Abstract
FDG PET/CT is sensitive to the metabolic, immune-related, and structural changes that can occur in tumors in the setting of cancer immunotherapy. However, unique mechanisms of immune checkpoint inhibitors (ICIs) can occasionally make response evaluation challenging, as tumors and inflammatory changes are both FDG avid. We discuss these response patterns and additional sequelae of ICI immunotherapy such as immune-related adverse events. We also review new immune-specific PET imaging probes that are either at the preclinical stage or in early clinical trials, which may help guide the clinical management of cancer patients treated with immunotherapy, and will likely have applications outside of oncology for other diseases in which the immune system plays a role.
Keywords: Immunotherapy, immune-related adverse events, FDG, PET/CT, immune imaging
INTRODUCTION
To promote their own proliferation and survival, cancer cells are known to escape immune surveillance and suppress the immune response.1,2 Although the processes by which tumors escape immune surveillance are not completely understood, some of these mechanisms have been elucidated.3 For example, tumors can express T cell suppressor proteins, either constitutively or in response to the initial immune response in the tumor microenvironment.4 As a result, drugs that target suppressors of cytotoxic T cells have been seen as attractive tools in immunotherapy.
Exemplary immunotherapeutic agents that have demonstrated survival benefit include immune checkpoint inhibitors such as ipilimumab, pembrolizumab, and nivolumab, which are usually administered every 2–3 weeks.5–7 Ipilimumab inhibits cytotoxic T lymphocyte-associated protein 4 (CTLA-4) by preventing CTLA-4 binding to the B7 ligand on antigen presenting cells (APCs) or even tumors.8–11 Nivolumab and pembrolizumab inhibit the membrane protein programmed cell death-1 receptor (PD-1) on cytotoxic T cells, preventing PD-1 binding to programmed cell death ligand 1 or 2 (PD-L1/PD-L2) (Figure 1).12,13 Similarly, PD-L1 inhibitors such as atezolizumab, avelumab, and durvalumab prevent PD-1 binding on cytotoxic T cells. In the absence of therapy, signaling through CTLA-4 or the PD-1 axis leads to suppression of cytotoxic T cell function and persistence of tumors. Treatment with checkpoint inhibitors blocks these inhibitory signals, and leads to activation of the immune response with T cell activation and expansion.
Figure 1.
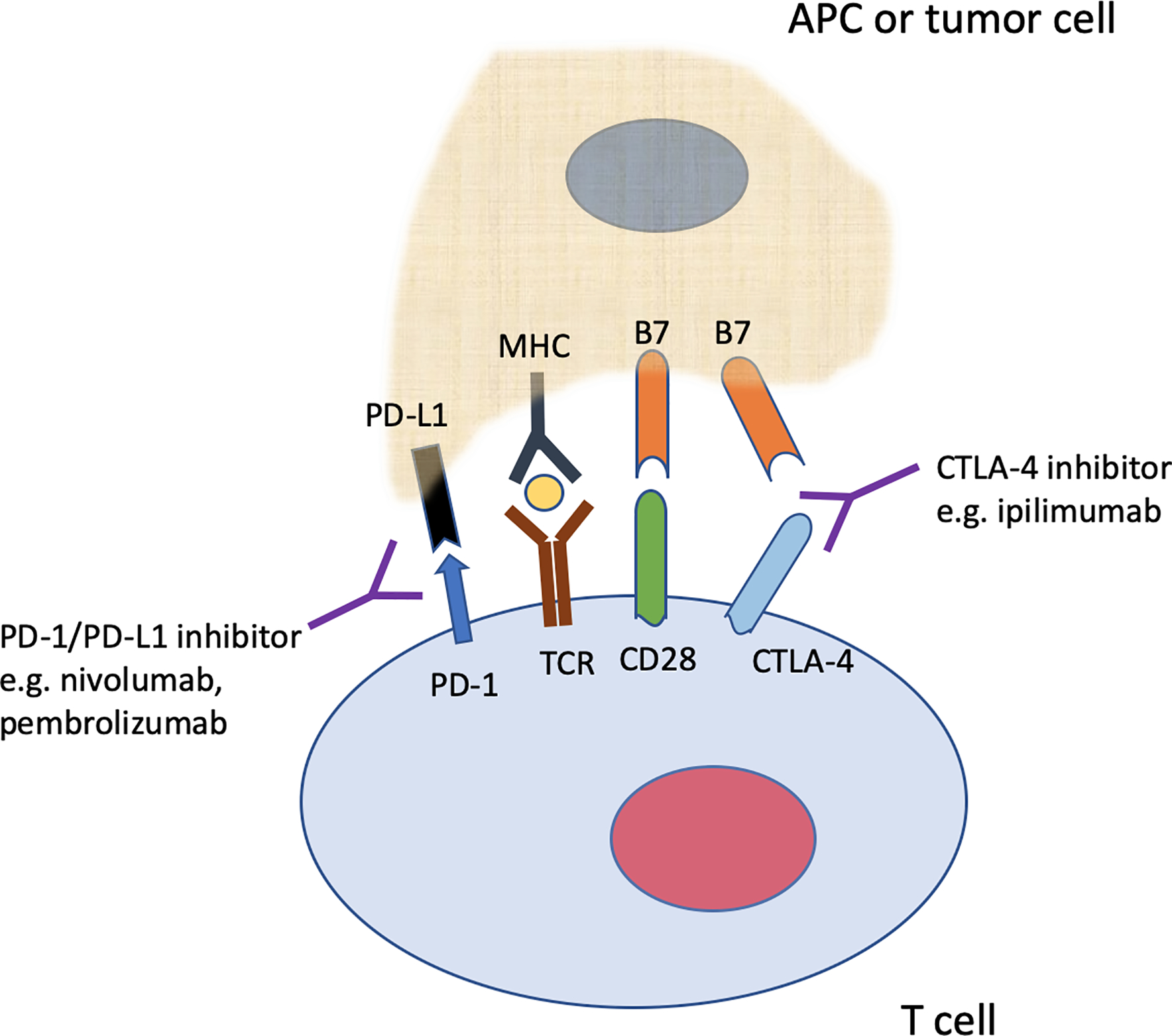
Illustration highlighting the interaction between CTLA-4 (on a T cell) and B7 (on an antigen presenting cell or tumor), and interaction between PD-1 (on a T cell) and PD-L1 (on an antigen presenting cell or tumor). Inhibitors of CTLA-4 (for example, ipilimumab) block the interaction between CTLA-4 and B7. Nivolumab and pembrolizumab are examples of immunotherapy agents that block PD-1, preventing the interaction between PD-1 and PD-L1. These inhibitors enhance anti-tumor activity through the aforementioned blockades.
APC = antigen presenting cell, TCR = T cell receptor, MHC = major histocompatibility complex, CD28 interacts with B7 to generate a co-stimulatory signal to T-cells. Yellow “oval” insert between MHC and TCR indicates processed peptide presented by MHC to TCR/T cell.
Immunotherapies have been used to re-engage and augment the immune response against a variety of malignancies such as melanoma, non-small cell lung cancer, renal cell cancer, urothelial cancer, head and neck squamous cell cancer, Merkel cell carcinoma, and Hodgkin lymphoma, and the role of immunotherapy in the treatment of cancer continues to expand.5–7,14–19 Although immunotherapy is generally associated with more frequent durable responses compared to chemotherapy or targeted therapy, more than 50% of patients do not respond.20–22 Given non-redundancy in immune checkpoint pathways, combination immunotherapy has been utilized to increase efficacy, although combination therapies are also associated with greater toxicity.7,23–27
Imaging is routinely used for diagnosis, staging, treatment planning and response assessment in oncology. Ultrasound, computed tomography (CT), and magnetic resonance imaging (MRI) can identify potential tumor lesions and assess changes in the size and density of lesions after treatment. In addition, functional imaging using positron emission tomography (PET) with 2-deoxy-2[18F]fluoro-D-glucose (FDG) is routinely used due to its high sensitivity for detecting malignancy and characterizing tumor metabolism. In this article, we discuss the role of FDG PET/CT in the assessment of treatment response after cancer immunotherapy, and identify a few approaches that utilize FDG PET/CT to evaluate the immune response. We also review new immune-specific PET imaging probes that are just beginning to be explored in early phase clinical trials.
ATYPICAL RESPONSE PATTERNS IN IMMUNOTHERAPY
The novel mechanism of action of immunotherapies, with immune and T cell activation, has the potential to lead to unusual patterns of response, such as pseudoprogression or hyperprogression, which are discussed below. However, it is important to note that these atypical responses are quite rare, and the vast majority of patients treated with current immunotherapy regimens have typical response patterns. In addition, it is important to be aware of potential immune-related adverse events (irAE), which can result in misleading findings on imaging.
Pseudoprogression
For some patients on immunotherapy, tumors can transiently increase in size, or new lesions may be seen.28 If follow-up evaluation shows resolution of the new lesions, and decreasing size or resolution of the lesions that had previously grown, this is termed pseudoprogression (Figure 2), and can be early or delayed.29 This phenomenon likely occurs as a result of tumor infiltration by immune cells, which has been confirmed by biopsy in a few cases.30–32 Many of these transiently increased/new lesions will also be avid on FDG PET/CT,33 and in some patients, pseudoprogression may be associated with clinical symptoms.34
Figure 2.
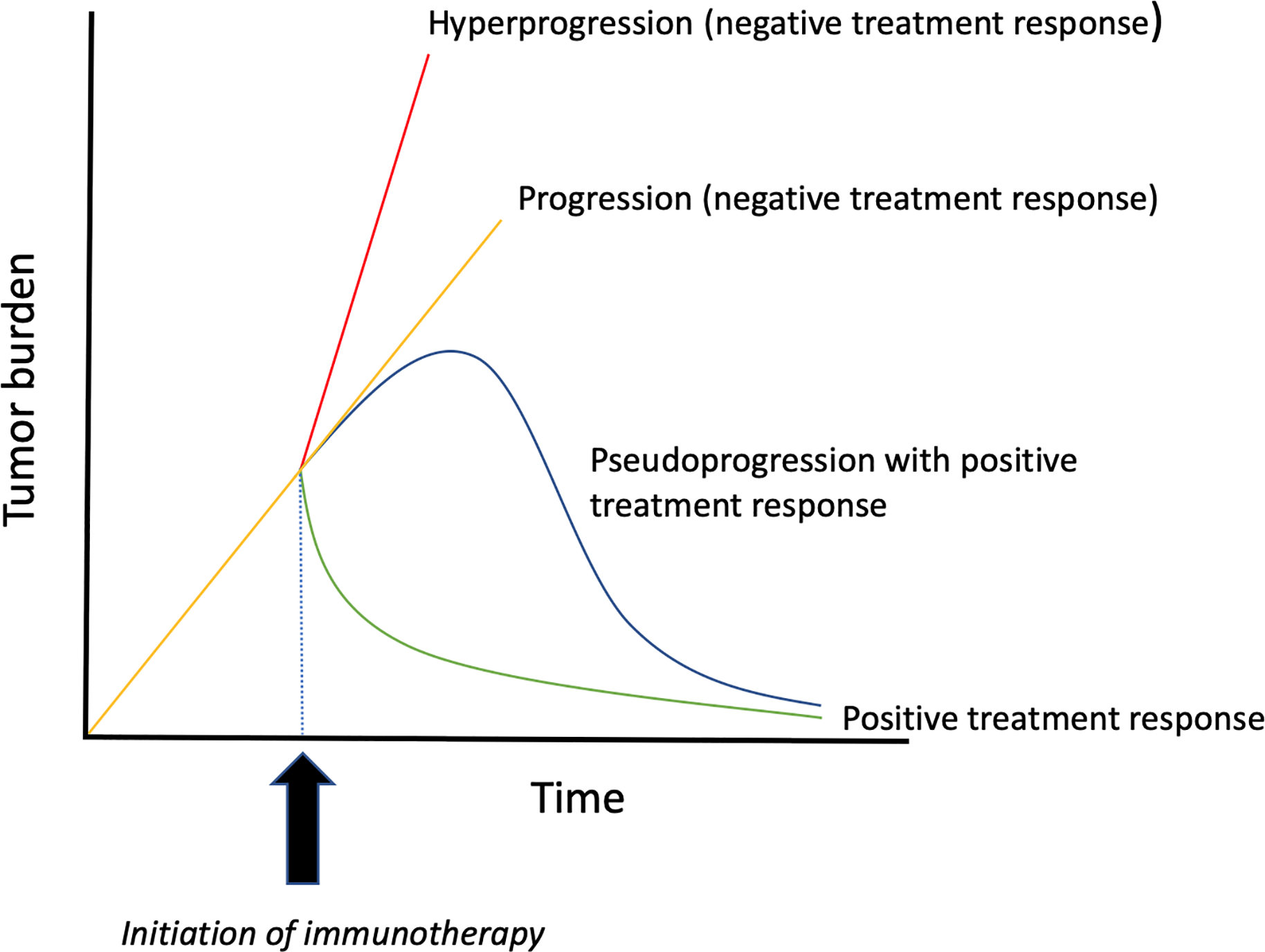
Schematic comparing response patterns following immunotherapy. Hyperprogression (red line) indicates a rapid increase in disease burden following immunotherapy, such that disease progresses at a significantly faster rate when compared to the pre-immunotherapy period. In routine progression (yellow line), tumor growth is grossly unchanged or only slightly diminished after initiation of immunotherapy. In pseudoprogression (blue line), tumors initially increase in size, however, subsequent anatomic or metabolic imaging demonstrates a decrease in disease burden. The green curve represents a typical response pattern, with tumor shrinkage following treatment.
Pseudoprogression has mainly been reported in melanoma patients treated with ipilimumab (occurring in up to 15% of cases), and appears much rarer with the use of anti-PD-1/PD-L1 agents.35–37 Billan and colleagues have compiled frequencies of pseudoprogression in pooled studies and clinical trials where anti-PD-1 axis immunotherapy agents were used to treat different cancers, and frequencies range from 1.3 to 9.3%.28 In the largest analysis to date involving 19 clinical trials and 2400 participants, nivolumab and pembrolizumab were investigated in various advanced solid tumors, and pseudoprogression was observed in 6.3% of patients.38 Thus, morphologic increase in tumor volume or metabolic activity on FDG PET/CT is much more likely to reflect true progressive disease.
Interestingly, pseudoprogression has also be seen with chimeric antigen receptor (CAR) T cell therapy when patients are imaged early; a case report of a patient with relapsed B-cell acute lymphoblastic leukemia noted pseudoprogression of extramedullary disease on MRI at 16 days post CAR T cell treatment, with subsequent response on day 30.39 In contrast, when patients with lymphoma were imaged at 1 month post CAR T cell therapy via FDG PET/CT, no evidence of pseudoprogression was identified even in patients that had cytokine release syndrome, suggesting that pseudoprogression should not be a confounding factor for routine follow-up scans in patients treated with CAR T cell therapy.40
Hyperprogression
Hyperprogression of cancer after the initiation of immune checkpoint inhibitors is a recently described response pattern in a subset of patients receiving PD-1/PD-L1 axis inhibitors.41 Hyperprogression is considered to be a therapy-induced acceleration of tumor growth kinetics (Figure 2), and has been defined as treatment failure of less than 2 months, or a two-fold or greater increase in tumor burden/growth rate during immunotherapy.42 However, the existence of hyperprogression continues to be controversial, given that it is difficult to establish if rapid progression is due to the natural history of the disease or an immunotherapy-induced process.42,43
In several reports, pre-baseline, baseline, and post-treatment scans were utilized, so that the tumor growth rate during immunotherapy could be compared with the growth rate prior to immunotherapy. Champiat and colleagues used this approach to show that 12/131 (9%) patients who received anti-PD-1/PD-L1 immunotherapy could be classified as hyperprogressors.41 In another study where pre-baseline, baseline and post-therapy scans were available, 6/155 (4%) of patients experienced hyperprogression.44 However, additional studies are needed to understand the biology driving hyperprogression, and provide more evidence for this controversial phenomenon.
Immune-Related Adverse Events
Immunotherapeutic agents can cause off-target side effects known as immune-related adverse events (irAEs), which result from inflammation of various organs/organ-systems. irAEs usually occur within 12 weeks of immunotherapy initiation, and commonly occur in the skin and gastrointestinal tract (Figure 3), although the pancreas (Figure 4), thyroid gland (Figure 5), pituitary gland, liver, lung (Figure 6), heart, and joints may also be affected.45,46 Sarcoid-like reactions can also occur as a manifestation of irAE (Figure 7).47 Although incidence rates vary by organ-system, irAEs may occur in over 50% of patients, and they appear to be more common in patients on anti-CTLA-4 monotherapy and combination immunotherapy.45,46,48 Fatality is rare and ranges from 0.3 to 1.3% in patients treated with PD-1/PD-L1 and CTLA-4 inhibitors, and is more frequently attributable to colitis-related toxicity in patients treated with ipilimumab, and pneumonitis when patients receive anti-PD-1/PD-L1 therapy.49
Figure 3.
Colitis in a 53-year-old woman with poorly differentiated adenocarcinoma of the lung treated with durvalumab (anti-PD-L1 therapy). MIP (A), CT (B) and fused FDG PET/CT images (C) acquired 3.5 months after treatment initiation revealed marked FDG uptake in the descending and sigmoid colon (arrowheads) with associated wall thickening and fat stranding consistent with colitis. The patient had bloody diarrhea at the time of the scan; sigmoidoscopy performed a week later showed acute colitis. Symptoms resolved following treatment with prednisone. Follow-up MIP (D), CT (E) and fused FDG PET/CT images (F) obtained 5 months later demonstrated marked improvement.
Figure 4.
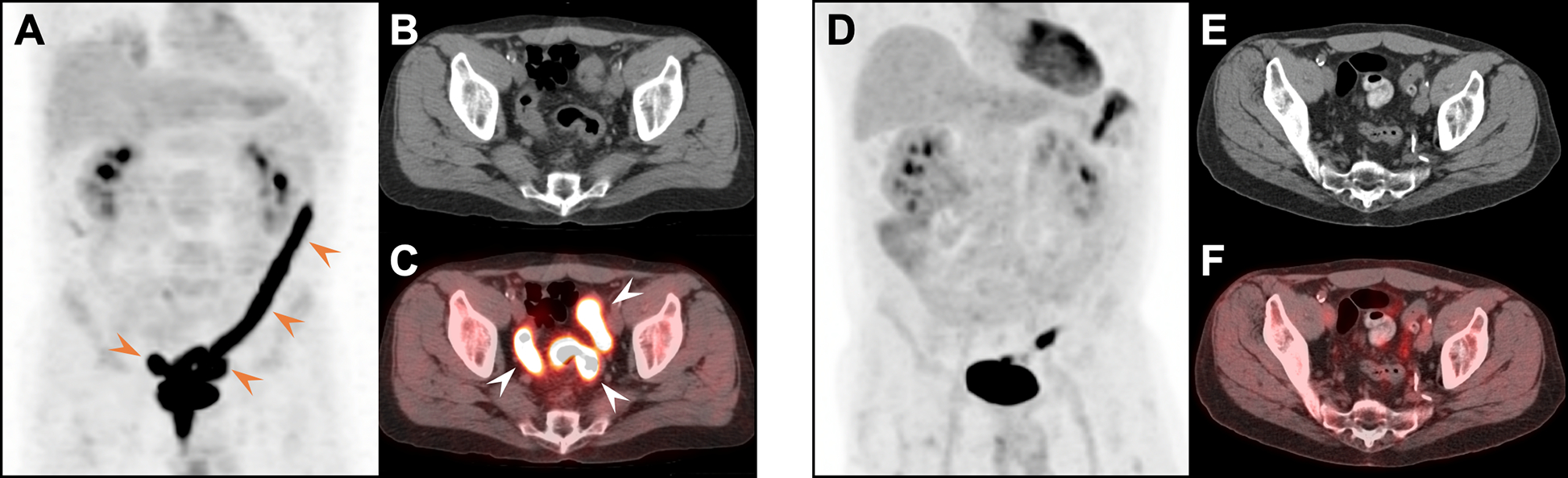
Pancreatitis in a 57-year-old woman with metastatic anal cancer on nivolumab. Fused FDG PET/CT (A) and CT (B) images acquired 7.5 months after initiation of treatment demonstrate increased FDG uptake in an edematous pancreas (arrows); the patient had diarrhea at the time of the scan. Nivolumab was held for one cycle and pancreatic enzyme supplementation was started. A follow up contrast-enhanced CT (C) performed 9 months later showed resolution with interval atrophy of the pancreas. Chronic right-sided hydronephrosis is also seen.
Figure 5.
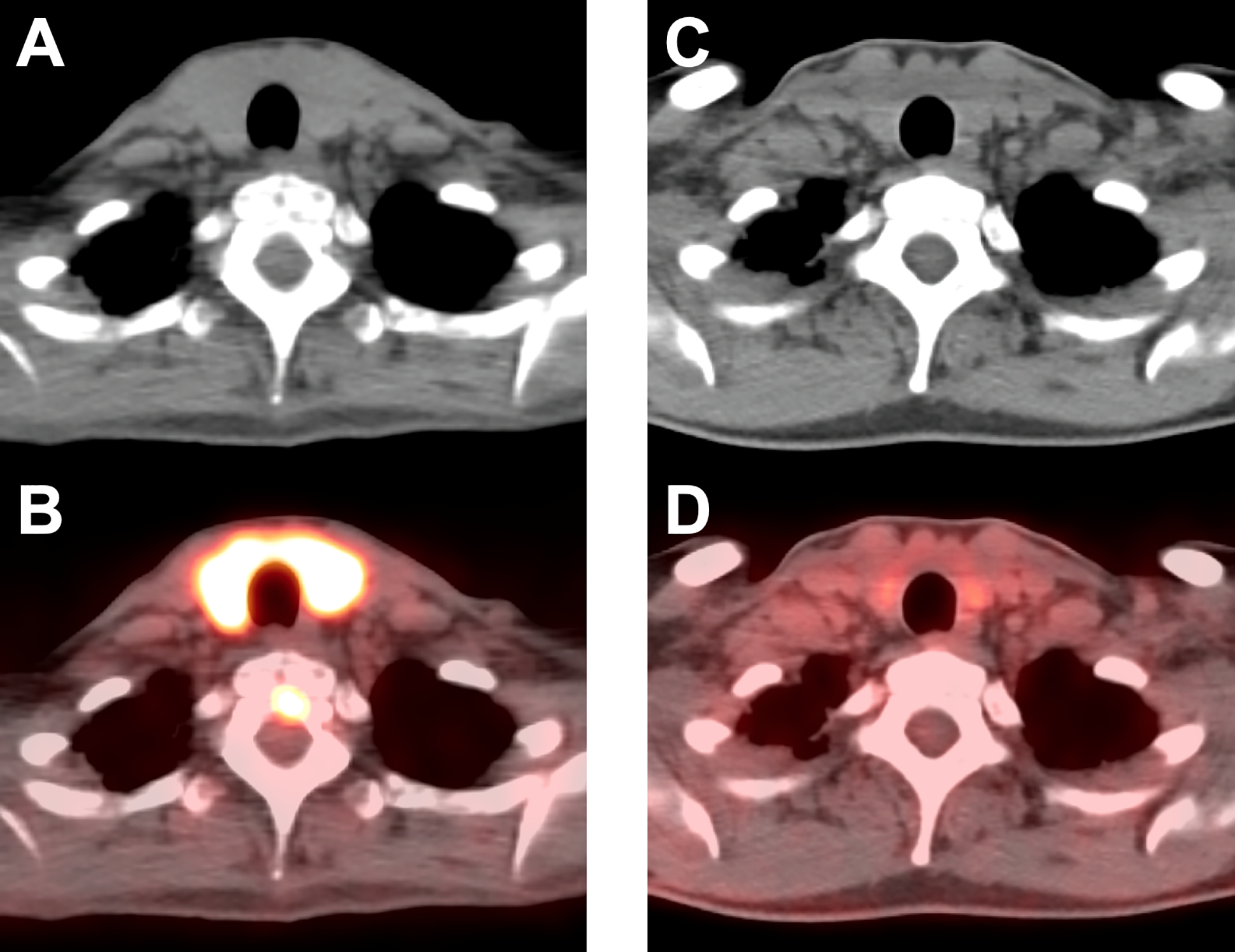
Thyroiditis in a 56-year-old woman with metastatic melanoma treated with ipilimumab / nivolumab. CT (A) and fused FDG PET/CT images (B) acquired 2.5 months after treatment initiation revealed marked FDG uptake in the thyroid gland consistent with thyroiditis; the patient had thyrotoxicosis at the time of the scan, which was followed by persistent hypothyroidism requiring levothyroxine replacement. Follow-up CT (C) and fused FDG PET/CT images (D) obtained 1 year later demonstrated resolution of the abnormal uptake in the thyroid gland.
Figure 6.
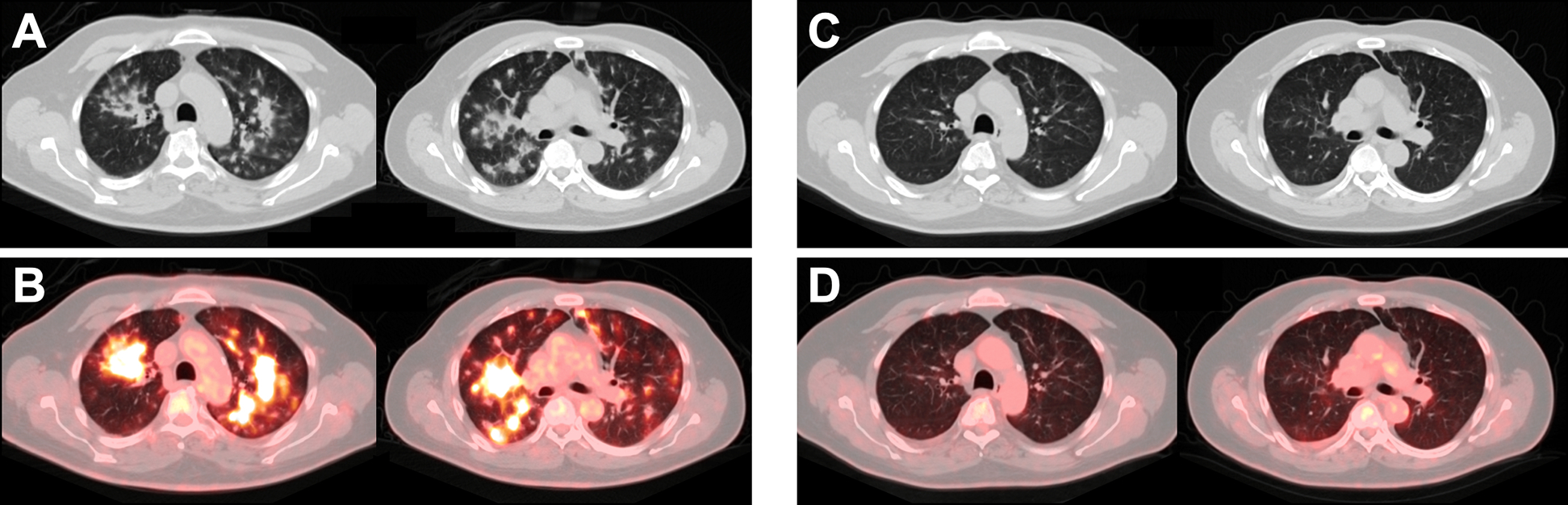
Pneumonitis in a 61-year-old man with metastatic squamous cell carcinoma of the left tonsil on pembrolizumab. CT (A) and fused FDG PET/CT images (B) acquired 7.5 months after initiation of treatment demonstrate FDG-avid nodular opacities in the lungs in a peribronchovascular distribution consistent with pneumonitis; at the time of the scan the patient had shortness of breath and cough. Pembrolizumab was held and steroids were initiated, and the patient’s symptoms improved. Follow-up CT (C) and fused FDG PET/CT images (D) acquired 4 months later demonstrate resolution of the pneumonitis.
Figure 7.
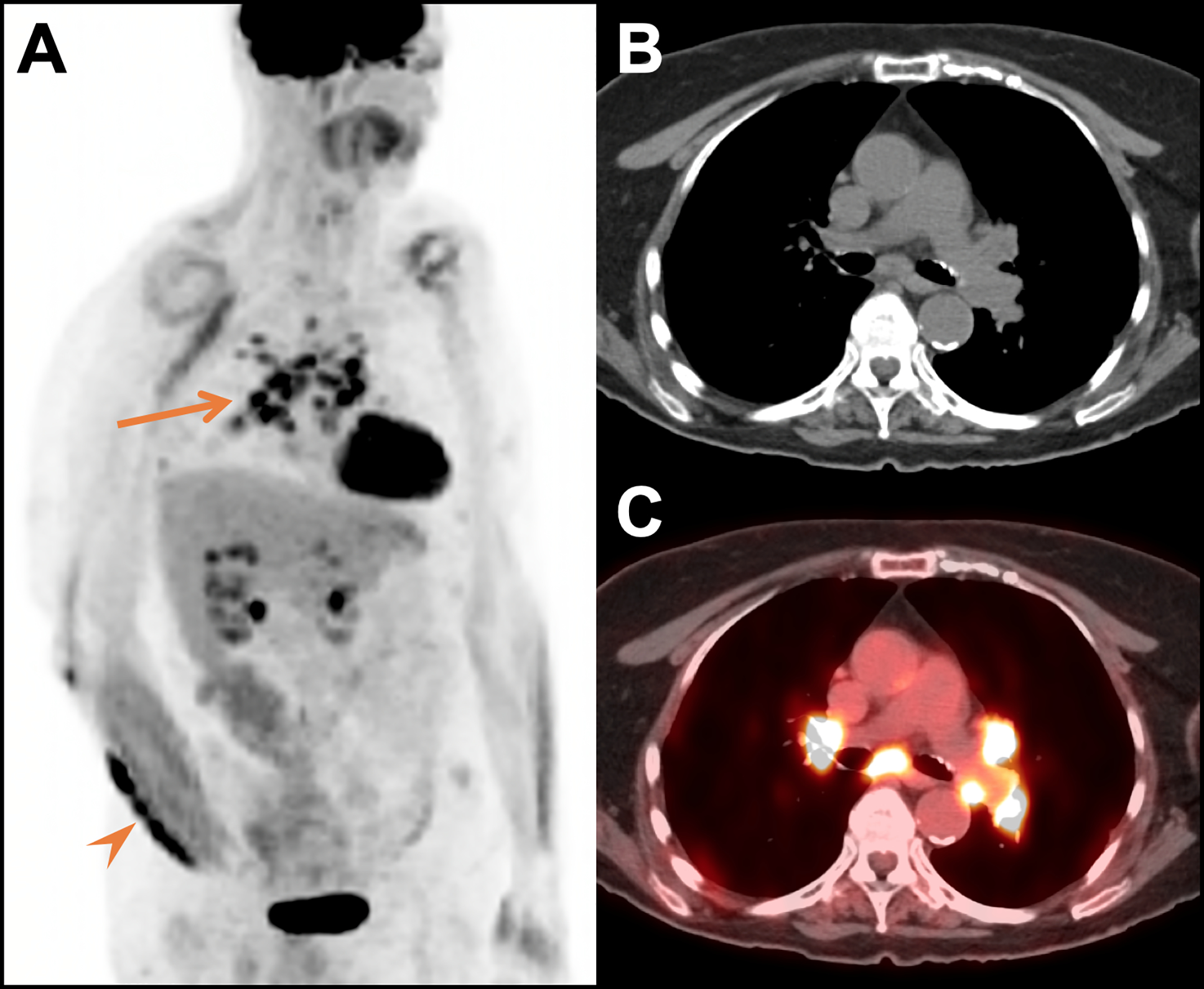
Sarcoid-like reaction in a 77-year-old woman with metastatic melanoma treated with pembrolizumab. Oblique MIP (A), CT (B) and fused FDG PET/CT images (C) acquired 13 months after treatment initiation revealed marked FDG uptake in mediastinal and bilateral hilar lymph nodes (arrow) which were new from the prior study and consistent with a sarcoid-like reaction. At the time of the scan there was also a new FDG avid right forearm mass (arrowhead); biopsy of the mass revealed granulomatous inflammation and no tumor. The mass persisted for an additional 12 months (including 5 months of pembrolizumab and 7 months of no therapy) and then spontaneously resolved.
irAEs can manifest on imaging in a range of organs and organ-systems, can precede clinical symptoms, and may even mimic metastatic disease,45,50–52 therefore it is important that radiologists are aware of this entity so that is included in the differential diagnoses for patients on immunotherapy. On FDG PET/CT, irAEs manifest as increased FDG uptake in the involved organs, and subsequent decreased uptake suggests resolution of acute inflammation.53–55 irAEs may also predict response to immunotherapy,56,57 although this may be organ/system dependent.46
RESPONSE EVALUATION
Response criteria in solid tumors (RECIST) and other metrics are routinely used to assess response to cancer therapy.58–61 However, the observation of pseudoprogression in a subgroup of patients treated primarily with ipilimumab motivated the development of new criteria for response assessment in the setting of cancer immunotherapy, in order to distinguish true progressive disease from pseudoprogression. In the majority of these new immune-related response criteria such as irRC, iRECIST and iPERCIST,33,37,62,63 an increase in size of lesions and/or appearance of new sites of disease on the first follow-up (relative to baseline imaging) reflects unconfirmed progressive disease (UPD). If follow-up anatomic imaging and/or FDG PET/CT after ≥4 weeks demonstrates no improvement or even worsening of disease, patients are classified as confirmed progressive disease (CPD). In the modified Lugano criteria for immunotherapy in lymphoma, biopsy or subsequent imaging can be performed.64 In addition, some investigators have combined anatomic and molecular imaging criteria to characterize response,65 while others have used thresholds of lesion size and number to determine progressive disease.66,67 Despite this wide variety of new immune-related response criteria, RECIST remains the primary method of response assessment for most clinical trials, including immunotherapy trials, with immune-related response criteria used for exploratory endpoints.
IMMUNE IMAGING WITH FDG PET/CT
FDG is known to be taken up by activated immune cells. In clinical FDG PET/CT scans this is reflected in inflammatory conditions such as infection, rheumatoid arthritis, and sarcoidosis, which demonstrate elevated FDG uptake.68–70 Additionally, in vitro studies have demonstrated markedly increased uptake in activated T cells compared to unstimulated T cells.71 In the routine clinical setting FDG activity in immune cells cannot be discriminated from FDG activity in tumor cells. However, if a baseline FDG PET/CT is compared with an early post-treatment FDG PET/CT over a short interval that minimizes changes in the tumor, any increase in FDG uptake should reflect tumor infiltration by activated immune cells. This metabolic “flare” phenomenon has been demonstrated in a preclinical mouse tumor model and reported in a few clinical cases, and is potentially an earlier and more sensitive measure of response to cancer immunotherapy.72–74 In fact, a recent clinical trial demonstrated that a metabolic flare could be detected in 2/16 (13%) patients with melanoma on pembrolizumab as early as 6–7 days post therapy, with dramatic increases in tumor maximum standardized uptake value (SUVMAX) that more than doubled and predicted a complete response to therapy; no tumor flare was seen in nonresponders.75 Future studies will need to test this approach in a larger cohort of patients, and explore the optimal posttreatment imaging time.
Other approaches to use FDG PET/CT imaging to predict response to immunotherapy have also been explored. For example, two studies have reported that an increased ratio of mean standardized uptake value (SUVMEAN) of bone marrow to liver (BLR) on baseline FDG PET/CT has been associated with decreased survival after anti-PD-1 immunotherapy in the setting of metastatic melanoma.76,77 This bone marrow hypermetabolism in patients with cancer is hypothesized to reflect a systemic inflammatory response, which leads to immunosuppression and is associated with cancer progression. Additional support for this hypothesis is provided by a significant positive correlation between FDG uptake in bone marrow and serum inflammatory markers including the white blood cell count and C-reactive protein.76
During immunotherapy, activation of the immune system can cause infiltration of lymphoid organs by immune cells. Sarcoid-like reaction, although considered to be an irAE, has been shown to reflect nodal infiltration by immune cells post-immunotherapy, and such nodal infiltration corresponds to associated FDG-avidity (Figure 7).78 In a recent study, all patients with FDG-avid sarcoid-like reactions following immunotherapy demonstrated positive response.79 Pseudoprogression also appears to indicate infiltration of tumors by immune cells which are FDG avid.31,80
FDG PET/CT has also been used to visualize the immune response following vaccination. Increased FDG uptake has been seen in ipsilateral axillary lymph nodes following the influenza vaccine for up to 2–4 weeks, with the highest uptake seen within the first week after the vaccine.81,82 In one case report, transiently increased FDG activity was also seen in the spleen at 2–3 days post vaccination, which resolved 12 days later.83 A similar pattern of increased FDG uptake in the deltoid muscle and ipsilateral axillary lymph nodes has been seen for up to several weeks following COVID-19 vaccination (Figure 8).84 These cases underscore the need for an accurate patient history, to ensure that FDG avid reactive axillary lymph nodes are not mistaken for metastatic disease.
Figure 8.
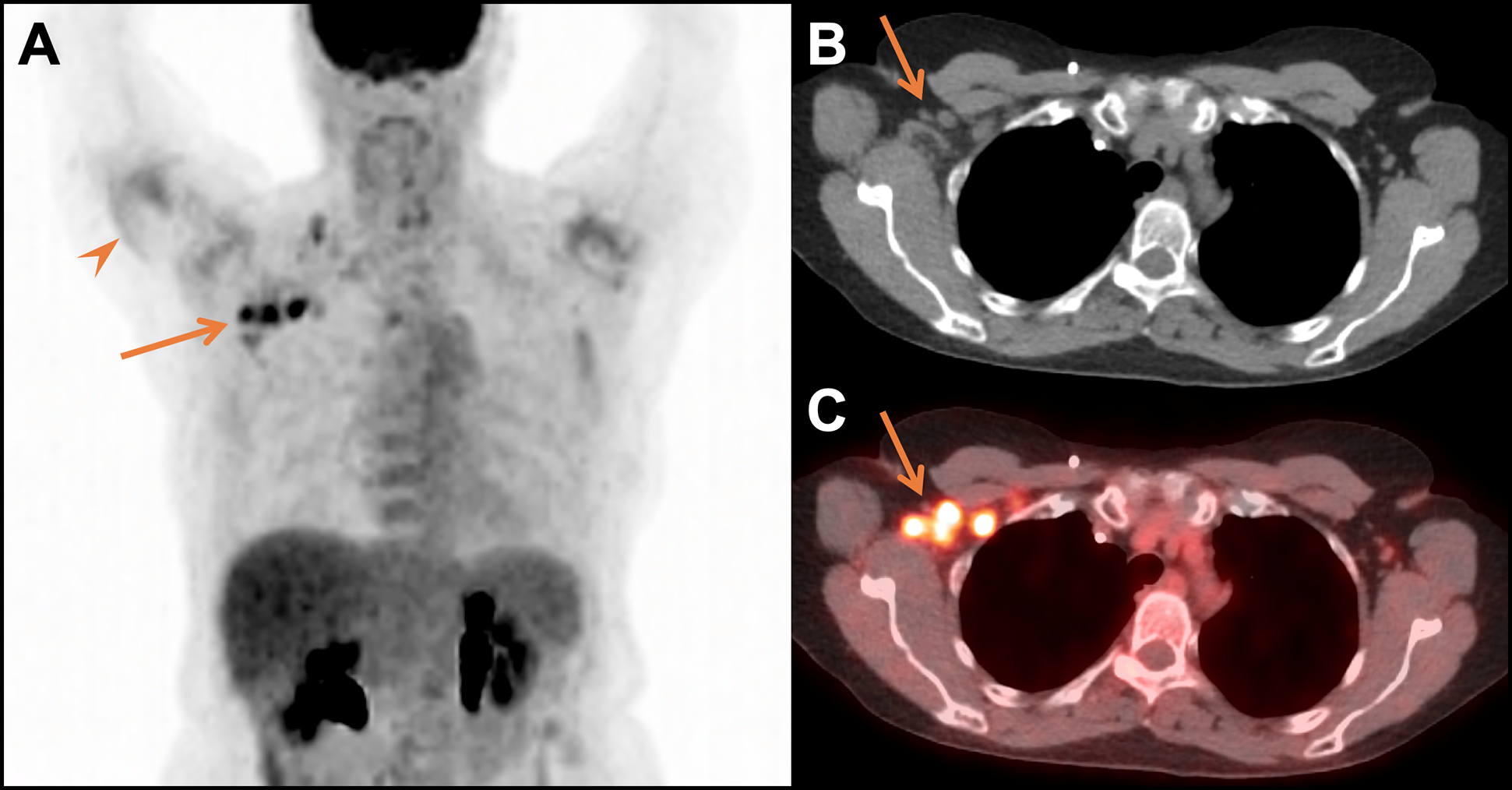
Imaging findings of COVID-19 vaccination. MIP (A), CT (B), and fused FDG PET/CT images (C) acquired 2 days after COVID-19 vaccination in the right arm revealed increased FDG uptake in the right deltoid muscle (arrowhead) and markedly increased uptake in right axillary lymph nodes (arrows) and a supraclavicular lymph node, which were normal in size. These findings were consistent with reactive changes from COVID-19 vaccination in a 70-year-old woman with a history of treated lung cancer and no evidence of recurrent disease for 4+years.
NEW IMMUNE-SPECIFIC PET IMAGING PROBES
Since FDG accumulates in both tumor cells and activated immune cells, FDG uptake can be nonspecific. In order to overcome this limitation, PET probes with higher specificity for immune-related targets are needed, which can be grouped into two different categories: 1) imaging probes that target general immune-related markers, or 2) probes designed to target markers that are more uniquely expressed in the setting of immune activation.
Given that tumor-infiltrating CD8+ T cells are predictive of response to immunotherapy,80 whole-body CD8 PET/CT imaging is of interest, as it has the potential to allow non-invasive assessment of temporal changes in CD8+ T cell concentration in tumors, both before and after immunotherapy. Although the majority of immune-specific probes are in preclinical development, a few are in early phase clinical trials. For example, a 89Zr-labeled anti-CD8 minibody (89Zr-Df-IAB22M2C) is currently in a phase 2 trial [NCT03802123] as a PET probe for imaging CD8+ T cells in patients with metastatic solid tumors, with the goal of correlating CD8 signal on PET/CT imaging to CD8+ T cell infiltration from biopsy samples, and response to cancer immunotherapy. Results from the phase 1 trial of 89Zr-Df-IAB22M2C demonstrated tracer uptake in tumors (Figure 9) and CD8 rich tissues (e.g. spleen, bone marrow, lymph nodes) with maximum uptake at 24–48 hours post injection and low background activity in non-T cell rich tissues (e.g. muscle, heart).85 In preclinical models of cancer immunotherapy, CD8- and CD3-specific imaging agents have both demonstrated greater trafficking and/or a more central distribution of tumor infiltrating T cells in responders versus nonresponders, which supports the potential utility of these agents as an early measure of response to immunotherapy.86–89 In addition, imaging agents that target immune cells have the potential to serve as noninvasive predictive biomarkers by differentiating patients with “hot” versus “cold” tumors, and their likelihood of responding to immunotherapy.90 CD8 PET/CT imaging could also be helpful in distinguishing pseudoprogression from treatment failure, and may complement FDG PET/CT as a problem-solving tool when immune-related changes need to be isolated from tumor growth.
Figure 9.
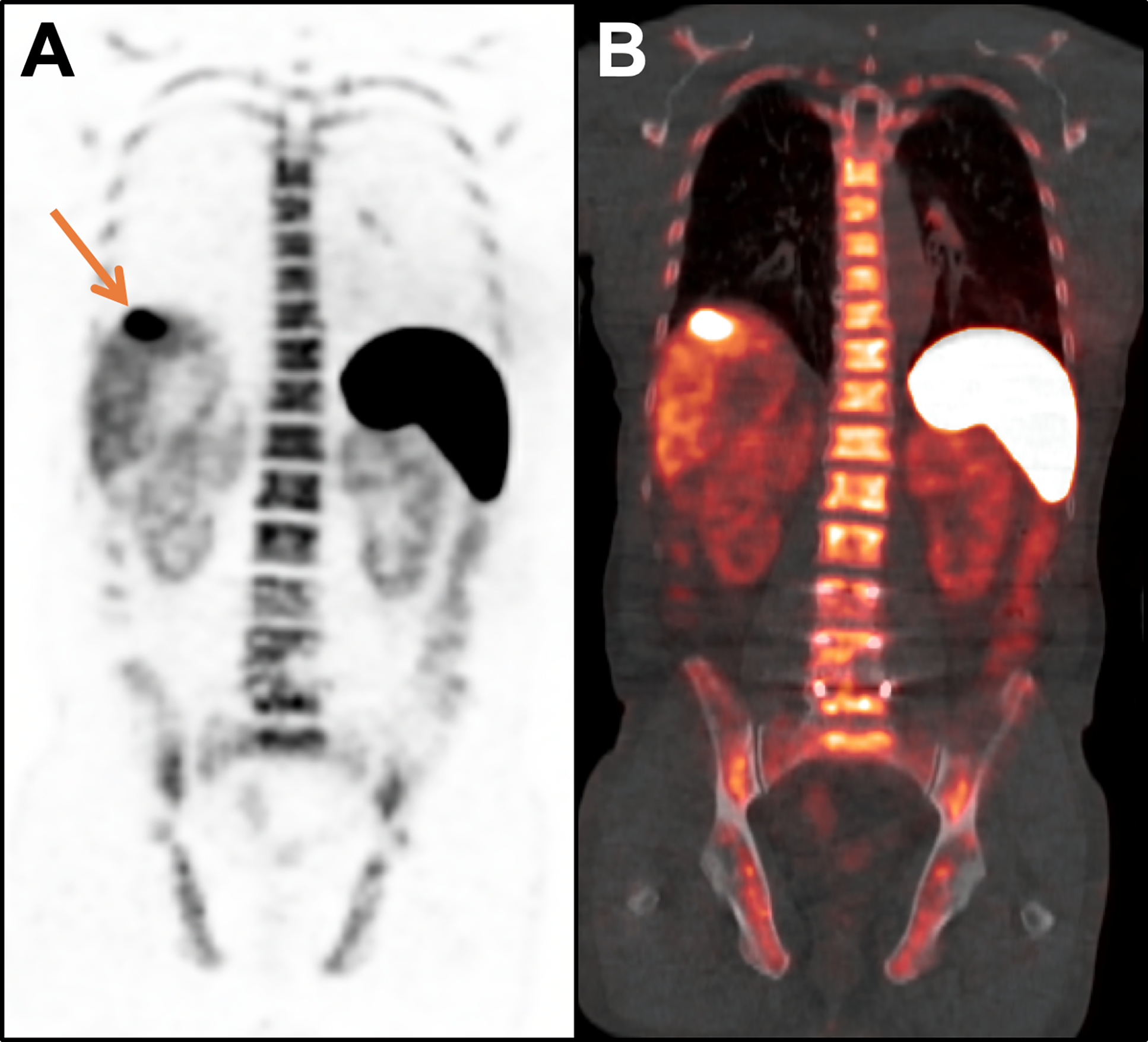
67-year-old man with metastatic hepatocellular carcinoma (HCC) treated with nivolumab. A CD8 PET/CT scan acquired 14 days after starting immunotherapy demonstrated increased tracer activity in the primary tumor (arrow; SUVMAX = 22.9) on the coronal PET (A) and fused PET/CT images (B), suggestive of tumor infiltration by CD8+ T cells and a productive anti-tumor immune response; physiologic tracer activity is seen in the spleen, liver, bone marrow, and kidneys. Follow-up imaging demonstrated a partial response to therapy, which has lasted 3+ years, with an associated drop in alpha-fetoprotein from 33.2 ng/mL at baseline to 1.4 ng/mL at 3 years.
Other immune-specific imaging agents that are in clinical trials include probes that target PD-L1 (89Zr-atezolizumab and 18F-BMS-986192) and PD-1 (89Zr-nivolumab).91–93 Given that PD-L1 expression levels have been shown to be a positive (albeit imperfect) predictive biomarker for patients undergoing immune checkpoint blockade therapy, these agents have the potential to serve as a noninvasive measure of PD-L1 expression, which would have particular utility in patients with lung cancer where assessment of PD-L1 expression is required prior to first-line treatment with anti-PD-1 therapy.94 A wide variety of other imaging probes that target immune-related markers such as CD4, CTLA-4, CD11b, CD47, VLA-4, and CXCR4 (and other chemokine receptors and ligands) are in preclinical development and may also prove to have utility in the setting of cancer immunotherapy in the future.95,96
Imaging agents that target immune activation are also being developed, which will be helpful in distinguishing activated immune cells present in the tumor microenvironment from quiescent immune cells. These include agents that are specific for key enzymes involved in T lymphocyte and other immune cell activation and proliferation (18F-FAC, 18F-CFA, and 18F-AraG), which are in early phase clinical trials, and have been used preclinically for detecting the location of activated T cells, monitoring graft-versus-host-disease, and evaluating auto-immune disorders.95,96 However, clinical data on the utility of these imaging agents in the setting of cancer immunotherapy have not yet been published. Other probes that are specific for activated immune cells include agents that target granzyme B (68Ga-NOTA-GZP), IL-2 (18F-FB-IL2), OX40 (64Cu-DOTA-AbOX40), and ICOS (89Zr-DFO-ICOS mAb).96 PET imaging probes that target granzyme B, OX40, and ICOS have all been tested in preclinical models of cancer immunotherapy, and demonstrated increased tumor uptake in responders versus nonresponders, suggesting that they could serve as an early measure of response.97–99 In addition, granzyme B PET/CT imaging using 68Ga-NOTA-hGZP is currently in a phase I clinical trial (NCT04169321).
The aforementioned studies indicate that the immune-specific PET imaging toolbox is likely to expand, and will provide information that supplements FDG PET/CT and anatomic imaging.100 These new imaging tools have the potential to have a major impact on patient management in the setting of cancer immunotherapy, and will likely have applications outside of oncology for other conditions in which the immune system plays a role, such as autoimmune and inflammatory diseases, transplant rejection, and infection.
Key Points:
Immunotherapy causes infiltration of tumors by immune cells and in rare cases is associated with unique response patterns such as pseudoprogression.
FDG PET/CT is frequently used to assess response to immunotherapy, and while it cannot distinguish immune-related activity from tumor growth it has the potential to provide insight into the immune response.
New probes for PET imaging of the immune system are likely to be helpful in predicting response to cancer immunotherapy and separating immune-related changes from progressive disease.
Acknowledgments
Funding Source: This work was supported by National Institutes of Health grants 5R01EB026892 and NIH 2T32EB004311–16
Footnotes
Conflicts of interest: None
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Clinics Care Points
- 1.Butt AQ, Mills KH. Immunosuppressive networks and checkpoints controlling antitumor immunity and their blockade in the development of cancer immunotherapeutics and vaccines. Oncogene. 2014;33(38):4623–4631. [DOI] [PubMed] [Google Scholar]
- 2.Liu Y, Cao X. Immunosuppressive cells in tumor immune escape and metastasis. J Mol Med (Berl). 2016;94(5):509–522. [DOI] [PubMed] [Google Scholar]
- 3.Leach DR, Krummel MF, Allison JP. Enhancement of antitumor immunity by CTLA-4 blockade. Science. 1996;271(5256):1734–1736. [DOI] [PubMed] [Google Scholar]
- 4.Jiang X, Wang J, Deng X, et al. Role of the tumor microenvironment in PD-L1/PD-1-mediated tumor immune escape. Mol Cancer. 2019;18(1):10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Hodi FS, O’Day SJ, McDermott DF, et al. Improved survival with ipilimumab in patients with metastatic melanoma. N Engl J Med. 2010;363(8):711–723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Topalian SL, Hodi FS, Brahmer JR, et al. Safety, activity, and immune correlates of anti-PD-1 antibody in cancer. N Engl J Med. 2012;366(26):2443–2454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Wolchok JD, Kluger H, Callahan MK, et al. Nivolumab plus ipilimumab in advanced melanoma. N Engl J Med. 2013;369(2):122–133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ni L, Dong C. New B7 Family Checkpoints in Human Cancers. Mol Cancer Ther. 2017;16(7):1203–1211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Rowshanravan B, Halliday N, Sansom DM. CTLA-4: a moving target in immunotherapy. Blood. 2018;131(1):58–67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Tirapu I, Huarte E, Guiducci C, et al. Low surface expression of B7–1 (CD80) is an immunoescape mechanism of colon carcinoma. Cancer Res. 2006;66(4):2442–2450. [DOI] [PubMed] [Google Scholar]
- 11.Walunas TL, Bakker CY, Bluestone JA. CTLA-4 ligation blocks CD28-dependent T cell activation. J Exp Med. 1996;183(6):2541–2550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Freeman GJ, Long AJ, Iwai Y, et al. Engagement of the PD-1 immunoinhibitory receptor by a novel B7 family member leads to negative regulation of lymphocyte activation. J Exp Med. 2000;192(7):1027–1034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Latchman Y, Wood CR, Chernova T, et al. PD-L2 is a second ligand for PD-1 and inhibits T cell activation. Nat Immunol. 2001;2(3):261–268. [DOI] [PubMed] [Google Scholar]
- 14.Ansell SM, Lesokhin AM, Borrello I, et al. PD-1 blockade with nivolumab in relapsed or refractory Hodgkin’s lymphoma. N Engl J Med. 2015;372(4):311–319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Armand P, Engert A, Younes A, et al. Nivolumab for Relapsed/Refractory Classic Hodgkin Lymphoma After Failure of Autologous Hematopoietic Cell Transplantation: Extended Follow-Up of the Multicohort Single-Arm Phase II CheckMate 205 Trial. J Clin Oncol. 2018;36(14):1428–1439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Brahmer J, Reckamp KL, Baas P, et al. Nivolumab versus Docetaxel in Advanced Squamous-Cell Non-Small-Cell Lung Cancer. N Engl J Med. 2015;373(2):123–135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Motzer RJ, Rini BI, McDermott DF, et al. Nivolumab for Metastatic Renal Cell Carcinoma: Results of a Randomized Phase II Trial. J Clin Oncol. 2015;33(13):1430–1437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Nghiem PT, Bhatia S, Lipson EJ, et al. PD-1 Blockade with Pembrolizumab in Advanced Merkel-Cell Carcinoma. N Engl J Med. 2016;374(26):2542–2552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.van den Bulk J, Verdegaal EM, de Miranda NF. Cancer immunotherapy: broadening the scope of targetable tumours. Open Biol. 2018;8(6). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Darvin P, Toor SM, Sasidharan Nair V, Elkord E. Immune checkpoint inhibitors: recent progress and potential biomarkers. Exp Mol Med. 2018;50(12):1–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Pons-Tostivint E, Latouche A, Vaflard P, et al. Comparative Analysis of Durable Responses on Immune Checkpoint Inhibitors Versus Other Systemic Therapies: A Pooled Analysis of Phase III Trials. JCO Precision Oncology. 2019(3):1–10. [DOI] [PubMed] [Google Scholar]
- 22.Rotte A Combination of CTLA-4 and PD-1 blockers for treatment of cancer. J Exp Clin Cancer Res. 2019;38(1):255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Gao J, Navai N, Alhalabi O, et al. Neoadjuvant PD-L1 plus CTLA-4 blockade in patients with cisplatin-ineligible operable high-risk urothelial carcinoma. Nat Med. 2020;26(12):1845–1851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Larkin J, Chiarion-Sileni V, Gonzalez R, et al. Combined Nivolumab and Ipilimumab or Monotherapy in Untreated Melanoma. N Engl J Med. 2015;373(1):23–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Long GV, Atkinson V, Lo S, et al. Combination nivolumab and ipilimumab or nivolumab alone in melanoma brain metastases: a multicentre randomised phase 2 study. Lancet Oncol. 2018;19(5):672–681. [DOI] [PubMed] [Google Scholar]
- 26.Rozeman EA, Menzies AM, van Akkooi ACJ, et al. Identification of the optimal combination dosing schedule of neoadjuvant ipilimumab plus nivolumab in macroscopic stage III melanoma (OpACIN-neo): a multicentre, phase 2, randomised, controlled trial. Lancet Oncol. 2019;20(7):948–960. [DOI] [PubMed] [Google Scholar]
- 27.van Dijk N, Gil-Jimenez A, Silina K, et al. Preoperative ipilimumab plus nivolumab in locoregionally advanced urothelial cancer: the NABUCCO trial. Nat Med. 2020;26(12):1839–1844. [DOI] [PubMed] [Google Scholar]
- 28.Billan S, Kaidar-Person O, Gil Z. Treatment after progression in the era of immunotherapy. Lancet Oncol. 2020;21(10):e463–e476. [DOI] [PubMed] [Google Scholar]
- 29.Hodi FS, Hwu WJ, Kefford R, et al. Evaluation of Immune-Related Response Criteria and RECIST v1.1 in Patients With Advanced Melanoma Treated With Pembrolizumab. J Clin Oncol. 2016;34(13):1510–1517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Di Giacomo AM, Danielli R, Guidoboni M, et al. Therapeutic efficacy of ipilimumab, an anti-CTLA-4 monoclonal antibody, in patients with metastatic melanoma unresponsive to prior systemic treatments: clinical and immunological evidence from three patient cases. Cancer Immunol Immunother. 2009;58(8):1297–1306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Kong BY, Menzies AM, Saunders CA, et al. Residual FDG-PET metabolic activity in metastatic melanoma patients with prolonged response to anti-PD-1 therapy. Pigment Cell Melanoma Res. 2016;29(5):572–577. [DOI] [PubMed] [Google Scholar]
- 32.Ledezma B, Binder S, Hamid O. Atypical clinical response patterns to ipilimumab. Clin J Oncol Nurs. 2011;15(4):393–403. [DOI] [PubMed] [Google Scholar]
- 33.Goldfarb L, Duchemann B, Chouahnia K, Zelek L, Soussan M. Monitoring anti-PD-1-based immunotherapy in non-small cell lung cancer with FDG PET: introduction of iPERCIST. EJNMMI Res. 2019;9(1):8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Sarfaty M, Moore A, Dudnik E, Peled N. Not only for melanoma. Subcutaneous pseudoprogression in lung squamous-cell carcinoma treated with nivolumab: A case report. Medicine (Baltimore). 2017;96(4):e5951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Aide N, Hicks RJ, Le Tourneau C, Lheureux S, Fanti S, Lopci E. FDG PET/CT for assessing tumour response to immunotherapy : Report on the EANM symposium on immune modulation and recent review of the literature. Eur J Nucl Med Mol Imaging. 2019;46(1):238–250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Wong ANM, McArthur GA, Hofman MS, Hicks RJ. The Advantages and Challenges of Using FDG PET/CT for Response Assessment in Melanoma in the Era of Targeted Agents and Immunotherapy. Eur J Nucl Med Mol Imaging. 2017;44(Suppl 1):67–77. [DOI] [PubMed] [Google Scholar]
- 37.Wolchok JD, Hoos A, O’Day S, et al. Guidelines for the evaluation of immune therapy activity in solid tumors: immune-related response criteria. Clin Cancer Res. 2009;15(23):7412–7420. [DOI] [PubMed] [Google Scholar]
- 38.Queirolo P, Spagnolo F. Atypical responses in patients with advanced melanoma, lung cancer, renal-cell carcinoma and other solid tumors treated with anti-PD-1 drugs: A systematic review. Cancer Treat Rev. 2017;59:71–78. [DOI] [PubMed] [Google Scholar]
- 39.Huang J, Rong L, Wang E, Fang Y. Pseudoprogression of extramedullary disease in relapsed acute lymphoblastic leukemia after CAR T-cell therapy. Immunotherapy. 2021;13(1):5–10. [DOI] [PubMed] [Google Scholar]
- 40.Shah NN, Nagle SJ, Torigian DA, et al. Early positron emission tomography/computed tomography as a predictor of response after CTL019 chimeric antigen receptor -T-cell therapy in B-cell non-Hodgkin lymphomas. Cytotherapy. 2018;20(12):1415–1418. [DOI] [PubMed] [Google Scholar]
- 41.Champiat S, Dercle L, Ammari S, et al. Hyperprogressive Disease Is a New Pattern of Progression in Cancer Patients Treated by Anti-PD-1/PD-L1. Clin Cancer Res. 2017;23(8):1920–1928. [DOI] [PubMed] [Google Scholar]
- 42.Understanding Hyperprogression in Cancer. Cancer Discov. 2019;9(7):821. [DOI] [PubMed] [Google Scholar]
- 43.Adashek JJ, Kato S, Ferrara R, Lo Russo G, Kurzrock R. Hyperprogression and Immune Checkpoint Inhibitors: Hype or Progress? Oncologist. 2020;25(2):94–98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Kato S, Goodman A, Walavalkar V, Barkauskas DA, Sharabi A, Kurzrock R. Hyperprogressors after Immunotherapy: Analysis of Genomic Alterations Associated with Accelerated Growth Rate. Clin Cancer Res. 2017;23(15):4242–4250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Martins F, Sofiya L, Sykiotis GP, et al. Adverse effects of immune-checkpoint inhibitors: epidemiology, management and surveillance. Nat Rev Clin Oncol. 2019;16(9):563–580. [DOI] [PubMed] [Google Scholar]
- 46.Xing P, Zhang F, Wang G, et al. Incidence rates of immune-related adverse events and their correlation with response in advanced solid tumours treated with NIVO or NIVO+IPI: a systematic review and meta-analysis. J Immunother Cancer. 2019;7(1):341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Tirumani SH, Ramaiya NH, Keraliya A, et al. Radiographic Profiling of Immune-Related Adverse Events in Advanced Melanoma Patients Treated with Ipilimumab. Cancer Immunol Res. 2015;3(10):1185–1192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Bertrand A, Kostine M, Barnetche T, Truchetet ME, Schaeverbeke T. Immune related adverse events associated with anti-CTLA-4 antibodies: systematic review and meta-analysis. BMC Med. 2015;13:211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Wang DY, Salem JE, Cohen JV, et al. Fatal Toxic Effects Associated With Immune Checkpoint Inhibitors: A Systematic Review and Meta-analysis. JAMA Oncol. 2018;4(12):1721–1728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Kwak JJ, Tirumani SH, Van den Abbeele AD, Koo PJ, Jacene HA. Cancer immunotherapy: imaging assessment of novel treatment response patterns and immune-related adverse events. Radiographics. 2015;35(2):424–437. [DOI] [PubMed] [Google Scholar]
- 51.Das JP, Postow MA, Friedman CF, Do RK, Halpenny DF. Imaging findings of immune checkpoint inhibitor associated pancreatitis. Eur J Radiol. 2020;131:109250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Das JP, Halpenny D, Do RK, Ulaner GA. Focal Immunotherapy-Induced Pancreatitis Mimicking Metastasis on FDG PET/CT. Clin Nucl Med. 2019;44(10):836–837. [DOI] [PubMed] [Google Scholar]
- 53.Mekki A, Dercle L, Lichtenstein P, et al. Detection of immune-related adverse events by medical imaging in patients treated with anti-programmed cell death 1. Eur J Cancer. 2018;96:91–104. [DOI] [PubMed] [Google Scholar]
- 54.Nawwar AA, Searle J, Lyburn ID. Pembrolizumab-Induced Thyroiditis and Colitis-Presentation and Resolution on Serial FDG PET/CT. Clin Nucl Med. 2021;46(2):e121–e122. [DOI] [PubMed] [Google Scholar]
- 55.Razzouk-Cadet M, Picard A, Grangeon-Chapon C, Lacour JP, Montaudie H. Nivolumab-Induced Pneumonitis in Patient With Metastatic Melanoma Showing Complete Remission on 18F-FDG PET/CT. Clin Nucl Med. 2019;44(10):806–807. [DOI] [PubMed] [Google Scholar]
- 56.Ayati N, Sadeghi R, Kiamanesh Z, Lee ST, Zakavi SR, Scott AM. The value of (18)F-FDG PET/CT for predicting or monitoring immunotherapy response in patients with metastatic melanoma: a systematic review and meta-analysis. Eur J Nucl Med Mol Imaging. 2021;48(2):428–448. [DOI] [PubMed] [Google Scholar]
- 57.Nobashi T, Baratto L, Reddy SA, et al. Predicting Response to Immunotherapy by Evaluating Tumors, Lymphoid Cell-Rich Organs, and Immune-Related Adverse Events Using FDG-PET/CT. Clin Nucl Med. 2019;44(4):e272–e279. [DOI] [PubMed] [Google Scholar]
- 58.Eisenhauer EA, Therasse P, Bogaerts J, et al. New response evaluation criteria in solid tumours: revised RECIST guideline (version 1.1). Eur J Cancer. 2009;45(2):228–247. [DOI] [PubMed] [Google Scholar]
- 59.Miller AB, Hoogstraten B, Staquet M, Winkler A. Reporting results of cancer treatment. Cancer. 1981;47(1):207–214. [DOI] [PubMed] [Google Scholar]
- 60.Wahl RL, Jacene H, Kasamon Y, Lodge MA. From RECIST to PERCIST: Evolving Considerations for PET response criteria in solid tumors. J Nucl Med. 2009;50 Suppl 1:122S–150S. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Young H, Baum R, Cremerius U, et al. Measurement of clinical and subclinical tumour response using [18F]-fluorodeoxyglucose and positron emission tomography: review and 1999 EORTC recommendations. European Organization for Research and Treatment of Cancer (EORTC) PET Study Group. Eur J Cancer. 1999;35(13):1773–1782. [DOI] [PubMed] [Google Scholar]
- 62.Nishino M, Giobbie-Hurder A, Gargano M, Suda M, Ramaiya NH, Hodi FS. Developing a common language for tumor response to immunotherapy: immune-related response criteria using unidimensional measurements. Clin Cancer Res. 2013;19(14):3936–3943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Seymour L, Bogaerts J, Perrone A, et al. iRECIST: guidelines for response criteria for use in trials testing immunotherapeutics. Lancet Oncol. 2017;18(3):e143–e152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Cheson BD, Ansell S, Schwartz L, et al. Refinement of the Lugano Classification lymphoma response criteria in the era of immunomodulatory therapy. Blood. 2016;128(21):2489–2496. [DOI] [PubMed] [Google Scholar]
- 65.Cho SY, Lipson EJ, Im HJ, et al. Prediction of Response to Immune Checkpoint Inhibitor Therapy Using Early-Time-Point (18)F-FDG PET/CT Imaging in Patients with Advanced Melanoma. J Nucl Med. 2017;58(9):1421–1428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Anwar H, Sachpekidis C, Winkler J, et al. Absolute number of new lesions on (18)F-FDG PET/CT is more predictive of clinical response than SUV changes in metastatic melanoma patients receiving ipilimumab. Eur J Nucl Med Mol Imaging. 2018;45(3):376–383. [DOI] [PubMed] [Google Scholar]
- 67.Sachpekidis C, Anwar H, Winkler J, et al. The role of interim (18)F-FDG PET/CT in prediction of response to ipilimumab treatment in metastatic melanoma. Eur J Nucl Med Mol Imaging. 2018;45(8):1289–1296. [DOI] [PubMed] [Google Scholar]
- 68.Hess S, Hansson SH, Pedersen KT, Basu S, Hoilund-Carlsen PF. FDG-PET/CT in Infectious and Inflammatory Diseases. PET Clin. 2014;9(4):497–519, vi–vii. [DOI] [PubMed] [Google Scholar]
- 69.Huber H, Hodolic M, Stelzmuller I, et al. Malignant disease as an incidental finding at (1)(8)F-FDG-PET/CT scanning in patients with granulomatous lung disease. Nucl Med Commun. 2015;36(5):430–437. [DOI] [PubMed] [Google Scholar]
- 70.Meller J, Sahlmann CO, Scheel AK. 18F-FDG PET and PET/CT in fever of unknown origin. J Nucl Med. 2007;48(1):35–45. [PubMed] [Google Scholar]
- 71.Patsoukis N, Bardhan K, Chatterjee P, et al. PD-1 alters T-cell metabolic reprogramming by inhibiting glycolysis and promoting lipolysis and fatty acid oxidation. Nat Commun. 2015;6:6692. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Chargari C, Le Moulec S, Bonardel G, Foehrenbach H, Vedrine L. Ipilimumab in cancer patients: the issue of early metabolic response. Anticancer Drugs. 2013;24(3):324–326. [DOI] [PubMed] [Google Scholar]
- 73.Escuin-Ordinas H, Elliott MW, Atefi M, et al. PET imaging to non-invasively study immune activation leading to antitumor responses with a 4–1BB agonistic antibody. J Immunother Cancer. 2013;1:14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Sachpekidis C, Larribere L, Pan L, Haberkorn U, Dimitrakopoulou-Strauss A, Hassel JC. Predictive value of early 18F-FDG PET/CT studies for treatment response evaluation to ipilimumab in metastatic melanoma: preliminary results of an ongoing study. Eur J Nucl Med Mol Imaging. 2015;42(3):386–396. [DOI] [PubMed] [Google Scholar]
- 75.Chang B, Huang A, Shang C, et al. Evaluation of the anti-PD-1 Flare Response in Patients with Advanced Melanoma Using FDG PET/CT Imaging and Hematologic Biomarkers. J Nucl Med. 2019;60:1270.30737300 [Google Scholar]
- 76.Nakamoto R, Zaba LC, Liang T, et al. Prognostic value of bone marrow metabolism on pretreatment (18)F-FDG PET/CT in patients with metastatic melanoma treated with anti-PD-1 therapy. J Nucl Med. 2021. [DOI] [PubMed] [Google Scholar]
- 77.Seban RD, Nemer JS, Marabelle A, et al. Prognostic and theranostic 18F-FDG PET biomarkers for anti-PD1 immunotherapy in metastatic melanoma: association with outcome and transcriptomics. Eur J Nucl Med Mol Imaging. 2019;46(11):2298–2310. [DOI] [PubMed] [Google Scholar]
- 78.Cheshire SC, Board RE, Lewis AR, Gudur LD, Dobson MJ. Pembrolizumab-induced Sarcoid-like Reactions during Treatment of Metastatic Melanoma. Radiology. 2018;289(2):564–567. [DOI] [PubMed] [Google Scholar]
- 79.Sachpekidis C, Larribere L, Kopp-Schneider A, Hassel JC, Dimitrakopoulou-Strauss A. Can benign lymphoid tissue changes in (18)F-FDG PET/CT predict response to immunotherapy in metastatic melanoma? Cancer Immunol Immunother. 2019;68(2):297–303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Tumeh PC, Harview CL, Yearley JH, et al. PD-1 blockade induces responses by inhibiting adaptive immune resistance. Nature. 2014;515(7528):568–571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Burger IA, Husmann L, Hany TF, Schmid DT, Schaefer NG. Incidence and intensity of F-18 FDG uptake after vaccination with H1N1 vaccine. Clin Nucl Med. 2011;36(10):848–853. [DOI] [PubMed] [Google Scholar]
- 82.Thomassen A, Lerberg Nielsen A, Gerke O, Johansen A, Petersen H. Duration of 18F-FDG avidity in lymph nodes after pandemic H1N1v and seasonal influenza vaccination. Eur J Nucl Med Mol Imaging. 2011;38(5):894–898. [DOI] [PubMed] [Google Scholar]
- 83.Mingos M, Howard S, Giacalone N, Kozono D, Jacene H. Systemic Immune Response to Vaccination on FDG-PET/CT. Nucl Med Mol Imaging. 2016;50(4):358–361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Eifer M, Eshet Y. Imaging of COVID-19 Vaccination at FDG PET/CT. Radiology. 2021:210030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Pandit-Taskar N, Postow MA, Hellmann MD, et al. First-in-Humans Imaging with (89)Zr-Df-IAB22M2C Anti-CD8 Minibody in Patients with Solid Malignancies: Preliminary Pharmacokinetics, Biodistribution, and Lesion Targeting. J Nucl Med. 2020;61(4):512–519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Kristensen LK, Christensen C, Alfsen MZ, Cold S, Nielsen CH, Kjaer A. Monitoring CD8a(+) T Cell Responses to Radiotherapy and CTLA-4 Blockade Using [(64)Cu]NOTA-CD8a PET Imaging. Mol Imaging Biol. 2020;22(4):1021–1030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Kristensen LK, Frohlich C, Christensen C, et al. CD4(+) and CD8a(+) PET imaging predicts response to novel PD-1 checkpoint inhibitor: studies of Sym021 in syngeneic mouse cancer models. Theranostics. 2019;9(26):8221–8238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Larimer BM, Wehrenberg-Klee E, Caraballo A, Mahmood U. Quantitative CD3 PET Imaging Predicts Tumor Growth Response to Anti-CTLA-4 Therapy. J Nucl Med. 2016;57(10):1607–1611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Tavare R, Escuin-Ordinas H, Mok S, et al. An Effective Immuno-PET Imaging Method to Monitor CD8-Dependent Responses to Immunotherapy. Cancer Res. 2016;76(1):73–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Vonderheide RH, Domchek SM, Clark AS. Immunotherapy for Breast Cancer: What Are We Missing? Clin Cancer Res. 2017;23(11):2640–2646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Bensch F, van der Veen EL, Lub-de Hooge MN, et al. (89)Zr-atezolizumab imaging as a non-invasive approach to assess clinical response to PD-L1 blockade in cancer. Nat Med. 2018;24(12):1852–1858. [DOI] [PubMed] [Google Scholar]
- 92.Huisman MC, Niemeijer AN, Windhorst AD, et al. Quantification of PD-L1 Expression with (18)F-BMS-986192 PET/CT in Patients with Advanced-Stage Non-Small Cell Lung Cancer. J Nucl Med. 2020;61(10):1455–1460. [DOI] [PubMed] [Google Scholar]
- 93.Niemeijer AN, Leung D, Huisman MC, et al. Whole body PD-1 and PD-L1 positron emission tomography in patients with non-small-cell lung cancer. Nat Commun. 2018;9(1):4664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Buttner R, Gosney JR, Skov BG, et al. Programmed Death-Ligand 1 Immunohistochemistry Testing: A Review of Analytical Assays and Clinical Implementation in Non-Small-Cell Lung Cancer. J Clin Oncol. 2017;35(34):3867–3876. [DOI] [PubMed] [Google Scholar]
- 95.Ponomarev V Advancing Immune and Cell-Based Therapies Through Imaging. Mol Imaging Biol. 2017;19(3):379–384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Wei W, Jiang D, Ehlerding EB, Luo Q, Cai W. Noninvasive PET Imaging of T cells. Trends Cancer. 2018;4(5):359–373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Alam IS, Mayer AT, Sagiv-Barfi I, et al. Imaging activated T cells predicts response to cancer vaccines. J Clin Invest. 2018;128(6):2569–2580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Larimer BM, Wehrenberg-Klee E, Dubois F, et al. Granzyme B PET Imaging as a Predictive Biomarker of Immunotherapy Response. Cancer Res. 2017;77(9):2318–2327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Xiao Z, Mayer AT, Nobashi TW, Gambhir SS. ICOS Is an Indicator of T-cell-Mediated Response to Cancer Immunotherapy. Cancer Res. 2020;80(14):3023–3032. [DOI] [PubMed] [Google Scholar]
- 100.Iravani A, Hicks RJ. Imaging the Cancer Immune Environment and Its Response to Pharmacologic Intervention, Part 2: The Role of Novel PET Agents. J Nucl Med. 2020;61(11):1553–1559. [DOI] [PubMed] [Google Scholar]


