Abstract
BACKGROUND:
To investigate a comprehensive array of MRI-based biomarkers of cerebrovascular disease in a cohort of PLWH and relate these imaging biomarkers to cognition.
SETTING:
Cross-sectional, community-based
METHODS:
Participants were PLWH in New York City ≥ 50 years old. They underwent a brain MRA/MRI to ascertain seven MRI markers of cerebrovascular disease: Silent brain infarcts, dilated perivascular spaces, microhemorrhages, white matter hyperintensity volume, white matter fractional anisotropy and mean diffusivity (measures of white matter integrity), and intracranial large artery stenosis. Participants underwent a battery of neurocognitive tests to obtain individual and global cognitive scores representative of various aspects of cognition.
RESULTS:
We included 85 participants (mean age 60 ± 6 years, 48% men, 78% non-Hispanic black), the majority with well controlled HIV (75% with CD4 count > 200 cell/mm3 and viral load < 400 copies/mL at or near the time of the MRI scan). Silent brain infarcts, intracranial large artery stenosis and poor white matter integrity were associated with poorer performance in at least one cognitive domain, but the sum of these three MRI markers of cerebrovascular disease was associated with lower working memory (B=−0.213, P=0.028), list learning (B=−0.275, P=0.019), global cognition (B=−0.129, P=0.007).
CONCLUSIONS:
We identified silent brain infarcts, intracranial large artery stenosis and poor white matter integrity as exposures that may be modifiable and may therefore influence cognitive decline. Additionally, these MRI markers of cerebrovascular disease may help identify PLWH at higher risk of cognitive decline, which may be more amenable to targeted therapies.
Keywords: HIV, HAND, cognition, silent brain infarcts, intracranial stenosis, dementia
INTRODUCTION
With the advent of combined antiretroviral therapy (cART), people living with HIV (PLWH) are living longer. Yet the aging of the HIV population parallels a rise of dementia in this population.1,2 Differentiating dementia and other HIV-associated neurocognitive disorders (HAND) has not been well achieved in PLWH. For example, there is evidence that in PLWH with persistent immunosuppression due to HIV, or PLWH who have had an AIDS diagnosis, HAND may be explained by direct brain damage from opportunistic infections, neoplasia or stroke.3–6 On the other hand, direct central nervous system infection by HIV appears directly related to central nervous system inflammation,7,8 and has been associated with HAND even with well-controlled HIV infection,9,10 suggesting that further investigation is warranted to understand the relationship between neurodegenerative disease and HIV.
In addition to neurodegenerative disease, PLWH are at a higher risk of cerebrovascular disease, overt (e.g. stroke) or covert (also known as silent or with no distinct symptoms). Cerebrovascular disease is an important consideration in the context of neurodegenerative disease because in non-HIV infected individuals, it is associated with a higher risk of dementia and poorer cognition. For example, people with intracranial large artery stenosis are at a higher risk of dementia.11–13 Similarly, silent brain infarcts and white matter hyperintensities are markers of cerebrovascular disease and have been associated with dementia, poorer cognition and neurodegeneration.14,15 People with stroke are at higher risk of dementia16 and those with dementia are at a higher risk of stroke.17 The mechanisms by which cerebrovascular disease contributes to dementia warrant further investigation. In fact, cerebrovascular disease may contribute to HAND, and markers of covert MRI-based cerebrovascular disease have been associated with HAND in PLWH.18,19
Most studies on the vascular contributions to HAND have not included brain arterial imaging. Because brain large artery disease, specifically intracranial large artery stenosis is an important predictor of stroke and cognition in people without HIV, we hypothesize that intracranial large artery stenosis may contribute to HAND in addition to other covert magnetic resonance imaging (MRI)-based biomarkers of cerebrovascular disease. The purpose of this study was to investigate a comprehensive array of covert MRI-based biomarkers of cerebrovascular disease in a cohort of PLWH and relate them to cognitive performance.
METHODS
Sample description:
Recruitment for the study began in January 2019 and ended in July 2019. Participants were recruited from a registry of individuals who had participated in prior studies and consented to be contacted for future studies, as well as through study flyers posted at HIV-related community-based organizations in New York City. Snowball sampling was also used as enrolled participants shared flyers with their peers. Inclusion criteria were the following: a) 50 years or older; b) having an HIV/AIDS diagnosis; c) taking antiretroviral therapy (ART); d) able to communicate and read in English; e) comfortable using a tablet to complete a survey; f) able to undergo a MRI safely; and g) a total error score of 20 or less on the Short Orientation Memory Concentration Test (to evaluate capacity to consent). Participants were excluded if they were pregnant, provided ineligible responses pertaining to the MRI criteria, or scored greater than 20 on the Short Orientation Memory Concentration Test. All research activities were reviewed and approved by the Columbia University Irving Medical Center Institutional Review Board (IRB). Demographic data such as age, sex and race/ethnicity were self-reported. Vascular risk factors were identified by a combination of self-report and evidence of medication use to treat a given risk factor. History of CD4 counts and viral load near the time of visit were self-reported or extracted from the local medical records for those participants that followed their care at our institution.
Procedures:
Eligible participants provided written informed consent at their initial in-person visit. Each participant attended two visits, one included a neuropsychological assessment, a blood draw, and a demographic survey, and the second visit included a magnetic resonance imaging (MRI) scan.
Neuropsychological Assessment, Survey Assessment Measures, & Biological Specimens
A combination of neuropsychological tests were put together at the guidance of a neuropsychologist (MP) to thoroughly assess mental status, verbal intelligence, attention, recall, processing speed, language, memory, depression symptoms, and adaptive function. The tests were administered in the following order: Craft Story 21 Recall, Benson Complex Figure Copy, Number Span Test: Forward, Number Span Test: Backward, Category Fluency (animals & vegetables), Trail Making Test (Part A & B), Craft Story Recall, Benson Complex Figure – Recall/Recognition, MINT (Multilingual Naming Test), Verbal Fluency (C, F, L), Buschke Selective Reminding Test – Learning Trials, Grooved Peg Board, Oral Trail Making Test (Part A & B), Neuro-QOL Depression Questionnaire, WHODAS 2.0 short form, Buschke Selective Remind Test – Delayed Recall/Recognition, NIH Toolbox Flanker Test, NIH Toolbox Pattern Comparison Test, NIH Toolbox Sorting Working Memory Task, Wide Range Achievement Test (WRAT- 4th edition) – Word Reading, and Montreal Cognitive Assessment (MoCA). Once participants completed the neuropsychological testing, they completed a demographic survey on an iPad.
Magnetic Resonance Imaging
MRI scans were obtained with a 3T scanner (Siemens MAGNETOM Prisma) with a 64- channel coil at the Columbia University Zuckerman Institute. The MRI scans were then administered by a certified MRI technologist conducted the following sequences: 3DT1 MP-RAGE (voxel size 1×1×1mm, isometric, TR/TE (ms) 2300/2.26, field of view 256 mm, echo spacing 6.8 ms); T2-weighted FLAIR (voxel size 0.45×0.45×0.90 mm, TR/TE (ms) 5000/387, field of view 230mm, echo spacing 3.62 ms); SWI (voxel size 0.86×0.86×1.50 mm, TE/TR 27/20, flip angle 15 degrees, field of view 220mm); MRA time of flight (voxel size 0.26×0.26×0.5mm, TR/TE (ms) 21/3.42, flip angle 18 degrees, field of view 200mm); and diffusion tensor images (voxel size 2×2×2.6mm, 64 non-collinear directions).
White matter hyperintensity volume was derived from T2-weighed FLAIR scans. Briefly, a Gaussian curve was fit to map voxel intensity values. All voxels that were above 1.5 standard deviations from the image mean intensity were labeled. After manual edits of false positives, remaining voxels were classified as WMH. The number of labeled voxels was summed and multiplied by voxel dimensions to yield total white matter hyperintensities volumes in cm3.20 From T1 and FLAIR images, silent brain infarcts and dilated perivascular spaces were ascertained using a pathology-informed algorithm previously described with good to excellent reliability.21 DTI images were eddy current corrected and fractional anisotropy and mean diffusivity maps were computed using the FMRIB Diffusion Toolbox (FSL v6.0 fsl.fmrib.ox.ac.uk/fsl/fslwiki/). Each subject’s FA and MD maps were normalized and projected onto a mean FA tract skeleton using the FSL Tract-Based Spatial Statistics (TBSS). Mean FA and mean diffusivity were computed from these skeletonized images using tracts-of-interest from the JHU-ICBM-DTI-81 white matter labels atlas. Abnormal white matter integrity was defined by the highest fractional anisotropy quartile and lowest mean diffusivity quartile. The brain MRA was read by two readers using the WASID criteria to define stenosis.22 Intracranial large artery stenosis was defined as any luminal stenosis > 50% in the major branches of the circle of Willis and vertebrobasilar system. Examples of these MRI phenotypes are shown in Figure 1.
Figure 1:
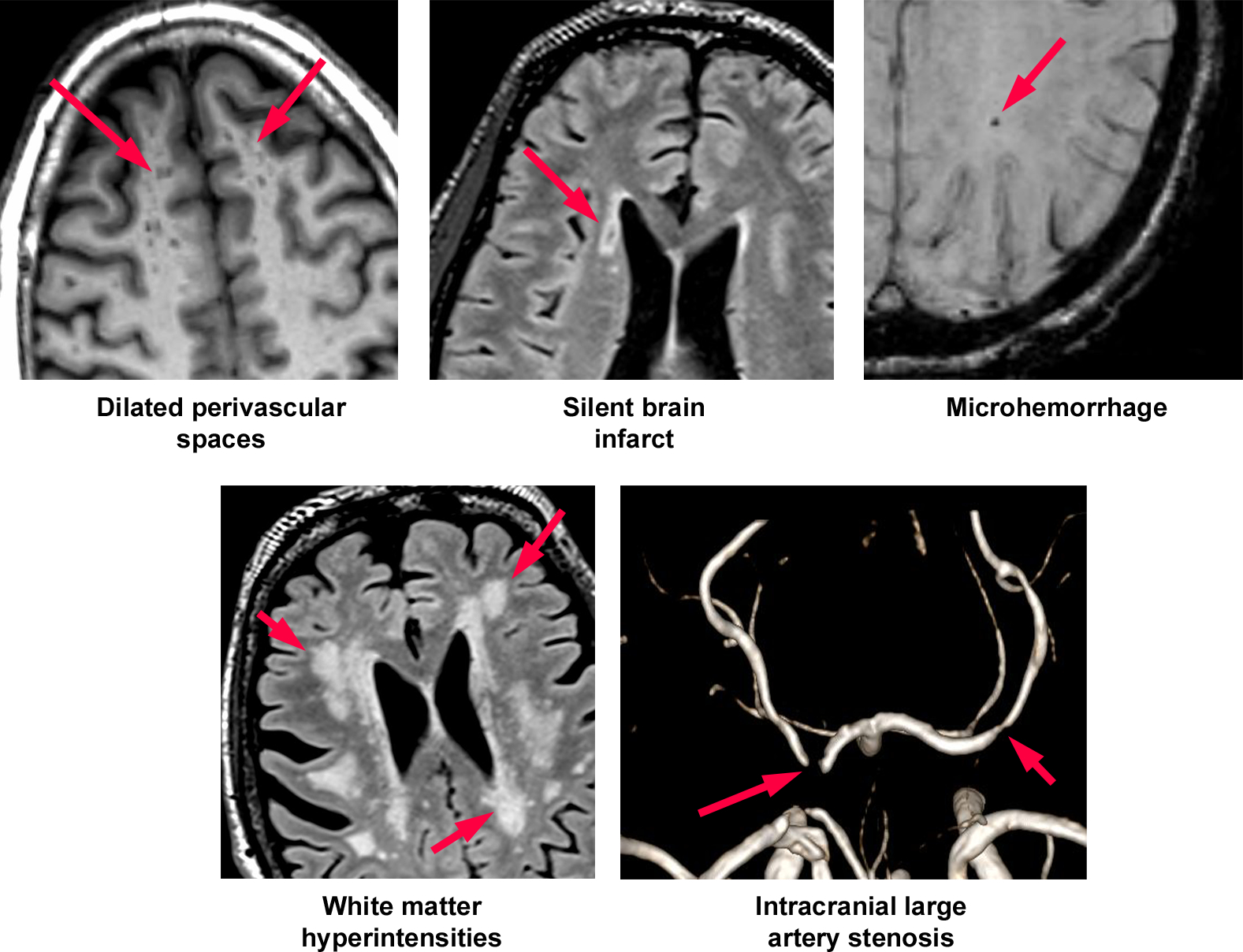
Examples of MRI-based biomaerks of covert cerebrovascular disease in this study.
Statistical Analysis
Descriptive statistics were used to calculate the demographic characteristics and vascular risk factors of the study participants.
Neurocognitive Domains: Participants’ scores on each of the neuropsychological measures were transformed into z-scores to permit comparison across the different domains of cognitive and neuropsychiatric functioning, using the means and SD’s of the study sample. SPSS package v20 (IBM SPSS Statistics) was used to conduct the factor analysis. Principal component analysis with oblimin rotation was used to explore the factors underlying the neurocognitive variables. All neurocognitive measures were considered for the initial analysis. The number of factors to be retained was guided by Kaiser’s criterion (eigenvalues > 1)23 and visual inspection of the scree plot. We labeled each factor with guidance from a neuropsychologist to characterize the items loading on a domain. We considered loadings of > .40 to reflect a variable selectively loading on a given factor to characterize the data. The reliability of the variables in each factor was assessed using Cronbach’s alpha coefficients > .80. The factor analysis yielded a 5-factor model that included Working Memory, Paragraph Memory/Naming, List Learning, Mental Sequencing, and Speed. We obtained a global cognitive score by averaging the z-scores of these five domains.
Following the factor analysis, we conducted linear regression models for each cognitive domain separately with the following exposure variable for each model: silent brain infarcts, upper quartile of white matter hyperintensities volume, abnormal white matter integrity, microhemorrhages, perivascular spaces and intracranial large artery stenosis. We adjusted for demographic and vascular risk factors (which included hypertension, diabetes, dyslipidemia and current smoking) in addition to status of HIV infection (controlled versus not controlled). Given small sample size, we considered a P value between 0.20 and 0.05 suggestive of a trend, and a P value of < 0.05 as statistically significant. We choose the exposure variable that emerged with at least a trend to form a cumulative cerebrovascular disease score to evaluate the impact of the cumulative cerebrovascular disease score in cognition.
RESULTS:
The sample was comprised of 85 participants (mean age 60 ± 6 years, 48% men, 78% non-Hispanic black). Demographic characteristics are provided in table 1. The majority had hypertension (66%) and well controlled HIV (75%, defined by CD4 count > 200 and viral load < 400 copies at or near the time of the MRI visit). A third of the sample (33%) had evidence of silent brain infarcts, 5% of microhemorrhages, 12% of intracranial large artery stenosis, and 44% had imaging evidence of abnormal white matter integrity as defined by a low fractional anisotropy and/or high mean diffusivity.
Table 1:
Sample characteristics (N=85)
| Age (in years, mean ± SD, range) | 60 ± 6, 50–75 | |
| Men | 48% | |
| Ethnicity | ||
| Non-Hispanic black | 78% | |
| Hispanic | 22% | |
| Education attainment | Incomplete high school or lower | 26% |
| Completed high school | 23% | |
| Some college | 25% | |
| College or higher | 26% | |
| Hypertension | 66% | |
| Diabetes | 18% | |
| Dyslipidemia | 37% | |
| Current smoking | 48% | |
| Viral load >400 copies/ML | 20% | |
| CD4 count (mean ± SD) | 636 ± 356 | |
| CD4 count < 200 | 10% | |
| Nadir CD4 < 200 | 65% | |
| Silent brain infarcts | 33% | |
| Microhemorrhages | 5% | |
| White matter hyperintensity volume(median, mean ± SD) | 4.34, 6.2 ± 6.1 | |
| White matter abnormal microstructure | 44% | |
| Intracranial large artery stenosis | 12% | |
In the univariate analysis, silent brain infarcts and intracranial large artery stenosis were associated with poorer cognition in at least one cognitive domain (table 2). The association between silent brain infarcts and poorer cognition attenuated after adjustment for vascular risk factors and demographic covariates, while the association between intracranial large artery stenosis and list learning remained significant. Abnormal white matter integrity was associated with poorer mental sequencing and global cognition only after adjusting for demographics and education. There was no statistical association or trends between white matter hyperintensities volume, microhemorrhages or perivascular spaces with cognitive performance.
Table 2:
Association between MRI-based biomarkers of covert cerebrovascular disease and cognitive performance among people living with HIV.
| Working Memory | Paragraph Memory/Naming | List Learning | Mental Sequencing | Speed | Global cognition | ||
|---|---|---|---|---|---|---|---|
| Silent brain infarcts | |||||||
| Model 1 | −0.34 ± 0.15* | −0.01 ± 0.19 | −0.22 ± 0.19 | −0.17 ± 0.09‡ | −0.01 ± 0.11 | −0.16 ± 0.08* | |
| Model 2 | −0.28 ± 0.16‡ | 0.08 ± 0.19 | −0.22 ± 0.19 | −0.14 ± 0.09 | −0.03 ± 0.12 | −0.12 ± 0.08 | |
| Model 3 | −0.29 ± 0.16‡ | 0.06 ± 0.19 | −0.23 ± 0.19 | −0.17 ± 0.09‡ | −0.02 ± 0.12 | −0.13 ± 0.08‡ | |
| White matter hyperintensity volume upper quartile | |||||||
| Model 1 | 0.08 ± 0.17 | 0.01 ± 0.19 | 0.16 ± 0.22 | −0.09 ± 0.11 | −0.11 ± 0.12 | 0.09 ± 0.09 | |
| Model 2 | 0.14 ± 0.18 | 0.01 ± 0.20 | 0.25 ± 0.21 | −0.04 ± 0.11 | −0.11 ± 0.13 | 0.04 ± 0.09 | |
| Model 3 | 0.15 ± 0.18 | −0.01 ± 0.20 | 0.22 ± 0.21 | −0.05 ± 0.11 | −0.14 ± 0.13 | −0.02 ± 0.09 | |
| Lowest FA quartile plus highest MD quartile | |||||||
| Model 1 | −0.03 ± 0.13 | 0.14 ± 0.16 | −0.20 ± 0.16 | −0.08 ± 0.08 | −0.09 ± 0.10 | −0.03 ± 0.07 | |
| Model 2 | −0.08 ± 0.15 | 0.08 ± 0.18 | −0.13 ± 0.17 | −0.17 ± 0.09‡ | −0.08 ± 0.12 | −0.09 ± 0.08 | |
| Model 3 | −0.13 ± 0.15 | 0.10 ± 0.18 | −0.10 ± 0.18 | −0.17 ± 0.09‡ | −0.05 ± 0.12 | −0.09 ± 0.07‡ | |
| Microhemorrhages | |||||||
| Model 1 | −0.26 ± 0.35 | 0.59 ± 0.42 | 0.42 ± 0.42 | −0.13 ± 0.20 | −0.16 ± 0.24 | 0.10 ± 0.18 | |
| Model 2 | −0.34 ± 0.35 | 0.50 ± 0.40 | 0.46 ± 0.41 | −0.15 ± 0.21 | −0.15 ± 0.25 | 0.07 ± 0.18 | |
| Model 3 | −0.27 ± 0.35 | 0.58 ± 0.41 | 0.45 ± 0.43 | −0.21 ± 0.21 | −0.14 ± 0.26 | 0.08 ± 0.18 | |
| Perivascular spaces | |||||||
| Model 1 | 0.03 ± 0.02 | <0.001 ± 0.02 | 0.02 ± 0.02 | 0.01 ± 0.01 | 0.003 ± 0.01 | 0.01 ± 0.01 | |
| Model 2 | 0.02 ± 0.02 | −0.01 ± 0.02 | 0.02 ± 0.02 | 0.01 ± 0.01 | 0.01 ± 0.01 | 0.01 ± 0.01 | |
| Model 3 | 0.02 ± 0.02 | −0.01 ± 0.02 | 0.02 ± 0.02 | 0.02 ± 0.01 | 0.005 ± 0.01 | 0.01 ± 0.01 | |
| Intracranial large artery stenosis | |||||||
| Model 1 | −0.06 ± 0.24 | −0.25 ± 0.29 | −0.74 ± 0.28* | 0.12 ± 0.15 | 0.21 ± 0.17 | −0.12 ± 0.12 | |
| Model 2 | −0.05 ± 0.24 | −0.26 ± 0.30 | −0.72 ± 0.27* | 0.08 ± 0.15 | 0.19 ± 0.17 | −0.13 ± 0.12 | |
| Model 3 | −0.02 ± 0.25 | −0.39 ± 0.28 | −0.69 ± 0.28* | 0.21 ± 0.16 | 0.20 ± 0.18 | −0.16 ± 0.12 | |
Analytic notes:
Model 1: Univariate analysis
Model 2: Adjusted for age, sex, education and ethnicity
Model 3: Adjusted for age, sex, education, ethnicity, vascular risk factors (hypertension, diabetes, smoking, dyslipidemia), ApoE4 genotype and well controlled HIV (suppressed viral load and CD4 > 200)
−All model for white matter disease were adjusted by total cranial volume
Identifies statistical significance with a p-value <0.05
Identifies an existing trend with a p-value <0.10
Abbreviations: FA, fractional anisotropy; MD, mean diffusivity.
In a post hoc analysis, we created a score that reflected the cumulative burden of MRI-based biomarkers of covert cerebrovascular disease in PLWH (CVD score) by adding on the covert MRI-based biomarkers of cerebrovascular disease that scored at last a trend of an association with any of the five cognitive domains. We used silent brain infarcts, abnormal white matter integrity and intracranial large artery stenosis to create the score (range 0–3) based on the results described in table 2. The CVD score was 0 in 36% of participants, 1 in 46%, 2 in 13% and 3 in 5%. In the adjusted models for age, sex, ethnicity, education attainment, vascular risk factors, ApoE4 and status of HIV infection, CVD score was associated with lower working memory (B=−0.213, P=0.028), list learning (B=−0.275, P=0.019), global cognition (B=−0.129, P=0.007) and a trend toward lower mental sequencing (B=−0.099, P=0.08). Other predictors of cognitive performance in these models are reported in table 3.
Table 3:
Role of cerebrovascular score in cognition among PLWH
| Working Memory | Paragraph Memory/Naming | List Learning | Mental Sequencing | Speed | Global cognition | |
|---|---|---|---|---|---|---|
| B estimate ± SE | ||||||
| Age (in year) | 0.006 ± 0.013 | 0.027 ± 0.015 | −0.030 ± 0.015‡ | 0.009 ± 0.008 | −0.005 ± 0.010 | −0.003 ± 0.006 |
| Men | 0.019 ± 0.179 | 0.115 ± 0.213 | −0.393 ± 0.214‡ | 0.079 ± 0.106 | −0.071 ± 0.137 | −0.015 ± 0.088 |
| Non-Hispanic black | −0.115 ± 0.181 | 0.073 ± 0.215 | 0.073 ± 0.217 | −0.166 ± 0.107 | −0.062 ± 0.137 | −0.050 ± 0.088 |
| Education attainment (ordinally) | 0.098 ± 0.048* | 0.097 ± 0.059 | 0.131 ± 0.062* | 0.068 ± 0.031* | −0.036 ± 0.037 | 0.069 ± 0.024* |
| ApoE4 | 0.294 ± 0.164‡ | 0.028 ± 0.195 | −0.142 ± 0.196 | 0.006 ± 0.097 | −0.025 ± 0.125 | 0.044 ± 0.081 |
| Sum of vascular risk factors (range 0–4) | −0.066 ± 0.091 | 0.129 ± 0.093 | −0.167 ± 0.094‡ | 0.018 ± 0.047 | 0.024 ± 0.059 | −0.004 ± 0.038 |
| Well controlled HIV | −0.092 ± 0.172 | 0.030 ± 0.204 | −0.024 ± 0.207 | −0.117 ± 0.102 | 0.122 ± 0.132 | −0.016 ± 0.084 |
| Cumulative cerebrovascular score (range 0–3) | −0.213 ± 0.095* | −0.053 ± 0.113 | −0.275 ± 0.114* | −0.099 ± 0.056‡ | 0.046 ± 0.076 | −0.129 ± 0.046* |
Analytical notes
Identifies statistical significance with a p-value <0.05
Identifies a trend defined by a p-value 0.05–0.10.
DISCUSSION:
We present data from a cross-sectional study of 85 PLWH who underwent a brain MRI to assess for MRI-based biomarkers of covert cerebrovascular disease and related these to cognitive performance. We found that the presence of silent brain infarcts, abnormal white matter integrity and intracranial large artery stenosis relate to some aspects of cognition, and that the cumulative prevalence of these three MRI biomarkers relates to poorer working memory, list learning, and global cognition. These associations were independent of sex, age, ethnicity, education, viral suppression, vascular risk factors and ApoE4. Expectedly, higher education attainment was associated with better overall cognition. Findings from this study identified: 1) vascular exposures that may be modifiable and may therefore influence cognition in PLWH; and 2) PLWH at higher risk of cognitive decline for testing targeted treatment.
Covert cerebrovascular disease represents an important contributor to cognition and dementia. This has been well-established in non-HIV infected cohorts, but this is the first study to provide evidence of this relationship in PLWH using a comprehensive array of covert MRI-based biomarkers of cerebrovascular disease including brain arterial imaging, an often neglected marker of brain health. Compared to clinically overt cerebrovascular disease like stroke, covert cerebrovascular disease is at least 3–4 times more frequent.24 Furthermore, covert cerebrovascular disease is a risk factor for stroke and dementia.14,25,26 Therefore, timely identification of covert cerebrovascular disease and understanding its role in stroke and dementia may help identify individuals at higher risk of future stroke and dementia with the potential for intervention to prevent further decline.
Although there is extensive evidence relating these imaging biomarkers of covert cerebrovascular disease to cognition and dementia risk among people without HIV, there is less evidence of the role of these combined imaging biomarkers in PLWH. Most studies of covert cerebrovascular disease do not include a brain MRA or other types of arterial imaging to inform the status of brain large artery disease such as intracranial large artery stenosis. The study examining intracranial large artery stenosis in PLWH is of great importance because ethnic minorities are at an increased risk of both intracranial large artery stenosis and HIV.27–29 Consequently, the inclusion of brain MRA in this work is a strength. The prevalence of intracranial stenosis has been studied mostly in the context of stroke and in hospital-based samples,30–33 but not in non-stroke or the general HIV-infected populations. The prevalence of intracranial large artery stenosis was 12%, half of what has been reported in samples of hospitalized patients.34 More importantly, intracranial large artery stenosis was a predictor of poorer verbal learning independent of vascular risk factors, HIV control and ApoE4. These findings mirror other non-HIV infected cohorts. 12,35,36 Nonetheless, the pathophysiology of the association remains uncertain, and there is partial evidence that intracranial large artery stenosis may decrease brain blood flow,37,38 which may trigger downstream mechanisms leading to neurodegeneration in addition to increased risk of stroke. Other non-atherosclerotic arteriopathies such as dolichoectasia (consisting of dilated brain arteries) are associated with poorer cognition, higher risk of dementia, and among PLWH, longer and deeper immunosuppression.11,31,39,40 Therefore, inclusion of brain MRA for systematic evaluation of such brain large artery phenotypes provides new insights into the role of brain large artery disease in HAND and other cerebral outcomes in PLWH.
White matter hyperintensities volume and/or integrity are imaging biomarkers of covert cerebrovascular disease frequently studied among PLWH. For example, White matter hyperintensities have been associated with decreased frontal cortical volume,26 longer duration of HIV infection, 41 and aging.42 Although we did not find any association between white matter hyperintensities volume and cognition, markers of abnormal integrity in white matter such as low fractional anisotropy and high mean diffusivity did show a trend towards poorer cognition, a finding replicated in some 43,44 but not all studies.45 The etiology of white matter disease in people with HIV has been attributed to aging, hypertension, historical effects of AIDS (with gliosis, which appears white in FLAIR images), and ongoing neuroinflammation.42,44,45 Intensive blood pressure control reduces the risk of mortality, vascular events (including stroke), and cognitive impairment in people without HIV.46–50 Therefore, it is likely that the same benefit would likely be achieved for hypertensive PLWH, especially for PLWH who suffer from disproportionate burden of traditional risk factors (e.g. hypertension, diabetes, dyslipidemia and smoking), and vascular disease.2,51 It is less clear, however, whether modifying other therapeutic targets such as neuroinflammation could prevent, delay or improve cognition in PLWH. This hypothesis may be worth exploring in future studies.
The prevalence of silent brain infarcts in this cohort is higher than what is reported elsewhere (33% versus 4%).52 The disparity may be in part due to differences in definition of silent brain infarcts, variability in healthcare systems across countries (United States versus France) and the underlying vascular health of the samples being compared. Prevalent silent brain infarcts in our study sample was associated with poorer language fluency and global cognition, and these associations attenuated after adjusting for vascular risk factors and demographics, suggesting that these confounders may partially mediate the effects of silent brain infarcts on cognition. The prevalence of silent brain infarcts has been extensively studied in people without HIV, and consistently reported to be associated with higher risk of vascular events,25 dementia15 and poorer cognition.53 Given the disparities in traditional risk factor among PLWH, it is not surprising that silent brain infarcts are more common among PLWH than in HIV negative controls. Although there are no findings from randomized trials to firmly support a specific clinical algorithm among people with incidentally found silent brain infarcts, independent of HIV status, the difference between silent brain infarcts and stroke is explained better by silent brain infarcts localization in more a clinically silent area (left hemisphere) and smaller infarct size than a different pathophysiology of silent brain infarcts compared to strokes.24 Further research on this topic would help guide clinical management of people with incidentally found silent brain infarcts, but the data from this study support the notion that silent brain infarcts are important determinants of poorer cognition in PLWH.
In post-hoc analyses, we found that a composite score representing the cumulative prevalence of silent brain infarcts, white matter abnormal integrity and intracranial large artery stenosis, was associated with poorer cognition in PLWH. Cumulative brain injury may decrease brain plasticity and remodeling to allow for compensation in the expected cognitive deficit. The cumulative prevalence of these imaging biomarkers may also reflect more severe vascular disease, which in turn could represent an epiphenomenon of poorer vascular health. Because of the heterogeneous prevalence of the individual component of the vascular score, we cannot infer the relative weight of each of them in the individual component of cognition. In a relatively small sample like ours, the additive model of effect size attributed to each biomarker may improve power to detect an association with cognition. The sample size made it difficult to evaluate the individual association between individual vascular risk factors and the imaging biomarkers. Similarly, results in table 2 would not survive multiple comparison adjustment and should be considered preliminary. Nonetheless, intense therapy of the four main modifiable vascular risks (e.g. hypertension, diabetes, dyslipidemia, and smoking) is a goal that should be pursued regardless of whether these risk factors were related to poorer cognition or not. Replication of these results in samples that include other racial and ethnic groups and less urban setting may increase the certainty of the association between covert MRI-based biomarkers of cerebrovascular disease and poorer cognition to a broader HIV population. Lastly, the relationship between cerebrovascular disease and Alzheimer pathology in the aging HIV population remains a relative underexplored area that may yield new insights into HAND.
ACKNOWLEDGEMENTS:
To our participants for their time and effort volunteering for this study.
Sources of funding:
NIH K24NR018621, Project 02 NR015737, AG R01057709
REFERENCES:
- 1.McArthur JC, Hoover DR, Bacellar H, et al. Dementia in AIDS patients: incidence and risk factors. Multicenter AIDS Cohort Study. Neurology. 1993;43(11):2245–2252. [DOI] [PubMed] [Google Scholar]
- 2.Gutierrez J, Albuquerque ALA, Falzon L. HIV infection as vascular risk: A systematic review of the literature and meta-analysis. PLOS ONE. 2017;12(5):e0176686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Gallant JE, Moore RD, Richman DD, Keruly J, Chaisson RE. Incidence and natural history of cytomegalovirus disease in patients with advanced human immunodeficiency virus disease treated with zidovudine. The Zidovudine Epidemiology Study Group. J Infect Dis 1992;166(6):1223–1227. [DOI] [PubMed] [Google Scholar]
- 4.Brightbill TC, Ihmeidan IH, Post MJ, Berger JR, Katz DA. Neurosyphilis in HIV-positive and HIV-negative patients: neuroimaging findings. AJNR Am J Neuroradiol 1995;16(4):703–711. [PMC free article] [PubMed] [Google Scholar]
- 5.Whiteman M, Espinoza L, Post MJ, Bell MD, Falcone S. Central nervous system tuberculosis in HIV-infected patients: clinical and radiographic findings. AJNR Am J Neuroradiol 1995;16(6):1319–1327. [PMC free article] [PubMed] [Google Scholar]
- 6.Gillams AR, Allen E, Hrieb K, Venna N, Craven D, Carter AP. Cerebral infarction in patients with AIDS. AJNR Am J Neuroradiol 1997;18(8):1581–1585. [PMC free article] [PubMed] [Google Scholar]
- 7.Williams DW, Eugenin EA, Calderon TM, Berman JW. Monocyte maturation, HIV susceptibility, and transmigration across the blood brain barrier are critical in HIV neuropathogenesis. J Leukoc Biol 2012;91(3):401–415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Valcour V, Chalermchai T, Sailasuta N, et al. Central nervous system viral invasion and inflammation during acute HIV infection. J Infect Dis 2012;206(2):275–282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kamat A, Lyons JL, Misra V, et al. Monocyte activation markers in cerebrospinal fluid associated with impaired neurocognitive testing in advanced HIV infection. J Acquir Immune Defic Syndr 2012;60(3):234–243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Yuan L, Qiao L, Wei F, et al. Cytokines in CSF correlate with HIV-associated neurocognitive disorders in the post-HAART era in China. J Neurovirol 2013;19(2):144–149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Gutierrez J, Guzman V, Khasiyev F, et al. Brain arterial dilatation and the risk of Alzheimer’s disease. Alzheimers Dement 2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Honig LS, Kukull W, Mayeux R. Atherosclerosis and AD: analysis of data from the US National Alzheimer’s Coordinating Center. Neurology. 2005;64(3):494–500. [DOI] [PubMed] [Google Scholar]
- 13.Kalback W, Esh C, Castano EM, et al. Atherosclerosis, vascular amyloidosis and brain hypoperfusion in the pathogenesis of sporadic Alzheimer’s disease. Neurol Res 2004;26(5):525–539. [DOI] [PubMed] [Google Scholar]
- 14.Brickman AM, Provenzano FA, Muraskin J, et al. Regional white matter hyperintensity volume, not hippocampal atrophy, predicts incident Alzheimer disease in the community. Arch Neurol 2012;69(12):1621–1627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Vermeer SE, Prins ND, den Heijer T, Hofman A, Koudstaal PJ, Breteler MM. Silent brain infarcts and the risk of dementia and cognitive decline. N Engl J Med. 2003;348(13):1215–1222. [DOI] [PubMed] [Google Scholar]
- 16.Tatemichi TK, Paik M, Bagiella E, et al. Risk of dementia after stroke in a hospitalized cohort: results of a longitudinal study. Neurology. 1994;44(10):1885–1891. [DOI] [PubMed] [Google Scholar]
- 17.Chi NF, Chien LN, Ku HL, Hu CJ, Chiou HY. Alzheimer disease and risk of stroke: a population-based cohort study. Neurology. 2013;80(8):705–711. [DOI] [PubMed] [Google Scholar]
- 18.Robinson-Papp J, Navis A, Dhamoon MS, Clark US, Gutierrez-Contreras J, Morgello S. The Use of Visual Rating Scales to Quantify Brain MRI Lesions in Patients with HIV Infection. J Neuroimaging. 2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Peluso MJ, Meyerhoff DJ, Price RW, et al. Cerebrospinal fluid and neuroimaging biomarker abnormalities suggest early neurological injury in a subset of individuals during primary HIV infection. J Infect Dis 2013;207(11):1703–1712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Brickman AM, Tosto G, Gutierrez J, et al. An MRI measure of degenerative and cerebrovascular pathology in Alzheimer disease. Neurology. 2018;91(15):e1402–e1412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Gutierrez J, Elkind MS, Cheung K, Rundek T, Sacco RL, Wright CB. Pulsatile and steady components of blood pressure and subclinical cerebrovascular disease: the Northern Manhattan Study. J Hypertens 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Chimowitz MI, Lynn MJ, Howlett-Smith H, et al. Comparison of warfarin and aspirin for symptomatic intracranial arterial stenosis. N Engl J Med 2005;352(13):1305–1316. [DOI] [PubMed] [Google Scholar]
- 23.Kaiser HF. The Application of Electronic Computers to Factor Analysis. Educational and Psychological Measurement. 1960;20(1):141–151. [Google Scholar]
- 24.Gutierrez J, Gil-Guevara A, Ramaswamy S, et al. Classification of Covert Brain Infarct Subtype and Risk of Death and Vascular Events. Stroke. 2020;51(1):90–98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Wright CB, Dong C, Perez EJ, et al. Subclinical Cerebrovascular Disease Increases the Risk of Incident Stroke and Mortality: The Northern Manhattan Study. Journal of the American Heart Association. 2017;6(9). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.McMurtray A, Nakamoto B, Shikuma C, Valcour V. Cortical atrophy and white matter hyperintensities in HIV: the Hawaii Aging with HIV Cohort Study. J Stroke Cerebrovasc Dis 2008;17(4):212–217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Qiao Y, Guallar E, Suri FK, et al. MR Imaging Measures of Intracranial Atherosclerosis in a Population-based Study. Radiology. 2016;280(3):860–868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Suri MFK, Qiao Y, Ma X, et al. Prevalence of Intracranial Atherosclerotic Stenosis Using High-Resolution Magnetic Resonance Angiography in the General Population. The Atherosclerosis Risk in Communities Study. 2016;47(5):1187–1193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Silverberg MJ, Leyden W, Quesenberry CP Jr., Horberg MA. Race/ethnicity and risk of AIDS and death among HIV-infected patients with access to care. J Gen Intern Med 2009;24(9):1065–1072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Edwards NJ, Grill MF, Choi HA, Ko NU. Frequency and Risk Factors for Cerebral Arterial Disease in a HIV/AIDS Neuroimaging Cohort. Cerebrovasc Dis 2016;41(3–4):170–176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Gutierrez J, Goldman J, Dwork AJ, Elkind MS, Marshall RS, Morgello S. Brain arterial remodeling contribution to nonembolic brain infarcts in patients with HIV. Neurology. 2015;85(13):1139–1145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Gutierrez J, Hatleberg CI, Evans H, Yin MT. Role of pre-stroke immunity in ischemic stroke mechanism among patients with HIV. AIDS Care. 2018:1–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Vinikoor MJ, Napravnik S, Floris-Moore M, Wilson S, Huang DY, Eron JJ. Incidence and Clinical Features of Cerebrovascular Disease Among HIV-Infected Adults in the Southeastern United States. Aids Res Hum Retrov 2013;29(7):1068–1074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Edwards NJ, Grill MF, Choi HA, Ko NU. Frequency and Risk Factors for Cerebral Arterial Disease in a HIV/AIDS Neuroimaging Cohort. Cerebrovascular Diseases. 2016;41(3–4):170–176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Dolan H, Crain B, Troncoso J, Resnick SM, Zonderman AB, Obrien RJ. Atherosclerosis, dementia, and Alzheimer disease in the Baltimore Longitudinal Study of Aging cohort. Ann Neurol 2010;68(2):231–240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Roher AE, Esh C, Rahman A, Kokjohn TA, Beach TG. Atherosclerosis of cerebral arteries in Alzheimer disease. Stroke. 2004;35(11 Suppl 1):2623–2627. [DOI] [PubMed] [Google Scholar]
- 37.Nixon AM, Gunel M, Sumpio BE. The critical role of hemodynamics in the development of cerebral vascular disease. J Neurosurg 2010;112(6):1240–1253. [DOI] [PubMed] [Google Scholar]
- 38.Kamouchi M, Kishikawa K, Okada Y, Inoue T, Ibayashi S, Iida M. Poststenotic Flow and Intracranial Hemodynamics in Patients with Carotid Stenosis: Transoral Carotid Ultrasonography Study. Am J Neuroradiol 2005;26(1):76–81. [PMC free article] [PubMed] [Google Scholar]
- 39.Gutierrez J, Menshawy K, Gonzalez M, et al. Brain large artery inflammation associated with HIV and large artery remodeling. AIDS. 2016;30(3):415–423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.De Alwis PM, Smith BR, Wu T, et al. In-vivo MRI Reveals Changes to Intracerebral Vasculature Caliber in HIV Infection. Frontiers in neurology. 2019;10(687). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Trentalange A, Prochet A, Imperiale D, et al. Cerebral white matter Hyperintensities in HIV-positive patients. Brain Imaging Behav 2020;14(1):10–18. [DOI] [PubMed] [Google Scholar]
- 42.Seider TR, Gongvatana A, Woods AJ, et al. Age exacerbates HIV-associated white matter abnormalities. J Neurovirol 2016;22(2):201–212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Ragin AB, Storey P, Cohen BA, Epstein LG, Edelman RR. Whole Brain Diffusion Tensor Imaging in HIV-Associated Cognitive Impairment. Am J Neuroradiol 2004;25(2):195–200. [PMC free article] [PubMed] [Google Scholar]
- 44.van Zoest RA, Underwood J, De Francesco D, et al. Structural Brain Abnormalities in Successfully Treated HIV Infection: Associations With Disease and Cerebrospinal Fluid Biomarkers. The Journal of Infectious Diseases. 2017;217(1):69–81. [DOI] [PubMed] [Google Scholar]
- 45.Su T, Caan MWA, Wit FWNM, et al. White matter structure alterations in HIV-1-infected men with sustained suppression of viraemia on treatment. AIDS. 2016;30(2):311–322. [DOI] [PubMed] [Google Scholar]
- 46.Lithell H, Hansson L, Skoog I, et al. The Study on Cognition and Prognosis in the Elderly (SCOPE): principal results of a randomized double-blind intervention trial. J Hypertens 2003;21(5):875–886. [DOI] [PubMed] [Google Scholar]
- 47.Forette F, Seux ML, Staessen JA, et al. Prevention of dementia in randomised double-blind placebo-controlled Systolic Hypertension in Europe (Syst-Eur) trial. Lancet. 1998;352(9137):1347–1351. [DOI] [PubMed] [Google Scholar]
- 48.Curb JD, Pressel SL, Cutler JA, et al. Effect of diuretic-based antihypertensive treatment on cardiovascular disease risk in older diabetic patients with isolated systolic hypertension. Systolic Hypertension in the Elderly Program Cooperative Research Group. JAMA. 1996;276(23):1886–1892. [PubMed] [Google Scholar]
- 49.Group TSMIftSR. Effect of Intensive vs Standard Blood Pressure Control on Probable Dementia: A Randomized Clinical Trial. JAMA. 2019;321(6):553–561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.SPRINT. A Randomized Trial of Intensive versus Standard Blood-Pressure Control. New England Journal of Medicine. 2015;373(22):2103–2116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Gutierrez J, Elkind MSV, Marshall RS. Cardiovascular profile and events of US adults 20–49 years with HIV: Results from the NHANES 1999–2008. AIDS Care. 2013:1–7. [DOI] [PubMed] [Google Scholar]
- 52.Moulignier A, Savatovsky J, Assoumou L, et al. Silent Cerebral Small-Vessel Disease Is Twice as Prevalent in Middle-Aged Individuals With Well-Controlled, Combination Antiretroviral Therapy–Treated Human Immunodeficiency Virus (HIV) Than in HIV-Uninfected Individuals. Clinical Infectious Diseases. 2017;66(11):1762–1769. [DOI] [PubMed] [Google Scholar]
- 53.Huijts M, Duits A, van Oostenbrugge RJ, Kroon AA, de Leeuw PW, Staals J. Accumulation of MRI Markers of Cerebral Small Vessel Disease is Associated with Decreased Cognitive Function. A Study in First-Ever Lacunar Stroke and Hypertensive Patients. Frontiers in aging neuroscience. 2013;5:72. [DOI] [PMC free article] [PubMed] [Google Scholar]


