Abstract
This cohort study investigates short-term reactions associated with COVID-19 vaccines among pregnant and lactating individuals vs individuals neither pregnant nor lactating but planning pregnancy.
Introduction
Vaccines against SARS-CoV-2 are highly effective in preventing COVID-19 illness.1,2 Research has found that COVID-19 is associated with adverse events in pregnancy,3 and recommendations therefore include offering SARS-CoV-2 vaccines to pregnant and lactating individuals, despite their lack of inclusion in initial clinical trials.4,5 To date, limited data on vaccine and pregnancy outcomes exist for SARS-CoV-2 vaccines in pregnancy and lactation.6 The objective of this study was to investigate experiences of pregnant and lactating individuals after receiving COVID-19 vaccines.
Methods
In January 2021, we launched an online prospective cohort study of adults primarily located in the United States who were pregnant, lactating, or planning pregnancy at the time of COVID-19 vaccination. This study was determined to be exempt from institutional review board review by the University of Washington (UW) Human Subjects Division (Common Rule category 2). We followed the Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) reporting guideline. Individuals who were recruited and enrolled online in the UW COVID-19 Vaccine in Pregnancy and Lactation Registry using chain-referral and snowball sampling were invited to participate. Electronic written informed consent was obtained, and self-reported demographic (including race and ethnicity), pregnancy, vaccination perception, and outcome data, including report of day 1 vaccine reactions, were entered via surveys into Research Electronic Data Capture (REDCap) software version 11.1.2 2021 (Vanderbilt University). Race and ethnicity data were collected to report diversity representation within our study population. Options for race and ethnicity were outlined following the Centers for Disease Control and Prevention’s National Health Interview Survey race and ethnicity categories. We performed statistical analysis (ie, χ2 tests and 1-way analysis of variance, odds ratios [ORs], and 95% CIs) using Stata statistical software version 16.1 (StataCorp). An α level of P ≤ .05, 2-sided, denoted significance. Data were analyzed from January through March 2021.
Results
As of March 16, 2021, 17 525 individuals (17 364 [99.7%] women among 17 418 individuals with sex data; mean [SD] age, 33.6 [3.6] years among 17 518 individuals with age data; 15 361 White individuals [87.6%] among all individuals) with known pregnancy status receiving at least 1 dose of a COVID-19 vaccine had enrolled in the study. Owing to missing data, percentages for participant characteristics are among those with data for that variable. There were 3 distinct groups: 7809 individuals who were pregnant (44.6%), 6815 individuals who were lactating (38.7%), and 2901 individuals who were neither pregnant nor lactating but planning pregnancy in the near future (16.5%) at the time of their first vaccine dose. Most individuals received the Pfizer-BioNTech BNT162b2 vaccine (10 790 of 17 431 individuals [61.9%] with data on vaccine type) or Moderna mRNA-1273 vaccine (6592 individuals [37.8%]). Most participants resided in the United States, were employed in health care, and had completed some college education (Table). Among all participants, 15 055 individuals (85.9%) reported receiving 2 doses.
Table. Baseline Characteristics Among 17 525 Participantsa.
| Characteristic | Pregnant individuals (n = 7809) | Lactating individuals (n = 6815) | Individuals planning pregnancyb (n = 2901) | P value | |||
|---|---|---|---|---|---|---|---|
| Totalc | No. (%) | Totalc | No. (%) | Totalc | No. (%) | ||
| Vaccine | |||||||
| Pfizer-BioNTech BNT162b2 | 7770 | 4777 (61.5) | 6775 | 4156 (61.3) | 2886 | 1857 (64.4) | .02 |
| Moderna mRNA-1273 | 2970 (38.2) | 2596 (38.3) | 1026 (35.6) | ||||
| Janssen JNJ-78436735 | 23 (0.3) | 23 (0.3) | 3 (0.1) | ||||
| Dose 1 to survey completion, mean (SD), d | 7565 | 25 (15.4) | 6452 | 27.8 (14.8) | 2833 | 31.2 (16.8) | <.001 |
| Dose 2 to survey completion, mean (SD), d | 6232 | 10.1 (10.6) | 5863 | 11.1 (11.7) | 2440 | 12.9 (11.9) | <.001 |
| Age, mean (SD), y | 7804 | 33.4 (3.6) | 6814 | 34.1 (3.7) | 2900 | 32.7 (3.5) | <.001 |
| Gravidity, mean (SD) | 5236 | 2.1 (1.3) | 4614 | 2.1 (1.3) | 1943 | 2.1 (1.3) | .56 |
| Parity, mean (SD) | 5245 | 1.2 (1.0) | 4619 | 1.2 (1.0) | 1948 | 1.2 (1.0) | .67 |
| Trimester of pregnancy at dose 1 | |||||||
| First | 7611 | 1822 (23.9) | NA | NA | NA | NA | NA |
| Second | 3694 (48.5) | NA | NA | ||||
| Third | 2095 (27.5) | NA | NA | ||||
| Race and ethnicityd | |||||||
| American Indian or Alaska Native | 7809 | 58 (0.7) | 6815 | 67 (1.0) | 2901 | 17 (0.6) | .09 |
| Asian | 640 (8.2) | 489 (7.2) | 183 (6.3) | .002 | |||
| Black or African American | 103 (1.3) | 104 (1.5) | 44 (1.5) | .53 | |||
| Native Hawaiian or other Pacific Islander | 34 (0.4) | 32 (0.5) | 7 (0.2) | .26 | |||
| White | 6802 (87.1) | 5883 (86.3) | 2676 (92.2) | <.001 | |||
| Othere | 118 (1.5) | 68 (1.0) | 45 (1.6) | .01 | |||
| Hispanic ethnicity | 7751 | 463 (6.0) | 6761 | 444 (6.6) | 2881 | 194 (6.7) | .21 |
| Education | |||||||
| Some college or less | 7756 | 281 (3.6) | 6761 | 377 (5.6) | 2885 | 121 (4.2) | <.001 |
| Bachelor's degree (eg, BA, AB, BS) | 1929 (24.9) | 1823 (27.0) | 899 (31.2) | ||||
| Master's degree | 2565 (33.1) | 2342 (34.6) | 1034 (35.8) | ||||
| Doctorate or professional degree | 2981 (38.4) | 2219 (32.8) | 831 (28.8) | ||||
| Area of employment | |||||||
| Health care | 7633 | 5310 (69.6) | 6539 | 4750 (72.6) | 2812 | 2205 (78.4) | <.001 |
| Academics or science | 823 (10.8) | 675 (10.3) | 228 (8.1) | ||||
| Teaching or childcare | 434 (5.7) | 343 (5.2) | 118 (4.2) | ||||
| Office work or tech industry | 406 (5.3) | 204 (3.1) | 76 (2.7) | ||||
| Otherf | 660 (8.6) | 567 (8.7) | 185 (6.6) | ||||
| Influenza vaccine this last season | 7755 | 7529 (97.1) | 6776 | 6461 (95.4) | 2878 | 2732 (94.9) | <.001 |
| Tdap vaccine in current or recent pregnancy | |||||||
| Yes | 7662 | 1995 (26.0) | 6755 | 6218 (92.0) | NA | NA | NA |
| Plan to get | 59 (0.8) | NA | NA | ||||
| Don’t know | 4698 (61.3) | 138 (2.0) | NA | ||||
| No | 910 (11.9) | 399 (5.9) | NA | ||||
| Should individuals receive the COVID-19 vaccine? | If pregnant? | If lactating? | If planning for pregnancy? | ||||
| Yes | 7456 | 6153 (82.5) | 6466 | 6080 (94.0) | 2669 | 2520 (94.4) | NA |
| Depends on circumstances | 1300 (17.4) | 382 (5.9) | 148 (5.6) | ||||
| No | 3 (0) | 4 (0.1) | 1 (0) | ||||
Abbreviations: NA, not applicable; Tdap, tetanus, diphtheria, and acellular pertussis.
All variables are based on pregnancy status at enrollment.
Participants in the planning group were neither pregnant nor lactating.
Totals differ from overall group numbers owing to missing data for some individuals.
Categories are not mutually exclusive. Missing numbers indicate participants who selected “Prefer not to answer.”
Participants could select other if they did not choose outlined race and ethnicity categories or in addition to other race and ethnicity categories. Those who selected other were given the opportunity to specify their own category.
Other includes military personnel, first responders, and individuals working in agriculture, manufacturing, construction, service, hospitality, retail industries, and other areas of employment.
Among all participants, 17 005 individuals (97.0%) reported any postvaccination reactions after the first dose, with the most common reactions being pain at injection site (16 019 individuals [91.4%]) and fatigue (5489 individuals [31.3%]). The frequency of reactions after the second dose was higher than after the first dose (eg, 10 399 individuals [69.2%] with fatigue after the second dose), but with similar distribution of symptoms (Figure). Odds of several reactions were statistically significantly decreased among individuals who were pregnant (eg, fever after BNT162b2 dose 2: OR, 0.44; 95% CI, 0.38-0.52; P < .001 and after mRNA-1273 dose 2: OR, 0.48; 95% CI, 0.40-0.57; P < .001) compared with individuals who were neither pregnant nor lactating (Figure). Mean (SD) maximum self-reported temperature was 38.1 (0.6) °C (100.6 [1.0] °F) among 499 participants with fever after dose 1 (including 131 pregnant individuals) and 38.2 (0.6) °C (100.7 [1.0] °F) among 3293 participants with fever after dose 2 (including 1051 pregnant individuals). Participants seeking medical care after vaccination included 100 individuals (0.6%) after dose 1 (including 50 pregnant individuals) and 221 individuals (1.5%) after dose 2 (including 156 pregnant individuals).
Figure. Reactions 1 Day Postvaccination.
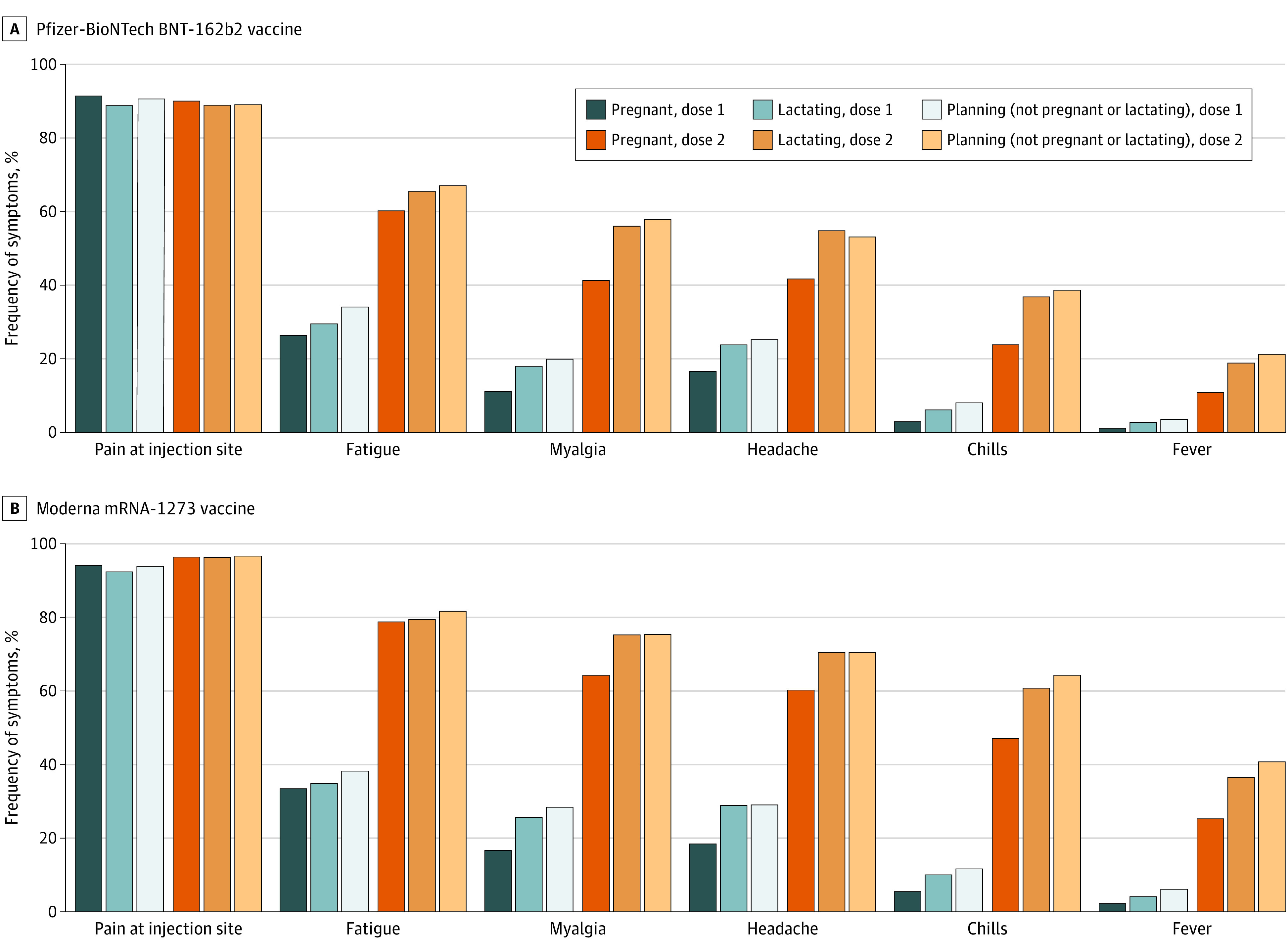
Among pregnant participants, any obstetrical symptoms were reported by 346 of 7809 individuals (4.4%) after the first dose and 484 of 6444 individuals (7.5%) after the second dose. Altogether, 6586 pregnant individuals (84.3%) had reported a second vaccine dose at the time of data analysis. Of these, 6244 individuals (94.8%) were still pregnant, while 288 individuals (4.3%) had delivered and 49 individuals (0.7%) reported miscarriages at the time of their second vaccine dose. Among lactating individuals, interrupted breastfeeding after vaccination was reported by 155 of 6815 individuals after the first dose (2.3%) and 130 of 6056 individuals after the second dose (2.2%), decreased milk supply for less than 24 hours by 339 individuals after the first dose (5.0%) and 434 individuals after the second dose (7.2%), and concerns about the infant after vaccination by 208 individuals after the first dose (3.0%) and 267 individuals after the second dose (4.4%).
Discussion
This large prospective cohort study found that COVID-19 vaccines were well-tolerated among individuals who were pregnant, lactating, or planning pregnancy. A strength of this study was the ability to compare vaccine reactions and perceptions in pregnant and lactating individuals vs individuals of similar age and fertility intentions who were neither pregnant nor lactating. Vaccination reactions for day 1 were similar among groups and comparable with findings among pregnant individuals previously reported.6 All groups reported increased reactions following dose 2 of BNT162b2 and mRNA-1273 vaccines.
Study limitations include that participants were drawn from a convenience sample with self-reported reactions and with limited perinatal outcome assessment, reflecting the first wave of vaccination, which largely consisted of health care workers owing to vaccine eligibility at the time of this ongoing study. As a result, our findings may be biased and not generalizable to all populations. In addition, there is potential participant overlap between our study and similar studies.6 Further studies are ongoing to investigate outcomes after receipt of COVID-19 vaccines among pregnant and lactating individuals.
References
- 1.Pfizer and BioNTech . Pfizer and BioNTech conclude Phase 3 Study of COVID-19 Vaccine Candidate, Meeting all primary efficacy endpoints. Accessed July 9, 2021. https://www.pfizer.com/news/press-release/press-release-detail/pfizer-and-biontech-conclude-phase-3-study-covid-19-vaccine
- 2.Moderna. Moderna announces primary efficacy analysis in phase 3 COVE Study for its COVID-19 Vaccine Candidate and Filing today with U.S. FDA for Emergency Use Authorization. Accessed July 9, 2021. https://investors.modernatx.com/news-releases/news-release-details/moderna-announces-primary-efficacy-analysis-phase-3-cove-study
- 3.Delahoy MJ, Whitaker M, O’Halloran A, et al. ; COVID-NET Surveillance Team . Characteristics and maternal and birth outcomes of hospitalized pregnant women with laboratory-confirmed COVID-19—COVID-NET, 13 states, March 1-August 22, 2020. MMWR Morb Mortal Wkly Rep. 2020;69(38):1347-1354. doi: 10.15585/mmwr.mm6938e1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.American College of Obstetricians and Gynecologists . COVID-19 vaccination considerations for obstetric–gynecologic care: practice advisory. Updated July 2, 2021. Accessed July 13, 2021. https://www.acog.org/clinical/clinical-guidance/practice-advisory/articles/2020/12/vaccinating-pregnant-and-lactating-patients-against-covid-19
- 5.Centers for Disease Control and Prevention . COVID-19 vaccines while pregnant or breastfeeding. Updated June 29, 2021. Accessed July 13, 2021. https://www.cdc.gov/coronavirus/2019-ncov/vaccines/recommendations/pregnancy.html
- 6.Shimabukuro TT, Kim SY, Myers TR, et al. ; CDC v-safe COVID-19 Pregnancy Registry Team . Preliminary findings of mRNA covid-19 vaccine safety in pregnant persons. N Engl J Med. 2021;384(24):2273-2282. doi: 10.1056/NEJMoa2104983 [DOI] [PMC free article] [PubMed] [Google Scholar]


