Abstract
Objective:
To evaluate clinical literature for direct oral anticoagulants (DOACs) therapy for non–Food and Drug Administration approved indications.
Data Sources:
Articles from MEDLINE, Cochrane Library, Google Scholar, and OVID databases were reviewed from 1946 through September 4, 2020.
Study Selection and Data Extraction:
Fully published studies assessing DOACs for atrial fibrillation (AF) with valvular heart disease (VHD), heart failure (HF), left ventricular thrombus (LVT), superficial vein thrombosis (SVT), or pulmonary hypertension (PH) were evaluated.
Data Synthesis:
Our review showed that DOACs are safe to use in patients with AF and VHD except for mitral stenosis or mechanical heart valve. Rivaroxaban 2.5 mg twice daily should be used with caution in patients with HF with reduced ejection fraction until further evaluation is performed. Four retrospective studies for DOAC use in patients with LVT showed conflicting results. One phase 3 randomized controlled trial showed noninferiority of rivaroxaban to fondaparinux for SVT treatment. The use of DOACs for pulmonary arterial hypertension was not evaluated in any clinical study, but 2 retrospective studies for the use of DOACs in patients with chronic thromboembolic PH (CTEPH) showed similar efficacy between DOACs and warfarin.
Relevance to Patient Care and Clinical Practice:
This review provides clinicians with a comprehensive literature review surrounding DOAC use in common off-label indications.
Conclusion:
DOACs can be considered for AF complicated by VHD except for mitral stenosis or mechanical valve replacement. DOACs (especially rivaroxaban) are considered as an alternative therapy for SVT and CTEPH. Further prospective studies for DOAC uses are needed for HF or LVT.
Keywords: direct oral anticoagulants, atrial fibrillation, pulmonary hypertension, heart failure, left ventricular thrombus, superficial vein thrombosis
Introduction
Direct oral anticoagulants (DOACs) are approved for various anticoagulation indications, including nonvalvular atrial fibrillation (NVAF), treatment of venous thromboembolism (VTE), and recurrent VTE prophylaxis. For the general patients, DOACs offer several advantages over vitamin K antagonists (VKAs), such as warfarin. DOACs offer patients a conveniently fixed dose without substantial laboratory monitoring and less dietary and drug interactions as compared with warfarin.1 However, the use of DOACs in non–Food and Drug Administration (FDA)-approved indications are still controversial because of lack of data and exclusion from major clinical trials. The major non-FDA indications include valvular atrial fibrillation (VAF), heart failure (HF), left ventricular thrombus (LVT), superficial vein thrombosis (SVT), and pulmonary hypertension (PH).
Although DOACs are approved for NVAF, pivotal clinical trials for this approval excluded patients with mechanical prosthetic valves or mitral stenosis. Currently, warfarin remains the sole approved oral anticoagulant for VAF. Because of the change in blood flow patterns in prosthetic valve recipients or decreased blood flow of heart chambers in patients with valvular heart disease (VHD), it is uncertain that DOACs are as effective or safe as warfarin in patients with AF and VHD.2 Additionally, LVT is a common complication of acute myocardial infarction (MI), and similarly, warfarin remains the standard of care.3 Since last guideline publications, multiple clinical studies were published to evaluate the use of DOACs in patients with VAF, LVT, HF with sinus rhythm, and patients with PH.4–11 Also, for patients with SVT, current guidelines recommend a 45-day course of fondaparinux or low-molecular-weight heparin for treatment in patients at an increased risk for deep-vein thrombosis (DVT).12,13 However, oral anticoagulation therapy with DOACs may be more advantageous in these patient populations. The purpose of this review is to evaluate contemporary clinical literature and help clinicians determine clinical appropriateness of a DOAC therapy for non–FDA-approved indications.
Methods
The 2 investigators performed database searches and study selection to identify relevant articles from MEDLINE (from 1946 through September 4, 2020), OVID, Cochrane Library, and Google Scholar (through September 4, 2020) using the following keywords: (dabigatran OR edoxaban OR rivaroxaban OR apixaban OR betrixaban) AND (heart failure OR pulmonary hypertension OR left ventricular thrombus OR superficial vein thrombosis OR valvular atrial fibrillation). Pertinent references from identified articles and guidelines were also reviewed via MEDLINE search.
Studies were included if they met the following criteria: (1) patients were receiving dabigatran, edoxaban, rivaroxaban, apixaban, or betrixaban; (2) for VAF, HF, PH, LVT, or SVT; and (3) studies were case series, case-control studies, cohort studies, meta-analyses, or randomized studies in the English language consisting of human subjects ≥18 years old. Off-label indications were selected based on expert opinions and clinical needs.
Studies were excluded if they focused on DOAC use in VTE prophylaxis, NVAF, DVT, or pulmonary embolism (PE) treatment; cardioversion for NVAF; ventricular assist device; or heparin-induced thrombocytopenia. Case reports, conference abstracts, or systematic reviews without meta-analysis were also excluded. A total of 37 studies were included in this analysis (Figure 1).
Figure 1.
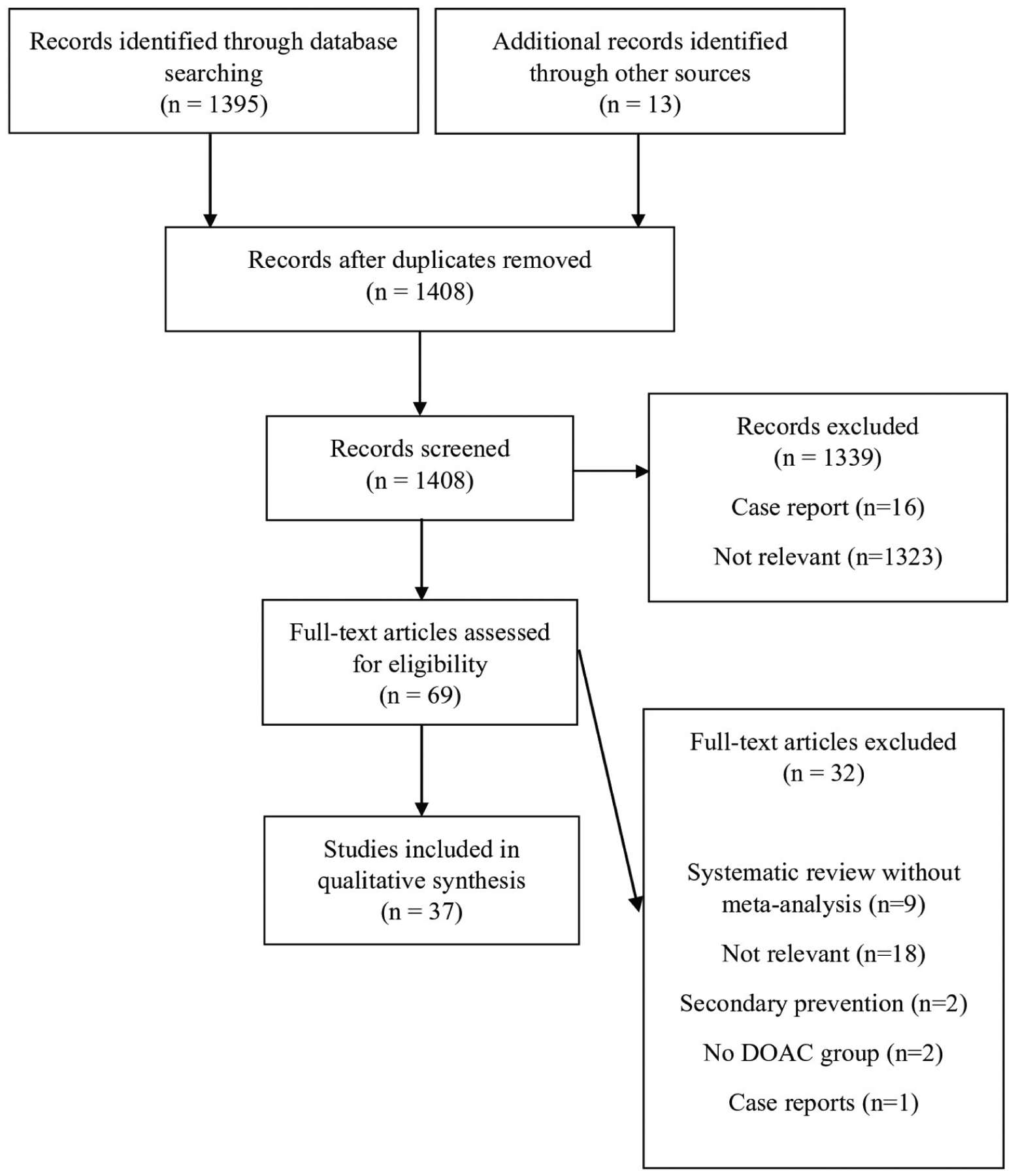
Flow diagram of direct oral anticoagulant (DOAC) off-label use literature search.
Data Extraction
Two investigators independently extracted the following data from the included studies: the study inclusion/exclusion criteria, study design, included patients’ baseline characteristics, the interventions, and efficacy and safety outcomes. The details in the included studies were also extracted for study quality assessment.
Quality Assessment
Two investigators independently rated the quality of the included studies. The Cochrane risk-of-bias tool version 2 for randomized controlled trials and Newcastle-Ottawa Scale for cohort studies were used for quality assessment (Supplemental Tables 1 and 2, available online).14,15
Results
Valvular Atrial Fibrillation
The American Heart Association (AHA)/American College of Cardiology (ACC)/Heart Rhythm Society guidelines recommend DOACs over VKA for stroke prevention in patients with NVAF.16 However, patients may also have other cardiac comorbidities, including the involvement of valve abnormalities or disease, and DOACs’ effectiveness and safety may not be reproduced in AF patients with VHD. For example, the RE-ALIGN trial showed that the dabigatran group had significantly higher thromboembolic and bleeding events in patients with mechanical heart valves compared with the warfarin group.17 Thus, clinical investigations of DOACs in patients with VHD needed to be further performed. However, the AHA/ACC 2019 updated guidelines currently define VAF as patients with moderate to severe mitral stenosis or a mechanical valve.16 The previous definitions also included bioprosthetic heart valves and valvular repair.18 Because there is a variety in the definitions of VAF, many of the pivotal randomized trials for DOACs included a variety of VHDs in their trials. Therefore, 6 post hoc analyses examining the efficacy and safety of DOAC use in AF patients with VHDs were conducted (Table 1).
Table 1.
Summary of Post Hoc Analyses of Randomized Studies or Real-World Observational Studies of DOACs in Patients With AF and VHD.
| Author/year | Study treatments and VHD definition | Study design | Baseline characteristics of study population | Efficacy outcome results | Safety outcome results |
|---|---|---|---|---|---|
| Avezum et al, 20157 | Apixaban 5 mg twice dailya (n = 2438) vs W with VHD (n = 2370) Included: all other VHD, including MR, TR, AR, AS, valve repair, bioprosthetic valve replacement Excluded: mechanical heart valves, moderate or severe MS |
Retrospective analysis of a randomized multicenter, double-blind, placebo-controlled trial (ARISTOTLE) | NR between VHD groups | Stroke or SEE apixaban vs W: 2.63% (n = 64) vs 3.76% (n = 89), HR = 0.70; 95% CI = 0.51–0.97 | Major bleeding: apixaban vs W: 4.06% (n = 99) vs 5.02% (n = 119), HR = 0.79; 95% CI = 0.61–1.04 |
| Breithardt et al, 20146 | Rivaroxaban 20 mg dailyb (n = 968) vs W (n = 1035) Included: all other native VHD (AS, AR, MR), annuloplasty with or without prosthetic ring, commissurotomy, and valvuloplasty Excluded: prosthetic heart valves, planned interventions that posed major uncontrolled bleeding risk, moderate to severe MS |
Retrospective analysis of a randomized multicenter, double-blind, placebo-controlled trial (ROCKET AF) | NR between VHD groups | Stroke or SEE: rivaroxaban vs W: 3.93% (n = 38) vs 4.83% (n = 50), HR = 0.83; 95% CI = 0.55–1.27 | Major or clinically relevant bleeding: rivaroxaban vs W 26.14% (n = 253) vs 23.12% (n = 240), HR = 1.25; 95% CI 1.05–1.49 |
| Briasoulis et al, 2018 (NOSd: S 3 stars, C 2 stars, and O 2 stars)29 | D150 twice daily (n = 1957) vs rivaroxaban 20 mg daily (n = 1957) vs W (n = 1957) Included: aortic valve disease, mitral valve disease (including mitral stenosis), tricuspid valve disease, pulmonary valve disease Excluded: mechanical or bioprosthetic valves |
Multicenter, retrospective cohort study using data from Centers for Medicare and Medicaid Services patient records and linking data sources using 3-way propensity-matched scores | Dabigatran vs rivaroxaban vs W group: average age (in years) 77 vs 77 vs 80 (P = 0.1); CHA2DS2-VASc score mean 5 vs 5 vs 5.6 (P = 0.2); HAS-BLED score mean 1.8 vs 1.8 vs 2 (P = 0.1) | Stroke: dabigatran (n = 20 [1.02%]) vs W(n = 18 [0.92%]; HR = 1.12; 95% CI = 0.59–1.1; P = 0.7); rivaroxaban (n = 22 [1.12%]) vs W (n = 18 [0.92%]; HR = 1.3; 95% CI = 0.7–2.4; P = 0.4); rivaroxaban (n = 22 [1.12%]) vs dabigatran (n = 20 [1.02%]; HR = 1.1; 95% CI = 0.64–2.1; P = 1.1) All-cause mortality: dabigatran (n = 63 [3.22%]) vs W (n = 90 [4.59%]; HR = 0.71; 95% CI = 0.52–0.98; P = 0.038); rivaroxaban (n = 60 [3.07%]) vs W (n = 90 [4.59%]; HR = 0.68; 95% CI = 0.49–0.95; P = 0.022); rivaroxaban (n = 60 [3.07%]) vs dabigatran (n = 63 [3.22%]; HR = 0.96; 95% CI = 0.67–1.37; P = 0.82) |
Non-GI bleeding: dabigatran (n = 4 [0.21%]) vs W (n = 23 [1.12%]; HR = 0.17; 95% CI = 0.06–0.49; P = 0.001); rivaroxaban (n = 8 [0.41%]) vs W (n = 23 [1.12%]; HR = 0.37; 95% CI = 0.17–0.84; P = 0.017); rivaroxaban (n = 8 [0.41%]) vs dabigatran (n = 4 [0.21%]; HR = 2.2; 95% CI = 0.66–7.3; P = 0.2) GI bleeding: dabigatran (n = 71 [3.63%]) vs W (n = 56 [2.87%]; HR = 1.27; 95% CI = 0.9–1.8; P = 0.17); rivaroxaban (n = 73 [3.73%]) vs W (n = 56 [2.87%]; HR = 1.4; 95% CI = 0.99–1.99; P = 0.05); rivaroxaban (n = 73 [3.73%]) vs dabigatran (n = 71 [3.63%]; HR = 1.1; 95% CI = 0.8–1.5; P = 0.5) |
| Carnicelli et al, 20175 | Edoxaban 60 mg (n = 63)c or edoxaban 30 mgc (n = 58) vs W (n = 70) Included: aortic or mitral bioprosthetic heart valves implanted >30 days prior to randomization Excluded: mechanical heart valve, unresected atrial myxoma, and moderate or severe MS |
Retrospective analysis of a randomized multicenter, double-blind, placebo-controlled trial (ENGAGE AF-TIMI 48) | NR between VHD groups | Stroke or SEE: edoxaban 60 mg vs W 4.76% vs 11.42% (HR = 0.37; 95% CI = 0.10–1.42; P = 0.15); edoxaban 30 mg vs W 6.89% vs 11.42% (HR = 0.53; 95% CI = 0.16–1.78; P = 0.31) | Major bleeding: edoxaban 60 mg vs W 6.35% vs 12.86% (HR = 0.50; 95% CI = 0.15–1.67; P = 0.26); edoxaban 30 mg vs W 1.72% vs 12.86% (HR = 0.12; 95% CI = 0.01–0.95; P = 0.045) |
| De Caterina et al, 201721 | Edoxaban 60 mgc (n = 917) or edoxaban 30 mgc (n = 952) vs W (n = 955) Included: prior echocardiographic evidence of moderate AR or MR, AS, bioprosthetic heart valves, prior valve repair Excluded: mechanical heart valve, unresected atrial myxoma, and moderate or severe MS |
Retrospective analysis of a randomized multicenter, double-blind, placebo-controlled trial (ENGAGE-TIMI-AF) | Edoxaban 60 mg or edoxaban 30 mg vs W group: age 72.27 vs 71.41 vs 71.85 years (P = 0.115); BMI 28.71 vs 28.95 vs 28.72 kg/m2 (P = 0.559); aspirin use at randomization 33.0% vs 32.6% vs 34.4% (P = 0.727); history of stroke/TIA 31.1 % vs 33.8% vs 35.0% (P = 0.649); CHA2DS2-VASc 4.58 vs 4.50 vs 4.59 (P = 0.549); HAS-BLED 2.59 vs 2.51 vs 2.54 (P = 0.114) | Stroke or SEE: edoxaban 60 mg vs W (HR = 0.69; 95% CI = 0.44–1.07; P = 0.097); edoxaban 30 mg vs W (HR = 0.97; 95% CI = 0.66–1.44; P = 0.893) | Major bleeding: edoxaban 60 mg vs W (HR = 0.74; 95% CI = 0.53–1.02; P = 0.068); edoxaban 30 mg vs W (HR = 0.41; 95% CI = 0.28–0.60; P < 0.001) |
| Durães et al, 20I631 | D110 twice daily (n = 15), vs W (n = 12)b; trial discontinued early because of low enrollment Included: bioprosthetic mitral and/or aortic valve replacement >3 months prior Excluded: concomitant use of antiplatelets, previous hemorrhagic stroke |
Single-center, open-label, randomized pilot study | Dabigatran vs W: average age 48.8 vs 45.7 years; hypertension 46.7% vs 50%; previous stroke 26.7% vs 33.3%; average LVEF 40 % vs 50%; HAS-BLED median 0 (0–1) vs 0 (0–1) | Dabigatran vs W: intracardiac thrombus 0% vs 8.3% (n = 1; RR 1.1; 95% CI = 0.9–1.3); stroke/SEE 0% vs 8.3% (n = 1; RR = 1.1; 95% CI = 0.9–1.3); death 0% vs 8.3% (n = 1; RR = 1.1; 95% CI = 0.9–1.3) | Dabigatran vs W: bleeding 6.7% (n = 1) vs 16.7% (n = 2; RR = 2.8; 95% CI = 0.2–35) |
| Ezekowitz et al, 20164 | VHD group: D110 twice daily (n = 1293) or D150 twice daily (n = 1354) vs W (n = 1305) Included: MR, TR, AR, AS, or mild MS Excluded: prosthetic heart valves, moderate or severe MS |
Retrospective analysis of multicenter, randomized controlled trial with blind dabigatran doses but open-label W use (RE-LY); propensity scores estimated | D110 or D150 vs W group: median age 74 or 74 vs 74 years (D110 vs W, P = 0.68; D150 vs W, P = 1.00); BMI 27.64 or 27.73 vs 27.64 kg/m2 (D110 vs W, P = 0.53; D150 vs W P = 1.00); history of SEE/TIA 21.6% or 22.9% vs 21.9% (D110 vs W, P = 1.00; D150 vs W, P = 1.00); median CHADS2 2.00 or 2.00 vs 2.00 (D110 vs W, P = 0.55; D150 vs W, P = 1.00) | Ischemic stroke or SE: D110 vs W: 3.63% (n = 47) vs 3.75% (n = 49); HR = 0.97 [95% CI = 0.65–1.45; P = 0.9]; D150 vs W: 2.22% (n = 30) vs 3.75% (n = 49); HR = 0.59 [95% CI = 0.37–0.93; P = 0.021] | Major bleeding: D110 vs W: 7.42% (n = 96) vs 10.11% (n = 132); HR = 0.73 [95% CI = 0.56–0.95; P = 0.017]; D150 vs W: 8.35% (n = 113) vs 10.11% (n = 132); HR = 0.82 [95% CI = 0.64–1.06; P = 0.12] |
| Guimarães et al, 201923 | Apixaban 5 mg twice dailya (n = 87) vs W (n = 69) Included: valve repair, bioprosthetic valve replacement Excluded: right-sided valve repair only, mechanical heart valves, moderate or severe MS |
Retrospective analysis of a randomized multicenter, double-blind, placebo-controlled trial (ARISTOTLE) | Apixaban vs W: median age in years 72 vs 74 (P = 0.5088); prior stroke, TIA, or SE 27.6% vs 17.4% (P = 0.1333); CHADS2 score <1: (35.6% vs 26.1%), 2: (29.9% vs 40.6%), >3: (34.5% vs 33.3%) [P = 0.3008]; HAS-BLED score <1: (27.6% vs 26.1%), 2 (36.8% vs 40.6%), >3 (35.6% vs 33.3%) [P = 0.8891] | Stroke or SEE: apixaban vs W 4.59% (n = 4) vs 2.89% (n = 2), HR = 1.714 (95% CI = 0.313–9.372; P = 0.53) | Major bleeding: apixaban vs W 8.05% (n = 7) vs 10.14% (n = 7; HR = 0.882; 95% CI = 0.309–2.519; P = 0.82) |
| Hampton et al, 2020 (NOSd: S 3 stars, C 0 stars, and O 3 stars)30 | Apixaban 2.5 or 5 mg bid (n = 133), vs rivaroxaban 15 or 20 mg daily (n = 50) vs dabigatran 75 or 150 mg bid (n = 17) Included: Bioprosthetic valves, annuloplasty ring, or moderate to severe mitral, tricuspid, or aortic valve disease documented by echocardiogram Excluded: mechanical valve, receiving dual antiplatelet therapy, receiving anticoagulation for another indication |
Single center, retrospective cohort study | Apixaban vs rivaroxaban vs dabigatran group: age 77.06 vs 74.73 vs 76.43 years (P = 0.26); weight (kg) 79.08 vs 87.16 vs 85.87 (P = 0.07); CHA2DS2-VASc score 4.35 vs 4.02 vs 4.06 (P = 0.38); HAS-BLED score 2.92 vs 2.66 vs 2.59 (P = 0.18) | Stroke or SEE: apixaban (n = 1 [0.8%]) vs rivaroxaban (n = 3 [6%]) vs dabigatran (n = 3 [17.6%]); P = 0.001 | Major bleeding: apixaban (n = 53.8%]) vs rivaroxaban (n = 4 [8%]) vs dabigatran (n = 2 [11.8%]); P = 0.264 |
| Kim et al, 2019 (NOSd: S 3 stars, C 2 stars, and O 2 stars)33 | Apixaban (n = 192), dabigatran (n = 367), rivaroxaban (n = 472), or edoxaban (n = 84) vs W (n = 1115) Included: any degree of MS, prescribed anticoagulation for ≥3 weeks Excluded: history of mitral valve surgery |
Multicenter, retrospective cohort study using data from the Republic of Korea Health Insurance Review and Assessment Service database; 1:1 propensity score matching | DOAC group vs W group: age 69.2 vs 70.2 years (P = 0.90); previous stroke 46.5 vs 46.7% (P = 0.90); mean CHA2DS2-VASc score was 5.2 (NR between groups) | DOAC group vs W group: ischemic stroke or SE 2.69% (n = 30) vs 13.09% (n = 140; HR = 0.28; 95% CI = 0.18–0.45) | DOAC group vs W group: ICH 0.63% (n = 7) vs 3.23% (n = 36; HR = 0.53; 95% CI = 0.22–1.26) |
| Strange et al, 2020 (NOSd: S 4 stars, C 2 stars, and O 2 stars)28 | Apixaban 2.5 or 5 mg bid (n = 942) or rivaroxaban 15 or 20 mg (n = 620) vs W (n = 1115) Included AS, AR, MR, bioprosthetic valves, mitral or aortic valve repairs Excluded: mechanical heart valves, MS, another indication for anticoagulation |
Multicenter, retrospective cohort study using nationwide Danish registries | Apixaban vs rivaroxaban vs W group: males 46.1 vs 49.7 vs 56.6% (P < 0.001); median age 81.0 vs 80.0 vs 77.0 years (P < 0.001); CHA2DS2-VASc score mean 3.9 vs 3.6 vs 3.5 (P = 0.023); HAS-BLED score mean: 2.7 vs 2.6 vs 2.6 (P = 0.023); previous stroke/SE n (%): 165 (17.5%) vs 109 (17.6%) vs 132 (11.8%), P < 0.001 | DOAC vs W Stroke or SEE: HR = 0.94 (95% CI = 0.56–1.59; P = 0.83) All-cause mortality: HR = 0.89 (95% CI = 0.74–1.07; P = 0.204) |
DOAC vs W major bleeding: HR = 0.77 (95% CI 0.53–1.10; P = 0.151) |
| Vinereanu et al, 201824 | Apixaban 5 mg twice dailya (n = 2438) vs W with VHD (n = 2370); MR (n = 3382), AR (n = 842), AS (n = 324) Included: MR, AR, AS Excluded: Right-sided valve repair only, mechanical heart valves, moderate or severe MS |
Retrospective analysis of a randomized multicenter, double-blind, placebo-controlled trial (ARISTOTLE) | NR between apixaban vs W groups | Stroke or SEE rate/100 patient-years (n = events), apixaban vs W: with MR, 1.25 (n = 39) vs 1.80 (n = 55; HR = 0.689; 95% CI = 0.457–1.039); with AR, 1.50 (n = 12) vs 2.38 (n = 17; HR = 0.573; 95% CI = 0.273–1.205); with AS, 2.16 (n = 7) vs 5.36 (n = 12; HR = 0.439; 95% CI = 0.171–1.128) | Major bleeding rate/100 patient-years (n = events), apixaban vs W: with MR, 2.01 (n = 57) vs 2.89 (n = 78; HR = 0.684; 95% CI = 0.486–0.963); with AR, 2.06 (n = 15) vs 3.34 (n = 21; HR = 0.609; 95% CI = 0.313–1.185); with AS, 4.03 (n = 11) vs 7.24 (n = 13; HR = 0.549; 95% CI = 0.245–1.231) |
Abbreviations: AF, atrial fibrillation; AR, aortic regurgitation; AS, aortic stenosis; BMI, body mass index; C, compatibility of study cohorts; CHADS2 or CHA2DS2-VASc, scoring system for measuring ischemic stroke risk; D110, dabigatran 110 mg twice daily; D150, dabigatran 150 mg twice daily; DOAC, direct oral anticoagulants; GI, gastrointestinal; HAS-BLED, scoring system for measuring major bleeding risk; HR, hazard ratio; ICH, intracranial hemorrhage; LVEF, left ventricular ejection fraction; MR, mitral regurgitation; MS, mitral stenosis; NOS, Newcastle-Ottawa Scale; NR, not reported; O, outcome ascertainment; RR, relative risk; S, selection of study cohorts; SE, systemic embolism; SEE, systemic embolic event; TIA, transient ischemic attack; TR, tricuspid regurgitation; VHD, valvular heart disease; VTE, venous thromboembolism; W, warfarin.
Apixaban dose adjusted to 2.5 mg twice daily for patients with >2 of the following criteria: age ≥80 years, body weight ≤60 kg, or serum creatinine level ≥1.5 mg/dL.
Rivaroxaban dose adjusted to 15 mg daily if CrCl =15–49 mL/min.
Edoxaban dose adjusted from 60 mg daily to 30 mg daily or from 30 mg daily to 15 mg daily if >1 of the following was present: creatinine clearance (CrCl) 30–49 mL/min, weight <60 kg, concomitant therapy with strong P-glycoprotein inhibitors.
The Newcastle-Ottawa Scale uses a star system to evaluate 3 perspectives: the selection of study cohorts, the comparability between 2 cohorts, and the ascertainment of outcomes for the included cohort studies.
The RE-LY (Randomized Evaluation of Long-Term Anticoagulant Therapy) trial evaluated dabigatran compared with warfarin in 18 113 patients with AF.19 A post hoc analysis of the RE-LY trial with VHD by Ezekowitz et al4 found that dabigatran 150 mg twice daily had lower event rates of stroke or systemic embolic event (SEE) compared with the warfarin group for those patients with VHD. Still, the major bleeding rates were comparable to those in the warfarin group. A limitation of this study was that the original trial for the post hoc analysis was an open-label trial, which could be subject to observer bias. Also, the study did not discuss severity of VHD.
The efficacy and safety of edoxaban 30 or 60 mg once daily was compared with warfarin in the ENGAGE AF-TIMI 48 trial (Effective Anticoagulation with Factor Xa Next Generation in Atrial Fibrillation Thrombolysis in Myocardial Infarction 48).20 The post hoc analysis of ENGAGE AF-TIMI 48 by Carnicelli et al5 showed that patients taking edoxaban had similar rates of stroke or SEE and major bleeding rates compared with warfarin. Of note, baseline characteristics were not reported between groups. Additionally, another post hoc analysis of ENGAGE AF-TIMI 48 by De Caterina et al21 looked at patients with VHD, defined as aortic stenosis, baseline evidence or history of at least moderate mitral or aortic regurgitation, or prior valvular surgery, including valve repair, valvuloplasty, and bioprosthetic valve placement. The edoxaban 30 and 60mg groups had similar rates of stroke/SEE and rates of major bleeding compared with the warfarin group. Although the study displayed the total number of events for each outcome in patients with VHD and mentioned that the low event occurrence may be a limitation, the number of events between each anticoagulation group was not clearly defined.
A post hoc study of the ROCKET AF trial22 (Rivaroxaban Once Daily Oral Direct Factor Xa Inhibition Compared With Vitamin K Antagonism for Prevention of Stroke Embolism Trial and Atrial Fibrillation) by Breithardt et al6 looked at rivaroxaban 20 mg daily compared with warfarin in a subgroup of patients with VAF. In patients with VAF, rivaroxaban and warfarin had comparable rates of stroke or SEE, whereas major and nonmajor clinically relevant bleeding rates were higher in the rivaroxaban group compared with the warfarin group. The limitation is that the study excluded bioprosthetic valves and did not quantify severity of VHD.
Apixaban 5 mg twice daily has also been analyzed for use in VAF. In a post hoc analysis of the ARISTOTLE trial by Avezum et al7 (Apixaban for Reduction in Stroke and Other Thromboembolic Events in Atrial Fibrillation) in patients with VHD, the stroke/SEE rate was significantly lower in the apixaban group than the warfarin group, and International Society of Thrombosis and Haemostasis major bleeding was not significantly different between groups.7 For patients in the ARISTOTLE trial who had a history of bioprosthetic valve replacement or native valve repair, a post hoc analysis by Guimarães et al23 found no significant differences in the occurrence of stroke/SEE or major bleeding between those taking apixaban or warfarin. However, this study had a low number of events occurring in either group, reflected in the wide CI and higher risk of type 2 error. Another post hoc analysis of ARISTOTLE by Vinereanu et al24 examined the relationship between 3 different subtypes of VHD (mitral regurgitation, aortic regurgitation, and aortic stenosis) and found no differences in effect of apixaban compared with warfarin for stroke/SEE or major bleeding in any of the subtypes of VHD.
Three meta-analyses were conducted based on clinical trials that included patients with AF and VHD receiving one of the DOACs (apixaban, dabigatran, edoxaban, or rivaroxaban).25–27 All 3 meta-analyses showed superiority of DOACs to warfarin for reducing stroke/SEE and intracranial hemorrhage, whereas there was no significant difference in major bleeding rates between the 2 groups. These meta-analyses offer support for the use of DOACs in patients with AF and VHD except for moderate or severe mitral stenosis or mechanical heart valves.
Five real-world retrospective studies have also been conducted concerning the use of DOACs for VAF (Table 1). The retrospective cohort study using the Danish National Patient Registry by Strange et al28 found no significant differences in stroke/SEE, major bleeding, and all-cause mortality rates between the DOAC (rivaroxaban or apixaban) and warfarin groups. However, this study relied on diagnostic codes for reporting outcomes. Also, the number of events between 2 anticoagulation groups was not clearly defined.
Another retrospective cohort study by Briasoulis et al29 analyzed Medicare beneficiaries with VAF (excluding mechanical or bioprosthetic valves) who were on chronic anticoagulation with rivaroxaban, dabigatran, or warfarin. Although this study included patients with mitral stenosis regardless of severity, only 323 of the 9960 patients initially identified with mitral valve disease had mitral stenosis, which was not independently analyzed. Overall, both rivaroxaban and dabigatran had a significantly lower event of death compared with warfarin, but ischemic stroke rates were similar between groups. Whereas gastrointestinal bleeding rates were similar between groups, DOACs had less nongastrointestinal bleeding rates compared with warfarin. However, this study did not clearly specify bleeding definitions, and event outcomes were based on diagnostic codes. The third single-center retrospective cohort study by Hampton et al30 followed patients with AF and VHD for at least 6 months after identification. Although the stroke/SEE rate was significantly higher in the dabigatran group compared with the rivaroxaban or apixaban groups, the dabigatran group was followed significantly longer than the apixaban and rivaroxaban groups (1464 vs 662.7 vs 1016.9 days; P < 0.001) and the stroke/SEE event may have been more easily detected in the longer follow-up period. The rates of major bleeding were similar between all 3 groups. This study enrolled patients based on type of VHD rather than DOAC prescribed, and because the majority of patients were on apixaban, it is challenging to draw comparisons between DOACs.
The only prospective study was a randomized controlled trial by Durães et al,31 which examined the outcomes of dabigatran compared with warfarin for patients with AF and/or bioprosthetic mitral or aortic valve replacement (Table 1). The trial was stopped early because of low enrollment but found similar occurrence of intracardiac thrombus, stroke/SEE, and bleeding after 90 days. However, because of the low number of enrolled patients and event rates between groups, there may be a strong possibility of a type 2 error. Another meta-analysis included this randomized trial in addition to post hoc analyses of the previously discussed pivotal DOAC phase 3 trials.32 It found that stroke/SEE and ICH rates were significantly reduced in patients with DOAC compared with warfarin (HR = 0.73, 95% CI = 0.60–0.90, and HR = 0.45, 95% CI = 0.24–0.87, respectively). Major bleeding occurrence was similar between groups (HR = 0.90; 95% CI = 0.68–1.20).
Whereas patients with mitral stenosis were excluded in the pivotal phase 3 trials for DOACs in AF and real-world studies discussed above, patients with mitral stenosis and AF were examined in a multicenter, big data-based retrospective study by Kim et al33 using 1:1 propensity score matching. After a mean follow-up of 27 months, the study found that stroke/SEE rate was significantly lower in the DOAC than the warfarin group. DOACs and warfarin also had similar ICH rates in this patient population, but other bleeding outcomes were not examined in the study. Although this is a retrospective study and it did not evaluate compliance, dosing, and time in therapeutic range for warfarin therapy, this was the first study evaluating the use of DOACs in patients with AF and mitral stenosis. Further prospective study investigation is needed for DOAC use in patients with AF and mitral stenosis prior to its routine use.
Heart Failure
HF is a risk factor for thromboembolism or stroke in patients with AF, but patients with HF with reduced ejection fraction (HFrEF) who remain in sinus rhythm also have an increased risk of stroke compared with those without HFrEF.34 Although the exact mechanisms are unclear, the hemostatic nature of HFrEF from decreased cardiac output could contribute to an increase in oxidative stress from tissue hypoperfusion.35 This may lead to an activation of proinflammatory cytokines and tumor necrosis factor, which can activate factor Xa and prothrombin. Therefore, inhibiting these elements of the coagulation system through DOACs is of keen interest. The current guideline recommendations by the ACA/AHA do not support routine chronic anticoagulation for HF without another compelling indication.10 Warfarin has not been shown to reduce death or thromboembolic events in patients with sinus rhythm, and HFrEF and may increase the risk of bleeding in patients with or without concurrent antithrombotic therapy.36–39 However, since the ACC/AHA HF guideline publication in 2013, 2 major trials evaluated the use of DOACs in patients with HF (Table 2).10
Table 2.
Summary of Studies of DOACs in Heart Failure Patients With Sinus Rhythm.
| Author/year | Study treatments and inclusion criteria | Study design | Baseline characteristics of the study population | Efficacy outcome results | Safety outcome results |
|---|---|---|---|---|---|
| Branch et al, 201944 | HFrEF cohort: rivaroxaban 2.5 mg twice daily and aspirin 100 mg daily (n = 236) or rivaroxaban 5 mg twice daily (n = 245) vs aspirin 100 mg daily (n = 240); LVEF between 30%−40% (n = 721), PAD or CAD and ≥65 years old or <65 years old and documented atherosclerosis or 2 risk factors (current smoker, HF, diabetes mellitus, nonlacunar ischemic stroke >1 month prior, eGFR <60 mL/min) | Retrospective analysis of multicenter, double-blind randomized clinical trial (COMPASS) | NR between aspirin and rivaroxaban groups with HF | Composite of cardiovascular death, stroke, or MI: rivaroxaban plus aspirin vs aspirin alone group: 10.2% vs 12.1% (HR = 0.82; 95% CI = 0.47–1.40); rivaroxaban alone vs aspirin alone group: 12.7% vs 12.1 % (HR = 1.07; 95% CI = 0.65–1.78) Stroke: rivaroxaban plus aspirin vs aspirin alone group: 2% vs 3% (HR = 0.74; 95% CI = 0.23–2.35), NR for rivaroxaban alone vs aspirin alone |
Major bleeding: rivaroxaban plus aspirin vs aspirin alone group: 4.7% vs 2.1% (HR = 2.30; 95% CI = 0.80–6.62); rivaroxaban alone vs aspirin alone group: 4.1% vs 2.1% (HR = 1.96; 95% CI = 0.67–5.75) |
| Greenberg et al, 201942 | Same as Zannad et al40 below | Retrospective analysis of multicenter, double-blind randomized clinical trial (COMMANDER HF) | NR; see Zannad et al below (COMMANDER HF) | Rivaroxaban vs placebo group: Thromboembolic composite (1): 13.1% vs 15.5% (HR = 0.83; 95% CI = 0.72–0.96; P = 0.01) Thromboembolic composite (2): 6.1% vs 7.6% (HR = 0.80; 95% CI = 0.64–0.98; P = 0.04) |
NR; see Zannad et al (COMMANDER HF) |
| Mehra et al, 201941 | Same as Zannad et al below | Retrospective analysis of multicenter, double-blind randomized clinical trial (COMMANDER HF) | NR; see Zannad et al below (COMMANDER HF) | Rivaroxaban vs placebo group: all-cause stroke or TIA: 2.4% vs 3.5%; 1.29 events vs 1.90 events/100 patient-years (HR = 0.68; 95% CI = 0.49–0.94; P = 0.02) | NR; see Zannad et al below (COMMANDER HF) |
| Zannad et al, 201840 | Rivaroxaban 2.5 mg twice daily (n = 2507) vs placebo (n = 2515); ≥3 months Hx HF, LVEF ≤40%, CAD, NSR, treated for HF episode <21 days prior to enrollment, BNP ≥200 pg/mL or NT-proBNP ≥800 pg/mL | Multicenter, double-blind randomized clinical trial (COMMANDER HF) | Rivaroxaban vs placebo group: average age 66.5 vs 66.3 years; average BMI 27.6 vs 27.8 kg/m2; previous stroke 8.3% vs 9.7%; NYHA: class 13.2% vs 2.7%; class II 44.8% vs 43.6%; class III 48.2% vs 49.9%; class IV 3.8% vs 3.8% | Rivaroxaban vs placebo group: composite of death from any cause, MI, or stroke: 25.0% vs 26.2% (HR = 0.94; 95% CI = 0.84–1.05; P = 0.27) Stroke: 2.0% vs 3.0% (HR = 0.66; 95% CI = 0.47–0.95) |
Rivaroxaban vs placebo group: fatal bleeding or bleeding into a critical space with potential for permanent disability: 0.7% vs 0.9% (HR = 0.80; 95% CI = 0.43–1.49; P = 0.48) |
Abbreviations: BNP, brain natriuretic peptide; BMI, body mass index; CAD, coronary artery disease; DOAC, direct oral anticoagulants; eGFR, estimated glomerular filtration rate; HF, heart failure; HFrEF, HF with reduced ejection fraction; HR, hazard ratio; Hx, history; LVEF, left ventricular ejection fraction; MI, myocardial infarction; NR, not reported; NSR, normal sinus rhythm; NT-proBNP, N-terminal pro-brain natriuretic peptide; NYHA, New York Heart Association; PAD, peripheral artery disease; TIA, transient ischemic attack.
The COMMANDER HF trial (A Study to Assess the Effectiveness and Safety of Rivaroxaban in Reducing the Risk of Death, Myocardial Infarction, or Stroke in Participants with Heart Failure and Coronary Artery Disease Following an Episode of Decompensated Heart Failure) was a randomized, double-blind, placebo-controlled trial that examined adding rivaroxaban 2.5 mg twice daily to standard care after an episode of worsening HF in order to reduce thromboembolic outcomes in patients with HFrEF, CAD, and sinus rhythm.40 There was no significant difference in the composite outcome of all-cause mortality, stroke, or MI between the rivaroxaban versus placebo groups, but the stroke rates were significantly lower in the rivaroxaban group. There was no significant difference in the primary composite safety outcome of fatal or potentially permanent disability causing bleeding. The major limitation was that these patients were enrolled after a HF exacerbation. The composite end point did include mortality, which may have been attributed to the HF itself. Patients may also have had paroxysmal AF in the absence of electrocardiographic monitoring.
Because treatment with rivaroxaban was associated with a reduced stroke rate compared with placebo without an increase in major bleeding, several post hoc analyses were conducted to investigate its use further in patients with HFrEF, CAD, and sinus rhythm for stroke prevention. One of these studies by Mehra et al41 specifically examined stroke outcomes and included all patients in the COMMANDER HF trial. The analysis of this population found that low-dose rivaroxaban significantly reduced rates of stroke or transient ischemic attack (TIA) compared with placebo. Of note, the benefits of adding low-dose rivaroxaban was seen in patients with CHA2DS2-VASc score >4.0 but not found in patients with scores ≤4.0. Also, because patients who had a prior stroke or TIA within 90 days of enrollment were excluded, the stroke risk may have been underestimated. Additionally, the CHA2DS2-VASc score is only validated to predict stroke risk in patients with AF, not in those with HF. Another post hoc analysis of COMMANDER HF by Greenberg et al42 also examined a composite thromboembolic end point (MI, ischemic stroke, symptomatic PE, or symptomatic DVT) with and without sudden/unwitnessed deaths as a component. Thromboembolic events were significantly less frequent in the rivaroxaban group compared with placebo, but none of the individual efficacy outcomes was significantly different between the 2 groups. Although neither study reported an additional safety end point, COMMANDER HF did not find a significant difference in major bleeding. Several of the end points in both studies were not adjudicated by an independent committee in COMMANDER HF. Although mortality was similar between groups, these analyses add support to the proposition that low-dose rivaroxaban may possibly reduce thromboembolic events in patients with recent worsening of chronic HF, CAD, and sinus rhythm without increasing the risk of bleeding. However, post hoc analyses should be interpreted as hypothesis generating, and prospective studies are needed.
The Cardiovascular Outcomes for People Using Anticoagulation Strategies (COMPASS) trial examined the impact of rivaroxaban, aspirin, or the combination on cardiovascular outcomes in patients with mild to moderate HF and chronic coronary or peripheral artery disease.43 However, this study excluded patients with EF <30% or New York Heart Association functional class III or IV, and the minority (12%) of the patients in the HF group had HFrEF. Additionally, this study did not record baseline AF, although patients who were chronically anticoagulated were excluded. The post hoc study in the patient subgroup with HFrEF by Branch et al44 showed that rivaroxaban plus aspirin had similar outcomes in the composite end point of cardiovascular death, stroke, or MI as well as stroke rates compared with those patients taking aspirin alone. The major bleeding rates were numerically higher in the rivaroxaban plus aspirin group but were not statistically significant. Similarly, no significant differences in the composite end point or major bleeding events were noted in the rivaroxaban alone group compared with the aspirin group. There were wide CIs because of the limited number of patients, and the risk of type 2 error cannot be dismissed. This study is also a post hoc analysis for hypothesis generating.
Overall, in patients with HFrEF, neither rivaroxaban 2.5 mg twice daily with aspirin nor rivaroxaban alone improved the composite outcome of stroke, MI, or cardiovascular death. However, for patients with HFrEF with worsening HF, CAD, and sinus rhythm, rivaroxaban 2.5 mg twice daily with or without aspirin did not have an increased risk of major bleeding compared with aspirin alone and may improve thromboembolic event rates. The post hoc analyses showed reduction in thromboembolism event rates, but major limitations exist in each study. Because all studies were post hoc analyses, future prospective studies need to reproduce the similar results and investigate the use of low-dose rivaroxaban 2.5 mg twice daily in patients with HFrEF prior to its routine use. To our knowledge, there is no study related to the use of anticoagulation therapy in patients with HF with preserved ejection fraction.
Left Ventricular Thrombus
LVT commonly develops in patients with HFrEF in the setting of acute anterior wall MI. Both the ACC/AHA STEMI (ST-elevation myocardial infarction) and AHA/American Stroke Association guidelines for the prevention of stroke in patients with stroke or TIA recommend VKAs as a first-line therapy for LVT treatment.3,45 Since the publication of the guidelines, multiple case series of DOACs investigated the efficacy and safety of DOACs in patients with LVT (Table 3) and showed LVT resolution rate with DOACs ranging from 80% to 100%.46–53 The meta-analysis of 52 case reports also found that 45 patients out of 49 patients (92%: 3 cases did not report LVT resolution) had LVT resolution, and rivaroxaban was most frequently used followed by apixaban.54 However, they did not compare DOACs with VKA controlled groups, and there were significant concerns of publication biases, which indicate that only positive outcome results could have been published. Thus, 4 recent retrospective real-world studies were published to compare the efficacy and safety between VKA and DOACs in patients with LVT (Table 3). Two small-scale single-center retrospective cohort studies showed that there were no significant differences in stroke or systemic embolism (SSE) or LVT resolution rates between the DOAC and VKA groups.9,55 Both these studies included very small sample sizes and did not have enough power to detect the difference in efficacy outcomes between the 2 groups. A multicenter retrospective cohort study by Robinson et al8 performed a multivariable analysis which found that DOAC use (n = 121) was associated with higher SSE than warfarin (n = 236). This is the largest study that compares DOACs with warfarin. The limitations were that LVT resolution was not evaluated, and compliance, time in therapeutic range, and dosing information were not collected in this study. In contrast, another retrospective study by Jones et al56 in patients with LVT after acute MI showed that DOAC use was associated with significantly higher LVT resolution rate compared with warfarin. Because of the conflicting study results, further investigation is needed before DOACs are recommended in routine practice setting.
Table 3.
DOAC Use for Left Ventricular Thrombus: Key Included Study Designs and Results.
| Study design | Participants | Follow-up period | Efficacy outcome | Safety outcome |
|---|---|---|---|---|
| Abdelnaby et al, 2019; case series50 | 8 Patients with ACS receiving dual antiplatelet therapy with rivaroxaban | NR | LVT resolution rate based on ECHO at 3 months: 87.5%. No TE event | No bleeding event |
| Bahmaid et al, 2019; case series51 | 7 Patients (rivaroxaban n = 6, dabigatran n = 1) | Mean follow-up 12.6 months | LVT resolution based on ECHO 100% | NR |
| Daher et al, 2020; single-center retrospective cohort study9 | DOAC 17 patients (apixaban n = 12, dabigatran n = 1, rivaroxaban n = 4) vs VKA 42 patients | NR | LVT resolution at 3 months: 70.6 vs 71.5% (P = 0.9) Stroke or SE: 11.8% vs 9.5% (P = 0.8) |
NR |
| Elikowski et al, 2019; case series47 | 7 Patients on apixaban | Mean follow-up 10.3 months | LVT resolution based on ECHO 100% | Major/minor bleeding 0% |
| Fleddermann et al, 2019; case series52 | 52 Patients total (apixaban n = 26, dabigatran n = 2, rivaroxaban n = 24); 35 patients with ECHO follow-up (apixaban n = 16, dabigatran n = 2, rivaroxaban n = 17) | Mean follow-up 11.7 months (for patients with ECHO follow-up) | LVT resolution based on ECHO: 83% (out of 35 patients); 1 cardioembolic event (TIA) | 3 GI bleeding events; 1 patient with epistaxis |
| Iqbal et al, 2020; single-center retrospective cohort study55 | DOAC 22 patients (apixaban n = 8, rivaroxaban n = 13, dabigatran n = 1) vs VKA 62 patients | Mean 3.0 ± 1.4 years | LVT resolution rate: 65.0% vs 75.0% (P = 0.33) Stroke: 0% vs 2.0% (P = 0.55) |
Clinically significant bleeding: 0% vs 10% (P = 0.13) |
| Jones et al, 2020; single-center retrospective cohort study56 | DOAC 41 patients (apixaban n = 15, rivaroxaban n = 24, edoxaban = 2) vs warfarin 60 patients | Median 2.2 years | LVT resolution rate: 82.0% vs 64.4% at 1 year (OR = 1.8; 95% CI = 1.2–2.9; P = 0.0018) Stroke or SSE: 2.4% vs 5% (P = 0.388) | Major bleeding 0% vs 6.7% (P = 0.030) |
| Makrides, 2016; case series53 | 3 Patients with anterior STEMI receiving dual antiplatelet therapy with rivaroxaban 15 mg daily | 3 Months of anticoagulation; 1 year follow-up | LVT resolution based on ECHO: 100% | No bleeding event |
| Robinson et al, 2020; multicenter retrospective cohort study8 | DOAC 121 patients (individual DOAC NR) vs warfarin 236 patients | Median 351 days (IQR 51–866 days) | LVT resolution rate: NR Stroke or SE: 14.0% vs 5.9% (HR = 2.64; 95% CI = 1.28–5.43; P = 0.01) | Bleeding (details in bleeding definition NR): 6.6% vs 8.1 % (further details in statistical analysis not performed) |
| Shokr et al, 2018; case series49 | 8 Patients (apixaban n = 4, rivaroxaban n = 4) | Mean duration of anticoagulation 4.6 months | LVT resolution based on ECHO: 100% | One GI bleeding |
| Smetana et al, 2017; case series46 | 10 Patients (apixaban n = 3, rivaroxaban n = 7) | 2 Years | Primary outcome LVT resolution rate at 3 months: 83% | 1 Patient on rivaroxaban had mild bleeding |
| Verma et al, 2019; case series48 | 15 Patients receiving dabigatran 110 mg twice daily for ischemic cardiomyopathy (n = 10) and 150 mg twice daily for nonischemic cardiomyopathy (n = 5) | Follow-up period of at least 6 months | LVT resolution at 3 months: 93%; 100% at 6 months | One GI bleeding |
Abbreviations: ACS, acute coronary syndrome; DOAC, direct oral anticoagulants; ECHO, echocardiogram; GI, gastrointestinal; HR, hazard ratio; IQR, interquartile range; LVT, left ventricular thrombus; NR, not reported; OR, odds ratio; SE, systemic embolism; SSE, stroke or systemic embolism; STEMI, ST-elevation myocardial infarction; TE, thromboembolism; TIA, transient ischemic attack; VKA, vitamin K antagonist.
Superficial Vein Thrombosis
Prior to DOAC availability, fondaparinux 2.5 mg subcutaneously once daily as a 45-day course was the gold standard therapy based on the only existing clinical trial, CALISTO, showing that fondaparinux significantly decreased the primary composite efficacy outcome of death from any cause, symptomatic PE, symptomatic DVT, or symptomatic extension to the saphenofemoral junction or symptomatic recurrence of SVT compared with placebo.57 However, parenteral injections for 45 days were not practical and convenient. Thus, rivaroxaban was investigated as an oral anticoagulant for SVT. The SURPRISE study is an open-label, randomized, noninferiority trial, which compared rivaroxaban 10 mg once daily with fondaparinux 2.5 mg once daily for 45 days in patients with symptomatic SVT.58 The primary efficacy outcome was a composite event rate of recurrence of SVT, symptomatic DVT, or PE and all-cause mortality. The primary safety outcome was a major bleeding rate. The primary efficacy outcome results were 3% in the rivaroxaban group (n = 211) and 2% in the fondaparinux group (n = 224; HR = 1.9, 95% CI = 0.6–6.4, P = 0.025 for noninferiority analysis), and neither group had a major bleeding event. Although this study was open label, it supports rivaroxaban 10 mg once daily as an alternative to fondaparinux for SVT treatment because of its noninferiority to fondaparinux and more convenient oral formulation over parenteral therapy for 45 days.
Pulmonary Hypertension
Patients with idiopathic pulmonary arterial hypertension (PAH) (group 1 PH) and chronic thromboembolic PH (CTEPH; group 4 PH) are often indicated for lifelong anticoagulation therapy, but anticoagulation therapy for other PH types (groups 2, 3, and 5) is not recommended. The ACC/AHA/American College of Chest Physicians guidelines suggest VKA with the therapeutic INR 1.5 to 2.5 in patients with idiopathic PAH (group 1 PH), and European Society of Cardiology PH guideline recommends lifelong oral anticoagulation therapy with either VKAs or DOACs for CTEPH.11,59 Since the publication of the last guidelines, DOACs have been utilized more in practice for PH.59 However, there is no published study available for DOAC use in patients with PAH (group 1 PH) that was identified within our comprehensive literature search. Only 2 studies (1 retrospective cohort study and 1 case series) are available for DOACs in patients with CTEPH. The first study is a case series of 20 CTEPH patients (16 patients on rivaroxaban, 3 patients on dabigatran, and 1 patient on apixaban) who were treated with DOACs.60 The mean follow-up period was 20.9 months, and 8 of 20 patients had undergone pulmonary endarterectomy. No patient developed a recurrent VTE episode during the follow-up. One patient developed major bleeding, but it occurred after a traumatic fall. Further evaluation in the study comparing DOACs with VKA was needed. Another study was the retrospective cohort study, which compared warfarin (n = 412) with rivaroxaban (n = 134) in patients with CTEPH.61 The major primary outcomes were death from any cause, recurrent VTE, and International Society of Thrombosis and Hemostasis major bleeding event. The mean follow-up period was 9.0 ± 8.5 years. The study was initially designed to compare warfarin and DOAC groups, but the majority of the DOACs was rivaroxaban. Therefore, events between warfarin and rivaroxaban groups were compared. There were no significant differences in recurrent VTE (8.9% vs 10.1%; HR = 1.21, 95% CI = 0.64–2.23, P = 0.55) and mortality rates (9.7% vs 13.8%; HR = 1.61, 95% CI = 0.89–2.99, P = 0.11) between the rivaroxaban and warfarin groups. The major bleeding rate was significantly lower in the rivaroxaban group than in the warfarin group (8.9% vs 14.8%; HR = 1.94, 95% CI = 1.05–3.62, P = 0.03). This study is the largest available for DOAC use in patients with CTEPH. A prospective study is needed, but no ongoing clinical trial is currently registered. It is unlikely that any clinical trial for the topic will be undertaken soon.
Future Directions
Multiple key clinical questions remain unanswered regarding off-label DOAC use. First, only 1 retrospective study evaluated the use of DOACs in patients with AF and mitral stenosis.33 Two ongoing/planned phase 4 clinical trials (dabigatran vs warfarin and rivaroxaban vs warfarin) will provide further insight into DOACs in patients with moderate to severe mitral stenosis complicated by AF.62,63 Second, the use of low-dose rivaroxaban therapy may be considered to prevent stroke events in patients with HFrEF, but future real-world studies need to verify the results from the post hoc studies of clinical trials for low-dose rivaroxaban therapy. Third, very limited data exist for DOACs in patients with PH. Especially, there are no published data on DOAC use in patients with PAH. There is no current ongoing clinical trial to answer the clinical question. Fourth, DOAC use in LVT treatment is still controversial based on conflicting real-world data. One ongoing multicenter randomized controlled trial is evaluating the efficacy and safety of rivaroxaban for post-STEMI LVT compared with warfarin.64 Another similarly designed, ongoing randomized controlled trial is investigating the use of apixaban in patients with STEMI who developed LVT compared with warfarin.65 Although they may answer the use of DOACs in the STEMI setting, uncertainty will still continue regarding the use of DOACs for LVT developed by nonischemic cardiomyopathy. Finally, no study evaluated the use of betrixaban in any off-label indication. Thus, this review is not appropriate to extrapolate results as a DOAC class effect.
Relevance to Patient Care and Clinical Practice
This review provides clinicians with useful information for 5 common clinical scenarios for DOAC off-label use: (1) VAF, (2) HFrEF with sinus rhythm, (3) LVT, (4) SVT, and (5) PH.
Conclusions
Our systematic review shows that DOACs should be considered in patients with AF for stroke prevention regardless of VHD status except for mitral stenosis or mechanical heart valve replacement. Oral anticoagulation therapy should generally be avoided in most patients with HF. If anticoagulation therapy is considered, rivaroxaban 2.5 mg twice daily may be considered to prevent stroke events for patients with HFrEF, CAD, and sinus rhythm. Still, prospective studies need to confirm the results from post hoc analyses of randomized controlled trials. DOAC use for LVT treatment is still controversial because of conflicting real-world study results, and further investigation is needed with ongoing clinical trials prior to its routine use for LVT. Rivaroxaban 10 mg once daily should be considered as an alternative to fondaparinux for SVT. Among DOACs for patients with CTEPH, rivaroxaban may be considered as an alternative to warfarin therapy, but warfarin is still the first-line therapy in patients with PAH because of paucity of clinical data for the use of DOACs in patients with PAH.
Supplementary Material
Funding
The authors received no financial support for the research, authorship, and/or publication of this article.
Footnotes
Declaration of Conflicting Interests
The authors declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Supplemental Material
Supplemental material for this article is available online.
References
- 1.Lip GYH, Banerjee A, Boriani G, et al. Antithrombotic therapy for atrial fibrillation: CHEST guideline and expert panel report. Chest. 2018;154:1121–1201. doi: 10.1016/j.chest.2018.07.040 [DOI] [PubMed] [Google Scholar]
- 2.Anderson SL, Marrs JC. Can direct oral anticoagulants be used for stroke prevention among patients with valvular atrial fibrillation? Curr Cardiol Rep. 2019;21:118. doi: 10.1007/s11886-019-1199-4 [DOI] [PubMed] [Google Scholar]
- 3.O’Gara PT, Kushner FG, Ascheim DD, et al. 2013 ACCF/AHA guideline for the management of ST-elevation myocardial infarction: a report of the American College of Cardiology Foundation/American Heart Association Task Force on Practice Guidelines. J Am Coll Cardiol. 2013;61:e78–e140. doi: 10.1016/j.jacc.2012.11.019 [DOI] [PubMed] [Google Scholar]
- 4.Ezekowitz MD, Nagarakanti R, Noack H, et al. Comparison of dabigatran and warfarin in patients with atrial fibrillation and valvular heart disease: the RE-LY Trial (Randomized Evaluation of Long-Term Anticoagulant Therapy). Circulation. 2016;134:589–598. doi: 10.1161/CIRCULATIONAHA.115.020950 [DOI] [PubMed] [Google Scholar]
- 5.Carnicelli AP, De Caterina R, Halperin JL, et al. Edoxaban for the prevention of thromboembolism in patients with atrial fibrillation and bioprosthetic valves. Circulation. 2017;135:1273–1275. doi: 10.1161/CIRCULATIONAHA.116.026714 [DOI] [PubMed] [Google Scholar]
- 6.Breithardt G, Baumgartner H, Berkowitz SD, et al. Clinical characteristics and outcomes with rivaroxaban vs. warfarin in patients with non-valvular atrial fibrillation but underlying native mitral and aortic valve disease participating in the ROCKET AF trial. Eur Heart J. 2014;35:3377–3385. doi: 10.1093/eurheartj/ehu305 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Avezum A, Lopes RD, Schulte PJ, et al. Apixaban in comparison with warfarin in patients with atrial fibrillation and valvular heart disease: findings from the Apixaban for Reduction in Stroke and Other Thromboembolic Events in Atrial Fibrillation (ARISTOTLE) Trial. Circulation. 2015;132:624–632. doi: 10.1161/CIRCULATIONAHA.114.014807 [DOI] [PubMed] [Google Scholar]
- 8.Robinson AA, Trankle CR, Eubanks G, et al. Off-label use of direct oral anticoagulants compared with warfarin for left ventricular thrombi. JAMA Cardiol. 2020;5:685–692. doi: 10.1001/jamacardio.2020.0652 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Daher J, Da Costa A, Hilaire C, et al. Management of left ventricular thrombi with direct oral anticoagulants: retrospective comparative study with vitamin K antagonists. Clin Drug Investig. 2020;40:343–353. doi: 10.1007/s40261-020-00898-3 [DOI] [PubMed] [Google Scholar]
- 10.Writing Committee Members; Yancy CW, Jessup M, et al. 2013 ACCF/AHA guideline for the management of heart failure: a report of the American College of Cardiology Foundation/American Heart Association Task Force on practice guidelines. Circulation. 2013;128:e240–e327. doi: 10.1161/CIR.0b013e31829e8807 [DOI] [PubMed] [Google Scholar]
- 11.McLaughlin VV, Archer SL, Badesch DB, et al. ACCF/AHA 2009 expert consensus document on pulmonary hypertension a report of the American College of Cardiology Foundation Task Force on Expert Consensus Documents and the American Heart Association developed in collaboration with the American College of Chest Physicians; American Thoracic Society, Inc.; and the Pulmonary Hypertension Association. J Am Coll Cardiol. 2009;53:1573–1619. doi: 10.1016/j.jacc.2009.01.004 [DOI] [PubMed] [Google Scholar]
- 12.Kearon C, Akl EA, Ornelas J, et al. Antithrombotic therapy for VTE disease: CHEST guideline and expert panel report. Chest. 2016;149:315–352. doi: 10.1016/j.chest.2015.11.026 [DOI] [PubMed] [Google Scholar]
- 13.Kearon C, Akl EA, Comerota AJ, et al. Antithrombotic therapy for VTE disease: Antithrombotic Therapy and Prevention of Thrombosis, 9th ed: American College of Chest Physicians Evidence-Based Clinical Practice Guidelines. Chest. 2012; 141(2, suppl):e419S–e496S. doi: 10.1378/chest.11-2301 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.The Ottawa Hospital. The Newcastle-Ottawa Scale (NOS) for assessing the quality of nonrandomised studies in meta-analyses. AccessedSeptember 13, 2020. http://www.ohri.ca/programs/clinical_epidemiology/oxford.asp
- 15.Sterne JAC, Savovic J, Page MJ, et al. RoB 2: a revised tool for assessing risk of bias in randomised trials. BMJ. 2019;366:l4898. doi: 10.1136/bmj.l4898 [DOI] [PubMed] [Google Scholar]
- 16.January CT, Wann LS, Calkins H, et al. 2019 AHA/ACC/HRS focused update of the 2014 AHA/ACC/HRS Guideline for the management of patients with atrial fibrillation: a report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines and the Heart Rhythm Society. J Am Coll Cardiol. 2019;74:104–132. doi: 10.1016/j.jacc.2019.01.011 [DOI] [PubMed] [Google Scholar]
- 17.Eikelboom JW, Connolly SJ, Brueckmann M, et al. Dabigatran versus warfarin in patients with mechanical heart valves. N Engl J Med. 2013;369:1206–1214. doi: 10.1056/NEJMoa1300615 [DOI] [PubMed] [Google Scholar]
- 18.January CT, Wann LS, Alpert JS, et al. 2014 AHA/ACC/HRS guideline for the management of patients with atrial fibrillation: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines and the Heart Rhythm Society. J Am Coll Cardiol. 2014;64:e1–e76. doi: 10.1016/j.jacc.2019.01.011 [DOI] [PubMed] [Google Scholar]
- 19.Connolly SJ, Ezekowitz MD, Yusuf S, et al. Dabigatran versus warfarin in patients with atrial fibrillation. N Engl J Med. 2009;361:1139–1151. doi: 10.1056/NEJMoa0905561 [DOI] [PubMed] [Google Scholar]
- 20.Giugliano RP, Ruff CT, Braunwald E, et al. Edoxaban versus warfarin in patients with atrial fibrillation. N Engl J Med. 2013;369:2093–2104. doi: 10.1056/NEJMoa1310907 [DOI] [PubMed] [Google Scholar]
- 21.De Caterina R, Renda G, Carnicelli AP, et al. Valvular heart disease patients on edoxaban or warfarin in the ENGAGE AF-TIMI 48 Trial. J Am Coll Cardiol. 2017;69:1372–1382. doi: 10.1016/j.jacc.2016.12.031 [DOI] [PubMed] [Google Scholar]
- 22.Patel MR, Mahaffey KW, Garg J, et al. Rivaroxaban versus warfarin in nonvalvular atrial fibrillation. N Engl J Med. 2011;365:883–891. doi: 10.1056/NEJMoa1009638 [DOI] [PubMed] [Google Scholar]
- 23.Guimarães PO, Pokorney SD, Lopes RD, et al. Efficacy and safety of apixaban vs warfarin in patients with atrial fibrillation and prior bioprosthetic valve replacement or valve repair: insights from the ARISTOTLE trial. Clin Cardiol. 2019;42: 568–571. doi: 10.1002/clc.23178 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Vinereanu D, Wang A, Mulder H, et al. Outcomes in anticoagulated patients with atrial fibrillation and with mitral or aortic valve disease. Heart. 2018;104:1292–1299. doi: 10.1136/heartjnl-2017-312272 [DOI] [PubMed] [Google Scholar]
- 25.Malik AH, Yandrapalli S, Aronow WS, Panza JA, Cooper HA. Oral anticoagulants in atrial fibrillation with valvular heart disease and bioprosthetic heart valves. Heart. 2019;105:1432–1436. doi: 10.1136/heartjnl-2019-314767 [DOI] [PubMed] [Google Scholar]
- 26.Pan KL, Singer DE, Ovbiagele B, Wu YL, Ahmed MA, Lee M. Effects of non-vitamin K antagonist oral anticoagulants versus warfarin in patients with atrial fibrillation and valvular heart disease: a systematic review and meta-analysis. J Am Heart Assoc. 2017;6:e005835. doi: 10.1161/JAHA.117.005835 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Renda G, Ricci F, Giugliano RP, De Caterina R. Non-vitamin K antagonist oral anticoagulants in patients with atrial fibrillation and valvular heart disease. J Am Coll Cardiol. 2017;69:1363–1371. doi: 10.1016/j.jacc.2016.12.038 [DOI] [PubMed] [Google Scholar]
- 28.Strange JE, Sindet-Pedersen C, Staerk L, et al. All-cause mortality, stroke, and bleeding in patients with atrial fibrillation and valvular heart disease. Eur Heart J Cardiovasc Pharmacother. Published online February 17, 2020. doi: 10.1093/ehjcvp/pvaa011 [DOI] [PubMed] [Google Scholar]
- 29.Briasoulis A, Inampudi C, Akintoye E, Alvarez P, Panaich S, Vaughan-Sarrazin M. Safety and efficacy of novel oral anticoagulants versus warfarin in Medicare beneficiaries with atrial fibrillation and valvular heart disease. J Am Heart Assoc. 2018;7:e008773. doi: 10.1161/JAHA.118.008773 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Hampton ML, Tellor KB, Armbruster AL, Theodos G, Schwarze MW. Evaluation of the Safety and Effectiveness of Direct-Acting Oral Anticoagulants in Patients with Atrial Fibrillation and Coexisting Valvular Heart Disease. Am J Cardiovasc Drugs. Published online February 11, 2020. doi: 10.1007/s40256-020-00398-x [DOI] [PubMed] [Google Scholar]
- 31.Durães AR, de Souza Roriz P, de Almeida Nunes B, et al. Dabigatran versus warfarin after bioprosthesis valve replacement for the management of atrial fibrillation postoperatively: DAWA Pilot Study. Drugs RD. 2016;16:149–154. doi: 10.1007/s40268-016-0124-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Caldeira D, David C, Costa J, Ferreira JJ, Pinto FJ. Non-vitamin K antagonist oral anticoagulants in patients with atrial fibrillation and valvular heart disease: systematic review and meta-analysis. Eur Heart J Cardiovasc Pharmacother. 2018;4:111–118. doi: 10.1093/ehjcvp/pvx028 [DOI] [PubMed] [Google Scholar]
- 33.Kim JY, Kim SH, Myong JP, et al. Outcomes of direct oral anticoagulants in patients with mitral stenosis. J Am Coll Cardiol. 2019;73:1123–1131. doi: 10.1016/j.jacc.2018.12.047 [DOI] [PubMed] [Google Scholar]
- 34.Lip GY, Piotrponikowski P, Andreotti F, et al. Thromboembolism and antithrombotic therapy for heart failure in sinus rhythm: an executive summary of a joint consensus document from the ESC Heart Failure Association and the ESC Working Group on Thrombosis. Thromb Haemost. 2012;108:1009–1022. doi: 10.1160/TH12-08-0578 [DOI] [PubMed] [Google Scholar]
- 35.Zannad F, Stough WG, Regnault V, et al. Is thrombosis a contributor to heart failure pathophysiology? Possible mechanisms, therapeutic opportunities, and clinical investigation challenges. Int J Cardiol. 2013;167:1772–1782. doi: 10.1016/j.ijcard.2012.12.018 [DOI] [PubMed] [Google Scholar]
- 36.Cleland JG, Findlay I, Jafri S, et al. The Warfarin/Aspirin Study in Heart failure (WASH): a randomized trial comparing antithrombotic strategies for patients with heart failure. Am Heart J. 2004;148:157–164. doi: 10.1016/j.ahj.2004.03.010 [DOI] [PubMed] [Google Scholar]
- 37.Cokkinos DV, Haralabopoulos GC, Kostis JB, Toutouzas PK; HELAS investigators. Efficacy of antithrombotic therapy in chronic heart failure: the HELAS study. Eur J Heart Fail. 2006;8:428–432. doi: 10.1016/j.ejheart.2006.02.012 [DOI] [PubMed] [Google Scholar]
- 38.Massie BM, Collins JF, Ammon SE, et al. Randomized trial of warfarin, aspirin, and clopidogrel in patients with chronic heart failure: the Warfarin and Antiplatelet Therapy in Chronic Heart Failure (WATCH) trial. Circulation. 2009;119:1616–1624. doi: 10.1161/CIRCULATIONAHA.108.801753 [DOI] [PubMed] [Google Scholar]
- 39.Homma S, Thompson JL, Pullicino PM, et al. Warfarin and aspirin in patients with heart failure and sinus rhythm. N Engl J Med. 2012;366:1859–1869. doi: 10.1056/NEJMoa1202299 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Zannad F, Anker SD, Byra WM, et al. Rivaroxaban in patients with heart failure, sinus rhythm, and coronary disease. N Engl J Med. 2018;379:1332–1342. doi: 10.1056/NEJMoa1808848 [DOI] [PubMed] [Google Scholar]
- 41.Mehra MR, Vaduganathan M, Fu M, et al. A comprehensive analysis of the effects of rivaroxaban on stroke or transient ischaemic attack in patients with heart failure, coronary artery disease, and sinus rhythm: the COMMANDER HF trial. Eur Heart J. 2019;40:3593–3602. doi: 10.1093/eurheartj/ehz427 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Greenberg B, Neaton JD, Anker SD, et al. Association of rivaroxaban with thromboembolic events in patients with heart failure, coronary disease, and sinus rhythm: a post hoc analysis of the COMMANDER HF Trial. JAMA Cardiol. 2019;4:515–523. doi: 10.1001/jamacardio.2019.1049 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Eikelboom JW, Connolly SJ, Bosch J, et al. Rivaroxaban with or without aspirin in stable cardiovascular disease. N Engl J Med. 2017;377:1319–1330. doi: 10.1056/NEJMoa1709118 [DOI] [PubMed] [Google Scholar]
- 44.Branch KR, Probstfield JL, Eikelboom JW, et al. Rivaroxaban with or without aspirin in patients with heart failure and chronic coronary or peripheral artery disease. Circulation. 2019;140: 529–537. doi: 10.1161/CIRCULATIONAHA.119.039609 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Kernan WN, Ovbiagele B, Black HR, et al. Guidelines for the prevention of stroke in patients with stroke and transient ischemic attack: a guideline for healthcare professionals from the American Heart Association/American Stroke Association. Stroke. 2014;45:2160–2236. doi: 10.1161/STR.0000000000000024 [DOI] [PubMed] [Google Scholar]
- 46.Smetana KS, Dunne J, Parrott K, et al. Oral factor Xa inhibitors for the treatment of left ventricular thrombus: a case series. J Thromb Haemost. 2017;44:519–524. doi: 10.1007/s11239-017-1560-7 [DOI] [PubMed] [Google Scholar]
- 47.Elikowski W, Małek-Elikowska M, Wróblewski D, et al. Apixaban in left ventricular thrombi treatment—a report of seven cases. Pol Merkur Lekarski. 2018;44:276–279. [PubMed] [Google Scholar]
- 48.Verma B, Singh A, Kumar M. Use of dabigatran for treatment of left ventricular thrombus: a tertiary care center experience. J Family Med Prim Care. 2019;8:2656–2660. doi: 10.4103/jfmpc.jfmpc_459_19 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Shokr M, Ahmed A, Abubakar H, et al. Use of direct oral anticoagulants in the treatment of left ventricular thrombi: a tertiary center experience and review of the literature. Clin Case Rep. 2018;7:135–142. doi: 10.1002/ccr3.1917 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Abdelnaby M, Almaghraby A, Abdelkarim O, Saleh Y, Hammad B, Badran H. The role of rivaroxaban in left ventricular thrombi. Anatol J Cardiol. 2019;21:47–50. doi: 10.14744/AnatolJCardiol.2018.48313 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Bahmaid RA, Ammar S, Al-Subaie S, Soofi MA, Mhish H, Yahia MA. Efficacy of direct oral anticoagulants on the resolution of left ventricular thrombus—a case series and literature review. JRSM Cardiovasc Dis. 2019;8:2048004019839548. doi: 10.1177/2048004019839548 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Fleddermann A, Eckert R, Muskala P, Hayes C, Magalski A, Main ML. Efficacy of direct acting oral anticoagulant drugs in treatment of left atrial appendage thrombus in patients with atrial fibrillation. Am J Cardiol. 2019;123:57–62. doi: 10.1016/j.amjcard.2018.09.026 [DOI] [PubMed] [Google Scholar]
- 53.Makrides CA. Resolution of left ventricular postinfarction thrombi in patients undergoing percutaneous coronary intervention using rivaroxaban in addition to dual antiplatelet therapy. BMJ Case Rep. 2016;2016:bcr2016217843. doi: 10.1136/bcr-2016-217843 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Tomasoni D, Sciatti E, Bonelli A, Vizzardi E, Metra M. Direct oral anticoagulants for the treatment of left ventricular thrombus—a new indication? A meta-summary of case reports. J Cardiovasc Pharmacol. 2020;75:530–534. doi: 10.1097/FJC.0000000000000826 [DOI] [PubMed] [Google Scholar]
- 55.Iqbal H, Straw S, Craven TP, Stirling K, Wheatcroft SB, Witte KK. Direct oral anticoagulants compared to vitamin K antagonist for the management of left ventricular thrombus. ESC Heart Fail. 2020;7:2032–2041. doi: 10.1002/ehf2.12718 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Jones DA, Wright P, Alizadeh MA, et al. The Use of Novel Oral Anti-Coagulant’s (NOAC) compared to vitamin K antagonists (warfarin) in patients with left ventricular thrombus after acute myocardial infarction (AMI). Eur Heart J Cardiovasc Pharmacother. Published online July 30, 2020. doi: 10.1093/ehjcvp/pvaa096 [DOI] [PubMed] [Google Scholar]
- 57.Decousus H, Prandoni P, Mismetti P, et al. Fondaparinux for the treatment of superficial-vein thrombosis in the legs. N Engl J Med. 2010;363:1222–1232. doi: 10.1056/NEJMoa0912072 [DOI] [PubMed] [Google Scholar]
- 58.Beyer-Westendorf J, Schellong SM, Gerlach H, et al. Prevention of thromboembolic complications in patients with superficial-vein thrombosis given rivaroxaban or fondaparinux: the open-label, randomised, non-inferiority SURPRISE phase 3b trial. Lancet Haematol. 2017;4:e105–e113. doi: 10.1016/S2352-3026(17)30014-5 [DOI] [PubMed] [Google Scholar]
- 59.Galiè N, Marc Humbert, Jean-Luc Vachiery, et al. 2015 ESC/ERS Guidelines for the diagnosis and treatment of pulmonary hypertension. The Joint Task Force for the Diagnosis and Treatment of Pulmonary Hypertension of the European Society of Cardiology (ESC) and the European Respiratory Society (ERS). Eur Respir J. 2015;46:903–975. doi: 10.1183/13993003.01032-2015 [DOI] [PubMed] [Google Scholar]
- 60.Gavilanes-Oleas FA, Alves JL Jr, Fernandes CJC, et al. Use of direct oral anticoagulants for chronic thromboembolic pulmonary hypertension. Clinics (Sao Paulo). 2018;73:e216. doi: 10.6061/clinics/2018/e216 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Sena S, Bulent M, Derya K, et al. Real-life data of direct anticoagulant use, bleeding risk and venous thromboembolism recurrence in chronic thromboembolic pulmonary hypertension patients: an observational retrospective study. Pulm Circ. 2020;10:2045894019873545. doi: 10.1177/2045894019873545 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Dabigatran for mitral stenosis atrial fibrillation. ClinicalTrials.gov identifier: NCT04045093. Updated September 17, 2020. AccessedOctober 20, 2020. https://clinicaltrials.gov/ct2/show/NCT04045093?term=warfarin&cond=mitral+stenosis&draw=2&rank=1
- 63.Rivoraxaban in mitral stenosis (RISE MS). ClinicalTrials.gov identifier: NCT03926156. Updated September 16, 2020. AccessedOctober 20, 2020. https://clinicaltrials.gov/ct2/show/NCT03926156?term=warfarin&cond=mitral+stenosis&draw=2&rank=3
- 64.Treatment of post-STEMI left ventricular thrombus with optimized anticoagulant (EARLYmyo-LVT). ClinicalTrials.gov identifier: NCT03764241. Updated March 12, 2020. AccessedOctober 20, 2020. https://clinicaltrials.gov/ct2/show/NCT03764241?cond=Left+Ventricular+Thrombus&draw=2&rank=1
- 65.Apixaban versus warfarin in patients with left ventricular (LV) thrombus. ClinicalTrials.gov identifier: NCT03232398. Updated August 7, 2019. AccessedOctober 20, 2020. https://clinicaltrials.gov/ct2/show/NCT03232398?cond=Left+Ventricular+Thrombus&draw=2&rank=8 [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


