Abstract
L-Leucine (L-Leu) in ovo administration was demonstrated to afford thermotolerance and modified amino acids metabolism in post-hatched broiler chicks under heat stress. This study aimed to investigate the changes in embryonic growth and amino acid metabolism after in ovo injection of L-Leu. Fertilized broiler eggs were subjected to in ovo injection of sterile water or L-Leu on embryonic day (ED) 7. The weight of embryos and yolk sacs were measured on ED 12, 14, 16, and 18. Plasma and livers were collected on ED 14 and 18 for free amino acid analysis. The weight and relative weight of embryos were significantly lowered by in ovo administration of L-Leu, but those of yolk sacs were not altered. Moreover, L-Leu in ovo injection significantly reduced the plasma proline concentration during embryogenesis and increased the plasma concentrations of tyrosine (Tyr) and lysine (Lys) in ED 18. Hepatic Lys concentration was also significantly increased by L-Leu in ovo injection. Interestingly, Leu concentrations in the plasma and liver were not affected by L-Leu administration. These results indicated that in ovo administered L-Leu was metabolized before ED 14 and affected embryonic growth and amino acid metabolism during embryogenesis.
Keywords: amino acid, chick, embryo, growth, L-leucine
Introduction
Heat stress is a severe problem that restricts the efficient and healthy production of broiler chickens for a long time (Zaboli et al., 2019). In order to reduce the negative impacts of heat stress on poultry production, many studies have been conducted to propose suitable and efficient strategies using broiler chicks and chickens (Lin et al., 2006; Renaudeau et al., 2012; Chowdhury, 2019). The utilization of feed additives and controlling the environment are the main strategies currently used for overcoming heat stress in the poultry industry (Lin et al., 2006). These are passive methods used to alleviate heat-related damage or to promote a lower room temperature; however, they have a low efficiency and a high cost associated (Wasti et al., 2020).
It has been demonstrated that thermal manipulation (TM), a technique that involves increasing the incubation temperature before hatching, affords thermotolerance in posthatched broiler chicks and chickens (Piestun et al., 2008; Loyau et al., 2014). In our previous studies, we found that TMaltered amino acid concentrations, including that of leucine (Leu), in the brain and liver of embryos (Han et al., 2017). Then, L-Leu was administered to the embryo, and it was found to improve thermotolerance and growth performance under heat stress in broiler chicks and chickens, respectively (Han et al., 2019a; 2020).
Amino acid metabolism was affected even after a short exposure to heat stress (Ito et al., 2014). Amino acids also play critical roles in the regulation of body temperature. For instance, L-citruline (Chowdhury et al., 2017) and Daspartic acid (D-Asp) (Erwan et al., 2014) have hypothermic functions that can afford thermotolerance in neonatal chicks. Recent studies showed that L-Leu in ovo injection influenced amino acid metabolism under acute or chronic heat stress in broilers without changes in Leu concentration in chicks or chickens (Han et al., 2019b, 2020). These results suggest that L-Leu may be a trigger, rather than a long-term regulator of L-Leu-mediated thermotolerance in broiler chicks. Thus, it is necessary to clarify the change in amino acid concentrations after L-Leu in ovo injection during embryogenesis. Therefore, the first aim of the current study was to investigate the effects of L-Leu in ovo injection on amino acid metabolism in broiler embryos.
Amino acids not only serve as building blocks of proteins, but also play important roles in growth (Wu, 2009). It has been demonstrated that in ovo administration of amino acids has the potential to improve post-hatch growth (Ohta et al., 1999) and the feed conversion ratio (Kadam et al., 2008) in neonatal chicks. L-Leu and its metabolites have been reported to suppress protein degradation and promote muscle growth (Duan et al., 2016) by activating the mechanistic target of rapamycin (mTOR) signaling pathway in skeletal muscle and adipose tissues (Shimomura et al., 2015). In ovo administration of L-Leu, but not L-isoleucine (L-Ile) and L-valine, on embryonic day (ED) 7 improved embryo weight on ED 14 without affecting the body weight on the hatching day (Kita et al., 2015). In our previous reports, L-Leu in ovo administration on ED 7 had no effect on the body weight at hatching (Han et al., 2017). However, the effects of L-Leu in ovo feeding on embryonic growth performance have not yet been fully elucidated. Thus, the second aim of this study was to investigate the changes in embryonic weight after in ovo administration of L-Leu in broiler embryos.
Materials and Methods
Experiment Design
One hundred and twenty fertilized broiler eggs (Ross 308 strain; 80-weeks old) were purchased from a local hatchery in Nanjing, China. Eggs were divided into two groups (control and L-Leu; n=60/ group) based on the egg weight, to form groups as uniform as possible. The average egg weights of the control and L-Leu groups were 66.9 g. Eggs were placed into an incubator (Hongde 2112 type incubator, Hongde Comp., Shandong, China) at 37.6°C with 60–70% relative humidity and auto-turning every 1.5 h. On ED 7, thirteen unfertilized eggs (4 eggs from the control group and 9 eggs from the L-Leu group) were detected via candling and discarded properly. The remaining eggs were subjected to in ovo injection of sterile water (0.5 mL/egg) for the control, and L-Leu solution (34.5 µmol/0.5 mL sterile water/egg) for the L-Leu group, as described elsewhere (Han et al., 2017, 2018). Briefly, a small hole was made at the blunt end of the egg after disinfecting the eggshell. The sterile water or L-Leu solution was injected into the yolk sac using a 1-mL disposable syringe with a 25-gauge needle. After injection, the small holes were immediately sealed with scotch tape, which was sterilized with 70% ethanol, and the eggs were returned to the incubator.
On ED 12, 14, 16, and 18, developing embryos (n=10–12) were randomly selected from each group to measure the weight of the egg, yolk-free embryo, and yolk sac. The sampling of the embryo and yolk sac was performed by the same person without knowing the grouping information. The relative embryo weight (%) was calculated as the ratio between yolk-free embryo weight and the initial egg weight. The relative yolk weight (%) was calculated as the ratio between the yolk sac weight and the initial egg weight. On ED 14 and 18, blood was collected from the umbilical vein using a heparinized 1-mL syringe with a 27-gauge needle, as described in our previous study (Han et al., 2018). The blood was placed into heparinized tubes immediately after sampling and centrifuged at 10,000×g and 4°C for 4 min to collect the plasma. After blood collection and measurement, the liver was collected, snap frozen using liquid nitrogen, and then stored in plasma at −80°C until further analysis.
This study was performed according to the Guidelines for the Care and Use of Laboratory Animals prepared by the Institutional Animal Care and Use Committee of Nanjing Agricultural University (permit number SYXK (Su) 2011-0036).
Analysis of Free Amino Acids in the Plasma and Liver
The free amino acid concentrations in the liver and plasma were analyzed using a fully automatic amino acid analyzer (L-8080 type, Hitachi, Japan), according to the method described by Zhang et al. (2017). The liver samples were weighed and homogenized in a 5% sulfonic acid solution and left for deproteinization on ice. The plasma was only well mixed with a 5% sulfonic acid solution for deproteinization. After 30 min, the liver or plasma samples were centrifuged at 4°C and 20,000×g for 20 min. The supernatant was collected and filtered using a 0.22-µm filter (Biosharp, Guangzhou saiguo biotech Co., LTD, Guangzhou, China). The filtrate and standard solution were incorporated into the amino acid analyzer. The amino acid concentrations were expressed as pmol/mg of wet tissue in the liver and as pmol/µL in the plasma. Since the system applied here could not separate the L- and D-forms of amino acids, the results of the determined amino acids only used the names of the amino acids.
Statistical Analysis
The data were statistically analyzed using a two-way analysis of variance (ANOVA) to determine the main effects of in ovo administration and embryo development age, as well as of their interaction. When a significant interaction was detected, the post hoc analysis of Holm–Sidak's multiple comparisons test was applied to examine the interaction effect of in ovo L-Leu feeding and age on the dependent variables. Statistical analysis was conducted using GraphPad Prism 6 (GraphPad Software, Inc., San Diego, CA, USA). A P<0.05 was used to denote significant differences. All data in each group were first subjected to a Thompson's rejection test, as described by Kobayashi and Pillai (2013), to eliminate the outliers (P<0.01), and the remaining data were subjected to analysis among groups. All results are expressed as mean±standard error of mean (SEM). The number of embryos used for statistical analysis in each group is shown in the figure legends and table notes.
Results
Changes of Embryonic Weight and YolkWeight During Incubation
Egg, embryo, and yolk weight changes, as well as the relative weights of the embryo and yolk are shown in Figs. 1 and 2. During embryonic development, egg weight did not change. The embryonic weight was significantly increased by the age of the embryo, with a reduction in the yolk weight. However, in ovo administration of L-Leu significantly reduced embryonic weight but had no effect on yolk weight (Fig. 1). The results of relative embryo and yolk weights showed a similar tendency as those of their weight, and L-Leu in ovo injection also significantly lowered the relative embryonic weight but showed no effect on the relative yolk weight (Fig. 2).
Fig. 1.
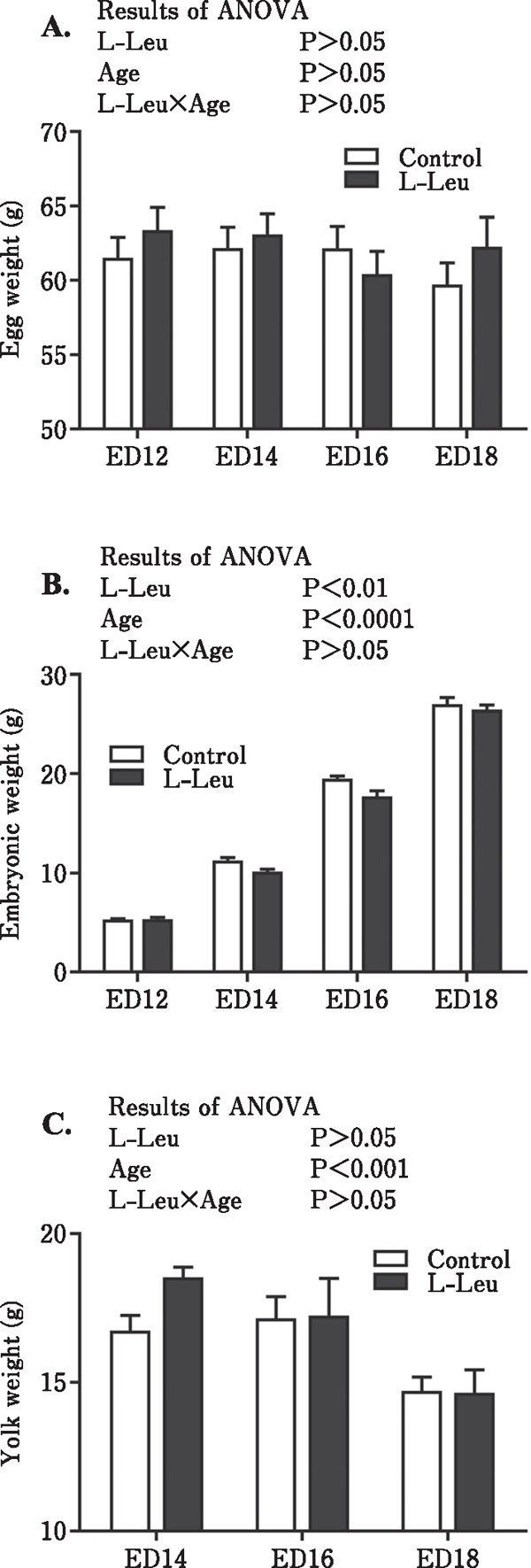
Effects of L-Leu in ovo injection on weight changes in egg (A), embryo (B), and yolk sac (C) during incubation. The number of chick embryos in each group was n=9 to 12. Values are represented as mean±SEM. L-Leu, L-leucine; ED, embryonic day.
Fig. 2.
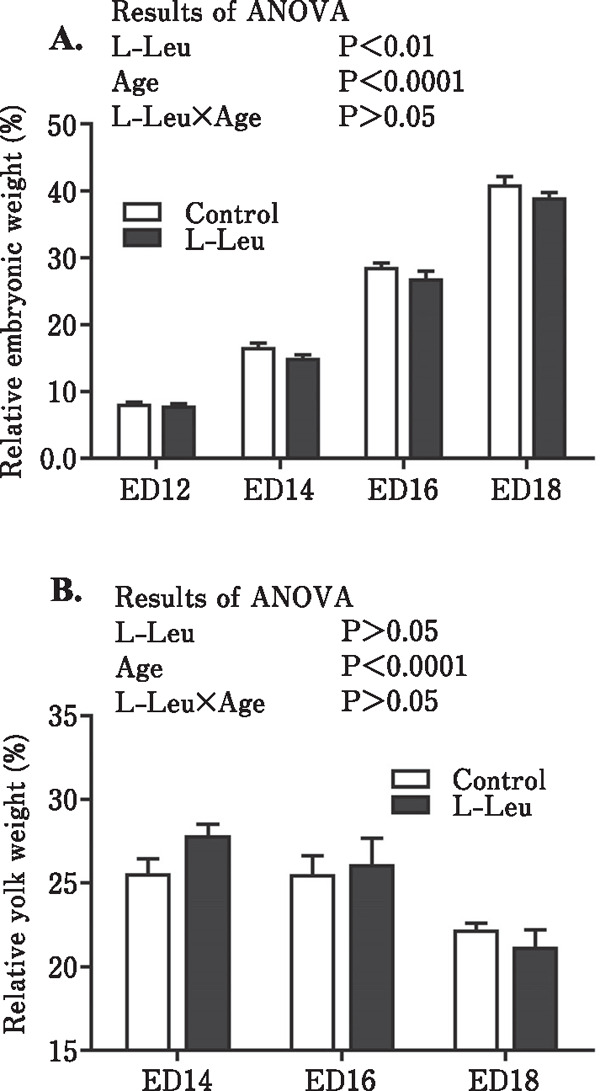
Effects of L-Leu in ovo injection on the relative weight changes of embryo (A) and yolk sac (B) during incubation. The number of chick embryos in each group was n=9 to 12. Values are represented as mean±SEM. L-Leu, L-leucine; ED, embryonic day.
Free Amino Acid Concentrations in the Plasma and Liver
The changes in free amino acid concentrations in the plasma and liver of broiler embryos are shown in Figs. 3 and 4 and Tables 1 and 2. Plasma tyrosine (Tyr) significantly (P<0.0001) decreased toward ED 18, but a significant interaction between L-Leu and age indicated that the decline did not occur so sharply in L-Leu in the in ovo-administered group compared to the control group (Fig. 3A). In ovo L-Leu administration caused a significant (P<0.01) increase in plasma lysine (Lys) concentration (Fig. 3B). The significant interaction between L-Leu and age that was observed in plasma Lys suggested that plasma Lys decreased with age in the control group; however, it increased with age in the L-Leu in ovo administration group (Fig. 3B). Embryonic development caused a significant (P<0.0001) increase in plasma proline (Pro) concentration during incubation (Fig. 3C). However, this increase was significantly (P<0.05) suppressed by L-Leu in ovo administration. In the liver, the significant interactions between L-Leu and age observed in Tyr (P<0.05) and Lys (P<0.01) concentrations, indicated that both amino acids decreased with the progression of age in the control group, but increased with age in the L-Leu in ovo administration group (Fig. 4). As shown in Tables 1 and 2, the concentrations of many amino acids in the plasma and liver were significantly increased by the embryonic age. However, only glycine (Gly) and Asp in the liver decreased with age. Notably, no effects of L-Leu in ovo administration were detected in these amino acids.
Fig. 3.
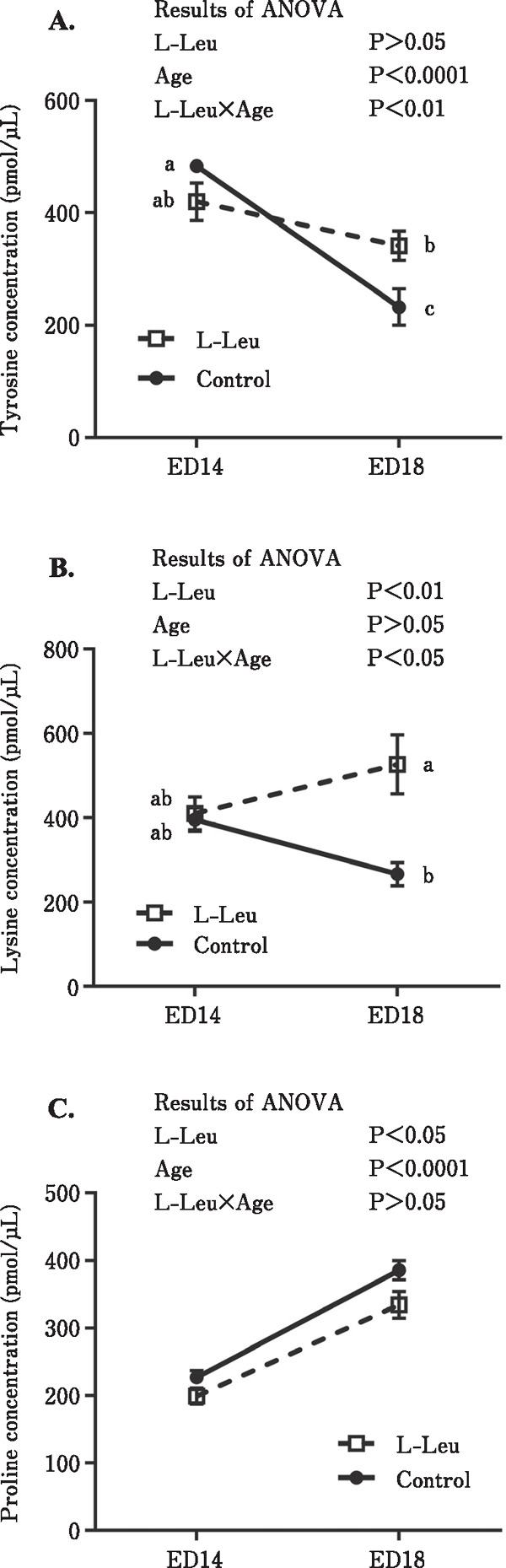
Effects of L-Leu in ovo injection on tyrosine (A), lysine (B), and proline (C) concentration changes in the plasma of chick embryos. The number of chick embryos in each group was n=6. Values are represented as mean±SEM. Different letters beside the symbols indicate significant (P<0.05) differences. L-Leu, L-leucine; ED, embryonic day.
Fig. 4.
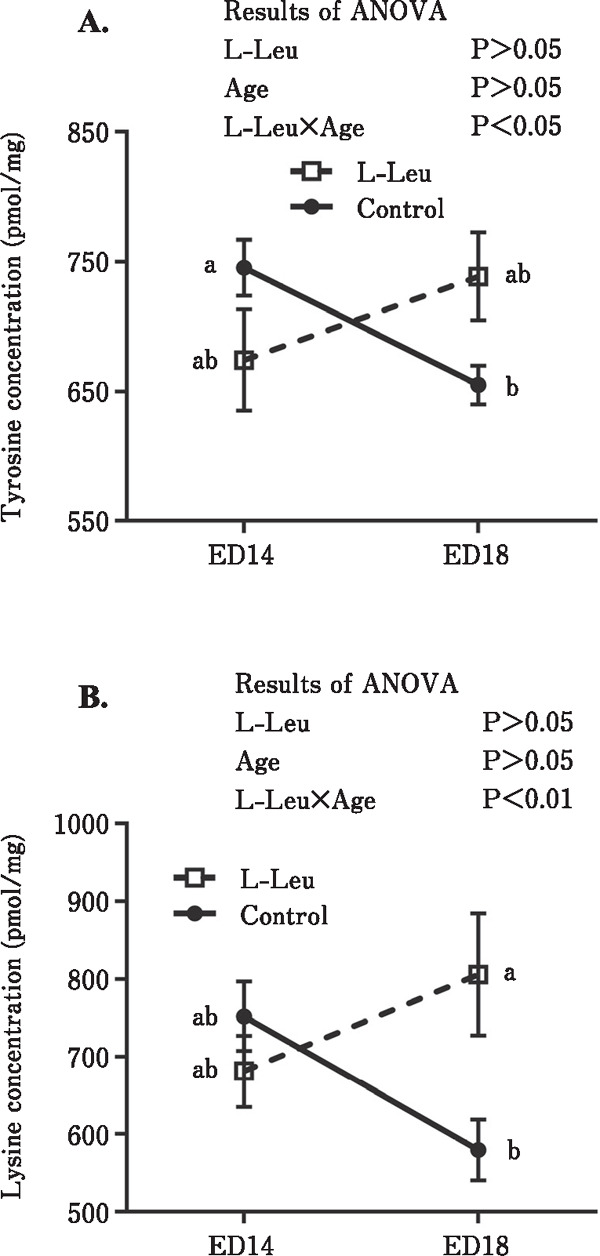
Effects of L-Leu in ovo injection on tyrosine (A) and lysine (B) concentration changes in the liver of chick embryos. The number of chick embryos in each group was n=6. Values are represented as mean±SEM. Different letters beside the symbols indicate significant (P<0.05) differences. L-Leu, L-leucine; ED, embryonic day.
Table 1. Plasma free amino acid concentration in chick embryos.
| Control | L-Leu | P value | |||||
|---|---|---|---|---|---|---|---|
| Amino acids | ED 14 | ED 18 | ED 14 | ED 18 | L-Leu | Age | L-Leu×Age |
| Histidine | 90±8 | 133±21 | 97±8 | 137±14 | NS | P<0.01 | NS |
| Threonine | 483±90 | 682±34 | 345±27 | 746±150 | NS | P<0.01 | NS |
| Valine | 449±16 | 611±37 | 450±39 | 528±27 | NS | P<0.001 | NS |
| Isoleucine | 219±10 | 218±24 | 235±16 | 237±5 | NS | NS | NS |
| Leucine | 175±9 | 199±24 | 199±12 | 216±14 | NS | NS | NS |
| Phenylalanine | 101±5 | 156±21 | 105±6 | 176±12 | NS | P<0.001 | NS |
| Methionine | 55±4 | 107±12 | 56±5 | 102±9 | NS | P<0.001 | NS |
| Lysine | 396±28ab | 266±27b | 410±39ab | 527±70a | P<0.01 | NS | P<0.05 |
| Tyrosine | 483±9a | 232±33c | 420±33ab | 341±26b | NS | P<0.0001 | P<0.01 |
| Proline | 227±10 | 386±14 | 199±12 | 335±20 | P<0.05 | P<0.0001 | NS |
| Arginine | 265±15 | 308±39 | 268±6 | 369±20 | NS | P<0.01 | NS |
| Glycine | 186±18 | 180±22 | 165±16 | 187±6 | NS | NS | NS |
| Aspartic acid | 13±2 | 16±3 | 13±4 | 15±2 | NS | NS | NS |
| Alanine | 273±17 | 318±37 | 245±18 | 328±7 | NS | P<0.05 | NS |
| Glutamic acid | 37±3 | 118±22 | 41±2 | 125±11 | NS | P<0.001 | NS |
| Cysteine | 43±4 | 102±12 | 38±3 | 94±9 | NS | P<0.001 | NS |
| Serine | 345±34 | 637±28 | 343±32 | 593±33 | NS | P<0.001 | NS |
Different superscripts in the same row indicate significant differences (P<0.05). The number of embryos used in each group was n=6. Values are represented as mean±SEM in pmol/µL. Control, sterile water injection; L-Leu, L-Leucine injection; ED, embryonic day; NS, not significant.
Table 2. Hepatic free amino acid concentration in chick embryos.
| Control | L-Leu | P value | |||||
|---|---|---|---|---|---|---|---|
| Amino acids | ED 14 | ED 18 | ED 14 | ED 18 | L-Leu | Age | L-Leu×Age |
| Histidine | 350±27 | 498±13 | 372±23 | 487±16 | NS | P<0.0001 | NS |
| Threonine | 2749±93 | 3149±417 | 2598±148 | 3514±56 | NS | P<0.05 | NS |
| Valine | 563±26 | 772±39 | 540±28 | 726±22 | NS | P<0.0001 | NS |
| Isoleucine | 218±12 | 244±13 | 213±5 | 258±11 | NS | P<0.01 | NS |
| Leucine | 559±34 | 681±22 | 539±28 | 719±30 | NS | P<0.0001 | NS |
| Phenylalanine | 320±15 | 381±13 | 309±10 | 385±14 | NS | P<0.0001 | NS |
| Methionine | 102±13 | 139±8 | 98±12 | 160±10 | NS | P<0.001 | NS |
| Lysine | 752±45ab | 579±39b | 681±46ab | 806±79a | NS | NS | P<0.01 |
| Tyrosine | 746±22a | 655±15b | 674±39ab | 739±34ab | NS | NS | P<0.05 |
| Proline | 234±25 | 367±14 | 205±1 | 305±5 | NS | P<0.001 | NS |
| Arginine | 380±21 | 506±28 | 367±26 | 547±34 | NS | P<0.0001 | NS |
| Glycine | 1228±55 | 951±10 | 1185±51 | 1000±29 | NS | P<0.0001 | NS |
| Aspartic acid | 2097±39 | 1310±91 | 1899±60 | 1476±114 | NS | P<0.0001 | P<0.05 |
| Alanine | 1094±25 | 1072±42 | 960±40 | 1175±74 | NS | NS | P<0.05 |
| Glutamic acid | 3071±133 | 2841±240 | 2960±106 | 3153±138 | NS | NS | NS |
| Serine | 1510±58 | 1637±96 | 1479±34 | 1650±73 | NS | P<0.05 | NS |
Different superscripts in the same row indicate significant differences (P<0.05). The number of embryos used in each group was n=6. Values are represented as mean±SEM in pmol/mg wet tissue. Control, sterile water injection; L-Leu, L-Leucine injection; ED, embryonic day; NS, not significant.
Discussion
In the current study, we administered L-Leu in ovo and examined the growth of embryos at different stages during incubation. The weight and relative weight of embryos were suppressed by L-Leu in ovo administration, especially during ED 14–18. Our previous results showed that the relative embryonic weight was significantly reduced by L-Leu in ovo administration on ED 18 and ED 19 (unpublished data). However, the body weight of neonatal chicks at hatching was not affected by L-Leu administration (Han et al., 2017), even with an increased dose of L-Leu (Han et al., 2019a). L-Leu in ovo administration stimulated oxygen consumption and heat production of embryos at ED 14. It was hypothesized that the in ovo administration of L-Leu might have stimulated the metabolic rate during the middle stages (Han et al., 2018) with a decline in the embryonic growth and metabolic rate at later stages of incubation. In the current study, a clearly lower level of embryonic weight was found at ED 14, 16, and 18, which suggested that the stimulation of L-Leu on metabolic activity might also have occurred on ED 12. Future studies will clarify the suppressed embryo growth by measuring the metabolic activity and growth-related hormones at different days after in ovo administration of L-Leu.
L-Leu is a branched chain amino acid (BCAA), and it has been demonstrated to improve muscle protein metabolism through the mTOR signaling pathway (Shimomura et al., 2015). Kita et al. (2015) reported that Leu in ovo injection caused an increase in the weight of whole embryos at ED 14 in layer chicks (Single Comb White Leghorn strain), in contrast with our current results in broiler chicks (Ross 308 strain). Similar results in turkey (hybrid converter strain) showed that in ovo injection of BCAAs on ED 22 reduced the (relative) weight of the embryo, but did not affect the yolk weight on ED 24 (Kop-Bozbay and Ocak, 2019). This difference could be explained by several decades of an intensive selection in chickens, which resulted in significant differences in growth, metabolite rate, and other physiological functions in embryos and chickens between layer and broiler strains (Havenstein et al., 2003; Sato et al., 2006; Druyan, 2010).
During embryogenesis, fatty acid oxidation contributes with more than 90% to the total energy requirements (Noble and Cocchi, 1990). The yolk is mainly the lipid source and is consumed by embryos during incubation. Leu was reported to increase the mitochondrial biogenesis (Duan et al., 2016) and hepatic mitochondrial function, and to be involved in fatty acid oxidation and lipid metabolism (Lindquist et al., 2017). L-Leu in ovo injection stimulated lipid metabolism and improved the metabolic activity on ED 14, as reported in a previous study (Han et al., 2018). However, in the current study, the yolk weight was not affected by L-Leu in ovo injection during embryogenesis, and the average relative yolk weight in the L-Leu group was higher than that in the control group on ED 14 (P=0.0495 using unpaired t-test between control and L-Leu groups, n=9/group). Future studies on the changes in L-Leu and its metabolites between ED 7 and ED 14 will be investigated to clarify this matter.
The free amino acid pool plays a critical role in embryonic growth. The different patterns of amino acid changes and lipid metabolism were related to the growth differences in broiler and layer embryos (Sato et al., 2006, 2009). In ovo injection of amino acids caused an increase in the growth of neonatal chicks by improving amino acid utilization and the development of digestive organs in embryos or chicks (Ohta et al., 1999; Gao et al., 2018). Most of the amino acid concentrations were increased and the free amino acid pool was enlarged during incubation to match the amino acid requirements for embryonic growth. This is in agreement with previous reports on broiler embryos (Ohta et al., 2004; Han et al., 2017). However, the levels of Ile, Leu, Gly, and Asp in the plasma did not significantly change with embryo development in the current study. Ohta et al. (2004) reported that the plasma Ile, Leu, Gly, and Asp concentrations increased with an increasing age in layer and broiler embryos. In ovo injection of an amino acid solution on ED 7 altered the plasma amino acid metabolism in neonatal chicks (Ohta et al., 2004), which suggested that the metabolism profiles of Ile, Leu, Gly, and Asp were altered by in ovo injected L-Leu, in this study. On the other hand, only Asp and Gly levels decreased, and that of glutamic acid did not change in the liver with the progress of embryonic age in the present study. This observation can be explained by uric acid synthesis, since uric acid synthesis in chicken embryos increases with the advancement of age (Fiske and Boyden, 1926) and these three amino acids are involved in uric acid synthesis.
In the current study, the amino acid profile was proposed to find some clues for L-Leu-mediated improvement of metabolism in the embryos of broiler chicks, as demonstrated in our previous studies (Han et al., 2018, 2019a, 2020). Tyr and Lys concentrations in the blood and liver of control embryos declined with embryonic development after ED 14 in the current study and in previous reports (Huether and Lajtha, 1991; Han et al., 2017). However, the changes in the concentration of Tyr and Lys were modified by L-Leu in ovo injection, resulting in an increase in Tyr and Lys levels in the blood and liver (unpaired t-test, P=0.0388 for Tyr in the liver) at ED 18. The developmental profiles of Tyr and Lys are almost identical in the blood and brain during embryogenesis (Huether and Lajtha, 1991). It could be predicted that the Tyr and Lys levels in the brain of the embryo might also be improved by L-Leu in ovo feeding at later stages of embryogenesis.
Tyr serves as a precursor of dopamine, epinephrine, and norepinephrine. Dopaminergic activity and norepinephrinergic metabolism were stimulated, and the Tyr concentration in the brain (mesencephalon) was also improved under fasting stress in neonatal chicks (Hamasu et al., 2012; Tran et al., 2015). The central administration of neuropeptide Y regulated monoamine metabolism and corticosterone response under heat stress, and reduced the body temperature without heat stress in chicks (Bahry et al., 2017). TM during incubation increased the Tyr level on ED 14 and reduced that of Tyr on ED 19 in broiler embryos (Han et al., 2017), affording thermotolerance in post-hatch chicks (Piestun et al., 2008). Thus, it could be hypothesized that the improved Tyr concentration may promote monoamine metabolism and influence brain function. The plasma Pro level was increased with embryo development (Ohta et al., 2004); however, the increase in plasma Pro was attenuated by L-Leu in ovo injection in the current study. Pro was reported to suppress dopamine and serotonin metabolism under stress conditions (Hamasu et al., 2009). The improved Tyr might be related to the reduced Pro via the alteration of monoamine metabolism. Future studies on monoamine analysis will be needed to clarify this matter.
In previous studies, Lys concentration was reduced via TM in the liver and brain of the embryo and decreased by an acute heat stress in the blood of broiler chicks (Han et al., 2017, 2019b). Moreover, in ovo administration of L-Leu caused an increase in hepatic and diencephalic Lys concentrations under acute heat stress in young broiler chicks (Han et al., 2019b), and improved the Lys concentration in the blood of broiler chickens after chronic heat stress (Han et al., 2020). Lys is an essential amino acid and the second limiting amino acid in broiler chicken diets based on corn and soybean. Dietary supplementation of Lys reduced the feed conversion ratio by increasing the plasma serotonin level in broiler chickens (Ishii et al., 2019). Both Leu and Lys are exclusively ketogenic amino acids, and their carbon skeletons can be broken down into acetyl-CoA and acetoacetate, a ketone body (Voet and Voet, 1995). L-Leu-mediated ketogenesis is expected to stimulate lipid metabolism during the middle stage of embryogenesis (Han et al., 2018). In the current study, the supplied L-Leu was totally catabolized before ED 14. Thus, the catabolism of supernumerary L-Leu might cause a reduction in Lys consumption during the middle stage of embryogenesis and increase Lys concentration at later stages. However, the reports about Lys supplementation in ovo are limited, and future studies on in ovo injection of Lys should be conducted to explore new functions of Lys.
In conclusion, L-Leu in ovo injection caused embryonic growth retardation and altered the metabolism of some amino acids in broiler embryos. Tyr and Lys may play potential roles in embryonic development. Unaffected Leu concentration in the blood and liver suggested that the injected L-Leu was totally metabolized before ED 14 and did not enhance the free amino acid pool.
Acknowledgments
This study was supported by the National Natural Science Foundation of China (No. 31772648) to C.L.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Bahry MA, Chowdhury VS, Yang H, Tran PV, Do PH, Han G, Ikeda H, Cockrem JF and Furuse M. Central administration of neuropeptide Y differentially regulates monoamines and corticosterone in heat-exposed fed and fasted chicks. Neuropeptides, 62: 93-100. 2017. [DOI] [PubMed] [Google Scholar]
- Chowdhury VS. Heat stress biomarker amino acids and neuropeptide afford thermotolerance in chicks. Journal of Poultry Science, 56: 1-11. 2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chowdhury VS, Han G, Bahry MA, Tran PV, Do PH, Yang H and Furuse M. L-Citrulline acts as potential hypothermic agent to afford thermotolerance in chicks. Journal of Thermal Biology, 69: 163-170. 2017. [DOI] [PubMed] [Google Scholar]
- Druyan S. The effects of genetic line (broilers vs. layers) on embryo development. Poultry Science, 89: 1457-1467. 2010. [DOI] [PubMed] [Google Scholar]
- Duan Y, Li F, Li Y, Tang Y, Kong X, Feng Z, Anthony TA, Watford M, Hou Y, Wu G and Yin Y. The role of leucine and its metabolites in protein and energy metabolism. Amino Acids, 48: 41-51. 2016. [DOI] [PubMed] [Google Scholar]
- Erwan E, Chowdhury VS, Nagasawa M, Goda R, Otsuka T, Yasuo S and Furuse M. Oral administration of D-aspartate, but not L-aspartate, depresses rectal temperature and alters plasma metabolites in chicks. Life Science, 109: 65-71. 2014. [DOI] [PubMed] [Google Scholar]
- Fiske CH and Boyden EA. Nitrogen metabolism in the chick embryo. Journal of Biological Chemistry, 70: 535-556. 1926. [Google Scholar]
- Gao T, Zhao M M, Li YJ, Zhang L, Li JL, Yu LL, Gao F and Zhou GH. Effects of in ovo feeding of L-arginine on the development of digestive organs, intestinal function and post-hatch performance of broiler embryos and hatchlings. Journal of Animal Physiology and Animal Nutrition, 102: e166-e175. 2018. [DOI] [PubMed] [Google Scholar]
- Hamasu K, Kabuki Y, Tomonaga S, Denbow DM and Furuse M. Changes in brain monoamine metabolism of neonatal chicks under two different acute stress conditions. British Poultry Science, 53: 145-149. 2012. [DOI] [PubMed] [Google Scholar]
- Hamasu K, Shigemi K, Kabuki Y, Tomonaga S, Denbow MD and Furuse M. Central l-proline attenuates stress-induced dopamine and serotonin metabolism in the chick forebrain. Neuroscience Letters, 460: 78-81. 2009. [DOI] [PubMed] [Google Scholar]
- Han G, Ouchi Y, Hirota T, Haraguchi S, Miyazaki T, Arakawa T, Masuhara N, Mizunoya W, Tatsumi R, Tashiro K, Bungo T, Furuse M and Chowdhury VS. Effects of L-leucine in ovo feeding on thermotolerance, growth and amino acid metabolism under heat stress in broilers. Animal, 14: 1701-1709. 2020. [Google Scholar]
- Han G, Yang H, Wang Y, Haraguchi S, Miyazaki T, Bungo T, Tashiro K, Furuse M and Chowdhury VS. L-Leucine increases the daily body temperature and affords thermotolerance in broiler chicks. Asian-Australasian Journal of Animal Sciences, 32: 842-848. 2019. a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Han G, Yang H, Wang Y, Zhang R, Tashiro K, Bungo T, Furuse M and Chowdhury VS. Effects of in ovo feeding of L-leucine on amino acids metabolism and heat-shock protein-70, and -90 mRNA expression in heat-exposed chicks. Poultry Science, 98: 1243-1253. 2019. b. [DOI] [PubMed] [Google Scholar]
- Han G, Yang H, Bungo T, Ikeda H, Wang Y, Nguyen LTT, Eltahan EM, Furuse M and Chowdhury VS. In ovo L-leucine administration stimulates lipid metabolisms in heat-exposed male, but not female, chicks to afford thermotolerance. Journal of Thermal Biology, 71: 74-82. 2018. [DOI] [PubMed] [Google Scholar]
- Han G, Yang H, Bahry MA, Tran PV, Do PH, Ikeda H, Furuse M and Chowdhury VS. L-Leucine acts as a potential agent in reducing body temperature at hatching and affords thermotolerance in broiler chicks. Comparative Biochemistry and Physiology Part A: Molecular & Integrative Physiology, 204: 48-56. 2017. [DOI] [PubMed] [Google Scholar]
- Havenstein GB, Ferket PR and Qureshi MA. Growth, livability, and feed conversion of 1957 versus 2001 broilers when fed representative 1957 and 2001 broiler diets. Poultry Science, 82: 1500-1508. 2003. [DOI] [PubMed] [Google Scholar]
- Huether G and Lajtha A. Changes in free amino acid concentrations in serum, brain, and CSF throughout embryogenesis. Neurochemical Research, 16: 145-150. 1991. [DOI] [PubMed] [Google Scholar]
- Ishii T, Shibata K, Kai S, Noguchi K, Hendawy AO, Fujimura S and Sato K. Dietary supplementation with lysine and threonine modulates the performance and plasma metabolites of broiler chicken. Journal of Poultry Science, 56: 204-211. 2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ito K, Erwan E, Nagasawa M, Furuse M and Chowdhury VS. Changes in free amino acid concentrations in the blood, brain and muscle of heat-exposed chicks. British Poultry Science, 55: 644-652. 2014. [DOI] [PubMed] [Google Scholar]
- Kadam MM, Bhanja SK, Mandal AB, Thakur R, Vasan P, Bhattacharyya A and Tyagi JS. Effect of in ovo threonine supplementation on early growth, immunological responses and digestive enzyme activities in broiler chickens. British Poultry Science, 49: 736-741. 2008. [DOI] [PubMed] [Google Scholar]
- Kita K, Ito KR, Sugahara M, Kobayashi M, Makino R, Takahashi N, Nakahara H, Takahashi K and Nishimukai M. Effect of in ovo administration of branched-chain amino acids on embryo growth and hatching time of chickens. Journal of Poultry Science, 52: 34-36. 2015. [Google Scholar]
- Kobayashi K and Pillai KS. A handbook of applied statistics in pharmacology. CRC Press, Boca Raton, FL, USA. 2013. [Google Scholar]
- Kop-Bozbay C and Ocak N. In ovo injection of branched-chain amino acids: Embryonic development, hatchability and hatching quality of turkey poults. Journal of Animal Physiology and Animal Nutrition, 103: 1135-1142. 2019. [DOI] [PubMed] [Google Scholar]
- Lin H, Jiao HC, Buyse J and Decuypere E. Strategies for preventing heat stress in poultry. World's Poultry Science Journal, 62: 71-86. 2006. [Google Scholar]
- Lindquist C, Bjorndal B, Rossmann CR, Tusubira D, Svardal A, Rosland GV, Tronstad KJ, Hallstrom S and Berge RK. Increased hepatic mitochondrial FA oxidation reduces plasma and liver TG levels and is associated with regulation of UCPs and APOC-III in rats. Journal of Lipid Research, 58: 1362-1373. 2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Loyau T, Métayer-Coustard S, Berri C, Crochet S, Cailleau-Audouin E, Sannier M, Chartrin P, Praud C, Hennequet-Antier C, Rideau N, Couroussé N, Mignon-Grasteau S, Everaert N, Duclos MJ, Yahav S, Tesseraud S and Collin A. Thermal manipulation during embryogenesis has long-term effects on muscle and liver metabolism in fast-growing chickens. PLoS One, 9: e105339. 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Noble RC and Cocchi M. Lipid metabolism and the neonatal chicken. Progress in Lipid Research, 29: 107-140. 1990. [DOI] [PubMed] [Google Scholar]
- Ohta Y, Yoshida T and Tsushima N. Comparison between broilers and layers for growth and protein use by embryos. Poultry Science, 83: 783-787. 2004. [DOI] [PubMed] [Google Scholar]
- Ohta Y, Tsushima N, Koide K, Kidd M and Ishibashi T. Effect of amino acid injection in broiler breeder eggs on embryonic growth and hatchability of chicks. Poultry Science, 78: 1493-1498. 1999. [DOI] [PubMed] [Google Scholar]
- Piestun Y, Shinder D, Ruzal M, Halevy O, Brake J and Yahav S. Thermal manipulations during broiler embryogenesis: effect on the acquisition of thermotolerance. Poultry Science, 87: 1516-1525. 2008. [DOI] [PubMed] [Google Scholar]
- Renaudeau D, Collin A, Yahav S, De Basilio V, Gourdine JL and Collier RJ. Adaptation to hot climate and strategies to alleviate heat stress in livestock production. Animal, 6: 707-728. 2012. [DOI] [PubMed] [Google Scholar]
- Sato M, Tomonaga S, Denbow DM and Furuse M. Changes in free amino acids in the brain during embryonic development in layer and broiler chickens. Amino Acids, 36: 303-308. 2009. [DOI] [PubMed] [Google Scholar]
- Sato M, Tachibana T and Furuse M. Heat production and lipid metabolism in broiler and layer chickens during embryonic development. Comparative Biochemistry and Physiology Part A: Molecular & Integrative Physiology, 143: 382-388. 2006. [DOI] [PubMed] [Google Scholar]
- Shimomura Y, Kitaura Y, Kadota Y, Ishikawa T, Kondo Y, Xu M, Ota M, Morishita Y, Bariuan JV and Zhen H. Novel physiological functions of branched-chain amino acids. Journal of Nutritional Science and Vitaminology, 61Suppl: S112-S114. 2015. [DOI] [PubMed] [Google Scholar]
- Tran PV, Chowdhury VS, Nagasawa M and Furuse M. Changes in free amino acid and monoamine concentrations in the chick brain associated with feeding behavior. Springer Plus, 4: 252. 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Voet D and Voet JG. Lipid metabolism. In: Biochemistry. 2nd ed. (Voet D and Voet JG eds.). pp. 662-762. John Wiley & Sons, Inc., USA. 1995. [Google Scholar]
- Wasti S, Sah N and Mishra B. Impact of heat stress on poultry health and performances, and potential mitigation strategies. Animals, 10: E1266. 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu G. Amino acids: metabolism, functions, and nutrition. Amino Acids, 37: 1-17. 2009. [DOI] [PubMed] [Google Scholar]
- Zaboli G, Huang X, Feng X and Ahn DU. How can heat stress affect chicken meat quality? - a review. Poultry Science, 98: 1551-1556. 2019. [DOI] [PubMed] [Google Scholar]
- Zhang S, Qiu W, Lu Q and Chen S. Determination of glutathione and free amino acids in muscles of four shellfish species by automatic amino acid analyzer. Food Science, 38: 170-176. 2017. (in Chinese with English abstract) [Google Scholar]


