Abstract
Objectives:
Elevated soluble urokinase Plasminogen Activator Receptor (suPAR) is a biomarker associated with adverse outcomes. We aimed to investigate the associations between plasma suPAR levels (testing the cut-offs ⩽4, 4-6, and ⩾6 ng/mL) with risk of 14-day mortality, and with the risk of mechanical ventilation in patients that tested positive for SARS-CoV-2.
Methods:
Observational cohort study of patients presenting with symptoms of COVID-19 at Department of Emergency Medicine, Amager and Hvidovre Hospital, Denmark from March 19th, 2020 to April 3rd, 2020. Plasma suPAR was measured using suPARnostic technologies. Patients were followed for development of mechanical ventilation and mortality for 14 days. Validation of our findings were carried out in a similar sized COVID-19 patient cohort from Mikkeli Central Hospital, Finland.
Results:
Among 386 patients with symptoms of COVID-19, the median (interquartile range) age was 64 years (46-77), 57% were women, median suPAR was 4.0 ng/mL (2.7-5.9). In total, 35 patients (9.1%) died during the 14 days follow-up. Patients with suPAR ⩽4 ng/mL (N = 196; 50.8%) had a low risk of mortality (N = 2; 1.0%; negative predictive value of 99.0%, specificity 55.3%, sensitivity 95.2%, positive predictive value 17.4%). Among patients with suPAR ⩾6 ng/mL (N = 92; 23.8%), 16 died (17.4%). About 99 patients (25.6%) tested positive for SARS CoV-2 and of those 12 (12.1%) developed need for mechanical ventilation. None of the SARS-CoV-2 positive patients with suPAR ⩽4 ng/mL (N = 28; 38.8%) needed mechanical ventilation or died. The Mikkeli Central Hospital validation cohort confirmed our findings concerning suPAR cut-offs for risk of development of mechanical ventilation and mortality.
Conclusions:
Patients with symptoms of COVID-19 and suPAR ⩽4 or ⩾6 ng/mL had low or high risk, respectively, concerning the need for mechanical ventilation or mortality. We suggest cut-offs for identification of risk groups in patients presenting to the ED with symptoms of or confirmed COVID-19.
Keywords: Prognosis, mechanical ventilation, biomarker, COVID-19, suPAR
Introduction
The coronavirus disease 2019 (COVID-19) pandemic, caused by the severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2), places extraordinary pressure on hospital resources. We have previously shown that soluble urokinase plasminogen activator receptor (suPAR) is a suitable biomarker to identify patients with COVID-19 symptoms who can be discharged from the Emergency Department (ED) at an early stage without risk of severe disease trajectory.1 In the present study, we seek to examine suPAR as a possible decision tool that may guide physicians to allocate patients to facilities with and without access to mechanical ventilation.
There are several reasons why the chronic inflammatory biomarker suPAR may be a suitable risk marker for patients with symptoms of COVID-19: Firstly, suPAR is a strong marker for readmission2 and mortality3 in unselected acute medical patients. Thus, even if the patient tests negative for SARS-CoV-2, the suPAR result still has clinical value. Secondly, suPAR is associated with clinical severity and/or mortality in several viral infections, including Human Immunodeficiency Virus (HIV)4 and Hepatitis C,5 Crimean-Congo hemorrhagic fever,6 Hantavirus7 infection, and in patients with SARS-CoV-2 infection.8,9 Thirdly, our target population was patients with symptoms of COVID-19: suPAR is elevated and associated with disease severity in patients with respiratory diseases including pediatric lower respiratory tract pneumonia,10 chronic obstructive pulmonary disease,11-13 and predicts risk of acute respiratory distress syndrome in patients with sepsis.14
Elevated suPAR (>6 ng/mL) has been reported to be a strong predictor for need of mechanical ventilation in COVID-19 patients: In a Greek study including 57 patients, positive predictive value (PPV) and negative predictive value (NPV) for prediction of severe respiratory failure defined as need of mechanical ventilation with an admission suPAR >6 ng/mL was 85.7% and 91.7%.8 The high PPV of suPAR in COVID-19 was confirmed in an International Study of Inflammation in COVID-19 (ISIC) including 352 COVID-19 patients where 45.8% of patients in the top suPAR tertile (>6.86 ng/mL) developed need of mechanical ventilation. In contrast, only 3 (2.6%) developed need of mechanical ventilation in the lowest suPAR tertile (<4.6 ng/mL).9
At Copenhagen University Hospital Hvidovre, Denmark, suPAR has been measured in acutely admitted medical patients at the Emergency Department (ED) since 2013.2,3 In studies conducted before the COVID-19 pandemic, among unselected acute medical patients, it was found that suPAR <3 ng/mL was associated with low risk of readmission and mortality (approximately half of the admitted acute care medical patients), 3 to 6 ng/mL as medium risk, and >6 ng/mL as high risk requiring clinical attention.2,3
As the COVID-19 pandemic has caused increased burden on hospital resources, we aimed to find relevant suPAR cut-offs that may aid in triage of patients seeking acute care. In the present study, we re-examine a previous study1 to test if baseline suPAR levels ⩽4 ng/mL in patients with symptoms of COVID-19 can identify patients with low risk of 14-day mortality and if baseline suPAR levels ⩾6 ng/mL can identify patients with high risk of mortality. Our secondary aim was to test if these suPAR cut-offs were associated with low and high risk, respectively, of mechanical ventilation and/or mortality in patients testing positive for SARS-CoV-2.
Methods
The study is a single center observational cohort study at Department of Emergency Medicine, Copenhagen University Hospital, Amager and Hvidovre, Hvidovre, Denmark.1 Patients included in the cohort were referred to the ED when primary sector-treatment due to COVID-19 symptoms and/or general health status was not feasible. Symptoms were mainly cough, fever, chest pain, arthralgia, shortness of breath, and headache.
Patient inclusion took place, during the first 3 weeks of the first COVID-19 wave in Denmark from March 19th, 2020, until April 3rd, 2020, and with follow-up until April 17th, 2020. Clinicians deemed whether patients were eligible for mechanical ventilation if necessary, during follow up. The data collection is described in detail elsewhere.1 In brief, we collected data on age, gender, number of comorbidities, national early warning score (NEWS), tobacco smoking habits, and duration of symptoms from electronic patient health records as well as blood biochemistry with the software Research Electronic Data Capture program (REDCap), and quality control of data was performed by 3 medical doctors (IA, MS, and JT).1
Furthermore, to validate our results, we included COVID-19 patients among acute medical patients from a prospective observational study at Mikkeli Central Hospital in Finland, from March 2020 to May 2021—the Finnish validation cohort.
On admission, these patients had a standard panel of blood tests analyzed and suPAR is part of these standard blood tests.
Laboratory Measurements
Coronavirus testing
Samples for SARS-CoV-2 testing were collected at the ED and analyzed at the Department of Clinical Microbiology, Copenhagen University Hospital, Hvidovre on material obtained from expectorate, nasopharyngeal suction, tracheal secretion, or swab from pharynx using a RealStar® SARS-CoV-2 RT-PCR Kit RUO (Altona Diagnostics, Hamburg, Germany) adapted to a Roche flow system. The limit of detection was reported to be 50 copies of RNA. The results of this test were not available to the ED physicians until 1 to 2 days after admission.
Biochemical analyses
Blood samples were obtained on admission at the ED (within the first 2 hours of hospital duration) and analyzed at the Department of Clinical Biochemistry, Copenhagen University Hospital, Hvidovre. White blood cell counts, hemoglobin, creatinine, C-reactive protein (CRP), alanine aminotransferase (ALAT), lactate dehydrogenase (LDH), and bilirubin were measured using a COBAS 8000 analyzer (Roche Diagnostics, Mannheim, Germany). Cell counts (leukocyte, thrombocyte, lymphocyte, and neutrophils) were measured using flow cytometry on a Sysmex XN 9000 (Sysmex Corporation, Kobe, Japan).
Plasma suPAR was measured using suPARnostic® Quick Triage point-of-care test (ViroGates, Birkerød, Denmark) according to the manufacturer’s instructions and quantified using an aLF reader (QIAGEN, Hilden, Germany). Blood for this test (EDTA, 4 mL) was drawn on arrival at the ED (within the first 2 hours of hospital duration) and centrifuged for 3 minutes.
suPAR was measured in real time 24/7, but the result was not available to the attending physicians as clinical cut-off values were not yet established, thus it was unknown whether suPAR added value in triage of patients with symptoms of COVID-19.
In the validation cohort from Mikkeli Central Hospital in Finland, EDTA plasma suPAR levels were analyzed as part of the standard admission blood samples at the Eastern Finland laboratory ISLAB with the using suPARnostic® Turbilatex assay (ViroGates A/S, Birkerød, Denmark) on a Cobas c501 clinical chemistry analyzer (Roche Diagnostics Ltd) according to the reagent manufacturer’s instructions.
Clinical Signs on Admission at ED
The Patient’s National Early Warning Score also known as NEWS (systolic and diastolic blood pressure, pulse, respiratory rate, O2-saturation, body temperature) and level of consciousness or new confusion were assessed upon arrival.3
Furthermore, patients were asked for the number of days they experienced symptoms of infection before presenting at the ED. Symptoms included sore throat, cough (productive, non-productive), body pain, fatigue, headache, dizziness, nausea/vomit, fever, abdominal pain, obstipation, diarrhea, dysuria, shortness of breaths, chest pain, arthralgia, cramps, chills, and hemoptysis.
Baseline comorbidities were recorded. The most common baseline comorbidities were heart and lung diseases, previous strokes, and inflammatory diseases. Details on comorbidities are described elsewhere.1
Incident Organ Dysfunction or Worsening in Chronic Organ Dysfunction at 14 Days Follow Up
Organ dysfunction during hospitalization was defined as kidney failure (defined as initiated dialysis), liver failure (defined as INR >1.6 for patients without anticoagulant therapy, and/or ALAT >400 U/L, and/or bilirubin >50 μmol/L, and/or albumin <15 g/L); lung failure (defined as need of >10 L oxygen/minutes and respiratory frequency ⩾28, need of non-invasive ventilation [NIV], or need of ventilator treatment), mechanical ventilation (defined as need of endotracheal intubation and ventilator treatment), heart failure (defined as Troponin T above 100 ng/mL, echocardiography with newly decreased pump function or lowering of pump function, or non-ischemic cardiac arrest).
Endpoints
The primary endpoint was the association between suPAR levels and 14-day mortality with the primary aim of testing if suPAR was associated with 14-day mortality in patients with symptoms of COVID-19.
The secondary endpoints were (1) to investigate baseline differences between patients that turn positive or negative in SARS-CoV-2 testing and (2) to determine the risk, according to suPAR cut-offs for developing the need for mechanical ventilation and other organ failures (see below) in SARS-CoV-2 positive or negative patients.
The secondary outcomes evaluated at 14 days follow-up were: discharged within 24 hours, still admitted, died, treatment with: oxygen, non-invasive ventilation (NIV), Continuous Positive Airway Pressure (CPAP), ventilator, Venovenous-Extra Corporal Membrane Oxygenation (VV-ECMO), vasopressor drugs, and development of organ failure for example, kidney failure, liver failure, lung failure, heart failure.
In the Mikkeli Central Hospital validation cohort, the same outcomes were obtained but follow-up was up to 90 days (range 20-90 days).
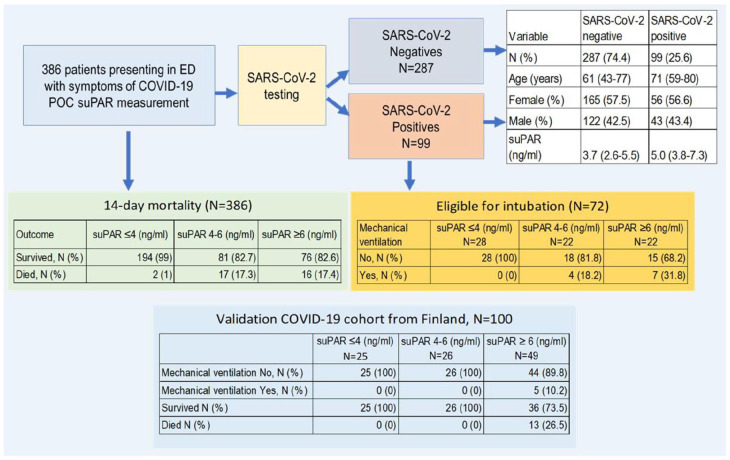
For schematic illustration of patients with symptom of COVID-19 (N = 386) and outcomes during 14 days follow-up (in the Danish cohort), and up to 90 days of follow-up in the Finnish cohort with COVID-19 patients (N = 100), please see Figure 1.
Figure 1.
Schematic illustration of the path in the ED given to patients who were SARS-CoV-2 positive (N = 99) and negative (N = 287) with symptoms of COVID-19 (N = 386) and followed during 14 days. Shown are the difference in variables among SARS-CoV-2 positive and negative test results, the need of mechanical ventilation in eligible confirmed COVID-19 patients (N = 72) according to suPAR cut-offs, and finally, outcomes indicated as a 14-day mortality or survival in the whole cohort among patients with symptoms of COVID-19 (N = 386) and comparison to the validation COVID-19 cohort from Finland (N = 100).
Abbreviations: POC, point of care technology; suPAR, soluble urokinase Plasminogen Activator Receptor.
Statistical Analysis
Continuous and categorical variables are presented as median (interquartile range [IQR]) and n (%), respectively. Calculations of negative predictive value (NPV), positive predictive value (PPV), sensitivity, and specificity were carried out.
Comparisons of baseline parameters between patients eligible/not eligible for intubation and SARS-CoV-2 status (positive/negative), and for the different suPAR cut-off categories were done by chi-squared test, Fishers exact test, or Wilcoxon sum rank test.
Because of multiple testing when comparing baseline or outcome variables, Bonferroni correction was applied, scaling the significance level according to the number of tests performed. The significance levels are listed in the tables. The statistical program R v3.60 (R Foundation for Statistical Computing, Vienna, Austria) was used for analyzes and figures.
Ethics
The database and collection of clinical data and entering in REDCap was approved by the Danish Data Protection Agency (record no. P-2020-513) and by the Danish Patient Safety Authority (record no. 31-1521-319). The Mikkeli Central Hospital validation cohort was approved by the South Savo social- and healthcare authority (study approval 684/13.02.03/2019).
Results
Baseline characteristics of patients presenting with symptoms of COVID-19
A total of 386 patients presenting at the ED with symptoms of COVID-19, mainly presenting with fever and cough, from March 19th, 2020 to April 3rd, 2020, were included in the study. The median age was 64 years (IQR 46-77), and 221 (57.3%) were women. The median admission suPAR level was 4.0 ng/mL (IQR 2.7-5.9). Baseline data, including number of comorbidities, National Early Warning Score (NEWS), tobacco smoking, duration of symptoms, most pronounced symptoms as cough and fever with standard panel of blood tests were analyzed; including electrolytes, blood counts, liver function, kidney function, and markers of infection and inflammation, for all 386 patients with symptoms of COVID-19 and stratified according to the suPAR cut-offs ⩽4, between 4 and 6, and ⩾6 ng/mL, are shown in Table 1.
Table 1.
Baseline data for all patients stratified according to suPAR levels.
| Variable* | suPAR ⩽4 | suPAR 4-6 | suPAR ⩾6 | Total | |
|---|---|---|---|---|---|
| N (%) | 196 (50.8) | 98 (25.4) | 92 (23.8) | 386 | |
| Age (y) | 52 (38-66) | 74 (61-82) | 75 (62-83.3) | 64 (46-77) | |
| Female | 123 (62.8) | 56 (57.1) | 42 (45.7) | 221 (57.3) | |
| Male | 73 (37.2) | 42 (42.9) | 50 (54.3) | 165 (42.7) | |
| Number of comorbidities | 1 (0-2) | 2 (1-4) | 3 (2-4) | 2 (1-3) | |
| Systolic blood pressure (mmHg), median (IQR) | 135 (124-150) | 136.5 (121.3-152) | 131 (117-148) | 135 (123-150) | |
| Diastolic blood pressure (mmHg) | 82 (75-91) | 81,5 (70.3-89) | 78 (67-86) | 81 (72.3-89) | |
| Pulse (beats/minute) | 86 (74-97) | 87 (81-100) | 83 (74-96) | 86 (76-97) | |
| Respiratory rate (breaths/minute) | 20 (16.8-22) | 20 (18-24) | 20 (18-26) | 20 (18-23) | |
| Saturation (%) | 98 (96-100) | 97 (95-98) | 96 (93-98) | 97 (95-99) | |
| Temperature (°C) | 37.3 (36.9-37.7) | 37.5 (36.9-38.2) | 37.5 (37-38.3) | 37.4 (36.9-38) | |
| NEWS on admission | 1 (0-3) | 2 (1-4.8) | 3 (1-6) | 2 (0-4) | |
| suPAR (ng/mL) | 2.7 (2.1-3.4) | 4.8 (4.5-5.5) | 8.1 (6.7-11.1) | 4 (2.7-5.9) | |
| Leukocyte count (E9/L) | 8 (6.3-10.4) | 8.3 (5.5-11.2) | 9.1 (6.3-11.4) | 8.3 (6.1-10.9) | |
| Thrombocyte count (E9/L) | 255 (202-301) | 225 (171.5-286.8) | 253 (188.5-357) | 249 (192-302) | |
| Lymphocyte count (E9/L) | 1.9 (1.3-2.5) | 1.2 (0.9-1.6) | 1.1 (0.7-1.8) | 1.5 (0.9-2.3) | |
| Neutrophil count (E9/L) | 4.9 (3.5-7.0) | 5.95 (3.6-9.1) | 6.65 (4.4-8.9) | 5.3 (3.6-8.1) | |
| Hemoglobin (mmol/L) | 8.7 (8.1-9.3) | 8.15 (7.5-8.9) | 7.85 (6.7-8.9) | 8.4 (7.6-9.1) | |
| CRP (mg/L) | 4.3 (1.0-22.5) | 27.5 (7.1-80.8) | 72 (19.8-130) | 15.5 (2.4-65.8) | |
| LDH (U/L) | 182 (162.8-205.3) | 227 (195.8-278.5) | 247 (195-317) | 199 (176-251) | |
| ALAT (U/L) | 23 (16-31) | 23 (17-35) | 24.5 (17-47.3) | 23 (17-35) | |
| Creatinine (μmol/L) | 68 (60-82) | 80 (66.3-100.8) | 96 (74.5-129.8) | 77 (62-96.5) | |
| Bilirubin (μmol/L) | 6 (4-10) | 8 (5-12) | 9 (6-14) | 7 (5-12 | |
| Active smokers | 51 (26) | 14 (14.3) | 19 (20.7) | 84 (21.8) | |
| Ex-smokers | 57 (29.1) | 41 (41.8) | 42 (45.7) | 140 (36.3) | |
| Never smoked | 82 (41.8) | 41 (41.8) | 29 (31.5) | 152 (39.4) | |
| Unknown | 6 (3.1) | 2 (2) | 2 (2.2) | 10 (2.6) | |
| Duration of symptoms, days | |||||
| 0-1 | 33 (17.2) | 23 (24.7) | 18 (20.2) | 74 (19.8) | |
| 2-3 | 38 (19.8) | 21 (22.6) | 21 (23.6) | 80 (21.4) | |
| 4-5 | 20 (10.4) | 16 (17.2) | 11 (12.4) | 47 (12.6) | |
| 6-7 | 30 (15.6) | 6 (6.5) | 11 (12.4) | 47 (12.6) | |
| 8-10 | 17 (8.9) | 5 (5.4) | 5 (5.6) | 27 (7.2) | |
| 11-13 | 6 (3.1) | 6 (6.5) | 4 (4.5) | 16 (4.3) | |
| 14-15 | 27 (14.1) | 6 (6.5) | 5 (5.6) | 38 (10.2) | |
| 15+ | 21 (10.9) | 10 (10.8) | 14 (15.7) | 45 (12) | |
| Cough | Yes | 137 (69.9) | 60 (61.2) | 55 (59.8) | 252 (65.3) |
| Fever | Yes | 84 (42.9) | 56 (57.1) | 51 (55.4) | 191 (49.5) |
Baseline data is defined as data available in the patient admission file during the first contact with the ED with symptoms of COVID-19. Data were stratified according to suPAR cut-off values. All biomarkers were measured at the first contact with ED.
Abbreviations: ALAT, alanine transaminase; CRP, C-reactive protein; LDH, lactate dehydrogenase; NEWS, national early warning score; suPAR, soluble urokinase Plasminogen Activator Receptor.
Abbreviations: ALAT, alanine transaminase; CRP, C-reactive protein; LDH, lactate dehydrogenase; NEWS, national early warning score; suPAR, soluble urokinase Plasminogen Activator Receptor.
Data showing as median (IQR) or N (%).
All variable has 386 data available, except LDH = 311, duration of symptoms = 374, temperature = 381, CRP = 382, ALAT = 382, creatinine = 383, hospital duration = 384, and for pulse, thrombocyte, hemoglobin, and bilirubin 385 data were available.
Fourteen-day mortality in patients with symptoms of COVID-19
Among the 386 patients presenting with symptoms of COVID-19 at the ED, a total of 35 patients died during a 14-day follow-up (Figure 1). Out of 196 (50.8%) patients with baseline suPAR ⩽4 ng/mL, 2 patients (1%) died and among 190 patients with baseline suPAR >4 ng/mL, 33 died (17%). A suPAR cut-off at 4 ng/mL will therefore result in a NPV for death of 99.0% for patients below cut-off and (PPV of 17.4% for patients above cut-off, a sensitivity of 94.3% and a specificity of 55.3%) (Table 2).
Table 2.
Outcomes for all patients admitted to ED with symptoms of COVID-19 regarding suPAR (ng/mL) cut-offs during 14 days of follow-up.
| Variable | suPAR ⩽4 | suPAR 4-6 | suPAR ⩾ 6 | P-value |
|---|---|---|---|---|
| N (%) | 196 (50.8) | 98 (25.4) | 92 (23.8) | |
| Discharged within 24 hours N (%) | 132 (67.3) | 30 (30.6) | 23 (25) | <.001 |
| Readmitted during follow up N (%) | 19 (9.7) | 12 (12.2) | 8 (8.7) | .68 |
| Still admitted N (%) | 6 (3.1) | 7 (7.1) | 17 (18.5) | <.001 |
| Need of oxygen N (%) | 69 (35.2) | 68 (69.4) | 67 (72.8) | <.001 |
| NIV N (%) | 4 (2) | 1 (1) | 2 (2.2) | .79 |
| CPAP N (%) | 6 (3.1) | 12 (12.2) | 9 (9.8) | .007 |
| Ventilator N (%) | 0 (0) | 5 (5.1) | 7 (7.6) | .001 |
| ECMO N (%) | 0 (0) | 0 (0) | 1 (1.1) | .201 |
| Pressor drugs N (%) | 0 (0) | 4 (4.1) | 8 (8.7) | <.001 |
| Kidney failure N (%) | 0 (0) | 2 (2) | 4 (4.3) | .019 |
| Liver failure N (%) | 0 (0) | 2 (2) | 2 (2.2) | .124 |
| Lung failure N (%) | 6 (3.1) | 13 (13.3) | 18 (19.6) | <.001 |
| Heart failure N (%) | 2 (1.0) | 2 (2.0) | 2 (2.2) | .688 |
| Died N (%) | 2 (1.0) | 17 (17.3) | 16 (17.4) | <.001 |
Abbreviations: CPAP, continuous positive airway pressure; ECMO, extra-corporal-membrane-oxygenation; NIV, non-invasive ventilation; suPAR, soluble urokinase Plasminogen Activator Receptor.
P value refers to differences between suPAR cut-off values. Bonferroni corrected significant level = .0036.
P-value compares differences in outcomes for all patients with symptom of COVD-19 according to different suPAR cut-off values during 14 days of follow-up.
Abbreviations: CPAP, continuous positive airway ventilation; ECMO, extra corporal membrane oxygenation; NIV, non-invasive ventilation.
Data are shown as N (%).
Bonferroni corrected significant level = .0036.
Out of 92 patients with baseline suPAR ⩾6 ng/mL, 16 (17.4%) died within 14 days, resulting in a NPV of 93.5%, PPV of 17.4%, a sensitivity of 48.6% and a specificity of 78.3% (Table 2).
Outcomes for patients with symptoms of COVID-19 as discharge, readmission, still admitted, types of organ failures, types of treatment, and death are stratified by baseline suPAR cut-offs and shown in Table 2. Patients presenting with symptom of COVID-19 with suPAR ⩽4 ng/mL were more likely to be discharged within 24 hours than patients with higher suPAR levels (P < .001), whereas patients presenting with suPAR >6 ng/mL were more likely to remain hospitalized at 14 days follow-up compared to medium or low suPAR cut-off levels (P < .001) (Table 2).
More patients with elevated suPAR (>6 ng/mL) received treatment with mechanical ventilation and pressor drugs, and increased mortality was observed in these patients compared to patients with suPAR below 4 ng/mL (P < .001); only few patients developed kidney and liver failure but there was a trend that this occurred more frequently among patients with suPAR >6 ng/mL compared to patients with suPAR <4 ng/mL (Table 2).
Baseline differences between SARS-CoV-2 positive and negative patients
Among the 386 patients with symptoms of COVID-19, 99 (25.6%) tested positive for SARS-CoV-2 in the RT-PCR assay. The patients who tested positive for SARS-CoV-2 differed on several clinical and biochemical baseline parameters from the 287 patients who tested negative (Table 3). Patients with a positive SARS-CoV-2 test were older, had lower blood saturation and higher NEWS, higher suPAR, CRP levels, LDH and ALAT meanwhile having lower leukocyte, thrombocyte, lymphocyte, neutrophil counts (all P < .01) (Table 3). There was a trend of higher measured creatinine among SARS-CoV-2 positive patients. The number of comorbidities did not differ between these 2 groups. Fever was more predominant among the SARS-CoV-2 negative compared to SARS-CoV-2 positive patients. There were fewer active smokers among patients who tested positive for SARS-CoV-2 (7.1% vs 27.0%, P < .001) (Table 3).
Table 3.
Baseline clinical parameters and biochemical markers according to SARS-CoV-2 testing result performed when patients presented at ED. Baseline data are defined as data available in the patient admission file during the first contact in ED presenting with symptoms of COVID-19.
| Variable | SARS-CoV-2 negative | SARS-CoV-2 positive | P-value | |
|---|---|---|---|---|
| N (%) | 287 (74.4) | 99 (25.6) | ||
| Age (y) | 61 (43-77) | 71 (59-80) | <.001 | |
| Female (%) | 165 (57.5) | 56 (56.6) | 1 | |
| Male (%) | 122 (42.5) | 43 (43.4) | ||
| Number of comorbidities | 2 (1-3) | 2 (1-3) | .29 | |
| Systolic blood pressure (mmHg) | 136 (124-152) | 130 (121-142) | .054 | |
| Diastolic blood pressure (mmHg) | 82 (73-91) | 80 (70-87) | .064 | |
| Pulse (bpm) | 86 (75-99) | 85 (77-95) | .703 | |
| Respiratory rate (breaths/minute) | 20 (17-23) | 20 (18-23) | .11 | |
| Saturation (%) | 98 (96-100) | 96 (95-98) | <.001 | |
| Temperature (°C) | 37.3 (36.8-37.7) | 38 (37.3-38.6) | <.001 | |
| National early warning score | 2 (0-4) | 3 (1-5) | .003 | |
| suPAR (ng/mL) | 3.7 (2.6-5.5) | 5.0 (3.8-7.3) | <.001 | |
| Leukocyte count (E9/L) | 9.0 (7-11.7) | 6.1 (4.6-7.7) | <.001 | |
| Thrombocyte count (E9/L) | 259 (207-311) | 198 (159-264) | <.001 | |
| Lymphocyte count (E9/L) | 1.7 (1.1-2.5) | 1.0 (0.7-1.4) | <.001 | |
| Neutrophil count (E9/L) | 5.9 (4.1-8.5) | 4.1 (3.0-5.9) | <.001 | |
| Hemoglobin (mmol/L) | 8.5 (7.6-9.2) | 8.3 (7.5-9.1) | .482 | |
| CRP (mg/L) | 8.4 (1.6-46.5) | 42 (16-94) | <.001 | |
| LDH (U/L) | 192 (174-229) | 248 (200-318) | <.001 | |
| ALAT (U/L) | 22 (16-31) | 28 (21.5-43.5) | <.001 | |
| Creatinine (μmol/L) | 74 (62-93) | 83.5 (65.3-102) | .016 | |
| Bilirubin (μmol/L) | 7 (5-12) | 7.5 (5.0-10.8) | .96 | |
| Active smoker | 77 (26.8) | 7 (7.1) | <.001 | |
| Ex-smoker | 101 (35.2) | 39 (39.4) | ||
| Never smoked | 102 (35.5) | 50 (50.5) | ||
| Unknown | 7 (2.4) | 3 (3) | ||
| Days with symptoms before admission | ||||
| 0-1 | 67 (24) | 7 (7.4) | <.001 | |
| 2-3 | 59 (21.1) | 21 (22.1) | ||
| 4-5 | 31 (11.1) | 16 (16.8) | ||
| 6-7 | 36 (12.9) | 11 (11.6) | ||
| 8-10 | 15 (5.4) | 12 (12.6) | ||
| 11-13 | 6 (2.2) | 10 (10.5) | ||
| 14-15 | 27 (9.7) | 11 (11.6) | ||
| 16+ | 38 (13.6) | 7 (7.4) | ||
| Cough | Yes | 180 (62.7) | 72 (72.7) | .093 |
| Fever | Yes | 124 (43.2) | 67 (67.7) | <.001 |
Abbreviations: ALAT, alanine aminotransferase; CRP, C-reactive protein; LDH, lactate dehydrogenase; suPAR, soluble urokinase Plasminogen Activator Receptor.
P-value compares differences in baseline clinical and biochemical markers according to the different SARS-CoV-2 result. Bonferroni-corrected significance level = .002.
Abbreviations: ALAT, alanine transaminase; CRP, C-reactive protein; LDH, lactate dehydrogenase; NEWS, national early warning score; suPAR, soluble urokinase Plasminogen Activator Receptor.
Parentheses refer to median (IQR) or N (%).
Bonferroni-corrected significance level = .002.
Outcomes according to suPAR levels in patients with a confirmed SARS-CoV-2 positive test and eligible for mechanical ventilation
At baseline, clinicians deemed whether patients were eligible for mechanical ventilation, if necessary. Among the 386 with symptoms of COVID-19, 310 were assessed eligible to receive mechanical ventilation if needed, while 76 were not, due to frailty and/or the patient requested not to receive mechanical ventilation (Figure 1). Those not eligible for mechanical ventilation were, in general, older (median age 82 [IQR 75-86]) patients with several comorbidities (median 3 [IQR 2-5]) (Table 3).
Of the 99 SARS-CoV-2 positive patients, a total of 72 (72.7%) patients were deemed eligible to receive mechanical ventilation if needed. Table 4 shows 14-day outcomes among the eligible COVID-19 patients stratified by baseline suPAR levels. During follow-up, 11 patients (15.3%) developed need for mechanical ventilation. Of these, 6 (8.3%) died during the 14-day follow-up. One patient with need for mechanical ventilation died before being intubated resulting in a total of 12 patients who developed need for mechanical ventilation and/or died (Table 4).
Table 4.
Outcomes (during 14-days follow-up) for SARS-CoV-2 positive patients who were eligible for mechanical ventilation. Bonferroni-corrected significance level = .003. Data are shown N (%). Treatment during in-hospitalization and outcomes during 14 days of follow-up among eligible for mechanical ventilation in COVID-19 patients. Furthermore, outcomes according to suPAR cut-offs is shown.
| Variable | suPAR ⩽4 ng/mL | suPAR 4-6 ng/mL | suPAR ⩾6 ng/mL | P value |
|---|---|---|---|---|
| N (%) | 28 (38.8) | 22 (30.6) | 22 (30.6) | |
| Discharged within 24 hours (%) | 16 (57.1) | 6 (27.3) | 5 (22.7) | .0219 |
| Readmitted during follow up (%) | 5 (18.5) | 2 (10) | 2 (10.5) | .629 |
| Still admitted (%) | 2 (7.1) | 3 (13.6) | 8 (36.4) | .0232 |
| Need of oxygen (%) | 10 (35.7) | 13 (59.1) | 15 (68.2) | .0573 |
| NIV (%) | 0 (0) | 0 (0) | 0 (0) | .607 |
| CPAP (%) | 1 (3.6) | 5 (22.7) | 5 (22.7) | .0884 |
| Ventilator (%) | 0 (0) | 4 (18.2) | 7 (31.8) | .00729 |
| ECMO (%) | 0 (0) | 0 (0) | 1 (4.5) | .316 |
| Pressor drugs (%) | 0 (0) | 3 (13.6) | 7 (31.8) | .00543 |
| Dialysis (%) | 0 (0) | 1 (4.5) | 3 (13.6) | .109 |
| Kidney failure (%) | 0 (0) | 2 (9.1) | 3 (13.6) | .152 |
| Liver failure (%) | 0 (0) | 2 (9.1) | 1 (4.5) | .278 |
| Lung failure (%) | 1 (3.6) | 4 (18.2) | 8 (36.4) | .0114 |
| Heart failure (%) | 0 (0) | 2 (9.1) | 0 (0) | .0966 |
| Died (%) | 0 (0) | 4 (18.2) | 2 (9.1) | .0687 |
Abbreviations: CPAP, continuous positive airway pressure; ECMO, extra-corporal-membrane-oxygenation; NIV, non-invasive ventilation; suPAR, soluble urokinase Plasminogen Activator Receptor.
Abbreviations: CPAP, continuous positive airway ventilation; CRP, C-reactive protein; ECMO, extra corporal membrane oxygenation; NIV, non-invasive ventilation; suPAR, soluble urokinase Plasminogen Activator Receptor.
Bonferroni-corrected significance level = .003. Data are shown N (%).
As shown in Table 4, 28 of the SARS-CoV-2 positive patients eligible for mechanical ventilation (38.8%) had a suPAR level ⩽4 ng/mL, and none of these patients developed need of mechanical ventilation or died. Of the 44 patients eligible for intubation with baseline suPAR >4 ng/mL, 12 died or developed need for mechanical ventilation. A suPAR cut-off equal to 4 ng/mL for COVID-19 patients eligible for mechanical ventilation therefore results in a PPV for death or mechanical ventilation for 27.2% and a NPV for 100%. The specificity is 46.7%, and sensitivity is 100%. Among the 22 SARS-CoV-2 positive patients eligible for mechanical ventilation with a suPAR ⩾6 ng/mL, 7 (31.8%) developed need for mechanical ventilation or died, resulting in a PPV of 31.8%, NPV of 90.0%, sensitivity for 58.3%, specificity for 81.7%.
The majority of SARS-CoV-2 positive patients (N = 28) discharged within 24 hours had a suPAR ⩽4 ng/mL. Regarding those patients that remained hospitalized at 14 days follow up, there was a trend that SARS-CoV-2 positive patients with suPAR ⩾6 ng/mL had longer hospitalizations (P = .02) (Table 4).
Among SARS-CoV-2 positive patients, there was a trend for those with suPAR ⩽4 ng/mL who had less need of treatment with oxygen, NIV, CPAP, ECMO, pressor drugs, and dialysis compared to SARS-CoV-2 positive patients with respectively suPAR 4 to 6 ng/mL and suPAR ⩾6 ng/mL (Table 4).
Validation of results from Mikkeli Central Hospital COVID-19 cohort
To validate the suPAR cut-offs for COVID-19, we obtained suPAR data from Mikkeli Central Hospital in Finland. A total of 100 acute medical patients presenting at the Emergency Department and testing positive for SARS-CoV-2 were included. Baseline and outcome data according to suPAR cut-offs are shown in Table 5.
Table 5.
Mikkeli Central Hospital, Finland, validation cohort table: treatment and outcomes such as AKI, need of ventilator treatment (mechanical ventilation) and death among COVID-19 patients are shown. The biomarkers, suPAR and CRP are given as median and IQR. Furthermore, are shown, admitted COVID-19 patients to hospital, ICU, discharged to home, length of stay, and when death occurred after patients tested positive for SARS-CoV-2 infection.
| Variable | All | suPAR ⩽4 ng/mL | suPAR 4-6 ng/mL | suPAR ⩾6 ng/mL | P value |
|---|---|---|---|---|---|
| N | 100 | 25 | 26 | 49 | NA |
| Sex, male N (%) | 56 (56) | 14 (56) | 13 (50) | 29 (59.2) | .75 |
| Age, Median (IQR) | 63 (42-76) | 35 (27-56) | 59 (36-66) | 75 (64-83) | <.001 |
| suPAR (ng/mL), Median (IQR) | 5.95 (4.1-8.6) | 3.5 (3.2-3.7) | 5.1 (4.4-5.6) | 8.8 (7.1-12.0) | NA |
| CRP, Median (IQR) | 19 (4-41) | 3.0 (3.0-3.7) | 22 (9.3-4.8) | 27 (16.5-80.0) | .001 |
| Admitted hospital N (%) | 61 (61) | 7 | 15 | 39 | <.001 |
| Admitted ICU N (%) | 4 (4) | 0 | 0 | 4 | .11 |
| Discharged to home N | 35 (35) | 18 | 11 | 6 | <.001 |
| Need of oxygen N (%) | 39 (39) (3 NA) | 0 (0) (1 NA) | 11 (42.3) (1 NA) | 28 (57.1) (1 NA) | <.001 |
| AKI N (%) | 2 (2.0) | 0 (0) | 0 (0) | 2 (4.1) | .35 |
| Ventilator N (%) | 5 (5.0) | 0 (0) | 0 (0) | 5 (10.2) | .06 |
| Hosp stay, Days (IQR) | 6 (1-11.3) | 1 (1-2) | 4 (1-7) | 8 (5-17) | <.001 |
| Mortality N (%) | 13 (13.0) | 0 (0) | 0 (0) | 13 (26.5) | <.001 |
Abbreviations: AKI, acute kidney injury; CRP, C-reactive protein; ICU, intensive care unit; NA, not available; suPAR, soluble urokinase plasminogen activator receptor.
Parentheses refer to median (IQR) or N (%).
In the Finnish COVID-19 patient cohort, there was an increase in suPAR with increasing age while no difference was observed with regard to sex. None of the 25 (25%) patients with a suPAR level ⩽4 ng/mL required oxygen therapy, developed AKI or need of mechanical ventilation or died resulting in a NPV below 4 ng/mL of 100%. In patients with suPAR above 6 ng/mL, 13 (26.5%) died, resulting in a PPV of 27.0%.
Discussion
Based on previous studies on the use of suPAR in the ED2,15 and in patients with COVID-198,9 we tested specific cut-offs for suPAR (⩽4, 4-6, ⩾6 ng/mL) for patients presenting with symptoms of COVID-19, before the SARS-CoV-2 test result was known. We found that a suPAR level ⩽4 ng/mL was associated with low risk of 14-day mortality (NPV = 99.0%). It has been shown that elevated suPAR is associated with disease severity, readmission,2 and mortality.3 However, as an unspecific biomarker reflecting overall patient disease severity, low suPAR seems useful as a potential discharge biomarker.2,3 In addition, a study found that more patients had been discharged when clinicians had access to suPAR.16
In this study, approximately 25% of the patients that presented with symptoms of COVID-19 during the first wave of the pandemic, tested positive for SARS-CoV-2, and approximately 80% of the positive patients were eligible for mechanical ventilation when required. In patients with a positive SARS-CoV-2 test, an elevated suPAR (⩾6 ng/mL) was associated with disease severity as shown by the higher number of long-term hospital admission, increased number of patients developing respiratory and organ failures (Table 4). This is in agreement with studies showing increased risk of mechanical ventilation in COVID-19 patients with suPAR >6 ng/mL compared to suPAR <6 ng/mL8 and increased risk of acute kidney injury in COVID-19 patients in the top tertile (>6.82 ng/mL) compared to lower tertiles.9 In fact, suPAR may be causative of acute kidney injury, as shown in studies before COVID-19.17,18
Our suggestion for specific cut-offs allows other researchers to test and validate these cut-offs. To have sufficient clinical relevance, in the general screening of acute medical patients admitted to the ED with or without SARS-CoV-2 test result, we propose that a suPAR cut-off level for identifying low-risk patients should have a NPV >95%, for example, for supporting the decision of discharge to own residence or transfer to other facilities. Although based on a small sample, we found that a suPAR cut-off of 4 ng/mL had a NPV of 99.0% for a 14-day mortality follow-up. In patients with a positive SARS-CoV-2 test result, our study suggests that suPAR, measured using a simple point-of-care device, may be useful for risk stratification according to the need of mechanical ventilation. A suPAR level <4 ng/mL had a NPV of 100% for the need for mechanical ventilation during the 14 days of follow-up among COVID-19 positive patients. However, transfer to facilities with isolation capacity but without the availability of mechanical ventilation should not only be based on a suPAR value alone, but also be carried out after thorough clinical examination and reviewing other relevant biomarkers such as CRP levels and NEWS, by ED physicians.1
In agreement with other studies, we found that suPAR ⩾6 ng/mL is associated with an elevated risk of need for mechanical ventilation or mortality.8,9 Currently, many potential treatments and drugs that may aid COVID-19 patients are in the pipeline or being tested. In trials that include patients at high risk of adverse outcomes, the possible benefit may outweigh the potential side effects. The first clinical trial to only include COVID-19 patients with suPAR >6 ng/mL has been carried out (suPAR-guided Anakinra Treatment for Validation of the Risk and Management of mechanical ventilation by COVID-19, ClinicalTrials.gov Identifier: NCT04357366).19 The study showed that the 14-day incidence of mechanical ventilation was 22.3% (95%CI 16.0-30.2) among suPAR-guided Anakinra-treated patients and 59.2% (95%CI 50.6-67.3; P 4.6 × 10−8) among patients with standard supportive treatment. A double-blinded randomized controlled trial, “suPAR-Guided Anakinra Treatment for Management of Severe Respiratory Failure by COVID-19 (SAVE-MORE),” is currently recruiting patients with suPAR above 6 ng/mL at 40 hospitals in Greece and Italy (ClinicalTrials.gov identifier: NCT04680949).19
Major differences between patients who tested positive or negative for SARS-CoV-2
We observed several differences with respect to clinical signs, biomarkers, and cell counts between patients who tested positive and negative for SARS-CoV-2, respectively. Patients who tested positive for SARS-CoV-2 were older, had lower blood saturation, and strikingly lower white cell counts as well as elevated suPAR, CRP, ALAT, and LDH levels, underpinning the severe impact of this viral infection on multiple parameters. These differences are worth noting as the SARS-CoV-2 testing may not always be immediately available, and testing may produce false negatives.20
Validation of suPAR cut-offs in COVID-19 patients from Mikkeli Central Hospital, Finland
To validate the proposed suPAR cut-offs, we reached out to our Finnish colleagues who measure suPAR as part of the routine biomarkers in acute medical patients seeking care in the Emergency Department. This independent validation confirmed that COVID-19 patients with suPAR ⩽4 ng/mL were in low risk of negative outcomes as none of the Finnish patients with suPAR below 4 ng/mL developed need of mechanical ventilation or died. In fact, the Finish COVID-19 patients that developed respiratory failure or died all had a baseline suPAR measurement of above 6 ng/mL. This confirms our proposed suPAR cut-offs and may aid in decision of triage and in decision taking by medical staff at the ED.
Perspectives of the findings
In this study, we show that patients tested positive for SARS-CoV-2 had elevated suPAR levels and that suPAR was associated with adverse outcomes. However, no specific suPAR cut-offs have previously been tested in patients with symptoms of COVID-19 or in patients with verified COVID-19. We suggest specific cut-offs for suPAR regarding risk of need for mechanical ventilation or mortality in COVID-19 patients by categorizing patients into 3 groups: suPAR ⩽4 ng/mL, suPAR 4 to 6 ng/mL, and suPAR ⩾6 ng/mL. These specific cut-offs would allow other researchers to test whether suPAR has relevance in their patient populations and could be used as a general screening tool in the ED.
Limitations
Most patients included in our study were of Caucasian ethnicity due to the homogeneous Danish and Finnish populations. Further studies for example, larger multi-center analyses of more ethnically diverse patient samples would be needed.
COVID-19 patients with suPAR levels between 4 and 6 ng/mL are in a gray area and our data does not provide any specific guidance for those. We suggest that COVID-19 patients with suPAR levels between 4 and 6 ng/mL whom are discharged should be informed to be aware of acute aggravation in symptoms and, to contact relevant health care services for re-evaluation by physicians at the hospital if needed, while in the meantime being aware of relevant rules concerning quarantine.
In conclusion, suPAR is a risk marker for patients presenting at the ED with symptoms of COVID-19, whether they get a positive or negative SARS-CoV-2 test result. Patients with baseline suPAR ⩾6 ng/mL were at increased risk of mortality during follow-up and patients with a positive SARS-CoV-2 test and suPAR >4 were at increased risk of developing need for mechanical ventilation compared to patients with suPAR <4 ng/mL. We suggest that patients with symptoms of COVID-19 and a suPAR level <4 ng/mL in need of hospital treatment can be transferred to facilities without ventilator access after a thorough clinical evaluation by the physician. In patients with symptoms of COVID-19, high admission suPAR level might be a potent indicator of imminent health risk, which has the potential to aid physicians to implement critical clinical decisions in a short time span.
We have suggested cut-offs for the use of suPAR and risk of adverse outcomes in acute medical patients with symptoms of COVID-19. Further studies are needed to validate the suPAR cut-offs when discharging or transferring patients with symptoms of COVID-19 to their own place of residence or to facilities without ventilator access.
Acknowledgments
We wish to thank all the patients involved in this study. We thank Marianne Falck for excellent technical assistance and Linda Camilla Andresen for effective coordination and organization.
Footnotes
Funding: The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: LJHR was supported by a postdoctoral fellowship through grant R288-2018-380 from the Lundbeck Foundation.
Declaration of Conflicting Interests: The author(s) declared the following potential conflicts of interest with respect to the research, authorship, and/or publication of this article: All authors have completed the ICMJE uniform disclosure form at www.icmje.org/coi_disclosure.pdf. JEO is a co-founder, shareholder, and CSO of ViroGates. JEO and OA are named inventors on patents on suPAR. The patents are owned by Copenhagen University Hospital Hvidovre, Denmark and licensed to ViroGates A/S. All other authors declare no financial relationships with any organization that might have an interest in the submitted work in the previous 3 years; no other relationships or activities that could appear to have influenced the submitted work.
Author Contributions: IA, JEO, JT, and OA have contributed to the conception of the study. IA, MS, HGJ, NOEC, SE, and JT have performed data collection. MBL, MS, and TK have performed data management. IA and TK have carried out the analyses and take responsibility for the integrity and accuracy of the data analysis. IA and JEO wrote the first draft. SS and HH provided data from the Mikkeli Central Hospital validation cohort. All authors contributed with interpretation of data, critical revision of the manuscript, and read and approved the final version. The corresponding author attests that all listed authors meet authorship criteria and that no others meeting the criteria have been omitted. IA is the guarantor of the study.
Ethical Approval: The study was approved by the Danish Data Protection Agency (record no. P-2020-513) and the Danish Patient Safety Authority (record no: 31-1521-319). There was no need of approval from the National Committee on Health Research Ethics since only registries were used.
Transparency Declaration: The lead authors (the manuscript’s guarantor) affirms that the manuscript is an honest, accurate, and transparent account of the study being reported; that no important aspects of the study have been omitted; and that any discrepancies from the study as planned (and, if relevant, registered) have been explained.
ORCID iDs: Izzet Altintas  https://orcid.org/0000-0001-6790-2218
https://orcid.org/0000-0001-6790-2218
Marius Ahm Stauning  https://orcid.org/0000-0002-5027-4435
https://orcid.org/0000-0002-5027-4435
Jan O Nehlin  https://orcid.org/0000-0001-6038-5027
https://orcid.org/0000-0001-6038-5027
References
- 1.Stauning MA, Altintas I, Kallemose T, et al. Soluble urokinase plasminogen activator receptor as a decision marker for early discharge of patients with COVID-19 symptoms in the emergency department. J Emerg Med. Published online 26 March 2021. doi: 10.1016/j.jemermed.2021.03.012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Rasmussen LJH, Ladelund S, Haupt TH, et al. Soluble urokinase plasminogen activator receptor (suPAR) in acute care: a strong marker of disease presence and severity, readmission and mortality. A retrospective cohort study. Emerg Med J. 2016;33:769-775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Rasmussen LJH, Ladelund S, Haupt TH, Ellekilde GE, Eugen-Olsen J, Andersen O. Combining national early warning score with soluble urokinase plasminogen activator receptor (suPAR) improves risk prediction in acute medical patients: a registry-based cohort study*. Crit Care Med. 2018;46:1961-1968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Oliveira I, Andersen A, Furtado A, et al. Assessment of simple risk markers for early mortality among HIV-infected patients in Guinea-Bissau: a cohort study. BMJ Open 2012;2:e001587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Berres M-L, Schlosser B, Berg T, Trautwein C, Wasmuth HE. Soluble urokinase plasminogen activator receptor is associated with progressive liver fibrosis in hepatitis C infection. J Clin Gastroenterol. 2012;46:334-338. [DOI] [PubMed] [Google Scholar]
- 6.Yilmaz G, Mentese A, Kaya S, Uzun A, Karahan SC, Koksal I. The diagnostic and prognostic significance of soluble urokinase plasminogen activator receptor in Crimean-Congo hemorrhagic fever. J Clin Virol. 2011;50:209-211. [DOI] [PubMed] [Google Scholar]
- 7.Outinen TK, Tervo L, Mäkelä S, et al. Plasma levels of soluble urokinase-type plasminogen activator receptor associate with the clinical severity of acute puumala hantavirus infection. PLoS One. 2013;8:e71335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Rovina N, Akinosoglou K, Eugen-Olsen J, Hayek S, Reiser J, Giamarellos-Bourboulis EJ. Soluble urokinase plasminogen activator receptor (suPAR) as an early predictor of severe respiratory failure in patients with COVID-19 pneumonia. Crit Care 2020;24:187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Azam TU, Shadid HR, Blakely P, et al. Soluble urokinase receptor (SuPAR) in COVID-19-related AKI. J Am Soc Nephrol. 2020;31:2725-2735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Çitlenbik H, Ulusoy E, Er A, et al. Levels of soluble urokinase plasminogen activator receptor in pediatric lower respiratory tract infections. Pediatr Allergy Immunol Pulmonol. 2019;32:121-127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Gumus A, Altintas N, Cinarka H, Kirbas A, Haziroglu M, Karatas M, Sahin U. Soluble urokinase-type plasminogen activator receptor is a novel biomarker predicting acute exacerbation in COPD. Int J Chron Obstruct Pulmon Dis. 2015;40:357-365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Håkansson KEJ, Ulrik CS, Godtfredsen NS, et al. High suPAR and low blood eosinophil count are risk factors for hospital readmission and mortality in patients with COPD. Int J Chron Obstruct Pulmon Dis. 2020;15:733-743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Godtfredsen NS, Jørgensen DV, Marsaa K, et al. Soluble urokinase plasminogen activator receptor predicts mortality in exacerbated COPD. Respir Res. 2018;19:97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Chen D, Wu X, Yang J, Yu L. Serum plasminogen activator urokinase receptor predicts elevated risk of acute respiratory distress syndrome in patients with sepsis and is positively associated with disease severity, inflammation and mortality. Exp Ther Med. 2019;18:2984-2992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Velissaris D, Dimopoulos G, Parissis J, et al. Prognostic role of soluble urokinase plasminogen activator receptor at the emergency department: a position paper by the Hellenic sepsis study group. Infect Dis Ther. 2020;9:407-416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Schultz M, Rasmussen LJ, Høi-Hansen T, et al. Early discharge from the emergency department based on soluble urokinase plasminogen activator receptor (suPAR) levels: a TRIAGE III substudy. Dis Markers. 2019;2019:3403549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hayek SS, Leaf DE, Samman Tahhan A, et al. Soluble urokinase receptor and acute kidney injury. N Engl J Med. 2020;382:416-426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Iversen E, Houlind MB, Eugen-Olsen J. Soluble urokinase receptor and acute kidney injury. N Engl J Med. 2020;382:2166-2168. [DOI] [PubMed] [Google Scholar]
- 19.Kyriazopoulou E, Panagopoulos P, Metallidis S, et al. An open label trial of anakinra to prevent respiratory failure in COVID-19. Elife. 2021;10:e66125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Bachelet VC. Do we know the diagnostic properties of the tests used in COVID-19? A rapid review of recently published literature. Medwave. 2020;20:e7891-e7891. [DOI] [PubMed] [Google Scholar]