Key Points
Question
Did gestational diabetes rates change from 2011 to 2019 among individuals at first live birth in the US, and were there differences by race and ethnicity subgroups?
Findings
In this serial, population-based, cross-sectional study of 12 610 235 individuals at first live birth aged 15 to 44 years, the age-standardized gestational diabetes rate increased from 47.6 to 63.5 per 1000 live births from 2011 to 2019. Rates increased in all racial and ethnic subgroups; in 2019, Asian Indian individuals had the highest gestational diabetes rate (129.1 per 1000 live births).
Meaning
Gestational diabetes rates among individuals with a singleton first live birth increased across all race and ethnicity subgroups in the US from 2011 to 2019.
Abstract
Importance
Gestational diabetes is associated with adverse maternal and offspring outcomes.
Objective
To determine whether rates of gestational diabetes among individuals at first live birth changed from 2011 to 2019 and how these rates differ by race and ethnicity in the US.
Design, Setting, and Participants
Serial cross-sectional analysis using National Center for Health Statistics data for 12 610 235 individuals aged 15 to 44 years with singleton first live births from 2011 to 2019 in the US.
Exposures
Gestational diabetes data stratified by the following race and ethnicity groups: Hispanic/Latina (including Central and South American, Cuban, Mexican, and Puerto Rican); non-Hispanic Asian/Pacific Islander (including Asian Indian, Chinese, Filipina, Japanese, Korean, and Vietnamese); non-Hispanic Black; and non-Hispanic White.
Main Outcomes and Measures
The primary outcomes were age-standardized rates of gestational diabetes (per 1000 live births) and respective mean annual percent change and rate ratios (RRs) of gestational diabetes in non-Hispanic Asian/Pacific Islander (overall and in subgroups), non-Hispanic Black, and Hispanic/Latina (overall and in subgroups) individuals relative to non-Hispanic White individuals (referent group).
Results
Among the 12 610 235 included individuals (mean [SD] age, 26.3 [5.8] years), the overall age-standardized gestational diabetes rate significantly increased from 47.6 (95% CI, 47.1-48.0) to 63.5 (95% CI, 63.1-64.0) per 1000 live births from 2011 to 2019, a mean annual percent change of 3.7% (95% CI, 2.8%-4.6%) per year. Of the 12 610 235 participants, 21% were Hispanic/Latina (2019 gestational diabetes rate, 66.6 [95% CI, 65.6-67.7]; RR, 1.15 [95% CI, 1.13-1.18]), 8% were non-Hispanic Asian/Pacific Islander (2019 gestational diabetes rate, 102.7 [95% CI, 100.7-104.7]; RR, 1.78 [95% CI, 1.74-1.82]), 14% were non-Hispanic Black (2019 gestational diabetes rate, 55.7 [95% CI, 54.5-57.0]; RR, 0.97 [95% CI, 0.94-0.99]), and 56% were non-Hispanic White (2019 gestational diabetes rate, 57.7 [95% CI, 57.2-58.3]; referent group). Gestational diabetes rates were highest in Asian Indian participants (2019 gestational diabetes rate, 129.1 [95% CI, 100.7-104.7]; RR, 2.24 [95% CI, 2.15-2.33]). Among Hispanic/Latina participants, gestational diabetes rates were highest among Puerto Rican individuals (2019 gestational diabetes rate, 75.8 [95% CI, 71.8-79.9]; RR, 1.31 [95% CI, 1.24-1.39]). Gestational diabetes rates increased among all race and ethnicity subgroups and across all age groups.
Conclusions and Relevance
Among individuals with a singleton first live birth in the US from 2011 to 2019, rates of gestational diabetes increased across all racial and ethnic subgroups. Differences in absolute gestational diabetes rates were observed across race and ethnicity subgroups.
This cross-sectional analysis uses data from the National Center for Health Statistics to quantify annual rates of gestational diabetes in individuals at first live birth among Hispanic/Latina, non-Hispanic Asian/Pacific Islander, non-Hispanic Black, and non-Hispanic White individuals in the US from 2011 to 2019.
Introduction
Gestational diabetes is a common disorder of pregnancy that is defined by the onset of glucose intolerance during pregnancy. Gestational diabetes is associated with adverse short- and long-term risks for women and their offspring. In mothers, hazard ratios for future diabetes after gestational diabetes ranged from 6.3 (95% CI, 5.0-7.9) in Asian individuals to 9.9 (95% CI, 7.5-13.1) in Black individuals in a retrospective cohort in Southern California,1 and a meta-analysis showed that gestational diabetes is associated with a relative risk of 2.0 (95% CI, 1.6-2.5) for future cardiovascular disease.2 In offspring, fetal exposure to gestational diabetes in utero has been linked to macrosomia and adiposity in newborns3 and impaired glucose tolerance and obesity in childhood,4,5 thereby increasing risks for adverse cardiometabolic outcomes for offspring across the lifespan.6
In 2016, the rate of gestational diabetes in the US was estimated to be 11.1% in Asian women, 6.6% in Hispanic women, 4.8% in non-Hispanic Black women, and 5.3% in non-Hispanic White women.7 However, it is unclear whether heterogeneity in rates of gestational diabetes exist within race and ethnicity groups (including Asian Indian, Chinese, Filipina, Japanese, Korean, and Vietnamese among non-Hispanic Asian populations and Cuban, Central/South American, Mexican, and Puerto Rican among Hispanic/Latina populations). Identifying gestational diabetes patterns in these disaggregated subgroups is necessary to accurately represent these diverse populations. In this study, data from the National Center for Health Statistics (NCHS) were examined to quantify annual rates of gestational diabetes in individuals at first live birth in race and ethnicity subgroups in the US from 2011 to 2019 to inform equitable strategies for clinical and population-level management and prevention.
Methods
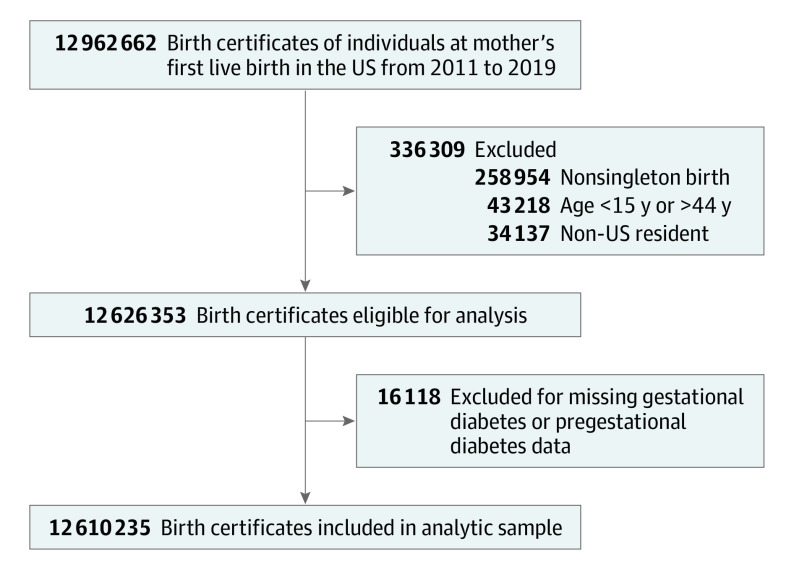
Maternal data from birth registration records collected by the NCHS that capture all first live births to individuals in the US were used to evaluate gestational diabetes rates from 2011 to 2019. In the NCHS data, “nulliparity” is defined as the “first live birth.” This activity was reviewed by the Centers for Disease Control and Prevention and was conducted consistent with applicable federal law and Centers for Disease Control and Prevention policy.8 This study was deemed to be exempt because it was not considered human subjects research due to the de-identified nature of the publicly available vital statistics data set. Records not using the 2003 revised certificate of birth were ineligible because previous versions did not distinguish gestational diabetes from pregestational diabetes. Records from individuals younger than 15 years or older than 44 years, records from nonsingleton births, and records from non-US residents were also ineligible and were removed. Records from nonsingleton deliveries (eg, twins) were removed to avoid duplication of maternal data, given that records could not be linked in the deidentified data set. Records missing data on gestational diabetes or pregestational diabetes were excluded from analysis (Figure 1). Data collection from live births is recommended to be completed by the attendant clinician at birth (eg, physician or midwife), who compiles data from various sources, including maternal self-report, prenatal records, labor and delivery triage records, admission history and physical examination, or delivery record, based on a standardized protocol from the NCHS.
Figure 1. Birth Records Included for Primary Analysis in a Study of the Trends in Gestational Diabetes at First Live Birth by Race and Ethnicity in the US from 2011 to 2019.
Records from the National Center for Health Statistics birth certificate files. Records that did not use the 2003 revised certificate of birth were not eligible for analysis because previous versions did not distinguish gestational diabetes from pregestational diabetes. Maternal characteristics for those whose records were excluded are shown in eTable 1 in the Supplement.
Outcomes and Maternal Characteristics
The primary outcome of interest was gestational diabetes, defined on the NCHS protocol worksheet as diabetes that is diagnosed during pregnancy. The secondary outcome of interest was pregestational diabetes, defined as diabetes diagnosed prior to pregnancy. In this data set, gestational diabetes and pregestational diabetes were mutually exclusive, such that individuals with pregestational diabetes could not be categorized as having gestational diabetes and individuals categorized as having gestational diabetes could not also be categorized as having pregestational diabetes.
Data were also collected on maternal demographic characteristics, such as age, race and ethnicity, education, and insurance (ie, primary source of payment for delivery: private, Medicaid, self-pay [no third party identified, ie, no insurance], or other), as well as prenatal characteristics, including pregestational body mass index (BMI) and receipt of prenatal care.
Race and ethnicity were evaluated as the primary stratification because previous regional data suggested differences in gestational diabetes rates by race and ethnicity9 and contemporary clinical guidelines identify race and ethnicity subgroups for enhanced gestational diabetes screening during pregnancy.10 Race is recognized as a social construct reflecting a range of factors and is not implied to be a biological determinant. Race and ethnicity were self-identified by the pregnant individual from fixed categories of race and ethnicity groups, which include disaggregated non-Hispanic Asian and Hispanic subgroups. Due to state-level inconsistencies in how multiple-race identification data were collected during the study period, the NCHS recommends use of bridged-race categories in analyses. The 4 bridged-race categories identified by the NCHS and used in our analysis are American Indian or Alaskan Native, Asian or Pacific Islander, Black, and White, which facilitate calculation and comparison of population-level rates and trends across time and states.11 Single-race categories, which were collected on the 2003 birth certificate revision, were used to identify non-Hispanic Asian and Pacific Islander and Hispanic subgroups. There were no records with missing race data in this data set, but 0.8% of records were missing ethnicity information, so these individuals were included in bridged-race categories but not in subgroup analyses.
Statistical Analysis
In the primary analysis, gestational diabetes data were evaluated for individuals with a first live birth, each year from 2011 to 2019, overall (comprising all race and ethnicity groups combined), among the 4 largest race and ethnicity groups (Hispanic/Latina, non-Hispanic Asian/Pacific Islander, non-Hispanic Black, and non-Hispanic White), among Hispanic/Latina subgroups (Central/South American, Cuban, Mexican, and Puerto Rican), and among non-Hispanic Asian subgroups (Asian Indian, Chinese, Filipina, Japanese, Korean, and Vietnamese). In a secondary analysis, gestational diabetes data were evaluated in the available smaller subgroups of individuals over the entire study period (2011-2019 combined due to small population size), including American Indian or Alaskan Native, Guamanian, Native Hawaiian, Samoan, Other Asian (not further specified), and Other Pacific Islander (not further specified) (per NCHS terminology).
Age-standardized rates of gestational diabetes per 1000 live births were calculated overall, among race and ethnicity groups, and among Hispanic and non-Hispanic Asian subgroups. Rates were age-standardized to the age distribution of US individuals who gave birth in 2011, the beginning of the study period. Age-specific rates of gestational diabetes at first live birth were also calculated in 5-year age strata (15-19 y, 20-24 y, 25-29 y, 30-34 y, 35-39 y, and 40-44 y), overall, and in each race and ethnicity subgroup. In secondary analyses of the same population of individuals aged 15 to 44 years with singleton first live births, age-standardized rates of pregestational diabetes were also calculated overall and stratified by race and ethnicity groups.
To quantify trends over time, Joinpoint Regression statistical software, version 4.7.0.0, was used to calculate mean annual percent change (APC) of age-standardized and age-specific rates of gestational diabetes from 2011 to 2019. Joinpoint Regression accounts for potential nonlinear trends by allowing for identification of up to 1 trend inflection point. Where an inflection was identified indicating a nonlinear trend, mean annual change was calculated based on the weight of the component trend segments. If no inflection point was identified, mean APC indicates the annual change in gestational diabetes rate across a linear trend.12 A secondary analysis was performed to evaluate trend magnitude and direction overall and in all race and ethnicity groups, specifically from 2016 to 2019, which are the years in which all US states and regions adopted the 2003 birth certificate revision. Standardized rate ratios (RRs) and 95% CIs were calculated using modified F intervals, as previously described by Tiwari et al,13 to compare annual age-standardized rates of gestational diabetes in individuals at first live birth in Hispanic/Latina, non-Hispanic Asian, and non-Hispanic Black subgroups, with non-Hispanic White individuals as the reference category. Age-standardized rate calculations and RR analyses were performed with Stata, version 15.1 (StataCorp). Because of the potential for type I error due to multiple comparisons, findings for secondary outcomes and analyses should be interpreted as exploratory. Two-sided P values <.05 indicate statistical significance.
Results
Maternal Characteristics
Of 12 962 662 birth certificates for the first live birth to individuals in the US from 2011 to 2019, a total of 43 218 (0.3%) were removed because the mothers were younger than 15 or older than 44 years, 258 954 (2.0%) were removed because they were non-singleton births, and 34 137 (0.3%) were removed because they were records from non-US residents; an additional 16 118 (0.1%) were excluded for missing data on gestational diabetes or pregestational diabetes status (Figure 1). Maternal characteristics from the excluded records are listed in eTable 1 in the Supplement.
Of the 12 610 235 individuals with singleton first live births in the primary analytic sample (mean [SD] age, 26.3 [5.8] years), 21% were Hispanic/Latina (mean [SD] age, 24.4 [5.8] years), 8% were non-Hispanic Asian/Pacific Islander (mean [SD] age, 29.8 years), 14% were non-Hispanic Black (mean [SD] age, 24.3 [5.7] years), and 56% were non-Hispanic White (mean [SD] age, 27.0 [5.6] years) (Table 1). The majority of individuals had completed at least a high school education (88%) and had insurance coverage (92% with Medicaid or private insurance). There were 144 861 records (1.1%) missing data on education, 107 899 (0.9%) missing insurance status, 367 616 (2.9%) missing prenatal care data, and 377 230 (3.0%) missing prepregnancy BMI data.
Table 1. Maternal Characteristics by Race and Ethnicity in Individuals Aged 15 to 44 Years With Singleton First Live Births in the US, 2011-2019.
| Characteristic | No. (%)a | ||||
|---|---|---|---|---|---|
| Overall (n = 12 610 235) |
Hispanic/Latina (n = 2 618 571) | Non-Hispanic Asian/Pacific Islander (n = 978 919) | Non-Hispanic Black (n = 1 774 263) | Non-Hispanic White (n = 7 035 544) | |
| Age, mean (SD), y | 26.3 (5.8) | 24.4 (5.8) | 29.8 (5.0) | 24.3 (5.7) | 27.0 (5.6) |
| Education | |||||
| Some high school or less | 1 450 871 (11.6) | 601 932 (23.2) | 52 326 (5.4) | 269 210 (15.3) | 499 828 (7.1) |
| High school graduate | 3 016 982 (24.2) | 837 820 (32.3) | 110 394 (11.4) | 578 488 (32.9) | 1 445 159 (20.6) |
| Any college | 7 997 521 (64.2) | 1 150 312 (44.4) | 802 458 (83.1) | 913 013 (51.9) | 5 055 176 (72.2) |
| Insurance | |||||
| Medicaid | 4 803 572 (38.4) | 1 471 205 (56.7) | 218 649 (22.5) | 1 087 815 (61.8) | 1 936 592 (27.8) |
| Private insurance | 6 753 250 (54.0) | 837 770 (32.3) | 661 143 (68.0) | 548 030 (31.2) | 4 616 167 (66.2) |
| Self-pay | 417 289 (3.3) | 155 144 (6.0) | 58 012 (6.0) | 50 073 (2.8) | 149 393 (2.1) |
| Other | 528 225 (4.2) | 130 693 (5.0) | 34 626 (3.6) | 73 296 (4.2) | 274 315 (3.9) |
| Prenatal care | |||||
| Started in 1st trimester | 9 549 047 (78.0) | 1 829 645 (71.9) | 765 279 (80.3) | 1 154 476 (68.2) | 5 655 660 (82.5) |
| Started in 2nd trimester | 2 011 583 (16.4) | 516 586 (20.3) | 135 188 (14.2) | 390 391 (23.1) | 932 401 (13.6) |
| Started in 3rd trimester | 523 193 (4.3) | 146 627 (5.8) | 44 104 (4.6) | 108 518 (6.4) | 212 681 (3.1) |
| None | 158 796 (1.3) | 51 382 (2.0) | 8002 (0.8) | 39 127 (2.3) | 56 897 (0.8) |
| Pregestational BMI, median (IQR) | 24.4 (21.5-29.0) | 24.9 (21.9-29.3) | 22.3 (20.1-25.2) | 25.8 (22.1-31.2) | 24.2 (21.5-28.9) |
| BMI ≥25 | 5 626 249 (46.0) | 1 247 991 (49.2) | 248 563 (26.3) | 942 200 (55.4) | 3 095 604 (45.1) |
| BMI ≥30 | 2 658 950 (21.7) | 569 500 (22.4) | 72 407 (7.7) | 508 110 (29.9) | 1 463 775 (21.3) |
Abbreviations: BMI, body mass index (calculated as weight in kilograms divided by height in meters squared); IQR, interquartile range.
Percentages for categorical maternal characteristics account for missing data. Characteristics in non-Hispanic Asian and Hispanic/Latina subgroups are detailed in eTables 1 and 2 in the Supplement.
Among non-Hispanic Asian subgroups, the mean (SD) maternal age ranged from 29.5 (4.0) years in Asian Indian individuals to 33.3 (4.7) years in Japanese individuals (eTable 2 in the Supplement). Among non-Hispanic Asian subgroups, 71% to 95% reported college attendance and 61% to 83% reported having private insurance. In Hispanic/Latina subgroups (eTable 3 in the Supplement), mean (SD) maternal age ranged from 23.8 (5.6) years in Mexican individuals to 27.1 (5.5) years in Cuban individuals. Cuban individuals had the highest frequency of college attendance (58%). Hispanic/Latina individuals predominantly reported having Medicaid insurance (49%-58%). From 2011 to 2019, among all individuals at first live birth overall, the mean (SD) age at delivery was 25.5 (5.9) years in 2011 and 27.0 (5.8) years in 2019, and the median (interquartile range) pregestational BMI was 24.0 (21.3-28.3) in 2011 and 25.0 (21.9-29.9) in 2019 (Table 2).
Table 2. Maternal Characteristics in Individuals Aged 15 to 44 Years With Singleton First Live Births From 2011 to 2019 in the US.
| Characteristic | No. (%)a | ||||
|---|---|---|---|---|---|
| 2011 | 2013 | 2015 | 2017 | 2019 | |
| No. | 1 312 431 | 1 360 253 | 1 459 015 | 1 421 125 | 1 395 829 |
| Age, mean (SD), y | 25.5 (5.9) | 25.9 (5.8) | 26.3 (5.8) | 26.7 (5.8) | 27.0 (5.8) |
| Race and ethnicity | |||||
| Hispanic | 275 449 (21.0) | 273 246 (20.1) | 300 055 (20.6) | 298 451 (21.0) | 303 338 (21.7) |
| Central/South American | 36 000 (2.7) | 36 821 (2.7) | 44 257 (3.0) | 48 080 (3.4) | 55 593 (16.1) |
| Cuban | 7291 (0.6) | 8218 (0.6) | 9416 (0.6) | 10 801 (0.8) | 10 691 (0.8) |
| Mexican | 163 229 (12.4) | 156 756 (11.5) | 170 010 (11.7) | 160 156 (11.3) | 162 937 (11.7) |
| Puerto Rican | 20 159 (1.5) | 21 086 (1.6) | 23 865 (1.6) | 25 911 (1.8) | 26 444 (1.9) |
| Other Hispanicb | 48 770 (3.7) | 50 365 (3.7) | 52 507 (3.6) | 53 503 (3.8) | 47 673 (3.4) |
| Non-Hispanic Asian/Pacific Islander | 88 478 (6.7) | 98 782 (7.3) | 113 168 (7.8) | 118 441 (8.3) | 114 138 (8.2) |
| Asian Indian | 22 917 (1.7) | 26 584 (2.0) | 31 921 (2.2) | 35 253 (2.5) | 33 030 (2.4) |
| Chinese | 19 029 (1.4) | 23 042 (1.7) | 25 471 (1.7) | 28 338 (2.0) | 26 771 (1.9) |
| Filipina | 11 043 (0.8) | 11 286 (0.8) | 12 528 (0.9) | 11 782 (0.8) | 11 514 (0.8) |
| Japanese | 2673 (0.2) | 2774 (0.2) | 3235 (0.2) | 2893 (0.2) | 2525 (0.2) |
| Korean | 6552 (0.5) | 6671 (0.5) | 7265 (0.5) | 6843 (0.5) | 6317 (0.5) |
| Vietnamese | 7361 (0.6) | 7829 (0.6) | 8592 (0.6) | 8588 (0.6) | 8810 (0.6) |
| Other Asian/Pacific Islanderc | 18 903 (1.4) | 20 596 (1.5) | 24 156 (1.7) | 24 744 (1.7) | 25 171 (1.8) |
| Non-Hispanic Black | 181 907 (13.9) | 193 426 (14.2) | 204 649 (14.0) | 201 876 (14.2) | 199 064 (14.3) |
| Non-Hispanic White | 746 421 (56.9) | 774 074 (56.9) | 817 525 (56.0) | 779 472 (54.8) | 755 848 (54.2) |
| Education | |||||
| Some high school or less | 197 935 (15.2) | 169 767 (12.6) | 165 257 (11.5) | 143 029 (10.2) | 128 149 (9.3) |
| High school graduate | 317 290 (24.4) | 326 004 (24.2) | 347 025 (24.1) | 338 756 (24.1) | 339 067 (24.6) |
| Any college | 783 205 (60.3) | 850 279 (63.2) | 930 107 (64.5) | 922 372 (65.7) | 910 090 (66.1) |
| Insurance | |||||
| Medicaid | 536 121 (41.5) | 534 672 (39.8) | 554 071 (38.2) | 527 789 (37.3) | 500 860 (36.1) |
| Private insurance | 652 147 (50.5) | 700 608 (52.1) | 786 882 (54.3) | 783 544 (55.4) | 789 469 (56.9) |
| Self-pay | 41 178 (3.2) | 45 054 (3.4) | 48 642 (3.4) | 47 302 (3.3) | 46 074 (3.3) |
| Other | 63 038 (4.9) | 63 601 (4.7) | 59 514 (4.1) | 54 805 (3.9) | 50 231 (3.6) |
| Prenatal care | |||||
| Started in 1st trimester | 959 777 (75.4) | 994 504 (75.9) | 1 113 988 (78.8) | 1 097 047 (79.1) | 1 087 687 (79.6) |
| Started in 2nd trimester | 244 676 (19.2) | 243 032 (18.6) | 222 152 (15.7) | 209 961 (15.1) | 199 249 (14.6) |
| Started in 3rd trimester | 52 267 (4.1) | 56 810 (4.3) | 59 881 (4.2) | 60 107 (4.3) | 59 220 (4.3) |
| None | 15 705 (1.2) | 15 279 (1.2) | 17 787 (1.3) | 19 466 (1.4) | 20 499 (1.5) |
| Pregestational BMI, median (IQR) | 24.0 (21.3-28.3) | 24.1 (21.3-28.3) | 24.3 (21.5-28.9) | 24.7 (21.6-29.3) | 25.0 (21.9-29.9) |
| BMI ≥25 | 538 746 (42.7) | 570 953 (43.8) | 643 635 (45.4) | 663 843 (47.8) | 692 774 (50.7) |
| BMI ≥30 | 244 712 (19.4) | 262 776 (20.2) | 302 500 (21.4) | 319 655 (23.0) | 341 168 (24.9) |
Abbreviations: BMI, body mass index (calculated as weight in kilograms divided by height in meters squared); IQR, interquartile range.
Percentages for categorical maternal characteristics account for missing data. Listed maternal characteristics data are from mothers of all race and ethnic groups.
Comprises other Hispanic subgroups not individually identified and unknown Hispanic origin.
Comprises Hawaiian, Guamanian, Samoan, and other Asian/Pacific Islander subgroups not individually identified, as well as non-Hispanic individuals reporting multiple races who were bridged to Asian/Pacific Islander race.
Age-Standardized Rates of Gestational Diabetes
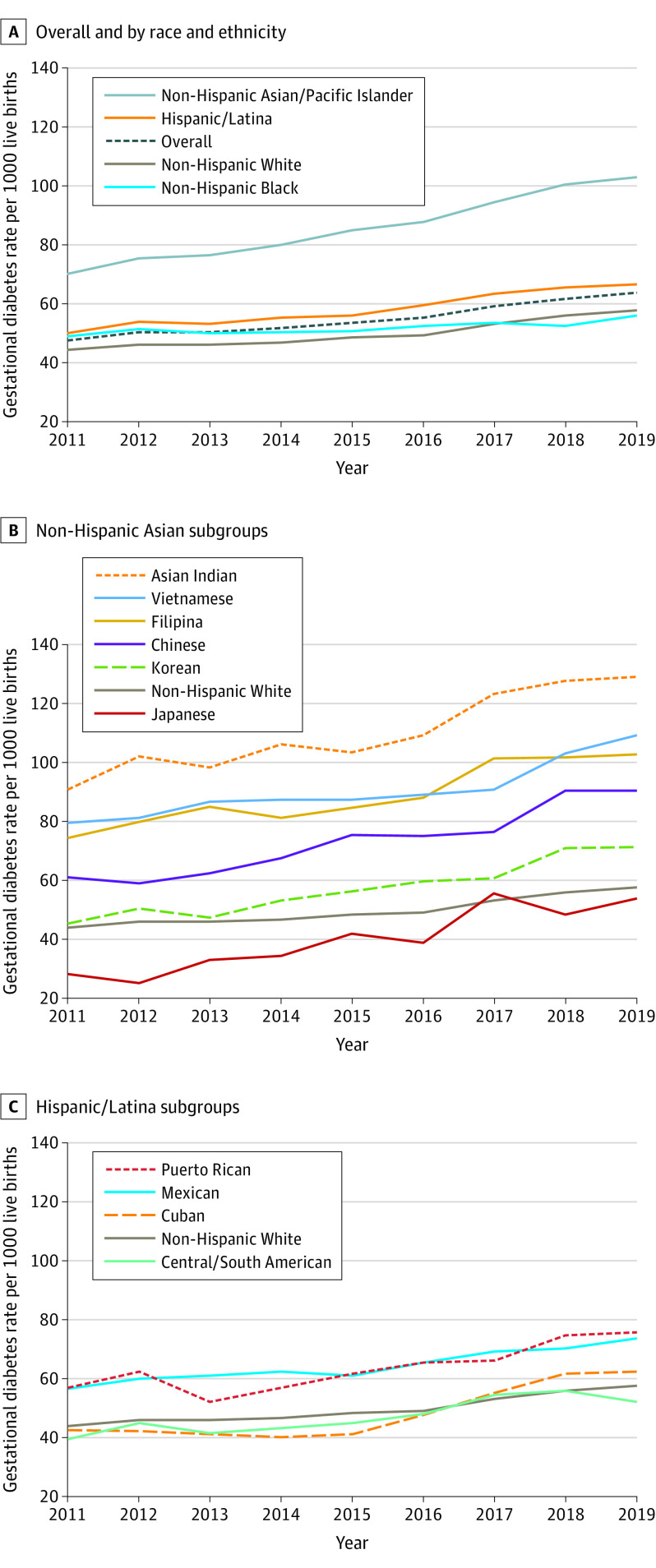
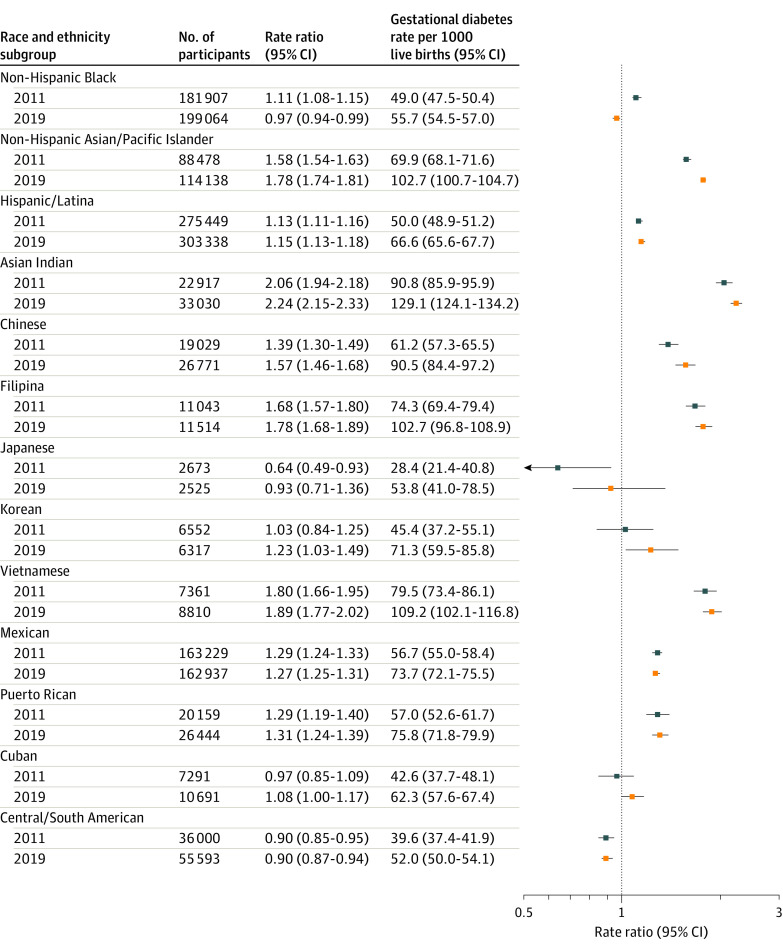
Overall age-standardized rates of gestational diabetes in individuals with a first live birth increased significantly from 47.6 (95% CI, 47.1-48.0) to 63.5 (95% CI, 63.1-64.0) per 1000 live births from 2011 to 2019 (Figure 2). The mean APC in age-standardized rates of gestational diabetes in this population was 3.7% (95% CI, 2.8-4.6) per year (eTable 4 in the Supplement). Gestational diabetes rates in non-Hispanic White individuals at first live birth increased significantly from 44.1 (95% CI, 43.6-44.6) to 57.7 (95% CI, 57.2-58.3) per 1000 live births from 2011 to 2019 (APC, 3.5% [95% CI, 2.8%-4.3%] per year). In non-Hispanic Black individuals at first live birth, gestational diabetes rates increased significantly from 49.0 (95% CI, 47.5-50.4) to 55.7 (95% CI, 54.5-57.0) per 1000 live births from 2011 to 2019 (APC, 1.3% [95% CI, 0.6%-1.9%] per year). Relative to the 2019 gestational diabetes rate in non-Hispanic White individuals (57.7 per 1000 live births), the rate of gestational diabetes in non-Hispanic Black individuals was statistically significantly lower (55.7 per 1000 live births; RR, 0.97 [95% CI, 0.94-0.99]; Figure 3). The RRs for gestational diabetes rates by race and ethnicity groups in 2011, 2015, and 2019 are shown in eTable 5 in the Supplement.
Figure 2. Age-Standardized Rates of Gestational Diabetes in Race and Ethnic Subgroups in Individuals Aged 15 to 44 Years With Singleton First Live Births in the US from 2011 to 2019.
Corresponding data, including annual percent change in gestational diabetes rates, are shown in eTable 3 in the Supplement.
Figure 3. Rate Ratios of Age-Standardized Rates of Gestational Diabetes Among Individuals Aged 15 to 44 Years With Singleton First Live Births in Race and Ethnic Minority Subgroups Relative to Non-Hispanic White Individuals in the US in 2011 and 2019.
Rate ratios in 2011 are relative to non-Hispanic White individuals in 2011 (gestational diabetes rate, 44.1 per 1000 live births). Rate ratios in 2019 are relative to non-Hispanic White individuals in 2019 (gestational diabetes rate, 57.7 per 1000 live births). Corresponding data are shown in eTable 4 in the Supplement.
Absolute age-standardized rates of gestational diabetes among non-Hispanic Asian/Pacific Islander individuals and non-Hispanic Asian subgroups at first live birth are shown in Figure 2. Specifically, among non-Hispanic Asian/Pacific Islander individuals in aggregate, gestational diabetes rates significantly increased from 69.9 (95% CI, 68.1-71.6) to 102.7 (95% CI, 100.7-104.7) per 1000 live births from 2011 to 2019 (APC, 5.0% [95% CI, 4.5%-5.5%] per year) (eTable 4 in the Supplement). Relative to the 2019 gestational diabetes rate among non-Hispanic White individuals, the rate of gestational diabetes among non-Hispanic Asian/Pacific Islander individuals was statistically significantly higher (102.7 per 1000 live births; RR, 1.78 [95% CI, 1.74-1.82]; Figure 3). Absolute gestational diabetes rates in nulliparous individuals at first live birth were highest among Asian Indian individuals, which increased significantly from 90.8 (95% CI, 85.9-95.9) to 129.1 (95% CI, 124.1-134.2) per 1000 live births from 2011 to 2019 (APC, 4.4% [95% CI, 3.1%-5.6%] per year). Although Japanese individuals had the fastest relative increase in absolute gestational diabetes rates, from 28.4 (95% CI, 21.4-40.8) to 53.8 (95% CI, 41.0-78.5) per 1000 live births from 2011 to 2019 (APC, 9.9% [95% CI, 6.4%-13.4%] per year), they had the lowest absolute gestational diabetes rates throughout most of the study period. Relative to non-Hispanic White individuals (gestational diabetes rate of 57.7 per 1000 live births in 2019), absolute gestational diabetes rates among non-Hispanic Asian subgroups ranged from not significantly different in Japanese individuals (53.8 per 1000 live births; 2019 RR, 0.93 [95% CI, 0.71-1.36]) to statistically significantly higher in Asian Indian individuals (129.1 per 1000 live births; 2019 RR, 2.24 [95% CI, 2.15-2.33]) (Figure 3).
Among Hispanic/Latina individuals at first live birth (Figure 2), age-standardized rates of gestational diabetes increased significantly from 50.0 (95% CI, 48.9-51.2) to 66.6 (95% CI, 65.6-67.7) per 1000 live births from 2011 to 2019 (APC, 3.7% [95% CI, 3.0%-4.3%] per year). Relative to the 2019 gestational diabetes rate in non-Hispanic White individuals, the rate of gestational diabetes among Hispanic/Latina individuals was statistically significantly higher (66.6 per 1000 live births; RR, 1.15 [95% CI, 1.13-1.18]) (Figure 3). Absolute gestational diabetes rates were highest in Puerto Rican individuals and increased significantly, from 57.0 (95% CI, 52.6-61.7) to 75.8 (95% CI, 71.8-79.9) per 1000 live births from 2011 to 2019, and lowest in Central/South American individuals and increased significantly, from 39.6 (95% CI, 37.4-41.9) to 52.0 (95% CI, 50.0-54.1) per 1000 live births from 2011 to 2019. Compared with non-Hispanic White individuals in 2019, gestational diabetes rates were significantly lower in Central/South American individuals (52.0 per 1000 live births; 2019 RR, 0.90 [95% CI, 0.97-0.94]), but significantly higher in Cuban (62.3 per 1000 live births; 2019 RR, 1.08 [95% CI, 1.00-1.17]), Mexican (73.7 per 1000 live births; 2019 RR, 1.27 [95% CI, 1.25-1.31]), and Puerto Rican (75.8 per 1000 live births; 2019 RR, 1.31 [95% CI, 1.24-1.39]) individuals (Figure 3). Secondary analyses showed similar magnitude and direction of gestational diabetes trends overall and in all race and ethnicity groups from 2016 to 2019 (eTable 6 in the Supplement).
Age-Specific Rates of Gestational Diabetes
Trends in age-specific rates of gestational diabetes in individuals at first live birth based on 5-year stratified age groups were calculated (eTables 7-9 in the Supplement). Overall gestational diabetes rates per 1000 live births significantly increased within all age strata from 2011 to 2019 (eTable 7 in the Supplement): from 16.0 to 22.4 (APC, 4.2% [95% CI, 3.2%-5.2%] per year) among individuals aged 15 to 19 years, 29.9 to 40.7 (APC, 4.0% [95% CI, 3.4%-4.6%] per year) among individuals aged 20 to 24 years, 46.6 to 61.1 (APC, 3.4% [95% CI, 2.5%-4.4%] per year) among individuals aged 25 to 29 years, 57.8 to 78.0 (APC, 3.8% [95% CI, 3.2%-4.4%] per year) among individuals aged 30 to 34 years, 76.9 to 100.8 (APC, 3.4% [95% CI, 2.8%-4.0%] per year) among individuals aged 35 to 39 years, and 92.5 to 127.5 (APC, 4.2% [95% CI, 3.5%-4.8%] per year) among individuals aged 40 to 44 years. Age-stratified gestational diabetes rates by race and ethnicity groups are shown in eTable 7 in the Supplement, among non-Hispanic Asian subgroups in eTable 8 in the Supplement, and among Hispanic/Latina subgroups in eTable 9 in the Supplement.
Age-Standardized Rates of Pregestational Diabetes
Pregestational diabetes rates in individuals at first live birth increased significantly from 7.3 (95% CI, 7.1-7.4) to 9.0 (95% CI, 8.9-9.2) per 1000 live births from 2011 to 2019 (APC, 2.8% [95% CI, 1.9%-3.6%] per year) (eTable 10 in the Supplement). Relative to non-Hispanic White individuals in 2019 (eTable 11 in the Supplement), in whom the rate of pregestational diabetes was 7.9 (95% CI, 7.7-8.1) per 1000 live births, rates of pregestational diabetes were significantly higher in 2019 among non-Hispanic Black individuals (14.2 per 1000 live births; RR, 1.80 [95% CI, 1.71-1.90]) and Hispanic/Latina individuals (10.8 per 1000 live births; RR, 1.38 [95% CI, 1.31-1.44]) and not statistically significantly different among non-Hispanic Asian/Pacific Islander individuals (7.6 per 1000 live births; RR 0.97 [95% CI, 0.89-1.04)]. Relative to non-Hispanic White individuals in 2019, the rates of pregestational diabetes among non-Hispanic Asian subgroups were significantly higher in Filipina individuals (10.1 per 1000 live births; 2019 RR, 1.29 [95% CI, 1.04-1.59]) and the rates of pregestational diabetes in Hispanic/Latina individuals were significantly higher in Mexican (12.8 [95% CI, 12.1-13.5] per 1000 live births; 2019 RR, 1.62 [95% CI, 1.53-1.73]) and Puerto Rican (14.5 [95% CI, 12.8-16.4] per 1000 live births; 2019 RR, 1.85 [95% CI, 1.62-2.09]) individuals. Secondary analyses showed similar magnitude and direction of pregestational diabetes trends overall and in all race and ethnic groups from 2016 to 2019 (eTable 6 in the Supplement).
Discussion
Among individuals at first live birth in the US, rates of gestational diabetes increased across all race and ethnicity groups and in all age groups from 2011 to 2019. Gestational diabetes rates were significantly higher among most non-Hispanic Asian and Hispanic/Latina subgroups relative to non-Hispanic White individuals. Asian Indian individuals had the highest absolute rates of gestational diabetes throughout the study period. Although absolute rates of pregestational diabetes were low, differences across race and ethnicity groups were present, with the highest rates in non-Hispanic Black and Hispanic/Latina individuals. Trends showed that rates of pregestational diabetes increased over time in most subgroups.
Increases in rates of gestational diabetes among individuals at first live birth of all race and ethnicity subgroups are likely multifactorial in etiology. Older maternal age during pregnancy is associated with a higher risk for gestational diabetes and may contribute, in part, to observed differences and trends; however, mean maternal age at delivery increased only modestly (<2 years) in the past decade.14 Certain groups (eg, Mexican and Puerto Rican individuals) had higher gestational diabetes rates despite lower maternal age compared with other groups, suggesting that factors beyond maternal age likely contribute to differences in gestational diabetes burden. Further, increasing gestational diabetes rates among individuals at first live birth were observed in all age subgroups, which suggests more widespread secular trends among all individuals.14,15 The observed trends in rates of gestational diabetes occurred in parallel with unfavorable increases in prevalence of obesity,16 physical inactivity,17 and poor diet quality,18 which are each known risk factors for gestational diabetes.19,20
Differences in gestational diabetes risk factors and differential exposure and vulnerability to social determinants of health between individuals by race and ethnicity group may also contribute to the wide heterogeneity in gestational diabetes rates. Differences in pregestational BMI, educational attainment, and insurance were observed that may influence risk of gestational diabetes and quality of prenatal care. Specifically, Hispanic/Latina individuals at first live birth had a relatively higher BMI and lower educational attainment than non-Hispanic White individuals. In contrast, Asian Indian individuals at first live birth had the highest rates of gestational diabetes despite lower BMI levels and higher educational attainment. Structural factors, such as access to and use of prenatal care, as well as the screening and gestational diabetes diagnostic criteria used by clinicians, have the potential to differentially influence both diagnosis and ascertainment of gestational diabetes across race and ethnicity groups.
Higher cardiometabolic risk may also be related to epigenetic and lifestyle factors as well as dysregulated visceral fat deposition at lower BMI values in Asian populations, contributing to the observed burden of gestational diabetes in non-Hispanic Asian American individuals.21 These patterns among subgroups with higher rates of gestational diabetes are similar to the epidemiology of type 2 diabetes in the US, with highest rates of type 2 diabetes in non-Hispanic Asian (Asian Indian) and Hispanic/Latino (Mexican American) adults.22 Known differences in behavioral, psychosocial, structural, and immigration-related factors between subgroups, such as culturally specific dietary patterns, physical activity levels, rates of depression, acculturation, and discrimination may further contribute to observed gestational diabetes differences.23,24,25,26 Several of these determinants may also contribute to the observed differences in gestational diabetes vs pregestational diabetes trends. For example, gestational diabetes rates were significantly lower, but pregestational diabetes rates were significantly higher, in non-Hispanic Black individuals compared with non-Hispanic White individuals, indicating that more non-Hispanic Black individuals had diabetes when becoming pregnant so were not “at risk” for gestational diabetes.
Given that gestational diabetes is associated with increased short-term and long-term risks for individuals and their offspring, the observed trends and disparities may portend a greater burden of future cardiometabolic disease. Gestational diabetes is associated with up to 10-times higher odds of development of maternal type 2 diabetes or prediabetes compared with individuals with a normoglycemic pregnancy,27,28 and an approximately 2-fold increased risk for incident cardiovascular disease.2,29,30 Recent trends in documenting increasing rates of premature mortality related to diabetes and coronary heart disease in young adults underscore the importance of targeting the postpartum period for cardiometabolic disease prevention.31,32,33 There is also evidence indicating intergenerational transmission of cardiometabolic risk related to gestational diabetes. Gestational diabetes is associated with childhood obesity and impaired glucose tolerance in offspring.27 One large observational study identified that offspring of women with gestational diabetes had a 29% higher rate of early-onset cardiovascular disease.6 However, further research is needed to understand the long-term cardiometabolic consequences of gestational diabetes in subgroups with the highest burden, who are less represented in previous research.
The observed heterogeneity in rates of gestational diabetes within subgroups of non-Hispanic Asian and Hispanic individuals at first live birth highlight the importance of ascertaining and reporting rates specific to these disaggregated non-Hispanic Asian and Hispanic subgroups.34,35 The reported trends herein may also guide strategies to equitably address increasing gestational diabetes rates by investigating the determinants of gestational diabetes and interventions to address them in populations with high burden.36 Addressing modifiable prenatal risk factors and implementing strategies to prevent gestational diabetes and postnatal diabetes in all individuals, with particular focus on groups with disproportionately high gestational diabetes rates, may help to reduce disparities in long-term cardiovascular and metabolic disease outcomes. Consideration for earlier screening prior to the universal second trimester screening for gestational diabetes (between 24 and 28 weeks) is currently recommended by the American College of Obstetrics and Gynecology in individuals with risk factors for gestational diabetes, including individuals who identify as being from racial or ethnic subgroups at increased risk.10
Limitations
This study has several limitations. First, it is possible that the assessment of the trends before 2016 may be affected by only including data from states that adopted the 2003 birth certificate revision. Individual-level data from birth certificates by state are not publicly available, so it was not possible to fully evaluate the degree to which staggered state-level adoption of the 2003 birth certificate revision contributed to observed trends. This issue may have particularly affected the results of the Native Hawaiian and Pacific Islander individuals, because Hawaii was not included until 2014. However, a secondary analysis showed similar magnitude and direction of trends from 2016 to 2019 (the period in which all states/regions adopted the revised certificate) as were observed from 2011 to 2019. Second, the analysis was only among individuals with singleton first live births. It is known that prior gestational diabetes is a risk factor for subsequent gestational diabetes, but the deidentified nature of this data set meant that individuals who had multiple children during the study period could not be distinguished. Because multiparity may be a risk factor for gestational diabetes, focusing on individuals at first live birth may actually underestimate total gestational diabetes rates.37
Third, the potential for miscoding or lack of awareness of pregestational diabetes that may be related to differences in screening or preconception care in individuals of childbearing age is possible. Previous research has shown that the sensitivity of birth certificates for identifying gestational diabetes is 46% to 83% (median, 65%) and the specificity is greater than 98%,38 with substantial agreement of gestational diabetes diagnosis between birth certificates and medical records.39 This study therefore focused primarily on identifying differences and trends in gestational diabetes, acknowledging that birth certificate data may underestimate the true burden of gestational diabetes and overt type 2 diabetes may be less likely to be diagnosed prior to pregnancy in certain subgroups. However, the NCHS data are the largest, most comprehensive source of vital statistics data capturing all live births in the US. Gestational diabetes diagnosis in these records is completed by the professional birth attendant, so identification of gestational diabetes does not rely on administrative coding. But, there may be inconsistency between clinicians in how gestational diabetes is diagnosed depending on which diagnostic criteria are used (eg, American College of Obstetrics and Gynecology vs the International Association of Diabetes and Pregnancy Study Groups criteria40), so these findings may, in part, reflect secular trends in gestational diabetes detection related to adoption of more liberal diagnostic criteria. Fourth, these estimates do not include individuals with fetal deaths, who were excluded given inconsistencies in state-level reporting. Fetal death is an event that has been associated with gestational diabetes, but represented less than 0.7% of all pregnancy records in the study period. Fifth, lack of data on other covariates, such as diet quality or physical activity, limit assessment of key risk factors that may be contributing to observed trends at a national level. Sixth, although these data include self-identification of several subgroups, options to identify as Southeast Asian or Middle Eastern/North African groups were not available.
Conclusions
Among individuals with a singleton first live birth in the US from 2011 to 2019, rates of gestational diabetes increased across all race and ethnicity subgroups. Differences in absolute gestational diabetes rates were observed across race and ethnicity subgroups.
eTable 1. Characteristics of individuals included versus excluded in analysis, 2011-2019
eTable 2. Maternal characteristics in individuals aged 15-44 years with singleton first live births in non-Hispanic Asian subgroups in the United States, 2011-2019
eTable 3. Maternal characteristics in individuals aged 15-44 years with singleton first live births in Hispanic/Latina subgroups, 2011-2019
eTable 4. Age-standardized rates of gestational diabetes mellitus in race/ethnic subgroups in individuals aged 15-44 years with singleton first live births in the United States, 2011-2019
eTable 5. Rate ratios of age-standardized rates of gestational diabetes mellitus in individuals aged 15-44 years with singleton first live births in racial/ethnic minority groups compared with non-Hispanic White individuals in the United States, 2011 to 2019
eTable 6. Secondary analysis of age-standardized rates of gestational diabetes mellitus and pre-gestational diabetes in females aged 15-44 years with singleton first live births in the United States, 2016-2019
eTable 7. Age-specific gestational diabetes rates per 1,000 live births in individuals at first live birth, by race/ethnicity
eTable 8. Age-specific gestational diabetes rates per 1,000 live births in non-Hispanic Asian subgroups
eTable 9. Age-specific gestational diabetes rates per 1,000 live births in Hispanic subgroups
eTable 10. Age-standardized rates of pre-gestational diabetes per 1,000 live births in individuals aged 15-44 years with singleton first live births by race/ethnicity in the United States, 2011-2019
eTable 11. Rate ratios of age-standardized rates of pre-gestational diabetes in individuals aged 15-44 years with singleton first live births in racial/ethnic subgroups compared with non-Hispanic White individuals in the United States, 2011 to 2019
eTable 12. Gestational diabetes rates in other non-Hispanic subgroups, 2011-2019 (pooled)
eFigure 1. Rates of gestational diabetes mellitus (per 1,000 live births) in individuals with singleton first live births in 5-year age strata, by race/ethnicity in the United States, 2011-2019
References
- 1.Xiang AH, Li BH, Black MH, et al. Racial and ethnic disparities in diabetes risk after gestational diabetes mellitus. Diabetologia. 2011;54(12):3016-3021. doi: 10.1007/s00125-011-2330-2 [DOI] [PubMed] [Google Scholar]
- 2.Kramer CK, Campbell S, Retnakaran R. Gestational diabetes and the risk of cardiovascular disease in women: a systematic review and meta-analysis. Diabetologia. 2019;62(6):905-914. doi: 10.1007/s00125-019-4840-2 [DOI] [PubMed] [Google Scholar]
- 3.HAPO Study Cooperative Research Group . Hyperglycemia and Adverse Pregnancy Outcome (HAPO) study: associations with neonatal anthropometrics. Diabetes. 2009;58(2):453-459. doi: 10.2337/db08-1112 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Lowe WL Jr, Scholtens DM, Kuang A, et al. ; HAPO Follow-up Study Cooperative Research Group . Hyperglycemia and Adverse Pregnancy Outcome Follow-up Study (HAPO FUS): maternal gestational diabetes mellitus and childhood glucose metabolism. Diabetes Care. 2019;42(3):372-380. doi: 10.2337/dc18-1646 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Nehring I, Chmitorz A, Reulen H, von Kries R, Ensenauer R. Gestational diabetes predicts the risk of childhood overweight and abdominal circumference independent of maternal obesity. Diabet Med. 2013;30(12):1449-1456. doi: 10.1111/dme.12286 [DOI] [PubMed] [Google Scholar]
- 6.Yu Y, Arah OA, Liew Z, et al. Maternal diabetes during pregnancy and early onset of cardiovascular disease in offspring: population based cohort study with 40 years of follow-up. BMJ. 2019;367:l6398. doi: 10.1136/bmj.l6398 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Deputy NP, Kim SY, Conrey EJ, Bullard KM. Prevalence and changes in preexisting diabetes and gestational diabetes among women who had a live birth: United States, 2012-2016. MMWR Morb Mortal Wkly Rep. 2018;67(43):1201-1207. doi: 10.15585/mmwr.mm6743a2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.US Government Code of Federal Regulations. 45 CFR part 46.102(l)(2); 21 CFR part 56; 42 USC §241(d); 5 USC §552a; 44 USC §3501 et seq.
- 9.Pu J, Zhao B, Wang EJ, et al. Racial/ethnic differences in gestational diabetes prevalence and contribution of common risk factors. Paediatr Perinat Epidemiol. 2015;29(5):436-443. doi: 10.1111/ppe.12209 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Committee on Practice Bulletins-Obstetrics . ACOG practice bulletin no. 190: gestational diabetes mellitus. Obstet Gynecol. 2018;131(2):e49-e64. doi: 10.1097/AOG.0000000000002501 [DOI] [PubMed] [Google Scholar]
- 11.User Guide to the 2014 Natality Public Use File. Centers for Disease Control and Prevention; 2014. Accessed November 15, 2020. http://data.nber.org/natality/2014/natl2014.pdf
- 12.Joinpoint Regression Program , version 4.7.0.0 [computer program]. National Cancer Institute; 2019.
- 13.Tiwari RC, Clegg LX, Zou Z. Efficient interval estimation for age-adjusted cancer rates. Stat Methods Med Res. 2006;15(6):547-569. doi: 10.1177/0962280206070621 [DOI] [PubMed] [Google Scholar]
- 14.Mathews TJ, Hamilton BE. Mean age of mothers is on the rise: United States, 2000-2014. NCHS Data Brief. 2016;(232):1-8. [PubMed] [Google Scholar]
- 15.Li Y, Ren X, He L, Li J, Zhang S, Chen W. Maternal age and the risk of gestational diabetes mellitus: a systematic review and meta-analysis of over 120 million participants. Diabetes Res Clin Pract. 2020;162:108044. doi: 10.1016/j.diabres.2020.108044 [DOI] [PubMed] [Google Scholar]
- 16.Ward ZJ, Bleich SN, Cradock AL, et al. Projected U.S. state-level prevalence of adult obesity and severe obesity. N Engl J Med. 2019;381(25):2440-2450. doi: 10.1056/NEJMsa1909301 [DOI] [PubMed] [Google Scholar]
- 17.An R, Xiang X, Yang Y, Yan H. Mapping the prevalence of physical inactivity in US states, 1984-2015. PLoS One. 2016;11(12):e0168175. doi: 10.1371/journal.pone.0168175 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Virani SS, Alonso A, Benjamin EJ, et al. ; American Heart Association Council on Epidemiology and Prevention Statistics Committee and Stroke Statistics Subcommittee . Heart disease and stroke statistics—2020 update: a report from the American Heart Association. Circulation. 2020;141(9):e139-e596. doi: 10.1161/CIR.0000000000000757 [DOI] [PubMed] [Google Scholar]
- 19.Chu SY, Callaghan WM, Kim SY, et al. Maternal obesity and risk of gestational diabetes mellitus. Diabetes Care. 2007;30(8):2070-2076. doi: 10.2337/dc06-2559a [DOI] [PubMed] [Google Scholar]
- 20.Oken E, Ning Y, Rifas-Shiman SL, Radesky JS, Rich-Edwards JW, Gillman MW. Associations of physical activity and inactivity before and during pregnancy with glucose tolerance. Obstet Gynecol. 2006;108(5):1200-1207. doi: 10.1097/01.AOG.0000241088.60745.70 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.WHO Expert Consultation . Appropriate body-mass index for Asian populations and its implications for policy and intervention strategies. Lancet. 2004;363(9403):157-163. doi: 10.1016/S0140-6736(03)15268-3 [DOI] [PubMed] [Google Scholar]
- 22.Cheng YJ, Kanaya AM, Araneta MRG, et al. Prevalence of diabetes by race and ethnicity in the United States, 2011-2016. JAMA. 2019;322(24):2389-2398. doi: 10.1001/jama.2019.19365 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Hassani Zadeh S, Boffetta P, Hosseinzadeh M. Dietary patterns and risk of gestational diabetes mellitus: a systematic review and meta-analysis of cohort studies. Clin Nutr ESPEN. 2020;36:1-9. doi: 10.1016/j.clnesp.2020.02.009 [DOI] [PubMed] [Google Scholar]
- 24.Gavin AR, Melville JL, Rue T, Guo Y, Dina KT, Katon WJ. Racial differences in the prevalence of antenatal depression. Gen Hosp Psychiatry. 2011;33(2):87-93. doi: 10.1016/j.genhosppsych.2010.11.012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Chen L, Shi L, Zhang D, Chao SM. Influence of acculturation on risk for gestational diabetes among Asian women. Prev Chronic Dis. 2019;16:E158. doi: 10.5888/pcd16.190212 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.MacGregor C, Freedman A, Keenan-Devlin L, et al. Maternal perceived discrimination and association with gestational diabetes. Am J Obstet Gynecol MFM. 2020;2(4):100222. doi: 10.1016/j.ajogmf.2020.100222 [DOI] [PubMed] [Google Scholar]
- 27.Lowe WL Jr, Scholtens DM, Lowe LP, et al. ; HAPO Follow-up Study Cooperative Research Group . Association of gestational diabetes with maternal disorders of glucose metabolism and childhood adiposity. JAMA. 2018;320(10):1005-1016. doi: 10.1001/jama.2018.11628 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Vounzoulaki E, Khunti K, Abner SC, Tan BK, Davies MJ, Gillies CL. Progression to type 2 diabetes in women with a known history of gestational diabetes: systematic review and meta-analysis. BMJ. 2020;369:m1361. doi: 10.1136/bmj.m1361 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kim C, Newton KM, Knopp RH. Gestational diabetes and the incidence of type 2 diabetes: a systematic review. Diabetes Care. 2002;25(10):1862-1868. doi: 10.2337/diacare.25.10.1862 [DOI] [PubMed] [Google Scholar]
- 30.Tobias DK, Stuart JJ, Li S, et al. Association of history of gestational diabetes with long-term cardiovascular disease risk in a large prospective cohort of US women. JAMA Intern Med. 2017;177(12):1735-1742. doi: 10.1001/jamainternmed.2017.2790 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Shah NS, Lloyd-Jones DM, Kandula NR, et al. Adverse trends in premature cardiometabolic mortality in the United States, 1999 to 2018. J Am Heart Assoc. 2020;9(23):e018213. doi: 10.1161/JAHA.120.018213 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Arora S, Stouffer GA, Kucharska-Newton AM, et al. Twenty year trends and sex differences in young adults hospitalized with acute myocardial infarction. Circulation. 2019;139(8):1047-1056. doi: 10.1161/CIRCULATIONAHA.118.037137 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Guo J, Chen JL, Whittemore R, Whitaker E. Postpartum lifestyle interventions to prevent type 2 diabetes among women with history of gestational diabetes: a systematic review of randomized clinical trials. J Womens Health (Larchmt). 2016;25(1):38-49. doi: 10.1089/jwh.2015.5262 [DOI] [PubMed] [Google Scholar]
- 34.Palaniappan LP, Araneta MR, Assimes TL, et al. ; American Heart Association Council on Epidemiology and Prevention; American Heart Association Council on Peripheral Vascular Disease; American Heart Association Council on Nutrition, Physical Activity, and Metabolism; American Heart Association Council on Clinical Cardiology; American Heart Association Council on Cardiovascular Nursing; Council on Cardiovascular Nursing . Call to action: cardiovascular disease in Asian Americans: a science advisory from the American Heart Association. Circulation. 2010;122(12):1242-1252. doi: 10.1161/CIR.0b013e3181f22af4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Rodriguez CJ, Allison M, Daviglus ML, et al. ; American Heart Association Council on Epidemiology and Prevention; American Heart Association Council on Clinical Cardiology; American Heart Association Council on Cardiovascular and Stroke Nursing . Status of cardiovascular disease and stroke in Hispanics/Latinos in the United States: a science advisory from the American Heart Association. Circulation. 2014;130(7):593-625. doi: 10.1161/CIR.0000000000000071 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Bower JK, Butler BN, Bose-Brill S, Kue J, Wassel CL. Racial/ethnic differences in diabetes screening and hyperglycemia among US women after gestational diabetes. Prev Chronic Dis. 2019;16:E145. doi: 10.5888/pcd16.190144 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Schwartz N, Green MS, Yefet E, Nachum Z. Modifiable risk factors for gestational diabetes recurrence. Endocrine. 2016;54(3):714-722. doi: 10.1007/s12020-016-1087-2 [DOI] [PubMed] [Google Scholar]
- 38.Devlin HM, Desai J, Walaszek A. Reviewing performance of birth certificate and hospital discharge data to identify births complicated by maternal diabetes. Matern Child Health J. 2009;13(5):660-666. doi: 10.1007/s10995-008-0390-9 [DOI] [PubMed] [Google Scholar]
- 39.Gregory ECW, Martin JA, Argov EL, Osterman MJK. Assessing the quality of medical and health data from the 2003 birth certificate revision: results from New York City. Natl Vital Stat Rep. 2019;68(8):1-20. [PubMed] [Google Scholar]
- 40.Metzger BE, Gabbe SG, Persson B, et al. ; International Association of Diabetes and Pregnancy Study Groups Consensus Panel . International association of diabetes and pregnancy study groups recommendations on the diagnosis and classification of hyperglycemia in pregnancy. Diabetes Care. 2010;33(3):676-682. doi: 10.2337/dc09-1848 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
eTable 1. Characteristics of individuals included versus excluded in analysis, 2011-2019
eTable 2. Maternal characteristics in individuals aged 15-44 years with singleton first live births in non-Hispanic Asian subgroups in the United States, 2011-2019
eTable 3. Maternal characteristics in individuals aged 15-44 years with singleton first live births in Hispanic/Latina subgroups, 2011-2019
eTable 4. Age-standardized rates of gestational diabetes mellitus in race/ethnic subgroups in individuals aged 15-44 years with singleton first live births in the United States, 2011-2019
eTable 5. Rate ratios of age-standardized rates of gestational diabetes mellitus in individuals aged 15-44 years with singleton first live births in racial/ethnic minority groups compared with non-Hispanic White individuals in the United States, 2011 to 2019
eTable 6. Secondary analysis of age-standardized rates of gestational diabetes mellitus and pre-gestational diabetes in females aged 15-44 years with singleton first live births in the United States, 2016-2019
eTable 7. Age-specific gestational diabetes rates per 1,000 live births in individuals at first live birth, by race/ethnicity
eTable 8. Age-specific gestational diabetes rates per 1,000 live births in non-Hispanic Asian subgroups
eTable 9. Age-specific gestational diabetes rates per 1,000 live births in Hispanic subgroups
eTable 10. Age-standardized rates of pre-gestational diabetes per 1,000 live births in individuals aged 15-44 years with singleton first live births by race/ethnicity in the United States, 2011-2019
eTable 11. Rate ratios of age-standardized rates of pre-gestational diabetes in individuals aged 15-44 years with singleton first live births in racial/ethnic subgroups compared with non-Hispanic White individuals in the United States, 2011 to 2019
eTable 12. Gestational diabetes rates in other non-Hispanic subgroups, 2011-2019 (pooled)
eFigure 1. Rates of gestational diabetes mellitus (per 1,000 live births) in individuals with singleton first live births in 5-year age strata, by race/ethnicity in the United States, 2011-2019