This economic evaluation assesses the cost-effectiveness of endoscopic screening for esophageal and gastric cancers in a hypothetical cohort of individuals aged 40 to 69 years in areas of China where the risk of upper gastrointestinal tract cancer is high.
Key Points
Question
Is endoscopic screening for esophageal and gastric cancers cost-effective in areas of China where the risk of these cancers is high?
Findings
In this model-based economic evaluation of a hypothetical closed cohort of 100 000 individuals aged 40 to 69 years from areas of China where the risk of upper gastrointestinal tract cancer is high, 5 endoscopic screening strategies with different frequencies had an incremental cost-effectiveness ratio of less than the per capita gross domestic product in China for each quality-adjusted life-year gained compared with no screening. Screening every 2 years would be the most cost-effective strategy.
Meaning
These findings suggest that combined endoscopic screening for esophageal cancer and gastric cancer would be cost-effective in areas of China where the risk of these cancers is high and that screening every 2 years would be the optimal strategy.
Abstract
Importance
Upper gastrointestinal tract cancer, including esophageal and gastric cancers, in China accounts for 50% of the global burden. Endoscopic screening may be associated with a decreased incidence of and mortality from upper gastrointestinal tract cancer.
Objective
To evaluate the cost-effectiveness of endoscopic screening for esophageal and gastric cancers among people aged 40 to 69 years in areas of China where the risk of these cancers is high.
Design, Setting, and Participants
For this economic evaluation, a Markov model was constructed for initial screening at different ages from a health care system perspective, and 5 endoscopic screening strategies with different frequencies (once per lifetime and every 10 years, 5 years, 3 years, and 2 years) were evaluated. The study was conducted between January 1, 2019, and October 31, 2020. Model parameters were estimated based on this project, government documents, and published literature. For each initial screening age (40-44, 45-49, 50-54, 55-59, 60-64, and 65-69 years), a closed cohort of 100 000 participants was assumed to enter the model and follow the alternative strategies.
Main Outcomes and Measures
Cost-effectiveness was measured by calculating the incremental cost-effectiveness ratio (ICER), and the willingness-to-pay threshold was assumed to be 3 times the per capita gross domestic product in China (US $10 276). Univariate and probabilistic sensitivity analyses were conducted to assess the robustness of model findings.
Results
The study included a hypothetical cohort of 100 000 individuals aged 40 to 69 years. All 5 screening strategies were associated with improved effectiveness by 1087 to 10 362 quality-adjusted life-years (QALYs) and increased costs by US $3 299 000 to $22 826 000 compared with no screening over a lifetime, leading to ICERs of US $1343 to $3035 per QALY. Screening at a higher frequency was associated with an increase in QALYs and costs; ICERs for higher frequency screening compared with the next-lower frequency screening were between US $1087 and $4511 per QALY. Screening every 2 years would be the most cost-effective strategy, with probabilities of 90% to 98% at 3 times the per capita gross domestic product of China. The model was the most sensitive to utility scores of esophageal cancer– or gastric cancer–related health states and compliance with screening.
Conclusions and Relevance
The findings suggest that combined endoscopic screening for esophageal and gastric cancers may be cost-effective in areas of China where the risk of these cancers is high; screening every 2 years would be the optimal strategy. These data may be useful for development of policies targeting the prevention and control of upper gastrointestinal tract cancer in China.
Introduction
The incidence of upper gastrointestinal tract cancer (UGIC), including esophageal cancer (EC) and gastric cancer (GC), is high in China, with an estimated 802 930 new cases (324 422 cases of EC and 478 508 cases of GC) and 674 924 deaths (301 135 due to EC and 373 789 due to GC) in 2020, accounting for approximately 50% of the global burden.1 However, the geographic distribution of UGIC is uneven in China, mortality rates in some areas being 2- to 3-fold higher than the national average.2,3,4
The prognosis of UGIC depends mainly on the disease stage at the time of diagnosis. In China, the overall 5-year survival rates for patients with EC and GC are 30.3% and 35.1%, respectively.5 However, the rates would be 86% and 90%, respectively, if disease were detected at an early stage.6,7 This potential for increased survival has provided justification for early detection programs. A series of nationwide screening programs has been established in several East Asian countries where the incidence rates are high. In Japan, radiographic screening for GC was developed in the 1960s, and a national screening program was established in 1983; currently, endoscopic screening every 2 to 3 years is usually recommended.8 South Korea introduced both radiographic screening and endoscopic screening for GC into national screening programs in 2000 and currently recommends endoscopic screening every 2 years.9 Over approximately the past 20 years, endoscopic examination has become a major screening method for UGIC because of its high accuracy.10 Several economic evaluation studies11,12,13,14,15,16 from South Korea, Singapore, Portugal, the US, and China showed that endoscopic screening for EC or GC was cost-effective compared with no screening. Another study17 conducted in the US suggested that the cost-effectiveness of combined endoscopic screening for EC and GC was comparable to that of funded screening programs for other cancers when it was integrated into the current colonoscopy screening program.
In China, several endoscopic screening programs for UGIC have been attempted in some areas with high incidence rates since 2005. Some observational studies18,19,20,21,22 have shown that endoscopic screening can reduce the incidence of and mortality associated with UGIC. To assess the feasibility and efficacy of endoscopic screening for EC and GC, a multicenter randomized controlled trial was launched in 2015, in which more than 140 000 persons aged 40 to 69 years were enrolled.23,24 A large amount of basic data has been obtained from this trial. The present study evaluated the cost-effectiveness of combined endoscopic screening for EC and GC in people aged 40 to 69 years in areas of China where the risk of these cancers is high. Furthermore, we evaluated the optimal initial age and frequency for screening.
Methods
This model-based economic evaluation was performed from the health care system perspective using TreeAge Pro (Healthcare Version) 2020 (TreeAge Software) and was conducted between January 1, 2019, and October 31, 2020. The project was registered with the Protocol Registration System in the Chinese Clinical Trial Registry and approved by the independent Ethics Committee of the National Cancer Center of China/Cancer Hospital, Chinese Academy of Medical Sciences. Because patient data were deidentified in the analysis, the requirement for informed consent was waived by the Ethics Committee of the National Cancer Center of China/Cancer Hospital, Chinese Academy of Medical Sciences. This evaluation followed the Consolidated Health Economic Evaluation Reporting Standards (CHEERS) reporting guideline.25
Markov Model
A Markov model was constructed for different initial screening ages (40-44, 45-49, 50-54, 55-59, 60-64, and 65-69 years) to simulate EC and GC progression and calculate related health and economic outcomes in a lifetime horizon. Five endoscopic screening strategies with different frequencies were considered, including once per lifetime and every 10 years, 5 years, 3 years, and 2 years. No screening was applied as a reference strategy. For each initial screening age, a closed cohort of 100 000 participants with a mean age of 42, 47, 52, 57, 62, or 67 years was assumed to enter the model and follow the alternative strategies.
The Markov model consisted of 26 health states. In addition to the normal and death states, series of EC and GC progression states were considered, and each progression state was divided into undetected and detected states. A posttreatment state was also considered separately from the states requiring medical treatment after diagnosis. Endoscopic screening, reexamination, and related treatment procedures followed the recommendations of the Chinese Expert Consensus on Screening and Endoscopic Diagnosis and Treatment of Early Esophageal Cancer/Gastric Cancer (2014 version).26,27 These recommendations were also followed in a randomized controlled trial by Chen et al.23 Details of the trial are given in the eMethods and eFigures 1 to 3 in the Supplement, details of the Markov model are given in eFigure 4 in the Supplement, and validation of the model is shown in eFigures 5 and 6 in the Supplement. Compliance with screening, reexamination, and treatment and complications associated with endoscopic screening were also considered in the model. The model was run with a 1-year cycle length and terminated when the mean age of the cohort reached 90 years. A half-cycle correction was applied.
Model Parameters
Compliance with endoscopic screening was determined to be 49% (range, 30%-80%) according to a pilot project concerning endoscopic screening for EC in several areas of China where the risk of EC is high (Table 1).18,28,29,30,31,32,33,34,35,36,37,38,39,40,41 Compliance with regular endoscopic reexamination among individuals who had mild esophageal dysplasia, moderate esophageal dysplasia, or low-grade gastric intraepithelial neoplasia detected on screening was assumed to be higher than compliance with endoscopic screening, which was 67% (range, 40%-90%) according to a previous report (Table 1).15 The model assumed that false-positive results would result in loss of quality of life but could be corrected with an endoscopic reexamination at an additional cost. The sensitivity and specificity of endoscopic examination for EC were 96% (range, 88%-99%) and 90% (range, 59%-100%), respectively, according to previous studies in areas in China where risk of EC is high.11,30,31 The sensitivity and specificity of endoscopic examination for GC were assumed to be 89% (range, 70%-98%) and 100% (range, 90%-100%), respectively, based on the published literature from other countries where risk for GC is high and other economic evaluation studies of GC8,12,32,33,34 because of the lack of available data in China. Clinically significant complications (eg, bleeding or perforation) are rare among individuals undergoing diagnostic endoscopic examination.42,43 The complication rate in the model was estimated to be 0.009% (range, 0%-0.2%) based on the trial by Chen et al23,24 and other published studies (Table 2).32,33,35
Table 1. Estimates of Parameters Used in the Model.
| Parameter | Base-case value | Range | Distribution | Source |
|---|---|---|---|---|
| Compliance with screening | 0.49 | 0.30-0.80 | Triangular (0.30, 0.49, 0.80) | Wei et al,18 2015; Feng et al,28 2015; Wang et al,29 2015 |
| Compliance with reexamination | 0.67 | 0.40-0.90 | Triangular (0.40, 0.67, 0.90) | Yang et al,15 2015 |
| Endoscopic examination characteristics | ||||
| Sensitivity for EC | 0.96 | 0.88-0.99 | Triangular (0.88, 0.96, 0.99) | Chang et al,11 2012; Dawsey et al,30 1998; Nagami et al,31 2014 |
| Specificity for EC | 0.90 | 0.59-1.00 | Triangular (0.59, 0.90, 1.00) | Chang et al,11 2012; Dawsey et al,30 1998; Nagami et al,31 2014 |
| Sensitivity for GC | 0.89 | 0.70-0.98 | Triangular (0.70, 0.89, 0.98) | Zhou et al,12 2013; Hamashima et al,8 2018; Yeh et al,32 2016; Lee et al,33 2007; Hamashima et al,34 2013 |
| Specificity for GC | 1.00 | 0.90-1.00 | Triangular (0.90, 1.00, 1.00) | Zhou et al,12 2013; Hamashima et al,8 2018; Yeh et al,32 2016; Lee et al,33 2007; Hamashima et al,34 2013 |
| Endoscopic examination complications | 0.00009 | 0-0.002 | Triangular (0, 0.00009, 0.002) | Zeng et al,24 2020; Yeh et al,32 2016; Lee et al,33 2007; Espino et al,35 2012 |
| Annual self-initiated examination | ||||
| Severe esophageal dysplasia and CIS or HGIN and CIS | 0.01 | 0.005-0.02 | Triangular (0.005, 0.01, 0.02) | Chang et al,11 2012 |
| Early EC or GC | 0.20 | 0.10-0.40 | Triangular (0.10, 0.20, 0.40) | Chang et al,11 2012 |
| Advanced EC or GC | 0.70 | 0.56-0.90 | Triangular (0.56, 0.70, 0.90) | Chang et al,11 2012 |
| Compliance with treatment | ||||
| Severe esophageal dysplasia or CIS | 0.7458 | 0.5625-0.9654 | β (31.72, 10.81) | Chen et al,23 2017 |
| Early EC | 0.9405 | 0.7149-1.0000 | β (8.17, 0.52) | Chen et al,23 2017 |
| Advanced EC | 0.9643 | 0.8393-1.0000 | β (16.99, 0.63) | Chen et al,23 2017 |
| HGIN or CIS | 0.5455 | 0.4425-0.7746 | β (92.43, 77.01) | Chen et al,23 2017 |
| Early GC | 0.9000 | 0.6792-1.0000 | β (12.41, 1.38) | Chen et al,23 2017 |
| Advanced GC | 0.9643 | 0.8393-1.0000 | β (16.99, 0.63) | Chen et al,23 2017 |
| Costs, $ | ||||
| Screening mobilization and administration per capita | 1.05 | ±50% | γ (0.16, 0.15) | Chen et al,23 2017 |
| Endoscopic examination | 47.87 | ±50% | γ (46.57, 0.97) | Chen et al,23 2017 |
| Treatment for endoscopic complications | 113.68 | ±50% | γ (5.82, 0.05) | Chen et al,23 2017 |
| Initial treatment | ||||
| Severe esophageal dysplasia or CIS | 1604 | ±50% | γ (3.33, 0.002) | Yang et al,36 2018 |
| Early EC | 7732 | ±50% | γ (2.33, 3.01) | Yang et al,36 2018 |
| Advanced EC | 7320 | ±50% | γ (3.33, 4.55) | Yang et al,36 2018 |
| HGIN or CIS | 1423 | ±50% | γ (1.41, 9.88) | Yang et al,36 2018 |
| Early GC | 7548 | ±50% | γ (4.61, 6.11) | Yang et al,36 2018 |
| Advanced GC | 7086 | ±50% | γ (5.96, 8.41) | Yang et al,36 2018 |
| Annual health care | ||||
| Severe esophageal dysplasia or CIS | 216 | ±50% | γ (1.22, 0.006) | Yang et al,36 2018 |
| Early EC | 367 | ±50% | γ (1.23, 0.003) | Yang et al,36 2018 |
| Advanced EC | 342 | ±50% | γ (2.05, 0.006) | Yang et al,36 2018 |
| HGIN or CIS | 243 | ±50% | γ (1.24, 0.005) | Yang et al,36 2018 |
| Early GC | 409 | ±50% | γ (1.18, 0.003) | Yang et al,36 2018 |
| Advanced GC | 435 | ±50% | γ (1.19, 0.003) | Yang et al,36 2018 |
| Utility scores | ||||
| Mild esophageal dysplasia | 1.00 | 0.98-1.00 | Triangular (0.98, 1.00, 1.00) | Sharaiha et al,37 2014; Inadomi et al,38 2009 |
| Moderate esophageal dysplasia | 1.00 | 0.98-1.00 | Triangular (0.98, 1.00, 1.00) | Sharaiha et al,37 2014; Inadomi et al,38 2009 |
| Severe esophageal dysplasia or CIS | 0.84 | 0.79-0.89 | β (3.57, 0.68) | Liu et al,39 2018 |
| Early EC | 0.70 | 0.66-0.74 | β (2.63, 1.13) | Liu et al,39 2018 |
| Advanced EC | 0.61 | 0.56-0.66 | β (1.12, 0.71) | Liu et al,39 2018 |
| LGIN | 1.00 | 0.98-1.00 | Triangular (0.98, 1.00, 1.00) | Sharaiha et al,37 2014; Inadomi et al,38 2009 |
| HGIN or CIS | 0.92 | 0.86-0.99 | β (2.53, 0.22) | Xia et al,40 2020 |
| Early GC | 0.75 | 0.71-0.78 | β (3.15, 1.05) | Xia et al,40 2020 |
| Advanced GC | 0.57 | 0.53-0.62 | β (1.35, 1.02) | Xia et al,40 2020 |
| Discount rate | 0.05 | 0-0.08 | NA | Weinstein et al,41 1996 |
Abbreviations: CIS, carcinoma in situ; EC, esophageal cancer; GC, gastric cancer; HGIN, high-grade intraepithelial neoplasia; LGIN, low-grade intraepithelial neoplasia; NA, not applicable.
Table 2. Base-Case Cost-effectiveness Results Compared Among Different Strategies by Initial Screening Age Among 100 000 Cohort Membersa.
| Initial screening age, strategy | QALYs | Incremental QALYs | Cost ($, thousand) | Incremental cost ($, thousand) | ICER ($/QALY) | |||
|---|---|---|---|---|---|---|---|---|
| Vs no screening | Vs the next most effective strategyb | Vs no screening | Vs the next most effective strategyb | Vs no screening | Vs the next most effective strategyb | |||
| 40-44 y | ||||||||
| No screening | 1 659 260 | NA | NA | 25 035 | NA | NA | NA | NA |
| Screening once per lifetime | 1 660 347 | 1087 | 1087 | 28 334 | 3299 | 3299 | 3035 | 3035 |
| Screening every 10 y | 1 663 677 | 4417 | 3330 | 31 954 | 6919 | 3620 | 1566 | 1087 |
| Screening every 5 y | 1 666 345 | 7085 | 2668 | 36 707 | 11 672 | 4753 | 1647 | 1781 |
| Screening every 3 y | 1 668 371 | 9111 | 2026 | 42 218 | 17 183 | 5511 | 1886 | 2720 |
| Screening every 2 y | 1 669 622 | 10 362 | 1251 | 47 861 | 22 826 | 5643 | 2203 | 4511 |
| 45-49 y | ||||||||
| No screening | 1 572 532 | NA | NA | 26 817 | NA | NA | NA | NA |
| Screening once per lifetime | 1 574 161 | 1629 | 1629 | 30 347 | 3530 | 3530 | 2167 | 2167 |
| Screening every 10 y | 1 576 941 | 4409 | 2780 | 33 961 | 7144 | 3614 | 1620 | 1300 |
| Screening every 5 y | 1 579 145 | 6613 | 2204 | 37 745 | 10 928 | 3784 | 1653 | 1717 |
| Screening every 3 y | 1 580 974 | 8442 | 1829 | 42 531 | 15 714 | 4786 | 1861 | 2617 |
| Screening every 2 y | 1 582 334 | 9802 | 1360 | 48 190 | 21 373 | 5659 | 2180 | 4161 |
| 50-54 y | ||||||||
| No screening | 1 468 506 | NA | NA | 30 229 | NA | NA | NA | NA |
| Screening once per lifetime | 1 470 890 | 2384 | 2384 | 34 142 | 3913 | 3913 | 1641 | 1641 |
| Screening every 10 y | 1 472 583 | 4077 | 1693 | 36 373 | 6144 | 2231 | 1507 | 1318 |
| Screening every 5 y | 1 474 773 | 6267 | 2190 | 40 356 | 10 127 | 3983 | 1616 | 1819 |
| Screening every 3 y | 1 476 325 | 7819 | 1552 | 44 120 | 13 891 | 3764 | 1777 | 2425 |
| Screening every 2 y | 1 477 651 | 9145 | 1326 | 49 010 | 18 781 | 4890 | 2054 | 3688 |
| 55-59 y | ||||||||
| No screening | 1 342 830 | NA | NA | 36 095 | NA | NA | NA | NA |
| Screening once per lifetime | 1 346 031 | 3201 | 3201 | 40 491 | 4396 | 4396 | 1373 | 1373 |
| Screening every 10 y | 1 347 264 | 4434 | 1233 | 42 811 | 6716 | 2320 | 1515 | 1882 |
| Screening every 5 y | 1 348 747 | 5917 | 1483 | 45 240 | 9145 | 2429 | 1546 | 1638 |
| Screening every 3 y | 1 350 365 | 7535 | 1618 | 49 171 | 13 076 | 3931 | 1735 | 2430 |
| Screening every 2 y | 1 351 491 | 8661 | 1126 | 52 843 | 16 748 | 3672 | 1934 | 3261 |
| 60-64 y | ||||||||
| No screening | 1 195 742 | NA | NA | 45 876 | NA | NA | NA | NA |
| Screening once per lifetime | 1 199 618 | 3876 | 3876 | 51 081 | 5205 | 5205 | 1343 | 1343 |
| Screening every 5 y | 1 201 091 | 5349 | 1473 | 53 970 | 8094 | 2889 | 1513 | 1961 |
| Screening every 3 y | 1 202 293 | 6551 | 1202 | 56 498 | 10 622 | 2528 | 1621 | 2103 |
| Screening every 2 y | 1 203 210 | 7468 | 917 | 58 779 | 12 903 | 2281 | 1728 | 2487 |
| 65-69 y | ||||||||
| No screening | 1 025 119 | NA | NA | 60 442 | NA | NA | NA | NA |
| Screening once per lifetime | 1 029 058 | 3939 | 3939 | 67 233 | 6791 | 6791 | 1724 | 1724 |
| Screening every 2 y | 1 030 594 | 5475 | 1536 | 70 810 | 10 368 | 3577 | 1894 | 2329 |
Abbreviations: ICER, incremental cost-effectiveness ratio; NA, not applicable; QALY, quality-adjusted life-year.
QALYs, costs, and ICERs are expressed as the values in 2019.
Compared with the next most effective strategy at the same initial screening age.
The prevalence rates of EC- and GC-related health states were estimated based on baseline screening reports from the trial by Chen et al23 in areas where the risk of these cancers is high (eTable 1 in the Supplement); these reports were used to determine initial distributions of cohort members across health states in the model. A wide range was set for each rate to cover the values reported in these areas by referring to previous studies from China.29,44,45,46 The annual transition probabilities were derived from published observational studies concerning the natural history of EC and GC and economic evaluation studies of EC and GC (eTable 2 in the Supplement).15,47,48,49
The model assumed that in the absence of active screening, individuals with severe esophageal dysplasia and carcinoma in situ, high-grade gastric intraepithelial neoplasia and carcinoma in situ, or EC or GC would receive a diagnosis on the basis of self-initiated examinations according to state-specific probabilities (Table 1).11 Individuals with these diagnoses would receive state-specific treatments, whereas individuals who did not receive these diagnoses would remain untreated. The rates of state-specific compliance with treatment were calculated for the proportion of screened patients who actually completed the entire treatment procedure in areas where the risk of UGIC is high in the trial by Chen et al23 (eTable 3 in the Supplement). The state-specific probabilities of recurrence after treatment and cancer-related mortality rates for advanced EC and GC were estimated based on the survival rates among patients with EC and GC (eTable 2 in the Supplement),15 whereas age-specific natural background death rates were obtained from the China Population & Employment Statistics Yearbook, 2019.50
Costs were converted from Chinese renminbi to 2019 US dollars (US $1 = ¥6.8968 in 2019). The cost of screening included screening mobilization and administration costs, endoscopic examination costs, and costs of treatment for endoscopic complications, all of which were obtained from the 7 study centers that participated in the trial by Chen et al23 (eTable 4 in the Supplement). The cost of EC- and GC-related treatment included the initial treatment cost after detection of the cancer and the subsequent annual health care cost after treatment, both of which were obtained from the survey included in the trial by Chen et al23 that was administered to assess the economic burden of UGIC in China.36 Calculation details are shown in eTable 5 in the Supplement.
The health outcome was utility-weighted life expectancy expressed as quality-adjusted life-years (QALYs). Utility scores of EC- and GC-related health states were obtained from the survey included in the trial by Chen et al23 that was administered to assess the quality of life of patients with UGIC in China (eTable 6 in the Supplement).39,40 Considering that patients diagnosed with mild esophageal dysplasia, moderate esophageal dysplasia, and low-grade gastric intraepithelial neoplasia do not have symptoms, we used a utility score of 1 in the base-case analysis and set a range of 0.98 to 1 in the sensitivity analyses.37,38 We assumed a discount rate of 5% (range, 0%-8%) for both QALYs and costs.41 The choice of distribution for all parameters was based on consideration of the properties of the parameters and the data informing the parameters.
Statistical Analysis
Effectiveness and Cost-effectiveness Evaluation
Expected QALYs and costs of each strategy were obtained from the model. We evaluated the cost-effectiveness of screening strategies at different initial screening ages with 2 approaches. First, we calculated the incremental cost-effectiveness ratio (ICER), defined as incremental costs per QALY gained of each screening strategy compared with no screening. Second, we calculated the ICER of each screening strategy compared with the next most effective screening strategy to identify the optimal strategy at each initial screening age. Because of the lack of data regarding the willingness to pay in the population of China, we adopted the cost-effectiveness definition used by the World Health Organization. Highly cost-effective was defined as an ICER less than 1 time the per capita gross domestic product (GDP) in China; cost-effective, an ICER of 1 to 3 times the per capita GDP; and not cost-effective, and an ICER greater than 3 times the per capita GDP.51 The per capita GDP in China in 2019 was US $10 276.
Uncertainty and Sensitivity Analyses
Univariate sensitivity analyses of all parameters within their respective ranges were performed to identify the main sensitivity parameters. Probabilistic sensitivity analyses were further conducted to determine the probability of each screening strategy being cost-effective compared with no screening and the probability of each strategy being optimal compared with all other strategies.
Results
Base-Case Results
The study included a hypothetical sample of 100 000 individuals aged 40 to 69 years. All 5 screening strategies increased QALYs and costs compared with no screening at the same initial screening age (Table 2) by 1087 to 10 362 QALYs and US $3 299 000 to $22 826 000 for a cohort of 100 000 participants over a lifetime, and the corresponding ICERs were US $1343 to $3035 per QALY, which were lower than the per capita GDP ($10 276). Therefore, all screening strategies would be more cost-effective than no screening.
Further comparisons of the screening strategies were performed, and more frequent screening would be associated with a gain of more QALYs but with higher costs (Table 2). When a screening strategy was compared with the next most effective screening strategy in the cohort with the same initial screening age, 917 to 3939 QALYs would be gained and an additional cost of US $2 231 000 to $6 791 000 gained for a cohort of 100 000 participants over a lifetime; the corresponding ICERs were US $1087 to $4511 per QALY, which was lower than the per capita GDP. Therefore, the high-frequency screening strategy would be more cost-effective than the low-frequency screening strategy, and screening every 2 years would be the optimal strategy at each initial screening age. Screening every 2 years and screening once per lifetime had the most incremental QALYs at the initial screening ages of 40 to 44 years and 65 to 69 years, respectively.
Sensitivity Analysis Results
Univariate sensitivity analyses revealed that the results remained largely unchanged over the plausible range of each parameter. The ICER upper limits of the most sensitive parameters of each screening strategy at different initial screening ages are shown in Table 3. When a screening strategy was compared with no screening, only varying utility scores of EC- and GC-related health states resulted in ICERs exceeding the per capita GDP at initial screening ages of 40 to 44 years and 65 to 69 years. When a screening strategy was compared with the next most effective screening strategy, varying utility scores of EC- and GC-related health states resulted in ICERs at some initial screening ages exceeding 3 times the per capita GDP, and varying compliance with screening resulted in ICERs of screening every 2 years vs screening every 3 years exceeding the per capita GDP. Univariate sensitivity analyses demonstrated the stability of the cost-effectiveness and ranking of the cost-effectiveness of the screening strategies.
Table 3. Upper Limits of Incremental Cost-effectiveness Ratio for Each Screening Strategy in Univariate Sensitivity Analyses by Initial Screening Agea.
| Initial screening age and screening strategy | Utility scoresb | Compliance with screening | Discount rate | Prevalences of EC- and GC-related health statesb | Costs of screeningc | Annual transition probability from severe esophageal dysplasia or CIS to early EC | ||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Vs no screening | Vs the next most effective strategy | Vs no screening | Vs the next most effective strategy | Vs no screening | Vs the next most effective strategy | Vs no screening | Vs the next most effective strategy | Vs no screening | Vs the next most effective strategy | Vs no screening | Vs the next most effective strategy | |
| 40-44 y | ||||||||||||
| Screening once per lifetime | 11 778d | 11 778d | 4016 | 4016 | 6671 | 6671 | 4711 | 4711 | 4422 | 4422 | 4592 | 4592 |
| Screening every 10 y | 4498 | 2877 | 1883 | 1271 | 3440 | 2144 | 1737 | 1129 | 2272 | 1570 | 2186 | 1658 |
| Screening every 5 y | 5116 | 6395 | 1845 | 2277 | 3467 | 3513 | 1811 | 1927 | 2397 | 2603 | 2308 | 2513 |
| Screening every 3 y | 6202 | 11 273d | 2085 | 5204 | 3871 | 5260 | 2082 | 3003 | 2758 | 4022 | 2609 | 3659 |
| Screening every 2 y | 7452 | 19 296d | 2619 | 13 156d | 4480 | 8663 | 2451 | 5127 | 3240 | 6747 | 2996 | 5740 |
| 45-49 | ||||||||||||
| Screening once per lifetime | 6153 | 6153 | 2760 | 2760 | 4486 | 4486 | 3448 | 3448 | 3092 | 3092 | 2677 | 2677 |
| Screening every 10 y | 5236 | 4571 | 1912 | 1473 | 3248 | 2358 | 1934 | 1389 | 2304 | 1842 | 2259 | 1986 |
| Screening every 5 y | 5310 | 5455 | 1853 | 2155 | 3246 | 3241 | 1949 | 1979 | 2370 | 2502 | 2284 | 2333 |
| Screening every 3 y | 6217 | 10 190 | 2020 | 4874 | 3583 | 4782 | 2206 | 3090 | 2687 | 3830 | 2539 | 3455 |
| Screening every 2 y | 7678 | 22 118d | 2552 | 10 504d | 4128 | 7457 | 2605 | 5002 | 3163 | 6125 | 2936 | 5387 |
| 50-54 y | ||||||||||||
| Screening once per lifetime | 3976 | 3976 | 1674 | 1974 | 3216 | 3216 | 2609 | 2609 | 2296 | 2296 | 2134 | 2134 |
| Screening every 10 y | 4253 | 4844 | 1564 | 1409 | 2897 | 2367 | 2010 | 1479 | 2122 | 1876 | 2069 | 1972 |
| Screening every 5 y | 5210 | 7982 | 1850 | 2752 | 2998 | 3197 | 2091 | 2219 | 2292 | 2609 | 2238 | 2560 |
| Screening every 3 y | 5803 | 8366 | 2233 | 6715 | 3252 | 4255 | 2320 | 3550 | 2542 | 3197 | 2424 | 3161 |
| Screening every 2 y | 7025 | 17 484d | 2821 | 12 807d | 3695 | 6278 | 2710 | 4887 | 2956 | 5401 | 2768 | 4787 |
| 55-59 y | ||||||||||||
| Screening once per lifetime | 3271 | 3271 | 1394 | 1394 | 2601 | 2601 | 2127 | 2127 | 1882 | 1882 | 1920 | 1920 |
| Screening every 10 y | 4841 | 52 896e | 1527 | 2043 | 2746 | 3194 | 2151 | 2191 | 2084 | 2608 | 2194 | 2954 |
| Screening every 5 y | 5026 | 5620 | 1680 | 2573 | 2777 | 2868 | 2186 | 2277 | 2155 | 2366 | 2210 | 2258 |
| Screening every 3 y | 6162 | 13 002d | 2076 | 5658 | 3046 | 4045 | 2476 | 3421 | 2443 | 3496 | 2455 | 3346 |
| Screening every 2 y | 6920 | 12 320d | 2523 | 13 669d | 3357 | 5377 | 2814 | 5008 | 2745 | 4770 | 2694 | 4255 |
| 60-64 y | ||||||||||||
| Screening once per lifetime | 3635 | 3635 | 1360 | 1360 | 2476 | 2476 | 1995 | 1995 | 1794 | 1794 | 2054 | 2054 |
| Screening every 5 y | 5310 | 31 253e | 1566 | 2611 | 2697 | 3324 | 2235 | 2736 | 2055 | 2741 | 2324 | 3033 |
| Screening every 3 y | 6082 | 11 374d | 1805 | 4871 | 2847 | 3515 | 2448 | 3321 | 2228 | 3001 | 2459 | 3044 |
| Screening every 2 y | 6492 | 9464 | 2076 | 11 769d | 3011 | 4159 | 2683 | 4334 | 2399 | 3621 | 2586 | 3459 |
| 65-69 y | ||||||||||||
| Screening once per lifetime | 8653 | 8653 | 1741 | 1741 | 3128 | 3128 | 2375 | 2375 | 2185 | 2185 | 3053 | 3053 |
| Screening every 2 y | 14 302d | 25 436d | 2019 | 4801 | 3372 | 4011 | 2747 | 3644 | 2456 | 3153 | 3313 | 3959 |
Abbreviations: CIS, carcinoma in situ; EC, esophageal cancer; GC, gastric cancer; ICER, incremental cost-effectiveness ratio.
Only main sensitive parameters are shown; ICERs are expressed as the value in 2019.
As a set of parameters, all the parameters changed simultaneously with a positive correlation in univariate sensitivity analyses.
Including screening mobilization and administration costs, endoscopic examination costs, and treatment costs for endoscopic complications. The 3 parameters changed simultaneously with a positive correlation in univariate sensitivity analyses.
The upper limit of ICER was higher than the per capita GDP but lower than 3 times the per capita GDP.
The upper limit of ICER was higher than 3 times the per capita GDP.
The results of the probabilistic sensitivity analyses of each screening strategy compared with no screening are shown in Figure 1. The probability of each screening strategy being cost-effective increased as the willingness-to-pay threshold increased and reached 91% to 98% even at a willingness-to-pay threshold of the per capita GDP. The results of the probabilistic sensitivity analyses conducted for each strategy compared with all other strategies are shown in Figure 2. Screening every 2 years maintained its dominance from a willingness-to-pay threshold of less than the per capita GDP to a willingness-to-pay threshold equal to 3 times the per capita GDP at each initial screening age, with probabilities of 82% to 93% and 90% to 98% for being optimal at 1 and 3 times per capita GDP, respectively. Therefore, all screening strategies would be more cost-effective than no screening, and screening every 2 years would be the optimal strategy at a willingness-to-pay threshold of 3 times the per capita GDP.
Figure 1. Cost-effectiveness Acceptability Curves of All Screening Strategies Compared With No Screening by Initial Screening Age.
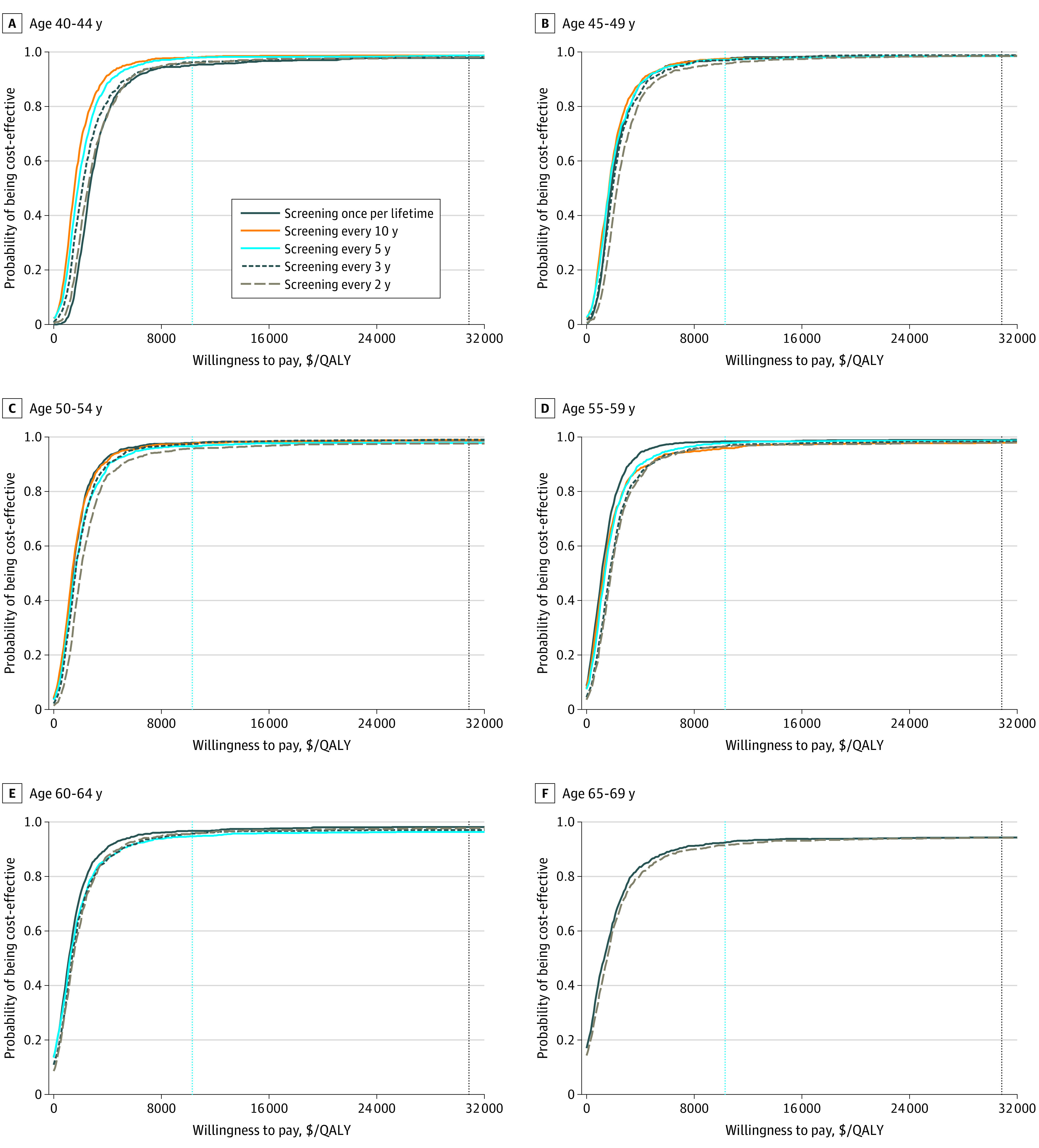
Dashed vertical blue lines represent per capita gross domestic product (GDP); dashed vertical black lines represent 3 times per capita GDP. QALY indicates quality-adjusted life-year.
Figure 2. Cost-effectiveness Acceptability Curves of All Strategies Competing With Each Other by Initial Screening Age.
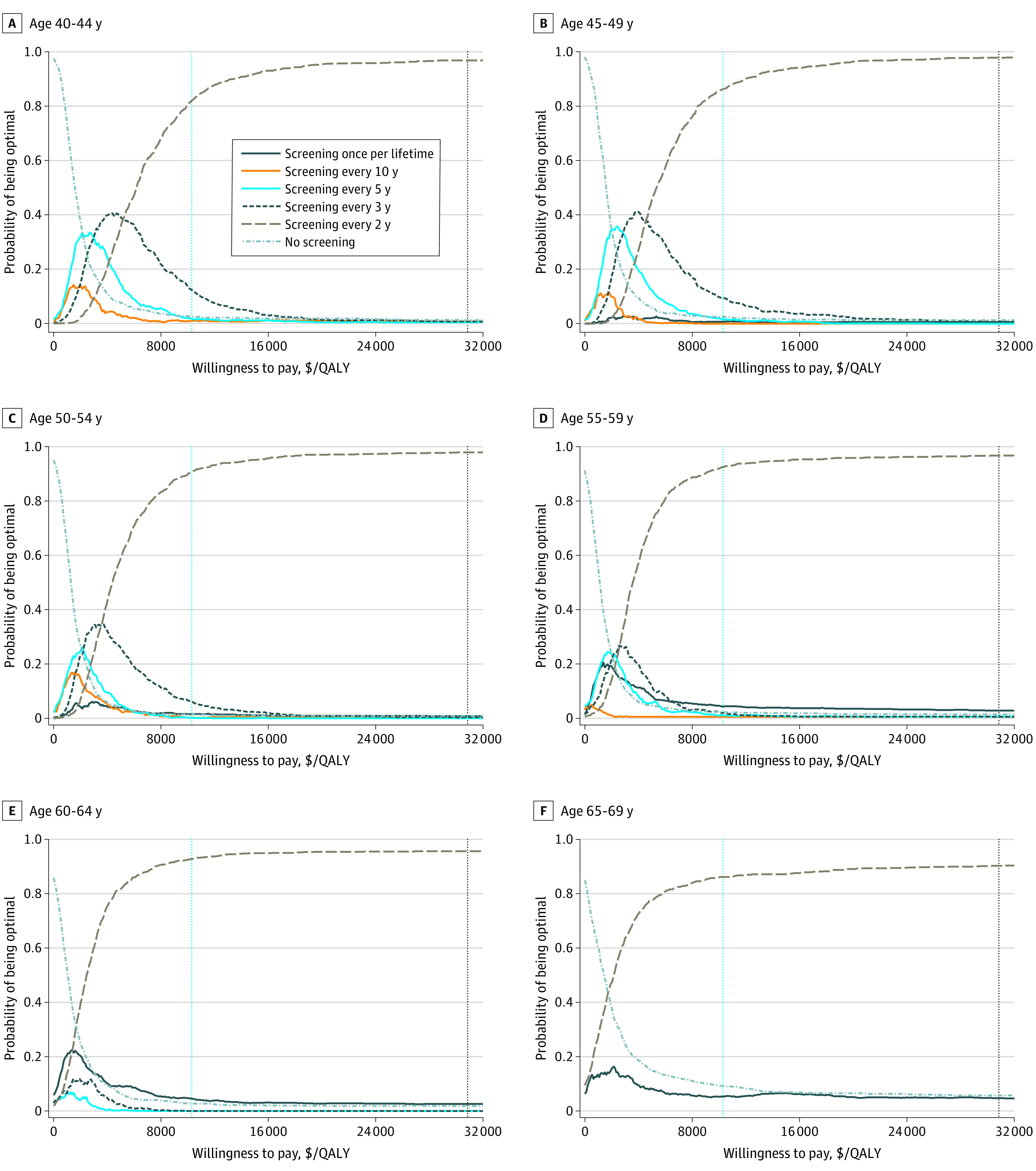
Dashed vertical blue lines represent per capita gross domestic product (GDP); dashed vertical black lines represent 3 times per capita GDP. QALY indicates quality-adjusted life-year.
Discussion
Upper gastrointestinal tract cancer continues to be a major public health burden in areas in China where incidence and mortality rate for this cancer are higher than those in other areas. The present study targeted local residents aged 40 to 69 years in areas where the risk of UGIC is high who were most likely to benefit from endoscopic screening according to the age characteristics of UGIC incidence and life expectancy in China, and this population accounts for a large proportion of patients with UGIC in China. Our base-case results suggest that combined endoscopic screening for EC and GC would be more cost-effective than no screening regardless of the initial screening age or screening frequency; this finding is consistent with the conclusion reported in a systematic review.52 Screening every 2 years would be the optimal strategy, and an initial screening age of 40 to 44 years was associated with the most health benefits. A screening frequency of 1 to 5 years has been previously proposed,53 with the optimal interval being less than 3 years.54 Most cases of early UGIC that were missed at the first endoscopic examination and subsequently identified in the second screening within 3 years were still amenable to curative surgery,55 which could theoretically increase the sensitivity of endoscopic examination after 2 consecutive screenings.13 The optimal strategy in this study was similar to the guidelines recommended in Japan and South Korea,8,9 where the incidence rates are as high as those in China. Areas in China where the risk of UGIC is high are usually rural and have limited health resources and an underdeveloped economy.56,57 Policy makers should consider the cost and effectiveness of the screening strategy, local economic level, and disease burden of UGIC when choosing appropriate screening strategies. If screening once per lifetime, which was the least expensive screening strategy, is preferable, the optimal initial screening age suggested by this study is 65 to 69 years.
Univariate sensitivity analyses revealed that utility scores of EC- and GC-related health states and compliance with screening were the 2 main sensitivity parameters. In this study, because most patients diagnosed with mild esophageal dysplasia, moderate esophageal dysplasia, or low-grade gastric intraepithelial neoplasia did not have symptoms, we used a utility score of 1 in the base-case analysis and a range of 0.98 to 1 in the sensitivity analyses according to other reports.37,38 Whether any decrements in the quality of life could be present in patients diagnosed with mild esophageal dysplasia, moderate esophageal dysplasia, or low-grade intraepithelial neoplasia if they were asymptomatic and received education regarding their true cancer risk is unknown.17 Although previous studies have found that patients with precancerous lesions have a poorer quality of life than the general population,58,59 whether the decrement in the quality of life is caused by coexisting symptoms (eg, esophagitis, gastric ulcers) or the patient’s perception of cancer risk remains unknown.60 Future studies should focus on the effect on the quality of life among those diagnosed with precancerous lesions. Compliance with screening affected whether the optimal strategy was screening every 2 years or screening every 3 years at a willingness-to-pay threshold of the per capita GDP. Endoscopic screening may be associated with reduced incidence and mortality of UGIC, and improving compliance among the target population is critical for achieving prevention effectiveness. The compliance with screening in this study was only 49%, which was derived from the areas with the highest incidence of UGIC in China18; these areas have the most longstanding promotion times and the most abundant experience with endoscopic screening and have residents with the highest cognitive understanding of endoscopic screening. Endoscopic screening is relatively expensive and slightly invasive, resulting in discomfort when individuals are examined. In addition, most of the target population, aged 40 to 69 years (especially men), in areas where there is high risk of UGIC are the primary sources of family income and migrate elsewhere for work. Therefore, compliance with endoscopic screening for UGIC is still low in China, and further popularization and promotion of endoscopic screening are needed to improve compliance.
Limitations
This study has limitations. The accuracy of the model depends on the accuracy of parameter estimates. First, base-case initial probabilities of EC- and GC-related health states were derived from the study by Chen et al.23 Residents of areas where there is high risk of UGIC enrolled in the project may have had a higher incidence of this cancer; thus, the estimated parameters may not represent the status of the entire region. Second, annual progression or regression transition probabilities of health states should increase or decrease with age in the real world. However, those between specific precancerous lesion states were fixed owing to the lack of relevant observational data. As a result, the cost-effectiveness of screening strategies may be overestimated in younger age groups and underestimated in older age groups. Third, compliance with different screening frequencies was assumed to be consistent in the model. In the real world, a higher screening frequency may be associated with a reduction in compliance because of patients’ concerns about pain and sedation and competing life demands.61,62 Fourth, annual screening was not considered as an alternative strategy in this study because it has not been recommended in any country until now. In addition, the burden may be difficult to address in countries with low GDP.
Conclusions
To our knowledge, this is the first comprehensive cost-effectiveness analysis of endoscopic screening for both EC and GC in China. The findings from the present study suggest that from the perspective of the health care system, combined endoscopic screening for EC and GC would be highly cost-effective for people aged 40 to 69 years in areas of China where there is a high risk of UGIC; screening every 2 years would be the optimal strategy. The findings provide important evidence for policies targeting the prevention and control of UGIC in China.
eMethods.
eFigure 1. Flowchart of the Overall Participants in the Multicenter Randomized Trial Project
eFigure 2. Flowchart of the Participants in the High-Risk Areas
eFigure 3. Flowchart of the Screening and Reexamination
eFigure 4. Markov Model of Upper Gastrointestinal Tract Cancer (Including Esophageal Cancer and Gastric Cancer) Progression
eFigure 5. Model Projected Age-Specific GC Incidence and Mortality Rates Compared to 2 Folds of Observed National GC Incidence and Mortality Rates in 2015
eFigure 6. Model Projected Age-Specific EC Incidence and Mortality Rates Compared to 2 Folds of Observed National EC Incidence and Mortality Rates in 2015
eTable 1. Prevalence Rates (%) of EC/GC-Related Health States Used in the Model, by Initial Screening Age
eTable 2. Annual Transition Probabilities Used in the Model
eTable 3. State-Specific Compliances with Treatment Estimated Based on Our Project
eTable 4. Costs of Screening Estimated Based on Our Project
eTable 5. Costs of EC/GC-Related Treatment in Different Disease Progression Stages Based on the Survey Included in Our Project (US$)
eTable 6. Utility Scores of EC/GC-Related Health States Based on the Survey Included in Our Project
eReferences.
References
- 1.Sung H, Ferlay J, Siegel RL, et al. Global Cancer Statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2021;71(3):209-249. doi: 10.3322/caac.21660 [DOI] [PubMed] [Google Scholar]
- 2.Wei WQ, Yang J, Zhang SW, Chen WQ, Qiao YL. Esophageal cancer mortality trends during the last 30 years in high-risk areas in China: comparison of results from national death surveys conducted in the 1970’s, 1990’s and 2004-2005. Asian Pac J Cancer Prev. 2011;12(7):1821-1826. [PubMed] [Google Scholar]
- 3.Lin Y, Totsuka Y, Shan B, et al. Esophageal cancer in high-risk areas of China: research progress and challenges. Ann Epidemiol. 2017;27(3):215-221. doi: 10.1016/j.annepidem.2016.11.004 [DOI] [PubMed] [Google Scholar]
- 4.Li JY, Liu BQ, Li GY, Chen ZJ, Sun XI, Rong SD. Atlas of cancer mortality in the People’s Republic of China: an aid for cancer control and research. Int J Epidemiol. 1981;10(2):127-133. doi: 10.1093/ije/10.2.127 [DOI] [PubMed] [Google Scholar]
- 5.Zeng H, Chen W, Zheng R, et al. Changing cancer survival in China during 2003-15: a pooled analysis of 17 population-based cancer registries. Lancet Glob Health. 2018;6(5):e555-e567. doi: 10.1016/S2214-109X(18)30127-X [DOI] [PubMed] [Google Scholar]
- 6.Wang GQ, Jiao GG, Chang FB, et al. Long-term results of operation for 420 patients with early squamous cell esophageal carcinoma discovered by screening. Ann Thorac Surg. 2004;77(5):1740-1744. doi: 10.1016/j.athoracsur.2003.10.098 [DOI] [PubMed] [Google Scholar]
- 7.Katai H, Ishikawa T, Akazawa K, et al. ; Registration Committee of the Japanese Gastric Cancer Association . Five-year survival analysis of surgically resected gastric cancer cases in Japan: a retrospective analysis of more than 100,000 patients from the nationwide registry of the Japanese Gastric Cancer Association (2001-2007). Gastric Cancer. 2018;21(1):144-154. doi: 10.1007/s10120-017-0716-7 [DOI] [PubMed] [Google Scholar]
- 8.Hamashima C; Systematic Review Group and Guideline Development Group for Gastric Cancer Screening Guidelines . Update version of the Japanese guidelines for gastric cancer screening. Jpn J Clin Oncol. 2018;48(7):673-683. doi: 10.1093/jjco/hyy077 [DOI] [PubMed] [Google Scholar]
- 9.Kim Y, Jun JK, Choi KS, Lee HY, Park EC. Overview of the National Cancer screening programme and the cancer screening status in Korea. Asian Pac J Cancer Prev. 2011;12(3):725-730. doi: 10.1097/01.cad.0000390767.85658.83 [DOI] [PubMed] [Google Scholar]
- 10.Hirota WK, Zuckerman MJ, Adler DG, et al. ; Standards of Practice Committee, American Society for Gastrointestinal Endoscopy . ASGE guideline: the role of endoscopy in the surveillance of premalignant conditions of the upper GI tract. Gastrointest Endosc. 2006;63(4):570-580. doi: 10.1016/j.gie.2006.02.004 [DOI] [PubMed] [Google Scholar]
- 11.Chang HS, Park EC, Chung W, et al. Comparing endoscopy and upper gastrointestinal x-ray for gastric cancer screening in South Korea: a cost-utility analysis. Asian Pac J Cancer Prev. 2012;13(6):2721-2728. doi: 10.7314/APJCP.2012.13.6.2721 [DOI] [PubMed] [Google Scholar]
- 12.Zhou HJ, Dan YY, Naidoo N, Li SC, Yeoh KG. A cost-effectiveness analysis evaluating endoscopic surveillance for gastric cancer for populations with low to intermediate risk. PLoS One. 2013;8(12):e83959. doi: 10.1371/journal.pone.0083959 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Areia M, Spaander MC, Kuipers EJ, Dinis-Ribeiro M. Endoscopic screening for gastric cancer: a cost-utility analysis for countries with an intermediate gastric cancer risk. United European Gastroenterol J. 2018;6(2):192-202. doi: 10.1177/2050640617722902 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Saumoy M, Schneider Y, Shen N, Kahaleh M, Sharaiha RZ, Shah SC. Cost effectiveness of gastric cancer screening according to race and ethnicity. Gastroenterology. 2018;155(3):648-660. doi: 10.1053/j.gastro.2018.05.026 [DOI] [PubMed] [Google Scholar]
- 15.Yang J, Wei WQ, Niu J, Liu ZC, Yang CX, Qiao YL. Cost-benefit analysis of esophageal cancer endoscopic screening in high-risk areas of China. World J Gastroenterol. 2012;18(20):2493-2501. doi: 10.3748/wjg.v18.i20.2493 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hur C, Choi SE, Kong CY, et al. High-resolution microendoscopy for esophageal cancer screening in China: a cost-effectiveness analysis. World J Gastroenterol. 2015;21(18):5513-5523. doi: 10.3748/wjg.v21.i18.5513 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Gupta N, Bansal A, Wani SB, Gaddam S, Rastogi A, Sharma P. Endoscopy for upper GI cancer screening in the general population: a cost-utility analysis. Gastrointest Endosc. 2011;74(3):610-624.e2. doi: 10.1016/j.gie.2011.05.001 [DOI] [PubMed] [Google Scholar]
- 18.Wei WQ, Chen ZF, He YT, et al. Long-term follow-up of a community assignment, one-time endoscopic screening study of esophageal cancer in China. J Clin Oncol. 2015;33(17):1951-1957. doi: 10.1200/JCO.2014.58.0423 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Chen R, Liu Y, Song G, et al. Effectiveness of one-time endoscopic screening programme in prevention of upper gastrointestinal cancer in China: a multicentre population-based cohort study. Gut. 2021;70(2):251-260. doi: 10.1136/gutjnl-2019-320200 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Zhang N, Li Y, Chang X, et al. Long-term effectiveness of one-time endoscopic screening for esophageal cancer: a community-based study in rural China. Cancer. 2020;126(20):4511-4520. doi: 10.1002/cncr.33119 [DOI] [PubMed] [Google Scholar]
- 21.Liu M, He Z, Guo C, et al. Effectiveness of intensive endoscopic screening for esophageal cancer in China: a community-based study. Am J Epidemiol. 2019;188(4):776-784. doi: 10.1093/aje/kwy291 [DOI] [PubMed] [Google Scholar]
- 22.Zhang X, Li M, Chen S, et al. Endoscopic screening in Asian countries is associated with reduced gastric cancer mortality: a meta-analysis and systematic review. Gastroenterology. 2018;155(2):347-354.e9. doi: 10.1053/j.gastro.2018.04.026 [DOI] [PubMed] [Google Scholar]
- 23.Chen W, Zeng H, Chen R, et al. Evaluating efficacy of screening for upper gastrointestinal cancer in China: a study protocol for a randomized controlled trial. Chin J Cancer Res. 2017;29(4):294-302. doi: 10.21147/j.issn.1000-9604.2017.04.02 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Zeng H, Sun K, Cao M, et al. Initial results from a multi-center population-based cluster randomized trial of esophageal and gastric cancer screening in China. BMC Gastroenterol. 2020;20(1):398. doi: 10.1186/s12876-020-01517-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Husereau D, Drummond M, Petrou S, et al. ; ISPOR Health Economic Evaluation Publication Guidelines-CHEERS Good Reporting Practices Task Force . Consolidated Health Economic Evaluation Reporting Standards (CHEERS)—explanation and elaboration: a report of the ISPOR Health Economic Evaluation Publication Guidelines Good Reporting Practices Task Force. Value Health. 2013;16(2):231-250. doi: 10.1016/j.jval.2013.02.002 [DOI] [PubMed] [Google Scholar]
- 26.Chinese Medical Association, Chinese Society of Digestive Endoscopy, Chinese Anti Cancer Association, The Society of Tumor Endoscopy . Chinese expert consensus on screening and endoscopic management of early esophageal cancer (Beijing, 2014). Clin J Gastroenterol. 2015;20(4):220-240. doi: 10.3969/j.issn.1008-7125.2015.04.006 [DOI] [Google Scholar]
- 27.Chinese Medical Association, Chinese Society of Digestive Endoscopy, Chinese Anti-Cancer Association, The Society of Tumor Endoscopy . Chinese consensus on screening and endoscopic diagnosis and management of early gastric cancer (Changsha, 2014). Article in Chinese. Clin J Gastroenterol. 2014;19(7):408-427. doi: 10.3969/j.issn.1008-7125.2014.07.006 [DOI] [Google Scholar]
- 28.Feng H, Song G, Yang J, et al. Cost-effectiveness analysis of esophageal cancer once-in-a-lifetime endoscopic screening in high-risk areas of rural China. Article in Chinese. Zhonghua Zhong Liu Za Zhi. 2015;37(6):476-480. doi: 10.3760/cma.j.issn.0253-3766.2015.06.017 [DOI] [PubMed] [Google Scholar]
- 29.Wang M, Hao C, Zhao D, et al. Distribution of esophageal squamous cell cancer and precursor lesions in high-risk areas, Linzhou in Henan province and Feicheng in Shandong province of China, 2005-2009. Article in Chinese. Zhonghua Yu Fang Yi Xue Za Zhi. 2015;49(8):677-682. [PubMed] [Google Scholar]
- 30.Dawsey SM, Fleischer DE, Wang GQ, et al. Mucosal iodine staining improves endoscopic visualization of squamous dysplasia and squamous cell carcinoma of the esophagus in Linxian, China. Cancer. 1998;83(2):220-231. doi: [DOI] [PubMed] [Google Scholar]
- 31.Nagami Y, Tominaga K, Machida H, et al. Usefulness of non-magnifying narrow-band imaging in screening of early esophageal squamous cell carcinoma: a prospective comparative study using propensity score matching. Am J Gastroenterol. 2014;109(6):845-854. doi: 10.1038/ajg.2014.94 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Yeh JM, Hur C, Ward Z, Schrag D, Goldie SJ. Gastric adenocarcinoma screening and prevention in the era of new biomarker and endoscopic technologies: a cost-effectiveness analysis. Gut. 2016;65(4):563-574. doi: 10.1136/gutjnl-2014-308588 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Lee YC, Lin JT, Wu HM, et al. Cost-effectiveness analysis between primary and secondary preventive strategies for gastric cancer. Cancer Epidemiol Biomarkers Prev. 2007;16(5):875-885. doi: 10.1158/1055-9965.EPI-06-0758 [DOI] [PubMed] [Google Scholar]
- 34.Hamashima C, Okamoto M, Shabana M, Osaki Y, Kishimoto T. Sensitivity of endoscopic screening for gastric cancer by the incidence method. Int J Cancer. 2013;133(3):653-659. doi: 10.1002/ijc.28065 [DOI] [PubMed] [Google Scholar]
- 35.Espino A, Garcia X, Mac-Namara M, et al. 805 complications of gastrointestinal endoscopy in 85,391 procedures. Gastrointest Endosc. 2012;75(4)(suppl S):AB170. doi: 10.1016/j.gie.2012.04.140 [DOI] [Google Scholar]
- 36.Yang Z, Zeng H, Xia R, et al. Annual cost of illness of stomach and esophageal cancer patients in urban and rural areas in China: A multi-center study. Chin J Cancer Res. 2018;30(4):439-448. doi: 10.21147/j.issn.1000-9604.2018.04.07 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Sharaiha RZ, Freedberg DE, Abrams JA, Wang YC. Cost-effectiveness of chemoprevention with proton pump inhibitors in Barrett’s esophagus. Dig Dis Sci. 2014;59(6):1222-1230. doi: 10.1007/s10620-014-3186-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Inadomi JM, Somsouk M, Madanick RD, Thomas JP, Shaheen NJ. A cost-utility analysis of ablative therapy for Barrett’s esophagus. Gastroenterology. 2009;136(7):2101-2114.e1-e6. doi: 10.1053/j.gastro.2009.02.062 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Liu Q, Zeng H, Xia R, et al. Health-related quality of life of esophageal cancer patients in daily life after treatment: a multicenter cross-sectional study in China. Cancer Med. 2018;7(11):5803-5811. doi: 10.1002/cam4.1817 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Xia R, Zeng H, Liu Q, et al. Health-related quality of life and health utility score of patients with gastric cancer: a multi-centre cross-sectional survey in China. Eur J Cancer Care (Engl). 2020;29(6):e13283. doi: 10.1111/ecc.13283 [DOI] [PubMed] [Google Scholar]
- 41.Weinstein MC, Siegel JE, Gold MR, Kamlet MS, Russell LB. Recommendations of the Panel on Cost-effectiveness in Health and Medicine. JAMA. 1996;276(15):1253-1258. doi: 10.1001/jama.1996.03540150055031 [DOI] [PubMed] [Google Scholar]
- 42.Sieg A, Hachmoeller-Eisenbach U, Eisenbach T. Prospective evaluation of complications in outpatient GI endoscopy: a survey among German gastroenterologists. Gastrointest Endosc. 2001;53(6):620-627. doi: 10.1067/mge.2001.114422 [DOI] [PubMed] [Google Scholar]
- 43.Levy I, Gralnek IM. Complications of diagnostic colonoscopy, upper endoscopy, and enteroscopy. Best Pract Res Clin Gastroenterol. 2016;30(5):705-718. doi: 10.1016/j.bpg.2016.09.005 [DOI] [PubMed] [Google Scholar]
- 44.Zheng X, Mao X, Xu K, et al. Massive endoscopic screening for esophageal and gastric cancers in a high-risk area of China. PLoS One. 2015;10(12):e0145097. doi: 10.1371/journal.pone.0145097 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Liang S, Li K, Gong J, Wang J, Ma H, Wang G. Results of the endoscopic screening program of esophageal and gastric cardia cancers using iodine staining in Feicheng, Shandong Province, from 2006 to 2012. Article in Chinese. Zhonghua Zhong Liu Za Zhi. 2015;37(7):549-553. [PubMed] [Google Scholar]
- 46.You WC. Intervention on gastric cancer and precancerous lesions—the practice of gastric cancer at high-risk area for 23 years. Journal of Peking University (Health Sciences). 2006;38:565-570. [Google Scholar]
- 47.Wang GQ, Abnet CC, Shen Q, et al. Histological precursors of oesophageal squamous cell carcinoma: results from a 13 year prospective follow up study in a high risk population. Gut. 2005;54(2):187-192. doi: 10.1136/gut.2004.046631 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Zhang Y, Zhang L, Pan KF, et al. . Prognosis of intestinal metaplasia and expressions of biomarkers in high-risk populations of gastric cancer in Shangdong province. Article in Chinese. World Chinese Journal of Digestology. 2006;14:2306-2310. doi: 10.11569/wcjd.v14.i23.2306 [DOI] [Google Scholar]
- 49.Wang LD, Yang HH, Fan ZM, et al. Cytological screening and 15 years’ follow-up (1986-2001) for early esophageal squamous cell carcinoma and precancerous lesions in a high-risk population in Anyang County, Henan Province, Northern China. Cancer Detect Prev. 2005;29(4):317-322. doi: 10.1016/j.cdp.2005.06.004 [DOI] [PubMed] [Google Scholar]
- 50.Department of Population and Employment Statistics, National Bureau of Statistics of China . China Population and Employment Statistics Yearbook. China Statistics Press; 2019. [Google Scholar]
- 51.Walker DG, Hutubessy R, Beutels P. WHO guide for standardisation of economic evaluations of immunization programmes. Vaccine. 2010;28(11):2356-2359. doi: 10.1016/j.vaccine.2009.06.035 [DOI] [PubMed] [Google Scholar]
- 52.Areia M, Carvalho R, Cadime AT, Rocha Gonçalves F, Dinis-Ribeiro M. Screening for gastric cancer and surveillance of premalignant lesions: a systematic review of cost-effectiveness studies. Helicobacter. 2013;18(5):325-337. doi: 10.1111/hel.12050 [DOI] [PubMed] [Google Scholar]
- 53.Fukao A, Tsubono Y, Tsuji I, HIsamichi S, Sugahara N, Takano A. The evaluation of screening for gastric cancer in Miyagi Prefecture, Japan: a population-based case-control study. Int J Cancer. 1995;60(1):45-48. doi: 10.1002/ijc.2910600106 [DOI] [PubMed] [Google Scholar]
- 54.Kim YS, Park HA, Kim BS, Yook JH, Lee MS. Efficacy of screening for gastric cancer in a Korean adult population: a case-control study. J Korean Med Sci. 2000;15(5):510-515. doi: 10.3346/jkms.2000.15.5.510 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Hosokawa O, Tsuda S, Kidani E, et al. Diagnosis of gastric cancer up to three years after negative upper gastrointestinal endoscopy. Endoscopy. 1998;30(8):669-674. doi: 10.1055/s-2007-1001386 [DOI] [PubMed] [Google Scholar]
- 56.He YT, Hou J, Chen ZF, et al. Trends in incidence of esophageal and gastric cardia cancer in high-risk areas in China. Eur J Cancer Prev. 2008;17(2):71-76. doi: 10.1097/CEJ.0b013e3282b6fd97 [DOI] [PubMed] [Google Scholar]
- 57.Wang JM, Xu B, Hsieh CC, Jiang QW. Longitudinal trends of stomach cancer and esophageal cancer in Yangzhong County: a high-incidence rural area of China. Eur J Gastroenterol Hepatol. 2005;17(12):1339-1344. doi: 10.1097/00042737-200512000-00012 [DOI] [PubMed] [Google Scholar]
- 58.Crockett SD, Lippmann QK, Dellon ES, Shaheen NJ. Health-related quality of life in patients with Barrett’s esophagus: a systematic review. Clin Gastroenterol Hepatol. 2009;7(6):613-623. doi: 10.1016/j.cgh.2009.02.024 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Wang L, Shi JF, Zhu J, et al. ; Health Economic Evaluation Working Group of the Cancer Screening Program in Urban China . Health-related quality of life and utility scores of patients with breast neoplasms in China: a multicenter cross-sectional survey. Breast. 2018;39:53-62. doi: 10.1016/j.breast.2018.03.004 [DOI] [PubMed] [Google Scholar]
- 60.Kruijshaar ME, Siersema PD, Janssens ACJW, Kerkhof M, Steyerberg EW, Essink-Bot ML; CYBAR Study Group . Patients with Barrett’s esophagus perceive their risk of developing esophageal adenocarcinoma as low. Gastrointest Endosc. 2007;65(1):26-30. doi: 10.1016/j.gie.2006.05.030 [DOI] [PubMed] [Google Scholar]
- 61.Oikonomidou E, Anastasiou F, Pilpilidis I, Kouroumalis E, Lionis C; Greek General Practice Dyspepsia Group . Upper gastrointestinal endoscopy for dyspepsia: exploratory study of factors influencing patient compliance in Greece. BMC Gastroenterol. 2011;11:11. doi: 10.1186/1471-230X-11-11 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Cao M, Li H, Sun D, et al. Cancer screening in China: the current status, challenges, and suggestions. Cancer Lett. 2021;506:120-127. doi: 10.1016/j.canlet.2021.02.017 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
eMethods.
eFigure 1. Flowchart of the Overall Participants in the Multicenter Randomized Trial Project
eFigure 2. Flowchart of the Participants in the High-Risk Areas
eFigure 3. Flowchart of the Screening and Reexamination
eFigure 4. Markov Model of Upper Gastrointestinal Tract Cancer (Including Esophageal Cancer and Gastric Cancer) Progression
eFigure 5. Model Projected Age-Specific GC Incidence and Mortality Rates Compared to 2 Folds of Observed National GC Incidence and Mortality Rates in 2015
eFigure 6. Model Projected Age-Specific EC Incidence and Mortality Rates Compared to 2 Folds of Observed National EC Incidence and Mortality Rates in 2015
eTable 1. Prevalence Rates (%) of EC/GC-Related Health States Used in the Model, by Initial Screening Age
eTable 2. Annual Transition Probabilities Used in the Model
eTable 3. State-Specific Compliances with Treatment Estimated Based on Our Project
eTable 4. Costs of Screening Estimated Based on Our Project
eTable 5. Costs of EC/GC-Related Treatment in Different Disease Progression Stages Based on the Survey Included in Our Project (US$)
eTable 6. Utility Scores of EC/GC-Related Health States Based on the Survey Included in Our Project
eReferences.


