Abstract
Vascular endothelial growth factor (VEGF) serves an important role in new blood vessel formation or angiogenesis, which is a critical event in tumor growth and metastasis. Bevacizumab is a humanized monoclonal antibody against VEGF-A, whereas S-1 is a fluoropyrimidine antineoplastic agent that induces apoptosis in various types of cancer cells. The present study evaluated the antitumor effects of bevacizumab in combination with 5-fluorouracil (5-FU) or S-1 against oral squamous cell carcinoma (OSCC) in vitro and in vivo. Two human OSCC cell lines were used, namely the high VEGF-A-expressing HSC-2 cells and the low VEGF-A-expressing SAS cells. MTT assay was used to evaluate the effect of bevacizumab and/or 5-FU against HSC-2 and SAS cell proliferation. Additionally, the antitumor effect of bevacizumab was evaluated alone and in combination with S-1 against HSC-2 tumors in nude mice. S-1 (6.9 mg/kg/day) was administered orally every day for 3 weeks, and bevacizumab (5 ml/kg/day) was injected intraperitoneally twice per week for 3 weeks. Apoptotic cells in mouse tumors were detected using the TUNEL method, and cell proliferation and microvessel density (MVD) were determined by immunohistochemical staining of Ki-67 and CD31, respectively. Bevacizumab alone did not inhibit OSCC cell proliferation in vitro, and did not exhibit any synergistic inhibitory effect in combination with 5-FU in vitro. However, combined bevacizumab and S-1 therapy exerted synergistic and significant antitumor effects in vivo on HSC-2 tumor xenografts, and induced apoptosis in tumor cells. Furthermore, this combination therapy led to decreased MVD and cell proliferative abilities, as well as increased apoptosis in residual tumors. The present findings suggested that the bevacizumab plus S-1 combination therapy may exert antitumor effects in high VEGF-A-expressing OSCC cells.
Keywords: vascular endothelial growth factor-A, bevacizumab, S-1, oral squamous cell carcinoma, combination therapy
Introduction
Recent advances in multidisciplinary treatments and reconstructive surgery have improved the overall survival (OS) of patients with oral cancer (1,2). The worldwide incidence rate of oral cancer is 300,400 cases annually and the 5-year OS rate is 50–60% (1,2). Nevertheless, the complexities of the process of tumor progression and tumor recurrence in inoperable cases often cause huge obstacles in cancer treatment (3). Tegafur, a component of the oral antitumor drug S-1, is the prodrug of 5-fluorouracil (5-FU) that inhibits DNA synthesis and causes RNA dysfunction in cells, thereby exerting strong antitumor effects against solid cancers of the head and neck region (4–7). S-1 with tegafur/uracil exhibited significantly improved treatment outcome and improved the 3-year survival rate in patients with head and neck squamous cell carcinoma (HNSCC) who underwent curative treatment compared with the patients who did not have S1 treatment (8). Although S-1 is associated with side effects, including severe leukopenia, neutropenia, nausea, vomiting and diarrhea, the frequency and degree of side effects are lower than those reported for other chemotherapeutic agents, such as docetaxel, taxol and cisplatin (4–11).
Angiogenesis has been identified as an important determinant of solid tumor growth and metastasis. Several regulatory factors are involved in tumor angiogenesis, among which vascular endothelial growth factor (VEGF)-A serves a particularly important role (12–15). In a clinical trial of HNSCC, strong VEGF-A expression was negatively associated with OS and disease-free survival (16–19). Similarly, high VEGF-A expression was significantly associated with cancer progression and a poor prognosis (based on T classification, N classification, grade, clinical stage and cumulative survival) among patients with oral squamous cell carcinoma (OSCC) (20,21). VEGF-A-targeting monoclonal antibody therapies have yielded successful results in various types of cancer including colorectal cancer and head and neck cancer (10–12,22–24).
Bevacizumab is a molecularly targeted drug with different properties compared with conventional chemotherapeutic agents (25). It directly binds to VEGF-A around the tumor and inhibits tumor angiogenesis (12). Moreover, bevacizumab exerts an antitumor effect by inhibiting the nutrient supply to tumor cells (13). Additionally, bevacizumab normalizes residual blood vessels and improves the drug delivery system into the tumor so that drugs reach the tumor at a higher concentration (10,11,22–24). In addition, the side effects of bevacizumab, which is administered by intravenous injection, differ from those of conventional chemotherapeutic agents (26,27). Common side effects of bevacizumab include bleeding, delayed wound healing, hypertension and proteinuria, which are rarely observed with conventional chemotherapy (27,28). Therefore, bevacizumab can be used in combination with conventional antitumor drugs with low side effects (10,11,22–24). A number of clinical trials with patients with recurrent and metastatic HNSCC and OSCC revealed that bevacizumab in combination with other molecular-targeted therapies, chemotherapies and/or radiation therapy led to improved OS and progression-free survival in numerous types of cancer, including HNSCC (10,11,22–24,29). Previous studies reported that bevacizumab in combination with S-1 yielded modest to high efficacy against a number of types of cancer, such as advanced recurrent or metastatic colorectal cancer, advanced gastric cancer, advanced lung cancer, advanced esophageal carcinoma with mild and acceptable toxicities, especially as a second- or third-line therapy (12,23,30–33). However, to the best of our knowledge, there are no available reports on the efficacy of bevacizumab plus S-1 treatment in HNSCC or OSCC.
In the present study, it was hypothesized that bevacizumab in combination with S-1 may exert stronger antitumor effect against OSCC cell lines compared with bevacizumab or S-1 alone. In addition, the possibility of fewer side effects of S-1 plus bevacizumab treatment in vivo compared with conventional chemotherapeutic agents was investigated.
Materials and methods
Cell lines, cell culture and reagents
The VEGF-A-expressing human oral or tongue cancer HSC-2, HSC-3, HSC-4 and SAS cell lines were obtained from the RIKEN BioResource Centre and maintained as monolayer cultures in DMEM/Ham's F12 with L-glutamine and phenol red medium (FUJIFILM Wako Pure Chemical Corporation) containing 10% FBS (Thermo Fisher Scientific, Inc.), 100 µg/ml streptomycin and 100 U/ml penicillin (Thermo Fisher Scientific, Inc.) at 37°C in a humidified incubator with 5% CO2.
FBS was not added to the cell culture medium subjected to western blotting, MTT assays and ELISA in order to avoid non-specific reactions with VEGF-A present in the FBS. S-1 was obtained from Taiho Pharmaceutical Co., Ltd., while bevacizumab was purchased from Chugai Pharmaceutical Co., Ltd.
Animals
A total of 12 female athymic BALB/c nu/nu nude mice (4-week-old) with an average body weight of 20 g were obtained from Japan SLC, Inc., and were kept under sterile conditions in a pathogen-free and temperature-controlled (average temperature, 22.1°C) environment with a 12 h light/dark cycle. The mice received sterilized water and food ad libitum. All manipulations were conducted aseptically inside a laminar flow hood. All in vivo experiments were approved by the Institutional Animal Care and Use Committee of Yamaguchi University (Ube, Japan) (approval no. 55-010).
Western blotting
HSC-2, HSC-3, HSC-4 and SAS cell lines were grown to 80% confluency in a 100-mm cell culture dish (BD Biosciences). After adding fresh FBS-free media, cells were incubated at 37°C for 24 and 48 h. The cells were washed, collected by scraping and lysed in 100 ml lysis buffer (1X formulation comprising 25 mM Tris-HCl pH 7.6, 150 mM NaCl, 1% NP-40, 1% sodium deoxycholate and 0.1% SDS). Protein concentrations in the whole cell lysates were determined using NanoDrop™ 1000 (Thermo Fisher Scientific, Inc.). A total of 80 µg protein/lane was loaded into NuPAGE™ 4–12% Bis-Tris gel (cat. no. NP0322; Thermo Fisher Scientific, Inc.) and was subjected to SDS-PAGE and then transferred onto PVDF membranes. A blocking solution was made from Blocker/Diluent part A (cat. no. 46-7003; Thermo Fisher Scientific, Inc.) and B (cat. no. 46-7004; Thermo Fisher Scientific, Inc.) according to the manufacturer's protocol, and the PVDF membranes were incubated in the blocking solution at room temperature for 30 min. Membranes were treated with VEGF-A (A-20 rabbit polyclonal; cat. no. SC-15;1:250; Santa Cruz Biotechnology, Inc.) (or α-tubulin (loading control; B-7 mouse monoclonal; cat. no. SC-5286; 1:1,000; Santa Cruz Biotechnology, Inc.) primary antibody at 4°C overnight. The membranes were washed using 1X wash solution (cat. no. 46-7005; Thermo Fisher Scientific, Inc.) 3 times at room temperature (5 min/wash) according to manufacturer's instruction, and then was incubated with either secondary antibody solution Alk-Phos. conjugated anti-rabbit (cat. no. 46-7006; neat; Thermo Fisher Scientific, Inc.) or Alk-Phos. conjugated anti-mouse (cat. no. 46-7006; neat; Thermo Fisher Scientific Inc.) at room temperature for 30 min. The membranes were washed 3 times with 1X wash solution at room temperature (5 min/wash) and protein bands were detected by incubating the membranes into Novex® AP Chromogenic substrate (BCIP/NBT) (cat. no. 100002902; Thermo Fisher Scientific, Inc.) at room temperature for 5–15 min. Quantification of protein bands was performed using ImageJ v1.51h software (National Institutes of Health). The fold-change of VEGF-A expression was calculated relative to the control (α-tubulin) and expressed as a percentage.
MTT assay
SAS and HSC-2 cells were seeded into a 96-well plate (BD Biosciences) at a density of 5×103 cells/well and incubated at 37°C for 24 h. Subsequently, cells were treated (at 37°C for 24 or 48 h) as follows: Control (serum-free medium without drugs), bevacizumab alone (1, 10 or 100 µg/ml)or 5-FU alone (0.5, 1 or 2 µg/ml) dissolved in serum-free medium. Cells were also treated with bevacizumab (1, 10 or 100 µg/ml) plus 5-FU (05, 1 or 2 µg/ml) dissolved in serum-free medium at 37°C for 48 h. Subsequently, MTT solution (5mg/ml) was added to each well (25 µl/well) and incubated for 4 h at 37°C. The purple formazan was dissolved in dimethyl sulfoxide (100 µl/well) and the absorbance was measured using a spectrophotometer (Bio-Rad Laboratories, Inc.) at a wavelength of 490 nm. All assays were performed in triplicate.
ELISA
SAS and HSC-2 cells were seeded into a 96-well plate (BD Biosciences) at a density of 5×103 cells/well, and wells were treated with one of the following treatments: Control (serum-free medium without drugs), bevacizumab alone (10 µg/ml), 5-FU alone (1 µg/ml) or bevacizumab (10 µg/ml) plus 5-FU (1 µg/ml). All cells were cultured at 37°C for 24 and 48 h, then VEGF-A concentrations in the supernatant from each well were measured via ELISA Human VEGF Quantikine ELISA kit (cat. no. DVE00; R&D Systems, Inc.) according to manufacturer's instructions.
Human cancer xenograft models
HSC-2 cells were used as a xenograft model in BALB/c nu/nu nude mice (n=12) as they have relatively higher tumorigenic potential than SAS cells. HSC-2 cell line batches were frozen in FBS supplemented with 5% dimethyl sulfoxide (v/v) prior to injection into mice, and then were thawed shortly before injection. In brief, HSC-2 cells (1×106/mice) were suspended into 0.1 ml PBS (0.05 M phosphate buffer containing 0.145 M sodium chloride, pH 7.4) and injected into the subcutaneous tissues of aforementioned nude mice with a 27-gauge needle when they were 5-weeks old. Tumors were allowed to grow for 10 days before treatment. These tumor-bearing mice were divided into four groups (n=3; tumor volumes, 40–50 mm3). The humane endpoints of this in vivo experiment were: Rapid weight loss in treated mice (≥20% body weight loss compared with the control group), very large tumor (tumor weight ≥10% of body weight), skin/tumor ulceration or necrosis, difficulty in ambulation and feeding or drinking disorder.
In vivo treatment protocol
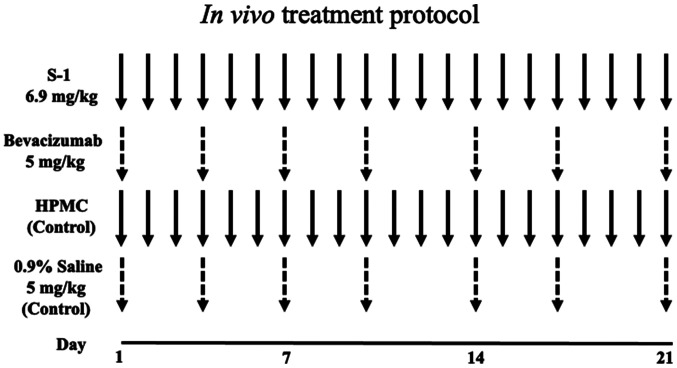
Fig. 1 shows the in vivo treatment protocol. The four treatment groups were: S-1, bevacizumab, combination therapy and control groups. S-1 was suspended in autoclaved 0.5% sodium hydroxypropyl methylcellulose (HPMC; Daiichi Kogyo Seiyaku Co., Ltd.) under sterile conditions to a concentration of 1.0 mg/ml and homogenized by stirring. The S-1 suspension was administered to mice via gastric lavage at a dosage of 6.9 mg/kg/day for 3 weeks (S-1 group). Bevacizumab was injected intraperitoneally (i.p) at a dosage of 5 mg/kg twice/week (on day 1 and 4 of each week) for 3 weeks (bevacizumab group). The combination group received both S-1 (6.9 mg/kg/day for 21 days) and bevacizumab 5 mg/kg twice/week (on day 1 and 4 of each week) for 3 weeks. The control mice group received either 5% HPMC (equal volume of S-1) via gastric lavage or 0.9% saline (i.p) at a dosage of 5 ml/kg twice/week for 3 weeks.
Figure 1.
In vivo treatment protocol. A total of 1×106 HSC-2 cells were injected into the subcutaneous tissues of 5-week-old nude mice. After 10 days of tumor growth (volume, 40–50 mm3), the mice were divided into four groups (n=3). The S-1 group received S-1 via gastric lavage (6.9 mg/kg/day) for 3 weeks, the bevacizumab group was injected with bevacizumab intraperitoneally (i.p; 5 mg/kg twice/week, on day 1 and 4 of each week) for 3 weeks, and the combination group received both S-1 (6.9 mg/kg/day) for 3 weeks and bevacizumab (5 mg/kg twice/week, on day 1 and 4 of each week) for 3 weeks. The control mice group received either 0.5% HPMC via gastric lavage or 0.9% saline (5 ml/kg twice/week) for 3 weeks. HPMC, hydroxypropyl methylcellulose.
Tumor measurements
HSC-2 ×enograft tumors were inspected twice/week and measured using a Vernier caliper. Tumor volume (mm3) was calculated using the standard formula a2 × b/2, where ‘a’ is the width and ‘b’ the length of the horizontal tumor. Body weights were also measured every 2 days. On day 21, 12 h after the final drug dosage, the experiment was terminated based on ethical considerations, and the mice were sacrificed using an overdose of Somnopentyl (sodium pentobarbital, 200 mg/kg; Merck & Co., Inc.). The tumors were dissected, fixed in 10% neutral-buffered formalin at room temperature for 24 h (Mildform® 10N; FUJIFILM Wako Pure Chemical Corporation) and embedded in paraffin for further study. The relative tumor volume was calculated using the following formula: Tumor volume of the respective tumor on day n/volume of the same tumor on day 0 (before the treatment started).
Immunohistochemistry
Avidin-biotin complex-based immunohistochemical methods were performed to detect CD31 and Ki-67 in tissue specimens using the EnVision kit™ (Dako; Agilent Technologies, Inc.). Paraffin-embedded tissue sections (4-µm-thick) were deparaffinized in xylene and rehydrated through a descending alcohol series at room temperature. Deparaffinized sections were immersed in absolute methanol containing 0.3% hydrogen peroxide for 20 min at room temperature to block endogenous peroxidases. The sections were then treated with Dako REAL™ peroxidase-blocking solution (cat. no. S2023 Dako; Agilent Technologies, Inc.,) for 30 min at room temperature to block non-specific reactions and were incubated overnight at 4°C with goat anti-mouse CD31 or PECAM-1 (cat. no. SC-376764; 1:100; Santa Cruz Biotechnology, Inc.) and mouse anti-human Ki-67 (clone MIB-1; cat. no. M7240; 1:100; Dako; Agilent Technologies, Inc.). After rinsing the tissue sections in PBS thrice for 5 min each, 100 µl secondary antibody (Dako REAL™ Envision™ detection system; horse radish peroxidase; rabbit/Mouse; cat. no. K5007; no dilution; Dako; Agilent Technologies, Inc.) was added at room temperature for 30 min. Tissue sections were rinsed thrice in PBS for 5 min each, and incubated with an chromogen 3,3′-diaminobenzidine tetrahydrochloride (DAB) solution for 7 min at room temperature using Dako REAL™ Envision™ detection system (cat. no. K5007; Dako; Agilent Technologies, Inc.). Tissue sections were then counterstained by hematoxylin at room temperature for 1 min. Tissue sections were subsequently dehydrated in graded ethanol, cleared using Histo-Clear (cat. no. HS200; National Diagnostics) and mounted with glass coverslips using D.P.X. (Millipore Sigma). Each experiment included positive controls (for Ki-67: human tongue squamous cell carnimona tissue; for CD31: human healthy tongue tissues) and negative controls (same tissue samples without primary antibody).
Evaluation of microvessel density (MVD)
Immunohistochemical techniques were used to evaluate MVD, and vascularity was determined as described previously (34,35). Unlike immunohistochemical analysis, tumor vessels were stained with the aforementioned anti-mouse CD31 antibody for 90 min at room temperature. Paraffin sections were pre-treated with 0.01% protease at 37°C for 20 min. The staining protocol was otherwise similar to that used for immunohistochemistry, as aforementioned. The number of vessels per field was counted in the area of highest vascular density. Vessel density was recorded as the number of CD31+ vessel points per field under ×200 magnification using a fluorescence microscope (BX51; Olympus Corporation). Vascularity was quantified in the stroma close to the epithelium, up to ~750 µm from the basal lamina, and microvessels in the tumors were counted. A total of 10 randomly selected fields from non-necrotic tumor tissue were examined per section, and the results are expressed as the mean percentage ± SD as previously described (36).
Evaluation of cell proliferation
Immunohistochemical techniques were also used to evaluate the cell proliferative ability. Tumor cells were stained with mouse anti-human Ki-67 by immunohistochemistry as aforementioned, and Ki-67+ nuclei were counted under ×200 magnification from a total of 1,000 cells to determine the distribution of cell proliferation using a fluorescence microscope (BX51; Olympus Corporation) as previously described (37).
Evaluation of apoptotic index
Tissue sections were subjected to TUNEL assay to examine apoptosis using the DeadEnd™ Fluorometric TUNEL system (cat. no. G3250; Promega, Corporation) according to manufacturer's instructions. Paraffin-embedded tumor sections (4-µm-thick) were deparaffinized in xylene, rehydrated using a graded ethanol series and incubated with 20 µg/ml proteinase K (Dako; Agilent Technologies, Inc.) at room temperature for 15 min. The sections were then rinsed with distilled water and incubated in a 3% hydrogen peroxide solution at room temperature for 5 min to block endogenous peroxidases. The sections were then washed with PBS, incubated in an equilibration buffer and treated with TdT enzyme in a humidified chamber at 37°C for 60 min. Slides were subsequently placed in working strength stop wash buffer at room temperature for 10 min, rinsed with PBS and incubated with an anti-digoxigenin-peroxidase conjugate at room temperature for 30 min. Diaminobenzidine (Peroxidase Substrate kit; Vector Laboratories, Inc.) was then applied to each section to reveal peroxidase activity. Hematoxylin was used at room temperature for 1 min as the counterstain. TUNEL-positive cells were counted under ×200 magnification from a minimum of 5 microscopic fields per section using a fluorescence microscope (BX51; Olympus Corporation) to determine the distribution of apoptotic cells. The apoptotic index was reported as the percentage of TUNEL-positive cells per 1,000 total cells as previously described (38).
Statistical analysis
MTT and ELISA data of drug-treated cells, tumor measurements, MVD, cell proliferative ability and apoptotic index were compared using one-way ANOVA followed by the post hoc Tukey's test. All data were presented as the mean ± SD. The Statcels2® 4-Step Excel Statistics software application was used for the analyses (OMS Publishing, Inc.; http://oms-publ.memo.jp/main/). P<0.05 was considered to indicate a statistically significant difference.
Results
Endogenous VEGF-A expression in four OSCC cell lines in vitro
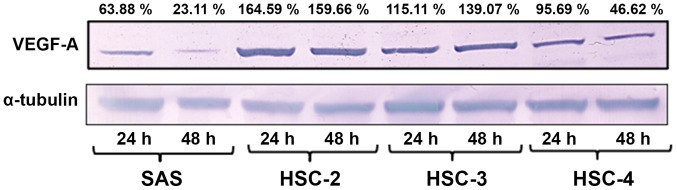
Western blotting of the cultured cell lines revealed the highest levels of VEGF-A expression in HSC-2 cells after 24 and 48 h of culture, followed by HSC-3, HSC-4 and SAS cells (Fig. 2).
Figure 2.
Endogenous VEGF-A expression in four OSCC cell lines in vitro. The OSCC SAS, HSC-2, HSC-3 and HSC-4 cell lines were incubated at 37°C for 24 or 48 h in FBS-free media. The highest VEGF-A expression was observed in HSC-2 cells, followed by HSC-3, HSC-4 and SAS at both time points. The fold-change of VEGF-A expression was calculated relative to the control (α-tubulin) and expressed as a percentage. OSCC, oral squamous cell carcinoma; VEGF-A, vascular endothelial growth factor A.
Inhibitory effects of 5-FU and bevacizumab on SAS and HSC-2 cell proliferation in vitro
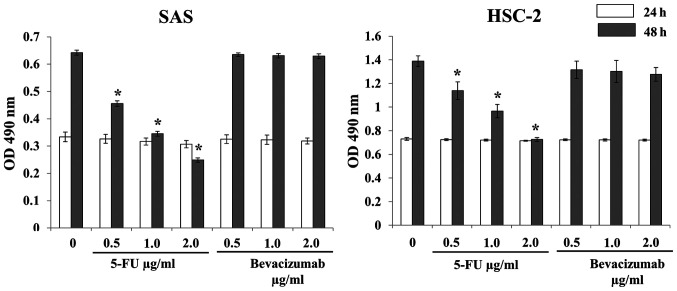
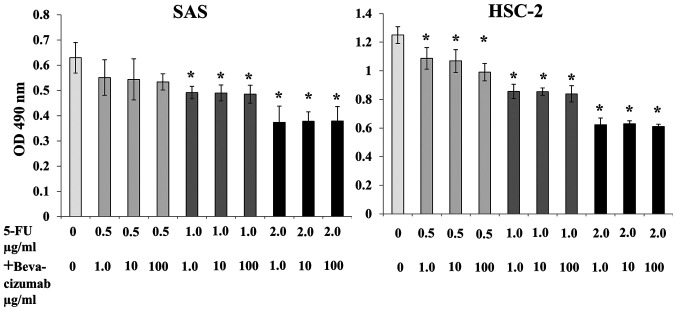
No significant decrease in SAS and HSC-2 cell proliferation was observed after treatment with 5-FU alone or bevacizumab alone for 24 h compared with no treatment (Fig. 3). Treatment with 5-FU alone for 48 h significantly inhibited the proliferation of SAS and HSC-2 cells compared with no treatment; however, bevacizumab treatment alone for 48 h did not exert any effect (Fig. 3). Although combined treatment with 5-FU and bevacizumab significantly inhibited the proliferation of both SAS and HSC-2 cells, no synergistic or additive effects were observed (Fig. 4).
Figure 3.
Inhibitory effects of 5-FU alone and bevacizumab alone on SAS and HSC-2 cell proliferation in vitro. 5-FU or bevacizumab alone did not inhibit cell proliferation in either cell line when treated for 24 h. For both cell lines, treatment with 5-FU alone for 48 h inhibited cell proliferation in a dose-dependent manner, and there was a significant difference between the control and treated cells. However, bevacizumab treatment alone did not have a similar effect. The data are presented as the mean ± SD. *P<0.05 vs. respective control (one-way ANOVA). OD, optical density; 5-FU, 5-fluorouracil.
Figure 4.
Inhibitory effects of 5-FU plus bevacizumab on SAS and HSC-2 cell proliferation in vitro. Compared with the control, combined treatment inhibited the proliferation of both SAS and HSC-2 cells. No synergistic or additive effects were observed. The data are presented as the mean ± SD. *P<0.05 vs. control (one-way ANOVA). OD, optical density; 5-FU, 5-fluorouracil.
Effects of 5-FU and bevacizumab on VEGF-A production by SAS and HSC-2 cells in vitro
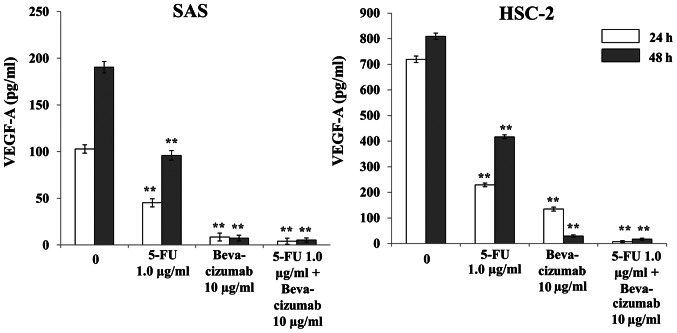
Treatment with 5-FU alone significantly decreased VEGF-A production in both SAS and HSC-2 cells compared with the control, and further decreases were observed with bevacizumab alone and in combination with 5-FU after 24 and 48 h of treatment (Fig. 5). In both cell lines, significant differences were observed after 24 and 48 h of treatment with either agent, alone or in combination, compared with the control (Fig. 5).
Figure 5.
Effects of 5-FU and bevacizumab on VEGF-A production by SAS and HSC-2 cells in vitro via ELISA. The amount of VEGF-A produced by both SAS and HSC-2 cells treated with 5-FU and bevacizumab alone or in combination differed significantly from the control. The data are presented as the mean ± SD. **P<0.01 vs. respective control (one-way ANOVA). F-FU, 5-fluorouracil; VEGF-A, vascular endothelial growth factor A.
Relative changes in tumor volume over time
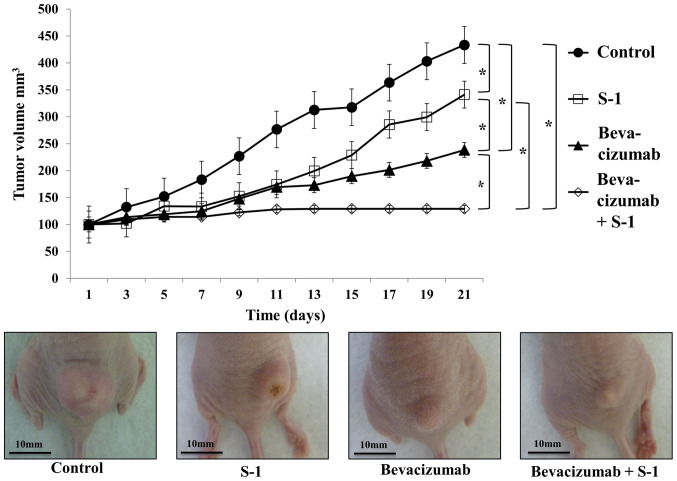
On average, tumor growth inhibition was observed with bevacizumab alone, S-1 alone or both agents in combination, and this inhibition was significantly different compared with that in the control group (Fig. 6). Bevacizumab was more effective than S-1 at decreasing tumor volume, and the combination of bevacizumab and S-1 was significantly more effective than either agent alone (Fig. 6). No increase in tumor volume was observed in mice treated with the combination of bevacizumab and S-1 after day 13 (Fig. 6). No loss of body weight was observed in any treated mice during the experimental period (data not shown). Furthermore, no abnormalities were observed in the heart, lung, liver and kidney of mice by the naked eye (data not shown).
Figure 6.
Inhibitory effects of S-1 and bevacizumab on tumor growth in vivo. The effects of bevacizumab alone, S-1 alone and combined therapy on HSC-2 tumor growth were observed in nude mice during a 21-day period of in vivo experiment. Bevacizumab, either alone or in combination with S-1, yielded significant growth inhibition compared with the control and S-1 alone. Combination treatment significantly decreased the tumor size compared with bevacizumab alone. No increase in tumor volume was observed in the group receiving combined therapy after day 13. Scale bar, 10 mm. The data are presented as the mean ± SD. *P<0.05 (one-way ANOVA).
Effects of S-1 and bevacizumab on residual tumor MVD in vivo
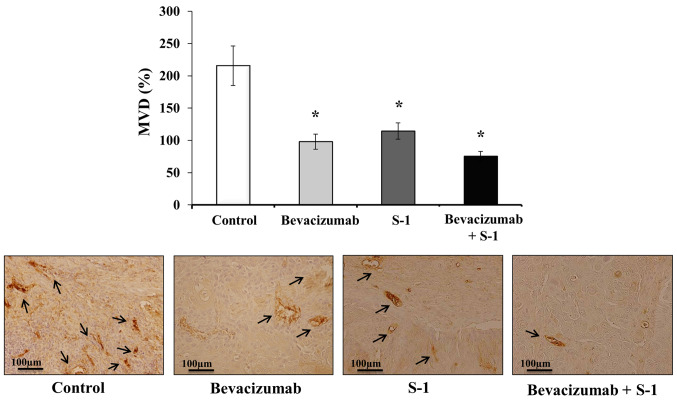
Mice tumor sections were subjected to immunohistochemical staining with CD31 to clarify the antitumor mechanisms affecting MVD. The following MVD values were recorded for each group: Control group, 215.67±30.55%; bevacizumab group, 98.00±11.79%; S-1 group, 114.33±12.66%; and combination group, 75.33±7.57% (Fig. 7). The MVDs of the S-1 alone, bevacizumab alone and combination groups were significantly lower than that of the control group (Fig. 7). Although the MVD value of bevacizumab alone was lower than that of S-1 alone, and the MVD of the combination group was lower than bevacizumab or S-1 alone, no significant differences were observed among the treatment groups (Fig. 7).
Figure 7.
Effects of S-1 and bevacizumab on residual tumor MVD in vivo. Bevacizumab alone, S-1 alone and combination therapy all significantly decreased the tumor MVD in vivo compared with the control. Scale bar, 100 µm. The data are presented as the mean ± SD. *P<0.05 vs. control (one-way ANOVA). MVD, microvessel density.
Inhibitory effects of S-1 and bevacizumab on cell proliferation in vivo
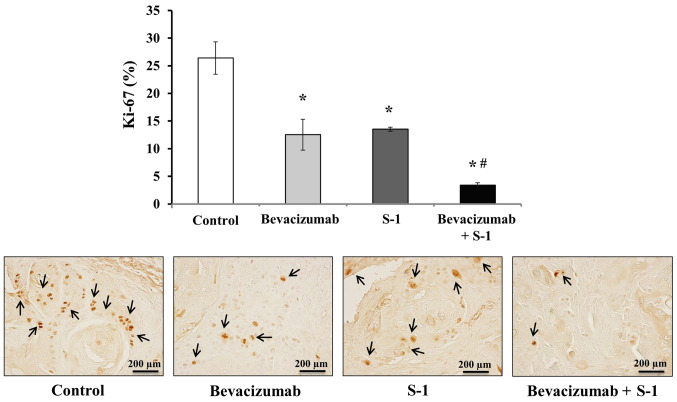
Tumor sections were stained with Ki-67 via immunohistochemistry, and the numbers of Ki-67+ nuclei were quantified to analyze the degree of inhibition of cell proliferation. Tumors treated with either bevacizumab or S-1 alone exhibited significantly decreased cell proliferative abilities compared with the control group (Fig. 8). Additionally, the number of Ki-67+ nuclei was significantly lower in tumors treated with both bevacizumab and S-1 compared with that of tumors in the control or individual treatment groups (Fig. 8). The number of Ki-67+ nuclei in different treatment groups was: Control group, 26.40±2.91%; bevacizumab group, 12.53±2.80%; S-1 group, 13.53±0.38%; combination group, 3.40±0.46% (Fig. 8).
Figure 8.
Inhibitory effects of S-1 and bevacizumab on cell proliferation in vivo. Bevacizumab alone, S-1 alone and bevacizumab plus S-1 all significantly decreased the cell proliferation in vivo compared with the control. Scale bar, 200 µm. The data are presented as the mean ± SD. *P<0.05 vs. control; #P<0.05 vs. Bevacizumab or S-1 (one-way ANOVA).
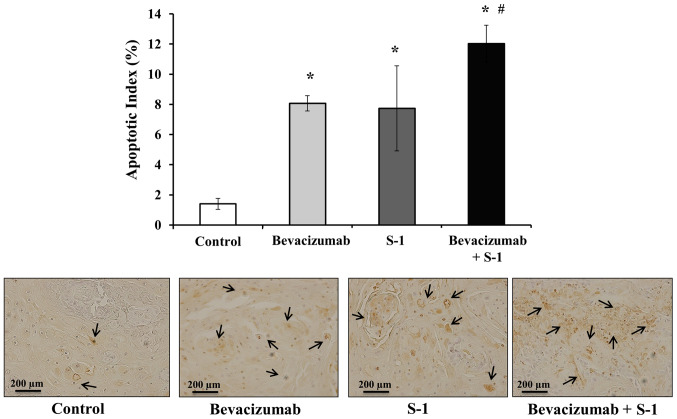
Effects of S-1 and bevacizumab on apoptosis induction in vivo
Next, the rate of apoptosis in nude mice tumors was analyzed after treatment with bevacizumab or S-1, alone or in combination. The number of apoptotic cells in each harvested tumor was quantified using a TUNEL assay. A significantly higher degree of apoptosis was observed in tumors treated with either bevacizumab or S-1 alone compared with the control group (Fig. 9). However, the highest number of apoptotic cells was observed in tumors treated with bevacizumab and S-1 in combination; this combination treatment significantly induced apoptosis compared with each agent alone, as well as compared with the control (Fig. 9). The apoptotic indexes were as follows: Control group, 1.4±0.36%; bevacizumab group, 8.07±0.5%; S-1 group, 7.73±2.82%; and combination group, 12.0±1.22% (Fig. 9).
Figure 9.
Effects of S-1 and bevacizumab on apoptosis induction in vivo. Treatment with bevacizumab alone, S-1 alone and bevacizumab plus S-1 led to a significantly higher apoptotic index in vivo compared with the control, as assessed by TUNEL assay. Scale bar, 200 µm. The data are presented as the mean ± SD. *P<0.05 vs. control; #P<0.05 vs. S-1 or bevacizumab (one-way ANOVA).
Discussion
A number of mice xenografts studies have revealed that 5-FU metabolized from S-1 exerts significant antitumor effects and inhibits tumor angiogenesis by inducing apoptosis of the tumor vascular endothelial cells in various types of tumor, including human OSCC (6–8,38–40). Additionally, bevacizumab monotherapy exhibited antitumor effects in nude mice with OSCC and HNSCC tumors (12). Moreover, this antitumor effect of bevacizumab was enhanced when administered in combination with paclitaxel, irinotecan, cisplatin, interleukin-8, irradiation or cetuximab (36,41–44).
In the present study, a synergistic and suppressive antitumor effect of bevacizumab plus S-1 combination therapy was observed against OSCC in vivo. Although the combined treatment with 5-FU and bevacizumab inhibited the proliferation of SAS and HSC-2 cells in vitro, it did not exhibit any synergistic effect. On the other hand, the results of the current in vivo experiments suggested that the anti-angiogenic action of bevacizumab and the antitumor effect of S-1 synergistically inhibited tumor cell proliferation and promoted apoptosis. Although VEGF-A expression was not analyzed in mice tumors, it may be possible that S-1/5-FU downregulated VEGF expression by suppressing NF-κB, which finally resulted in decreased MVD and inhibited angiogenesis (8,45).
Similar results were obtained in two types of OSCC cell lines in vitro: HSC-2, which expressed high levels of VEGF-A, and SAS, which expressed low levels of VEGF-A; however, there was no synergistic effect of the combination treatment. HSC-2 cells are derived from squamous cell carcinoma of the mouth; it has an epithelial cell-like morphology and no metastatic potential (46). On the other hand, SAS is a poorly differentiated squamous cell carcinoma cell line from a human tongue primary lesion (47). 5-FU inhibited cell proliferation and directly decreased VEGF-A expression. By contrast, bevacizumab alone did not inhibit cell proliferation and did not exhibit a synergistic suppressive effect when administered in combination with 5-FU; in other words, it did not have a direct antitumor effect in vitro. However, bevacizumab neutralized VEGF-A expression in the culture supernatant, thus decreasing the concentration of VEGF-A around the neoplastic cells. The reason why bevacizumab alone did not exert antitumor effect or apoptosis in vitro remains unknown and should be further clarified in the future.
Based on the present results, it can be assumed that bevacizumab alone cannot exert antitumor effects in an in vitro environment (48). However, it can possibly exert antitumor effects in an in vivo environment, which is a complex mixture of tumor cells, tumor blood vessels and interstitial cells. In the present study, HSC-2 cells were used for the in vivo experiments, as this cell line had a higher tumorigenic potential and it strongly expressed VEGF-A compared with the SAS cell line.
It has been reported that 5-FU inhibits tumor cell proliferation and has an anti-angiogenic effect, which is not as strong as bevacizumab (32,33,36,38,40,41). In the present study, it was observed that in the S-1 alone group, 5-FU metabolized from S-1 exerted an antitumor effect by inhibiting cell proliferation and inducing apoptosis in vivo, as well as by decreasing MVD. However, we assumed that bevacizumab alone led to the regression of immature tumor blood vessels and a negative feedback loop involving continuous reduction of the nutrient supply to cancer cells; these factors ultimately led to the inhibition of cell proliferation and induction of apoptosis.
Tumor progression was slowed down by S-1 or bevacizumab alone, but was not completely inhibited in mice. It can be hypothesized that, in the combination group, the marked decreases in tumor progression, MVD and cell proliferative ability, and the increase in the apoptotic index may be attributed to an improved ability to maintain homeostasis, which was absent in the S-1 or bevacizumab alone groups. Hence, we suggest the following mechanism behind the antitumor effect of S-1 and bevacizumab combination therapy. Bevacizumab binds to VEGF-A secreted from tumor cells and may induce the regression of immature tumor blood vessels and a negative feedback cycle that disrupts the continuous nutrient supply to cancer cells, thus inhibiting cell proliferation and promoting apoptosis. At the same time, it may be assumed that S-1 exerts direct antitumor effects, as well as promoting continuous indirect antitumor angiogenesis inhibition, thereby exerting marked antitumor effects. This may be a reason for the marked antitumor effect of the combination treatment on cell proliferation and apoptosis, but not for the direct effect on angiogenesis. However, the underlying mechanisms of S-1 and bevacizumab combination treatment in OSCC are not completely understood, and there may be other unknown underlying factors responsible for the efficacy of this combination treatment. Additionally, the present study did not analyze VEGF-A expression in mice tumors nor the concentrations of drugs within tumor tissues, adjacent tissues or plasma. Therefore, this should be further investigated in future studies.
Recent findings have indicated that once a solid tumor reaches a certain size, it begins to secrete various angiogenic factors to promote the neovasculature (49,50). Tumor growth can be suppressed by blocking blood vessels that feed tumors, which can then disappear via tumor dormancy without metastasis (51,52). Given the lack of further increase in tumor volume after day 13 of the bevacizumab and S-1 combination therapy in the present study, the potential need for tumor dormancy therapy in OSCC should be considered, as proposed by Folkman and Hochberg (53). In other words, bevacizumab inhibits VEGF-A secretion from tumor cells and causes simultaneous inhibition of tumor growth and angiogenesis. Bevacizumab exerts a significant antitumor effect by improving drug delivery and thus allowing high concentrations of 5-FU to diffuse into the tumor (54). Therefore, conventional chemotherapy can directly exert antitumor effect on tumor cells, whereas bevacizumab can indirectly inhibit cell proliferation and increase apoptosis by decreasing nutrition supply to the tumor (55). In addition, bevacizumab can be expected to have further antitumor effects if combined with conventional antitumor agents (56). Hence, the current combination therapy may be used as a new treatment for OSCC due to its enhanced antitumor effects.
In conclusion, the combined treatment of S-1 and bevacizumab was effective against OSCC cells in vitro and exhibited marked synergistic antitumor effects in vivo. This combination therapy seemed to inhibit cell proliferation and promote apoptosis by controlling tumor angiogenesis. The present results indicated that S-1 and bevacizumab combination therapy may be a useful and promising treatment for refractory, highly angiogenic OSCC tumors.
Acknowledgements
Not applicable.
Funding Statement
The present study was supported in part by Grant-in-Aid from the Japanese Ministry of Education, Science and Culture (grant nos. 21890168 and 15K11292).
Funding
The present study was supported in part by Grant-in-Aid from the Japanese Ministry of Education, Science and Culture (grant nos. 21890168 and 15K11292).
Availability of data and materials
The datasets used and/or analyzed during the current study are available from the corresponding author on reasonable request.
Authors' contributions
YI and KH designed the study. YI and TT performed the experiments. YI, TF and KH analyzed the data, wrote and revised the manuscript. YI, TF and KH confirmed the authenticity of all the raw data. YU and KM helped in analysis and interpretation of data, revised the manuscript and provided valuable suggestions during the study. All authors have read and approved the final version of the manuscript and are fully responsible for its content.
Ethics approval and consent to participate
All in vivo experiments were approved by the Institutional Animal Care and Use Committee of Yamaguchi University (approval no. 55-010; Ube, Japan).
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
References
- 1.Joo YH, Cho JK, Koo BS, Kwon M, Kwon SK, Kwon SY, Kim MS, Kim JK, Kim H, Nam I, et al. Guidelines for the surgical management of oral cancer: Korean society of thyroid-head and neck surgery. Clin Exp Otorhinolaryngol. 2019;12:107–144. doi: 10.21053/ceo.2018.01816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Chi AC, Day TA, Neville BW. Oral cavity and oropharyngeal squamous cell carcinoma - an update. CA Cancer J Clin. 2015;65:401–421. doi: 10.3322/caac.21293. [DOI] [PubMed] [Google Scholar]
- 3.Vermorken JB, Remenar E, van Herpen C, Gorlia T, Mesia R, Degardin M, Stewart JS, Jelic S, Betka J, Preiss JH, et al. EORTC 24971/TAX 323 Study Group Cisplatin, fluorouracil, and docetaxel in unresectable head and neck cancer. N Engl J Med. 2007;357:1695–1704. doi: 10.1056/NEJMoa071028. [DOI] [PubMed] [Google Scholar]
- 4.Tsukuda M, Kida A, Fujii M, Kono N, Yoshihara T, Hasegawa Y, Sugita M, Chemotherapy Study Group of Head and Neck Cancer Randomized scheduling feasibility study of S-1 for adjuvant chemotherapy in advanced head and neck cancer. Br J Cancer. 2005;93:884–889. doi: 10.1038/sj.bjc.6602804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Harada K, Sato M, Ueyama Y, Nagayama M, Hamakawa H, Nagahata S, Yoshimura Y, Osaki T, Ryoke K, Oral Cancer Study Group of Chugoku-Shikoku Multi-institutional phase II trial of S-1 in patients with oral squamous cell carcinoma. Anticancer Drugs. 2008;19:85–90. doi: 10.1097/CAD.0b013e3282f0ce4d. [DOI] [PubMed] [Google Scholar]
- 6.Yokoe H, Kasamatsu A, Ogawara K, Ishigami T, Sato Y, Shibata M, Tanzawa H, Uzawa K. Neoadjuvant chemotherapy with S-1 for patients with oral squamous cell carcinoma. J Cancer Sci Ther. 2010;2:132–135. doi: 10.4172/1948-5956.1000038. [DOI] [Google Scholar]
- 7.Tsukahara K, Kubota A, Hasegawa Y, Takemura H, Terada T, Taguchi T, Nagahara K, Nakatani H, Yoshino K, Higaki Y, et al. Randomized phase III trial of adjuvant chemotherapy with S-1 after curative treatment in patients with squamous-cell carcinoma of the head and neck (ACTH-HNC) PLoS One. 2015;11:1–15. doi: 10.1371/journal.pone.0116965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Harada K, Ferdous T, Ueyama Y. Therapeutic strategies with oral fluoropyrimidine anticancer agent, S-1 against oral cancer. Jpn Dent Sci Rev. 2017;53:61–77. doi: 10.1016/j.jdsr.2016.11.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Sakuramoto S, Sasako M, Yamaguchi T, Kinoshita T, Fujii M, Nashimoto A, Furukawa H, Nakajima T, Ohashi Y, Imamura H, et al. ACTS-GC Group Adjuvant chemotherapy for gastric cancer with S-1, an oral fluoropyrimidine. N Engl J Med. 2007;357:1810–1820. doi: 10.1056/NEJMoa072252. [DOI] [PubMed] [Google Scholar]
- 10.Yamada Y, Takahari D, Matsumoto H, Baba H, Nakamura M, Yoshida K, Yoshida M, Iwamoto S, Shimada K, Komatsu Y, et al. Leucovorin, fluorouracil, and oxaliplatin plus bevacizumab versus S-1 and oxaliplatin plus bevacizumab in patients with metastatic colorectal cancer (SOFT): An open-label, non-inferiority, randomised phase 3 trial. Lancet Oncol. 2013;14:1278–1286. doi: 10.1016/S1470-2045(13)70490-X. [DOI] [PubMed] [Google Scholar]
- 11.Presta LG, Chen H, O'Connor SJ, Chisholm V, Meng YG, Krummen L, Winkler M, Ferrara N. Humanization of an anti-vascular endothelial growth factor monoclonal antibody for the therapy of solid tumors and other disorders. Cancer Res. 1997;57:4593–4599. [PubMed] [Google Scholar]
- 12.Yoshida H, Yoshimura H, Matsuda S, Ryoke T, Kiyoshima T, Kobayashi M, Sano K. Effects of peritumoral bevacizumab injection against oral squamous cell carcinoma in a nude mouse xenograft model: A preliminary study. Oncol Lett. 2018;15:8627–8634. doi: 10.3892/ol.2018.8399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Willett CG, Boucher Y, di Tomaso E, Duda DG, Munn LL, Tong RT, Chung DC, Sahani DV, Kalva SP, Kozin SV, et al. Direct evidence that the VEGF-specific antibody bevacizumab has antivascular effects in human rectal cancer. Nat Med. 2004;10:145–147. doi: 10.1038/nm0604-649c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Tong M, Lloyd B, Pei P, Mallery RS. Human head and neck squamous cell carcinoma cells are both targets and effectors for the angiogenic cytokine, VEGF. J Cell Biochem. 2008;105:1202–1210. doi: 10.1002/jcb.21920. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Vassilakopoulou M, Psyrri A, Argiris A. Targeting angiogenesis in head and neck cancer. Oral Oncol. 2015;51:409–415. doi: 10.1016/j.oraloncology.2015.01.006. [DOI] [PubMed] [Google Scholar]
- 16.Mineta H, Miura K, Ogino T, Takebayashi S, Misawa K, Ueda Y, Suzuki I, Dictor M, Borg A, Wennerberg J. Prognostic value of vascular endothelial growth factor (VEGF) in head and neck squamous cell carcinomas. Br J Cancer. 2000;83:775–781. doi: 10.1054/bjoc.2000.1357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Smith BD, Smith GL, Carter D, Sasaki CT, Haffty BG. Prognostic significance of vascular endothelial growth factor protein levels in oral and oropharyngeal squamous cell carcinoma. J Clin Oncol. 2000;18:2046–2052. doi: 10.1200/JCO.2000.18.10.2046. [DOI] [PubMed] [Google Scholar]
- 18.Tse GM, Chan AW, Yu KH, King AD, Wong KT, Chen GG, Tsang RK, Chan AB. Strong immunohistochemical expression of vascular endothelial growth factor predicts overall survival in head and neck squamous cell carcinoma. Ann Surg Oncol. 2007;14:3558–3565. doi: 10.1245/s10434-007-9632-0. [DOI] [PubMed] [Google Scholar]
- 19.O-charoenrat P, Rpys-Evans P, Eccles S. Expression of vascular endothelial growth family members in head and neck squamous cell carcinoma correlates with lymph node metastasis. Cancer. 2001;92:556–568. doi: 10.1002/1097-0142(20010801)92:3<556::AID-CNCR1355>3.0.CO;2-Q. [DOI] [PubMed] [Google Scholar]
- 20.Shang ZJ, Li JR, Li ZB. Circulating levels of vascular endothelial growth factor in patients with oral squamous cell carcinoma. Int J Oral Maxillofac Surg. 2002;31:495–498. doi: 10.1054/ijom.2002.0284. [DOI] [PubMed] [Google Scholar]
- 21.Cheng SJ, Lee JJ, Kok SH, Chou CH, Chang HH, Yang H, Chiang ML, Kuo MY. Expression of vascular endothelial growth factor is significantly associated with progression and prognosis of oral squamous cell carcinomas in Taiwan. J Formos Med Assoc. 2011;110:50–57. doi: 10.1016/S0929-6646(11)60008-9. [DOI] [PubMed] [Google Scholar]
- 22.Hurwitz H, Fehrenbacher L, Novotny W, Cartwright T, Hainsworth J, Heim W, Berlin J, Baron A, Griffing S, Holmgren E, et al. Bevacizumab plus irinotecan, fluorouracil, and leucovorin for metastatic colorectal cancer. N Engl J Med. 2004;350:2335–2342. doi: 10.1056/NEJMoa032691. [DOI] [PubMed] [Google Scholar]
- 23.Seiwert TY, Haraf DJ, Cohen EE, Stenson K, Witt ME, Dekker A, Kocherginsky M, Weichselbaum RR, Chen HX, Vokes EE. Phase I study of bevacizumab added to fluorouracil- and hydroxyurea-based concomitant chemoradiotherapy for poor-prognosis head and neck cancer. J Clin Oncol. 2008;26:1732–1741. doi: 10.1200/JCO.2007.13.1706. [DOI] [PubMed] [Google Scholar]
- 24.Yao M, Galanopoulos N, Lavertu P, Fu P, Gibson M, Argiris A, Rezaee R, Zender C, Wasman J, Machtay M, et al. Phase II study of bevacizumab in combination with docetaxel and radiation in locally advanced squamous cell carcinoma of the head and neck. Head Neck. 2015;37:1665–1671. doi: 10.1002/hed.23813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Ferrara N, Davis-Smyth T. The biology of vascular endothelial growth factor. Endocr Rev. 1997;18:4–25. doi: 10.1210/edrv.18.1.0287. [DOI] [PubMed] [Google Scholar]
- 26.Monk BJ, Sill MW, McMeekin DS, Cohn DE, Ramondetta LM, Boardman CH, Benda J, Cella D. Phase III trial of four cisplatin-containing doublet combinations in stage IVB, recurrent, or persistent cervical carcinoma: A Gynecologic Oncology Group study. J Clin Oncol. 2009;27:4649–4655. doi: 10.1200/JCO.2009.21.8909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Wright JD, Viviano D, Powell MA, Gibb RK, Mutch DG, Grigsby PW, Rader JS. Bevacizumab combination therapy in heavily pretreated, recurrent cervical cancer. Gynecol Oncol. 2006;103:489–493. doi: 10.1016/j.ygyno.2006.03.023. [DOI] [PubMed] [Google Scholar]
- 28.Monk BJ, Sill MW, Burger RA, Gray HJ, Buekers TE, Roman LD. Phase II trial of bevacizumab in the treatment of persistent or recurrent squamous cell carcinoma of the cervix: A gynecologic oncology group study. J Clin Oncol. 2009;27:1069–1074. doi: 10.1200/JCO.2009.21.8909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Suzuki S, Shimazaki J, Morishita K, Koike N, Harada N, Hayashi T, Suzuki M. Efficacy and safety of oxaliplatin, bevacizumab and oral S-1 for advanced recurrent colorectal cancer. Mol Clin Oncol. 2016;5:391–394. doi: 10.3892/mco.2016.977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Fang J, Wang H, Xu Q. Bevacizumab combined with low-dose S-1 as maintenance therapy with a long progression-free survival in an elderly patient with heavily pre-treated advanced gastric cancer: A case report. Biomed Rep. 2013;1:239–242. doi: 10.3892/br.2012.37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Yamada K, Ichiki M, Takahashi K, Hisamatsu Y, Takeoka H, Azuma K, Shukuya T, Nishikawa K, Tokito T, Ishii H, et al. A multicenter phase II trial of S-1 combined with bevacizumab after platinum-based chemotherapy in patients with advanced non-squamous non-small cell lung cancer. Cancer Chemother Pharmacol. 2016;78:501–507. doi: 10.1007/s00280-016-3101-z. [DOI] [PubMed] [Google Scholar]
- 32.Nie K, Geng C, Zhang L, Liu S, Zhang Z, Wang R, Zou X, Ji Y. Clinical observation of bevacizumab combined with S-1 in the treatment of pretreated advanced esophageal carcinoma. Chin Med Sci J. 2016;31:221–227. doi: 10.1016/S1001-9294(17)30004-4. [DOI] [PubMed] [Google Scholar]
- 33.Yoshida M, Takagane A, Miyake Y, Shimada K, Nagata N, Sato A, Ogata Y, Fukunaga M, Otsuka K, Takahashi T, et al. A phase II study of third-line combination chemotherapy with bevacizumab plus S-1 for metastatic colorectal cancer with mutated KRAS (SAVIOR Study) Oncology. 2016;91:24–30. doi: 10.1159/000446372. [DOI] [PubMed] [Google Scholar]
- 34.Weidner N, Semple JP, Welch WR, Folkman J. Tumor angiogenesis and metastasis - correlation in invasive breast carcinoma. N Engl J Med. 1991;324:1–8. doi: 10.1056/NEJM199101033240101. [DOI] [PubMed] [Google Scholar]
- 35.Harada K, Baillie R, Lu S, Syrjänen S, Schor AM. VEGF expression in skin warts. Relevance to angiogenesis and vasodilation. Arch Dermatol Res. 2001;293:233–238. doi: 10.1007/s004030100222. [DOI] [PubMed] [Google Scholar]
- 36.Fujita K, Sano D, Kimura M, Yamashita Y, Kawakami M, Ishiguro Y, Nishimura G, Matsuda H, Tsukuda M. Anti-tumor effects of bevacizumab in combination with paclitaxel on head and neck squamous cell carcinoma. Oncol Rep. 2007;18:47–51. [PubMed] [Google Scholar]
- 37.Roland NJ, Caslin AW, Bowie GL, Jones AS. Has the cellular proliferation marker Ki67 any clinical relevance in squamous cell carcinoma of the head and neck? Clin Otolaryngol Allied Sci. 1994;19:13–18. doi: 10.1111/j.1365-2273.1994.tb01140.x. [DOI] [PubMed] [Google Scholar]
- 38.Itashiki Y, Harada K, Ferdous T, Yoshida H. Effects of tumor necrosis factor-related apoptosis-inducing ligand alone and in combination with fluoropyrimidine anticancer agent, S-1, on tumor growth of human oral squamous cell carcinoma xenografts in nude mice. Anticancer Res. 2007;27:2365–2375. [PubMed] [Google Scholar]
- 39.Harada K, Supriatno, Kawashima Y, Yoshida H, Sato M. S-1 inhibits tumorigenicity and angiogenesis of human oral squamous cell carcinoma cells by suppressing expression of phoshorylated Akt, vascular endothelial growth factor and fibroblast growth factor-2. Int J Oncol. 2007;30:365–374. [PubMed] [Google Scholar]
- 40.Shirasaka T, Nakano K, Takechi T, Satake H, Uchida J, Fujioka A, Saito H, Okabe H, Oyama K, Takeda S, et al. Antitumor activity of 1 M tegafur-0.4 M 5-chloro-2,4-dihydroxypyridine-1 M potassium oxonate (S-1) against human colon carcinoma orthotopically implanted into nude rats. Cancer Res. 1996;56:2602–2606. [PubMed] [Google Scholar]
- 41.Yoshida M, Muro K, Tsuji A, Hamamoto Y, Yoshino T, Yoshida K, Shirao K, Miyata Y, Takahari D, Takahash T, et al. Combination chemotherapy with bevacizumab and S-1 for elderly patients with metastatic colorectal cancer (BASIC trial) Eur J Cancer. 2015;51:935–941. doi: 10.1016/j.ejca.2015.03.007. [DOI] [PubMed] [Google Scholar]
- 42.Cao S, Durrani FA, Toth K, Rustum YM, Seshadri M. Bevacizumab enhances the therapeutic efficacy of Irinotecan against human head and neck squamous cell carcinoma xenografts. Oral Oncol. 2011;47:459–466. doi: 10.1016/j.oraloncology.2011.04.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Wang Y, Dong L, Bi Q, Li X, Wu D, Ge X, Zhang X, Fu J, Zhang C, Wang C, et al. Investigation of the efficacy of a bevacizumab-cetuximab-cisplatin regimen in treating head and neck squamous cell carcinoma in mice. Target Oncol. 2010;5:237–243. doi: 10.1007/s11523-010-0164-3. [DOI] [PubMed] [Google Scholar]
- 44.Gyanchandani R, Sano D, Ortega Alves MV, Klein JD, Knapick BA, Oh S, Myers JN, Kim S. Interleukin-8 as a modulator of response to bevacizumab in preclinical models of head and neck squamous cell carcinoma. Oral Oncol. 2013;49:761–770. doi: 10.1016/j.oraloncology.2013.03.452. [DOI] [PubMed] [Google Scholar]
- 45.Ferdous T, Harada K, Kin T, Harada T, Ueyama Y. Efficacy of schedule-dependent metronomic S-1 chemotherapy in human oral squamous cell carcinoma cells. Int J Oncol. 2013;43:271–279. doi: 10.3892/ijo.2013.1950. [DOI] [PubMed] [Google Scholar]
- 46.CELL BANK Website: HSC-2 cell, corp-author. https://cellbank.brc.riken.jp/cell_bank/CellInfo/?cellNo=RCB1945 [Google Scholar]
- 47.Takahashi K, Kanazawa H, Akiyama Y, Tazaki S, Takahara M, Muto T, Tanazawa H, Sato K. Establishment and characterization of a cell line (SAS) from poorly differentiated human squamous cell carcinoma of the tongue. J Jpn Stomatol Soc. 1989;38:20–28. (In Japanese) [Google Scholar]
- 48.Heydar H, Mansouri K, Norooznezhad M, Norooznezhad F, Mohamadnia A, Bahrami N. Bevacizumab inhibits angiogenic cytokines in head and neck squamous cell carcinoma: From gene to the protein. Int J Hematol Oncol Stem Cell Res. 2018;12:136–141. [PMC free article] [PubMed] [Google Scholar]
- 49.Wary KK. Molecular targets for anti-angiogenic therapy. Curr Opin Mol Ther. 2004;6:54–70. [PubMed] [Google Scholar]
- 50.Gimbrone MA, Jr, Leapman SB, Cotran RS, Folkman J. Tumor dormancy in vivo by prevention of neovascularization. J Exp Med. 1972;136:261–276. doi: 10.1084/jem.136.2.261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Holmgren L, O'Reily MS, Folkman J. Dormancy of micrometastasis, balanced proliferation and apoptosis in the presence of angiogenesis suppression. Nat Med. 1995;1:149–153. doi: 10.1038/nm0295-149. [DOI] [PubMed] [Google Scholar]
- 52.O'Reilly MS, Holmgren L, Chen C, Folkman J. Angiostatin induces and sustains dormancy of human primary tumors in mice. Nat Med. 1996;2:689–692. doi: 10.1038/nm0696-689. [DOI] [PubMed] [Google Scholar]
- 53.Folkman J, Hochberg M. Self-regulation of growth in three dimensions. J Exp Med. 1973;138:745–753. doi: 10.1084/jem.138.4.745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Turley RS, Fontanella AN, Padussis JC, Toshimitsu H, Tokuhisa Y, Cho EH, Hanna G, Beasley GM, Augustine CK, Dewhirst MW, Tyler DS. Bevacizumab-induced alterations in vascular permeability and drug delivery: A novel approach to augment regional chemotherapy for in-transit melanoma. Clin Cancer Res. 2012;18:3328–3339. doi: 10.1158/1078-0432.CCR-11-3000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Iwasaki J, Nihira S. Anti-angiogenic therapy against gastrointestinal tract cancers. Jpn J Clin Oncol. 2009;39:543–551. doi: 10.1093/jjco/hyp062. [DOI] [PubMed] [Google Scholar]
- 56.Falcetta F, Bizzaro F, D'Agostini E, Bani MR, Giavazzi R, Ubezio P. Modeling cytostatic and cytotoxic responses to new treatment regimens for ovarian cancer. Cancer Res. 2017;77:6759–6769. doi: 10.1158/0008-5472.CAN-17-1099. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The datasets used and/or analyzed during the current study are available from the corresponding author on reasonable request.