Abstract
Stroke is a leading cause of disability and mortality, and the incidence of ischemic stroke is projected to continue to rise in coming decades. These projections emphasize the need for improved imaging techniques for accurate diagnosis allowing effective treatments for ischemic stroke. Ischemic stroke is commonly evaluated with CT or MRI. Noncontrast CT is typically utilized within 4.5 hours of symptom onset to identify candidates for thrombolysis. Beyond this time window, thrombolytic therapy may lead to poor outcomes if patients are not optimally selected using appropriate imaging. MRI provides an accurate method for the earliest identification of core infarct, and MR perfusion can identify salvageable hypoperfused penumbra. The prognostic value for a better outcome in these patients lies in the ability to distinguish between core infarct and salvageable brain at risk—the ischemic penumbra—which is a function of the degree of ischemia and time. Many centers underutilize MRI for acute evaluation of ischemic stroke. This review will illustrate how perfusion-diffusion mismatch calculated from diffusion-weighted MRI and MR perfusion is a reliable approach for patient selection for stroke therapy and can be performed in timeframes that are comparable to CT-based algorithms while providing potentially superior diagnostic information.
Keywords: Diffusion-Perfusion Mismatch, Penumbra, Mechanical Thrombectomy, Large Vessel Occlusion Ischemic Stroke
INTRODUCTION
Stroke is a leading cause of morbidity and mortality. The incidence of stroke in the United States is currently approximately 800,000 cases annually.1 Among stroke subtypes, ischemic stroke encompasses 87% of all strokes in the United States.2 Clinical outcome after stroke is dependent on timely detection and intervention. Accepted definitions and guidelines for management exist.3 In spite of such guidance, variability in individual physiology, risk factors, time of onset, and presentation can confound evaluation. It is often difficult to properly assess the many factors at play, particularly when seeking to minimize delays given the need for timely action in patients suitable for therapy. While diffusion and perfusion techniques have been available for many years, more recent advancements in interventional therapy and results from multicenter trials have solidified their use in patient selection and treatment planning.
PATHOPHYSIOLOGY OF STROKE PROGRESSION
Stroke incidence and the resultant societal burden continue to increase such that now every 40 seconds a person suffers a stroke.4 Stroke is the leading cause of disability worldwide and ranks fifth among all causes of death in the United States.5 The mantra ‘time is brain’ has been augmented with more physiologically refined concepts like the ‘tissue clock’6 and ‘collateral clock’7 as research trials and understanding of the complex interplay between time, brain tissue, and collateral flow improves. This change in conceptualization was brought about by results from multiple trials that demonstrated the importance of penumbral brain tissue surrounding the infarct core. The definition of this brain tissue has changed over the last three decades. The importance of the penumbra was first published by Astrup in 1977.8 He defined the penumbra in terms of the critical cerebral blood flow below which it can progress to irreversible infarction. Later Hossman9 in 1994 described the maintained functional energy status of the penumbra. By 2000 the definition was refined to a molecular basis by expressing the region between the infarct core and area with normal blood flow to have multiple penumbral layers in a gradient as the hypoxia-induced neuronal death spreads from the core to the periphery.10, 11 These definitions have improved our understanding of the physiological evolution of the penumbra as a dynamic interplay between blood flow, cerebrovascular reserve, and neuronal cytotoxicity with time.
The fundamental pathophysiological understanding of the penumbra is quite simple. It is the region of the brain in which the neurons are deprived of their vascular supply but can survive if early reperfusion is established.12 The viability of the penumbra can persist up to 24 hours in some patients, and possibly even longer in some individuals, providing a window for therapeutic interventions in many stroke patients.13–15 It is of diagnostic, therapeutic, neuroprotective, and biochemical importance for current and future studies of stroke care.16 The hemodynamics within the core and penumbra are distinct and form the basis of perfusion imaging. However, before considering perfusion imaging, it is helpful to describe imaging techniques to identify infarcted tissue and provides information against which perfusion imaging will be compared.
DETECTION AND TIMING OF CORE INFARCTION
Diffusion weighted imaging (DWI) sequences are the most sensitive and specific for identification of infarcted tissue in the acute setting.17 DWI is highly sensitive to changes in the diffusion of water molecules within tissue.17 In the setting of cytotoxic edema from acute ischemic stroke, restriction in the diffusion of water is identified as hyperintense signal on DWI18 and corresponding hypointensity on the apparent diffusion coefficient (ADC) map.19 This ‘restriction’ of water/proton motion is due to loss of cellular energy, which causes loss of Na/K pump activity and results in cellular swelling that reduces diffusion of water within and between cells.17 The accuracy of DWI in detecting core infarction is highly dependent on the B value used and the number of gradient directions. Multidirectional DTI (diffusion tensor imaging) and the generated DTI trace image is the most accurate measure of acute infarct and outperforms conventional diffusion-weighted sequences.20 The DTI parameters for the acute ischemic stroke protocol at our center are 2D, 128×128 matrix, 3mm slice thickness, B value=2000, 20 directions. An apparent diffusion coefficient less than 620×10−6 mm2/s has been shown to be the optimal threshold for detection of the ischemic core.21 It is important to observe that the ADC value remains darker than surrounding brain parenchyma during the first week, pseudonormalizes at 7–10 days, and thereafter becomes brighter than surrounding tissue. This late stage, when high signal is present on both DWI and ADC, termed ‘T2-shine through’ indicates a subacute infarct in which high T2 signal related to vasogenic edema outweighs the diffusion restriction. Figure 1 demonstrates evolution of DWI, ADC, and T2 signal in an infarct in the same patient over the course of months. Studies have shown that lower baseline ADC was associated with T2 shine-through on 1-month follow-up imaging, indicating that baseline restricted diffusion was associated with slower recovery and ongoing slow progression of ischemia to infarction.22 It should also be noted that while DWI core infarcts rarely totally reverse, systematic reviews have shown that DWI core infarct volume can reduce in up to 26.5% of patients, depending on the method of follow up (FLAIR or DWI).23, 24
Figure 1.
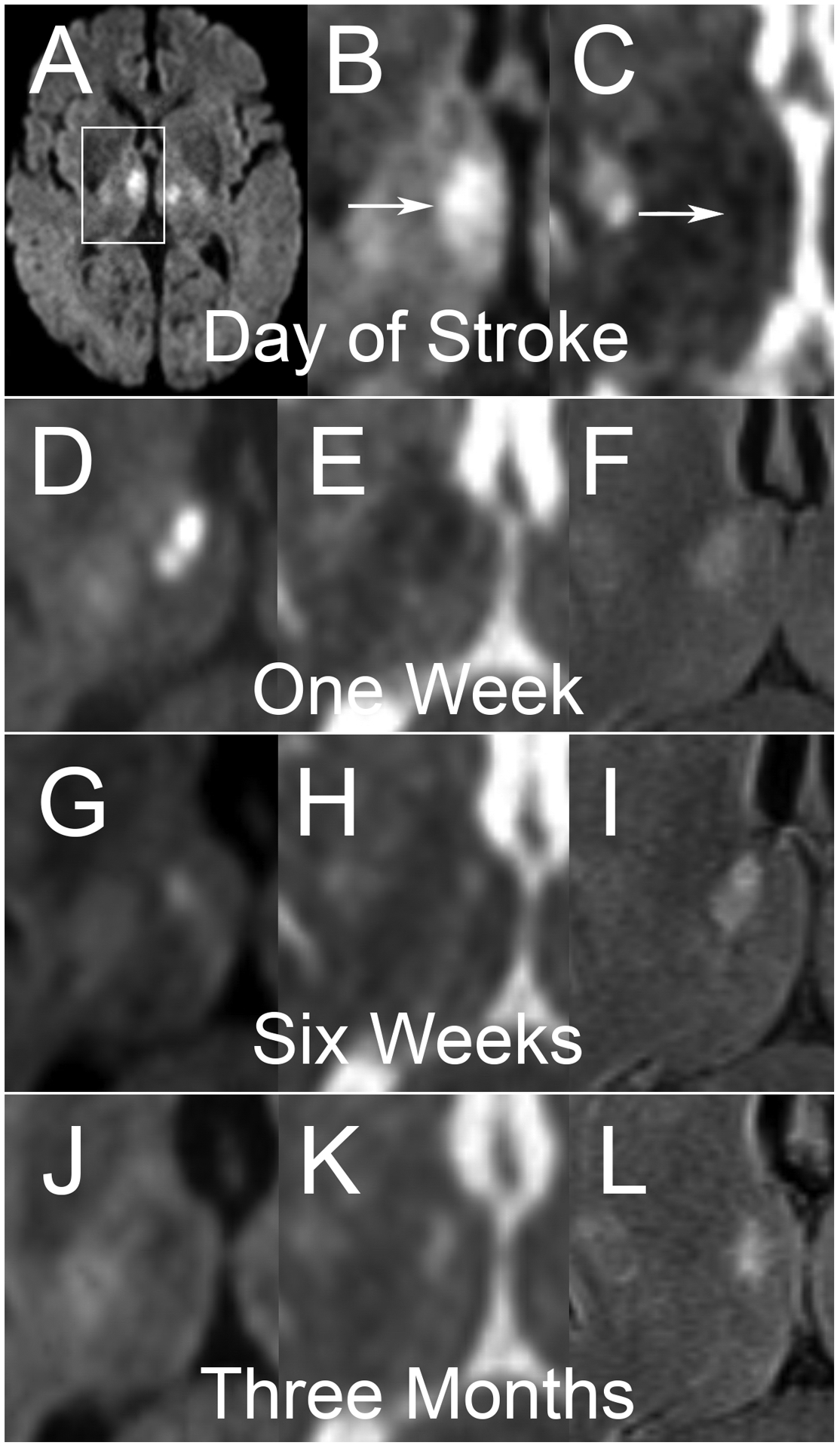
A middle-aged man presented with altered mental status and was found to have bilateral thalamic infarcts on DWI (A). White box demonstrates region of interest for subsequent cropped axial images of the right deep gray structures. Sequential studies with DWI (B, D, G, J), ADC (C, E, H, K) and FLAIR (F, I, L) sequences are shown. Imaging on the day of the infarct (A, B, C) demonstrates hyperintense DWI signal with hypointense ADC signal. Of note, FLIAR imaging was not performed at this time. At one week, DWI (D) demonstrates more pronounced hyperintensity with clear corresponding hypointensity on ADC (E) and hyperintensity on FLAIR. At six weeks, very little DWI (G) hyperintensity persists, which matches corresponding ADC (H) that is now hyperintense, consistent with T2 shine-through confirmed on FLAIR (I). At three months, All DWI (J) hyperintensity has resolved, with some hypointensity seen in the area of the chronic infarct. ADC (K) and FLAIR (L) both demonstrate hyperintense signal.
Clinical trials have taken advantage of FLAIR positivity lagging behind diffusion signal in acute ischemic stroke. FLAIR signal becomes positive in nearly 100% of patients after 6 hours, though most patients convert within 4.5h.25 Thus DWI-FLAIR mismatch in which there is high signal on DWI and low signal on FLAIR approximates the 4.5h timepoint. Results of the MR WITNESS26 study suggested that, in patients with unknown time of symptoms onset, intravenous tissue Plasminogen Activator (IV tPA) administration was safe in patients with a DWI-FLAIR mismatch. This trial allowed treatment within 4.5 hours of symptom discovery, even if last known normal was up to 24 hours prior. Thus, MRI imaging with DWI and FLAIR can play a significant role in characterizing the tissue clock in patient selection, providing accurate results on core infarct size and timing of the infarct. MRI allows selection criteria to be personalized to patient presentation and clinical condition, and MRI perfusion imaging identifies salvageable brain, which can guide treatment planning and provide helpful prognostic information to family members who may be asked to quickly make difficult decisions. In addition to high sensitivity in identifying core infarct, MRI also does not have the ionizing radiation inherent to CT.27–29 Despite the superiority of MRI over CT in detecting core infarction, the most common reason for MRI not being the initial test is patient contraindications such as excessive patient motion, implanted devices or metal, lack of availability at some centers, and perceived time advantages found with CT.30
IMAGING TISSUE AT RISK
Cerebral hemodynamic homeostasis is maintained across a wide range of perfusion pressures. The cerebral perfusion pressure (CPP), defined as the difference between the mean arterial pressure (MAP) and intracranial pressure (ICP) is responsible for maintaining adequate oxygen supply for tissue survival. This in turn is due to the adaptive vasodilation of the cerebral blood vessels that lead to an increase in blood volume whenever there is compromised blood flow to brain tissue. When this compensatory system is overwhelmed, the tissue’s ability to extract oxygen reduces, leading to metabolic instability. This derangement leads to cessation of electrical activity of the neurons, which causes neurological deficits, and is eventually followed by neuronal death. Reversible and irreversible cytotoxic injury forms the pathological basis of our understanding of the core and penumbra and its assessment with perfusion imaging.
In addition to assessing infarct core, it is important to assess the potentially salvageable tissue that constitutes the penumbra. Imaging of the ischemic penumbra is possible through multiple techniques, the most commonly used being CT or MR perfusion imaging. There are multiple perfusion metrics to consider when evaluating the penumbra. These include cerebral blood flow (CBF), cerebral blood volume (CBV), mean transit time (MTT), time to drain (TTD), the time at which the deconvolved residue function reaches its maximum value (Tmax), and time to peak contrast concentration (TTP). Time-concentration relationships are summarized in Figure 2. The central volume theorem combines three parameters—MTT, CBV, and CBF—into a single equation that is useful in determining the two areas of infarct core and penumbra.31
Figure 2.
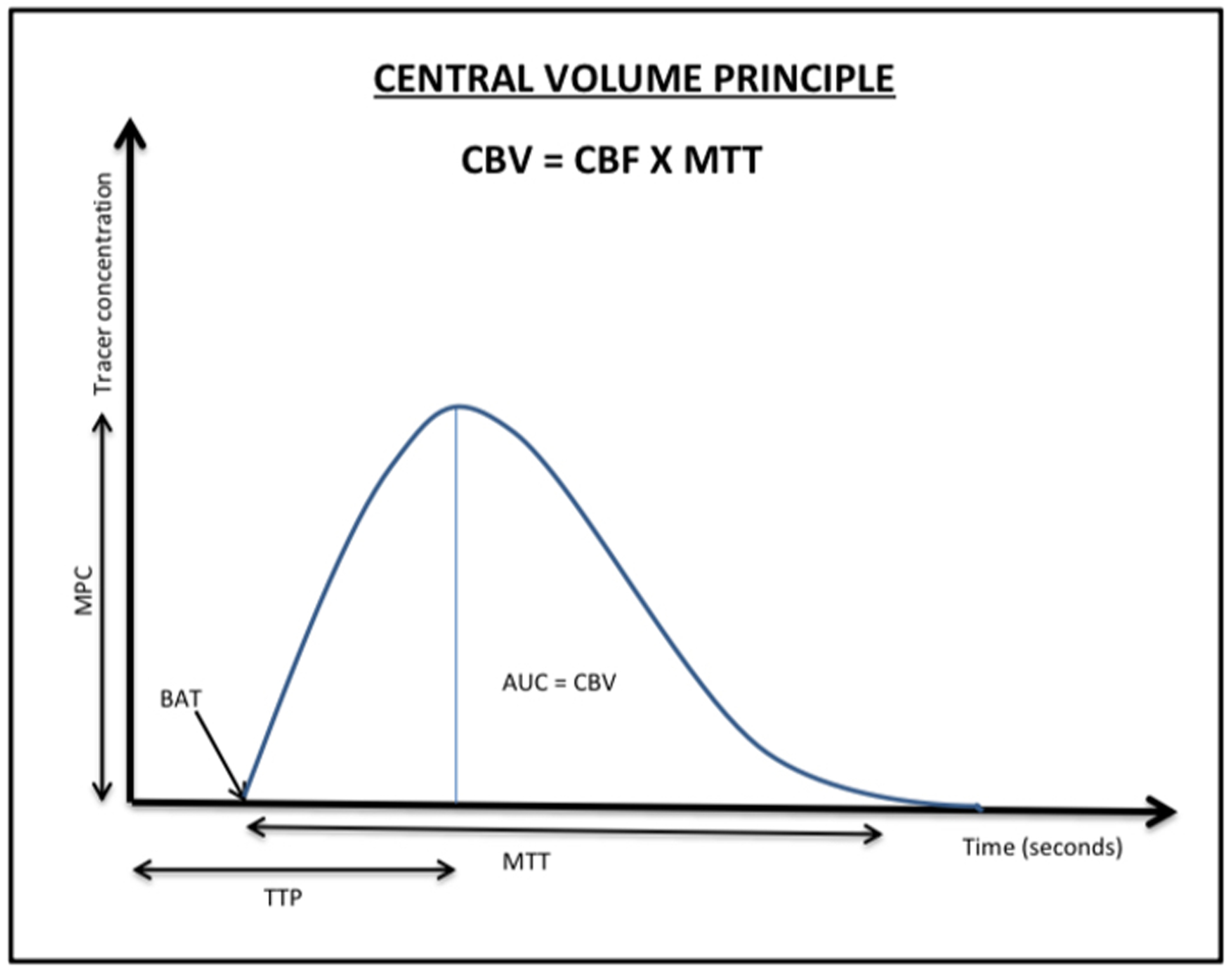
A concentration-time curve demonstrates the central volume principle, plotting time on the x-axis against tracer concentration on the y-axis. Area under the curve (AUC) quantifies to cerebral blood volume at a given time. Beginning of arrival of tracer (BAT) is the time at which contrast agent enters the arterial input. Time to peak (TTP) represents the time between the tracer injection and maximum tissue enhancement. Mean transit time (MTT) is the total time the contrast takes to move from the arterial to venous phases. Peak concentration (MPC) is the highest measured tracer concentration.
The gold standard for core infarction is identified on DWI, but core infarction also has a very low rCBF and CBV. In contrast, the penumbra, despite having some reduction in rCBF, has no DWI signal abnormality and normal or increased CBV due to hemodynamic compensatory dilation of arteries and vascular contribution via surrounding collaterals. The different perfusion states as captured by imaging are summarized in Table 1.32 It is important to keep in mind the concept of ‘luxury perfusion’ of the infarct core to prevent false negative results in identifying infarct core if DWI is not available. This occurs due to the abnormal autoregulation of the cerebral vasculature during the acute and subacute period following an ischemic stroke. It is imperative to evaluate the infarct core on anatomic sequences and especially DWI, particularly in cases of luxury perfusion where the perfusion parameters can appear normal.33,34
Table 1:
Distinguishing abnormal perfusion states of the core and penumbra using perfusion imaging.
| PERFUSION PARAMETER | CORE* | PENUMBRA |
|---|---|---|
| MTT or Tmax | ↑ | ↑ |
| CBF | ↓↓ | ↓ |
| CBV | ↓↓ | Normal/↑ |
Core infarct can also demonstrate so-called “luxury perfusion” in which infarcted tissue has normal perfusion imaging appearance.
MR perfusion imaging can be performed with gadolinium contrast-enhanced techniques using dynamic susceptibility contrast (DSC) or dynamic contrast enhancement (DCE) sequences, or via a noncontrast technique using arterial spin labeling (ASL) sequences.35 Intravenous gadolinium results in T1 and T2/T2* shortening effects. In DSC, multiple T2*-weighted images are obtained before, during and after intravenous injection of gadolinium. Due to the T2* shortening effects of gadolinium, the signal intensity curve of DSC perfusion shows a drop in signal that can be used to produce a concentration time curve.36 The area under the concentration time curve represents the CBV in each voxel. Other parameters derived from DSC are MTT, Tmax, CBF, Time to peak (TTP-representing highest signal loss).36 DSC is the perfusion technique most frequently used for acute ischemic stroke imaging, and the parameter Tmax of >6 seconds most accurately represents the total ischemic area, from which the core infarct identified by DWI is subtracted to calculate the ischemic penumbra.36 While both low CBV and very low CBF serve as good measures of core infarct volume, the most accurate measurement of core infarction is identified on DWI.36 On the other hand, the DCE technique is T1-weighted and due to the T1-shortening effects of gadolinium, produces a signal intensity curve with the contrast bolus corresponding to increased signal and perfusion metrics include measurement of vascular permeability.36 One metric in this technique is the contrast transfer constant (Ktrans ), which is a measure of both vascular permeability and microvessel density.36
ASL can be considered as an alternative to methods using gadolinium contrast agents. This technique exploits the inherent motion of blood flow in the brain and has gained popularity in cerebral perfusion and collateral flow imaging. ASL uses a radiofrequency pulse to spin-label a percentage of flowing protons in water molecules in the bloodstream, and these are used as the radiotracer and compared to a control acquisition.37 The latter is subtracted from the former to obtain perfusion imaging and visualize the ischemic penumbra surrounding the core infarct. While still early in its clinical adoption, ASL has been shown to increase stroke detection by 5% when used together with DWI and perfusion imaging in areas with small stroke lesions.38 ASL has been found to have an association with good neurological outcome and reduced Modified Rankin Score on hospital discharge.38
STROKE IMAGING IN THERAPEUTIC TRIALS
Early trials that studied the use of IV tPA in stroke management were NINDS39 followed by the MR WITNESS26, EXTEND40 and WAKE-UP41 trials that used DWI-FLAIR mismatch as a patient selection criteria for treatment. The NINDS trial was a 2-phase trial. This trial studied the 24hr and 3-month outcome in patients that were administered IV tPA within 3 hours of stroke onset. Results proved better 3-month outcome in these patients compared to the placebo group. MR WITNESS trial used DWI-FLAIR mismatch in patients with stroke within 4.5 to 24 hours after onset, and found that IV tPA was safe in treating patients within 4.5 hours of arrival. A longer time interval of 4.5 hours to 9 hours for IV tPA use was tested in the EXTEND trial. This trial utilized abnormal perfusion imaging to select patients for treatment with a mismatch ratio of at least 1.2. This trial reported a significant benefit for functional independence at 90 days but had higher rates of symptomatic intracranial hemorrhage. The limitation of this study was that it was stopped early due to the release of the results of the WAKE-UP trial therefore losing its clinical importance and results were not applicable to lacunar stroke. Results of the WAKE-UP trial showed the benefit of using IV-tPA within 4.5 hours if used in conjunction with MRI DWI-FLAIR.
Multiple randomized control trials have confirmed the substantial benefits of mechanical thrombectomy compared to medical therapy alone, although this did not occur until after several trials with negative results provided useful lessons for the design of subsequent trials. Among these early negative trials, the most important were Mechanical Retrieval and Recanalization of Stroke Clots Using Embolectomy (MR RESCUE)42 and International Management of Stroke (IMS III)43. MR RESCUE studied the outcome difference in thrombectomy versus standard care in patients using pre-treatment imaging of the penumbra volume. It included 118 patients with an anterior circulation ischemic stroke that were all treated with IV-tPA. The primary outcome of mRS at 90 days was found to be the same in both treatment groups, showing that penumbral imaging as a diagnostic test for treatment selection was not associated with a superior outcome. A major limitation of this study was apparent prolongation of time to treatment, which was often attributed to utilization of MRI over CT. While a valid critique in general, minimization of delays was accomplished in subsequent trials that demonstrated benefit of mechanical thrombectomy for large vessel occlusion. This was affected by improvements in efficiency in stroke care delivery and progress made in endovascular devices rather than avoidance of MRI-based evaluation.
2015 proved to be a turning point in stroke care with publication of the results of the MR CLEAN trial.44 This was followed by the ESCAPE, REVASCAT, SWIFT PRIME and EXTEND IA trials, all of which confirmed the benefit of mechanical thrombectomy for large vessel occlusions in the internal carotid artery or M1 segment of the middle cerebral artery.45,46,47,48 These trials were subsequently assessed in a patient-level meta-analysis known as the HERMES study, which is summarized in Table 2.49 Of these positive trials, SWIFT PRIME and EXTEND-IA used perfusion imaging for patient selection. In addition to standard and angiographic imaging, ESCAPE also included collateral status.45 The consensus after these trials was the clear benefit of mechanical thrombectomy for treatment of large vessel occlusion (LVO) within 6 hours of last known normal.49 The combined HERMES cohort included a total of 1287 patients with a mean age of 68 years.49 There was a significant benefit for the functional independence outcome (46% versus 26.5% (OR, 2.49; 95% CI, 1.76–3.53; p < 0.0001)).49 HERMES results showed that beyond 7.3 hours, the benefit of treatment seemed to decline, although benefit could exist for some patients.49
Table 2:
Summary of the 5 trials included in HERMES
| TRIAL | Treatment window | Vascular imaging | Perfusion imaging criteria (If used) |
|---|---|---|---|
| MR CLEAN | 6 hours | CTA | No |
| ESCAPE | 12 hours | CTA | No |
| REVASCAT | 8 hours | CTA or MRA | Perfusion CT-CBV ASPECTS or perfusion CT-SI ASPECTS in patients >4.5 hours from onset |
| SWIFT PRIME | 6 hours | CTA or MRA | Perfusion CT or perfusion MRI Core<50mL, mismatch >1.8 |
| EXTEND IA | 6 hours | CTA | Perfusion CT Core <70mL Absolute mismatch volume >10 mL Mismatch ratio >1.2 |
The results of the 5 trials included in HERMES answered the question of utility of stroke intervention within six hours, but they also shed light on the need to address patients in extended timeframes. This led to two subsequent trials, DAWN50 and DEFUSE-351, that expanded guidelines for the treatment of acute ischemic stroke. The DAWN trial was a prospective trial of 206 patients, whose last known well was between 6–24 hours.50 Patients were categorized into three subgroups based on patient age, infarct volume, and NIHSS score.50 Patients were randomized to mechanical thrombectomy using the Trevo device plus standard medical care versus only medical care.50 The trial was stopped early due to the early evidence that thrombectomy in this late window was highly efficacious in patients with a mismatch between clinical status and infarct size measured using diffusion-weighted MR or CT perfusion and post processing with the RAPID software.50 After adjusting for baseline characteristics it was found that the absolute difference in the primary and secondary endpoints of utility weighted mRS and functional independence at 90 days were 2.1 (1.2–3.1) and 36 (24–47), respectively.50 This large difference in positive outcomes in the mechanical thrombectomy group and no significant difference between secondary complications of intracranial hemorrhage changed the possibilities for the management of strokes in the late window period.50 DEFUSE-3 criteria for mismatch were initial infarct volume <70ml plus the ratio of hypoperfused/infarct >1.7 and a mismatch of hypoperfused-core of at least 15ml in patients last known well 6–16 hours.51 A good functional outcome was more frequent in the thrombectomy group (45% versus 17%, p<0.0001), and the trial was stropped after an early interim analysis triggered by the positive results of DAWN.51 A sub study of the DEFUSE-3 trial compared the outcomes between males and females and found that females were found to have smaller cores, slower progression of infarct and better collateral circulation.51 This shows the difference in hemodynamic properties between sex that contributes to cerebral perfusion.52
PERFUSION PROCESSING WITH AN EYE TOWARD TREATMENT
The penumbral imaging selection criteria utilized in DAWN and DEFUSE-3 have been adapted for clinical use. While these two trials had similar criteria, there were differences, and the post-processing of these diagnostic studies images is carried out by software that varies across medical centers. As such, criteria for the hypoperfusion threshold and methods for processing images have not been standardized to date.
The current guidelines for acute stroke management include IV tPA within 4.5 hours of stroke onset using noncontrast CT to exclude intracranial hemorrhage and contraindication to treatment. A study done on 225 patients that were ineligible for thrombectomy was done to test outcome of treatment with tPA between 4.5 to 9 hours window period showed 35.4% of patients with mRS 0–1 at 90 days (versus 29.5% in placebo group), but no difference in functional independence and significantly higher risk of symptomatic intracranial hemorrhage during this extended window period.40 For patients presenting with emergent LVO, guidelines have been established to determine candidacy for mechanical thrombectomy based on imaging findings, clinical status based, and symptom timeframe.53 Thrombectomy is currently recommended up to 24 hours from last known normal which has been extended from the earlier guidelines. During the <6 hours interval, a CT ASPECTS ≥6, DWI MRI ASPECTS ≥6, small infarct volume (<70ml) with a target mismatch ratio and moderate to good collateral circulation is a good indication for mechanical thrombectomy.53 Studies have shown that patients with a large infarct core and mismatch ratio that are deemed ineligible for thrombectomy might still benefit from treatment if carefully chosen according to individual patient characteristics and perfusion characteristics, and this will be assessed in future trials.52, 53 Guidelines suggest that in patients presenting with an LVO within 6–24 hours, the decision for thrombectomy should be based on DWI-PWI mismatch and CT perfusion criteria from DAWN and DEFUSE-3 trials. In patients that do not fit these criteria but have a favorable CT-ASPECTS of 6–10 and good collaterals, thrombectomy may also show favorable long term outcomes53.
Studies have been performed to externally validate the criteria set in the DAWN and DEFUSE-3 trials. Results from one such study of patients with unknown time of stroke onset showed that patients that were deemed ineligible for treatment based on DAWN and DEFUSE-3 selection criteria had a favorable mRS of <2 after undergoing thrombectomy, showing that stringent volume thresholds might exclude potential patients with a favorable outcome.56 A retrospective study was conducted on 1705 patients with imaging profiles compatible with DAWN/DEFUSE-3 criteria and extended the criteria to include lower NIHHS scale and lower CT-ASPECT score. Results showed that among these patients, 5.6% were eligible by either of the trial criteria and 11.1% were eligible by the extended criteria.57 This further supports the need for a wide volume threshold on imaging or more accurate measurement of core volume to avoid missing target patients for treatment using endovascular thrombectomy.
A limitation to instituting strict thresholds guidelines for perfusion imaging is the difference in software used for the post processing of imaging obtained on CT or MRI. A study done to compare two different processing software packages—RAPID® (iSchema View) and Olea Sphere®(Olea Medical)—showed differences between the measured volumes of hypoperfused tissue (median 91.0 ml versus 102.2 ml; p < 0.05) and abnormality on ADC (median 30.0 ml versus 23.9 ml; p < 0.05).58 It is important to note that different MRI software may affect patient evaluation, particularly with respect to mechanical thrombectomy candidacy. Semi-automated MRI perfusion-diffusion mismatch calculation will help clinicians to correctly stratify patients for treatment options, particularly those that are eligible for mechanical thrombectomy, and will be a goal for future innovation.59
ELEMENTS OF AN MRI PROTOCOL FOR ACUTE ISCHEMIC STROKE EVALUATION
While CT is often utilized for its fast acquisition time, MRI provides superior diagnostic accuracy that may warrant its selection, particularly when utilizing truncated rapid protocols at centers with well-developed workflows. Such a protocol can be acquired in less than 6 minutes and includes DWI, fluid attenuated inversion recovery (FLAIR), susceptibility weighted imaging (SWI) or gradient recalled echo (GRE), and can be coupled with MRI perfusion imaging and MR angiogram (MRA) while still remaining within 10 minutes. Figure 3 provides images from a patient selected for mechanical thrombectomy following use of this protocol at our center. Compared to slightly less scanning time for CT protocols, MRI can be considered superior given that it is most sensitive for the detection of early stroke. One study has tried to implement an MRI Stroke protocol to obtain MRI imaging in stroke patients in less than the normal time of 20 mins for a conventional MRI brain protocol.60 This study proved that with an efficient work team and fast imaging technologies it is fairly possible to obtain MRI imaging with superior quality and reproducibility than CT.60 Further efficiency gains can be accomplished by developing a truncated protocol that emphasizes rapid acquisition. At our institution, the rapid acute stroke protocol includes axial GRE, DWI, FLAIR, DSC perfusion, and postcontrast MRA; this is all acquired in fewer than ten minutes, which is comparable to the CT-based algorithm that includes noncontrast CT, CTA, and CT perfusion. At our institution, 1.5T scanners are most often utilized due to greater availability for acute stroke patients, yet even greater efficiency gains can be achieved with 3T scanners that require even less time to complete the protocol.
Figure 3.
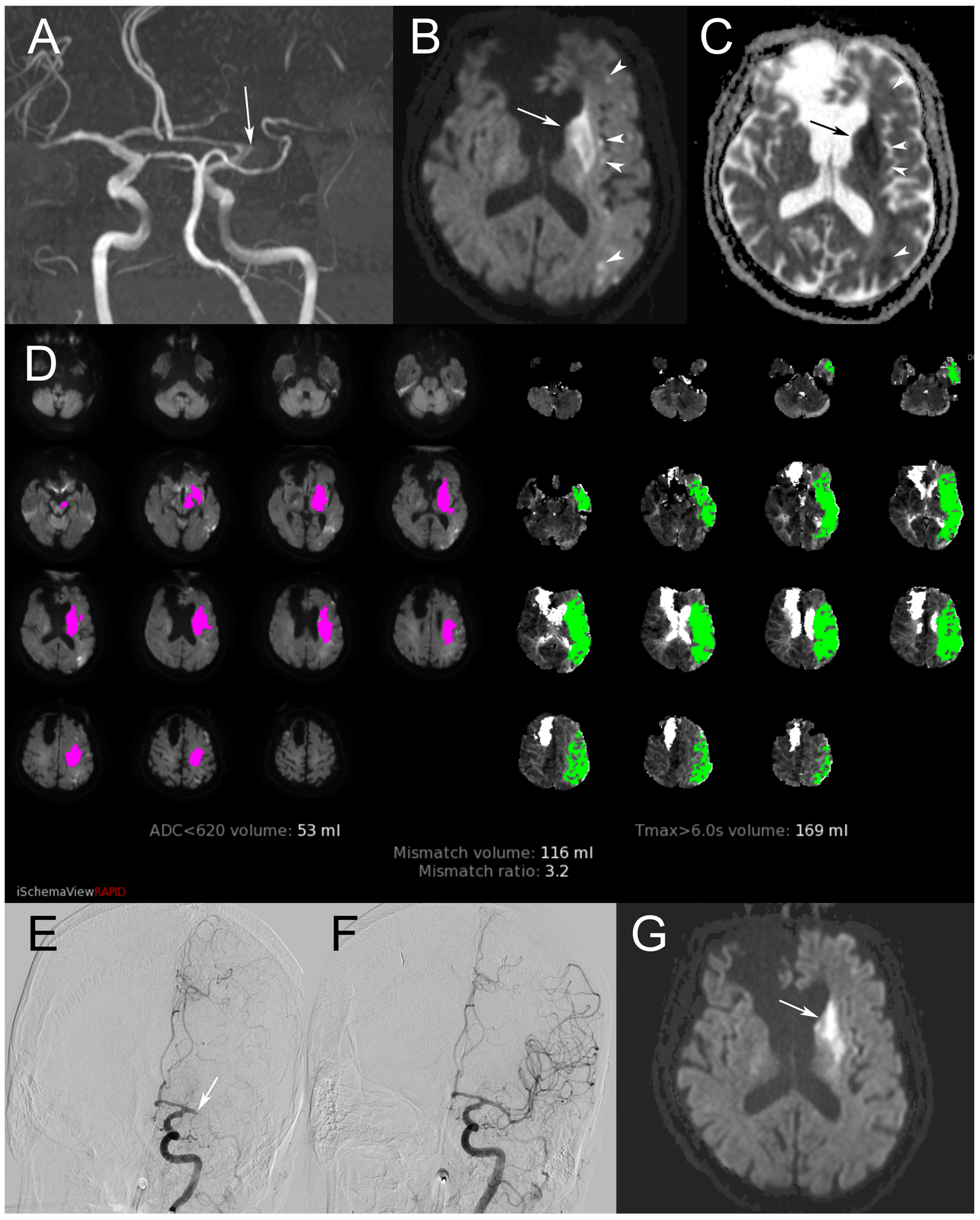
A retirement-aged man presented with right hemiplegia and sensation loss, raising concern for left MCA occlusion. Symptom onset was greater than six hours at the time of presentation. Maximum intensity projection in the coronal plane of time of flight MRA (A) confirms occlusion of the left M1 segment (arrow). DWI (B) shows infarct in the caudate head and lentiform nuclei (arrow), as well as scattered areas of restricted diffusion in the left MCA territory (arrowheads). These areas are confirmed with corresponding hypointensity on ADC (C). Perfusion imaging summary image (D) demonstrates a large volume of tissue with elevated Tmax>6s, indicated by green shading. From this, volume of infarct, indicated by magenta shading, is subtracted to calculate penumbra. This patient’s calculated core and penumbra were favorable for mechanical thrombectomy. Digital subtraction angiography in the Towne’s projection during injection of the left ICA (E) confirms left M1 occlusion (arrow). Complete recanalization was achieved, seen on repeat angiography after thrombectomy (F). Stability MRI obtained 24 hours after treatment (G) again demonstrates infarct in the left deep gray structures, although there has been reversal in previously visualized areas of restricted diffusion in most other areas of the left MCA territory.
CONCLUSION
Imaging of the penumbra is a highly debated topic. Research is still underway for validating a standardized threshold for patient selection using MRI perfusion imaging despite variations in software and post-processing algorithms. Future research must concentrate on ways to address the time and financial constraints of implementing an MRI stroke protocol for patient selection for endovascular thrombectomy. Large scale randomized controlled trials to validate standardized threshold values for imaging as well as testing mechanical thrombectomy outcomes in patients with stroke onset beyond 24 hours will be of vital importance for patient care. A collaborative effort between neuroradiologists, interventionalists, and neurologists will not only lead to better and faster implementation of MRI perfusion imaging but also improved success of mechanical thrombectomy in eligible patients.
Sources of Support:
NIH K23NS105924
REFERENCES:
- 1.Virani Salim S, Alonso Alvaro, Benjamin Emelia J., et al. Heart Disease and Stroke Statistics—2020 Update: A Report From the American Heart Association. Circulation. 2020;141(9):e139–e596. doi: 10.1161/CIR.0000000000000757 [DOI] [PubMed] [Google Scholar]
- 2.Benjamin EJ, Blaha MJ, Chiuve SE, et al. Heart Disease and Stroke Statistics—2017 Update. Circulation. 2017;135(10):e146–e603. doi: 10.1161/CIR.0000000000000485 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Powers William J, Rabinstein Alejandro A, Ackerson Teri, et al. Guidelines for the Early Management of Patients With Acute Ischemic Stroke: 2019 Update to the 2018 Guidelines for the Early Management of Acute Ischemic Stroke: A Guideline for Healthcare Professionals From the American Heart Association/American Stroke Association. Stroke. 2019;50(12):e344–e418. doi: 10.1161/STR.0000000000000211 [DOI] [PubMed] [Google Scholar]
- 4.Stroke Facts | cdc.gov. Published September 9, 2020. AccessedNovember 6, 2020. https://www.cdc.gov/stroke/facts.htm
- 5.Virani SS, Alonso A, Benjamin EJ, et al. Heart Disease and Stroke Statistics—2020 Update: A Report From the American Heart Association. Circulation. 2020;141(9). doi: 10.1161/CIR.0000000000000757 [DOI] [PubMed] [Google Scholar]
- 6.Nawabi J, Flottmann F, Kemmling A, et al. Elevated early lesion water uptake in acute stroke predicts poor outcome despite successful recanalization - When “tissue clock” and “time clock” are desynchronized. Int J Stroke Off J Int Stroke Soc. Published online October 26, 2019:1747493019884522. doi: 10.1177/1747493019884522 [DOI] [PubMed] [Google Scholar]
- 7.Achala Vagal, Richard Aviv, Heidi Sucharew, et al. Collateral Clock Is More Important Than Time Clock for Tissue Fate. Stroke. 2018;49(9):2102–2107. doi: 10.1161/STROKEAHA.118.021484 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Astrup J, Symon L, Branston NM, Lassen NA. Cortical evoked potential and extracellular K+ and H+ at critical levels of brain ischemia. Stroke. 1977;8(1):51–57. doi: 10.1161/01.STR.8.1.51 [DOI] [PubMed] [Google Scholar]
- 9.Hossmann KA. Viability thresholds and the penumbra of focal ischemia. Ann Neurol. 1994;36(4):557–565. doi: 10.1002/ana.410360404 [DOI] [PubMed] [Google Scholar]
- 10.Tagaya M, Haring H-P, Stuiver I, et al. Rapid Loss of Microvascular Integrin Expression during Focal Brain Ischemia Reflects Neuron Injury. J Cereb Blood Flow Metab. 2001;21(7):835–846. doi: 10.1097/00004647-200107000-00009 [DOI] [PubMed] [Google Scholar]
- 11.Sharp FR, Lu A, Tang Y, Millhorn DE. Multiple molecular penumbras after focal cerebral ischemia. J Cereb Blood Flow Metab Off J Int Soc Cereb Blood Flow Metab. 2000;20(7):1011–1032. doi: 10.1097/00004647-200007000-00001 [DOI] [PubMed] [Google Scholar]
- 12.Yuh WTC, Alexander MD, Ueda T, et al. Revisiting Current Golden Rules in Managing Acute Ischemic Stroke: Evaluation of New Strategies to Further Improve Treatment Selection and Outcome. Am J Roentgenol. 2016;208(1):32–41. doi: 10.2214/AJR.16.16557 [DOI] [PubMed] [Google Scholar]
- 13.Markus R, Reutens DC, Kazui S, et al. Hypoxic tissue in ischaemic stroke: persistence and clinical consequences of spontaneous survival. Brain J Neurol. 2004;127(Pt 6):1427–1436. doi: 10.1093/brain/awh162 [DOI] [PubMed] [Google Scholar]
- 14.Darby DG, Barber PA, Gerraty RP, et al. Pathophysiological Topography of Acute Ischemia by Combined Diffusion-Weighted and Perfusion MRI. Stroke. 1999;30(10):2043–2052. doi: 10.1161/01.STR.30.10.2043 [DOI] [PubMed] [Google Scholar]
- 15.Heiss WD, Huber M, Fink GR, et al. Progressive derangement of periinfarct viable tissue in ischemic stroke. J Cereb Blood Flow Metab Off J Int Soc Cereb Blood Flow Metab. 1992;12(2):193–203. doi: 10.1038/jcbfm.1992.29 [DOI] [PubMed] [Google Scholar]
- 16.Ramos-Cabrer P, Campos F, Sobrino T, Castillo J. Targeting the Ischemic Penumbra. Stroke. 2011;42(1, Supplement 1):S7–S11. doi: 10.1161/STROKEAHA.110.596684 [DOI] [PubMed] [Google Scholar]
- 17.Le Bihan D, Iima M. Diffusion Magnetic Resonance Imaging: What Water Tells Us about Biological Tissues. PLoS Biol. 2015;13(7). doi: 10.1371/journal.pbio.1002203 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Schlaug G, Siewert B, Benfield A, Edelman RR, Warach S. Time course of the apparent diffusion coefficient (ADC) abnormality in human stroke. Neurology. 1997;49(1):113–119. doi: 10.1212/wnl.49.1.113 [DOI] [PubMed] [Google Scholar]
- 19.Warach S, Chien D, Li W, Ronthal M, Edelman RR. Fast magnetic resonance diffusion-weighted imaging of acute human stroke. Neurology. 1992;42(9):1717–1723. doi: 10.1212/wnl.42.9.1717 [DOI] [PubMed] [Google Scholar]
- 20.Chou MC, Tzeng WS, Chung HW, et al. T2-enhanced tensor diffusion trace-weighted image in the detection of hyper-acute cerebral infarction: Comparison with isotropic diffusion-weighted image. Eur J Radiol. 2010;74(3). doi: 10.1016/j.ejrad.2009.04.023 [DOI] [PubMed] [Google Scholar]
- 21.Purushotham A, Campbell BCV, Straka M, et al. Apparent Diffusion Coefficient Threshold for Delineation of Ischemic Core. Int J Stroke. 2015;10(3):348–353. doi: 10.1111/ijs.12068 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Rivers CS, Wardlaw JM, Armitage PA, et al. Persistent Infarct Hyperintensity on Diffusion-Weighted Imaging Late After Stroke Indicates Heterogeneous, Delayed, Infarct Evolution. Stroke. 2006;37(6):1418–1423. doi: 10.1161/01.STR.0000221294.90068.c4 [DOI] [PubMed] [Google Scholar]
- 23.Kranz PG, Eastwood JD. Does Diffusion-Weighted Imaging Represent the Ischemic Core? An Evidence-Based Systematic Review. Am J Neuroradiol. 2009;30(6):1206–1212. doi: 10.3174/ajnr.A1547 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Nagaraja N, Forder JR, Warach S, Merino JG. Reversible diffusion-weighted imaging lesions in acute ischemic stroke: A systematic review. Neurology. 2020;94(13):571–587. doi: 10.1212/WNL.0000000000009173 [DOI] [PubMed] [Google Scholar]
- 25.Thomalla G, Cheng B, Ebinger M, et al. DWI-FLAIR mismatch for the identification of patients with acute ischaemic stroke within 4·5 h of symptom onset (PRE-FLAIR): a multicentre observational study. Lancet Neurol. 2011;10(11):978–986. doi: 10.1016/S1474-4422(11)70192-2 [DOI] [PubMed] [Google Scholar]
- 26.Schwamm LH, Wu O, Song SS, et al. Intravenous Thrombolysis in Unwitnessed Stroke Onset: MR WITNESS Trial Results. Ann Neurol. 2018;83(5):980–993. doi: 10.1002/ana.25235 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.mnysiwalla A, Richard IA, P S. Radiation dose from multidetector row CT imaging for acute stroke. Neuroradiol 2009. 51(10):635–640. doi: 10.1007/s00234-009-0543-6 [DOI] [PubMed] [Google Scholar]
- 28.Jauch Edward C, Saver Jeffrey L, Adams Harold P, et al. Guidelines for the Early Management of Patients With Acute Ischemic Stroke. Stroke. 2013;44(3):870–947. doi: 10.1161/STR.0b013e318284056a [DOI] [PubMed] [Google Scholar]
- 29.Chalela JA, Kidwell CS, Nentwich LM, et al. Magnetic resonance imaging and computed tomography in emergency assessment of patients with suspected acute stroke: a prospective comparison. The Lancet. 2007;369(9558):293–298. doi: 10.1016/S0140-6736(07)60151-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Briley DP, Meagher T, King D. Practical limitations of acute stroke MRI due to patient-related problems. Neurology. 2005;64(2):400–401. doi: 10.1212/WNL.64.2.400 [DOI] [PubMed] [Google Scholar]
- 31.Muizelaar JP, Fatouros PP, Schröder ML. A new method for quantitative regional cerebral blood volume measurements using computed tomography. Stroke. 1997;28(10):1998–2005. doi: 10.1161/01.str.28.10.1998 [DOI] [PubMed] [Google Scholar]
- 32.Copen WA, Schaefer PW, Wu O. MR Perfusion Imaging in Acute Ischemic Stroke. Neuroimaging Clin N Am. 2011;21(2):259–283. doi: 10.1016/j.nic.2011.02.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Sotoudeh H, Shafaat O, Singhal A, Bag A. Luxury perfusion: A paradoxical finding and pitfall of CT perfusion in subacute infarction of brain. Radiol Case Rep. 2018;14(1):6–9. doi: 10.1016/j.radcr.2018.08.031 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Nagar VA, McKinney AM, Karagulle AT, Truwit CL. Reperfusion Phenomenon Masking Acute and Subacute Infarcts at Dynamic Perfusion CT: Confirmation by Fusion of CT and Diffusion-Weighted MR Images. Am J Roentgenol. 2009;193(6):1629–1638. doi: 10.2214/AJR.09.2664 [DOI] [PubMed] [Google Scholar]
- 35.Williams DS, Detre JA, Leigh JS, Koretsky AP. Magnetic resonance imaging of perfusion using spin inversion of arterial water. Proc Natl Acad Sci U S A. 1992;89(1):212–216. doi: 10.1073/pnas.89.1.212 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Welker K, Boxerman J, Kalnin XA, Kaufmann T, Shiroishi M, Wintermark M. ASFNR Recommendations for Clinical Performance of MR Dynamic Susceptibility Contrast Perfusion Imaging of the Brain. :11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Greg Zaharchuk. Arterial Spin–Labeled Perfusion Imaging in Acute Ischemic Stroke. Stroke. 2014;45(4):1202–1207. doi: 10.1161/STROKEAHA.113.003612 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.deHavenon Adam, Haynor David R., Tirschwell DaDavid L., et al. Association of Collateral Blood Vessels Detected by Arterial Spin Labeling Magnetic Resonance Imaging With Neurological Outcome After Ischemic Stroke. JAMA Neurol. 74(4):453–458. doi: 10.1001/jamaneurol.2016.4491 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.The National Institute of Neurological Disorder and Stroke rt-PA Stroke Stdy Group. Tissue Plasminogen Activator for Acute Ischemic Stroke. 333:1581–1588. doi: 10.1056/nejm199512143332401 [DOI] [Google Scholar]
- 40.Ma H, Campbell BCV, Parsons MW, et al. Thrombolysis Guided by Perfusion Imaging up to 9 Hours after Onset of Stroke. N Engl J Med. 2019;380(19):1795–1803. doi: 10.1056/NEJMoa1813046 [DOI] [PubMed] [Google Scholar]
- 41.Thomalla G, Simonsen CZ, Boutitie F, et al. MRI-Guided Thrombolysis for Stroke with Unknown Time of Onset. N Engl J Med. 2018;379(7):611–622. doi: 10.1056/NEJMoa1804355 [DOI] [PubMed] [Google Scholar]
- 42.Kidwell CS, Jahan R, Gornbein J, et al. A Trial of Imaging Selection and Endovascular Treatment for Ischemic Stroke. N Engl J Med. 2013;368(10):914–923. doi: 10.1056/NEJMoa1212793 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Broderick JP, Palesch YY, Demchuk AM, Yeatts SD, et al. Endovascular Therapy after Intravenous t-PA versus t-PA Alone for Stroke. N Engl J Med. 368:893–903. doi: 10.1056/nejmoa1214300 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Mulder MJHL, Jansen IGH, Goldhoorn R-JB, et al. Time to Endovascular Treatment and Outcome in Acute Ischemic Stroke: MR CLEAN Registry Results. Circulation. 2018;138(3):232–240. doi: 10.1161/CIRCULATIONAHA.117.032600 [DOI] [PubMed] [Google Scholar]
- 45.Goyal M, Demchuk AM, Menon BK, et al. Randomized Assessment of Rapid Endovascular Treatment of Ischemic Stroke. N Engl J Med. 2015;372(11):1019–1030. doi: 10.1056/NEJMoa1414905 [DOI] [PubMed] [Google Scholar]
- 46.Tudor G. Jovin, Angel Chamorro, Erik Cobo, et al. Thrombectomy within 8 Hours after Symptom Onset in Ischemic Stroke. N Engl J Med. 2015;372:2296–2306. doi: 10.1056/nejmoa1503780 [DOI] [PubMed] [Google Scholar]
- 47.Saver JL, Goyal M, Bonafe A, et al. Stent-Retriever Thrombectomy after Intravenous t-PA vs. t-PA Alone in Stroke. N Engl J Med. 2015;372(24):2285–2295. doi: 10.1056/NEJMoa1415061 [DOI] [PubMed] [Google Scholar]
- 48.Campbell BCV, Mitchell PJ, Kleinig TJ, et al. Endovascular Therapy for Ischemic Stroke with Perfusion-Imaging Selection. 10.1056/NEJMoa1414792. doi: 10.1056/NEJMoa1414792 [DOI] [PubMed]
- 49.Goyal M, Menon BK, van Zwam WH, et al. Endovascular thrombectomy after large-vessel ischaemic stroke: a meta-analysis of individual patient data from five randomised trials. The Lancet. 2016;387(10029):1723–1731. doi: 10.1016/S0140-6736(16)00163-X [DOI] [PubMed] [Google Scholar]
- 50.Nogueira RG, Jadhav AP, Haussen DC, et al. Thrombectomy 6 to 24 Hours after Stroke with a Mismatch between Deficit and Infarct. N Engl J Med. 2018;378(1):11–21. doi: 10.1056/NEJMoa1706442 [DOI] [PubMed] [Google Scholar]
- 51.Albers GW, Marks MP, Kemp S, et al. Thrombectomy for Stroke at 6 to 16 Hours with Selection by Perfusion Imaging. N Engl J Med. 2018;378:708–718. doi: 10.1056/NEJMoa1713973 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Dula AN, Mlynash M, Zuck ND, Albers GW, Warach SJ, DEFUSE 3 Investigators. Neuroimaging in Ischemic Stroke Is Different Between Men and Women in the DEFUSE 3 Cohort. Stroke. 2020;51(2):481–488. doi: 10.1161/STROKEAHA.119.028205 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Mokin M, Ansari SA, McTaggart RA, et al. Indications for thrombectomy in acute ischemic stroke from emergent large vessel occlusion (ELVO): report of the SNIS Standards and Guidelines Committee. J NeuroInterventional Surg. 2019;11(3):215–220. doi: 10.1136/neurintsurg-2018-014640 [DOI] [PubMed] [Google Scholar]
- 54.Kerleroux B, Janot K, Dargazanli C, et al. Perfusion Imaging to Select Patients with Large Ischemic Core for Mechanical Thrombectomy. J Stroke. 2020;22(2):225–233. doi: 10.5853/jos.2019.02908 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Rebello LC, Bouslama M, Haussen DC, et al. Endovascular Treatment for Patients With Acute Stroke Who Have a Large Ischemic Core and Large Mismatch Imaging Profile. JAMA Neurol. 2017;74(1):34. doi: 10.1001/jamaneurol.2016.3954 [DOI] [PubMed] [Google Scholar]
- 56.Ducroux C, Khoury N, Lecler A, et al. Application of the DAWN clinical imaging mismatch and DEFUSE 3 selection criteria: benefit seems similar but restrictive volume cut-offs might omit potential responders. Eur J Neurol. 2018;25(8):1093–1099. doi: 10.1111/ene.13660 [DOI] [PubMed] [Google Scholar]
- 57.Nannoni S, Strambo D, Sirimarco G, et al. Eligibility for late endovascular treatment using DAWN, DEFUSE-3, and more liberal selection criteria in a stroke center. J Neurointerventional Surg. 2020;12(9):842–847. doi: 10.1136/neurintsurg-2019-015382 [DOI] [PubMed] [Google Scholar]
- 58.Deutschmann H, Hinteregger N, Wießpeiner U, et al. Automated MRI perfusion-diffusion mismatch estimation may be significantly different in individual patients when using different software packages. Eur Radiol. Published online August 21, 2020. doi: 10.1007/s00330-020-07150-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Cereda CW, Christensen S, Campbell BC, et al. A benchmarking tool to evaluate computer tomography perfusion infarct core predictions against a DWI standard. J Cereb Blood Flow Metab. 2016;36(10):1780–1789. doi: 10.1177/0271678X15610586 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Nael K, Khan R, Gagandeep C, et al. Six-Minute Magnetic Resonance Imaging Protocol for Evaluation of Acute Ischemic Stroke. AHA J. 2014(45):1985–1991. doi: 10.1161/STROKEAHA.114.005305 [DOI] [PubMed] [Google Scholar]


