Abstract
Background:
Overweight men with prostate cancer are more likely to suffer from recurrence and death following prostatectomy compared with healthy weight men. This study tested the feasibility of delivering a comprehensive program to foster weight loss before and weight maintenance after surgery in overweight men with localized prostate cancer.
Methods:
Twenty overweight men scheduled for prostatectomy elected either the intervention (n = 15) or the nonintervention (n = 5). Anthropometrics, biomarkers, diet quality, nutrition literacy, quality of life, and long-term follow-up were assessed in both groups.
Results:
The intervention led to 5.55 kg of weight loss including 3.88 kg of fat loss from baseline to surgery (mean = 8.3 weeks). The intervention significantly increased fiber, protein, fruit, nut, and vegetable intake; and decreased trans fats intake during weight loss. The intervention significantly reduced insulin, C-peptide, systolic blood pressure, leptin:adiponectin ratio, and visceral adiposity compared to the nonintervention. Post-surgically, weight loss was maintained. Changes in lipid profiles, nutrition literacy, and follow-up were not statistically significant in either group.
Conclusion:
Significant weight loss (≥5%) is feasible with a coaching intervention in overweight men preparing for prostatectomy and is associated with favorable cardiometabolic effects. This study is registered under NCT02252484 (www.clinicaltrials.gov).
Introduction
Prostate cancer is the leading cancer and cause of cancer-related death among American men (1). Although most men with localized prostate cancer are cancer-free for 5 years after prostatectomy, 23–27% will have recurrence after 5-years (2,3). Data suggests that obesity (4,5) and weight gain (6–8) are associated with higher recurrence rates. Furthermore, obesity increases the risk of cardiovascular disease (CVD), a major cause of death among prostate cancer survivors (9).
Immediately following cancer diagnosis, patients are motivated to transition toward a healthy lifestyle (10,11). One key factor is the desire to retain quality-of-life (11). Poor general health, in addition to urinary dysfunction and sexual dysfunction, has been found to be an independent predictor for regret after prostatectomy (12). Physical limitations and increased severity of common side effects after prostatectomy are associated with reduced physical activity (10). Timing an intervention before prostatectomy provides a coping strategy and capitalizes on a window of opportunity between diagnosis and surgery when men express a readiness for lifestyle change to improve their health.
Whether weight loss or fat loss will reduce prostate cancer recurrence and how much weight/fat loss is needed to change biomarkers related to prostate cancer is unknown. A 5% weight loss decreases the risk of obesity-related cardiovascular disease (13–15). Visceral adipose tissue is a strong driver for insulin resistance and hypoadiponectinemia. By reducing visceral adipose tissue through diet and exercise, there is a potential to reverse hyperinsulinemia and leptin:adiponectin ratio. Therefore, weight loss prior to prostatectomy could be a strategy to reduce the risk of prostate cancer recurrence.
Four pre-prostatectomy weight loss intervention trials lasting 6–8 weeks have been conducted, reporting 1.7 to 5.3 kg of weight loss (16–20). Two of these trials evaluated body composition before and after the intervention and the weight lost from those interventions; Henning et al. reported a statistically significant decrease in body fat and insulin, but no change in leptin:adiponectin ratio or trunk fat (18). Demark-Wahnefried et al. reported a statistically significant decrease in leptin, but did not elicit a statistically significant change in body fat or insulin compared to a control group (17). Demark-Wahnefried et al. reported improved vitality and erection frequency in the weight loss intervention group (17). None of these trials reported changes in visceral adipose tissue, weight maintenance after prostatectomy, or long-term outcomes.
We tested the feasibility of delivering a comprehensive weight management program with a pre-surgical weight loss phase followed by a post-surgical weight maintenance phase to overweight men (BMI 25–45 kg/m2) with prostate cancer. Unique to our study in patients with prostate cancer, we employed a weight loss intervention that emphasized competition, autonomy, and technology while addressing male-specific barriers to change as these themes have been shown to be effective for behavior change interventions in men (21). Given that prior evidence indicates that men are less likely to perceive themselves as overweight (22), part of this feasibility study was to offer the men a self-select option for the nutrition and exercise intervention arm compared to a nonintervention arm without a structured weight loss program, once they were informed of the relationship between obesity and prostate cancer. We hypothesized that the intervention would lead to clinically meaningful weight loss (≥5% loss in body weight), successful weight maintenance (≤3% regain) (15), and favorable modulation of body composition and biomarkers. The primary aim was to develop a novel weight management intervention tailored for men to induce weight/fat loss before prostatectomy while minimizing adverse effects. Exploratory aims were to prevent weight regain after prostatectomy and to determine how the intervention affects diet quality, nutrition literacy, biomarkers, quality of life, and long-term outcomes.
Materials and Methods
Participants
Participants were recruited from the University of Kansas (KU) Health System Urologic Clinic and the KU Cancer Center. Inclusion criteria included histologically confirmed, clinically localized prostate cancer; planned robotic-assisted laparoscopic radical prostatectomy; BMI of 25 to 45 kg/m2; age 50 to 72; and reliable internet access. Exclusion criteria included prior prostate cancer treatment, 5-alpha reductase inhibitor-use within 3 mo, of study enrollment, or the presence of a high-risk medical condition.
Design
The pilot study was conducted at the Clinical and Translational Science Unit at KU Medical Center from October 2014 to June 2016. The study protocol was approved by the protocol review monitoring committee of the KU Cancer Center and the Institutional Review Board. All study participants provided written informed consent.
The primary aim was to determine if the intervention would lead to significant weight reduction (≥5% body weight loss) from baseline to approximately 1 week prior to surgery. It was unknown if men, newly diagnosed with prostate cancer, would choose assistance with weight loss. To ascertain this information, participants self-selected the weight-loss intervention (n = 15) or the nonintervention (n = 5).
Intervention
The intervention consisted of a minimum of 3. Five to a maximum of 16-weeks of a weight loss period prior to prostatectomy (pragmatically depending on the surgical scheduling) and a 12-week weight maintenance period postoperatively (Fig. 1). Sessions focused on four-components for weight management tailored toward men: lifestyle coaching, healthy diet with meal replacements, physical activity, and self-monitoring technology. Coaching sessions were monitored for fidelity by the principal investigator (JHR) using a nutritionist observation form (≥80% adherence was acceptable).
Figure 1.
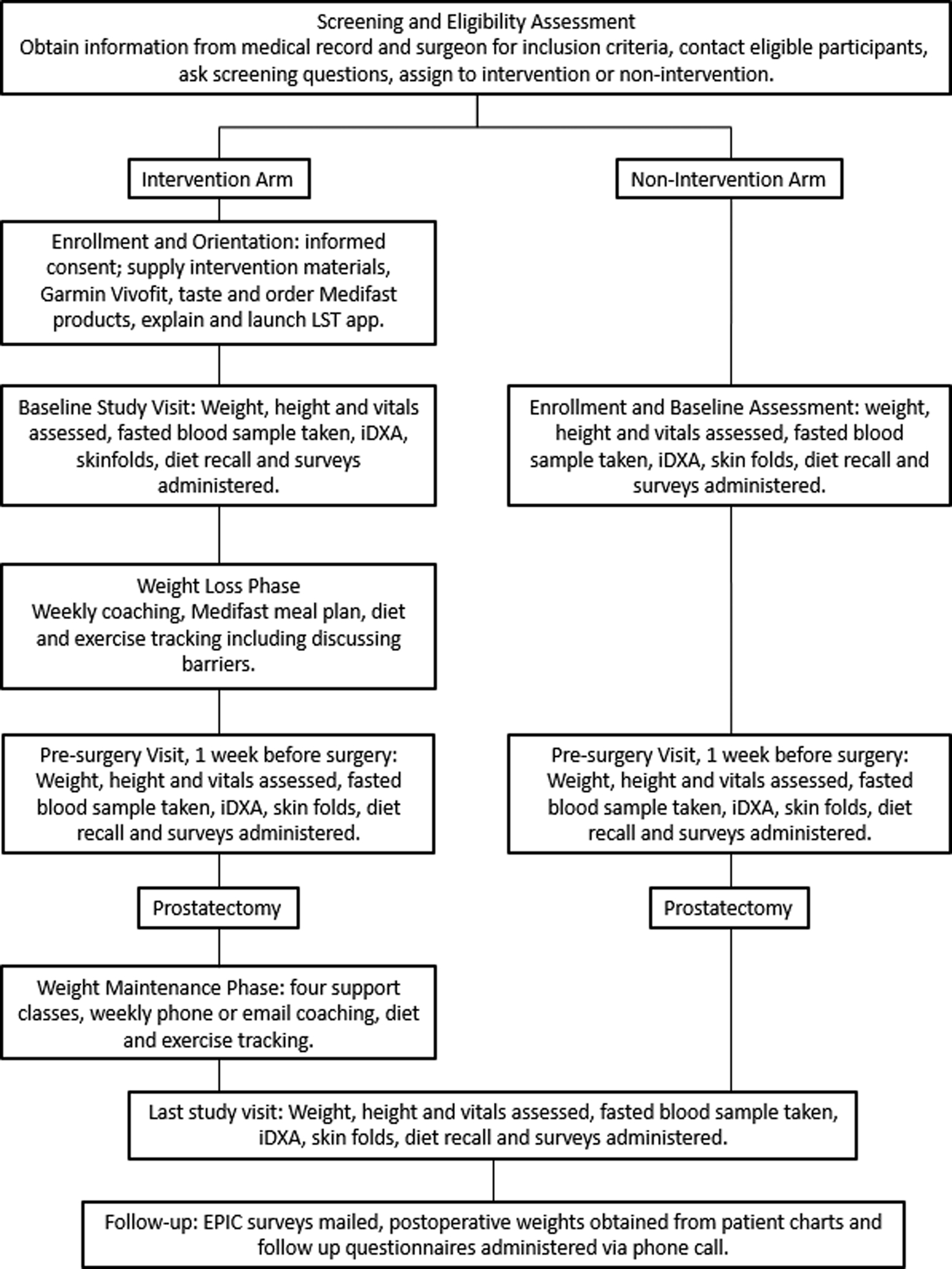
Study timeline and schema – Flow diagram showing the study timeline from screening and to follow-up.
Lifestyle Coaching
Coaching sessions delivered in person or via Zoom Video conferencing (Zoom, San Jose, California, USA) were used to educate on healthy diet guidelines and exercise strategies and set weekly goals as well as discuss overall weight loss progress. During the weight loss phase, sessions occurred weekly; during the weight maintenance phase, sessions occurred every three weeks for twelve weeks. Weekly coaching was reinforced with a mid-week check in to track progress, reinforce goals, discuss weight trends, reinforce education, and discuss strategies to overcome obstacles. As the study progressed, many men chose virtual meetings to reduce the burden of the study when traveling from a long distance or to accommodate their work and travel schedules better. LST AtHome application (LifeScience Technologies, LLC, Leawood, KS, USA) was used to monitor dietary intake, physical activity, and overall adherence to goals throughout the course of the study. To incentivize friendly competition, a leaderboard on the application showed participants their rank for cumulative steps taken; participants were listed by aliases to protect identity. Coaching was grounded in Social Cognitive Theory (23) (interactions with nutritionists) and Problem-Solving Theory (24) (identifying and removing barriers related to diet and exercise); see Table 1 for how the behavioral strategies were implemented. Early in the study, participants requested a peer mentorship program. Peer mentorship offered a way for participants to share their experience with each other. The study team did not monitor these calls. The dashboard on the app showed body weight trends to reinforce self-efficacy and accountability.
Table 1.
Conceptual framework for the intervention tailored for men with localized prostate cancer.
| Behavioral strategy | How the behavioral strategy is implemented |
|---|---|
| Self-efficacy | The app and seamless technology are critically important to target men’s self-efficacy and intention to perform physical activity particularly in male-tailored interventions. |
| Perceived barriers | Each session, participants identify personal barriers & potential solutions for behaviors. |
| Behavior shaping | Each session, participants compare their eating pattern to the eating plan and are taught how to modify their eating and physical activity behaviors according to the recommendations. |
| Goal setting | Coaches teach and guide sensible goal setting for eating and physical activity each session. |
| Self-monitoring | Participants track their diet, steps (accelerometer), and body weight through a seamless app. |
| Direct reinforcement and feedback | Participants receive feedback including reinforcement and strategies to overcome barriers. The app also provides a dashboard to show participants and the health educator progress in real time. |
| Peer/social support | Group sessions during weight maintenance provide opportunities to support, interact, and teach. The peer mentor program facilitates one-on-one support for participants during the program. |
| Relapse prevention | Coaches facilitate contingency plans to address high risk situations. |
Diet
The dietary intervention targeted ~1 kg per week weight loss based on individual metrics for estimated energy expenditure and caloric deficit and aimed at ~5% pre-surgical weight loss. During the weight loss phase, participants followed the Medifast 5&2&2® Plan (Jason Pharmaceuticals, Inc., Owings Mills, MD, USA) (Table 2). Each day, participants combined self-selected foods along with meal replacements. The Medifast 5&2&2® plan instructed participants to include five meal replacements (each 90–110 kcals, 11–15 g protein), 2 “lean and green” self-prepared meals (each 5–7 oz. lean protein, three servings of non-starchy vegetables and up to two healthy fat servings) and two self-prepared healthy snacks (each one serving of fruit, dairy, or grain). After surgery, participants transitioned to the Medifast 3 & 3 Plan® (Table 3) to incorporate calories from whole-grains, low-fat dairy, and fruits during weight maintenance and reduce meal replacements from 5 to three daily. The rationale for using meal replacements in our approach was that they lead to greater weight loss in short-term interventions (25,26); we needed to replace unhealthy snacks with healthy ones, and we needed a palatable delivery of plant-based protein to displace red meat intake. Daily food intake was tracked through the app with a seamless interface into the USDA food database. The app tallied each food group to reinforce self-efficacy.
Table 2.
Medifast 5 & 2 & 2 Plan® nutrient composition.a
| Component | Range |
|---|---|
| Caloriesb (kcals) | 1300–1500 |
| Carbs (%) | >26% |
| Protein (%) | >26% |
| Fatc (%) | ≥6% |
| Sodium (g) | 1.25–2.30 |
| Fiber (g) | 25–35 |
Reproduced with permission from Medifast®. Edited for clarity.
Calories were adjusted based on the rate of weight loss.
≤35% calories from fat.
Table 3.
Medifast 3&3 Plan® nutrient composition.a
| Component | Range |
|---|---|
| Caloriesb (kcals) | 1200–3000 per day |
| Carbs (%) | ~37–60% |
| Protein (%) | ~25–45% |
| Fat (%) | ~10–40% |
Reproduced with permission from Medifast®.
Calories were adjusted based on the rate of weight loss.
Physical Activity
Intervention participants were given a Vivofit® wrist-wearable accelerometer (Garmin Ltd., Olathe, KS, USA) and were instructed to continue normal physical activity while tracking their steps from orientation to baseline. From baseline to study completion, physical activity goals were customized to either increase time of moderate intensity physical activity or steps/week by 10% or adapted to maintain as much physical activity as tolerated. Many participants in this pilot study also requested specific exercise instruction for functional or home strength training. Our team created short exercise videos to meet the participants’ needs that were customizable with our technology by adding or removing exercises to the patients’ app as indicated. Participants logged intentional exercise and synced the accelerometer in the LST AtHome App. The dashboard on the app showed daily steps and ranked participants on a weekly leaderboard as a means of motivating the participants.
Self-Monitoring Technology
In this feasibility study, we collaborated with LifeScience Technologies to customize a seamless self-monitoring system for body weight, diet, physical activity, and adverse events specific to our prostate cancer patient preferences. In this customized program, both participants and health educators could input and view data in real-time. The dashboard showed the participants their energy target, weight progress, a daily food group tally, and physical activity tracking. Each card on the dashboard opens an applet to manage nutrition, physical activity, body weight, or obtain education (exercise videos, preparing/recovering from surgery, recipes). The applets presented to the participant were selected by the study team based on the needs of the patient at specific timepoints related to their surgery. LST AtHome captured and reported data, rewarded patients for healthy activity through points and leaderboards and offered communication in a HIPAA compliant manner between patient and the study team. The dashboard for the application was continually evolving during this study to meet the requests of the participants to track food items and physical activity more simply. Participants without access to a scale were provided a Withings™ wireless scale (Cambridge, MA, USA) to track their weight which uploaded seamlessly into the dashboard via Bluetooth technology
Group Support
Skill building sessions at the Clinical Research Center Demonstration Kitchen were an important part of the weight maintenance phase of the intervention. For each of the 4 in-person sessions, participants and their spouse/caregiver(s) interacted through cooking demonstrations and hands-on educational sessions. The modules focused on dietary strategies for prostate cancer survivorship with the topics listed in Table 4. The groups met every 3 weeks and included interactive activities at each session to engage attendees. Some examples of these activities included the attendees competing to see who can form a meat patty closest to three ounces and guessing how many sugar cubes are in some of their favorite drinks. The hands-on learning engaged sensory concepts with tasting, measuring portions, exercising, and interpreting food labels. Our approach re-emphasized the nutrition education covered during the pre-surgical phase and helped participants transition from meal replacements to home-made meals. In addition, the group sessions were lively and fun as the participants, spouses, and our team shared recipes, tactics, and pearls of wisdom. Participants who joined by videoconference could also view and interact with the group, but they did miss the tastings and hands-on activities. Group sessions and peer support helped participants brainstorm ways to keep engaged by listening to audiobooks while exercising or signing up for run/walks that support charities or causes that resonated with them. The group classes and the peer mentoring also facilitated how to safely return to physical activity after surgery.
Table 4.
Curriculum tailored for men with localized prostate cancer (5th–7th grade reading level).
| Curriculum for prostate cancer surgery: weight loss before surgery (3.5–16 week individual program) | ||
|---|---|---|
| Introduction to Meal Plan and Technology | Define the link between obesity and prostate cancer, taste & order meal replacements; setup technology | Wear and sync accelerometer; Start tracking nutrition, physical activity, and weight |
| Energy Balance | Show principles of energy balance | Eat less; increase physical activity; identify personal barriers & potential solutions |
| The Meal Plan | Personalize the Medifast® 5&2&2 Plan | List foods that you prefer in each food group; identify personal barriers & potential solutions to following the meal plan |
| Portion Distortion | Identify healthy portion sizes | Develop strategies for controlling portions |
| Physical Activity: FITT | List ways to increase physical activity using Frequency, Intensity, Time, and Type & activities of daily living | Plan ways to increase intentional physical activity and activities of daily living; identify personal barriers & potential solutions |
| Goal Setting: SMART | Share values that are motivating to be in the program; set appropriate weight goal | Write long-term goal and match to values; identify the short-term goals to achieve the long-term goal |
| Self-Monitoring | Self-monitoring | |
| Meal Planning | Meal planning | |
| Eating Out | List strategies to limit intake when eating out | Identify meals that fit the meal plan at favorite restaurants |
| Social Situations | Review strategies for social situations | Identify personal barriers & potential solutions |
| Energy Density | Identify foods high and low in energy density | Identify foods high in energy density that are barriers & list low energy dense foods that can help |
| Fruits & Vegetables | Define benefits of fruits & vegetables | Identify favorite fruits and vegetables; plan strategies to increase daily consumption of fruits and non-starchy vegetables |
| Curriculum for prostate cancer surgery: weight maintenance after surgery (12 week group support program) | ||
| Lesson | Objectives | Key Messages |
| Shaken, Not Stirred | Identify sugary beverages; define empty calories Choose fluids using good, better, best guides. | Limit added sugars & alcohol, read food labels Consume ≤ 1 sugary beverage/day |
| Eye of the Tiger | List physical activity guidelines Choose exercise plan and record it Fuel activity without undoing workout | Exercise increases energy expenditure Exercise ≥ 30 mins,/day |
| Veg out | Describe and limit energy dense foods Identify foods high in phytochemicals Choose your vegetables and fruits | Consume ≥ 5 servings fruits & vegetables/day |
| Where’s the Beef | List how red, processed, or charred meat affects health Make healthy choices at restaurants Choose your protein | Choose lean chicken, fish/seafood, plant proteins Make ¼ of the plate protein Follow healthy choices guide for dining out |
Nonintervention
The nonintervention group had the same study timeline as the intervention group, 3.5 – 16 weeks prior to surgery and 12 weeks after surgery. Nonintervention participants were educated about the relationship between obesity and prostate cancer without receiving weight management coaching.
Assessments
Intervention and nonintervention participants were assessed in person at baseline, one week prior to scheduled surgery, and 12 weeks post-surgery at research visits. Follow-up data were collected from patient medical records at 3, 6, 9, 12, 24, and 36 mo, post-surgery. Age, smoking history, medications, comorbidities, tumor characteristics, and clinical outcomes were obtained from the medical record and verified with participants and surgeons. The National Cancer Institute Common Terminology Criteria for Adverse Events (AE), Version 4.0 was used for AE reporting. Study data were collected and managed in a research electronic data capture database (27).
Anthropometrics and Vitals
Anthropometric measurements and vitals were assessed at each research visit. Vitals (blood pressure, heart rate and body temperature) were taken after the participant rested for 5 mins,. Height (without shoes) was measured using a wall-mounted stadiometer (SECA Model #216 1814009); body weight (without shoes and in light clothing) was taken on the same scale (Detecto® Model 758 C); waist circumference was obtained immediately below the last floating rib; and hip circumference was measured at the widest part of the hip. Body composition was assessed by dual-energy X-ray absorptiometry (GE iDXA®, Lunar Corporation, Madison, WI, software version 13.6).
Diet Adherence and Diet Quality
Dietary adherence was assessed with diet recalls using the multiple pass approach. Two 24-hr dietary recalls (one weekday and one weekend) obtained by phone or in person the week of each research visit were averaged and analyzed with Nutrition Data System for Research (NDSR Version 2014, University of Minnesota, Minneapolis) software. The diet quality of intervention and nonintervention participants was scored using the Alternative Healthy Eating Index (AHEI). Nutrition Literacy Assessments (NLits) were given to participants at the first and last research visit to evaluate their understanding of a healthy diet (28).
Tracking Physical Activity
Physical activity data during the intervention were collected from self-recorded activity in the LST app and Vivofit® accelerometers.
Cardiometabolic Biomarkers
Cardiometabolic biomarkers were analyzed at research visits after a 9-hour fast. Whole blood was collected in sodium ethylenediaminetetraacetic acid and serum separator tubes. Glucose, lipids (total cholesterol; HDL and LDL cholesterol; and triglycerides), insulin, C-peptide and high sensitivity C-reactive protein (hsCRP) were measured by Quest Diagnostics Laboratories (Lenexa, KS). Adiponectin, resistin and leptin from serum samples were batch processed using MILLIPLEX® MAP kits (MilliporeSigma, Billerica, MA, USA) and analyzed on a Luminex 200™ instrument with xPONENT™ software. Human Adipokine Magnetic Bead Panel 1 (cat. # HADK1MAG-61K) was used to measure adiponectin and resistin; Human Adipokine Magnetic Bead Panel 2 (cat. # HADK2MAG-61K) was used to measure leptin.
Quality of Life & Long-Term Outcomes
A Short Form 8 (SF8) survey was given at each research visit to assess participant health. Expanded Prostate Cancer Index Composite (EPIC) surveys were mailed to participants a year after study completion to assess urinary continence and erectile function. Postoperative weights were collected from physician visits at 3, 6, 9 and 12 mo, after surgery. Phone calls were used to track prostate cancer recurrence and to obtain self-reported body weights at 12, 24 and 36 mo, after surgery.
Statistical Analysis
The primary goal of this study was to evaluate the feasibility of delivering a comprehensive coaching program to men with prostate cancer designed to achieve significant weight loss (≥5kg, ~5%, ~1kg/week) before prostatectomy. A sample size of 20 (15 intervention, five control) allows 85% power to detect a between group difference of >4.5 kg weight loss with a significance level of 0.05 using a two-sided two-sample unequal-variance T-test. The observed weight loss standard deviations used in the power calculation for intervention and control participants were 5.02 kg and 1.17 kg respectively. The sample size of 15 subjects in the intervention group allowed us to detect weight loss of 3.4 kg or higher with over 80% power at 0.05 level of significance. Given the small sample size and non-normal distributions of the observed data, non-parametric methods were used for analyses. Within-arm differences were assessed using the Wilcoxon signed-rank test and between arm differences were assessed using the Wilcoxon rank-sum test. The tests were considered statistically significant if P < 0.05. Since these exploratory analyses were from a feasibility study, no multiple comparison adjustments were completed. R Version 3.5.0 was used for statistical analyses of all data.
Results
Feasibility and Baseline Characteristics
The accrual target was met, and 85% retention was achieved. Twenty participants enrolled (intervention, n = 15; nonintervention, n = 5); thirteen (87%) completed the intervention and four (80%) completed the nonintervention (Fig. 2). Reasons for electing the nonintervention group included one participant’s perception that the timeframe was insufficient to lose to his goal body weight, another felt the distance was too far to travel for visits, one participant reported a disinterest in losing weight, one participant did not like the meal replacements, and one participant was overwhelmed by making lifestyle changes within the context of his busy life. Of the intervention participants, one withdrew shortly after baseline due to study burden, and one was removed before weight maintenance due to treatment change. Of the nonintervention participants, one was lost to follow-up after baseline assessment, and one missed his pre-surgery visit but remained in the study. The pre-surgical phase averaged 8.3 weeks for the intervention and 3.6 weeks for the nonintervention. Baseline PSA, weight and BMI did not differ significantly (P > 0.1) between the two arms (Table 5).
Figure 2.
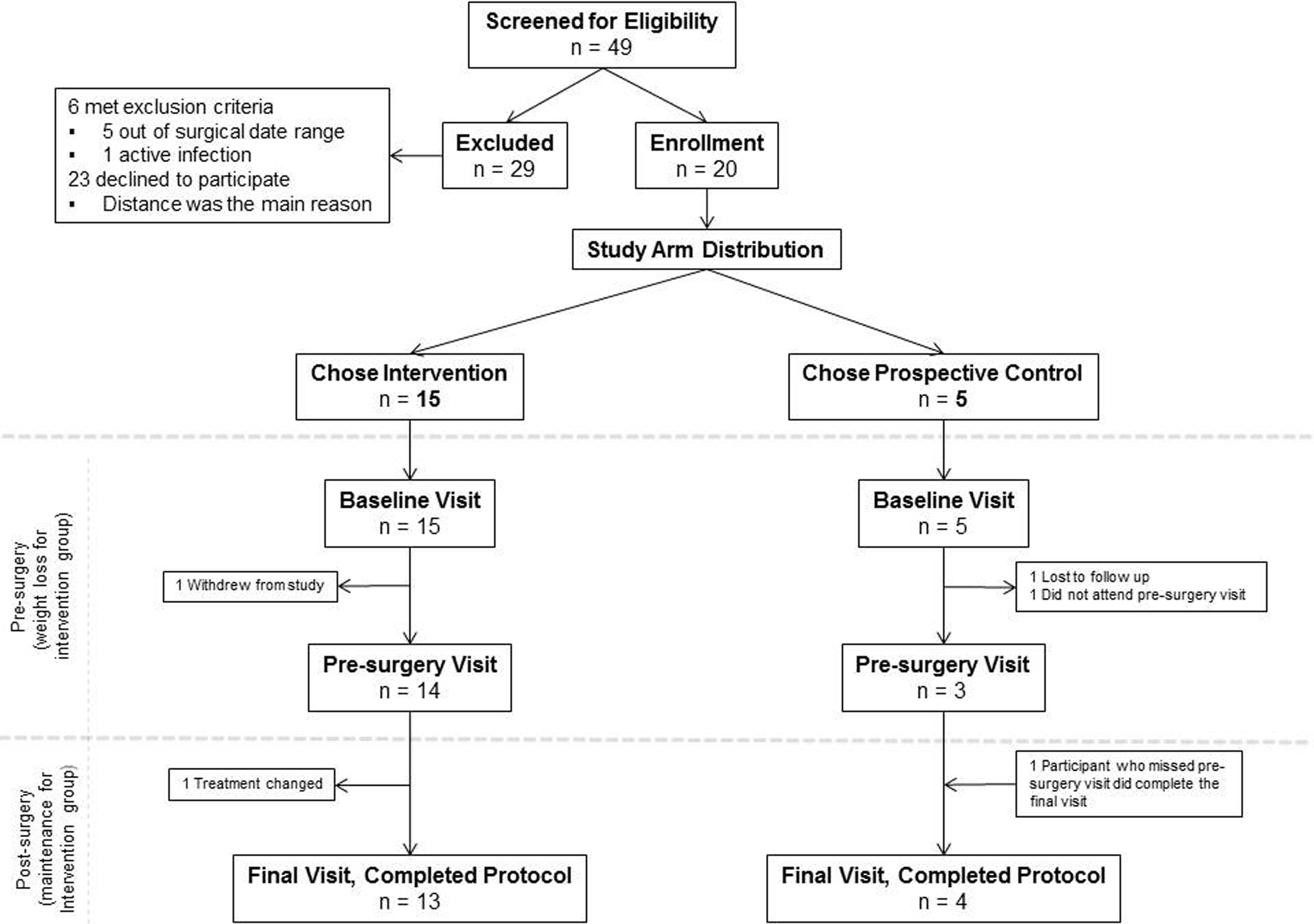
Consort diagram – Flow diagram showing the number of men who were recruited, enrolled and finished the study.
Table 5.
Baseline characteristics.a
| Characteristic | Intervention | Nonintervention |
|---|---|---|
| Age | 60.9 ± 5.7 (15) | 58.6 ± 3.4 (5) |
| Race | ||
| White | 12/15 (80.0%) | 4/5 (80.0%) |
| Black | 3/15 (20%) | 1/5 (20.0%) |
| Ethnicity | ||
| Hispanic | 0/15 (0%) | 0/5 (0%) |
| Non-Hispanic | 15/15 (100%) | 5/5 (100%) |
| Highest education level | ||
| High school/GED | 1/15 (6.67%) | 0/4 (0%) |
| Some college/associate’s | 3/15 (20%) | 3/4 (75%) |
| Bachelor’s | 7/15 (46.67%) | 1/4 (25%) |
| Master’s | 3/15 (20%) | 0/4 (0%) |
| Doctoral | 1/15 (6.67%) | 0/4 (0%) |
| RUCA Codes | ||
| 1 | 11/15 (73.33%) | 2/5 (40%) |
| 2 | 2/15 (13.33%) | 3/5 (60%) |
| 4 | 1/15 (6.67%) | 0/5 (0%) |
| 5 | 1/15 (6.67%) | 0/5 (0%) |
| Smoking status | ||
| Current smoker | 2/15 (13.33%) | 0/5 (0%) |
| Former smoker | 7/13 (53.85%) | 1/5 (20%) |
| Clinical stage | ||
| T1c | 14 (93.3%) | 3 (60.0%) |
| T2a | 1 (0.07%) | 1 (20.0%) |
| T2c | 0 (0%) | 1 (20.0%) |
| PSA (ng/mL2) | 5.83 ± 1.9 (15) | 6.00 ± 1.1 (5) |
| Weight (kg2)b | 98.6 ± 11.4 (15) | 89.6 ± 11.7 (5) |
| BMI (kg/m2)b | 30.2 ± 2.9 (15) | 1 ± 2.2 (5) |
Values are mean ± SD (n) or n (%). PSA, prostate-specific antigen; BMI, body mass index. Prostate cancer must be T1 or T2 based on the American Joint Committee (7th edition) to meet study inclusion criteria. RUCA codes are based on 2010 Rural-Urban Commuting Area Codes – 1, Metropolitan area core: primary flow within an urbanized area (UA); 2, Metropolitan area high commuting: primary flow 30% or more to a UA; 3, Metropolitan area low commuting: primary flow 10% to 30% to a UA; 4, Micropolitan area core: primary flow within an Urban Cluster of 10,000 to 49,999 (large UC); 5, Micropolitan high commuting: primary flow 30% or more to a large UC.
P value between arms > 0.1.
Adverse Events
No intervention participant withdrew from the study due to AEs, and no AE was greater than grade 2. AEs reported (n reports = relation to intervention) were nausea (1 = possibly), dizziness (1 = unlikely), gum sensitivity (1 = unlikely), lip angioedema (1 = unrelated), fluid retention (1 = unrelated), migraine (1 = possibly), back pain (1 = unlikely, 1 = possibly), abdominal pain (1 = unlikely, 1 = definitely) constipation (1 = possibly, 1 = probably, 1 = definitely), bloating (2 = unrelated, 1 = definitely) and flatulence (1 = unlikely, 1 = probably, 1 = definitely). AEs from the nonintervention were not collected.
Anthropometrics & Vitals
Anthropometrics are shown in Table 6. During the weight loss phase, intervention participants lost an average of 5.55 kg (5.4%) body weight including 3.88 kg (11.8%) fat; during the pre-surgery phase, nonintervention participants gained an average of 0.12 kg (0.1%) body weight while losing an average of 0.294 kg (0.8%) fat. Between group differences were significant (weight, P = 0.01; fat, P = 0.03). Over the course of the study, eight out of 13 intervention group men lost ≥5% of their body weight (Fig. 3) from baseline to 12 weeks post-surgery. Weight was maintained in the intervention (P = 0.8) and the nonintervention (P = 0.3) for 12 weeks postoperatively.
Table 6.
Anthropometric data among men in intervention and nonintervention groups at baseline, 1 week before and 12 weeks after prostatectomy.a
| Intervention | Nonintervention | Between arm changes | |||||||
|---|---|---|---|---|---|---|---|---|---|
| Anthropometric | Baseline | Pre-surgery | Post-surgery | Pwithinb | Baseline | Pre-surgery | Post-surgery | Pwithinb | Pbetweenc |
| Body weight (kg) | 98.6 ± 11.4 (15) | 94.1 ± 9.8 (14) | 95.5 ± 11.6 (13)d | 0.0002 | 89.6 ± 11.7 (5) | 92.0 ± 15.2 (3) | 87.5 ± 13.9 (4) | 1.00 | 0.01 |
| RSMI (kg/m2) | 9.2 ± 0.8 (15) | 9.0 ± 0.8 (14) | 9.0 ± 1.0 (13)d | 0.01 | 9.0 ± 0.9 (5) | 9.3 ± 0.4 (3) | 9.0 ± 0.3 (3) | 1.00 | 0.36 |
| Total BFM (kg) | 30.6 ± 5.5 (15) | 27.2 ± 4.7 (14) | 28.1 ± 6.0 (13)d | 0.001 | 26.5 ± 8.4 (5) | 25.5 ± 11.2 (3) | 25.2 ± 1.1 (3) | 0.50 | 0.03 |
| nbLBM (kg) | 63.5 ± 6.6 (15) | 62.8 ± 6.6 (14) | 63.1 ± 7.3 (13)d | 0.01 | 58.8 ± 5.6 (5) | 61.5 ± 5.0 (3) | 59.5 ± 5.8 (3) | 0.75 | 0.36 |
| FFMI (kg/m2) | 19.5 ± 1.5 (15) | 19.3 ± 1.5 (14) | 19.2 ± 1.7 (13)d | 0.01 | 19.0 ± 1.9 (5) | 19.5 ± 1.1 (3) | 19.0 ± 1.1 (3) | 1.00 | 0.36 |
| VAT (g) | 1993.5 ± 889.2 (15) | 1705.6 ± 834.4 (14) | 1805.1 ± 946.5 (13)d | 0.003 | 2018.4 ± 1051.7 (5) | 1933.3 ± 1341.3 (3) | 1819.7 ± 1174.0 (3) | 0.25 | 0.20 |
| WC (cm) | 102.1 ± 8.8 (15) | 97.5 ± 8.7 (12) | 99.3 ± 10.0 (13)d | 0.001 | 100.6 ± 9.9 (5) | 101.9 ± 14.0 (2) | 97.0 ± 10.0 (4) | 0.50 | 0.66 |
| HC (cm) | 107.3 ± 4.8 (15) | 104.7 ± 3.5 (12) | 106.0 ± 5.9 (13)d | 0.03 | 102.2 ± 6.5 (5) | 103.4 ± 7.9 (2) | 101.7 ± 7.1 (4) | 1.00 | 0.71 |
| wbBT (g) | 3776.9 ± 519.7 (15) | 3818.1 ± 508.5 (14) | 3856.2 ± 487.8 (13) | 0.90 | 3601.6 ± 399.3 (5) | 3869.7 ± 85.0 (3) | 3860.7 ± 91.2 (3) | 0.50 | 0.36 |
Values are mean ± SD (n). RSMI, relative skeletal muscle index; BFM, body fat mass; nbLBM, non-bone lean body mass; FFMI, fat free mass index; VAT, visceral adipose tissue; WC, waist circumference; HC, hip circumference; wbBT, whole body bone tissue.
P value within arms; change from baseline to pre-surgery.
P value between arms; change from baseline to pre-surgery.
P value < 0.05 from baseline value; all other net changes were not significant.
Figure 3.
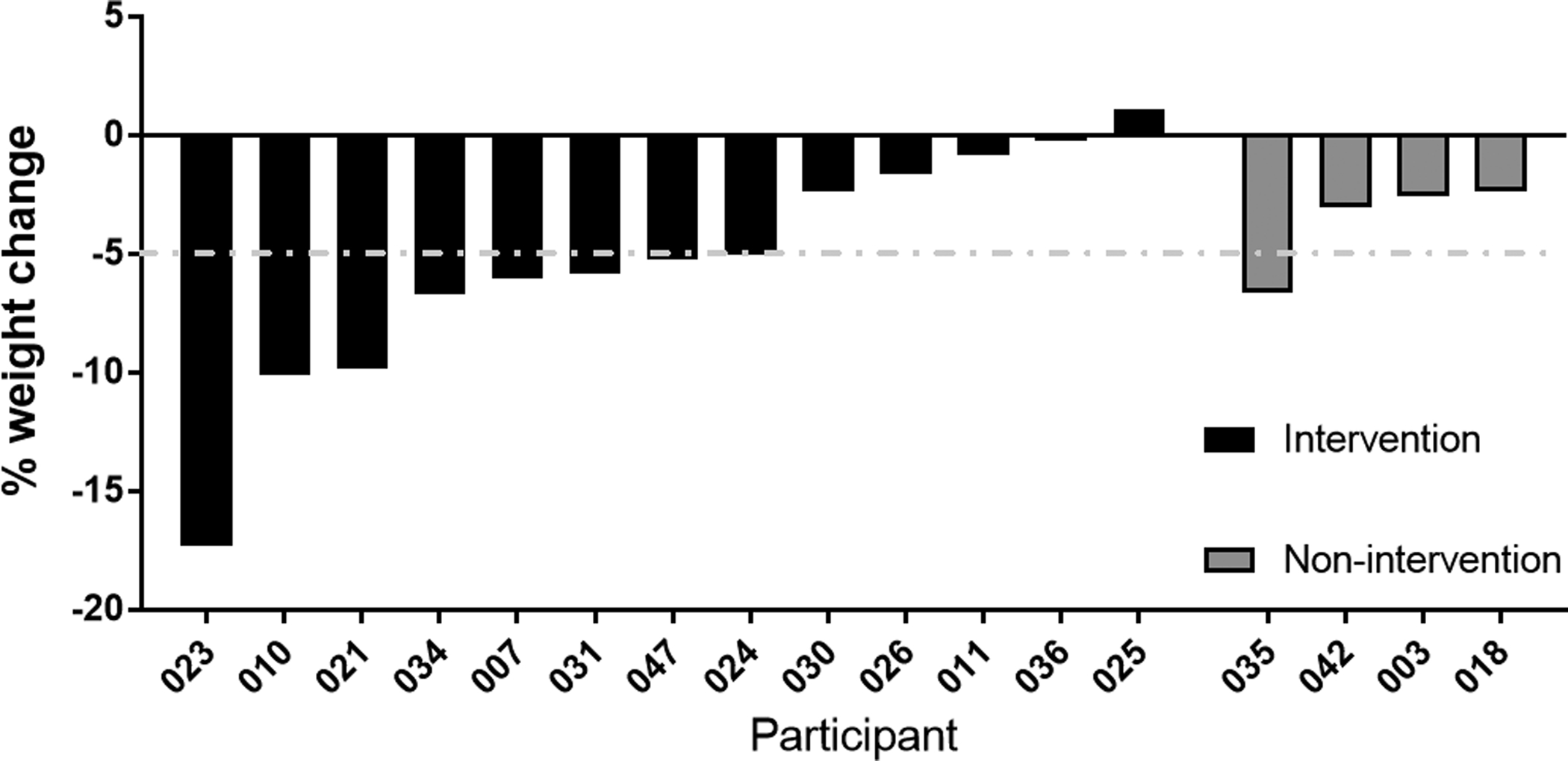
Change in perioperative weight – Bar graph showing the percent change in weight of men from baseline to 12 weeks post-surgery in both the intervention and nonintervention arms.
The intervention reduced waist and hip circumferences; waist:hip ratio; and visceral adipose tissue mass from baseline to pre-surgery (P = 0.001, 0.03, 0.06, 0.003, respectively) and baseline to 12 weeks post-surgery (P = 0.008, 0.02, 0.07, 0.002, respectively). Systolic blood pressure decreased with the intervention from baseline to pre-surgery (P = 0.02; between group, P = 0.7).
Dietary Adherence and Diet Quality
The AHEI is divided into separate domain scores, which are combined to yield a total score. In the intervention group, fruit (P = 0.04), nut (P = 0.04), and vegetable (P = 0.009) intake increased while the consumption of trans fats (P = 0.002) decreased during weight loss, which is shown by their increasing AHEI domain scores (Table 7). The higher AHEI domain scores in the aforementioned categories indicate greater amounts of these foods eaten, except in the case of trans fat, where the higher score indicates less trans fat eaten. Total AHEI scores trended toward improvement in the intervention during weight loss (P = 0.09) and remained stable 12 weeks post-surgery (P = 0.8); AHEI domain scores and dietary recall data remained unchanged in the nonintervention group. There were no significant changes in NLit scores (Table 8) from baseline to 12 weeks post-surgery in either group. In the intervention group, both fiber (P = 0.002) and protein (P = 0.009) intake increased during weight loss as well.
Table 7.
AHEI scores among men in intervention and nonintervention groups at baseline, 1 week before and 12 weeks after prostatectomy.a
| Intervention score | Nonintervention score | Between arm changes | |||||||
|---|---|---|---|---|---|---|---|---|---|
| Component | Baseline | Pre-surgery | 12 weeks post-surgery | Pwithinb | Baseline | Pre-surgery | 12 weeks post-surgery | Pwithinb | Pwithoutc |
| AHEI Scores | |||||||||
| Alcohol score | 4.5 ± 3.0 (15) | 6.3 ± 3.9 (14) | 5.4 ± 3.9 (13) | 0.05 | 1.9 ± 1.3 (4) | 2.5 ± 0.0 (3) | 4.4 ± 3.8 (4) | N/A | 0.38 |
| EPA/DHA score | 6.1 ± 4.3 (15) | 4.8 ± 3.4 (14) | 5.8 ± 3.4 (13) | 0.37 | 3.9 ± 4.1 (4) | 4.5 ± 4.9 (3) | 9.1 ± 1.9 (4) | 0.5 | 0.38 |
| Fruit score | 2.5 ± 2.2 (15) | 4.5 ± 3.2 (14) | 2.7 ± 2.5 (13) | 0.04 | 2.2 ± 3.1 (4) | 1.9 ± 1.7 (3) | 2.2 ± 1.5 (4) | 1.00 | 0.23 |
| Nuts, legumes score | 6.9 ± 4.0 (15) | 10.0 ± 0.0 (14) | 9.0 ± 2.8 (13) | 0.04 | 7.5 ± 4.4 (4) | 3.4 ± 3.7 (3) | 3.9 ± 4.3 (4) | 1.00 | 0.35 |
| PUFA score | 7.6 ± 2.5 (15) | 6.8 ± 2.5 (14) | 6.8 ± 1.9 (13) | 0.26 | 8.0 ± 1.6 (4) | 5.9 ± 3.7 (3) | 8.7 ± 1.8 (4) | 1.00 | 0.94 |
| Red meat score | 4.9 ± 4.2 (15) | 4.1 ± 4.3 (14) | 5.7 ± 3.6 (13) | 0.48 | 1.4 ± 2.9 (4) | 2.4 ± 3.3 (3) | 2.0 ± 2.4 (4) | 1.00 | 0.62 |
| Sodium score | 6.4 ± 2.0 (15) | 5.6 ± 2.0 (14) | 6.4 ± 2.0 (13) | 0.20 | 4.5 ± 1.0 (4) | 4.8 ± 2.0 (3) | 4.6 ± 1.7 (4) | 1.00 | 0.52 |
| Trans fat scored | 7.6 ± 1.6 (15) | 9.2 ± 0.92 (14) | 9.0 ± 1.1 (13)e | 0.002 | 8.4 ± 1.0 (4) | 7.7 ± 2.2 (3) | 8.9 ± 1.2 (4) | 0.50 | 0.07 |
| Vegetable score | 5.2 ± 3.1 (15) | 8.3 ± 2.0 (14) | 7.3 ± 2.9 (13) | 0.01 | 5.4 ± 2.4 (4) | 5.8 ± 3.6 (3) | 5.4 ± 2.5 (4) | 1.00 | 0.47 |
| Whole grain score | 2.6 ± 2.2 (15) | 1.1 ± 1.3 (14) | 1.9 ± 2.2 (13) | 0.09 | 1.8 ± 0.90 (4) | 2.0 ± 1.1 (3) | 4.8 ± 4.3 (4) | 0.5 | 0.23 |
| SSBs & juice score | 8.7 ± 2.9 (15) | 7.5 ± 3.7 (14) | 10.0 ± 0.04 (13) | 0.55 | 8.1 ± 3.7 (4) | 6.7 ± 5.8 (3) | 7.5 ± 5.0 (4) | N/A | 0.93 |
| Total score | 63.0 ± 8.2 (15) | 68.1 ± 7.5 (14) | 70.0 ± 9.9 (13) | 0.09 | 53.0 ± 12.0 (4) | 47.6 ± 9.0 (3) | 61.4 ± 15.3 (4) | 0.50 | 0.20 |
Values are mean ± SD (n). Each component yields a separate score and are calculated together to yield a total score. Higher AHEI scores indicates greater adherence to Dietary Guidelines or a more healthful diet, and not necessarily greater consumption of the item (i.e., trans fat, SSB, and alcohol). AHEI, Alternative Healthy Eating Index; EPA, eicosapentaenoic acid; DHA, docosahexanoic acid; SSB, sugar-sweetened beverage.
P value within arms; change from baseline to pre-surgery.
P value between arms; change from baseline to pre-surgery.
A higher trans fat score indicates less trans-fat grams consumed.
P value < 0.05 from baseline value; all other net changes were not significant.
Table 8.
NLit number correct among men in intervention and nonintervention groups at baseline and 12 weeks post-surgery.a
| Intervention (n = 11) | Nonintervention (n = 3) | Between arm changes | Between arm differences at baseline | |||||
|---|---|---|---|---|---|---|---|---|
| Component | Baseline | 12 weeks post-surgery | Pwithinb | Baseline | 12 weeks post-surgery | Pwithinb | Pbetweenc | Pbetweend |
| Nutrition & health | 6.18 ± 0.75 | 6.18 ± 0.87 | 1.00 | 4.67 ± 2.52 | 7.00 ± 0.37 | 0.37 | 0.09 | 0.33 |
| Energy sources in food | 5.27 ± 0.79 | 5.45 ± 0.69 | 0.42 | 3.67 ± 2.52 | 4.67 ± 1.53 | 0.37 | 0.16 | 0.28 |
| Household food measurement | 4.73 ± 0.90 | 5.09 ± 0.83 | 0.24 | 4.33 ± 1.53 | 4.67 ± 0.58 | 1.00 | 0.94 | 0.63 |
| Food label and numeracy | 4.09 ± 0.83 | 4.45 ± 0.82 | 0.24 | 3.67 ± 0.58 | 4.00 ± 1.00 | 1.00 | 0.94 | 0.45 |
| Food groups | 6.72 ± 1.35 | 7.00 ± 1.18 | 0.39 | 6.00 ± 1.00 | 6.33 ± 1.53 | 1.00 | 0.86 | 0.34 |
| Consumer skills | 8.45 ± 0.52 | 8.54 ± 0.52 | 0.77 | 7.67 ± 1.15 | 8.00 ± 1.00 | 1.00 | 0.66 | 0.20 |
| Total | 35.45 ± 2.66 | 36.72 ± 2.05 | 0.12 | 30.00 ± 9.00 | 34.67 ± 3.79 | 0.37 | 0.64 | 0.34 |
Values are mean ± SD; NLit, Nutrition Literacy Assessment Instrument.
P value within arms; change from baseline to 12 weeks post-surgery.
P value between arms; change from baseline to 12 weeks post-surgery.
P value between arms; differences between groups at baseline.
Tracking Physical Activity
Daily step counts recorded by intervention participants did not change significantly from baseline to pre-surgery. Intervention participants recorded more minutes/week of ≥3 Metabolic Equivalents (METs) during weight maintenance compared to weight loss.
Cardiometabolic Biomarkers
Cardiometabolic biomarker results are recorded in Table 9. In the intervention arm, insulin, C-peptide and the leptin:adiponectin ratio significantly decreased by 3.37 μIU/mL (P = 0.03), 0.73 ng/mL (P = 0.01) and 0.70 (P = 0.008) respectively, during the weight loss phase. Fasting glucose concentration decreased by 11.1 mg/dL (P = 0.06) during weight loss in the intervention arm. Lipid profiles, hsCRP, and resistin did not show a statistically significant change in either group during the weight loss phase (P > 0.05). From baseline to 12 weeks post-surgery, resistin levels increased by 4.12 ng/mL (P = 0.0) in the intervention arm without a significant change in the nonintervention arm.
Table 9.
Biomarker results among men in intervention and nonintervention groups at baseline, 1 week before and 12 weeks after prostatectomy.a
| Intervention | Nonintervention | Between arm changes | |||||||
|---|---|---|---|---|---|---|---|---|---|
| Biomarker/component | Baseline | Pre-surgery | Post-surgery | Pwithinb | Baseline | Pre-surgery | Post-surgery | Pwithinb | Pbetweenc |
| Hs-CRP (mg/L) | 1.5 ± 1.1 (15) | 1.7 ± 1.5 (14) | 2.6 ± 4.8 (13) | 0.97 | 4.3 ± 5.1 (5) | 1.7 ± 0.6 (3) | 2.6 ± 1.9 (4) | 0.75 | 0.43 |
| Total cholesterol (mg/dL) | 185.6 ± 37.7 (15) | 177.6 ± 47.8 (14) | 181.2 ± 36.1 (13) | 0.54 | 201.6 ± 32.4 (5) | 215.7 ± 46.1 (3) | 185.3 ± 31.9 (4) | 0.25 | 0.21 |
| HDL cholesterol (mg/dL) | 53.2 ± 14.2 (15) | 54.7 ± 14.0 (14) | 52.9 ± 11.6 (13) | 0.43 | 54.2 ± 13.5 (5) | 46.3 ± 8.5 (3) | 54.8 ± 14.7 (4) | 1.00 | 0.28 |
| LDL cholesterol (mg/dL) | 107.6 ± 28.8 (14) | 104.2 ± 38.6 (14) | 106.9 ± 26.4 (13) | 0.53 | 120.0 ± 29.1 (5) | 130.5 ± 57.3 (2) | 100.3 ± 27.4 (4) | 0.50 | 0.17 |
| Triglycerides (mg/dL) | 132.3 ± 154.6 (15) | 92.8 ± 35.7 (14) | 106.9 ± 52.1 (13) | 0.94 | 137.4 ± 73.6 (5) | 220.7 ± 165.0 (3) | 151.8 ± 135.8 (4) | 0.59 | 0.57 |
| C-peptide (ng/mL) | 2.3 ± 1.4 (15) | 1.5 ± 0.6 (14) | 1.8 ± 1.0 (13) | 0.01 | 2.4 ± 1.4 (5) | 2.4 ± 2.0 (3) | 2.1 ± 1.2 (4) | 0.75 | 0.30 |
| FBG (mg/dL) | 108.8 ± 26.9 (15) | 98.6 ± 15.9 (14) | 104.7 ± 28.7 (13) | 0.06 | 103.4 ± 14.3 (5) | 102.0 ± 8.9 (3) | 104.3 ± 11.0 (4) | 1.00 | 0.49 |
| Fasting insulin (μIU/mL) | 8.8 ± 7.0 (15) | 5.5 ± 2.7 (14) | 8.3 ± 8.1 (13) | 0.03 | 10.1 ± 6.3 (5) | 8.4 ± 5.5 (3) | 7.7 ± 3.4 (4) | 0.75 | 0.71 |
| Leptin:adiponectin ratio | 1.325 ± 1.424 (15) | 0.794 ± 0.981 (13) | 1.339 ± 2.191 (13) | 0.008 | 0.914 ± 0.606 (5) | 0.799 ± 0.629 (3) | 0.834 ± 0.841 (4) | 0.75 | 0.30 |
| Resistin (ng/mL) | 24.7 ± 9.9 (15) | 24.7 ± 10.9 (13) | 28.3 ± 8.0 (13)d | 0.50 | 25.9 ± 7.1 (5) | 28.8 ± 3.4 (3) | 35.0 ± 1.5 (4) | 1.00 | 0.80 |
| Metabolic syndrome | 33.0% (5/15) | 14.3% (2/14) | 30.1% (4/13) | N/A | 40% (2/5) | 66.7% (2/3) | 25% (1/4) | N/A | N/A |
Values represent mean ± SD (n) or % (participants affected/total participants). hs-CRP, high sensitivity C-reactive protein; HDL, high-density lipoprotein; LDL, low-density lipoprotein; FBG, fasting blood glucose.
P value within arms; from baseline to pre-surgery.
P value between arms; change from baseline to pre-surgery.
P value < 0.05 from baseline value; all other net changes were not significant.
Quality-of-Life & Long-Term Outcomes
SF8 scores are shown in Table 10. There were not any significant differences in SF8 scores during weight loss. The difference in change of general health scores between groups was statistically significant from baseline to week 12 (P = 0.02). EPIC surveys did not reveal any significant between group differences in quality-of-life one year after study completion. Modest weight changes occurred in both groups after study completion. One year after study completion, 64% of intervention participants were still wearing their Vivofit® accelerometer and 18% were tracking their nutrition and exercise. Two and three years after study completion, one intervention and none of the nonintervention participants experienced prostate cancer recurrence.
Table 10.
SF8 scores among men in intervention and nonintervention groups at baseline, 1 week before and 12 weeks after prostatectomy.a
| Intervention score | Nonintervention score | Between arm changes | |||||||
|---|---|---|---|---|---|---|---|---|---|
| SF8 component | Baseline | Pre-surgery | 12 weeks post-surgery | Pwithinb | Baseline | Pre-surgery | 12 weeks post-surgery | Pwithinb | Pbetweenc |
| General health | 49.7 ± 6.8 (15) | 52.9 ± 4.4 (14) | 52.3 ± 6.5 (13) | 0.13 | 51.6 ± 2.9 (5) | 50.7 ± 3.7 (3) | 46.4 ± 0 (4)4 | 1.0 | 0.02 |
| Physical functioning | 50.2 ± 7.4 (15) | 52.2 ± 4.1 (14) | 49.6 ± 7.8 (13) | 0.13 | 52.9 ± 2.6 (5) | 52.1 ± 3.3 (3) | 44.2 ± 4.8 (4) | 1.0 | 0.01 |
| Role physical | 50.6 ± 7.7 (15) | 52.7 ± 4.6 (14) | 50.0 ± 7.6 (13) | 0.18 | 51.2 ± 3.9 (5) | 51.6 ± 4.1 (3) | 43.6 ± 8.4 (4) | 1.0 | 0.23 |
| Bodily pain | 52.6 ± 8.0 (15) | 53.7 ± 7.1 (14) | 57.5 ± 4.6 (13) | 0.44 | 52.9 ± 7.2 (5) | 56.4 ± 7.6 (3) | 51.9 ± 8.6 (4) | NA | 0.10 |
| Vitality | 54.4 ± 5.2 (15) | 54.3 ± 5.4 (14) | 54.5 ± 4.5 (13) | 1.0 | 49.3 ± 5.7 (5) | 52.1 ± 6.0 (3) | 47.8 ± 5.2 (4) | 1.0 | 0.43 |
| Social functioning | 50.7 ± 8.9 (15) | 51.5 ± 5.4 (14) | 50.5 ± 6.2 (13) | 0.79 | 47.0 ± 6.5 (5) | 51.4 ± 3.3 (3) | 43.7 ± 11.2 (4) | 0.34 | 0.61 |
| Mental health | 50.0 ± 8.7 (15) | 50.9 ± 6.1 (14) | 50.8 ± 8.1 (13) | 0.27 | 46.2 ± 6.9 (5) | 49.3 ± 7.6 (3) | 46.7 ± 12.4 (4) | NA | 0.59 |
| Role emotional | 49.0 ± 6.9 (15) | 50.4 ± 4.3 (14) | 50.3 ± 4.4 (13) | 0.37 | 45.5 ± 5.1 (5) | 47.9 ± 3.9 (3) | 41.4 ± 10.0 (4) | 1.0 | 0.05 |
| Physical | 52.1 ± 7.4 (15) | 54.6 ± 4.8 (14) | 53.6 ± 7.8 (13) | 0.17 | 54.1 ± 3.4 (5) | 54.6 ± 3.5 (3) | 45.8 ± 5.4 (4) | 0.25 | 0.04 |
| Mental | 51.4 ± 10.1 (15) | 52.1 ± 5.5 (14) | 52.7 ± 8.0 (13) | 0.86 | 44.7 ± 7.1 (5) | 49.5 ± 6.0 (3) | 45.2 ± 12.7 (4) | 0.50 | 0.55 |
Values are mean ± SD (n); SF8, Short-form 8.
P value within arms; change from baseline to surgery.
P value between arms; change from baseline to study end.
Discussion
This trial demonstrates the feasibility of implementing a male-tailored weight management program to achieve ≥5% weight loss and ≤3% regain in overweight men preparing for prostatectomy. Men are less likely to perceive themselves as overweight, and research suggests that effective weight loss regimens for men should be less restrictive and provide autonomy. Our study allowed for self-selection to study arms, which did introduce inherent bias; yet, the self-selection process showed men are actually willing to adopt a weight loss program (22). Feasibility was demonstrated; our study confirmed that men are not only willing to adopt a weight loss program but can succeed in losing significant amounts of weight. Our study supports findings that this timeframe immediately following prostate cancer diagnosis constitutes an opportune time when men are motivated to modify their lifestyle (11,29–31) as indicated by data showing that three quarters of men opted for the weight loss regimen. Additionally, favorable changes in visceral adipose and other risk biomarkers were observed following the program.
The 5.4% pre-prostatectomy weight loss achieved in our study is comparable to the 2 to 6% (17,20) and the 0.8 to 6.1 kg (32) reported in similar trials. Participants in our study lost similar amounts of fat and lean tissue mass as Demark-Wahnefried et al. (3.88 kg vs 3.12 kg and 1.6 kg vs 1.19 kg respectively). Demark-Wahnefried et al. reported 4.7 kg weight loss whereas we report 5.55 kg weight loss (17). Changes in lean tissue mass noted in our intervention are also similar to results by Henning et al. in which participants lost 1.6 kg of lean tissue in concomitance with 3.7 kg weight (18). While significant weight and fat loss were achieved, these results suggest greater attention to lean mass preservation may be needed.
Similar to results reported by Wright et al. (20), consumption of fruits and vegetables increased in our intervention group, which may reduce the risk for CVD (33). In addition, we report intervention-specific increases in fiber, protein and nut intake along with a decrease in the consumption of trans fats prior to prostatectomy. While dietary adherence increased and ≥5% weight loss was achieved, stagnant NLit scores during the intervention demonstrate that participants were able to achieve weight loss simplified by the provision of meal replacements and one on one coaching; yet, the scores reflect where the nutrition curriculum may be bolstered to improve nutrition education and comprehension.
Increasing step counts was selected as the primary physical activity goal based on formative work from our team, which found that men in our region identified walking as their preferred mode of physical activity (34). We also used steps to facilitate competition through a de-identified step leaderboard within the LST app. We report modest step-count improvements from baseline to surgery; however, changes varied dramatically between participants, contributing to a small net change. Importantly, participants were not blinded to their steps and started wearing the accelerometer one week prior to their baseline visit, which may have inflated baseline counts. Recorded METs in the intervention group were higher during the post-operative phase compared to the pre-operative phase despite the coinciding decline in steps.
Abdominal obesity has been specifically linked with prostate cancer progression (35–37). Abdominal adiposity and elevated leptin:adiponectin ratios are associated with a higher risk of aggressive pathological features (38,39). We report significant reductions in both visceral adiposity and leptin:adiponectin ratios from baseline-to-surgery among intervention participants. These reductions may be protective; however, longitudinal survival data are required to provide a target for future research.
Hyperinsulinemia caused by excessive adiposity is associated with prostate cancer development and aggressiveness (40,41). While higher levels of insulin-resistance biomarkers are linked to more aggressive cancers (39,42), it is currently inconclusive if a reduction of these biomarkers decreases prostate cancer progression. Pre-surgical intervention improvements in fasting insulin, fasting glucose and C-peptide levels reported in our study suggest weight loss benefits on insulin sensitivity. Similarly, Henning et al. reported a reduction in insulin levels in men who lost weight before prostatectomy (18) and Wright et al. reported improvements in glycemic biomarkers, notably a significant increase in serum IGFBP-3 following 6 weeks of caloric restriction in prostate cancer patients (20).
Given that prostate cancer survivors are more likely to die from heart disease than prostate cancer recurrence (43), the observed improvements in C-peptide, insulin, glucose, central adiposity and systolic blood pressure in our intervention suggest a pre-surgical weight loss-mediated benefit on cardiometabolic health. These results suggest potential long-term overall health benefits of weight management programs to overweight men undergoing prostatectomy.
Higher levels of resistin are linked to insulin resistance and obesity. Similarly, mean baseline resistin levels were above the normal range of 7–22 ng/mL in our participants (44). Although we report an increase in serum resistin by 12 weeks post-surgery in the intervention arm, our results are similar to a 4 mo, weight loss intervention trial by Koebnick et al., suggesting that resistin may not be affected by weight change alone (45).
We report a significant difference between groups in the difference in change of general health scores over the course of the study. Demark-Wahnefried et al. reported significant improvements in vitality scores (one subdomain of general health) in the weight loss intervention group compared to control (17). Focusing on improving overall health around the time of prostatectomy may offer a coping strategy that helps men feel better more globally.
Given that only one intervention participant recurred during the follow up period, it is difficult to draw conclusions from these data given the one recurrence in follow-up coincided with weight regain and smoking, which are both associated with a higher risk for prostate cancer recurrence (46). The follow-up period currently reported was only three years and not long enough to obtain an adequate number of prostate cancer recurrence events. We recognize this as a limitation to the follow-up data.
Several other limitations to our study exist. The purpose of this project was to assess feasibility, so the sample size is small. The exploratory aims were analyzed without adjustments for multiple comparisons with the intent to inform the design of a larger trial and are not intended to be conclusive. The preoperative duration was not identical across participants and was shorter in the nonintervention than the intervention. While non-randomization is also a limitation, the self-selection method for group assignment resulted in a larger intervention group compared to the nonintervention, indicating a readiness for overweight men to be willing to lose weight before their prostatectomy. We recognize that men choosing the intervention over the nonintervention may inherently have a higher motivation for health overall. Lastly, the weight maintenance period was only 3 mo, which did not significantly increase nutrition literacy. These data and limitations informed the design of a larger-scale trial (NCT03261271) currently underway.
Our study has several strengths. Our nonintervention group did not receive diet or lifestyle counseling before or after prostatectomy which reduces bias from unintended behavior changes in the comparison group. Significant weight loss only occurred in the intervention group, strengthening the validity of between group differences. Moreover, our study included a weight maintenance period after prostatectomy as well as 3-year outcomes to track recurrence and participant status. These data may guide the design of future trials to facilitate sustained behavior changes for long term health. Interestingly, Wilson et al. reported a significant reduction in visceral adipose tissue and fat mass in a retrospective analysis of patients referred to an allied health clinic for a very similar program for up to 12 weeks (average 29 day) before prostatectomy (47), demonstrating the feasibility of incorporating programming like this into clinical pathways for better health outcomes.
The men in our intervention achieved clinically meaningful weight loss along with improvements in body composition during the preoperative window, and weight was successfully maintained during the 12-week postoperative weight maintenance phase. We demonstrate that short-term diet and lifestyle changes coupled with coaching support and self-tracking technology prior to prostatectomy have the capacity to favorably modify biomarkers. These data supported further investigation in a larger, randomized trial (NCT03261271) to evaluate the effects of a peri-pros-tatectomy weight management program to improve disease-specific biomarkers in overweight men undergoing prostatectomy.
Acknowledgments
The authors would like to thank the CTSU staff for assisting with study visits, operating the iDXA machine, and performing phlebotomy; Tanner Isaacson, Cole Chana and Abigail Stanley for health coaching; Lucas Bider, Katie Erickson, Heather Gibbs, and Kelsey Nicholson for contributions to the program curriculum; Areej Bawajeeh for help with data extraction; Thomas Yankee and Rich Hastings for managing the equipment used to run the Luminex assays; Craig Lanio at MilliporeSigma for support with Luminex analysis; Brent Kevern and Jody Loyd at LST for customizing the self-tracking application and assisting with participant training; and Medifast® for donating meal replacements.
Funding
The Prostate Cancer (PCa) Guys Resilient by Individualized Training (PCaGRIT) trial was supported by the University of Kansas Cancer Center Cancer Prevention Pilot Grant program (JHR), the National Cancer Institute Cancer Center Support Grant (P30 CA16852), in part by an NIH Clinical and Translational Science Award grant (UL1 TR000001, formerly UL1RR033179), and used the Nutrition Shared Resource. Support for J. Hamilton-Reeves (JHR) was provided by KL2 training grant KL2TR000119 a CTSA grant from NCATS awarded to the University of Kansas Medical Center for Frontiers: The Heartland Institute for Clinical and Translational Research (JHR). Research used the Nutrition Shared Resource. The contents are solely the responsibility of the authors and do not necessarily represent the official views of the NIH or NCATS. Research reported in this publication was supported by the National Cancer. Institute Cancer Center Support Grant P30 CA168524 and used the Biospecimen Shared Resource, the Nutrition Shared Resource, and the Biostatistics and Informatics Shared Resource. Jason Pharmaceuticals, a wholly-owned subsidiary of Medifast, Inc. provided an in-kind donation of the meal replacements used in the study.
Abbreviations:
- AE
adverse events
- AHEI
Alternative Healthy Eating Index
- BMI
body mass index
- CTSU
Clinical and Translational Science Unit
- CVD
cardiovascular disease
- DXA
dual energy x-ray absorptiometry
- EPIC
expanded prostate cancer index
- HDL
high density lipoprotein
- hsCRP
high sensitivity C-reactive protein
- IGBFBP
insulin-like growth factor binding protein
- KU
University of Kansas
- LST
LifeScience Technologies
- LDL
low density lipoprotein
- MET
metabolic equivalent
- NCI
National Cancer Institute
- NDSR
Nutrition Data System for Research
- NLit
Nutrition Literacy Assessment
- PSA
prostate specific antigen
Footnotes
Disclosure Statement
None of the authors had conflicts of interests to report.
References
- 1.Siegel RL, Miller KD, Jemal A. Cancer statistics, 2019. CA A Cancer J Clin. 2019;69(1):7–34. doi: 10.3322/caac.21551 [DOI] [PubMed] [Google Scholar]
- 2.Roehl KA, Han M, Ramos CG, Antenor JA, Catalona WJ. Cancer progression and survival rates following anatomical radical retropubic prostatectomy in 3,478 consecutive patients: long-term results. J Urol. 2004; 172(3):910–914. doi: 10.1097/01.ju.0000134888.22332.bb [DOI] [PubMed] [Google Scholar]
- 3.Pound CR, Partin AW, Eisenberger MA, Chan DW, Pearson JD, Walsh PC. Natural history of progression after PSA elevation following radical prostatectomy. JAMA. 1999;281(17):1591–1597. doi: 10.1001/jama.281.17.1591 [DOI] [PubMed] [Google Scholar]
- 4.Chalfin HJ, Lee SB, Jeong BC, Freedland SJ, Alai H, Feng Z, Trock BJ, Partin AW, Humphreys E, Walsh PC, et al. Obesity and long-term survival after radical prostatectomy. J Urol. 2014;192(4):1100–1104. doi: 10.1016/j.juro.2014.04.086 [DOI] [PubMed] [Google Scholar]
- 5.Ho T, Gerber L, Aronson WJ, Terris MK, Presti JC, Kane CJ, Amling CL, Freedland SJ. Obesity, prostate-specific antigen nadir, and biochemical recurrence after radical prostatectomy: biology or technique? Results from the search database. Eur Urol. 2012; 62(5):910–916. doi: 10.1016/j.eururo.2012.08.015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Cao Y, Ma J. Body mass index, prostate cancer-specific mortality, and biochemical recurrence: a systematic review and meta-analysis. Cancer Prev Res (Philadelphia, PA). 2011;4(4):486–501. doi: 10.1158/1940-6207.CAPR-10-0229 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Joshu CE, Mondul AM, Menke A, Meinhold C, Han M, Humphreys EB, Freedland SJ, Walsh PC, Platz EA. Weight gain is associated with an increased risk of prostate cancer recurrence after prostatectomy in the PSA era. Cancer Prev Res (Phila). 2011;4(4): 544–551. doi: 10.1158/1940-6207.CAPR-10-0257 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Whitley BM, Moreira DM, Thomas JA, Aronson WJ, Terris MK, Presti JC Jr., Kane CJ, Amling CL, Freedland SJ. Preoperative weight change and risk of adverse outcome following radical prostatectomy: results from the shared equal access regional cancer hospital database. Prostate Cancer Prostat Dis. 2011; 14(4):361–366. doi: 10.1038/pcan.2011.42 [DOI] [PubMed] [Google Scholar]
- 9.Zaorsky NG, Churilla TM, Egleston BL, Fisher SG, Ridge JA, Horwitz EM, Meyer JE. Causes of death among cancer patients. Ann Oncol. 2017;28(2): 400–407. doi: 10.1093/annonc/mdw604 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Bluethmann SM, Basen-Engquist K, Vernon SW, Cox M, Gabriel KP, Stansberry SA, Carmack CL, Blalock JA, Demark-Wahnefried W. 2015. Grasping the ‘teachable moment’: time since diagnosis, symptom burden and health behaviors in breast, colorectal and prostate cancer survivors. Psychooncology. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Satia JA, Walsh JF, Pruthi RS. Health behavior changes in white and African American prostate cancer survivors. Cancer Nurs. 2009;32(2):107–117. doi: 10.1097/NCC.0b013e3181982d4c [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Schroeck FR, Krupski TL, Sun L, Albala DM, Price MM, Polascik TJ, Robertson CN, Tewari AK, Moul JW. Satisfaction and regret after open retropubic or robot-assisted laparoscopic radical prostatectomy. Eur Urol. 2008;54(4):785–93. doi: 10.1016/j.eururo.2008.06.063 [DOI] [PubMed] [Google Scholar]
- 13.Donnelly JE, Blair SN, Jakicic JM, Manore MM, Rankin JW, Smith BK, American College of Sports Medicine. American College of Sports Medicine Position Stand. Appropriate physical activity intervention strategies for weight loss and prevention of weight regain for adults. Med Sci Sports Exerc. 2009; 41(2):459–471. doi: 10.1249/MSS.0b013e3181949333 [DOI] [PubMed] [Google Scholar]
- 14.Wing RR, Lang W, Wadden TA, Safford M, Knowler WC, Bertoni AG, Hill JO, Brancati FL, Peters A, Wagenknecht L, the Look AHEAD Research Group. Benefits of modest weight loss in improving cardiovascular risk factors in overweight and obese individuals with type 2 diabetes. Diabetes Care. 2011;34(7): 1481–1486. doi: 10.2337/dc10-2415 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Stevens J, Truesdale KP, McClain JE, Cai J. The definition of weight maintenance. Int J Obes. 2006;30(3): 391–399. doi: 10.1038/sj.ijo.0803175 [DOI] [PubMed] [Google Scholar]
- 16.Demark-Wahnefried W, Nix JW, Hunter GR, Rais-Bahrami S, Desmond RA, Chacko B, Morrow CD, Azrad M, Fruge AD, Tsuruta Y, et al. Feasibility outcomes of a presurgical randomized controlled trial exploring the impact of caloric restriction and increased physical activity versus a wait-list control on tumor characteristics and circulating biomarkers in men electing prostatectomy for prostate cancer. BMC Cancer. 2016;16:61. doi: 10.1186/s12885-016-2075-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Demark-Wahnefried W, Rais-Bahrami S, Desmond RA, Gordetsky JB, Hunter GR, Yang ES, Azrad M, Frugé AD, Tsuruta Y, Norian LA, et al. Presurgical weight loss affects tumour traits and circulating biomarkers in men with prostate cancer. Br J Cancer. 2017;117(9):1303–1313. doi: 10.1038/bjc.2017.303 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Henning SM, Galet C, Gollapudi K, Byrd JB, Liang P, Li Z, Grogan T, Elashoff D, Magyar CE, Said J, et al. Phase ii prospective randomized trial of weight loss prior to radical prostatectomy. Prostate Cancer Prostatic Dis. 2018;21(2):212–220. doi: 10.1038/s41391-017-0001-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Lin DW, Neuhouser ML, Schenk JM, Coleman IM, Hawley S, Gifford D, Hung H, Knudsen BS, Nelson PS, Kristal AR. Low-fat, low-glycemic load diet and gene expression in human prostate epithelium: a feasibility study of using CDNA microarrays to assess the response to dietary intervention in target tissues. Cancer Epidemiol Biomarkers Prev. 2007;16(10): 2150–2154. doi: 10.1158/1055-9965.EPI-07-0154 [DOI] [PubMed] [Google Scholar]
- 20.Wright JL, Plymate S, D’Oria-Cameron A, Bain C, Haugk K, Xiao L, Lin DW, Stanford JL, McTiernan A. A study of caloric restriction versus standard diet in overweight men with newly diagnosed prostate cancer: a randomized controlled trial. Prostate. 2013; 73(12):1345–1351. doi: 10.1002/pros.22682 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Morgan PJ, Callister R, Collins CE, Plotnikoff RC, Young MD, Berry N, McElduff P, Burrows T, Aguiar E, Saunders KL. The shed-it community trial: a randomized controlled trial of internet- and paper-based weight loss programs tailored for overweight and obese men. Ann Behav Med. 2013;45(2):139–152. doi: 10.1007/s12160-012-9424-z [DOI] [PubMed] [Google Scholar]
- 22.Lewis S, Thomas SL, Hyde J, Castle DJ, Komesaroff PA. A qualitative investigation of obese men’s experiences with their weight. Am J Health Behav. 2011; 35(4):458–469. [DOI] [PubMed] [Google Scholar]
- 23.Bandura A Health promotion by social cognitive means. Health Educ Behav. 2004;31(2):143–164. doi: 10.1177/1090198104263660 [DOI] [PubMed] [Google Scholar]
- 24.Murawski ME, Milsom VA, Ross KM, Rickel KA, DeBraganza N, Gibbons LM, Perri MG. Problem solving, treatment adherence, and weight-loss outcome among women participating in lifestyle treatment for obesity. Eating Behav. 2009;10(3):146–151. doi: 10.1016/j.eatbeh.2009.03.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Heymsfield SB, van Mierlo CA, van der Knaap HC, Heo M, Frier HI. Weight management using a meal replacement strategy: meta and pooling analysis from six studies. Int J Obes. 2003;27(5):537–549. doi: 10.1038/sj.ijo.0802258 [DOI] [PubMed] [Google Scholar]
- 26.Young MD, Lubans DR, Collins CE, Callister R, Plotnikoff RC, Morgan PJ. Behavioral mediators of weight loss in the shed-it community randomized controlled trial for overweight and obese men. Ann Behav Med. 2015;49(2):286–292. doi: 10.1007/s12160-014-9657-0 [DOI] [PubMed] [Google Scholar]
- 27.Harris PA, Taylor R, Thielke R, Payne J, Gonzalez N, Conde JG. Research electronic data capture (REDCap)-a metadata-driven methodology and work-flow process for providing translational research informatics support. J Biomed Inform. 2009;42(2): 377–381. doi: 10.1016/j.jbi.2008.08.010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Gibbs HD, Ellerbeck EF, Gajewski B, Zhang C, Sullivan DK. The nutrition literacy assessment instrument is a valid and reliable measure of nutrition literacy in adults with chronic disease. J Nutr Educ Behav. 2018;50(3):247–257. doi: 10.1016/j.jneb.2017.10.008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Avery KN, Donovan JL, Gilbert R, Davis M, Emmett P, Down L, Oliver S, Neal DE, Hamdy FC, Lane JA. Men with prostate cancer make positive dietary changes following diagnosis and treatment. Cancer Causes Control. 2013;24(6):1119–1128. doi: 10.1007/s10552-013-0189-x [DOI] [PubMed] [Google Scholar]
- 30.Patterson RE, Neuhouser ML, Hedderson MM, Schwartz SM, Standish LJ, Bowen DJ. Changes in diet, physical activity, and supplement use among adults diagnosed with cancer. J Am Dietetic Assoc. 2003;103(3):323–328. [DOI] [PubMed] [Google Scholar]
- 31.Avery KN, Donovan JL, Horwood J, Neal DE, Hamdy FC, Parker C, Wade J, Lane A. The importance of dietary change for men diagnosed with and at risk of prostate cancer: a multi-centre interview study with men, their partners and health professionals. BMC Fam Pract. 2014;15:81. doi: 10.1186/1471-2296-15-81 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Mohamad H, McNeill G, Haseen F, N’Dow J, Craig LC, Heys SD. The effect of dietary and exercise interventions on body weight in prostate cancer patients: a systematic review. Nutr Cancer. 2015;67(1):43–60. doi: 10.1080/01635581.2015.976313 [DOI] [PubMed] [Google Scholar]
- 33.Jochems SHJ, Van Osch FHM, Bryan RT, Wesselius A, van Schooten FJ, Cheng KK, Zeegers MP. Impact of dietary patterns and the main food groups on mortality and recurrence in cancer survivors: a systematic review of current epidemiological literature. BMJ Open. 2018;8(2):e014530. doi: 10.1136/bmjopen-2016-014530 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Diggett B, Holzbeierlein J, Klemp J, Glennon C, Hamilton-Reeves JM. Patient-centered perspectives on the access to educational opportunities specific to lifestyle modification in men at risk for primary or secondary prostate cancer. J Cancer Educ. 2014;29(2): 252–257. doi: 10.1007/s13187-013-0583-9 [DOI] [PubMed] [Google Scholar]
- 35.De Nunzio C, Albisinni S, Freedland SJ, Miano L, Cindolo L, Finazzi Agro E, Autorino R, De Sio M, Schips L, Tubaro A. Abdominal obesity as risk factor for prostate cancer diagnosis and high grade disease: a prospective multicenter Italian cohort study. Urol Oncol. 2013;31(7):997–1002. doi: 10.1016/j.urolonc.2011.08.007 [DOI] [PubMed] [Google Scholar]
- 36.van Kruijsdijk RC, van der Wall E, Visseren FL. Obesity and cancer: the role of dysfunctional adipose tissue. Cancer Epidemiol Biomarkers Prev. 2009; 18(10):2569–2578. doi: 10.1158/1055-9965.EPI-09-0372 [DOI] [PubMed] [Google Scholar]
- 37.Lysaght J, van der Stok EP, Allott EH, Casey R, Donohoe CL, Howard JM, McGarrigle SA, Ravi N, Reynolds JV, Pidgeon GP. Pro-inflammatory and tumour proliferative properties of excess visceral adipose tissue. Cancer Lett. 2011;312(1):62–72. doi: 10.1016/j.canlet.2011.07.034 [DOI] [PubMed] [Google Scholar]
- 38.Qu YY, Dai B, Kong YY, Chang K, Ye DW, Yao XD, Zhang SL, Zhang HL, Yang WY. Influence of obesity on localized prostate cancer patients treated with radical prostatectomy. Asian J Androl. 2013;15(6): 747–752. doi: 10.1038/aja.2013.104 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Di Sebastiano KM, Pinthus JH, Duivenvoorden WC, Patterson L, Dubin JA, Mourtzakis M. Elevated c-peptides, abdominal obesity, and abnormal adipokine profile are associated with higher gleason scores in prostate cancer. Prostate. 2017;77(2):211–221. doi: 10.1002/pros.23262 [DOI] [PubMed] [Google Scholar]
- 40.Hammarsten J, Damber JE, Peeker R, Mellstrom D, Hogstedt B. A higher prediagnostic insulin level is a prospective risk factor for incident prostate cancer. Cancer Epidemiol. 2010;34(5):574–579. doi: 10.1016/j.canep.2010.06.014 [DOI] [PubMed] [Google Scholar]
- 41.Hammarsten J, Hogstedt B. Hyperinsulinaemia: a prospective risk factor for lethal clinical prostate cancer. Eur J Cancer (Oxford, England). 2005;41(18): 2887–2895. doi: 10.1016/j.ejca.2005.09.003 [DOI] [PubMed] [Google Scholar]
- 42.Ma J, Li H, Giovannucci E, Mucci L, Qiu W, Nguyen PL, Gaziano JM, Pollak M, Stampfer MJ. Prediagnostic body-mass index, plasma c-peptide concentration, and prostate cancer-specific mortality in men with prostate cancer: a long-term survival analysis. Lancet Oncol. 2008;9(11):1039–1047. doi: 10.1016/S1470-2045(08)70235-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Epstein MM, Edgren G, Rider JR, Mucci LA, Adami H-O. Temporal trends in cause of death among Swedish and US men with prostate cancer. J Natl Cancer Inst. 2012;104(17):1335–1342. doi: 10.1093/jnci/djs299 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Acquarone E, Monacelli F, Borghi R, Nencioni A, Odetti P. Resistin: a reappraisal. Mech Ageing Dev. 2019;178:46–63. doi: 10.1016/j.mad.2019.01.004 [DOI] [PubMed] [Google Scholar]
- 45.Koebnick C, Wagner K, Garcia AL, Gruendel S, Lahmann PH, Weickert MO, Möhlig M, Harsch IA, Einig C, Speth M, et al. Increase in serum resistin during weight loss in overweight subjects is related to lipid metabolism. Int J Obes. 2006;30(7):1097–1103. doi: 10.1038/sj.ijo.0803242 [DOI] [PubMed] [Google Scholar]
- 46.Kenfield SA, Stampfer MJ, Chan JM, Giovannucci E. Smoking and prostate cancer survival and recurrence. JAMA. 2011;305(24):2548–2555. doi: 10.1001/jama.2011.879 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Wilson RL, Shannon T, Calton E, Galvao DA, Taaffe DR, Hart NH, Lyons-Wall P, Newton RU. Efficacy of a weight loss program prior to robot assisted radical prostatectomy in overweight and obese men with prostate cancer. Surg Oncol. 2020;35:182–188. doi: 10.1016/j.suronc.2020.08.006 [DOI] [PubMed] [Google Scholar]


