Abstract
Background & objectives:
Examine maternal gestational diabetes mellitus (GDM), macrosomia and DNA methylation in candidate genes IGF1, IGF2, H19, ARHGRF11, MEST, NR3C1, ADIPOQ and RETN.
Materials & methods:
1145 Children (572 GDM cases and 573 controls) from The Tianjin GDM study, including 177 with macrosomia, had blood DNA collection at median age 5.9 (range: 3.1–10.0). We used logistic regression to screen for associations with GDM and model macrosomia on 37 CpGs, and performed mediation analysis.
Results:
One CpG was associated with macrosomia at false discovery rate (FDR) <0.05 (cg14428359 in MEST); two (cg19466922 in MEST and cg26263166 in IGF2) were associated at p < 0.05 but mediated 26 and 13%, respectively.
Conclusion:
MEST and IGF2 were previously identified for potential involvement in fetal growth and development (Trial Registration number: NCT01554358 [ClinicalTrials.gov]).
Keywords: : epigenetic epidemiology, epigenetics, macrosomia, metabolic diseases, pediatrics
Lay abstract
Many women who get gestational diabetes during pregnancy go on to give birth to larger (macrosomic) babies. These babies then grow up to have greater risk of being overweight or obese, and all the health concerns this entails. We sought to examine whether epigenetic factors could help explain this link, by examining the blood of some children whose mothers were enrolled in a gestational diabetes study in China. We identified three sites on two different genes as being associated with both gestational diabetes and macrosomia. The way these genes work suggest a mechanism for how they contribute to macrosomia, providing a promising new avenue for future research, early detection and precision prevention (Trial Registration number: NCT01554358 [ClinicalTrials.gov]).
Fetal macrosomia (defined as birth weight >4000–4500 g, or greater than 90% for gestational age) is estimated to affect 7% of all births in the US as of 2015 [1], with the crude rate of macrosomia having increased nearly 24-times from 1979 to 2010 [2]. Macrosomia is associated with increased rates of maternal [3,4] and fetal [5–7] complications as well as increased risks of obesity and Type 2 diabetes [8,9] and potentially other aging-related diseases as well [10]. Gestational diabetes mellitus (GDM) is a known risk factor for macrosomia [11], with recent estimates suggesting that between 15 and 45% of newborns of mothers with GDM are macrosomic compared with 12% in children of non-GDM mothers [12]. Epigenetic reprogramming in the in utero environment is one suspected driver of this relationship.
Inflammatory cells (including white blood cells) may participate in the etiology of pediatric obesity [13] by sustaining metabolic changes such as those due to GDM exposure in offspring [14], and contributing to obesity [15]. GDM [16–18] can affect the child’s blood DNA methylation, which can in turn influence metabolic risks [17–19]. For example, West et al. [20] linked blood DNA methylation in children 8–12 years old to prenatal GDM exposure. Talens et al. found that methylation at loci sensitive to prenatal exposures was associated with myocardial infarction later in life [19]. Even if these methylation changes do not persist into later life, that can still perturb cardiometabolic phenotypes in childhood (e.g., macrosomia) and in turn influence health later in life. Thus, blood DNA derived from circulating leukocytes provides an ideal tissue for methylomic biomarkers of GDM exposure and potentially its subsequent adverse metabolic impacts.
Most studies to date of DNA methylation associated with macrosomia have focused on Beckwith–Wiedemann syndrome, a congenital disorder characterized by macrosomia and caused by genetic and epigenetic abnormalities in chromosomal region 11p15.5, which includes the imprinting genes IGF2/H19 and CDKN1C/KCNQ1OT1 [21]. To date, few studies have examined DNA methylation in children with macrosomia but without Beckwith–Wiedemann syndrome. Two that examined DNA methylation in cord blood found altered methylation in IGF2/H19 [22] and ARHGEF11 [23] associated with macrosomia in offspring exposed to in utero hyperglycemia. This study’s objectives were to explore associations between both GDM and macrosomia and DNA methylation of eight genes selected for analysis based on a literature review of candidates potentially involved in GDM and obesogenic pathways [12,24].
Materials & methods
Study population & data collection
The full study population and methods have been described previously [25]. Briefly, data were taken from the Tianjin GDM Observational Study, in the 4th-largest city in China; mothers were aged 20–49 and diagnosed with GDM between 2005 and 2009 using the 1998 WHO criteria [26]. All enrolled pregnant women at 26–30 gestational weeks first underwent a 1-h oral glucose test (OGTT) with a 50-g glucose load; women with a glucose reading ≥7.8 mmol/l were invited to a 2-h OGTT with a 75 g glucose load and had a result confirming either diabetes (fasting glucose >7 nmol/l or 2-h OGTT >11.1 nmol/l) or impaired glucose tolerance (2-h glucose >7.8 and <11.1 nmol/l) were defined as having GDM.
The present study included 580 women with GDM and their children; an additional 580 mother–child pairs without GDM and frequency were matched on child sex and birth date were also included [27,28]. Of these, only 15 (eight from GDM mothers, seven from the control group) mother-child pairs were excluded from epigenetic analyses during data preprocessing. This left a total of 1145 mothers for analysis, with baseline visit between 2013 and 2016 which included self-administered questionnaires and a physical examination. Blood draws for DNA methylation analysis (using the Illumina 850 K array [Illumina, CA, USA]) were collected from children at median age 5.9 years, range 3.1–10.2.
Covariate measurement
All mothers completed a questionnaire on sociodemographic variables (age, marital status, education, income and occupation), history of GDM (family history of diabetes, coronary heart disease, stroke, cancer and hypertension), medical history (hypertension, diabetes and hypercholesterolemia), prepregnancy and postpartum weight and weight gain during pregnancy, alcohol intake, smoking habits, physical activity, dietary habits (via self-administered food frequency questionnaire) and sleep status. Bodyweight, height, waist and hip circumferences, blood pressure, and heart rate were all measured according to standard protocols. Body fat was measured by a body composition analyzer (InBody J20 [InBody UK, Coalville, UK]) to the nearest 0.1% and triceps, subscapular and suprailiac skinfolds by skinfold caliper to the nearest 0.5 cm. BMI was calculated as the bodyweight in kilograms divided by the square of the height in meters. Children’s information was obtained by another questionnaire completed by their mothers and included age, sex, birth date, birth length, birth weight, gestational age (copied from birth certificate), infant feeding patterns, lactation duration, routine activities (physical activities and sleep duration), dietary habits and history of diseases.
White blood cell type proportion estimation & principal components for non-negative probes
To account for cell composition variability we estimated the proportions of CD4+ T lymphocytes, CD8+ T lymphocytes, B lymphocytes, natural killer cells, monocytes and granulocytes using the method published by Houseman et al. [29] To account for experimental batch effects and other technical biases, we derived surrogate variables from intensity data for nonnegative internal control probes using principal components analysis [30]. The top 16 principal components explain 95.03% of the variation across the non-negative internal control probes and can be adjusted for in multivariate analyses to account for potential batch effects.
Illumina EPIC array methylation data quality control & preprocessing
Infinium MethylationEPIC BeadChip raw data (IDAT files [Illumina]) were loaded into the R package minfi. Quality control and data preprocessing were conducted using the R package ENmix (Bioconductor, NY, USA) [30] with default parameter settings. In the quality control step, low-quality methylation measurements were identified by detection p-value < 10-6 or number of beads <3 [30]. We excluded 10,861 CpGs with a detection rate <95% and 13 samples with a percentage of low-quality methylation measurements with >5% or extremely low intensity of bisulfite conversion probes (less than 3 × standard deviation of the intensity across samples below the mean intensity) [30]. We identified no sample outliers based on total intensity across CpGs. Dye bias was corrected using regression on logarithm of internal control probes (RELIC), which utilizes the intensity values of paired internal control probes that monitor the two-color channels [31]. We then separately quantile-normalized M or U intensities for Infinium I or II probes, respectively. At last, low-quality methylation values (detection p < 10-6 or number of beads <3) and extreme β-value outliers across samples (defined by Tukey’s method) were set as missing. The final clean methylation working dataset contains 856,146 CpG probes and 1145 samples.
Statistical methods
First, we conducted a descriptive analysis comparing all variables of interest between macrosomia cases and controls; continuous variables were compared using a t-test for normally distributed variables and a Wilcoxon rank-sum test for non-normally distributed variables (i.e., skinfolds), and categorical variables using a chi-squared test. Next, based on a literature review we identified eight candidate genes with a total of 488 CpG sites available in our dataset: ARHGEF11 (52 CpG sites), MEST (89 CpGs), NR3C1 (86 CpGs), ADIPOQ (13 CpGs), RETN (11 CpGs), IGF1 (27 CpGs), IGF2 (151 CpGs) and H19 (59 CpGs). For the single-CpG analysis we initially screened 488 CpG sites in a logistic regression model for associations with methylation as the outcome and GDM as the independent variable of interest at p < 0.05; 37 of these were significant and tested in a second logistic regression model with macrosomia as the outcome (and methylation as the exposure). Both of these models adjusted for maternal height, age, smoking status, passive smoking exposure, prepregnancy BMI, pregnancy weight gain, parity, pregnancy hypertension, child sex and gestational age at delivery, child age and weight for age z-score at methylation measurement, blood cell type proportions and 10 principal components representing processing batch effects (>90% of variance). We used FDR adjustment to correct for multiple testing in these final macrosomia models, with FDR-adjusted q <0.05 considered statistically significant. We also conducted a sensitivity analysis exploring the effect of replacing macrosomia as our outcome with birth weight as a continuous variable in our single-CpG analysis. Next, we used two methods (the Fisher method and the Empirical Brown method) [32] to combine the single-CpG raw p-values and conduct regional analyses of the promoter and body regions of each of our target genes, testing their associations with macrosomia. Finally, we conducted separate mediation analyses of our three most significant hits using the R package ‘mediation’ [33]. Specifically, two models are fit: one modeling the effects of GDM on DNA methylation, and a second one jointly modeling the effects of GDM (directly) and DNA methylation (indirectly) on macrosomia. Using Monte Carlo simulations, a mediation proportion is estimated, indicating how much of the effects of GDM on macrosomia could be explained by the indirect path in which GDM drives a change in DNA methylation, which then affects macrosomia. This effect is estimated in a model including all confounding variables that affect both the mediating variable (methylation) and the outcome (macrosomia).
Results
Participant characteristics by maternal GDM status have been previously reported [34]. Table 1 shows our participant characteristics and descriptive analysis by child macrosomia status. Briefly, we confirmed the association between GDM and macrosomia. Of 573 children born to mothers without GDM, 63 met the criteria for macrosomia and 510 did not; of 572 children born to mothers with GDM, 114 met the criteria for macrosomia and 458 did not (p ≤ 0.001). Mothers with greater pregnancy weight gain and prepregnancy BMI also tended to give birth to macrosomic children (p < 0.001 for both). Additionally, female children tended to be macrosomic (p = 0.03) as did those with an older gestational age at delivery (p < 0.001); median birth weight was 765 g heavier for macrosomic children (p < 0.001). Finally, macrosomic children tended to have greater BMI z-score (p < 0.001), weight for age z-score (p < 0.001), body fat percentage (p = 0.02) and suprailiac skinfold (p = 0.03) at the time of methylation measurement. We identified no other significant differences in participant characteristics by macrosomia status.
Table 1. . Participant characteristics by child macrosomia status.
| Non-macrosomia (n = 968) | Macrosomia (n = 177) | p-value | |
|---|---|---|---|
| Maternal characteristics | |||
| GDM status | <0.001 | ||
| – No | 510 (51.26%) | 63 (35.59%) | |
| – Yes | 458 (48.74%) | 114 (64.41%) | |
| Total parity | 0.17 | ||
| – 1 | 952 (98.35%) | 177 (100%) | |
| – 2 | 16 (1.65%) | 0 (0%) | |
| Smoking status | 0.09 | ||
| – Never | 925 (95.56%) | 166 (93.79%) | |
| – Former | 15 (1.55%) | 7 (3.95%) | |
| – Current | 28 (2.89%) | 4 (2.26%) | |
| Pregnancy-induced hypertension | 0.56 | ||
| – No | 931 (96.18%) | 168 (94.92%) | |
| – Yes | 37 (3.82%) | 9 (5.08%) | |
| Maternal height (cm) | 117.5 (93.5–155.4) | 118.5 (97.0–144.8) | 0.17 |
| Age at delivery | 29.58 (22.6–49.4) | 29.43 (23.5–41.2) | 0.35 |
| Pregnancy weight gain (kg) | 15 (4–45) | 20 (10–43) | <0.001 |
| Prepregnancy BMI (kg/m2) | 21.37 (15.4–45.0) | 23.28 (17.7–33.2) | <0.001 |
| Child characteristics at birth | |||
| Child sex | 0.03 | ||
| – Male | 492 (50.83%) | 63 (35.59%) | |
| – Female | 476 (49.17%) | 114 (64.41%) | |
| Child’s birth weight (g) | 3385 (1500–3980) | 4150 (4000–5300) | <0.001 |
| Gestational age at delivery (weeks) | 39 (28–43) | 40 (35–43) | <0.001 |
| Child characteristics at methylation measurement | |||
| Age (years, mean [SD]) | 5.89 (1.26) | 5.81 (1.20) | 0.437 |
| Z-score for BMI (mean [SD]) | 0.11 (1.30) | 0.60 (1.43) | <0.001 |
| Weight for age Z-score (mean [SD]) | 0.50 (1.23) | 1.01 (1.19) | <0.001 |
| Bodyfat (%, mean [SD]) | 19.77 (7.63) | 21.33 (8.61) | 0.015 |
| Skinfolds at triceps (mm, median [IQR]) | 11.00 [9.00, 15.00] | 11.00 [9.50, 17.00] | 0.222 |
| Skinfolds at subscapular (mm, median [IQR]) | 6.00 [5.00, 8.00] | 6.50 [5.50, 9.00] | 0.07 |
| Skinfolds at suprailiac (mm, median [IQR]) | 8.50 [6.00, 12.50] | 9.00 [7.00, 14.00] | 0.034 |
GDM: Gestational diabetes mellitus; IQR: Interquartile range; SD: Standard deviation.
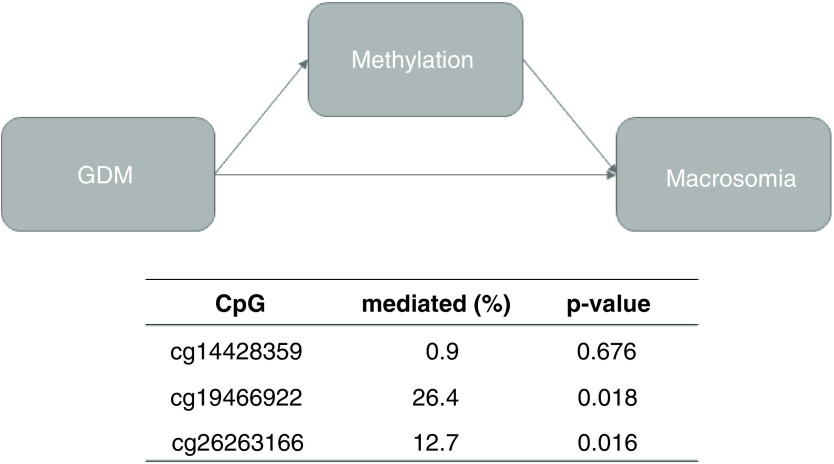
Table 2 shows the most significant CpG sites in our single-CpG analysis. One CpG site on MEST was significantly associated with macrosomia at q <0.05; an additional two CpGs (one on each of MEST and IGF2) were associated with macrosomia at p < 0.05 but q >0.05. Replacing macrosomia with birth weight in our single-CpG analysis did not substantially change our results (data available upon request). Table 3 shows the results of our regional analyses. Using the Fisher method, we identified differential methylation in the promoter region of IGF2 (p = 0.04), and marginally differential methylation in the body region of MEST (p = 0.06). Of note, one of the hits in our single-CpG analysis was located in the body of MEST (cg19466922). Figure 1 shows the results of the mediation analyses of our three significant single-CpG hits. Interestingly, both cg19466922 and cg26263166 were significant mediators of the GDM-Macrosomia association (p = 0.02 for both), mediating 26% and 13% of the relationship, respectively. Our third hit, cg14428359, was not a significant mediator (p = 0.68).
Table 2. . Top CpGs associated with gestational diabetes mellitus at p < 0.05 and their associations with macrosomia.
| CpG information | Macrosomia n = 37 CpGs |
GDM n = 488 CpGs |
||||||
|---|---|---|---|---|---|---|---|---|
| CpG | Gene | Region | β | SE | p-value | q | β | SE |
| cg14428359 | MEST | 3’UTR | -0.012 | 0.003 | <0.001† | <0.001‡ | -0.006 | 0.003 |
| cg19466922 | MEST | Body | -0.010 | 0.004 | 0.006† | 0.089 | -0.017 | 0.004 |
| cg26263166 | IGF2 | 3′UTR | 0.003 | 0.001 | 0.017† | 0.169 | 0.003 | 0.001 |
| cg05345888 | ARHGEF11 | Body | 0.001 | 0.001 | 0.06 | 0.324 | 0.003 | 0.001 |
| cg15886040 | H19 | TSS1500 | 0.006 | 0.003 | 0.064 | 0.324 | 0.008 | 0.004 |
| cg07492680 | ARHGEF11 | Body | -0.004 | 0.002 | 0.064 | 0.324 | -0.006 | 0.002 |
| cg21585493 | ARHGEF11 | TSS200 | 0.001 | 0.001 | 0.09 | 0.341 | 0.002 | 0.001 |
| cg11322849 | INS-IGF2 | TSS1500 | -0.004 | 0.002 | 0.09 | 0.341 | -0.006 | 0.003 |
| cg07589972 | NR3C1 | TSS1500 | 0.002 | 0.001 | 0.147 | 0.492 | -0.004 | 0.001 |
| cg01785096 | ARHGEF11 | Body | 0.003 | 0.003 | 0.187 | 0.563 | 0.007 | 0.003 |
| cg18146873 | NR3C1 | 1st Exon | 0.000 | 0.000 | 0.21 | 0.576 | 0.001 | 0.000 |
| cg15922305 | H19 | TSS200 | -0.004 | 0.004 | 0.298 | 0.685 | 0.01 | 0.004 |
| cg04811924 | ARHGEF11 | TSS200 | 0.001 | 0.001 | 0.33 | 0.685 | 0.001 | 0.001 |
| cg20148994 | MEST | TSS1500 | 0.001 | 0.001 | 0.375 | 0.685 | 0.004 | 0.002 |
| cg25587594 | IGF1 | Body | 0.001 | 0.001 | 0.377 | 0.685 | 0.002 | 0.001 |
| cg12381613 | ARHGEF11 | Body | -0.002 | 0.002 | 0.39 | 0.685 | -0.007 | 0.003 |
| cg12888360 | NR3C1 | Body | 0.001 | 0.001 | 0.444 | 0.685 | 0.004 | 0.002 |
| cg15495409 | ADIPOQ | 3’UTR | 0.000 | 0.000 | 0.468 | 0.685 | 0.001 | 0.001 |
| cg22648984 | ARHGEF11 | Body | -0.001 | 0.002 | 0.474 | 0.685 | -0.005 | 0.002 |
| cg21167159 | H19 | Body | 0.001 | 0.002 | 0.496 | 0.685 | 0.005 | 0.002 |
| cg22424892 | H19 | Body | 0.000 | 0.000 | 0.539 | 0.685 | -0.001 | 0.000 |
| cg25852472 | H19 | Body | 0.002 | 0.003 | 0.543 | 0.685 | -0.007 | 0.003 |
| cg00629244 | NR3C1 | TSS200 | 0.000 | 0.000 | 0.554 | 0.685 | 0.000 | 0.000 |
| cg19457823 | NR3C1 | Body | -0.002 | 0.003 | 0.555 | 0.685 | -0.008 | 0.003 |
| cg16224829 | NR3C1 | 5’UTR | -0.001 | 0.002 | 0.569 | 0.685 | -0.006 | 0.002 |
| cg20101237 | ARHGEF11 | Body | -0.001 | 0.002 | 0.661 | 0.712 | -0.005 | 0.003 |
| cg02166532 | INS-IGF2 | Body | -0.001 | 0.001 | 0.672 | 0.712 | -0.003 | 0.001 |
| cg13791131 | IGF2AS | Body | -0.001 | 0.001 | 0.675 | 0.712 | -0.004 | 0.001 |
| cg07342901 | H19 | Body | 0.000 | 0.001 | 0.726 | 0.712 | -0.003 | 0.001 |
| cg16641322 | INS-IGF2 | Body | 0.000 | 0.001 | 0.75 | 0.712 | -0.003 | 0.001 |
| cg15508379 | IGF2AS | Body | 0.000 | 0.001 | 0.751 | 0.712 | 0.002 | 0.001 |
| cg00286878 | MEST | TSS1500 | 0.001 | 0.002 | 0.756 | 0.712 | 0.007 | 0.002 |
| cg12347392 | MEST | TSS1500 | -0.001 | 0.006 | 0.805 | 0.735 | 0.012 | 0.006 |
| cg11607484 | ARHGEF11 | 3’UTR | 0.000 | 0.001 | 0.829 | 0.735 | 0.002 | 0.001 |
| cg08162473 | INS-IGF2 | Body | 0.000 | 0.000 | 0.937 | 0.807 | 0.000 | 0.000 |
| cg13993218 | INS-IGF2 | Body | 0.00E + 00 | 0.003 | 0.979 | 0.81 | -0.008 | 0.004 |
| cg07409197 | MEST | TSS1500 | 0.00E + 00 | 0.003 | 0.995 | 0.81 | 0.006 | 0.003 |
Significant at p < 0.05.
Significant at q <0.05.
All models adjusted for maternal height, age, smoking status, passive smoking exposure, prepregnancy BMI, pregnancy weight gain, parity, pregnancy hypertension; child sex and gestational age at delivery; child age and weight for age z-score at methylation measurement; blood cell type proportions; and ten principal components representing processing batch effects (>90% of variance).
GDM: Gestational diabetes mellitus; SE: Standard error.
Table 3. . Gene regional methylation associated with gestational diabetes mellitus and macrosomia.
| Gene/region | CpGs | pEBM | pFisher |
|---|---|---|---|
| IGF2_promoter | 133 | 0.16 | 0.04† |
| MEST_Body | 8 | 0.08 | 0.06 |
| ARHGEF11_promoter | 20 | 0.13 | 0.09 |
| IGF2_Body | 18 | 0.21 | 0.12 |
| ARHGEF11_Body | 32 | 0.38 | 0.34 |
| IGF1_Body | 12 | 0.38 | 0.38 |
| ADIPOQ_Body | 2 | 0.45 | 0.45 |
| MEST_promoter | 81 | 0.48 | 0.53 |
| ADIPOQ_promoter | 11 | 0.71 | 0.75 |
| NR3C1_Body | 30 | 0.69 | 0.83 |
| NR3C1_promoter | 56 | 0.73 | 0.85 |
| RETN_promoter | 11 | 0.64 | 0.86 |
| H19_Body | 12 | 0.82 | 0.88 |
| H19_promoter | 47 | 0.78 | 0.96 |
| IGF1_promoter | 15 | 0.96 | 0.97 |
Significant at p < 0.05.
All models adjusted for maternal height, age, smoking status, passive smoking exposure, prepregnancy BMI, pregnancy weight gain, parity, pregnancy hypertension; child sex and gestational age at delivery; child age and weight for age z-score at methylation measurement; blood cell type proportions; and ten principal components representing processing batch effects (>90% of variance).
EBM: Empirical Brown method; GDM: Gestational diabetes mellitus.
Figure 1. . Results of mediation analysis of three CpGs associated with macrosomia and GDM at p < 0.05.
All models adjusted for maternal height, age, smoking status, passive smoking exposure, prepregnancy BMI, pregnancy weight gain, parity, pregnancy hypertension, child sex and gestational age at delivery, child age and weight for age z-score at methylation measurement, blood cell type proportions and ten principal components representing processing batch effects (>90% of variance).
GDM: Gestational diabetes mellitus.
Discussion
In this study we found limited evidence of an epigenetic basis for part of the well-documented association between GDM and macrosomia. While little research has been done examining epigenetics in association with GDM and macrosomia, a recent meta-analysis found limited evidence for associations between cord blood DNA methylation and maternal GDM [35]. Our single-CpG analysis found three CpGs in the genes MEST and IGF2 to be associated with both GDM and macrosomia, limited evidence for regional associations with GDM and macrosomia in the promoters of IGF2 and ARHGEF11 as well as the body of MEST, and moderate mediation effects for two of the CpGs identified in our single-CpG analysis. These findings indicate that MEST and IGF2 may be particularly important for maternal GDM phenotypes to influence macrosomia and potentially similar cardiometabolic outcomes (e.g., childhood obesity) later in life. Additional research is needed to further characterize this association (e.g., in terms of maternal hyperglycemia or glycemic control, when in pregnancy potential epigenetic reprogramming may occur), and assess potential interventions to ‘interrupt’ the epigenetic mechanism and prevent macrosomia.
IGF2 is an imprinted gene that manufactures the protein IGF-2 and is heavily involved in fetal growth and development. We found hypermethylation of one locus in the 3’UTR region of IGF2 may be associated with both GDM and macrosomia, and an association with IGF2 promoter methylation in our regional analysis. However, the link between methylation in the 3’UTR gene region and gene expression is unclear, thus without gene expression data we cannot posit a direct mechanistic link. Other studies have found changes in IGF2 expression and/or methylation in association with GDM and macrosomia. A previous study by Su et al. in another Chinese population found increased expression of IGF2 in placental tissue and cord blood of fetuses whose mothers had been diagnosed with GDM, and in children born with macrosomia [22]. Their results also suggested a joint action between IGF2 and the gene H19 also located in the IC1 complex; however, our analysis suggests that DNA methylation of H19 is not involved. Another study by Zhang et al. found reduced IGF2 expression in children born small for gestational age but no association with IGF2 methylation [36]. Hoyo et al. likewise found an association between lower IGF2 methylation in cord blood and elevated IGF2 plasma protein concentrations, which were in turn associated with higher birth weight [37]. Our study found one CpG in the 3’UTR region of IGF2 to be associated with macrosomia; until a relationship to gene expression can be established for this region (or CpG), the extent to which our finding reflects those of prior studies will be unclear. Nonetheless our findings add to the evidence that exposure to GDM in utero may alter IGF2 methylation, and potentially increase IGF2 expression and therefore fetal growth, ultimately facilitating increased birth weight. Data are needed to compare our DNA methylation results with IGF2 expression, thus confirming the functional impact of our associations; however, if consistent with the prior literature our findings in childhood methylation suggest that the previously identified effects of GDM (and/or related variables such as maternal hyperglycemia) on IGF2 methylation may persist after birth.
MEST is another imprinted gene that encodes a member of the alpha/beta hydrolase superfamily, which may play a role in fetal development. We found that hypomethylation of two loci, one in the 3’UTR region and one in the body of MEST, was associated with both GDM and macrosomia. A prior study by Kappil et al. found increased MEST expression in placental tissue of children born small for gestational age [38], while Deyssenroth et al. found a differentially methylated region in MEST that was common to children in both small and large for gestational age groups [39]. Gonzalez-Nahm et al. found a similar, hypermethylated differentially methylated region in MEST associated with lower weight [40]. El Hajj et al. also found hypomethylation of MEST in newborns of mothers with GDM [41]. As with IGF2, gene expression data are needed to confirm the mechanistic nature of our findings, but as one of our identified loci is located in the body of MEST, the inverse association identified is generally consistent with the literature. Functional validation of our findings with gene expression data is necessary to confirm a mechanistic hypothesis, but if confirmed the timing of our methylation data collection suggests that there are persistent effects of GDM (and/or related variables such as maternal hyperglycemia/glycemic control) on the epigenome into childhood. As this population matures we can also explore the possibility that these mechanisms translate into elevated BMI in adolescence and/or adulthood, and potentially related phenotypes such as obesity and diabetes.
Also of note is our mediation analysis, which found that the loci cg26263166 (in the 3’UTR of IGF2) and cg19466922 (in the body of MEST), mediated 13 and 26%, respectvely, of the association between GDM and macrosomia, which has been well-documented elsewhere in the literature. However in light of our weak results after FDR adjustment, and the timing of blood sample collection in our study, this finding should be interpreted with caution until it can be confirmed in additional larger populations. One of the assumptions upon which this analysis rests is that DNA methylation remains correlated between birth and the age of DNA collection (median 5.9 years). To our knowledge, few studies have repeatedly examined DNA methylation in children; however, genetic influences on DNA methylation have been shown to be stable throughout the life course [42]. If environmental influences remain relatively stable as well, then it is possible that maternal epigenetic influences can still be detected years after birth. Further epigenetic studies in children are warranted to test this possibility. Our mediation findings may also reflect methylation at other loci correlated with those we identified. Thus, we cannot rule out the possibility that these results represent other epigenetic effects, or the results of other childhood exposures such as diet. Additional, better powered research using cord blood DNA and/or DNA collected at or soon after birth is warranted to confirm our mediation findings, but if successful this would point to a potential mechanism for the effects of GDM and related variables on growth and a potential point of intervention to prevent macrosomia and/or other downstream effects (e.g., elevated BMI).
This study has many strengths: a unique, well-characterized population, a prospective design and rigorous confounder control. However, this study is also subject to limitations. As described above, the lack of gene expression data and timing of the DNA methylation measurement relative to our phenotypes of interest limit our ability to make causal inferences on the basis of our findings. The lack of DNA methylation data taken from cord blood (or at birth) is a limitation of this study, as unmeasured confounders occurring during childhood may influence our results. Additionally, this was a Chinese population, which may be subject to genetic and/or environmental effects that prevent our findings from being generalizable to other populations. This study was also under powered to detect epigenome-wide effects due to the relatively small number of macrosomia cases (n = 177 across GDM and non-GDM groups). This prevented an epigenome-wide study of DNA methylation markers of macrosomia, and reduced our ability to detect effects even in our candidate gene study after multiple testing and robust covariate adjustment. Future research with additional macrosomia cases will help address these limitations, and may yield more findings due to improved statistical power. Another limitation is the lack of data on maternal hyperglycemia or glycemic control during pregnancy. This prevented us from doing a more in-depth study of important GDM-related variables which may be the primary drivers of the epigenetic relationships observed in this study. Future research should examine these possibilities. Additionally, researchers should explore other potential unmeasured confounders from this study (e.g., diet), and seek to confirm these findings using DNA methylation measured in cord blood and/or closer to birth. Finally, particularly given our findings in two untranslated regions, future research using gene expression data is necessary to place our results in their proper mechanistic context.
Conclusion
Nonetheless, this unique cohort enabled us to identify DNA methylation loci that may play a role in the relationship between GDM (or related variables) and macrosomia. These loci were associated with GDM despite being measured during childhood, and if confirmed in future studies (in particular, using blood DNA from closer to birth, and using gene expression data to confirm functional effects) this may point to mechanisms for the development of macrosomia and potentially other obesity-related phenotypes. Further investigation with more macrosomia cases, gene expression data, DNA methylation collected closer to birth, more detailed maternal data, and phenotypes collected later in life will help place our findings in an appropriate context and could potentially facilitate the development of early detection, prevention, and treatment initiatives.
Future perspective
Traditional interventions remain the best choice for controlling gestational diabetes, and may help mitigate risk of macrosomia in children. However the persistently strong associations between GDM and macrosomia suggest a form of biological transmission. This study provides modest evidence of that transmission being mediated in part by DNA methylation changes. These changes occur on genes known to be associated with fetal growth and development, further strengthening the case for a mechanistic association. Nonetheless these associations are difficult to measure and subject to a great many potentially confounding factors. Further studies to completely explore this topic are necessary to strengthen the case for an epigenetic basis for GDM-macrosomia transmission. However, if validated, these findings could lead to useful early detection tools and/or therapeutic and preventive targets in the epigenome for macrosomia in particular but potentially other related metabolic conditions as well such as obesity and type two diabetes.
Summary points.
Fetal macrosomia is an important birth complication associated with later-life cardiometabolic diseases, as well as in utero exposure to gestational diabetes mellitus (GDM).
DNA methylation of white blood cells is a plausible mechanism through which GDM exposure may influence macrosomia development.
For the first time, this study examined DNA methylation changes in childhood associated with both GDM and macrosomia, to assess whether epigenetic mechanisms potentially involved in this process can still be detected years after birth.
We found that one CpG (cg14428359) in MEST was hypomethylated among macrosomic children; two others (cg19466922 in MEST and cg26263166 in IGF2) were also associated before adjustment for multiple testing.
Of these three CpGs, one (cg19466922) is located in the gene body of MEST and thus may be associated with gene expression. The other two are located in untranslated regions; gene expression studies are needed to assess potential functional effects.
Our regional analysis identified associations between macrosomia and the promoter of IGF2, suggesting an overall functional effect for this gene despite the lack of significant single-CpG findings. The body of MEST was also marginally associated.
Our mediation analysis of these three CpGs identified two significant mediators-cg19466922 and cg26263166 in MEST and IGF2, respectively. They were found to mediate 26.4 and 12.7% of the association between GDM and macrosomia, respectively, in separate models.
If validated, these findings suggest potential utility for methylomic biomarkers in the early detection and prevention of macrosomia, and potentially related metabolic diseases, particularly in cases of in utero GDM exposure.
Footnotes
Financial & competing interests disclosure
This study was supported by grants from the European Foundation for the Study of Diabetes (EFSD)/Chinese Diabetes Society (CDS)/Lilly programme for Collaborative Research between China and Europe, and the National Institute of Diabetes and Digestive and Kidney Diseases (R01DK100790). This project also used core facilities supported by the NORC Center Grant P30 DK072476, and the COBRE Center Grant P30 GM118430. G Hu was partly supported by the grant from the National Institute of General Medical Sciences (U54GM104940). A Baccarelli was partially supported by a grant from the National Institute of Environmental Health Studies (P30ES009089). L Hou was partially supported by the American Heart Association Children’s Strategically Focused Research Network. BT Joyce was partially supported by an American Heart Association Career Development Award (Grant number 19CDA34630050). H Liu was supported by the Natural Science Foundation of Tianjin, China (19JCYBJC28000). The authors have no other relevant affiliations or financial involvement with any organization or entity with a financial interest in or financial conflict with the subject matter or materials discussed in the manuscript apart from those disclosed.
No writing assistance was utilized in the production of this manuscript.
Ethical conduct of research
This study was approved by the Human Subjects Committee of the Tianjin Women's and Children's Health Center, and all participants provided written informed consent. This original trial is registered with ClinicalTrials.gov, number NCT01554358.
Data sharing statement
The authors certify that this manuscript reports the secondary analysis of clinical trial data that have been shared with them, and that the use of this shared data is in accordance with the terms (if any) agreed upon their receipt. The source of this data is: Tianjin Gestational Diabetes Prevention Program (NCT01554358).
References
Papers of special note have been highlighted as: • of interest; •• of considerable interest
- 1.Martin JA, Hamilton BE, Osterman MJ, Driscoll AK, Mathews TJ. Births: Final Data for 2015. Natl Vital. Stat. Rep. 66(1), 1 (2017). [PubMed] [Google Scholar]
- 2.Lavery JA, Friedman AM, Keyes KM, Wright JD, Ananth CV. Gestational diabetes in the United States: temporal changes in prevalence rates between 1979 and 2010. BJOG 124(5), 804–813 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Turkmen S, Johansson S, Dahmoun M. Foetal macrosomia and foetal-maternal outcomes at birth. J. Pregnancy 2018, 4790136 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Crosby DA, Ahmed S, Razley A, Morrison JJ. Obstetric and neonatal characteristics of pregnancy and delivery for infant birthweight >/= 5.0 kg. J. Matern. Fetal Neonatal. Med. 30(24), 2961–2965 (2017). [DOI] [PubMed] [Google Scholar]
- 5.Mendez-Figueroa H, Truong VTT, Pedroza C, Chauhan SP. Large for gestational age infants and adverse outcomes among uncomplicated pregnancies at term. Am. J. Perinatol. 34(7), 655–662 (2017). [DOI] [PubMed] [Google Scholar]
- 6.Yamamoto JM, Kallas-Koeman MM, Butalia S, Lodha AK, Donovan LE. Large-for-gestational-age (LGA) neonate predicts a 2.5-fold increased odds of neonatal hypoglycaemia in women with type 1 diabetes. Diab. Metab. Res. Rev. 33(1), (2017). [DOI] [PubMed] [Google Scholar]
- 7.Cordero L, Paetow P, Landon MB, Nankervis CA. Neonatal outcomes of macrosomic infants of diabetic and non-diabetic mothers. J. Neonatal Perinat. Med. 8(2), 105–112 (2015). [DOI] [PubMed] [Google Scholar]
- 8.Johnsson IW, Haglund B, Ahlsson F, Gustafsson J. A high birth weight is associated with increased risk of type 2 diabetes and obesity. Pediatric Obesity 10(2), 77–83 (2015). [DOI] [PubMed] [Google Scholar]
- 9.Yu Z, Han S, Zhu Get al. Birth weight and subsequent risk of obesity: a systematic review and meta-analysis. Obesity Rev. 12(7), 525–542 (2011). [DOI] [PubMed] [Google Scholar]
- 10.Vaiserman AM. Birth weight predicts aging trajectory: a hypothesis. Mech. Ageing Dev. 173, 61–70 (2018). [DOI] [PubMed] [Google Scholar]
- 11.Collier A, Abraham EC, Armstrong J, Godwin J, Monteath K, Lindsay R. Reported prevalence of gestational diabetes in Scotland: the relationship with obesity, age, socioeconomic status, smoking and macrosomia, and how many are we missing? J. Diabetes Investig. 8(2), 161–167 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kc K, Shakya S, Zhang H. Gestational diabetes mellitus and macrosomia: a literature review. Ann. Nutr. Metab. 66(Suppl. 2), 14–20 (2015). [DOI] [PubMed] [Google Scholar]
- 13.Inzaugarat ME, Billordo LA, Vodanovich Fet al. Alterations in innate and adaptive immune leukocytes are involved in paediatric obesity. Pediatr. Obes. 9(5), 381–390 (2013). [DOI] [PubMed] [Google Scholar]
- 14.Frias AE, Grove KL. Obesity: a transgenerational problem linked to nutrition during pregnancy. Semin. Reprod. Med. 30(6), 472–478 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]; • Review articles discussing the link between GDM (gestational diabetes mellitus) andmacrosomia, its consequences, and potential mechanisms.
- 15.Hotamisligil GS. Inflammation and metabolic disorders. Nature 444(7121), 860–867 (2006). [DOI] [PubMed] [Google Scholar]
- 16.Ruchat SM, Houde AA, Voisin Get al. Gestational diabetes mellitus epigenetically affects genes predominantly involved in metabolic diseases. Epigenetics 8(9), 935–943 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Heijmans BT, Tobi EW, Stein ADet al. Persistent epigenetic differences associated with prenatal exposure to famine in humans. Proc. Natl Acad. Sci. USA 105(44), 17046–17049 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Tobi EW, Lumey LH, Talens RPet al. DNA methylation differences after exposure to prenatal famine are common and timing- and sex-specific. Hum. Mol. Genet. 18(21), 4046–4053 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Talens RP, Jukema JW, Trompet Set al. Hypermethylation at loci sensitive to the prenatal environment is associated with increased incidence of myocardial infarction. Int. J. Epidemiol. 41(1), 106–115 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.West NA, Kechris K, Dabelea D. Exposure to maternal diabetes in utero and DNA methylation patterns in the offspring. Immunometabol. 1, 1–9 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Soejima H, Higashimoto K. Epigenetic and genetic alterations of the imprinting disorder Beckwith-Wiedemann syndrome and related disorders. J. Hum. Genet. 58(7), 402–409 (2013). [DOI] [PubMed] [Google Scholar]
- 22.Su R, Wang C, Feng Het al. Alteration in expression and methylation of IGF2/H19 in placenta and umbilical cord blood are associated with macrosomia exposed to intrauterine hyperglycemia. PLoS ONE 11(2), e0148399 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Yan J, Su R, Zhang Wet al. Epigenetic alteration of Rho guanine nucleotide exchange factor 11 (ARHGEF11) in cord blood samples in macrosomia exposed to intrauterine hyperglycemia. J. Maternal Fetal Neonat. Med. 34(3), 422–431 (2021). [DOI] [PubMed] [Google Scholar]
- 24.Chiavaroli V, Derraik JG, Hofman PL, Cutfield WS. Born large for gestational age: bigger is not always better. J. Pediat. 170, 307–311 (2016). [DOI] [PubMed] [Google Scholar]
- 25.Hu G, Tian H, Zhang Fet al. Tianjin Gestational Diabetes Mellitus Prevention Program: study design, methods, and 1-year interim report on the feasibility of lifestyle intervention program. Diab. Res. Clin. Pract. 98(3), 508–517 (2012). [DOI] [PubMed] [Google Scholar]
- 26.Alberti KGMM, Zimmet PF. Definition, diagnosis and classification of diabetes mellitus and its complications. Part 1: diagnosis and classification of diabetes mellitus. Provisional report of a WHO consultation. Diab. Med. 15(7), 539–553 (1998). [DOI] [PubMed] [Google Scholar]
- 27.Wang J, Wang L, Liu Het al. Maternal gestational diabetes and different indicators of childhood obesity - a large study. Endocr. Connect. 7(12), 1464–1471 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Liang Z, Liu H, Wang Let al. Maternal MTNR1B genotype, maternal gestational weight gain, and childhood obesity. Am. J. Clin. Nutrit. 111(2), 360–368 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Houseman EA, Accomando WP, Koestler DCet al. DNA methylation arrays as surrogate measures of cell mixture distribution. BMC Bioinformat. 13, 86 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Xu Z, Niu L, Li L, Taylor JA. ENmix: a novel background correction method for Illumina HumanMethylation450 BeadChip. Nucleic Acids Res. 44(3), e20 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Xu Z, Langie SA, De Boever P, Taylor JA, Niu L. RELIC: a novel dye-bias correction method for Illumina Methylation BeadChip. BMC Genomics 18(1), 4 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Poole W, Gibbs DL, Shmulevich I, Bernard B, Knijnenburg TA. Combining dependent P-values with an empirical adaptation of Brown's method. Bioinformat. 32(17), i430–i436 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Tingley D, Yamamoto T, Hirose K, Keele L, Imai K. Mediation: r package for causal mediation analysis. 59(5), (2014). [Google Scholar]
- 34.Shiau S, Wang L, Liu Het al. Prenatal gestational diabetes mellitus exposure and accelerated offspring DNA methylation age in early childhood. Epigenetics 16(2), 186–195 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Howe CG, Cox B, Fore Ret al. Maternal gestational diabetes mellitus and newborn DNA methylation: findings from the pregnancy and childhood epigenetics consortium. Diabetes Care 43(1), 98–105 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]; •• Recent meta-analysisfinding associations between GDM and DNA methylation in cord blood.
- 36.Zhang S, Zhai G, Wang J, Shi W, Zhang R, Chen C. IGF-II expression and methylation in small for gestational age infants. J. Pediat. Endocrinol. Metabol. 28(5–6), 613–618 (2015). [DOI] [PubMed] [Google Scholar]
- 37.Hoyo C, Fortner K, Murtha APet al. Association of cord blood methylation fractions at imprinted insulin-like growth factor 2 (IGF2), plasma IGF2, and birth weight. Cancer Causes Control 23(4), 635–645 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Kappil MA, Green BB, Armstrong DAet al. Placental expression profile of imprinted genes impacts birth weight. Epigenetics 10(9), 842–849 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Deyssenroth MA, Marsit CJ, Chen J, Lambertini L. In-depth characterization of the placental imprintome reveals novel differentially methylated regions across birth weight categories. Epigenetics 15(1–2), 47–60 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Gonzalez-Nahm S, Mendez MA, Benjamin-Neelon SEet al. DNA methylation of imprinted genes at birth is associated with child weight status at birth, 1 year, and 3 years. Clin. Epigenetics 10, 90 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.El Hajj N, Pliushch G, Schneider Eet al. Metabolic programming of MEST DNA methylation by intrauterine exposure to gestational diabetes mellitus. Diabetes 62(4), 1320–1328 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Gaunt TR, Shihab HA, Hemani Get al. Systematic identification of genetic influences on methylation across the human life course. Genome Biol. 17, 61 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]