Abstract
Whole-brain radiotherapy (WBRT) is the mainstay of therapy in treating cancer patients with brain metastases, but unfortunately, it might also lead to decline in neurocognitive function. This study aims to investigate the preservation of long-term neurocognitive function in patients after hippocampal avoidance whole-brain radiotherapy (HA-WBRT). Retrospectively, 47 patients diagnosed with brain metastases of non-small cell lung cancer (NSCLC) between 2015-01-01 and 2017-12-31 at the Department of Oncology, XXX Hospital were selected and divided into 2 groups. Group A (n = 27) received HA-WBRT, whereas group B (n = 20) received WBRT. Neurocognitive function was analyzed at baseline and at 3, 6, 9, 12 and 24 months after radiotherapy, using Mine-Mental State Examination (MMSE) scales and Montreal Cognitive Assessment (MoCA) scales. The OS, PFS and tumor recurrence sites were also analyzed. When evaluated at 12 and 24 months after radiotherapy, the cognitive function scores of the hippocampal avoidance group were significantly higher than those of the non-hippocampal avoidance group (P < 0.001). In terms of patient survival, there was no significant difference in OS (P = 0.2) and PFS (P = 0.18) between these 2 groups. Fourteen patients in group A and 12 patients in group B had brain tumor recurrence after radiation, only one patient in group A occurred within 5 mm from the edge of the hippocampus (P > 0.05). In conclusion, HA-WBRT might have a protective effect on long-term neurocognitive function and did not affect patient survival.
Keywords: neurocognitive function, non-small cell lung cancer, whole-brain radiotherapy, hippocampal avoidance whole-brain radiotherapy, brain metastases
Introduction
Up to 40 % of patients with systemic malignancies would be diagnosed with brain metastases.1 The treatments of brain metastases include surgery, stereotactic radiosurgery (SRS), whole-brain radiotherapy (WBRT), chemotherapy and targeted therapy. Until recently, brain metastases were treated in a generally homogeneous manner, with WBRT being the primary treatment. Surgical resection of brain metastases has been limited to large isolated lesions; SRS is reserved for smaller lesions in locations which were difficult to access.2 Although WBRT is the major treatment for brain metastases, it causes long-term and irreversible sequelae of central nervous system, such as cerebellar dysfunction and dementia, cognitive deterioration, and leukoencephalopathy.3 Moreover, recent clinical trials reported a dose-response-related risk of decline in neurocognitive function (NCF) related to hippocampal radiation dose volume that may interfere quality of life of patients.4 A phase III trial conducted at MD Anderson Cancer Center showed that adding WBRT to SRS increased the risk of a decline in learning and memory compared to SRS alone (52% vs 24%, respectively).5
Strong evidence suggested that radiation-induced damage to the hippocampus was responsible for decline in NCF of the patients received WBRT.6 Hippocampal avoidance whole-brain radiotherapy (HA-WBRT) has been proposed to preserve NCF by limiting the dose to the hippocampus.7 HIPPO is an ongoing randomized Phase II trial comparing hippocampal sparing to conventional WBRT after surgical resection or radiosurgery. HIPPO hypothesized that irradiation of the bilateral hippocampi may cause part of the adverse neurocognitive effect from WBRT, and reducing dosage to the hippocampi may help preserve NCF.8 HA-WBRT may delay onset, or reduce severity of NCF decline without decreased intracranial disease control, therefore it improves outcome of the patients.9 Previous studies revealed that cells of the hippocampus were highly sensitive to radiation even at low doses.10 Several studies have also found that oligometastatic disease was relatively spared from metastasis in hippocampus.11-13 Complex IMRT plans have been developed sparing the hippocampus to preserve the NCF during WBRT.14
To date, the role of HA-WBRT in long-term NCF preservation has not been completely elucidated. In this 2-year retrospective study, our purpose is to evaluate the NCF of the patients, who underwent WBRT with or without hippocampal avoidance. Overall survival (OS) and progress free survival (PFS) was also calculated to verify the impact of HA-WBRT on survival. Finally, recurrence rate in the brain or in the hippocampus avoidance area was evaluated to elucidate the influence of HA-WBRT on intracranial recurrence.
Methods
Between 2015-01-01 and 2017-12-31 in Department of Oncology, Jiangsu Subei People’s Hospital, 47 patients met the inclusion criteria with non-small cell lung cancer (NSCLC) brain metastases who received WBRT or HA-WBRT, including 33 males and 14 females. The median age was 65 years with range from 45 to 83 years. The enrollment criteria were: 1) age ≥ 18 years; 2) KPS score ≥ 70 points; 3) pathological tissue basis of the primary lesion and enhanced contrast of the skull 3.0 T MRI suggesting at least one metastasis in the brain. Exclusion criteria were: 1) Recent (≤3 months) cerebral hemorrhage or cerebral thrombosis that may affect the cognitive history of cerebrovascular disease; 2) diagnosed mental illness or organic mental illness; 3) congenital recognition dysfunction; 4) cognitive disorders caused by brain metastasis before radiotherapy or by metastasis outside the brain, such as Alzheimer’s disease, Parkinson’s disease and brain trauma dementia; 5) patients with definitive leptomeningeal metastases; 6) brain metastases in the brainstem or other life-threatening parts, or located in the hippocampus avoidance area. MRI images of each patient have been reviewed by 2 experienced radiologist together to determine if the patient had metastases in hippocampus avoidance area. Patients had any suspected metastases near hippocampus avoidance area were excluded from the study. Signed informed consent was obtained from all the patients. There were 27 cases in the hippocampus avoidance group and 20 cases in the non-hippocampal avoidance group. The brain metastases of all patients were measured. The longest diameter of single tumor was measured according to the RECIST standard. The maximum length of 2 largest measurable lesions was measured for multiple tumors. Statistical analysis was performed using GraphPad Prism 8 software or R software version 3.22. Comparisons were done with t test where appropriate. There was no significant difference in the size of tumor metastases between the 2 groups before radiotherapy.
Procedures
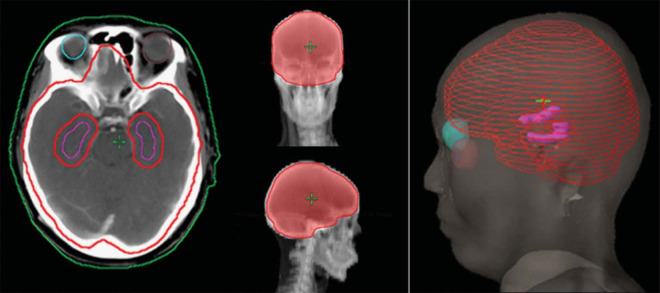
Patients were placed with a thermoplastic mask in a neutral head position. CT scan with contrast was acquired at 2.5 mm slice. With the same body position, MRI scan with gadolinium contrast enhanced T1-weighted sequence was acquired at 1.5 mm slice. After a skull and other bony anatomy-based fusion of the CT and MRI images, images were transferred to the Eclipse Treatment Planning System (TPS, Version 8.6, Varian Medical Systems). An experienced neuroradiologist contoured the clinical target volume (CTV) throughout the brain and OAR (hippocampus, eyes, lens, optic nerve). Contouring of hippocampus was carried out in accordance with the contouring Atlas of the RTOG 0933 trial15 and defined the area around the 5 mm edge of the hippocampus Hippocampal avoidance (HA) to achieve the best design plan; Planned target volume (PTV) including isotropic CTV plus 5 mm edge, and subtracting HA from PTV to obtain PTV-HA (Figure 1).
Figure 1.
PTV-HA and OARs (purple represents hippocampus, and red represents PTV-HA).
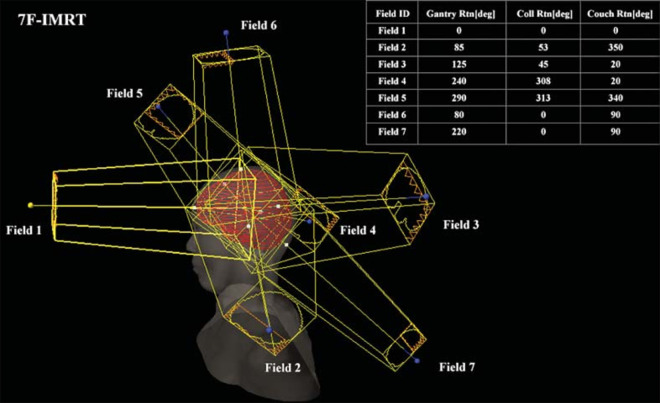
The 7F-IMRT and dual arc VMAT plans were designed with the Varian IX medical electron linac. The delivered dose was 300 cGy x 10 times. The dose rate in the field was 300 MU/ min. There were 7 planning areas and isocenter illumination. The angle of view was adjusted according to the actual situation (Figure 2).
Figure 2.
Beam-on fields of 7F-IMRT for one patient.
The optimization parameters for the 2 plans were the same. The maximum dose of OAR was defined as 50 Gy for optic nerve, 45 Gy for eye and 10 Gy for lens.16 The maximum dose of hippocampus cannot exceed 20 Gy.17 MU was not limited to the optimization process of the VMAT program. The dose was calculated using an Anisotropic Analytical Algorithm (AAA) algorithm with a dose calculation grid of 2.5 mm.
NCF, OS and PFS
The oncology department of our hospital routinely used MMSE and MoCA to measure the NCF of patients before and after brain radiotherapy. Following-up NCF was performed at baseline and at 3, 6, 9, 12 and 24 months after radiotherapy. The long-term NCF of patients with or without hippocampus avoidance was recorded, including overall orientation, registration, attention and calculation, recall, language and praxis. The patient’s OS and PFS were also recorded to assess the impact of hippocampal avoidance on survival.
Statistical Analysis
Patients receiving WBRT with or without HA in this study were divided into 2 groups. Group comparisons between continuous and categorical variables were performed by t-test and chi-square test, respectively. The MMSE score and MoCA score were treated as continuous variable at different time points. Age, gender, education, metastasis were all plausible factors that could affect the long-term effect of cognitive function. Thus, simple linear regression was conducted to figure out the possible factors. One way analysis of covariance (ANCOVA) was applied to further validate whether group factor was an independent factor that would affect patient cognitive decline and its interaction effect with time. Kaplan-Meier survival analysis and log-rank test were used to evaluate the difference of OS and PFS between groups. Statistical analysis was performed using GraphPad Prism 8 software or R software version 3.22, and the P-values were all 2-sided and considered significant when less than 0.05.
Results
Between 2015-01-01 and 2017-12-31, we enrolled and analyzed 47 patients who met the inclusion criteria. Patient characteristics are summarized in Table 1. There were no significant differences between the 2 arms in terms of age, gender, education, types of lung cancer, number and size of brain metastasis, prognostic score, RPA grade and base line of MMSE and MoCA.
Table 1.
Pretreatment Characteristics of All Patients.
| Patient character | WBRT + HA (n = 27) | WBRT (n = 20) | P value |
|---|---|---|---|
| Age | 60.9 ± 7.4 | 63.7 ± 10.3 | 0.28 |
| Gender | 0.32 | ||
| F | 6 (22.2) | 8 (40) | |
| M | 21 (77.8) | 12 (60) | |
| Education | 0.98 | ||
| College/University | 1 (3.7) | 1 (5) | |
| None | 2 (7.4) | 1 (5) | |
| Primary | 17 (63) | 13 (65) | |
| Secondary | 7 (25.9) | 5 (25) | |
| Types of lung cancer | 0.40 | ||
| Adenocarcinoma | 15 (55.6) | 13 (65) | |
| Squamous cell carcinoma | 12 (44.4) | 7 (35) | |
| Metastasis | 0.10 | ||
| Multiple | 19 (70.4) | 10 (50) | |
| Single | 8 (29.6) | 10 (50) | |
| Mean metastasis number | 3.19 ± 3.03 | 4.55 ± 4.98 | 0.25 |
| Metastasis size (mm) | 32.7 ± 14.4 | 28.4 ± 15.5 | 0.33 |
| Other metastasis | 0.24 | ||
| Yes | 14 (51.9) | 14 (70.0) | |
| No | 13 (48.1) | 6 (30.0) | |
| Prognostic score (PS) | 0.72 | ||
| <2 | 22 (81.5) | 15 (75.0) | |
| ≥2 | 5 (18.5) | 5 (25.0) | |
| RPA grade | >0.99 | ||
| <II | 13 (48.1) | 10 (50.0) | |
| ≥II | 14 (51.9) | 10 (50.0) | |
| MMSE base line | 29.2 ± 0.8 | 28.9 ± 1.1 | 0.18 |
| MoCA base line | 28.7 ± 0.8 | 29.0 ± 0.9 | 0.30 |
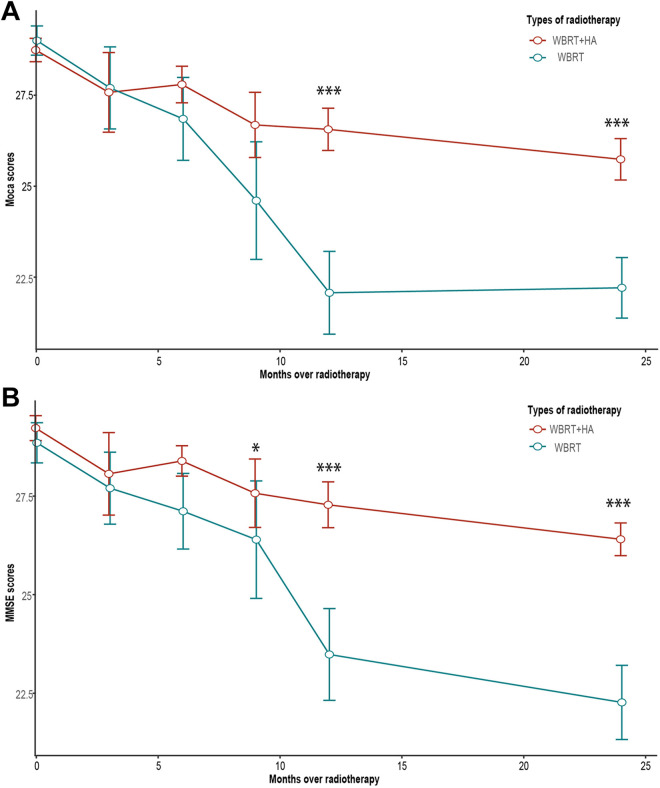
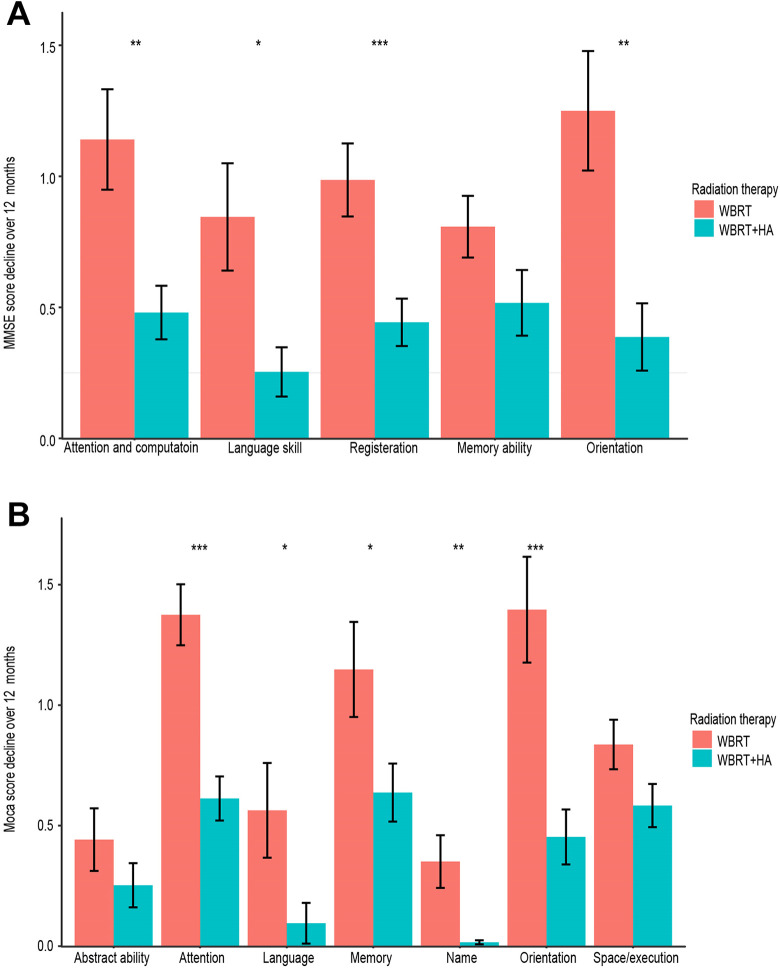
This research evaluated the NCF by the MMSE and MoCA scales of 47 brain metastases NSCLC patients who received either WBRT or HA-WBRT. The number of patients alive at baseline and at 3, 6, 9, 12 and 24 months after radiation is shown in Table 2. The long-term cognitive function of the hippocampal avoidance group was significantly higher than that of the non-hippocampal avoidance group (Figure 3). Statistical differences were found by the MMSE scale, but were not by the MoCA scale at 9 months after radiotherapy. There were significant differences between the 2 groups at 12 and 24 months after radiotherapy. Moreover, single factor linear regression showed that PS score and irradiation methods had significant impacts on cognitive function. Other factors, including gender, age, RPA, education, and metastasis, had no significant effect on the results (Table 3). It is worth noting that after 12 months of radiotherapy, statistically significant differences were observed between these 2 groups in Attention and Calculation, Language and Praxis, Registration, and Orientation in the MMSE scale. In the MoCA scale, differences were also found in Attention, Language, Memory, Name, and Orientation, of which differences in Attention and Orientation were statistically significant (Figure 4).
Table 2.
Number of Patients Alive Before and After Radiation.
| Time (month) | 0 | 3 | 6 | 9 | 12 | 24 |
|---|---|---|---|---|---|---|
| Alive patients | ||||||
| WBRT | 20 | 20 | 19 | 19 | 17 | 4 |
| WBRT + HA | 27 | 26 | 24 | 23 | 20 | 6 |
Figure 3.
A, MoCA scale score comparison of WBRT and WBRT + HA arms, P value < 0.001 (at 12 and 24 months). B, MMSE scale score comparison of 2 arms, P value < 0.05 (at 9 months), P value < 0.001 (at 12 and 24 months).
Table 3.
PS Score and Irradiation Methods Had a Significant Impact on Cognitive Function.
| MMSE | MoCA | |||
|---|---|---|---|---|
| t value | P value | t value | P value | |
| Gender | −0.49 | 0.624 | −0.44 | 0.655 |
| Agea | 0.21 | 0.832 | 0.10 | 0.913 |
| PSb | 2.13 | 0.038 | 2.23 | 0.030 |
| RPAb | 0.48 | 0.629 | 0.63 | 0.529 |
| Educationb | −0.34 | 0.732 | −0.57 | 0.568 |
| Metastasis (multiple vs single) | 0.39 | 0.696 | 0.41 | 0.681 |
| WBRT + HA vs WBRT | −3.02 | 0.004 | −4.10 | 0.000 |
a Represents continuous variable.
b Represents ordered factorial variables.
Figure 4.
A, MMSE scale scores of attention and computation, language and praxis, registration, and orientation of 2 arms had statistically significant difference at 12 months. B, MoCA scale scores of attention, language, memory, name, and orientation of 2 arms had statistically significant difference at 12 months (*P < 0.05, **P < 0.01, ***P < 0.001).
As we described in Figure 3 that cognitive function trajectory may be associated with time and group, one way analysis of covariance (ANCOVA) model was applied to further evaluate the time, group and their interaction effect on patient cognitive function decline after controlling for potential confounding effect derived by invariable linear regression (Table 3). The result exhibited that there was significant group effect (F = 59.74, P < 0.01 for MMSE; F = 47.88, P < 0.01 for MoCA), time effect (F = 143.69, P < 0.01 for MMSE; F = 131.25, P < 0.01 for MoCA), and the interaction effect (F = 31.41, P < 0.01 for MMSE; F = 33.27, P < 0.01 for MoCA) in cognitive decline in both measurements (Tables 4 and 5).
Table 4.
Significant Group Effect, Time Effect and Interaction Effect in Cognitive Decline by MMSE Scale.
| MMSE | MMSE | MMSE | |||
|---|---|---|---|---|---|
| Baseline | 1-year | 2-year | F value | P value | |
| Radiation therapy | 59.74 | <0.01 | |||
| WBRT | 29.22 ± 0.31 | 27.23 ± 0.59 | 26.45 ± 0.39 | ||
| WBRT + HA | 28.85 ± 0.50 | 23.44 ± 1.16 | 22.33 ± 0.92 | ||
| Time | 143.69 | <0.01 | |||
| Group × time | 31.41 | <0.01 |
Table 5.
Significant Group Effect, Time Effect and Interaction Effect in Cognitive Decline by MoCA Scale.
| MoCA | MoCA | MoCA | ||||
|---|---|---|---|---|---|---|
| Baseline | 1-year | 2-year | F value | P value | ||
| Radiation therapy | 47.88 | <0.01 | ||||
| WBRT | 28.74 ± 0.32 | 26.56 ± 0.58 | 25.61 ± 0.55 | |||
| WBRT + HA | 29.00 ± 0.40 | 22.04 ± 1.14 | 22.22 ± 0.86 | |||
| Time | 131.25 | <0.01 | ||||
| Group × time | 33.27 | <0.01 |
For HA-WBRT arm, MRI images of brain recurrence were compared to original OAR delineation one slice by one slice to distinguish if the recurrence was within 5 mm from the hippocampus and minimize the underestimation of metastases. For WBRT arm, MRI images of brain recurrence had been reviewed by 2 experienced radiologists to determine if the patient had recurrence in hippocampus avoidance area. We found that among the 27 patients in HA-WBRT arm, 14 patients (51.9%) had tumor recurrence in the brain, 13 patients had a recurrence outside the brain, and only one brain recurrence was within 5 mm from the hippocampus. Among the 20 patients in WBRT arm, 12 patients (60%) had a recurrence in the brain, 8 patients had a recurrence outside the brain. No patient in WBRT arm had brain tumor recurrence within 5 mm from the hippocampus. These results have shown that hippocampal avoidance didn’t cause higher recurrence rate in the brain (P = 0.2) or in the hippocampus avoidance area (P = 0.9) (Table 6).
Table 6.
Hippocampal Avoidance Didn’t Lead to Higher Recurrence Rate.
| Recurrence site | HA-WBRT (%) | WBRT (%) | P value |
|---|---|---|---|
| Inside the brain | 14 (51.9) | 12 (60.0) | 0.2 |
| Outside the brain | 13 (48.1) | 8 (40.0) | 0.8 |
| Within 5 mm from the hippocampus | 1 (3.7) | 0 | 0.9 |
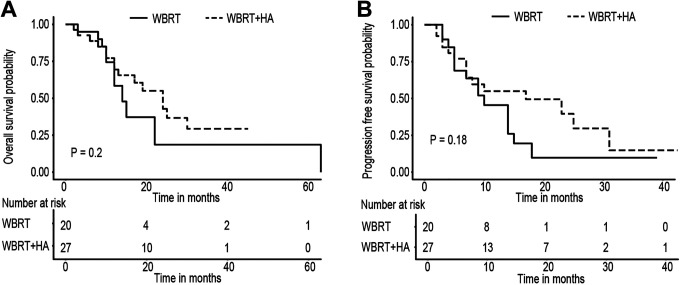
The safety of HA-WBRT was further confirmed by the prognosis of the 2 groups. The results showed that after 2 years of follow up, there was no significant difference in OS (P = 0.2) or PFS (P = 0.18). Hippocampal avoidance did not reduce the OS and PFS of the patients; therefore, it did not affect the patient’s survival (Figure 5).
Figure 5.
A, OS of WBRT and WBRT + HA arms had no statistically significant difference, P value = 0.2 (log-rank). B, PFS of WBRT and WBRT + HA arms had no statistically significant difference, P value = 0.18 (log-ran).
Discussion
Historically, surgical resection and SRS with or without WBRT is used to treat patients with solitary or limited number of brain metastasis. WBRT alone is used to treat those with multiple brain metastases.18,19 In patients with brain metastasis, WBRT was the gold standard treatment and improves tumor control and patient survival. But WBRT was also associated with considerable neurotoxicity and may reduce patients’ quality of life.20,21 In this study, the main objective was to investigate whether HA-WBRT protects long-term NCF for the patients and whether this technique had an effect on survival, disease progression, and recurrence. MMSE scale and MoCA scale were used to evaluate the NCF of NSCLC patients with brain metastasis receiving WBRT or HA-WBRT at baseline and at 3, 6, 9, 12, and 24 months after radiotherapy. OS and PFS were also included in the study. There was a significant difference of NCF scores between these 2 groups at 12 and 24 months after radiotherapy (P < 0.001).There were no significant differences between the 2 groups in OS (P = 0.2) and PFS (P = 0.18). Only one patient in the 2 groups had tumor occurred within 5 mm from the edge of the hippocampus (P = 0.9).
NRG CC001, a recently published randomized, multi-center, phase III trial compared conventional WBRT with memantine to HA-WBRT with memantine. It showed that HA-WBRT with memantine had better cognitive preservation with no difference in intracranial PFS and OS. HA-WBRT with memantine arm showed less deterioration of executive function at 4 months (P = 0.01) and learning (P = 0.049), memory (P = 0.02) at 6 months.22 However, in our study, a significant difference of NCF scores was observed at 9 months by MMSE scale (P < 0.05, Figure 3) and at 12 and 24 months by both MMSE and MoCA scales (P < 0.001, Figure 3). NRG CC001 showed a difference in short term cognitive function, but this study showed a difference only in long term cognitive function. This difference may be attributable to the small patient number of this study, and a trend of higher scores of HA-WBRT can be observed at 6 months (Figure 3). Other than that, different NCF test batteries were used in both studies. In NRG CC001, it included tests of memory (Hopkins Verbal Learning Test-Revised [HVLT-R]), verbal fluency (Controlled Oral Word Association [COWA]), processing speed (Trail Making Test Part A [TMT-A]), and executive function (Trail Making Test Part B [TMT-B]). Although MMSE and MoCA scales were widely used for detection of NCF,23 it was also showed that MMSE had relative lower sensitivity in detection of NCF changes than HVLT test.24 The lower sensitivity of MMSE may also hampered early detection of neurocognitive failure.
Oehlke et al conducted a similar study in 2015 and concluded that hippocampal avoidance had a potential impact on neurocognitive function but did not affect patient survival. However, due to the fact that the survival rate of lung cancer patients was generally low at that time and their follow-up time was only42 weeks, conclusion remains to be verified.20 In NRG CC001 trial, data of decline in NCF was only reported before 12 months after radiation.22 In our work, we showed that significance could be observed after long-term (12 months and later) follow-up. In addition, our data showed that no differences in OS and PFS were observed whether patients received WBRT or HA-WBRT, indicating that HA-WBRT did not affect the efficacy of WBRT. Our results suggested that reducing the dose in the hippocampal might not compromise intracranial tumor control while it could protect patients’ NCF. Therefore, HA-WBRT is a safe approach for patients with brain metastasis.
In contrast to the majority of the studies, our retrospective study is a long-term follow-up process rather than a short-term one. In the past, long-term follow-up was not possible due to relative short survival for patients with brain metastasis, so the effect of hippocampal avoidance technology on long-term cognitive function could not be examined. Nowadays, due to the advance of tumor therapy such as new anti-tumor targeted drugs, the prognosis of brain metastasis has dramatically improved.25 Therefore, the long-term cognitive function protection is more important than ever for patients’ quality of life.
There are some limitations to our study, including its retrospective nature, a small patient number, single-center experience, and lack of Quality of Life data. Because of the retrospective nature of the study, the results could be impacted by selection bias. There would be confounding factors for survival, such as a heterogeneous group of patients, disease status, and systemic treatment plan. As described above, MMSE may have lower sensitivity. The NCF test batteries suggested by NRG CC001 may be better options for further research. It is a long-term follow-up study, 17/20 patients in WBRT arm and 20/27 patients in HA-WBRT arm survived at 12 months; however, only 4/20 patients in WBRT arm and 6/27 patients in HA-WBRT arm survived at 24 months. The limited number of patients survived at 24 months may affect the reliability of the results.
It is reported that patients with limited number of brain metastases had better local control and survival under the treatment of SRS than WBRT.26,27 After the linear accelerator equipped with cone beam computed tomography (CBCT) in 2018, patients of brain metastases <4 in our hospital were preferred to receive SRS. But in this study, patients of brain metastases <4 were treated with WBRT or HA-WBRT, due to equipment limitations.
Conclusion
At present, hippocampal avoidance technology has not yet been widely applied because of the uncertainty of its safety and effect. Results from this study demonstrated that HA-WBRT might have a protective effect on long-term neurocognitive function. Furthermore, there was no statistically significant difference in OS and PFS between patients who underwent HA-WBRT or WBRT. HA-WBRT did not increase the risk of brain recurrence close to the edge of the hippocampus.
Acknowledgments
The authors wish to thank the staff at the Department of Radiation Oncology in Northern Jiangsu People’s Hospital for their work in carrying out radiation therapy and long-time follow-up of patients after radiation.
Abbreviations
- WBRT
whole-brain radiotherapy
- HA-WBRT
hippocampal avoidance whole-brain radiotherapy
- NCF
neurocognitive function
- MMSE
Mine-Mental State Examination
- MoCA
Montreal Cognitive Assessment
- OS
overall survival
- PFS
progress free survival.
Authors’ Note: Buhai Wang and Shiwei Fu contributed equally to the work. This study did not require an ethical board approval because it did not contain human or animal trials. No human tissue or blood sample was involved in this study. It was a retrospective study, only collected clinical data of patients and did not interfere with the treatment plan of the patients. It would not bring risks to the physiology of the patients. Personal privacy of the patients would not be revealed in this study.
Declaration of Conflicting Interests: The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: national key research and development program/ Fund No: hxkt2017-7; health and family planning commission of Jiangsu province of China/ Fund No: S2017009.
ORCID iD: Buhai Wang, PhD  https://orcid.org/0000-0001-6391-4502
https://orcid.org/0000-0001-6391-4502
References
- 1.Cairncross JG, Kim JH, Posner JB. Radiation therapy for brain metastases. Ann Neurol. 1980;7(6):529–541. [DOI] [PubMed] [Google Scholar]
- 2.Thiagarajan A, Yamada Y. Radiobiology and radiotherapy of brain metastases. Clin Exp Metastasis. 2017;34(6-7):411–419. [DOI] [PubMed] [Google Scholar]
- 3.Monaco EA, III, Faraji AH, Berkowitz O, et al. Leukoencephalopathy after whole-brain radiation therapy plus radiosurgery versus radiosurgery alone for metastatic lung cancer. Cancer. 2013;119(1):226–232. [DOI] [PubMed] [Google Scholar]
- 4.Gondi V, Hermann BP, Mehta MP, Tomé WA. Hippocampal dosimetry predicts neurocognitive function impairment after fractionated stereotactic radiotherapy for benign or low-grade adult brain tumors. Int J Radiat Oncol Biol Phys. 2013;85(2):348–354. [DOI] [PubMed] [Google Scholar]
- 5.Chang EL, Wefel JS, Hess KR, et al. Neurocognition in patients with brain metastases treated with radiosurgery or radiosurgery plus whole-brain irradiation: a randomised controlled trial. Lancet Oncol. 2009;10(11):1037–1044. [DOI] [PubMed] [Google Scholar]
- 6.Gondi V, Tomé WA, Mehta MP. Why avoid the hippocampus? A comprehensive review. Radiother Oncol. 2010;97(3):370–376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ma TM, Grimm J, McIntyre R, et al. A prospective evaluation of hippocampal radiation dose volume effects and memory deficits following cranial irradiation. Radiother Oncol. 2017;125(5):234–240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Megias D, Phillips M, Clifton-Hadley L, et al. Dose specification for hippocampal sparing whole brain radiotherapy (HS WBRT): considerations from the UK HIPPO trial QA programme. Br J Radiol. 2017;90(1071):20160829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Korkmaz Kirakli E, Oztekin O. Is hippocampal avoidance during whole-brain radiotherapy risky for patients with small-cell lung cancer? Hippocampal metastasis rate and associated risk factors. Technol Cancer Res Treat. 2017;16(6):1202–1208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Mizumatsu S, Monje ML, Morhardt DR, et al. Extreme sensitivity of adult neurogenesis to low doses of X-irradiation. Cancer Res. 2003;63(14):4021–4027. [PubMed] [Google Scholar]
- 11.Gondi V, Tome WA, Marsh J, et al. Estimated risk of perihippocampal disease progression after hippocampal avoidance during whole-brain radiotherapy: safety profile for RTOG 0933. Radiother Oncol. 2010;95(3):327–331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hong AM, Suo C, Valenzuela M, et al. Low incidence of melanoma brain metastasis in the hippocampus. Radiother Oncol. 2014;111(1):59–62. [DOI] [PubMed] [Google Scholar]
- 13.Sun B, Huang Z, Wu S, et al. Incidence and relapse risk of intracranial metastases within the perihippocampal region in 314 patients with breast cancer. Radiother Oncol. 2016;118(1):181–186. [DOI] [PubMed] [Google Scholar]
- 14.Lawson JD, Wang JZ, Nath SK, et al. Intracranial application of IMRT based radiosurgery to treat multiple or large irregular lesions and verification of infra-red frameless localization system. J Neurooncol. 2010;97(1):59–66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Gondi V, Pugh SL, Tome WA, et al. Preservation of memory with conformal avoidance of the hippocampal neural stem-cell compartment during whole-brain radiotherapy for brain metastases (RTOG 0933): a phase II multi-institutional trial. J Clin Oncol. 2014;32(34):3810–3816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Scoccianti S, Detti B, Gadda D, et al. Organs at risk in the brain and their dose-constraints in adults and in children: a radiation oncologist’s guide for delineation in everyday practice. Radiother Oncol. 2015;114(2):230–238. [DOI] [PubMed] [Google Scholar]
- 17.Gondi V, Tolakanahalli R, Mehta MP, et al. Hippocampal-sparing whole-brain radiotherapy: a “how-to” technique using helical tomotherapy and linear accelerator-based intensity-modulated radiotherapy. Int J Radiat Oncol Biol Phys. 2010;78(4):1244–1252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Koay E, Sulman EP. Management of brain metastasis: past lessons, modern management, and future considerations. Curr Oncol Rep. 2012;14(1):70–78. [DOI] [PubMed] [Google Scholar]
- 19.Maclean J, Fersht N, Singhera M, et al. Multi-disciplinary management for patients with oligometastases to the brain: results of a 5 year cohort study. Radiat Oncol. 2013;8:156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Oehlke O, Wucherpfennig D, Fels F, et al. Whole brain irradiation with hippocampal sparing and dose escalation on multiple brain metastases: local tumour control and survival. Strahlenther Onkol. 2015;191(6):461–469. [DOI] [PubMed] [Google Scholar]
- 21.Tsai PF, Yang CC, Chuang CC, et al. Hippocampal dosimetry correlates with the change in neurocognitive function after hippocampal sparing during whole brain radiotherapy: a prospective study. Radiat Oncol. 2015;10:253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Brown PD, Gondi V, Pugh S, et al. for NRG Oncology. Hippocampal avoidance during whole-brain radiotherapy plus memantine for patients with brain metastases: phase III trial NRG Oncology CC001. J Clin Oncol. 2020;38(10):1019–1029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Siqueira G, Hagemann P, Coelho DS, et al. Can MoCA and MMSE be interchangeable cognitive screening tools? A systematic review. Gerontologist. 2019;59(6):e743–e763. [DOI] [PubMed] [Google Scholar]
- 24.Meyers CA, Wefel JS. The use of the mini-mental state examination to assess cognitive functioning in cancer trials: no ifs, ands, buts, or sensitivity. J Clin Oncol. 2003;21(19):3557–3558. [DOI] [PubMed] [Google Scholar]
- 25.Sperduto PW, Yang TJ, Beal K, et al. Estimating survival in patients with lung cancer and brain metastases: an update of the graded prognostic assessment for lung cancer using molecular markers (Lung-molGPA). JAMA Oncol. 2017;3(6):827–831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Guckenberger M, Andratschke N, Dieckmann K, et al. ESTRO ACROP consensus guideline on implementation and practice of stereotactic body radiotherapy for peripherally located early stage non-small cell lung cancer. Radiother Oncol. 2017;124(1):11–17. [DOI] [PubMed] [Google Scholar]
- 27.Kocher M, Maarouf M, Bendel M, et al. Linac radiosurgery versus whole brain radiotherapy for brain metastases. A survival comparison based on the RTOG recursive partitioning analysis. Strahlenther Onkol. 2004;180(5):263–267. [DOI] [PubMed] [Google Scholar]