Abstract
Objectives: Atopic dermatitis (AD) is one of the most common skin disorders in infants and children and is often aggravated by increased Staphylococcus aureus (S. aureus) colonization. An inhibitory effect of a specific egg yolk antibody (IgY) on S. aureus growth was demonstrated in this study. Furthermore, the effects of water- or oil-based adjuvants on the preparation of anti-S. aureus IgY and hen immunization were compared.
Methods: Hens were immunized intramuscularly with formalin-killed S. aureus mixed with either a water-soluble polysaccharide λ-carrageenan, oil-based Freund's complete adjuvant (FCA), or Freund's incomplete adjuvant (FIA). Anti-S. aureus IgYs (FIA-IgY, FCA/FIA-IgY, and λCarra-IgY) were purified from the egg yolk of immunized hen eggs, and the activity of the IgY against S. aureus antigen was measured by ELISA. The proportion of each IgY that was absorbed by S. aureus was also determined. Then, the effect of purified anti-S. aureus IgY on S. aureus growth inhibition was investigated in vitro.
Results: The yolk of eggs and purified FIA-IgY from the FIA group showed the highest antibody activity, followed by FCA/FIA-IgY and λCarra-IgY. The proportion of each IgY that was absorbed by S. aureus antigen was as follows: FIA-IgY (18.1%), FCA/FIA-IgY (12.9%), and λCarra-IgY (7.0%). Only FIA-IgY significantly inhibited S. aureus growth in liquid medium.
Conclusion: A specific IgY that was produced using the FIA adjutant inhibited S. aureus growth. Although water-soluble λ-carrageenan showed an adjuvant effect on anti-S. aureus IgY induction in egg yolk, but did not inhibit S. aureus growth. The use of the oil adjuvant FIA was necessary in the preparation of anti-S. aureus IgY as a treatment for AD symptoms.
Keywords: adjuvant, atopic dermatitis, λ-carrageenan, IgY, Staphylococcus aureus
Introduction
Atopic dermatitis (AD) is an itchy inflammatory skin disorder that follows a chronic relapsing-remitting course (Spergel and Paller, 2003; Leung et al., 2004). AD is one of the most common skin disorders observed in infants and children; and in some patients, the disorder persists into adulthood. Generally, non-pathogenic Staphylococcus epidermidis is a dominant commensal bacterium on normal skin; however, the skin of patients with AD is colonized by Staphylococcus aureus (S. aureus) in high densities (Baker, 2006). Leyden et al. (1974) reported that S. aureus was found on the skin of 90% of 50 AD patients with chronic plaques. Furthermore, studies that used animal models and observed children in the clinic have reported decreased bacterial diversity on the skin, with increased S. aureus colonization during the active phase of AD (Kong et al., 2012; Kobayashi et al., 2015).
AD is characterized by dry skin due to water and lipid deficiencies; and therefore, bacteria can easily invade epidermal cells (Baker, 2006). With increased S. aureus colonization, the immune reaction to S. aureus promotes skin inflammation in patients with AD (Baker, 2006). The use of steroids is a common treatment for AD, to control skin inflammation; however, steroids do not inhibit bacterial colonization (Katoh et al., 2019). Growth inhibitory effects have been reported for a specific IgY against bacteria (Sugita-konishi et al., 1996; Hatta et al., 1997; Lee et al., 2002; Sunwoo et al., 2002). IgY is an immunoglobulin found in avian egg yolk (Schade et al., 2005); and in avian species, a maternal antibody is transferred to offspring via egg yolk. In chickens, only IgY (MW: 180 kDa), which is equivalent to mammalian IgG (MW: 150 kDa), is transferred to the egg yolk. Therefore, pure IgY can be easily isolated by removing yolk lipoproteins followed by salting-out IgY from water-soluble proteins.
Generally, an adjuvant is applied to animals with an antigen to increase animal immunity and the production of a sufficient amount of specific antibodies against the antigen. In this study, λ-carrageenan was tested as an adjuvant. Carrageenan is a polysaccharide extracted from red algae that is used as a thickening agent in food processing (Necas and Bartosikova, 2013). It is formed by alternate D-galactose and 3,6-anhydro-galactose units jointed by α-1,3 and β-1,4-glycosidic linkages. Carrageenan is classified into three types, kappa (k), iota (i), and lambda (λ) carrageenan, based on the number of 3, 6-anhydros galactose molecules and the presence of the ester sulfate group (−O-SO3−) on repeating galactose units (Fig. 1). Carrageenan has been used as an inflammatory induction agent in animal experiments (Morris, 2003; Silva et al., 2010). Notably, when injected to rat footpad, carrageenan produces acute paw edema, and experimental inflammation has also been induced in chickens footpads by carrageenan injection (Ito et al., 1989; Roach and Sufka, 2003). Furthermore, Elfaki et al. (1992 & 1993) reported that the injection ofi-carrageenan with mycoplasma into chickens protected them against airsacculitis.
Fig. 1.
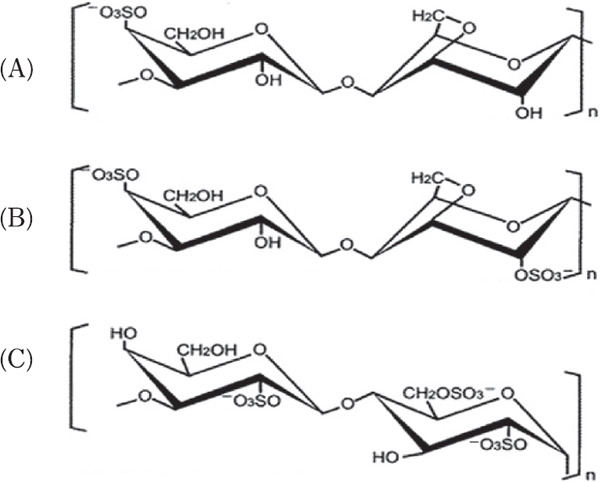
Structures of (A) κ-, (B) ι-, and λ-carrageenan (C).
Generally, Freund's complete adjuvant (FCA) and incomplete adjuvant (FIA) have been frequently used in animal immunological experiments (Stils, 2005). Since carrageenan has inflammatory and immune response enhancement properties, it can be used as a water-soluble adjuvant to intramuscularly immunize hens for the production of specific IgY antibodies on a large scale. In this study, the adjuvant effect of the most water-soluble carrageenan, λ-carrageenan, on anti-S. aureus IgY production was compared with that of oil-based FCA and FIA adjuvants.
The first purpose of this study was to evaluate the inhibitory effects of highly pure anti-S. aureus IgY on S. aureus growth, as a potential therapeutic for patients with AD. The second purpose was to examine the adjuvant effect of λ-carrageenan in inducing anti-S. aureus IgY production in egg yolks of immunized hens.
Materials and Methods
Animal Care
All procedures involving animals and their care conformed to the Guidelines for Proper Conduct of Animal Experiments (Science Council of Japan, 2006).
Materials
Food-ingredient-grade λ-carrageenan was obtained from Taiyo Kagaku Co. Ltd. (Mie, Japan). Freund's complete adjuvant (FCA) and Freund's incomplete adjuvant (FIA) were purchased from Becton, Dickinson and Company (MD, USA). BacTiter-Glo™ Microbial Cell Viability Assay was obtained from Promega (Madison, WI, USA). The other chemicals that were used were of suitable grade.
Immunization of Hens
Antigens were prepared by emulsifying/homogenizing formalin-killed S. aureus (8.4×109 cf u/mL) with an equal volume of either FIA, FCA, 2% λ-carrageenan, or 0.9% NaCl solution. Hens (Boris Brown, 276 days old) were immunized by intramuscular injection, with a total of 1 mL of antigen (4.2×109 cfu/mL) at 3–4 sites in the right side of the underwing muscle, per hen. Three hens in each group received subsequent injections three times, at two-week intervals, after an initial immunization in the same manner. Hens that received FCA at the initial immunization were subsequently injected with FIA as an adjuvant (FCA/FIA group). In control group, an antigen was not injected into the hens. Eggs were collected, and the number of eggs laid was recorded in each group every two weeks. Egg yolk from eggs collected every two weeks after the initial immunization were diluted two-fold with 0.05% sodium azide and stored at 4°C until antibody activity measurement. Other egg yolks were pooled together, every two weeks/hen, frozen at −30 °C, and used to purify IgY antibody.
Determination of Anti-S. aureus IgY Antibody Activity
The activity of IgY in egg yolk was measured by ELISA. A 96-well microplate (Sumitomo Bakelite, Tokyo, Japan) was coated with 50 µL/well off ormalin-killed S. aureus (8.4 ×109 cfu/mL) in an ELISA coating buffer (pH9.6). The plate was incubated at 37°C for 1 h and washed four times with Tris-buffered saline containing 0.05% Tween20, pH7.4 (TBS-T). Then, the plate was filled with 200 µL/well of TBS-T containing 10 mg/mL of edible collagen (E-CAN) (Nippon Syoji, Okayama, Japan), followed by 1 h incubation at 37°C to block the plate. The plate was further washed with TBS-T four times. The two-fold diluted egg yolk solution that contained the primary antibody was further diluted with TBS-T (1:1000). This egg yolk solution was then added to the wells (50 µL/well) at a final dilution of 1:2000, followed by 1 h incubation at 37°C. After the plate was washed with TBS-T, 200 µL/well of diluted (1:2000) secondary antibody, alkaline phosphatase-conjugated rabbit anti-chicken IgY (H + L) (Abnova, Taipei, Taiwan), was added, and the plate was incubated and washed in the same manner. 50 µL of p-nitrophenyl phosphate disodium salt (1 mg/mL), as a substrate, was added to each well, followed by incubation for 15 min at 37°C. The enzyme reaction was stopped by the addition of 50 µL/well of 2 M NaOH, and absorbance at 405 nm was measured using a microplate reader (Infinite 200 PRO, Tecan Japan, Kanagawa, Japan).
IgY Purification
Pooled egg yolk from eggs that were collected two weeks prior to the highest ELISA value in each group (Fig. 2) were used for IgY purification, according to the λ-carrageenan method described by Hatta et al. (1990) with slight modification. After thawing the frozen egg yolk, 100 g of egg yolk was homogenized with 700 mL of 0.36% sodium chloride using a homomixer (TK HomoMixer Mark II, Tokushukika, Osaka, Japan), and 200 mL of 0.4% λ-carrageenan solution was gently added to the yolk homogenate while stirring to flocculate lipoproteins with carrageenan. The mixture was held at room temperature for 1 h, followed by centrifugation (20°C, 7500 rpm, 20 min) to isolate the supernatant (water soluble protein fraction). Sodium sulfate (anhydrous powder) was added to the supernatant at 15% (w/v). After the overnight salting-out procedure, with gentle stirring at room temperature, the solution was centrifuged (20°C, 8,000 rpm, 30 min). The resulting precipitate was dissolved in 50 mL of 10 mM phosphate buffer (pH 7.2). The sample was then dialyzed in 10 mM phosphate buffer (pH 7.2) for 6 h in a cold room, at least four times. The dialyzed solution was filtered through a 0.45 µm membrane filter, and the filtrate was lyophilized and stored at −30°C until use. The following IgY antibodies were purified: FIA-IgY (FIA as an adjuvant); FCA/FIA-IgY (FCA and FIA as adjuvants); λCarra-IgY: (λ-carrageenan as an adjuvant); and Cont-IgY (control). The purified IgY concentration was estimated from the absorption at 280 nm, using the value of 15.8 for a 1% solution in a light path of 1 cm (Gallagher, and Voss, 1969).
Fig. 2.
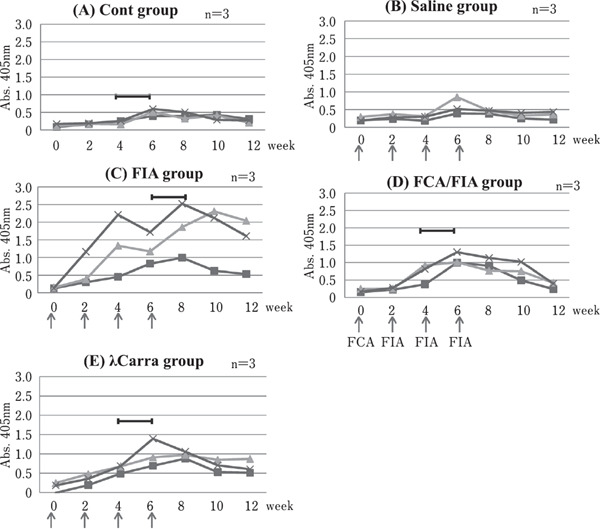
Changes in anti-S. aureus IgY activity in Egg Yolk. The changes of IgY activity in diluted egg yolk by ELISA of three individual hens are shown as -×-, -■- and -▲- in each group. The arrows indicate the times of immunization. FIA: Freund's incomplete adjuvant, FCA: Freund's complete adjuvant, λCarra: λ-carrageenan. The bar ( ) indicates the period in which hen egg yolk was pooled (-×-) and used for IgY purification.
) indicates the period in which hen egg yolk was pooled (-×-) and used for IgY purification.
IgY Purity Evaluation
IgY purity was measured by HPLC and SDS-PAGE. The HPLC SCL-10A system (SHIMADZ, Kyoto, Japan) equipped with a TSK gel G3000SW (Tosoh, Tokyo, Japan) column was used. Phosphate buffer (0.1 M, pH 6.8) containing 0.1 M sodium sulfate was used as the mobile phase at a flow rate of 0.5 mL/min. The IgY concentration was detected by the absorbance at 280 nm. SDS-PAGE was performed according to the Laemmli method (Laemmli, 1970), using the slab type vertical gel system (ATTO, Tokyo, Japan) with a 5–20% gradient gel (c-PAGEL HR, ATTO). The EzProtein Ladder (ATTO) was used as a molecular weight marker.
ELISA Activity of Purified IgY
Purified IgY (5 µg/mL) diluted with TBS-T was used as a primary antibody for the ELISA analysis described above, using a 96-well microplate coated with 50 µL/well formalin-killed S. aureus antigen (7.4×107 cfu/mL).
Estimated Specific IgY Antibody Ratio
The proportion of specific IgY antibody among the total purified polyclonal antibody was determined by gel filtration using HPLC. Purified IgY (2 mg/mL) in 10 mM phosphate buffer (pH 6.8) was mixed with an equal amount of formalin-killed S. aureus (9.3×108 cfu/mL). Then, the mixture was shaken at 37°C for more than 30 min, to absorb the specific antibody with the antigen. The resultant mixture was centrifuged and the supernatant (unbound IgY to the antigen) was collected. The unbound IgY samples were applied to the HPLC system, according to the method described above. The peak area of the purified IgY (1 mg/mL) was set to 100%. The peak area of unbound IgY solution was compared with that of the corresponding purified IgY protein (1 mg/mL), and the ratio of the difference was calculated to estimate the anti-S. aureus specific IgY ratio (%).
S. aureus Growth Inhibition
A 96-well microplate was used to study S. aureus growth inhibition of purif ied anti-S. aureus IgY. S. aureus (5.8×106 cfu/mL) diluted with doubly concentrated LB broth, Lennox (Sigma-Aldrich Japan, Tokyo, Japan) was mixed with an equal amount of IgY samples (20 mg/mL) from each group. The resultant mixture was added to each well (100 µL/well) of a 96-well cell culture plate (TPP, Trasadingen, Switzerland). As a negative control, the bacteria solution was mixed with an equal amount of sterilized distilled water. The plate was incubated at 37°C for 24 h, and then, 80 µL of sample from each well was periodically transferred to the wells of a 96-well transparent microplate (Greiner bio-one, Kremsmünster, Austria), and turbidity was measured at 600 nm. Then, 30 µL of the turbidity-measured sample from each well was transferred to the wells of a 96-well black plate (Sumitomo Bakelite, Tokyo, Japan). Luminescence due to ATP was measured using the BacTiter-Glo™ Microbial Cell Viability Assay kit, to monitor bacterial growth. The absorbance and luminescence activity were measured using a SpectraMax i3x (Molecular Device Japan, Tokyo, Japan).
Statistical Analyses
The number of laid eggs, turbidity, and ATP luminescence activity data (at 8, 10, and 24 h) were analyzed by one-way analysis of variance (ANOVA), and the Tukey-Kramer test was performed as a post-hoc test. These analyses were performed using BellCurve for Excel software (Social Survey Research Information, Tokyo, Japan).
Results
Changes in Anti-S. aureus IgY Activity in Egg Yolk and Egg-laying Rate
Figure 2A–E shows the changes in ELISA values of diluted egg yolk from each hen following immunization. In the saline group (without an adjuvant), only one of three hens showed a slight increase in IgY activity. On the other hand, each hen in the FIA group showed a large increase in IgY activity, although the values fluctuated. In the FCA/FIA group, all three hens showed increased IgY activity. Furthermore, all three hens in the λ-carrageenan (λCarra) group also showed increased IgY activity, at levels similar to the FCA/FIA group.
Table 1 shows the changes in the number of eggs laid in two weeks and egg-laying rates. When the number of eggs laid every two weeks in the Cont group was set to 100%, the egg-laying rate in the FIA group decreased to 59.1% (p=0.19) on days 15–28 (weeks 2–4); while in the FCA/FIA group, the rate decreased to 60.5% (p=0.07) on days 29–42 (weeks 4–6). In the λCarra group, the minimum egg-laying rate (81.6%) was observed on days 29–42 (weeks 4–6). Although there were not statistically significant differences between the egg-laying rates of each group over that of the Cont group, the smallest egg-laying rate decrease after immunization injections was observed in the λCarra group, on days 29–42 (weeks 4–6).
Table 1. Changes in the number of eggs laid in 2 weeks and egg-laying rates.
| Cont | Saline | FIA | FCA/FIA | λCarra | |
|---|---|---|---|---|---|
| group | group | group | group | group | |
| Day 1∼14 (0–2w) |
14.0 (100%) | 11.7 (83.3%) | 12.0 (85.7%) | 13.0 (92.9%) | 13.0 (92.9%) |
| Day 15∼28 (2–4w) |
14.7 (100%) | 12.7 (86.4%) | 8.7 (59.1%) [p=0.19] |
12.3 (84.1%) | 12.0 (81.8%) |
| Day 29∼42 (4–6w) |
12.7 (100%) | 12.0 (94.7%) | 10.7 (84.2%) | 7.7 (60.5%) [p=0.07] |
10.3 (81.6%) |
| Day 43∼56 (6–8w) |
13.3 (100%) | 12.0 (90.0%) | 12.3 (92.5%) | 11.7 (87.5%) | 12.7 (95.0%) |
| Day 57∼70 (8–10w) |
13.7 (100%) | 13.0 (95.1%) | 13.0 (95.1%) | 12.3 (90.2%) | 12.0 (87.8%) |
| Day 71∼84 (10–12w) |
13.3 (100%) | 12.7 (95.0%) | 12.3 (92.5%) | 11.3 (85.0%) | 11.7 (87.5%) |
| Average | 13.6 (100%) | 12.3 (90.6%) | 11.5 (84.5%) | 11.4 (83.7%) | 11.9 (87.8%) |
The egg laying rate (%) for 2 weeks in each group (n=3) was calculated as the number of eggs laid (in 2 weeks). The Cont group was set to 100%.
IgY Purification
When the λ-carrageenan method was used for IgY purification, the amounts of isolated Cont-IgY, FIA-IgY, FCA/FIA-IgY, and λCarra-IgY proteins were 427.3 mg, 460.7 mg, 473.0 mg, and 451.1 mg from 100 g of egg yolk, respectively. When the IgY content of 100 g of egg yolk was considered to be 1,000 mg (Hatta et al., 1996), the IgY recoveries of each purified IgY were 42.7%, 46.1%, 47.3%, and 45.1%, respectively. According to HPLC measurements, each IgY was between 99.0% and 100% pure (Table 2). In the SDS-PAGE analysis, each purified IgY showed two main bands, the heavy (H) and light (L) chains, with a few faint bands due to impurities (data not shown).
Table 2. Summary of IgY purification.
| The amount of IgY (mg) | Purity measured by HPLC | Proportion of IgY attaching to S.aureus | |
|---|---|---|---|
| Cont-IgY | 427.3 | 99.0% | 0% |
| FIA-IgY | 460.7 | 100% | 18.1% |
| FCA/FIA-IgY | 473.0 | 100% | 12.9% |
| λCarra-IgY | 451.1 | 100% | 7.0% |
Reaction of Purified IgY with Antigen
Figure 3 shows the anti-S. aureus activity of each purified IgY, measured by ELISA. FIA-IgY showed the highest activity, followed by FCA/FIA-IgY, and then, λCarra-IgY.
Fig. 3.
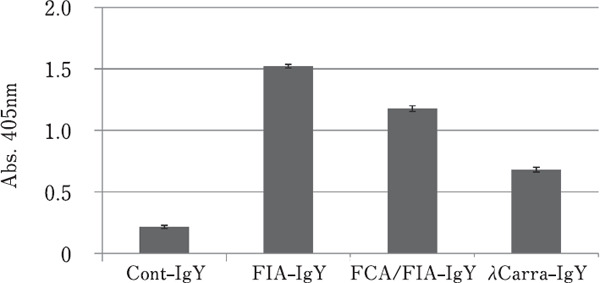
Reactivity of each purified IgY with S. aureus (by ELISA). Data represent means±SD (n=3).
Proportion of Specific IgY in the Purified IgY
Based on the peak area calculation determined by HPLC, the proportions of specific IgY absorbed by S. aureus antigen were estimated as follows: 0% (Cont-IgY), 18.1% (FIA-IgY), 12.9% (FCA/FIA-IgY), and 7.0% (λCarra-IgY), as shown in Table 2.
Growth Inhibitory Effect of Purified Anti-S. aureus IgY
Figures 4A and 4B show the S. aureus growth inhibition due to purified anti-S. aureus IgY, by monitoring solution turbidity (absorbance at 600 nm) and luminescence activity due to ATP content, respectively. Each purified IgY (10 mg/mL), with freshly cultured S. aureus, was inoculated into LB medium. There was significant growth suppression, based on solution in turbidity at 24 h, in the presence of FIA-IgY (p<0.05), compared to Cont-IgY, FCA/FIA-IgY, and λCarra-IgY. FCA/FIA-IgY and λCarra-IgY showed slight suppression of S. aureus growth (at 24 h) compared to Cont-IgY; however, this difference was not statistically significant (Fig. 4A). Fig. 4B shows the ATP assay results. FIA-IgY showed significantly lower luminescence activity compared to Cont-IgY, FCA/FIA-IgY, and λCarra-IgY (p<0.05) at 24 h, and this indicates a growth inhibitory effect of purified anti-S. aureus IgY (FIA-IgY). At 8 h and 10 h, similar low turbidity and luminescence activity was apparent for FIAIgY (p<0.05).
Fig. 4.
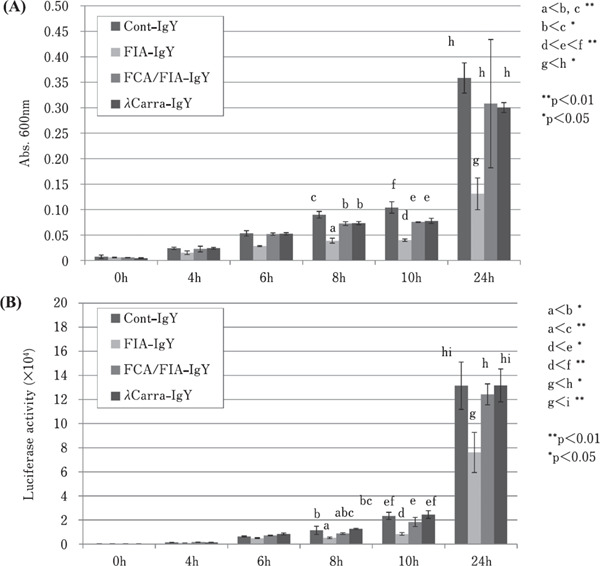
Changes in S. aureus growth monitored by turbidity (A) and ATP luminescence activity (B). The amount of S. aureus incubated with each individual IgY was measured by turbidity and luminescence ATP activity. Data represent mean±SD (n=3). Different letters indicate statistically significant differences.
Discussion
The present study has shown that the antibody activity of an anti-S. aureus IgY was increased using either FIA, a combination of FCA/FIA, or λ-carrageenan as an adjuvant. The adjuvant activities were compared based on the IgY sample ELISA values. The FIA adjuvant induced the highest level of specific IgY activity, followed by FCA/FIA, and λ-carrageenan. FCA and FIA are both oil-based adjuvants that gradually release the antigen from the injection site (Stils, 2005). FCA is composed of inactivated Mycobacterium tuberculosis, whereas the mycobacterial component is absent in FIA. The strong immunostimulatory property of FCA is due to the inactivated mycobacteria, which enhances immune response and results in severe local inflammation in experimental animals (Stils, 2005). However, in this study, FIA alone induced a higher antibody activity against the S. aureus antigen than a combination of FCA/FIA. Shimizu et al. (1989) reported a similar result in preparation of anti-E. coli IgY, in which the presence of mycobacteria components in FCA with bacterial antigen suppressed the induction of specific IgY, even when FCA was used only at the first injection, followed by FIA for the boosting immunization. Therefore, emulsion composed of bacterial antigens with oil-based adjuvants, such as FIA, enhances specific IgY activity against bacterial antigens, probably through slowly releasing the antigen in the hen's muscle.
It has been known that carrageenan induces inflammatory responses. In this study, the adjuvant effect of 2% λ-carrageenan was slightly lower than that of the oil-based FCA/FIA adjuvant. However, water soluble λ-carrageenan induced specific IgY antibodies against S. aureus; therefore, it can be used as a water-soluble adjuvant to immunize hens. Generally, oil based FCA and FIA adjuvants need to be emulsified with an antigen solution when immunizing animals (Stils, 2005), and such emulsions become extremely thick viscous mixtures. Therefore, it is impossible to inject the viscous emulsions into a large number of hens for large-scale IgY production. Since λ-carrageenan does not form a gel, it is the least viscous among the three types of carrageenan (Necas and Bartosikova, 2013). Thus, a high concentration of λ-carrageenan solution, such as 2%, can be injected with antigens continually into hens. In addition, in this study, it was shown that the egg-laying rate (%) in the λ-carrageenan group had the least decrease, compared to those from FIA and FCA/FIA groups (Table 1). Therefore, λ-carrageenan provides a promising adjuvant for the intramuscular vaccination of a large number of hens for IgY production. Furthermore, it may be used as a common adjuvant for the vaccination of laying hens on an industrial scale, like aluminum hydroxide and other water soluble adjuvants.
According to the turbidity and ATP assay results, there was a significant reduction in S. aureus growth in the presence of FIA-IgY, after the 24 h incubation, compared to Cont-IgY. In addition, the proportion of specific anti-S. aureus IgY antibody was the largest in the FIA-IgY group (18.1%), of all purified IgY. This suggests that when the specific IgY ratio (%) and ELISA-measured IgY antibody activity values are greater than those of the purified FIA-IgY, anti-S. aureus IgY inhibits the growth of S. aureus.
The mechanism of the inhibitory effect of specific anti-bodies on bacteria growth is not yet known. Lin et al. (1998) reported that a monoclonal antibody specific for the Enterobacter ligand-binding site of FepA that is exposed on the bacteria surface inhibited the growth of E. coli. It is possible that a polyclonal antibody could similarly block a specific site that is exposed on the bacteria surface and inhibit bacteria growth. In this study, S. aureus (5.8×106 cfu/mL) was inoculated into LB medium, which initially contained 10 mg/mL anti-S. aureus IgY, and incubated at 37°C. Leyden et al. (1974) reported that the amount of S. aureus in patients with AD with chronic plaques and exudative lesions ranged from 1×106–1.4×107 cfu/cm2. Therefore, the present investigated a concentration of S. aureus that is similar to that found on the skin of patients with AD. On the other hand, the S. aureus growth conditions that were experimentally investigated in this study involved an ideal LB medium culture. This experimental setup assumes that the anti-S. aureus IgY will also be able to inhibit S.aureus growth on patient skin.
In conclusion, the present study showed that the S. aureus immunization of hens with λ-carrageenan adjuvant induced anti-S.aureus IgY antibodies. The λ-carrageenan adjuvant will make intramuscular injection simpler and easier, even on an industrial scale, with a low risk of egg laying rate (%) degradation. In terms of the growth inhibitory effect against S. aureus, however, the specific IgY that were raised in this study using an FIA adjuvant were the only effective IgY, in which 18.1% of the specific antibody bound to S. aureus. For the production of an effective anti-S. aureus IgY, the use of oil adjuvant FIA was necessary for inhibiting S. aureus growth, and such an antibody formulation can be expected to prevent the exacerbation of AD symptoms.
Acknowledgments
This study was supported by JSPS KAKENHI Grant Number JP16K12712. The authors also thank Miss Momoka Umehara, Miss Yuka Shimoide, and Miss Hiromi Yoshika for their excellent laboratory work on the present research project.
Conflicts of Interest
The authors declare no conflict of Interest.
References
- Baker BS. The role of microorganisms in atopic dermatitis. Clinical and Experimental Immunology, 144: 1-9. 2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elfaki MG, Kleven SH, Jin LH and Ragland WL. Sequential intracoelomic and intrabursal immunization of chickens with inactivated Mycoplasma gallisepticum bacterin and iota carrageenan adjuvant. Vaccine, 10: 655-662. 1992. [DOI] [PubMed] [Google Scholar]
- Elfaki MG, Kleven SH, Jin LH and Ragland WL. Protection against airsacculitis with sequential systemic and local immunization of chickens using killed Mycoplasma gallisepticum bacterin with iota carrageenan adjuvant. Vaccine, 11: 311-317. 1993. [DOI] [PubMed] [Google Scholar]
- Gallagher JS and Voss EW Jr.. Molecular weight of a purified chicken antibody. Immunochemistry, 6: 199-206. 1969. [DOI] [PubMed] [Google Scholar]
- Hatta H, Kim M and Yamamoto T. A novel isolation method for hen egg yolk antibody, “IgY”, Agricultural and Biological Chemistry, 54: 2531-2535. 1990. [PubMed] [Google Scholar]
- Hatta H, Ozeki M and Tsuda K. Egg yolk antibody IgY and its application. In: Hen eggs: their basic and applied science (Yamamoto T, Juneja LR, Hatta H, and Kim M eds.). pp. 151-178. CRC Press. Boca Raton, FL, USA. 1996. [Google Scholar]
- Hatta H, Tsuda K, Ozeki M, Kim M, Yamamoto T, Otake S, Hirasawa M, Katz J, Childers NK and Michalek SM. Passive immunization against dental plaque formation in humans: effect of a mouth rinse containing egg yolk antibodies (IgY) specific to Streptococcus mutants. Caries Research, 31: 268-274. 1997. [DOI] [PubMed] [Google Scholar]
- Ito NMK, Noronha AMB and Bohm GM. Carrageenan-induced acute inflammatory response in chicks. Research in Veterinary Science, 46: 192-195. 1989. [PubMed] [Google Scholar]
- Katoh N, Ohya Y, Ikeda M, Ebihara T, Katayama I, Saeki H, Shimojo N, Tanaka A, Nakahara T, Nagao M, Hide M, Fujita Y, Fujisawa T, Futamura M, Masuda K, Murota H and Yamamoto-hanada K. Clinical practice guidelines for the management of atopic dermatitis 2018. Journal of Dermatology, 46: 1053-1101. 2019. [DOI] [PubMed] [Google Scholar]
- Kobayashi T, Glatz M, Horiuchi K, Kawasaki H, Akiyama H, Kaplan DH, Kong HH, Amagai M, Nagao K, and Nagao K. Dysbiosis and Staphylococcus aureus colonization drives inflammation in atopic dermatitis. Immunity, 42: 756-766. 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kong HH, Oh J, Deming C, Conlan S, Grice EA, Beatson MA, Nomicos E, Polley EC, Komarow HD, NISC Comparative Sequence Program, Murray PR, Turner ML and Segre JA. Temporal shifts in the skin microbiome associated with disease flares and treatment in children with atopic dermatitis. Genome Research, 22: 850-859. 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laemmli UK. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature, 227: 680-685. 1970. [DOI] [PubMed] [Google Scholar]
- Lee EN, Sunwoo HH, Menninen K and Sim JS. In vitro studies of chicken egg yolk antibody (IgY) against Salmonella enteritidis and Salmonella typhimurium. Poultry Science, 81: 632-641. 2002. [DOI] [PubMed] [Google Scholar]
- Leung DYM, Boguniewicz M, Howell MD, Nomura I and Hamid QA. New insights into atopic dermatitis. Journal of Clinical Investigation, 113: 651-657. 2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leyden JJ, Marples RR and Kligman AM. Staphylococcus aureus in the lesions of atopic dermatitis. British Journal of Dermatology, 90: 525-530. 1974. [DOI] [PubMed] [Google Scholar]
- Lin J, Hogan JS and Smith KL. Inhibition of in vitro growth of coliform bacteria by a monoclonal antibody directed against ferric enterobactin receptor FepA. Journal of Dairy Science, 81: 1267-1274. 1998. [DOI] [PubMed] [Google Scholar]
- Morris CJ. Carrageenan-induced paw edema in the rat and mouse. In: Inflammation protocols (Winyard PG and Willoughby DA eds.). Methods in Molecular Biology, vol 225 pp. 115-121. Humana Press. Totowa, NJ, US. 2003. [DOI] [PubMed] [Google Scholar]
- Necas J and Bartosikova L. Carrageenan: a review: Veterinarni Medicina, 58: 187-205. 2013. [Google Scholar]
- Roach JT and Sufka KJ. Characterization of the chick carrageenan response. Brain Research, 994: 216-225. 2003. [DOI] [PubMed] [Google Scholar]
- Schade R, Calzado EG, Sarmiento R, Chacana PA, Porankiewiczasplund J and Terzolo HR. Chicken egg yolk antibodies (IgY-technology): a review of progress in production and use in research and human and veterinary medicine. Alternatives to Laboratory Animals, 33: 129-154. 2005. [DOI] [PubMed] [Google Scholar]
- Shimizu M, Fitzsimmons RC and Nakai S. Serum and egg antibody responses in chickens to Escherichia coli. Agricultural and Biological Chemistry, 53: 3233-3238. 1989. [Google Scholar]
- Silva FRF, Dore CMPG, Marques CT, Nascimento MS, Benevides NMB, Rocha HAO, Chavante SF and Leite EL. Anticoagulant activity, paw edema and pleurisy induced carrageenan: action of major types of commercial carrageenans. Carbohydrate Polymers, 79: 26-33. 2010. [Google Scholar]
- Spergel JM and Paller AS. Atopic dermatitis and the atopic march. Journal of Allergy and Clinical Immunology, 112: 118-127. 2003 [DOI] [PubMed] [Google Scholar]
- StilsHF, Jr.Adjuvants and antibody production: dispelling the myths associated with Freund's complete and other adjuvants. ILAR Journal, 46: 280-293. 2005. [DOI] [PubMed] [Google Scholar]
- Sugita-konishi Y, Shibata K, Yun SS, Hara-kudo Y, Yamaguchi K and Kumagai S. Immune functions of immunoglobulin Y isolated from egg yolk of hens immunized with various infectious bacteria. Bioscience, Biotechnology, and Biochemistry, 60: 886-888. 1996. [DOI] [PubMed] [Google Scholar]
- Sunwoo HH, Lee EN, Menninen K, Suresh MR and Sim JS. Growth inhibitory effect of chicken egg yolk antibody (IgY) on Escherichia coli O157:H7. Journal of Food Science, 67: 1486-1494. 2002. [Google Scholar]


