Abstract
Background:
Skin prick testing (SPT) is an important investigation in the evaluation of allergy to fungal pathogens. However, the background sensitivity to fungal allergens among healthy people in Uganda is unknown. Our aim was to assess the background prevalence of Aspergillus fumigatus SPT positivity in apparently healthy adults without known atopic disease in Uganda.
Methods:
For this pilot study, we recruited 50 healthy volunteers using convenience sampling, 56% of whom were health workers. We performed the SPT for A. fumigatus according to manufacturer’s instructions. A wheal diameter of ⩾3 mm was considered positive.
Results:
The prevalence of A. fumigatus skin positivity was 60% (30/50). Participants with a positive A. fumigatus SPT were significantly younger than those with a negative result [median age (years): 28 versus 35; p = 0.005].
Conclusion:
There is a high skin positivity against A. fumigatus among non-atopic healthy Ugandan adults. There is an urgent need to establish a normal wheal cut-off value for this population. SPT alone may be an unreliable test for the diagnosis of A. fumigatus associated allergic syndromes. More studies are needed to define the prevalence of A. fumigatus skin positivity among non-atopic healthy population in Africa.
Keywords: Aspergillus sensitisation, atopy, fungal allergy, skin prick testing, Uganda
Introduction
Fungal allergy in the context of asthma,1 non-cystic fibrosis bronchiectasis,2 tuberculosis,3 chronic obstructive pulmonary disease (COPD),4 or fungal rhinosinusitis is one of the most common health problems globally.5 However, the pathogenic significance of fungi from the genera Alternaria, Cladosporium, Penicillium and Aspergillus is poorly described in Africa.6 The burden of serious fungal diseases in Uganda is high, with an estimated 2.5 million cases per year,7 yet the index of clinical suspicion remains low,8 despite recent evidence showing that Aspergillus species are significant causes of morbidity in different at-risk populations in Uganda.3,6–10
The lungs are the primary site of infection by Aspergillus species, causing disorders including allergic, chronic, sub-acute and invasive pulmonary aspergillosis,11 with Aspergillus fumigatus the most commonly implicated species.12 Human allergic disorders associated with A. fumigatus include allergic asthma, severe asthma with fungal sensitisation (SAFS), allergic rhinosinusitis, allergic bronchopulmonary aspergillosis (ABPA), and hypersensitivity pneumonitis.13 In addition, A. fumigatus is isolated frequently from the respiratory tract of patients with asthma who do not meet the criteria for ABPA or SAFS, and occasionally in the respiratory tract of healthy individuals.14 Diagnosis of Aspergillus sensitization requires demonstration of evidence of allergic sensitization to Aspergillus either by skin prick testing (SPT) or Aspergillus-specific IgE immunoassays.1
There is a paucity of data on the frequency of SPT reactivity against A. fumigatus in healthy adults without known atopic disease. We therefore aimed to describe the distribution of A. fumigatus SPT positivity among healthy adults without known atopic disease in Uganda, which will, in turn, generate hypotheses to encourage further research in this field of fungal allergy in Uganda.
Methods
This was a cross-sectional study evaluating the frequency of A. fumigatus skin prick positivity among healthy adults without known atopic disease in Uganda. It was carried out between March and October 2019. This study was nested within the African Severe Asthma Program (ASAP) clinical study [ClinicalTrials.gov identifier: NCT03065920] at the Makerere University Lung Institute.15 ASAP is a clinical study with the primary objective being to identify and characterize severe asthma in Uganda, Kenya, and Ethiopia. Participants provided written informed consent to participate in this study. Ethics approval for this sub-study was obtained from the School of Biomedical Sciences Research and Ethics Committee (SBS 598), the Uganda National Council for Science and Technology (HS 2532), and the Uganda National Drug Authority (9464). All participants in the current study were healthy adults (⩾18 years), without known atopic disease or respiratory conditions, and not taking steroids or oral antihistamines for any reason in the last 7 days. Since there is limited literature on the prevalence of Aspergillus skin positivity among healthy population, to understand prevalence of Aspergillus sensitisation in Ugandan healthy population, we consented and tested healthy volunteers. We used healthy individuals from the general population, including medical workers, medical students, and support staff at Makerere University and Kiruddu National Referral Hospital. A convenience sampling method was employed for this pilot study.
A. fumigatus SPT [Immunospec (Pty) Ltd, Johannesburg, Gauteng, South Africa] was performed and the results interpreted according to international guidelines.16 Normal saline served as a negative control while histamine was the positive control, with a mean wheal diameter of at least 3 mm being considered positive, after 15 min of allergen application. We did not perform testing for total serum or A. fumigatus-specific IgE.
Data were analyzed using STATA® version 16 (STATA, College Station, TX, USA). Primary data analysis aimed to describe the distribution of A. fumigatus skin prick positivity at a 95% confidence interval (CI).
Results
Between March and October 2019, we enrolled 50 eligible participants, of whom 28 (56%) were female; the median age for all participants was 30 years [interquartile range (IQR) = 27–35). A total of 28 (56%) of the participants were health workers (Table 1).
Table 1.
Baseline characteristics of the study population.
| Demographics | Frequency (%) |
|---|---|
| Age, median (IQR) | 30 (27–35) |
| Female, n (%) | 28 (56) |
| Occupation, n (%) | |
| Health worker | 28 (56) |
| Volunteer | 5 (10) |
| Counsellor | 4 (8) |
| Records officer | 4 (8) |
| Student | 4 (8) |
| Information technology specialist | 3 (6) |
| Self employed | 1 (2) |
| Project administrator | 1 (2) |
IQR, interquartile range.
The prevalence of A. fumigatus skin positivity was 60% (30/50) (95% CI = 45.6–72.8). There was a significant difference in age between participants who were positive for A. fumigatus skin test and those who were negative (p = 0.005) (Figure 1). There was no significant difference in A. fumigatus reactivity by gender (p = 0.907) or occupation (p = 0.612). None of the participants reacted to the negative control. Only one participant had a wheal diameter of 2 mm and the rest had zero against the negative control. Figure 2 is a histogram of wheal sizes for A. fumigatus skin prick test for this population.
Figure 1.
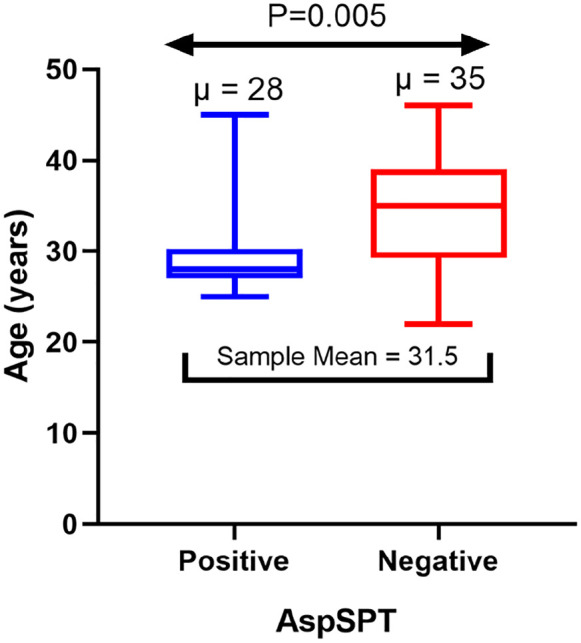
Distribution of age by Aspergillus fumigatus skin positivity.
AspSPT, A. fumigatus skin prick test.
Figure 2.
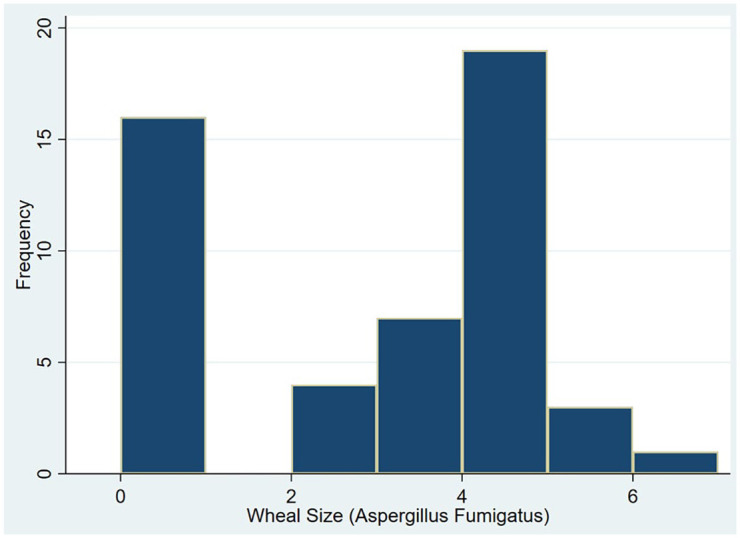
Distribution of wheal size for Aspergillus fumigatus. Wheal size (µ) is measured in millimeters.
Discussion
In the present study, we report a high prevalence (60%) of A. fumigatus skin positivity among healthy adults without known atopic disease in Uganda. This could indicate the fact that apparently healthy people have an undiagnosed fungal atopy. Alternatively, this could also indicate that A. fumigatus is the commonest fungal allergen in Uganda, similar to what is reported in the developed countries. However, a study from India showed a 2% prevalence of A. fumigatus skin positivity among healthy controls.17 In addition, our previous work among adult asthmatics in Uganda showed a lower prevalence (48%) of A. fumigatus skin reactivity than the 60% observed in this study despite the atopic nature of asthmatics.10 We cannot rule out the possibility of cross-reactivity to A. fumigatus crude extracts, which often do not indicate genuine sensitization.13,18 Besides, products secreted after conidial germination into hyphae can be differentially recognized by protective T-cells in healthy non-atopic individuals.19 Therefore, SPT should not be used alone to diagnose A. fumigatus sensitivity in Uganda since the background prevalence in healthy populations is apparently very high based on the results of this pilot study. However, we recommend that larger studies be carried out to confirm these data. The in vitro A. fumigatus-specific IgE may be more informative to reflect the actual burden. Those with skin positivity were significantly younger. However, the reason for this observation is unclear and not really clinically relevant.
Our study is limited by its very small sample size and sampling frame, which limits its generalization to the general population. The optimal cut-off wheal size to define A. fumigatus in our population is unknown. This could have led to an over-estimation of the prevalence of A. fumigatus SPT reactivity in this population. One uncertainty is whether the 3 mm cut-off is appropriate for Uganda; a 5 mm cut-off would reduce the prevalence of A. fumigatus sensitisation to 8%, which is more reasonable. Therefore, we propose to define a suitable cut-off wheal size in healthy adults. However, our estimate provides baseline information to encourage further research in the field of fungal allergy in Africa. More studies are needed to define the prevalence of A. fumigatus skin positivity among non-atopic healthy population in Africa. There is an urgent need to establish a normal wheal cut-off value for this population.
Conclusion
In conclusion, we found a high prevalence of A. fumigatus skin positivity in apparently healthy non-atopic individuals in Uganda. Those with skin positivity were significantly younger. Given a very high background A. fumigatus skin positivity rate in our setting, SPT alone may be an unreliable test for the diagnosis of A. fumigatus-associated allergic syndromes. We propose to define a suitable cut-off wheal size in healthy adults.
Supplemental Material
Supplemental material, sj-xlsx-1-tai-10.1177_20499361211039040 for Prevalence of Aspergillus fumigatus skin positivity in adults without an apparent/known atopic disease in Uganda by Richard Kwizera, Felix Bongomin, Ronald Olum, William Worodria, Freddie Bwanga, David B. Meya, Bruce J. Kirenga, Robin Gore, Stephen J. Fowler and David W. Denning in Therapeutic Advances in Infectious Disease
Acknowledgments
We would like to acknowledge all our participants. We thank the IDI research department for providing the PhD student with office space, internet access, printing services, research peer support, health insurance, and salary support during the period of study. David Denning and Stephen Fowler receive funding from the Manchester NIHR Biomedical Research Centre.
Footnotes
Author contributions: RK conceived the study. RK designed concept/protocol. RK, WW, DWD, and BJK participated in getting project funding. RK collected data. RK and RO reviewed, curated and analysed data. RK participated in initial manuscript drafting. RK, FB, RO, DBM, WW, FB, SJF, DWD, RG, and BJK participated in critical revisions for intellectual content. BJK participated in project administration. DBM, WW, FB, SJF, and RG participated in supervision.
Availability of data and materials: All data are available in the manuscript and supporting information.
Conflict of interest statement: The authors declare that there is no conflict of interest.
Funding: The authors disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: This work was supported through the DELTAS Africa Initiative grant # DEL-15-011 to THRiVE-2. The DELTAS Africa Initiative is an independent funding scheme of the African Academy of Sciences (AAS)’s Alliance for Accelerating Excellence in Science in Africa (AESA) and supported by the New Partnership for Africa’s Development Planning and Coordinating Agency (NEPAD Agency) with funding from the Wellcome Trust grant # 107742/Z/15/Z and the UK government. ASAP [ClinicalTrials.gov identifier: NCT03065920] under the Makerere University Lung Institute provided SPT testing kits to the student. ASAP was funded by the GlaxoSmithKline (GSK) African Non-Communicable Disease (NCD) Open Lab (Project number: 8019) to Bruce Kirenga. Funders had no role in data collection, analysis, or decision to publish. Authors retained control of the final content of the publication.
ORCID iDs: Richard Kwizera  https://orcid.org/0000-0002-5270-3539
https://orcid.org/0000-0002-5270-3539
Felix Bongomin  https://orcid.org/0000-0003-4515-8517
https://orcid.org/0000-0003-4515-8517
Ronald Olum  https://orcid.org/0000-0003-1289-0111
https://orcid.org/0000-0003-1289-0111
Supplemental material: Supplemental material for this article is available online.
Contributor Information
Richard Kwizera, Department of Research, Infectious Diseases Institute, College of Health Sciences, Makerere University, P.O. BOX 22418, Kampala, Central, Uganda,Makerere University Lung Institute, College of Health Sciences, Makerere University, Kampala, Uganda.
Felix Bongomin, Department of Medical Microbiology, Faculty of Medicine, Gulu University, Gulu, Uganda, Department of Medicine, School of Medicine, College of Health Sciences, Makerere University, Kampala, Uganda.
Ronald Olum, Department of Medicine, School of Medicine, College of Health Sciences, Makerere University, Kampala, Uganda.
William Worodria, Department of Medicine, School of Medicine, College of Health Sciences, Makerere University, Kampala, Uganda, Division of Pulmonology, Mulago National Referral Hospital, Kampala, Uganda.
Freddie Bwanga, Department of Medical Microbiology, School of Biomedical Sciences, College of Health Sciences, Makerere University Kampala, Uganda.
David B. Meya, Infectious Diseases Institute, College of Health Sciences, Makerere University, Kampala, Uganda, Department of Medicine, School of Medicine, College of Health Sciences, Makerere University, Kampala, Uganda
Bruce J. Kirenga, Makerere University Lung Institute, College of Health Sciences, Makerere University, Kampala, Uganda, Department of Medicine, School of Medicine, College of Health Sciences, Makerere University, Kampala, Uganda, Division of Pulmonology, Mulago National Referral Hospital, Kampala, Uganda
Robin Gore, Cambridge University Hospitals NHS Foundation Trust, Cambridge, Cambridgeshire, UK.
Stephen J. Fowler, Division of Infection, Immunity and Respiratory Medicine, School of Biological Sciences, Faculty of Biology, Medicine and Health, The University of Manchester, NIHR Biomedical Research Centre, Manchester University Hospitals NHS Foundation Trust, UK
David W. Denning, Division of Infection, Immunity and Respiratory Medicine, School of Biological Sciences, Faculty of Biology, Medicine and Health, The University of Manchester, NIHR Biomedical Research Centre, Manchester University Hospitals NHS Foundation Trust, UK
References
- 1.Denning DW, Pashley C, Hartl D, et al. Fungal allergy in asthma–state of the art and research needs. Clin Transl Allergy 2014; 4: 14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Moss RB.Fungi in cystic fibrosis and non–cystic fibrosis bronchiectasis. Semin Respir Crit Care Med 2015; 36: 207–216. [DOI] [PubMed] [Google Scholar]
- 3.Kwizera R, Parkes-Ratanshi R, Page ID, et al. Elevated Aspergillus-specific antibody levels among HIV infected Ugandans with pulmonary tuberculosis. BMC Pulm Med 2017; 17: 149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Tiew PY, Ko FWS, Pang SL, et al. Environmental fungal sensitisation associates with poorer clinical outcomes in COPD. Eur Respir J 2020; 56: 2000418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ferguson BJ, Barnes L, Bernstein JM, et al. Geographic variation in allergic fungal rhinosinusitis. Otolaryngol Clin North Am 2000; 33: 441–449. [DOI] [PubMed] [Google Scholar]
- 6.Kwizera R, Musaazi J, Meya DB, et al. Burden of fungal asthma in Africa: a systematic review and meta-analysis. PLoS One 2019; 14: e0216568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Parkes-Ratanshi R, Achan B, Kwizera R, et al. Cryptococcal disease and the burden of other fungal diseases in Uganda; where are the knowledge gaps and how can we fill them? Mycoses 2015; 58(Suppl. 5): 85–93. [DOI] [PubMed] [Google Scholar]
- 8.Kwizera R, Bongomin F, Lukande R.Deep fungal infections diagnosed by histology in Uganda: a 70-year retrospective study. Med Mycol 2020; 58: 1044–1052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kwizera R, Katende A, Teu A, et al. Algorithm-aided diagnosis of chronic pulmonary aspergillosis in low- and middle-income countries by use of a lateral flow device. Eur J Clin Microbiol Infect Dis 2020; 39: 1–3. [DOI] [PubMed] [Google Scholar]
- 10.Kwizera R, Wadda V, Mugenyi L, et al. Skin prick reactivity among asthmatics in East Africa. World Allergy Organ J 2020; 13: 100130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kousha M, Tadi R, Soubani AO.Pulmonary aspergillosis: a clinical review. Eur Respir Rev 2011; 20: 156–174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Garnacho-Montero J, Olaechea P, Alvarez-Lerma F, et al. Epidemiology, diagnosis and treatment of fungal respiratory infections in the critically ill patient. Revista Espanola De Quimioterapia 2013; 26: 173–188. [PubMed] [Google Scholar]
- 13.Fukutomi Y, Taniguchi M.Sensitization to fungal allergens: resolved and unresolved issues. Allergol Int 2015; 64: 321–331. [DOI] [PubMed] [Google Scholar]
- 14.Lass-Florl C, Salzer GM, Schmid T, et al. Pulmonary Aspergillus colonization in humans and its impact on management of critically ill patients. Br J Haematol 1999; 104: 745–747. [DOI] [PubMed] [Google Scholar]
- 15.Kirenga B, Chakaya J, Yimer G, et al. Phenotypic characteristics and asthma severity in an East African cohort of adults and adolescents with asthma: findings from the African severe asthma project. BMJ Open Respir Res 2020; 7: e000484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Dreborg S.The skin prick test in the diagnosis of atopic allergy. J Am Acad Dermatol 1989; 21: 820–821. [DOI] [PubMed] [Google Scholar]
- 17.Dhooria S, Kumar P, Saikia B, et al. Prevalence of Aspergillus sensitisation in pulmonary tuberculosis-related fibrocavitary disease. Int J Tuberc Lung Dis 2014; 18: 850–855. [DOI] [PubMed] [Google Scholar]
- 18.Mari A, Schneider P, Wally V, et al. Sensitization to fungi: epidemiology, comparative skin tests, and IgE reactivity of fungal extracts. Clin Exp Allergy 2003; 33: 1429–1438. [DOI] [PubMed] [Google Scholar]
- 19.Chaudhary N, Staab JF, Marr KA.Healthy human T-Cell responses to Aspergillus fumigatus antigens. PLoS One 2010; 5: e9036. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental material, sj-xlsx-1-tai-10.1177_20499361211039040 for Prevalence of Aspergillus fumigatus skin positivity in adults without an apparent/known atopic disease in Uganda by Richard Kwizera, Felix Bongomin, Ronald Olum, William Worodria, Freddie Bwanga, David B. Meya, Bruce J. Kirenga, Robin Gore, Stephen J. Fowler and David W. Denning in Therapeutic Advances in Infectious Disease


