Abstract
Helicobacter pylori (H. pylori) is an infectious agent influencing as much as 50% of the world’s population. It is the causative agent for several diseases, most especially gastric and duodenal peptic ulcer, gastric adenocarcinoma and mucosa-associated lymphoid tissue lymphoma of the stomach. A number of other, extragastric manifestations also are associated with H. pylori infection. These include neurological disorders, such as Alzheimer’s disease, demyelinating multiple sclerosis and Parkinson’s disease. There is also evidence for a relationship between H. pylori infection and such dermatological diseases as psoriasis and rosacea as well as a connection with infection and open-angle glaucoma. Generally little is known about the relationship between H. pylori infection and diseases of the pancreas. Most evidence about H. pylori and its potential role in the development of pancreatic diseases concerns pancreatic adenocarcinoma and autoimmune forms of chronic pancreatitis. There is data (albeit not fully consistent) indicating modestly increased pancreatic cancer risk in H. pylori-positive patients. The pathogenetic mechanism of this increase is not yet fully elucidated, but several theories have been proposed. Reduction of antral D-cells in H. pylori-positive patients causes a suppression of somatostatin secretion that, in turn, stimulates increased secretin secretion. That stimulates pancreatic growth and thus increases the risk of carcinogenesis. Alternatively, H. pylori, as a part of microbiome dysbiosis and the so-called oncobiome, is proven to be associated with pancreatic adenocarcinoma development via the promotion of cellular proliferation. The role of H. pylori in the inflammation characteristic of autoimmune pancreatitis seems to be explained by a mechanism of molecular mimicry among several proteins (mostly enzymes) of H. pylori and pancreatic tissue. Patients with autoimmune pancreatitis often show positivity for antibodies against H. pylori proteins. H. pylori, as a part of microbiome dysbiosis, also is viewed as a potential trigger of autoimmune inflammation of the pancreas. It is precisely these relationships (and associated equivocal conclusions) that constitute a center of attention among pancreatologists, immunologists and pathologists. In order to obtain clear and valid results, more studies on sufficiently large cohorts of patients are needed. The topic is itself sufficiently significant to draw the interest of clinicians and inspire further systematic research. Next-generation sequencing could play an important role in investigating the microbiome as a potential diagnostic and prognostic biomarker for pancreatic cancer.
Keywords: Helicobacter pylori, Pancreatic cancer, Autoimmune pancreatitis, Carcinogenesis, Microbiome, Molecular mimicry
Core Tip: Helicobacter pylori is the causative agent for several gastrointestinal diseases and a number of extragastric manifestations. The role of Helicobacter pylori in the inflammation characteristic of autoimmune pancreatitis seems to be explained by a mechanism of molecular mimicry between several proteins (mostly enzymes) of Helicobacter pylori and pancreatic tissue. The topic is itself sufficiently significant to draw the interest of clinicians and inspire further systematic research. Next-generation sequencing could play an important role in investigating the microbiome as a potential diagnostic and prognostic biomarker for pancreatic cancer.
INTRODUCTION
Helicobacter pylori (H. pylori) is a bacterium colonizing gastric mucosa and has been a center of researchers’ attention for more than three decades. The bacterium has been identified in biological samples even several tens of thousands of years old. For example, it was discovered in the body of a mummified man approximately 50-years-old at death and found in a frozen Alpine glacier between Italy and Austria. This mummy, named the “Iceman,” is estimated to have lived 5000–6000 years ago. Researchers searching for the presence of H. pylori infection found a bacterial strain from Asia, thereby helping to prove that several thousands of years ago people migrated from Asia to Europe[1,2].
H. pylori has been confirmed as the cause of chronic active gastritis, which may progress into peptic ulcer or even gastric carcinoma. The infection is often associated with extragastric manifestations such as hypochromic anemia or immune thrombocytopenia[3,4]. There are, however, a number of other neurological, cardiovascular, metabolic, allergic and hepatobiliary diseases, including diseases of the eye, that are associated with the presence of H. pylori infection[5]. There are two forms of H. pylori gastric colonization. One of these manifests as body predominant infection (or pangastritis) with hypoacidity and atrophic gastritis. These patients are predisposed to gastric ulcers and gastric adenocarcinoma. The second form of colonization is associated with predominantly antral gastritis, leading to increased gastrin production, probably via local impairment of somatostatin release and reduction of antral D-cells. This results in hypersecretion of acid and, thereby, predisposition to prepyloric and duodenal ulcer. Moreover, it is specifically this second form of colonization of the antral gastric mucosa by H. pylori that has been identified as a potential risk factor for the development of pancreatic carcinoma. In addition, H. pylori-positive antral gastritis is described as a primarily infectious disease[6-9].
The H. pylori bacterium is associated with several virulence factors (Table 1)[6-11].
Table 1.
Helicobacter pylori virulence factors–peptic ulcer disease and gastric carcinoma
|
Virulence factor
|
Classification
|
Disease association
|
| CagA | Cytotoxin-associated gene A | Peptic gastric/duodenal ulcer |
| DupA | Duodenal ulcer promoting gene | Duodenal peptic ulcer |
| VacA | Vacuolating cytotoxin A | Peptic gastric ulcer, premalignant disease progression |
| OipA | Outer inflammatory protein A | Peptic ulcer disease, gastric cancer |
The global prevalence of H. pylori infection remains high. An especially strong prevalence is reported in Japan and China, where it reaches 60%-90%, but similar prevalence data has been recorded in Russia and certain countries in Eastern Europe. In Western and Central Europe, the reported prevalence ranges between 30% and 40%[12]. This begs the question whether such a frequently observed bacterium, which is of course connected mainly to diseases of the stomach and duodenum, could also play a part in the pathogenesis of other diseases beyond the gastroduodenal ones.
H. PYLORI AND OTHER BACTERIA IN PANCREATIC CARCINOMA
Cancer and cardio-cerebrovascular diseases are major causes of mortality worldwide[13,14]. Pancreatic cancer is one of the most lethal malignancies, its incidence is rising, and its prognosis is extremely poor[15,16]. Pancreatic cancer continues to have the lowest 5-year relative survival rate among solid tumors (at 7%-9%) and is projected to become the second leading cause of cancer-related death by 2030 in western countries[17].
There is general agreement that any role H. pylori plays in inducing pancreatic carcinoma is based on pathophysiological changes. Currently, there are two hypotheses[18,19]: (1) Patients with H. pylori infection have a reduced number of antral D-cells, which causes a suppression of somatostatin secretion and that in turn stimulates increased secretion of secretin and pancreatic bicarbonate. In a mouse model, secretin stimulates pancreatic growth and DNA synthesis in the cells of pancreatic ducts, and this may induce proliferation of epithelial cells; and (2) H. pylori growth in the gastric corpus mucosa leads to atrophic gastritis and hypoacidity causing bacterial overgrowth and increased production of bacterially catalyzed N-nitrosamines and transportation of these endogenous carcinogens to the host pancreas via the bloodstream. Proliferation mediated through carcinogens such as N-nitrosamines leads to the development of pancreatic carcinoma.
Clinical observations have shown it is crucial to identify the concrete virulence factor possessed by the bacterium. Risk of pancreatic carcinoma has been shown to be associated with H. pylori strains that are negative for cytotoxin-associated gene A (CagA)[20-22]. These findings are universally accepted, even though there have been some smaller studies that did not quite so unequivocally confirm these results. A study published in 2012 reported only insignificantly increased risk of pancreatic carcinoma in persons with CagA-positive H. pylori and only slightly decreased risk of pancreatic carcinoma development in persons with CagA-positive H. pylori[23].
A meta-analysis from Liu et al[24] showed no evidence for higher pancreatic cancer risk in patients with atrophic gastritis and confirmed just a slight association for higher risk with CagA-negative H. pylori strains. By contrast, in a case-control study, Huang et al[25] showed that atrophic gastritis in H. pylori-negative patients might bring increased risk of pancreatic cancer.
Gastric acid secretion is what regulates the function of pancreatic duct cells and their production of bicarbonate and the watery portion of pancreatic juices. This mechanism may influence the state and function of pancreatic duct epithelial cells and lead to pancreatic duct cell dysplasia, the final result of which may be the development of pancreatic carcinomas. Eradication of H. pylori in patients with peptic duodenal ulcers leads to a decrease in gastric hyperacidity and even to restoration of normal gastric secretion[26]. Similarly, in patients with atrophic gastritis, eradication of the bacteria leads to improvement or even normalization of hypo- or anacidity[27].
Nilsson et al[28] reported finding H. pylori present in pancreatic tissue of patients with pancreatic adenocarcinomas, pancreatic neuroendocrine tumors, multiple endocrine neoplasia type 1 and chronic pancreatitis. H. pylori DNA was detected in 75% of patients with pancreatic adenocarcinomas, and H. pylori infection was present in 57% of patients with neuroendocrine tumors, 38% of patients with multiple endocrine neoplasia type 1 and surprisingly 60% of patients with chronic pancreatitis. The samples obtained from other benign pancreatic diseases were all negative. In gastroduodenal biopsies, the positivity of H. pylori was detected in 33% of cases, but surprisingly 60% of the samples tested positive for the presence of Helicobacter bilis. H. pylori DNA was not detected in any of the tissue samples from the gallbladder and common bile duct. Those authors conclude that H. pylori is often present in pancreatic carcinoma tissues, and it is highly probable that the infection plays a part not only in the development and progression of pancreatic carcinoma but also of chronic pancreatitis.
Despite the rather ambiguous clinical results for the association between H. pylori infection and pancreatic carcinomas as well as the unclear pathogenetic mechanism for the induction of pancreatic carcinomas, most of the published meta-analytical studies do support the conclusion that the bacterium plays a role in inducing pancreatic carcinomas[19,20,29-31]. A recent Japanese study evaluated the risk of pancreatic carcinoma along with concurrent H. pylori infection and atrophic gastritis on a large cohort of more than 20000 patients. Although in a normal Japanese population no statistically significant relationship was observed between pancreatic carcinoma and atrophic gastritis, in a subset of H. pylori-positive smokers with atrophic gastritis there was a significant risk for the induction of pancreatic carcinomas[32].
It is clear that the role of the microbiome is becoming one of the crucial research areas regarding risk of pancreatic carcinoma[33]. There can be no doubt that pancreatic adenocarcinoma is often associated with microbiome dysbiosis and the oncobiome[34,35]. Oncobiosis alters the immune system because the oncobiome usually has a different immunogenic profile than the eubiome[36].
The oncobiome and transformation of the microbiome support cellular proliferation, invasion and metastasis. Published studies show that these processes are the main targets of oncobiosis and oncobiotic bacterial metabolites[37].
Although H. pylori colonization is associated with pancreatic cancer[38], as mentioned, so too are oral flora. Porphyromonas gingivalis and Capnocytophaga gingivalis, in particular, play an important role in pancreatic cancer development[39-41]. The availability of next-generation sequencing has contributed to improving our understanding of the orointestinal microbiome and shown that this has potential to become a noninvasive diagnostic and prognostic biomarker for pancreatic cancer[42].
Even though bacteria are found in pancreatic tumor tissues, the mechanism of their colonization remains unknown[43]. A study from Japan showed the detection rate for Fusobacterium species in pancreatic cancer tissue to be 8.8%[44].
A meta-analysis published in 2012[45] and another study[46] confirmed the hypothesis that general and abdominal fatness is associated with an increased risk of pancreatic cancer. Moreover, it has been shown that alterations in the bacterial composition, also known as dysbiosis, contribute to various gastrointestinal and metabolic disorders such as obesity and diabetes[42,47]. Smoking or dietary sources could also play roles and could enhance pathophysiological actions related to carcinogenic effect in the pancreas of N-nitrosamines associated with H. pylori[18]. The possible roles of bacteria, including H. pylori, and other factors in pancreatic cancer development are illustrated in Figure 1 (prepared in accordance with Li et al[43] and created in collaboration with the Service Center for E-Learning at Masaryk University, Faculty of Informatics).
Figure 1.
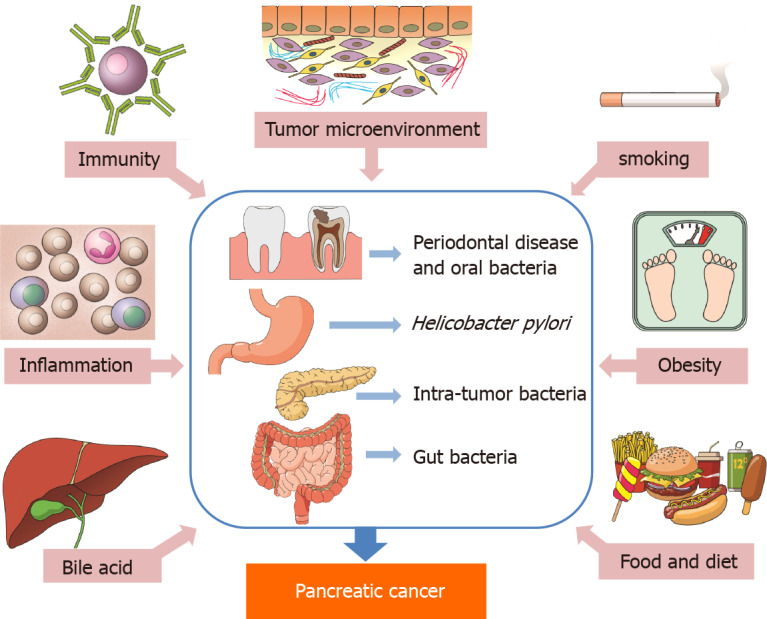
The possible roles of bacteria, including Helicobacter pylori, and other factors in pancreatic cancer development.
H. PYLORI AND AUTOIMMUNE PANCREATITIS
Autoimmune pancreatitis (AIP) type-1 belongs to the group of immunoglobulin G4 (IgG4)-related diseases. The characteristic signs of this disease group are chronic inflammatory reaction, pronounced fibrotization of tissues and presence of mononuclear inflammatory infiltrate with plasma cells positive for IgG4[48]. IgG4 is an immunoglobulin that is among the least represented across the immunoglobulin spectrum, making up less than 5% of that spectrum overall[49]. A current hypothesis explaining the mechanism of the disease is based on the effect of molecular mimicry between a pathogen, most commonly H. pylori, and an antigen, most commonly carbonic anhydrase II, serine protease inhibitor Kazal type 1 or lactoferrin[50].
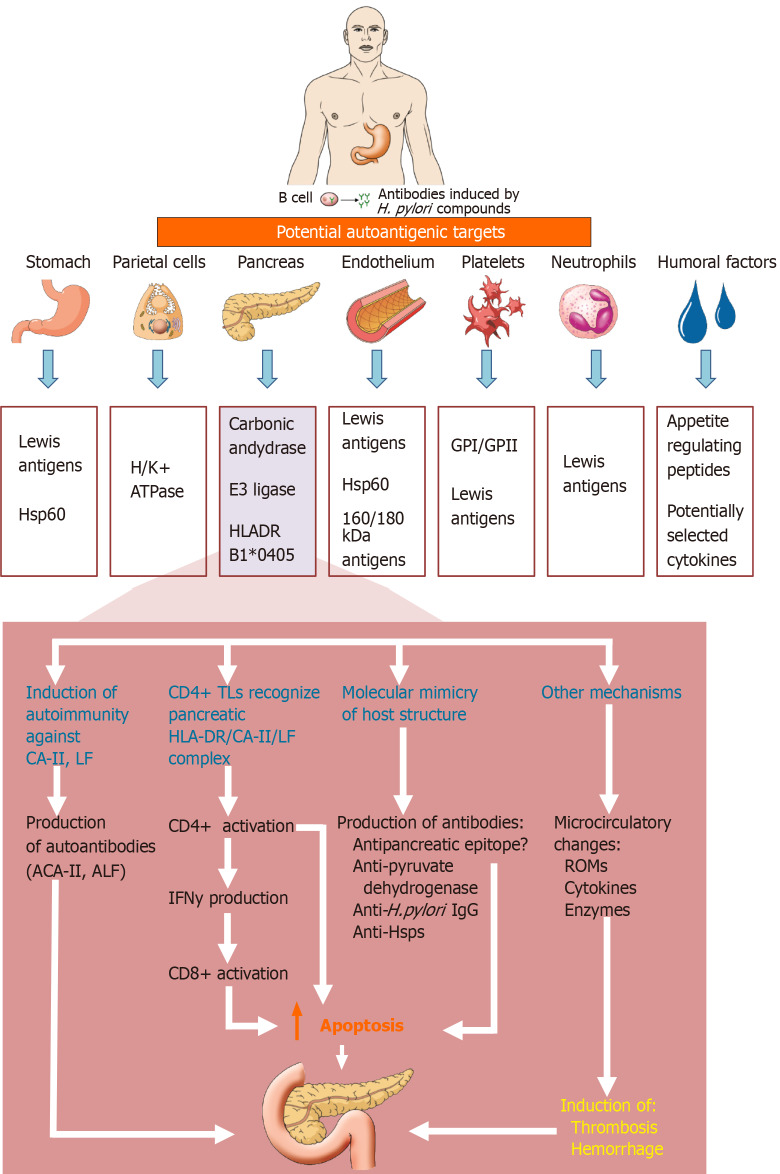
In 2005, Guarneri et al[51] described a significant resemblance between human carbonic anhydrase II and H. pylori alfa-carbonic anhydrase, an enzyme vital for the bacteria’s survival in the severely acidic environment of the stomach. Human carbonic anhydrase II is produced by the pancreatic duct epithelial cells. As such, H. pylori may therefore directly influence the process of AIP development, given that it carries a protein very similar to carbonic anhydrase II. In 2009, Frulloni et al[52] described H. pylori plasminogen-binding protein (PBP). Patients with AIP show positivity for antibodies against this protein in up to 95% of cases. PBP was not detected in patients with chronic alcohol-induced pancreatitis or in patients with intraductal papillary mucinous neoplasia. H. pylori PBP is molecularly very similar to the enzyme ubiquitin-protein ligase E3 component n-recognin 2. This enzyme is substantially produced by pancreatic acinar cells and therefore, hypothetically, may represent another mechanism through which H. pylori infection induces the development of AIP. The potential mechanism by which H. pylori might contribute to AIP is presented in Figure 2 (edited in accordance with Chmiela and Gonciarz[53] and Kountouras et al[54] and created in collaboration with the Service Center for E-Learning at Masaryk University, Faculty of Informatics).
Figure 2.
The potential mechanism by which Helicobacter pylori might contribute to autoimmune pancreatitis. H. pylori: Helicobacter pylori; GP: Glycoproteins; Hsp: Heat shock protein; H+/K+ ATPase: H+/K+-adenosine triphosphatase; HLA-DR: Human leukocyte class II DR antigens; CA-II: Carbonic anhydrase type II antigens; ACA-II: Anticarbonic anhydrase II antibody; LF: Lactoferrin; ALF: Antilactoferrin antibody; TLs: T lymphocytes; IFN-ɣ: Interferon-ɣ; anti-Hsps: Antibodies against heat shock proteins; ROMs: Reactive oxygen metabolites.
Dore et al[55] found that the levels of pancreatic enzymes in patients with chronic pancreatitis and concurrent positivity of H. pylori infection can be influenced by eradication of the bacteria[55]. Detection of H. pylori in the pancreatic tissue of patients with chronic pancreatitis is a significant finding, and it poses a question as to the role of the bacteria in the development of chronic pancreatitis[28].
Similar to the question about the relationship between H. pylori and pancreatic carcinoma, just as equivocal are the conclusions and opinions as to the role of H. pylori infection in the development of AIP. When evaluating the influence of H. pylori infection but also the presence of IgG4, cytokine and PBP, one prospective English study found no differences among a group of patients with AIP, a group of patients with IgG4-associated diseases and healthy controls[56]. The results of that study, therefore, do not support the presumption that H. pylori plasminogen plays a role in AIP.
In 2018, Backhus et al[48] published a paper summarizing new pathways in the pathogenesis of IgG4-related diseases[48]. One mechanism may be the transformation from beta cells to plasma cells and activation of eosinophilic granulocytes associated with the secretion of proinflammatory cytokines. As a result of this process, elevated IgE levels and eosinophilia are detected in some individuals with IgG4-related disease[57]. IgG4-expressing plasmablasts appear to play a key role in the pathogenesis of IgG4-related disease. Plasmablasts that express CD19, CD20-CD27+ and CD38++ are most likely the precursors of tissue antibodies produced by plasma cells[58]. It would be valuable to determine the role of intestinal dysbiosis, which may be a mediator of the experimental autoimmune form of pancreatitis through the activation of plasmacytoid dendritic cells[59]. Innate immune responses against intestinal microflora are probably involved in the development of experimental AIP. Intestinal dysbiosis increases sensitivity to experimental AIP via the activation of plasmacytoid dendritic cells that mediate chronic fibroinflammatory responses.
Contrary data also have been found regarding AIP as a potential risk factor for pancreatic cancer. A multicentric study from the Mayo Clinic including a total of 1064 patients with AIP did not show a significant increased risk of malignancies[60]. Other studies, however, suggest that AIP slightly increases the risk of pancreatic cancer. Macinga et al[61] assessed the occurrence of AIP in pancreatic masses resected for focal pancreatic enlargement. In 295 pancreatic resections, AIP was diagnosed in 15 patients (5.1%). Within this group of patients with AIP, pancreatic adenocarcinoma was diagnosed in 6 cases (40%). Moreover, Ikeura et al[62] showed a trend towards greater pancreatic cancer development in patients with AIP (4.8%) against patients with other chronic pancreatitis (2.4%).
CONCLUSION
H. pylori infection is an important etiological factor for diseases of the stomach and duodenum. Its role in the induction of peptic gastroduodenal ulcer disease, gastritis (especially antral), carcinomas and mucosa-associated lymphoid tissue lymphoma of the stomach has already been sufficiently described. Its role in pancreatic carcinogenesis or in the induction of AIP remains unclear. However, there are a number of research findings and conclusions tending to confirm and others to deny its effect and involvement. Nonetheless, it can be stated in summary that H. pylori infection is one of many factors within a complex that plays a role in the development of both of these diseases.
Oral bacteria, gut bacteria, H. pylori and intratumor bacteria must contribute to the etiology and pathogenesis of pancreatic cancer. Four mechanisms are important for this process: (1) bacteria stimulate chronic inflammation (with inflammatory mediators facilitating cell proliferation, mutagenesis, oncogene activation and angiogenesis); (2) bacteria may influence the pathogenesis of cancer by activating NF-kappa B and inhibiting cellular apoptosis; (3) bacteria can produce some substances that act in a carcinogenic manner; and (4) bacteria may overgrow, bacterial dysbiosis may occur, and oncobiome interactions may arise.
The oncobiome and bacterial metabolites (short-chain fatty acids, secondary bile acids, polyamines and indole derivatives) are important factors in the induction of pancreatic cancer. Next-generation sequencing could play an important role in investigating the microbiome as a potential future, noninvasive diagnostic and prognostic biomarker for pancreatic cancer.
Recently, evidence has suggested a positive association between bacteria and pancreatic cancer. Activation of related immune inflammation and increased nitrosamine exposure could be the most important mechanisms. Bacterial stimulation of chronic inflammation and the oncobiome could be related to autoimmune mechanisms.
In the case of AIP, the role of H. pylori seems to be explained by inflammation stimulation via mechanisms of molecular mimicry between several proteins (mostly enzymes) of H. pylori and pancreatic tissue. Patients with AIP often show positivity for antibodies against H. pylori proteins. Being a part of microbiome dysbiosis, H. pylori is also being considered as a potential trigger of autoimmune inflammation of the pancreas. Moreover, according to some sources, AIP slightly increases the risk of pancreatic cancer and is regarded as a potential risk factor.
Footnotes
Conflict-of-interest statement: All of the authors declare having nothing to disclose.
Manuscript source: Invited manuscript
Peer-review started: January 13, 2021
First decision: February 24, 2021
Article in press: July 5, 2021
Specialty type: Oncology
Country/Territory of origin: Czech Republic
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): 0
Grade C (Good): C
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Ahmadi Hedayati M S-Editor: Fan JR L-Editor: Filipodia P-Editor: Wang LYT
Contributor Information
Lumir Kunovsky, Department of Surgery, University Hospital Brno and Faculty of Medicine, Masaryk University, Brno 62500, Czech Republic; Department of Gastroenterology and Internal Medicine, University Hospital Brno and Faculty of Medicine, Masaryk University, Brno 62500, Czech Republic.
Petr Dite, Department of Gastroenterology and Internal Medicine, University Hospital Brno and Faculty of Medicine, Masaryk University, Brno 62500, Czech Republic; Department of Gastroenterology and Internal Medicine, University Hospital Ostrava, Ostrava 70800, Czech Republic; Faculty of Medicine, University of Ostrava, Ostrava 70300, Czech Republic.
Petr Jabandziev, Department of Pediatrics, University Hospital Brno and Faculty of Medicine, Masaryk University, Brno 61300, Czech Republic; Central European Institute of Technology, Masaryk University, Brno 62500, Czech Republic.
Jiri Dolina, Department of Gastroenterology and Internal Medicine, University Hospital Brno and Faculty of Medicine, Masaryk University, Brno 62500, Czech Republic.
Jitka Vaculova, Department of Gastroenterology and Internal Medicine, University Hospital Brno and Faculty of Medicine, Masaryk University, Brno 62500, Czech Republic.
Martin Blaho, Department of Gastroenterology and Internal Medicine, University Hospital Ostrava, Ostrava 70800, Czech Republic; Faculty of Medicine, University of Ostrava, Ostrava 70300, Czech Republic.
Martina Bojkova, Department of Gastroenterology and Internal Medicine, University Hospital Ostrava, Ostrava 70800, Czech Republic; Faculty of Medicine, University of Ostrava, Ostrava 70300, Czech Republic.
Jana Dvorackova, Department of Intensive Medicine, Emergency Medicine and Forensic Studies, University Hospital Ostrava, Ostrava 70800, Czech Republic; Faculty of Medicine, University of Ostrava, Ostrava 70300, Czech Republic.
Magdalena Uvirova, CGB Laboratory a.s. Ostrava, Ostrava 70300, Czech Republic.
Zdenek Kala, Department of Surgery, University Hospital Brno and Faculty of Medicine, Masaryk University, Brno 62500, Czech Republic.
Jan Trna, Department of Gastroenterology and Internal Medicine, University Hospital Brno and Faculty of Medicine, Masaryk University, Brno 62500, Czech Republic; Department of Gastroenterology and Digestive Endoscopy, Masaryk Memorial Cancer Institute, Brno 65653, Czech Republic; Department of Internal Medicine, Hospital Boskovice, Boskovice 68001, Czech Republic. jan.trna@mou.cz.
References
- 1.Müller W, Fricke H, Halliday AN, McCulloch MT, Wartho JA. Origin and migration of the Alpine Iceman. Science. 2003;302:862–866. doi: 10.1126/science.1089837. [DOI] [PubMed] [Google Scholar]
- 2.Maixner F, Krause-Kyora B, Turaev D, Herbig A, Hoopmann MR, Hallows JL, Kusebauch U, Vigl EE, Malfertheiner P, Megraud F, O'Sullivan N, Cipollini G, Coia V, Samadelli M, Engstrand L, Linz B, Moritz RL, Grimm R, Krause J, Nebel A, Moodley Y, Rattei T, Zink A. The 5300-year-old Helicobacter pylori genome of the Iceman. Science. 2016;351:162–165. doi: 10.1126/science.aad2545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Malfertheiner P, Megraud F, O'Morain CA, Atherton J, Axon AT, Bazzoli F, Gensini GF, Gisbert JP, Graham DY, Rokkas T, El-Omar EM, Kuipers EJ European Helicobacter Study Group. Management of Helicobacter pylori infection--the Maastricht IV/ Florence Consensus Report. Gut. 2012;61:646–664. doi: 10.1136/gutjnl-2012-302084. [DOI] [PubMed] [Google Scholar]
- 4.Malfertheiner P, Link A, Selgrad M. Helicobacter pylori: perspectives and time trends. Nat Rev Gastroenterol Hepatol. 2014;11:628–638. doi: 10.1038/nrgastro.2014.99. [DOI] [PubMed] [Google Scholar]
- 5.Gravina AG, Zagari RM, De Musis C, Romano L, Loguercio C, Romano M. Helicobacter pylori and extragastric diseases: A review. World J Gastroenterol. 2018;24:3204–3221. doi: 10.3748/wjg.v24.i29.3204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Yamaoka Y, Ojo O, Fujimoto S, Odenbreit S, Haas R, Gutierrez O, El-Zimaity HM, Reddy R, Arnqvist A, Graham DY. Helicobacter pylori outer membrane proteins and gastroduodenal disease. Gut. 2006;55:775–781. doi: 10.1136/gut.2005.083014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.González CA, Figueiredo C, Lic CB, Ferreira RM, Pardo ML, Ruiz Liso JM, Alonso P, Sala N, Capella G, Sanz-Anquela JM. Helicobacter pylori cagA and vacA genotypes as predictors of progression of gastric preneoplastic lesions: a long-term follow-up in a high-risk area in Spain. Am J Gastroenterol. 2011;106:867–874. doi: 10.1038/ajg.2011.1. [DOI] [PubMed] [Google Scholar]
- 8.Liu J, He C, Chen M, Wang Z, Xing C, Yuan Y. Association of presence/absence and on/off patterns of Helicobacter pylori oipA gene with peptic ulcer disease and gastric cancer risks: a meta-analysis. BMC Infect Dis. 2013;13:555. doi: 10.1186/1471-2334-13-555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Schulz C, Schütte K, Malfertheiner P. Helicobacter pylori and Other Gastric Microbiota in Gastroduodenal Pathologies. Dig Dis. 2016;34:210–216. doi: 10.1159/000443353. [DOI] [PubMed] [Google Scholar]
- 10.Brenner H, Rothenbacher D, Arndt V. Epidemiology of stomach cancer. Methods Mol Biol. 2009;472:467–477. doi: 10.1007/978-1-60327-492-0_23. [DOI] [PubMed] [Google Scholar]
- 11.Braga LLBC, Batista MHR, de Azevedo OGR, da Silva Costa KC, Gomes AD, Rocha GA, Queiroz DMM. oipA "on" status of Helicobacter pylori is associated with gastric cancer in North-Eastern Brazil. BMC Cancer. 2019;19:48. doi: 10.1186/s12885-018-5249-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Haarstad H, Petersen H. Short- and long-term effects of secretin and a cholecystokinin-like peptide on pancreatic growth and synthesis of RNA and polyamines. Scand J Gastroenterol. 1989;24:721–732. doi: 10.3109/00365528909093114. [DOI] [PubMed] [Google Scholar]
- 13.Wu X, Yan F, Wang L, Sun G, Liu J, Qu M, Wang Y, Li T. MicroRNA: Another Pharmacological Avenue for Colorectal Cancer? Front Cell Dev Biol. 2020;8:812. doi: 10.3389/fcell.2020.00812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Zhang F, Wang K, Du P, Yang W, He Y, Li T, Mei Z. Risk of Stroke in Cancer Survivors: A Meta-analysis of Population-Based Cohort Studies. Neurology. 2021;96:e513–e526. doi: 10.1212/WNL.0000000000011264. [DOI] [PubMed] [Google Scholar]
- 15.Deng Z, Li X, Shi Y, Lu Y, Yao W, Wang J. A Novel Autophagy-Related IncRNAs Signature for Prognostic Prediction and Clinical Value in Patients With Pancreatic Cancer. Front Cell Dev Biol. 2020;8:606817. doi: 10.3389/fcell.2020.606817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Zhu X, Cao Y, Su T, Zhu X, Ju X, Zhao X, Jiang L, Ye Y, Cao F, Qing S, Zhang H. Failure patterns and outcomes of dose escalation of stereotactic body radiotherapy for locally advanced pancreatic cancer: a multicenter cohort study. Ther Adv Med Oncol. 2020;12:1758835920977155. doi: 10.1177/1758835920977155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Simionato F, Zecchetto C, Merz V, Cavaliere A, Casalino S, Gaule M, D'Onofrio M, Malleo G, Landoni L, Esposito A, Marchegiani G, Casetti L, Tuveri M, Paiella S, Scopelliti F, Giardino A, Frigerio I, Regi P, Capelli P, Gobbo S, Gabbrielli A, Bernardoni L, Fedele V, Rossi I, Piazzola C, Giacomazzi S, Pasquato M, Gianfortone M, Milleri S, Milella M, Butturini G, Salvia R, Bassi C, Melisi D. A phase II study of liposomal irinotecan with 5-fluorouracil, leucovorin and oxaliplatin in patients with resectable pancreatic cancer: the nITRO trial. Ther Adv Med Oncol. 2020;12:1758835920947969. doi: 10.1177/1758835920947969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Risch HA. Etiology of pancreatic cancer, with a hypothesis concerning the role of N-nitroso compounds and excess gastric acidity. J Natl Cancer Inst. 2003;95:948–960. doi: 10.1093/jnci/95.13.948. [DOI] [PubMed] [Google Scholar]
- 19.Bulajic M, Panic N, Löhr JM. Helicobacter pylori and pancreatic diseases. World J Gastrointest Pathophysiol. 2014;5:380–383. doi: 10.4291/wjgp.v5.i4.380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Risch HA, Yu H, Lu L, Kidd MS. ABO blood group, Helicobacter pylori seropositivity, and risk of pancreatic cancer: a case-control study. J Natl Cancer Inst. 2010;102:502–505. doi: 10.1093/jnci/djq007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Schulte A, Pandeya N, Fawcett J, Fritschi L, Risch HA, Webb PM, Whiteman DC, Neale RE. Association between Helicobacter pylori and pancreatic cancer risk: a meta-analysis. Cancer Causes Control. 2015;26:1027–1035. doi: 10.1007/s10552-015-0595-3. [DOI] [PubMed] [Google Scholar]
- 22.Chen XZ, Wang R, Chen HN, Hu JK. Cytotoxin-Associated Gene A-Negative Strains of Helicobacter pylori as a Potential Risk Factor of Pancreatic Cancer: A Meta-Analysis Based on Nested Case-Control Studies. Pancreas. 2015;44:1340–1344. doi: 10.1097/MPA.0000000000000414. [DOI] [PubMed] [Google Scholar]
- 23.Gawin A, Wex T, Ławniczak M, Malfertheiner P, Starzyńska T. [Helicobacter pylori infection in pancreatic cancer] Pol Merkur Lekarski. 2012;32:103–107. [PubMed] [Google Scholar]
- 24.Liu H, Chen YT, Wang R, Chen XZ. Helicobacter pylori infection, atrophic gastritis, and pancreatic cancer risk: A meta-analysis of prospective epidemiologic studies. Medicine (Baltimore) 2017;96:e7811. doi: 10.1097/MD.0000000000007811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Huang J, Zagai U, Hallmans G, Nyrén O, Engstrand L, Stolzenberg-Solomon R, Duell EJ, Overvad K, Katzke VA, Kaaks R, Jenab M, Park JY, Murillo R, Trichopoulou A, Lagiou P, Bamia C, Bradbury KE, Riboli E, Aune D, Tsilidis KK, Capellá G, Agudo A, Krogh V, Palli D, Panico S, Weiderpass E, Tjønneland A, Olsen A, Martínez B, Redondo-Sanchez D, Chirlaque MD, Hm Peeters P, Regnér S, Lindkvist B, Naccarati A, Ardanaz E, Larrañaga N, Boutron-Ruault MC, Rebours V, Barré A, Bueno-de-Mesquita HB, Ye W. Helicobacter pylori infection, chronic corpus atrophic gastritis and pancreatic cancer risk in the European Prospective Investigation into Cancer and Nutrition (EPIC) cohort: A nested case-control study. Int J Cancer. 2017;140:1727–1735. doi: 10.1002/ijc.30590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Parente F, Maconi G, Sangaletti O, Minguzzi M, Vago L, Bianchi Porro G. Behaviour of acid secretion, gastrin release, serum pepsinogen I, and gastric emptying of liquids over six months from eradication of helicobacter pylori in duodenal ulcer patients. A controlled study. Gut. 1995;37:210–215. doi: 10.1136/gut.37.2.210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Haruma K, Mihara M, Okamoto E, Kusunoki H, Hananoki M, Tanaka S, Yoshihara M, Sumii K, Kajiyama G. Eradication of Helicobacter pylori increases gastric acidity in patients with atrophic gastritis of the corpus-evaluation of 24-h pH monitoring. Aliment Pharmacol Ther. 1999;13:155–162. doi: 10.1046/j.1365-2036.1999.00459.x. [DOI] [PubMed] [Google Scholar]
- 28.Nilsson HO, Stenram U, Ihse I, Wadstrom T. Helicobacter species ribosomal DNA in the pancreas, stomach and duodenum of pancreatic cancer patients. World J Gastroenterol. 2006;12:3038–3043. doi: 10.3748/wjg.v12.i19.3038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Xiao M, Wang Y, Gao Y. Association between Helicobacter pylori infection and pancreatic cancer development: a meta-analysis. PLoS One. 2013;8:e75559. doi: 10.1371/journal.pone.0075559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Ai F, Hua X, Liu Y, Lin J, Feng Z. Preliminary study of pancreatic cancer associated with Helicobacter pylori infection. Cell Biochem Biophys. 2015;71:397–400. doi: 10.1007/s12013-014-0211-2. [DOI] [PubMed] [Google Scholar]
- 31.Guo Y, Liu W, Wu J. Helicobacter pylori infection and pancreatic cancer risk: A meta-analysis. J Cancer Res Ther. 2016;12:C229–C232. doi: 10.4103/0973-1482.200744. [DOI] [PubMed] [Google Scholar]
- 32.Hirabayashi M, Inoue M, Sawada N, Saito E, Abe SK, Hidaka A, Iwasaki M, Yamaji T, Shimazu T, Tsugane S. Helicobacter pylori infection, atrophic gastritis, and risk of pancreatic cancer: A population-based cohort study in a large Japanese population: the JPHC Study. Sci Rep. 2019;9:6099. doi: 10.1038/s41598-019-42365-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Sethi V, Vitiello GA, Saxena D, Miller G, Dudeja V. The Role of the Microbiome in Immunologic Development and its Implication For Pancreatic Cancer Immunotherapy. Gastroenterology. 2019;156:2097–2115.e2. doi: 10.1053/j.gastro.2018.12.045. [DOI] [PubMed] [Google Scholar]
- 34.Kiss B, Mikó E, Sebő É, Toth J, Ujlaki G, Szabó J, Uray K, Bai P, Árkosy P. Oncobiosis and Microbial Metabolite Signaling in Pancreatic Adenocarcinoma. Cancers (Basel) 2020;12 doi: 10.3390/cancers12051068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Picardo SL, Coburn B, Hansen AR. The microbiome and cancer for clinicians. Crit Rev Oncol Hematol. 2019;141:1–12. doi: 10.1016/j.critrevonc.2019.06.004. [DOI] [PubMed] [Google Scholar]
- 36.Gopalakrishnan V, Spencer CN, Nezi L, Reuben A, Andrews MC, Karpinets TV, Prieto PA, Vicente D, Hoffman K, Wei SC, Cogdill AP, Zhao L, Hudgens CW, Hutchinson DS, Manzo T, Petaccia de Macedo M, Cotechini T, Kumar T, Chen WS, Reddy SM, Szczepaniak Sloane R, Galloway-Pena J, Jiang H, Chen PL, Shpall EJ, Rezvani K, Alousi AM, Chemaly RF, Shelburne S, Vence LM, Okhuysen PC, Jensen VB, Swennes AG, McAllister F, Marcelo Riquelme Sanchez E, Zhang Y, Le Chatelier E, Zitvogel L, Pons N, Austin-Breneman JL, Haydu LE, Burton EM, Gardner JM, Sirmans E, Hu J, Lazar AJ, Tsujikawa T, Diab A, Tawbi H, Glitza IC, Hwu WJ, Patel SP, Woodman SE, Amaria RN, Davies MA, Gershenwald JE, Hwu P, Lee JE, Zhang J, Coussens LM, Cooper ZA, Futreal PA, Daniel CR, Ajami NJ, Petrosino JF, Tetzlaff MT, Sharma P, Allison JP, Jenq RR, Wargo JA. Gut microbiome modulates response to anti-PD-1 immunotherapy in melanoma patients. Science. 2018;359:97–103. doi: 10.1126/science.aan4236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Kovács T, Mikó E, Vida A, Sebő É, Toth J, Csonka T, Boratkó A, Ujlaki G, Lente G, Kovács P, Tóth D, Árkosy P, Kiss B, Méhes G, Goedert JJ, Bai P. Cadaverine, a metabolite of the microbiome, reduces breast cancer aggressiveness through trace amino acid receptors. Sci Rep. 2019;9:1300. doi: 10.1038/s41598-018-37664-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Michaud DS. Role of bacterial infections in pancreatic cancer. Carcinogenesis. 2013;34:2193–2197. doi: 10.1093/carcin/bgt249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Michaud DS, Izard J. Microbiota, oral microbiome, and pancreatic cancer. Cancer J. 2014;20:203–206. doi: 10.1097/PPO.0000000000000046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Gaiser RA, Halimi A, Alkharaan H, Lu L, Davanian H, Healy K, Hugerth LW, Ateeb Z, Valente R, Fernández Moro C, Del Chiaro M, Sällberg Chen M. Enrichment of oral microbiota in early cystic precursors to invasive pancreatic cancer. Gut. 2019;68:2186–2194. doi: 10.1136/gutjnl-2018-317458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Karpiński TM. Role of Oral Microbiota in Cancer Development. Microorganisms. 2019;7 doi: 10.3390/microorganisms7010020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Ammer-Herrmenau C, Pfisterer N, Weingarten MF, Neesse A. The microbiome in pancreatic diseases: Recent advances and future perspectives. United European Gastroenterol J. 2020;8:878–885. doi: 10.1177/2050640620944720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Li P, Shu Y, Gu Y. The potential role of bacteria in pancreatic cancer: a systematic review. Carcinogenesis. 2020;41:397–404. doi: 10.1093/carcin/bgaa013. [DOI] [PubMed] [Google Scholar]
- 44.Mitsuhashi K, Nosho K, Sukawa Y, Matsunaga Y, Ito M, Kurihara H, Kanno S, Igarashi H, Naito T, Adachi Y, Tachibana M, Tanuma T, Maguchi H, Shinohara T, Hasegawa T, Imamura M, Kimura Y, Hirata K, Maruyama R, Suzuki H, Imai K, Yamamoto H, Shinomura Y. Association of Fusobacterium species in pancreatic cancer tissues with molecular features and prognosis. Oncotarget. 2015;6:7209–7220. doi: 10.18632/oncotarget.3109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Aune D, Greenwood DC, Chan DS, Vieira R, Vieira AR, Navarro Rosenblatt DA, Cade JE, Burley VJ, Norat T. Body mass index, abdominal fatness and pancreatic cancer risk: a systematic review and non-linear dose-response meta-analysis of prospective studies. Ann Oncol. 2012;23:843–852. doi: 10.1093/annonc/mdr398. [DOI] [PubMed] [Google Scholar]
- 46.Park J, Morley TS, Kim M, Clegg DJ, Scherer PE. Obesity and cancer--mechanisms underlying tumour progression and recurrence. Nat Rev Endocrinol. 2014;10:455–465. doi: 10.1038/nrendo.2014.94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Hall AB, Tolonen AC, Xavier RJ. Human genetic variation and the gut microbiome in disease. Nat Rev Genet. 2017;18:690–699. doi: 10.1038/nrg.2017.63. [DOI] [PubMed] [Google Scholar]
- 48.Backhus J, Seufferlein T, Perkhofer L, Hermann PC, Kleger A. IgG4-Related Diseases in the Gastrointestinal Tract: Clinical Presentation, Diagnosis and Treatment Challenges. Digestion. 2019;100:1–14. doi: 10.1159/000492814. [DOI] [PubMed] [Google Scholar]
- 49.van der Neut Kolfschoten M, Schuurman J, Losen M, Bleeker WK, Martínez-Martínez P, Vermeulen E, den Bleker TH, Wiegman L, Vink T, Aarden LA, De Baets MH, van de Winkel JG, Aalberse RC, Parren PW. Anti-inflammatory activity of human IgG4 antibodies by dynamic Fab arm exchange. Science. 2007;317:1554–1557. doi: 10.1126/science.1144603. [DOI] [PubMed] [Google Scholar]
- 50.de Buy Wenniger LJ, Culver EL, Beuers U. Exposure to occupational antigens might predispose to IgG4-related disease. Hepatology. 2014;60:1453–1454. doi: 10.1002/hep.26999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Guarneri F, Guarneri C, Benvenga S. Helicobacter pylori and autoimmune pancreatitis: role of carbonic anhydrase via molecular mimicry? J Cell Mol Med. 2005;9:741–744. doi: 10.1111/j.1582-4934.2005.tb00506.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Frulloni L, Lunardi C, Simone R, Dolcino M, Scattolini C, Falconi M, Benini L, Vantini I, Corrocher R, Puccetti A. Identification of a novel antibody associated with autoimmune pancreatitis. N Engl J Med. 2009;361:2135–2142. doi: 10.1056/NEJMoa0903068. [DOI] [PubMed] [Google Scholar]
- 53.Chmiela M, Gonciarz W. Molecular mimicry in Helicobacter pylori infections. World J Gastroenterol. 2017;23:3964–3977. doi: 10.3748/wjg.v23.i22.3964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Kountouras J, Zavos C, Chatzopoulos D. A concept on the role of Helicobacter pylori infection in autoimmune pancreatitis. J Cell Mol Med. 2005;9:196–207. doi: 10.1111/j.1582-4934.2005.tb00349.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Dore MP, Sepulveda AR, Pedroni A, Realdi G, Delitala G. Reversal of elevated pancreatic enzymes after Helicobacter pylori eradication. Intern Emerg Med. 2008;3:269–270. doi: 10.1007/s11739-008-0117-3. [DOI] [PubMed] [Google Scholar]
- 56.Culver EL, Smit WL, Evans C, Sadler R, Cargill T, Makuch M, Wang LM, Ferry B, Klenerman P, Barnes E. No evidence to support a role for Helicobacter pylori infection and plasminogen binding protein in autoimmune pancreatitis and IgG4-related disease in a UK cohort. Pancreatology. 2017;17:395–402. doi: 10.1016/j.pan.2017.04.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Wynn TA. Fibrotic disease and the T(H)1/T(H)2 paradigm. Nat Rev Immunol. 2004;4:583–594. doi: 10.1038/nri1412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Mattoo H, Mahajan VS, Della-Torre E, Sekigami Y, Carruthers M, Wallace ZS, Deshpande V, Stone JH, Pillai S. De novo oligoclonal expansions of circulating plasmablasts in active and relapsing IgG4-related disease. J Allergy Clin Immunol. 2014;134:679–687. doi: 10.1016/j.jaci.2014.03.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Kamata K, Watanabe T, Minaga K, Hara A, Yoshikawa T, Okamoto A, Yamao K, Takenaka M, Park AM, Kudo M. Intestinal dysbiosis mediates experimental autoimmune pancreatitis via activation of plasmacytoid dendritic cells. Int Immunol. 2019;31:795–809. doi: 10.1093/intimm/dxz050. [DOI] [PubMed] [Google Scholar]
- 60.Hart PA, Kamisawa T, Brugge WR, Chung JB, Culver EL, Czakó L, Frulloni L, Go VL, Gress TM, Kim MH, Kawa S, Lee KT, Lerch MM, Liao WC, Löhr M, Okazaki K, Ryu JK, Schleinitz N, Shimizu K, Shimosegawa T, Soetikno R, Webster G, Yadav D, Zen Y, Chari ST. Long-term outcomes of autoimmune pancreatitis: a multicentre, international analysis. Gut. 2013;62:1771–1776. doi: 10.1136/gutjnl-2012-303617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Macinga P, Pulkertova A, Bajer L, Maluskova J, Oliverius M, Smejkal M, Heczkova M, Spicak J, Hucl T. Simultaneous occurrence of autoimmune pancreatitis and pancreatic cancer in patients resected for focal pancreatic mass. World J Gastroenterol. 2017;23:2185–2193. doi: 10.3748/wjg.v23.i12.2185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Ikeura T, Miyoshi H, Uchida K, Fukui T, Shimatani M, Fukui Y, Sumimoto K, Matsushita M, Takaoka M, Okazaki K. Relationship between autoimmune pancreatitis and pancreatic cancer: a single-center experience. Pancreatology. 2014;14:373–379. doi: 10.1016/j.pan.2014.04.029. [DOI] [PubMed] [Google Scholar]