. All groups were significantly different from each other in the overall survival and progression-free survival curves and Group 1 showed better overall survival and progression-free survival than others. CA19-9: Carbohydrate antigen 19-9; NLR: Neutrophil-to-lymphocyte ratio.
First response of the chemotherapy and association of CA19-9 18% decline and post-treatment NLR
We compared the first response evaluation of the cohort by RECIST criteria 1.1 according to post-treatment NLR and CA19-9 response. Patients with post-treatment NLR < 2.62 showed statistically better clinical response than patients with post-treatment NLR ≥ 2.62 did. CA19-9 responders showed better clinical response than non-responders did (Supplementary Table 3).
In CA19-9 responders and non-responders, post-treatment NLR discriminated better clinical response of post-treatment NLR < 2.62 group from ≥ 2.62 group. In CA19-9 responders, the response evaluation of post-treatment NLR < 2.62 vs ≥ 2.62 was as follows: Partial response (PR) 52.2% vs 26.3%, stable disease (SD) 43.3% vs 55.3%, progressive disease (PD) 4.5% vs 18.4% (P = 0.002). In CA19-9 non-responders, the response evaluation of post-treatment NLR < 2.62 vs ≥ 2.62 was as follows: PR 10.3% vs 6.7%, SD 53.8% vs 28.0% and PD 35.9% vs 65.3% (P = 0.009). The combination of CA19-9 decline and post-treatment NLR revealed a significant correlation with clinical response (Table 3).
Table 3.
The association of the first response evaluation and post-treatment neutrophil-to-lymphocyte ratio according to carbohydrate antigen 19-9 response in carbohydrate antigen 19-9 positive group
|
|
Decline of CA19-9 ≥ 18%
|
Decline of CA19-9 < 18%
|
||||||||
|
Post-treatment NLR
|
< 2.62
|
|
≥ 2.62
|
|
|
< 2.62
|
|
≥ 2.62
|
|
|
|
|
n
= 67
|
%
|
n
= 38
|
%
|
P
value
1
|
n
= 39
|
%
|
n
= 75
|
%
|
P
value
1
|
| 1st Response | 0.002 | 0.009 | ||||||||
| CR/PR | 35 | 52.2 | 10 | 26.3 | 4 | 10.3 | 5 | 6.7 | ||
| SD | 29 | 43.3 | 21 | 55.3 | 21 | 53.8 | 21 | 28.0 | ||
| PD | 3 | 4.5 | 7 | 18.4 | 14 | 35.9 | 49 | 65.3 | ||
P value from linear-by-linear association test. NLR: Neutrophil-to-lymphocyte ratio; CA19-9: Carbohydrate antigen 19-9; CR: Complete response; PR: Partial response; SD: Stable disease; PD: Progressive disease.
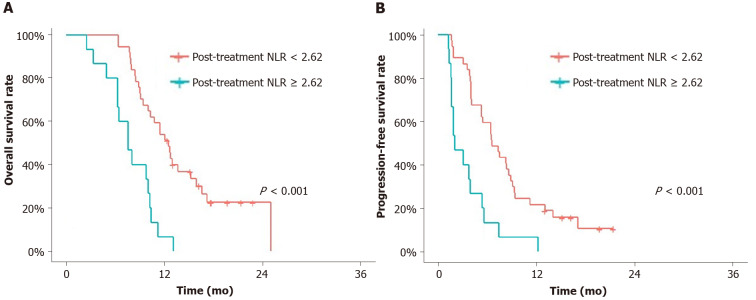
Post-treatment NLR as a prognostic marker in CA19-9-negative group
First response of the chemotherapy and association of post-treatment NLR in CA19-9-negative group: In the CA19-9-negative cohort (n = 52), the post-treatment NLR < 2.62 group showed better OS and PFS than the NLR ≥ 2.62 group (median OS 12.7 mo vs 7.7 mo, P < 0.001; PFS 6.7 mo vs 2.1 mo, P < 0.001) (Figure 3). In each CA19-9 true-negative and false-negative group, post-treatment NLR discriminated better prognosis of NLR < 2.62 group from ≥ 2.62 group with statistical significance.
Figure 3.
Overall survival and progression-free survival. A and B: Overall survival (A) and progression-free survival (B) of the carbohydrate antigen 19-9-negative groups according to post-treatment neutrophil-to-lymphocyte ratio (NLR). Post-treatment NLR < 2.62 group showed significantly better overall survival and progression-free survival than post treatment NLR ≥ 2.62. NLR: Neutrophil-to-lymphocyte ratio.
The post-treatment NLR < 2.62 group showed a better clinical response at the first response evaluation than the NLR ≥ 2.62 group (P = 0.032) (Table 4).
Table 4.
The association of the first response evaluation and post-treatment neutrophil-to-lymphocyte ratio in carbohydrate antigen 19-9 negative group
|
|
CA19-9 negative group n = 52
|
||||
|
Post-treatment NLR
|
< 2.62
|
|
≥ 2.62
|
|
|
|
|
n
= 37
|
%
|
n
= 15
|
%
|
P
value
1
|
| 1st Response | 0.032 | ||||
| CR/PR | 8 | 21.6 | 3 | 20.0 | |
| SD | 25 | 67.6 | 4 | 26.7 | |
| PD | 4 | 10.8 | 8 | 53.3 | |
P value from linear-by-linear association test. NLR: Neutrophil-to-lymphocyte ratio; CA19-9: Carbohydrate antigen 19-9; CR: Complete response; PR: Partial response; SD: Stable disease; PD: Progressive disease.
DISCUSSION
In this study, we showed that decline of CA19-9 and post-treatment NLR were independent prognostic factors in advanced pancreatic cancer treated with the first line systemic chemotherapy. Post-treatment NLR 2.62 could discriminate better prognosis in both CA19-9-positive and -negative groups. Combination of 18% decline of CA19-9 and post-treatment NLR < 2.62 could improve prognostic accuracy in CA19-9 positive group.
Baseline CA19-9 level is a prognostic marker in both resectable and advanced pancreatic cancer[22]. Change in CA19-9 Level is also prognostic; a decline in CA19-9 Level from pre- to post-operation is a predictive marker for improved survival in patients who underwent surgical resection[23,24]. In patients undergoing systemic chemotherapy, a decline in CA19-9 Level from baseline to the time of response evaluation is considered prognostic[9,25-30]. However, most studies investigating the prognostic role of CA19-9 kinetics were retrospective and study cohorts were heterogeneous; some included all the patients regardless of baseline CA19-9 level and others included only patients with elevated CA19-9. The cut-off values of decline of CA 19-9 for prognosis was determined by different methods, and they varied from 0 to 50% in their studies[25-30].
In this study, we divided the patients into CA19-9-positive and -negative groups and analyzed CA19-9 response in the CA19-9-positive group. We compared the different cut-off values of CA19-9 decline at the first response evaluation in the CA19-9 positive group(n = 219), and 18% decline of the CA19-9 level which maximized the You-den index in the ROC curve for one-year survival was determined as cut-off value. Patients with ≥ 18% and < 18% declines showed good discriminating power using a log-rank test between the groups. This result is similar with previous studies with large cohort which showed 20% decline of CA19-9 is prognostic[28-30]. And 18% reduction of CA19-9 was associated with better radiologic response at the first response evaluation.
The prognostic significance of NLR in pancreatic cancer was explored by many studies, and different cut-off values from two to five were used[31]. This study adapted NLR 2.60 and 2.62 for pre- and post-treatment NLR as the optimal cut-off. Post-treatment NLR was more prognostic than pre-treatment NLR. Post-treatment NLR 2.62 showed the area under the curve of 0.73 in ROC curve for 1- year survival. This is as high as that of 18% decline of CA 19-9 with AUC of 0.75 (Supplementary Figure 1).
A few studies have shown that NLR kinetics could predict treatment outcome in pancreatic cancer patients during systemic chemotherapy[32-34]. To evaluate the prognostic significance of NLR, Chen et al[34] divided the patients by increased or decreased NLR from the baseline to the response evaluation, and Luo et al[33] divided the patients by baseline NLR 3.1 and further subdivided by increased or decreased NLR from the baseline to the response evaluation.
In this study, we simply divided the patients by post-treatment NLR 2.62. Although there has been no consensus for a normal NLR value, dividing patients within the cut-off range of NLR (< 2.62 vs ≥ 2.62) showed better predictive power than by dividing patients based on change of NLR (increased vs decreased) between pre- and post-treatment in this study.
We showed that a combination of the two most significant factors in survival, namely CA19-9 response and post-treatment NLR < 2.62, showed well-established groups based on their prognosis. And they showed positive correlation with radiologic response at the first response evaluation. Among the patients in post-treatment NLR < 2.62 and CA19-9 responder group, only 4.3% had radiological PD. These two factors could be used as prognostic markers and adjunctive tools for response evaluation in the patients with elevated CA19-9 levels.
In addition, we divided CA19-9-negative groups into true-negative and false-negative groups. In the baseline characteristics, true-negative group showed no difference from positive group but, false-negative group showed relatively better clinical variables. Moreover, survival was better in false-negative group than in CA19-9-positive or true-negative groups. Considering CA19-9 correlates with stage of disease, false-negative CA19-9 group might indicate less aggressive disease than CA19-9 positive group[24]. The patients with CA19-9-true-negativity was 7.8% in this study, and their median survival was similar to that of CA19-9 positive group. Although, Lewis blood type or genotype was not evaluated in this study, some of these patients might be Lewis negative group considering CA19-9 was not elevated with disease progression. The proportion of CA19-9-true-negativity is similar to that of Lewis negative group in previous studies[35,36]. Further study is needed to confirm the relationship between CA19-9 level and prognosis with evaluation of Lewis blood type.
We showed that post treatment NLR (< 2.62) group could discriminate better prognosis in both false-positive and true-positive group. In addition, response evaluation was more dependent on radiologic evaluation in CA19-9 negative group than in CA19-9 positive group. CEA could be helpful; however, about 30%-60% of patients are CEA-positive[37-39]. In this study, only 19.2% (n = 10) of CA19-9-negative patients were CEA-positive. Post-treatment NLR could be particularly helpful to predict prognosis, and could be applied as an adjunctive tool for response evaluation in CA19-9 negative group.
Limitations of the study
This study aimed to assess the prognostic significance of NLR and CA19-9 in patients with advanced pancreatic adenocarcinoma who receive first-line chemotherapy; its potential has been confirmed. However, due to the retrospective nature of the study and the use of single-center data, our study was limited by selection bias. Despite several efforts to reduce selection bias, including the use of multivariate analyses, unadjusted bias may still be present.
Lewis blood type or genotype was not evaluated, and the CA19-9-negative group had a small sample size (n = 52); thus, results obtained within the group should be interpreted with caution.
CONCLUSION
In advanced pancreatic cancer patients positive for CA19-9 and treated with systemic chemotherapy, the combination of post-treatment NLR < 2.62 and 18% decline of CA19-9 at the first response evaluation is a good prognostic marker. Post-treatment NLR < 2.62 alone could be used as a prognostic marker and an adjunctive tool for response evaluation in CA19-9-negative patients.
ARTICLE HIGHLIGHTS
Research background
A decline of serum carbohydrate antigen 19-9 (CA19-9) levels during systemic chemotherapy is considered as a prognostic marker in advanced pancreatic cancer. Neutrophil-to-lymphocyte ratio (NLR) has been studied as a simple and useful prognostic marker.
Research motivation
This study investigated prognostic significance of pre- and post-treatment NLR and decline of CA19-9 in advanced pancreatic cancer.
Research objectives
To assess the prognostic significance of NLR and CA19-9 in patients with advanced pancreatic adenocarcinoma received first-line chemotherapy.
Research methods
We retrospectively analyzed patients with advanced pancreatic cancer who received first-line chemotherapy. Patients were divided according to CA19-9 positivity and pre-and post-treatment NLR levels. We evaluated survival analysis and response of the treatment according to the cut-off value of post-treatment NLR and decline of CA19-9 determined by time-dependent receiver operating characteristic curve.
Research results
We included 271 patients in this study. Cut-off value of NLR and CA19-9 reduction was determined as 2.62 and 18%. Multivariate analysis showed that post-treatment NLR and CA19-9 decline were significantly associated with survival. The combination of CA19-9 decline and post-treatment NLR showed a significant correlation with clinical response in CA19-9 positive group. Within the CA19-9-negative group, the post-treatment NLR < 2.62 group showed better survival and better clinical response.
Research conclusions
In advanced pancreatic cancer patients, the combination of post-treatment NLR and decline of CA19-9 is a good prognostic marker. Post-treatment NLR alone could be used as a prognostic marker and an adjunctive tool for response evaluation in CA19-9-negative patients.
Research perspectives
Future clinical trials for metastatic pancreatic cancer need to include post-treatment NLR and decline CA19-9 as clinical indicators.
Footnotes
Institutional review board statement: This study was reviewed and approved by the Ethics Committees of The Institutional Review Board of the Catholic University of Seoul Saint Mary’s Hospital (approval No. KC20RASI0321).
Informed consent statement: The requirement for informed consent was waived because the study was based on retrospective analyses of existing administrative and clinical data.
Conflict-of-interest statement: All the authors have no conflict of interest related to the manuscript.
STROBE statement: The authors have read the STROBE Statement-checklist of items, and the manuscript was prepared and revised according to the STROBE Statement-checklist of items.
Manuscript source: Unsolicited manuscript
Peer-review started: February 24, 2021
First decision: April 19, 2021
Article in press: July 9, 2021
Specialty type: Oncology
Country/Territory of origin: South Korea
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): 0
Grade C (Good): C, C
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Liu Y S-Editor: Gao CC L-Editor: A P-Editor: Ma YJ
Contributor Information
Kabsoo Shin, Division of Medical Oncology, Department of Internal Medicine, Seoul St. Mary's Hospital, College of Medicine, The Catholic University of Korea, Seoul 06591, South Korea; Catholic Cancer Research Institute, College of Medicine, The Catholic University of Korea, Seoul 06591, South Korea.
Eun-Kyo Jung, Division of Medical Oncology, Department of Internal Medicine, Seoul St. Mary's Hospital, College of Medicine, The Catholic University of Korea, Seoul 06591, South Korea; Catholic Cancer Research Institute, College of Medicine, The Catholic University of Korea, Seoul 06591, South Korea.
Se Jun Park, Division of Medical Oncology, Department of Internal Medicine, Seoul St. Mary's Hospital, College of Medicine, The Catholic University of Korea, Seoul 06591, South Korea; Catholic Cancer Research Institute, College of Medicine, The Catholic University of Korea, Seoul 06591, South Korea.
Sangwoon Jeong, Division of Medical Oncology, Department of Internal Medicine, Seoul St. Mary's Hospital, College of Medicine, The Catholic University of Korea, Seoul 06591, South Korea; Catholic Cancer Research Institute, College of Medicine, The Catholic University of Korea, Seoul 06591, South Korea.
In-Ho Kim, Division of Medical Oncology, Department of Internal Medicine, Seoul St. Mary's Hospital, College of Medicine, The Catholic University of Korea, Seoul 06591, South Korea; Catholic Cancer Research Institute, College of Medicine, The Catholic University of Korea, Seoul 06591, South Korea.
Myung-ah Lee, Division of Medical Oncology, Department of Internal Medicine, Seoul St. Mary's Hospital, College of Medicine, The Catholic University of Korea, Seoul 06591, South Korea; Catholic Cancer Research Institute, College of Medicine, The Catholic University of Korea, Seoul 06591, South Korea. angelamd@catholic.ac.kr.
Data sharing statement
No additional data are available.
References
- 1.Siegel RL, Miller KD, Jemal A. Cancer statistics, 2018. CA Cancer J Clin. 2018;68:7–30. doi: 10.3322/caac.21442. [DOI] [PubMed] [Google Scholar]
- 2.Bray F, Ferlay J, Soerjomataram I, Siegel RL, Torre LA, Jemal A. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2018;68:394–424. doi: 10.3322/caac.21492. [DOI] [PubMed] [Google Scholar]
- 3.Gillen S, Schuster T, Meyer Zum Büschenfelde C, Friess H, Kleeff J. Preoperative/neoadjuvant therapy in pancreatic cancer: a systematic review and meta-analysis of response and resection percentages. PLoS Med. 2010;7:e1000267. doi: 10.1371/journal.pmed.1000267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Conroy T, Desseigne F, Ychou M, Bouché O, Guimbaud R, Bécouarn Y, Adenis A, Raoul JL, Gourgou-Bourgade S, de la Fouchardière C, Bennouna J, Bachet JB, Khemissa-Akouz F, Péré-Vergé D, Delbaldo C, Assenat E, Chauffert B, Michel P, Montoto-Grillot C, Ducreux M Groupe Tumeurs Digestives of Unicancer; PRODIGE Intergroup. FOLFIRINOX vs gemcitabine for metastatic pancreatic cancer. N Engl J Med. 2011;364:1817–1825. doi: 10.1056/NEJMoa1011923. [DOI] [PubMed] [Google Scholar]
- 5.Von Hoff DD, Ervin T, Arena FP, Chiorean EG, Infante J, Moore M, Seay T, Tjulandin SA, Ma WW, Saleh MN, Harris M, Reni M, Dowden S, Laheru D, Bahary N, Ramanathan RK, Tabernero J, Hidalgo M, Goldstein D, Van Cutsem E, Wei X, Iglesias J, Renschler MF. Increased survival in pancreatic cancer with nab-paclitaxel plus gemcitabine. N Engl J Med. 2013;369:1691–1703. doi: 10.1056/NEJMoa1304369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Tempero MA. NCCN Guidelines Updates: Pancreatic Cancer. J Natl Compr Canc Netw. 2019;17:603–605. doi: 10.6004/jnccn.2019.5007. [DOI] [PubMed] [Google Scholar]
- 7.Brambs HJ, Claussen CD. Pancreatic and ampullary carcinoma. Ultrasound, computed tomography, magnetic resonance imaging and angiography. Endoscopy. 1993;25:58–68. doi: 10.1055/s-2007-1009126. [DOI] [PubMed] [Google Scholar]
- 8.Goonetilleke KS, Siriwardena AK. Systematic review of carbohydrate antigen (CA 19-9) as a biochemical marker in the diagnosis of pancreatic cancer. Eur J Surg Oncol. 2007;33:266–270. doi: 10.1016/j.ejso.2006.10.004. [DOI] [PubMed] [Google Scholar]
- 9.Ballehaninna UK, Chamberlain RS. The clinical utility of serum CA 19-9 in the diagnosis, prognosis and management of pancreatic adenocarcinoma: An evidence based appraisal. J Gastrointest Oncol. 2012;3:105–119. doi: 10.3978/j.issn.2078-6891.2011.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Tempero MA, Uchida E, Takasaki H, Burnett DA, Steplewski Z, Pour PM. Relationship of carbohydrate antigen 19-9 and Lewis antigens in pancreatic cancer. Cancer Res. 1987;47:5501–5503. [PubMed] [Google Scholar]
- 11.Marrelli D, Caruso S, Pedrazzani C, Neri A, Fernandes E, Marini M, Pinto E, Roviello F. CA19-9 serum levels in obstructive jaundice: clinical value in benign and malignant conditions. Am J Surg. 2009;198:333–339. doi: 10.1016/j.amjsurg.2008.12.031. [DOI] [PubMed] [Google Scholar]
- 12.Grivennikov SI, Greten FR, Karin M. Immunity, inflammation, and cancer. Cell. 2010;140:883–899. doi: 10.1016/j.cell.2010.01.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Dunn GP, Old LJ, Schreiber RD. The immunobiology of cancer immunosurveillance and immunoediting. Immunity. 2004;21:137–148. doi: 10.1016/j.immuni.2004.07.017. [DOI] [PubMed] [Google Scholar]
- 14.Mantovani A, Allavena P, Sica A, Balkwill F. Cancer-related inflammation. Nature. 2008;454:436–444. doi: 10.1038/nature07205. [DOI] [PubMed] [Google Scholar]
- 15.Yu SL, Xu LT, Qi Q, Geng YW, Chen H, Meng ZQ, Wang P, Chen Z. Serum lactate dehydrogenase predicts prognosis and correlates with systemic inflammatory response in patients with advanced pancreatic cancer after gemcitabine-based chemotherapy. Sci Rep. 2017;7:45194. doi: 10.1038/srep45194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Yamada Y, Fujii H, Watanabe D, Kato-Hayashi H, Ohata K, Kobayashi R, Ishihara T, Uemura S, Iwashita T, Shimizu M, Suzuki A. Severe Neutropenia is Associated with Better Clinical Outcomes in Patients with Advanced Pancreatic Cancer Who Receive Modified FOLFIRINOX Therapy. Cancers (Basel) 2018;10 doi: 10.3390/cancers10110454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Howard R, Kanetsky PA, Egan KM. Exploring the prognostic value of the neutrophil-to-lymphocyte ratio in cancer. Sci Rep. 2019;9:19673. doi: 10.1038/s41598-019-56218-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hwang I, Kang J, Ip HNN, Jeong JH, Kim KP, Chang HM, Yoo C, Ryoo BY. Prognostic factors in patients with metastatic or recurrent pancreatic cancer treated with first-line nab-paclitaxel plus gemcitabine: implication of inflammation-based scores. Invest New Drugs. 2019;37:584–590. doi: 10.1007/s10637-018-0681-y. [DOI] [PubMed] [Google Scholar]
- 19.Asaoka T, Miyamoto A, Maeda S, Tsujie M, Hama N, Yamamoto K, Miyake M, Haraguchi N, Nishikawa K, Hirao M, Ikeda M, Sekimoto M, Nakamori S. Prognostic impact of preoperative NLR and CA19-9 in pancreatic cancer. Pancreatology. 2016;16:434–440. doi: 10.1016/j.pan.2015.10.006. [DOI] [PubMed] [Google Scholar]
- 20.Song JY, Chen MQ, Guo JH, Lian SF, Xu BH. Combined pretreatment serum CA19-9 and neutrophil-to-lymphocyte ratio as a potential prognostic factor in metastatic pancreatic cancer patients. Medicine (Baltimore) 2018;97:e9707. doi: 10.1097/MD.0000000000009707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Sakamoto T, Saito H, Uchinaka EI, Morimoto M, Amisaki M, Tokuyasu N, Honjo S, Ashida K, Fujiwara Y. The Combination of Neutrophil-to-lymphocyte Ratio and Serum Carbohydrate Antigen 19-9 Level as a Prognostic Indicator in Patients with Recurrent Pancreatic Cancer. Anticancer Res. 2018;38:5497–5503. doi: 10.21873/anticanres.12883. [DOI] [PubMed] [Google Scholar]
- 22.Boeck S, Stieber P, Holdenrieder S, Wilkowski R, Heinemann V. Prognostic and therapeutic significance of carbohydrate antigen 19-9 as tumor marker in patients with pancreatic cancer. Oncology. 2006;70:255–264. doi: 10.1159/000094888. [DOI] [PubMed] [Google Scholar]
- 23.Kondo N, Murakami Y, Uemura K, Hayashidani Y, Sudo T, Hashimoto Y, Nakashima A, Sakabe R, Shigemoto N, Kato Y, Ohge H, Sueda T. Prognostic impact of perioperative serum CA 19-9 Levels in patients with resectable pancreatic cancer. Ann Surg Oncol. 2010;17:2321–2329. doi: 10.1245/s10434-010-1033-0. [DOI] [PubMed] [Google Scholar]
- 24.Ferrone CR, Finkelstein DM, Thayer SP, Muzikansky A, Fernandez-delCastillo C, Warshaw AL. Perioperative CA19-9 Levels can predict stage and survival in patients with resectable pancreatic adenocarcinoma. J Clin Oncol. 2006;24:2897–2902. doi: 10.1200/JCO.2005.05.3934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Bauer TM, El-Rayes BF, Li X, Hammad N, Philip PA, Shields AF, Zalupski MM, Bekaii-Saab T. Carbohydrate antigen 19-9 is a prognostic and predictive biomarker in patients with advanced pancreatic cancer who receive gemcitabine-containing chemotherapy: a pooled analysis of 6 prospective trials. Cancer. 2013;119:285–292. doi: 10.1002/cncr.27734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Tabernero J, Chiorean EG, Infante JR, Hingorani SR, Ganju V, Weekes C, Scheithauer W, Ramanathan RK, Goldstein D, Penenberg DN, Romano A, Ferrara S, Von Hoff DD. Prognostic factors of survival in a randomized phase III trial (MPACT) of weekly nab-paclitaxel plus gemcitabine vs gemcitabine alone in patients with metastatic pancreatic cancer. Oncologist. 2015;20:143–150. doi: 10.1634/theoncologist.2014-0394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Ko AH, Hwang J, Venook AP, Abbruzzese JL, Bergsland EK, Tempero MA. Serum CA19-9 response as a surrogate for clinical outcome in patients receiving fixed-dose rate gemcitabine for advanced pancreatic cancer. Br J Cancer. 2005;93:195–199. doi: 10.1038/sj.bjc.6602687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Halm U, Schumann T, Schiefke I, Witzigmann H, Mössner J, Keim V. Decrease of CA 19-9 during chemotherapy with gemcitabine predicts survival time in patients with advanced pancreatic cancer. Br J Cancer. 2000;82:1013–1016. doi: 10.1054/bjoc.1999.1035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Chiorean EG, Von Hoff DD, Reni M, Arena FP, Infante JR, Bathini VG, Wood TE, Mainwaring PN, Muldoon RT, Clingan PR, Kunzmann V, Ramanathan RK, Tabernero J, Goldstein D, McGovern D, Lu B, Ko A. CA19-9 decrease at 8 wk as a predictor of overall survival in a randomized phase III trial (MPACT) of weekly nab-paclitaxel plus gemcitabine vs gemcitabine alone in patients with metastatic pancreatic cancer. Ann Oncol. 2016;27:654–660. doi: 10.1093/annonc/mdw006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Robert M, Jarlier M, Gourgou S, Desseigne F, Ychou M, Bouché O, Juzyna B, Conroy T, Bennouna J. Retrospective Analysis of CA19-9 Decrease in Patients with Metastatic Pancreatic Carcinoma Treated with FOLFIRINOX or Gemcitabine in a Randomized Phase III Study (ACCORD11/PRODIGE4) Oncology. 2017;93:367–376. doi: 10.1159/000477850. [DOI] [PubMed] [Google Scholar]
- 31.Zhou Y, Wei Q, Fan J, Cheng S, Ding W, Hua Z. Prognostic role of the neutrophil-to-lymphocyte ratio in pancreatic cancer: A meta-analysis containing 8252 patients. Clin Chim Acta. 2018;479:181–189. doi: 10.1016/j.cca.2018.01.024. [DOI] [PubMed] [Google Scholar]
- 32.Xue P, Kanai M, Mori Y, Nishimura T, Uza N, Kodama Y, Kawaguchi Y, Takaori K, Matsumoto S, Uemoto S, Chiba T. Neutrophil-to-lymphocyte ratio for predicting palliative chemotherapy outcomes in advanced pancreatic cancer patients. Cancer Med. 2014;3:406–415. doi: 10.1002/cam4.204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Luo G, Guo M, Liu Z, Xiao Z, Jin K, Long J, Liu L, Liu C, Xu J, Ni Q, Yu X. Blood neutrophil-lymphocyte ratio predicts survival in patients with advanced pancreatic cancer treated with chemotherapy. Ann Surg Oncol. 2015;22:670–676. doi: 10.1245/s10434-014-4021-y. [DOI] [PubMed] [Google Scholar]
- 34.Chen Y, Yan H, Wang Y, Shi Y, Dai G. Significance of baseline and change in neutrophil-to-lymphocyte ratio in predicting prognosis: a retrospective analysis in advanced pancreatic ductal adenocarcinoma. Sci Rep. 2017;7:753. doi: 10.1038/s41598-017-00859-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Park HD, Park KU, Song J, Ki CS, Han KS, Kim JQ. The relationship between Lewis/Secretor genotypes and serum carbohydrate antigen 19-9 Levels in a Korean population. Korean J Lab Med. 2010;30:51–57. doi: 10.3343/kjlm.2010.30.1.51. [DOI] [PubMed] [Google Scholar]
- 36.Park KU, Song J, Han KS, Kim JQ. The fusion allele of the FUT2 (secretor type alpha(1,2)-fucosyltransferase) gene at a high frequency and a new se385 allele in a Korean population. Ann Hematol. 2005;84:656–660. doi: 10.1007/s00277-005-1041-5. [DOI] [PubMed] [Google Scholar]
- 37.Imaoka H, Mizuno N, Hara K, Hijioka S, Tajika M, Tanaka T, Ishihara M, Hirayama Y, Hieda N, Yoshida T, Okuno N, Shimizu Y, Niwa Y, Yamao K. Prognostic impact of carcinoembryonic antigen (CEA) on patients with metastatic pancreatic cancer: A retrospective cohort study. Pancreatology. 2016;16:859–864. doi: 10.1016/j.pan.2016.05.007. [DOI] [PubMed] [Google Scholar]
- 38.Lee KJ, Yi SW, Chung MJ, Park SW, Song SY, Chung JB, Park JY. Serum CA 19-9 and CEA levels as a prognostic factor in pancreatic adenocarcinoma. Yonsei Med J. 2013;54:643–649. doi: 10.3349/ymj.2013.54.3.643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Reitz D, Gerger A, Seidel J, Kornprat P, Samonigg H, Stotz M, Szkandera J, Pichler M. Combination of tumour markers CEA and CA19-9 improves the prognostic prediction in patients with pancreatic cancer. J Clin Pathol. 2015;68:427–433. doi: 10.1136/jclinpath-2014-202451. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
No additional data are available.