Abstract
Background
Testis expressed 19 (TEX19) is a specific human stem cell gene identified as cancer‐testis antigen (CTA), which emerged as a potential therapeutic drug target. TEX19.1, a mouse paralog of human TEX19, can interact with LINE‐1 retrotransposable element ORF1 protein (LIRE1) and subsequently restrict mobilization of LINE‐1 elements in the genome.
Aim
This study aimed to predict the interaction of TEX19 with LIRE1 and analyze TEX19 missense polymorphisms. TEX19 model was generated using I‐TASSER and the interaction between TEX19 and LIRE1 was studied using the HADDOCK software.
Methods
The stability of the docking formed complex was studied through the molecular dynamic simulation using GROMACS. Missense SNPs (n=102) of TEX19 were screened for their potential effects on protein structure and function using different software.
Results
Outcomes of this study revealed amino acids that potentially stabilize the predicted interaction interface between TEX19 and LIRE1. Of these SNPs, 37 were predicted to play a probably damaging role for the protein, three of them (F35S, P61R, and E55L) located at the binding site of LIRE1 and could disturb this binding affinity.
Conclusion
This information can be verified by further in vitro and in vivo experimentations and could be exploited for potential therapeutic targets.
Keywords: LINE‐1, MD simulation, molecular docking, SNPs analysis, TEX19
interaction pattern between TEX19 and LIRE1 proteins Formation of Van der Waals, electrostatic interactions and hydrogen bonding and formation of TEX19 and LIRE1complex
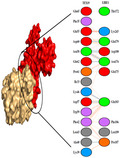
1. INTRODUCTION
Cancer/testis (CT) genes are mainly expressed in the testis with a significant upregulation during oncogenesis (Wang et al., 2016) Testis‐expressed 19 (TEX19) (OMIM# 615647) is one such mammalian‐specific CT genes that is unique for humans and expressed in adult testis and undifferentiated embryonic stem cells and primordial germ cells (Kuntz et al., 2008; Wang et al., 2001). This gene was duplicated in mouse and rat genomes giving rise to TEX19.1 and TEX19.2 paralogs. Among these paralogs, mouse Tex19.1 is more similar to human TEX19 and both genes are expressed throughout pluripotent cycle and their expression is lost when pluripotent stem cells differentiate (Hawsawi et al., 2018; Kuntz et al., 2008). Multiple sequence alignment of TEX19 proteins resulted in two conserved domains, which do not share homologies with known proteins. Therefore, it was unable to predict their functions (Kuntz et al., 2008). In two separate studies, Feichtinger et al. (2012) and Planells‐Palop et al., 2017, studied the expression profiles of human meiotic genes in different types of cancer and conducted meta‐analyses of clinical data sets, however, the role of human TEX19 in cancer was not well reflected both studies (Feichtinger et al., 2012; Planells‐Palop et al., 2017).
Mice with TEX19 double knockout (TEX19DKO) or single TEX19.1KO exhibited a fully penetrant phenotype with impaired spermatogenesis, testis degeneration, small testes (Yang et al., 2010), in oogenesis (Reichmann et al., 2020) defects in meiotic chromosome synapsis, persistence of DNA double‐strand breaks during meiosis, lack of post‐meiotic germ cells, and upregulation of MMERVK10C expression (Ollinger et al., 2008; Tarabay et al., 2013, 2017). However, TEX19.2KO mice presented only a subtle phenotype with discrete seminiferous tubule degeneration in adult male testes (Hawsawi et al., 2020; Tarabay et al., 2017). TEX19.1 is the only transcripts present in developing and adult ovaries as well as in the placenta and TEX19.1KO mouse embryos exhibit intrauterine growth retardation and have small placentas due to reduced number of spongiotrophoblast, glycogen trophoblast and sinusoidal trophoblast giant cells (Reichmann et al., 2013; Tarabay et al., 2013). TEX19 was also identified for its role in the progression of bladder and ovarian cancer and was considered as a potential immunotherapeutic target for cancer treatment (Xu et al., 2020; Zhong et al., 2016).
Retrotransposons are mobile genetic elements which act as significant driver of the evolution of the mammalian genome, but their mobilization can also make the genome vulnerable to genetic disorders and cancers (Al‐Amer et al., 2020; Alsohime et al., 2021; Garcia‐Perez et al., 2016; Kotb et al., 2018). In humans, the majorities of retrotransposition events are activated by long interspersed element class 1 (known as LINE‐1 or L1) (OMIM# 151626) gene which encodes ORF1 protein (also known as LIRE1 which stands for LINE‐1 retrotransposable element ORF1 protein) (Beck et al., 2011). The human TEX19 protein has been shown experimentally to interact with human LINE‐1 ORF1p and promotes polyubiquitylation of hL1‐ORF1p as it restricts mobilization of both human LINE‐1 (MacLennan et al., 2017).
LIRE1 is a nucleic acid‐binding protein that plays an essential role in the retrotransposition of LINE‐1 elements in the genome (Martin & Bushman, 2001). To maintain stability of mammalian genomes and minimizing incidence of mutation and cancer, our cells release factors to restrict the mobilization of L1 through binding and subsequently inhibiting LIRE1. Among these factors, TEX19.1 can act as retrotransposon inhibitor gene which suppresses L1 expression in mice spermatocytes (Reichmann et al., 2012). Mice TEX19.1 protein can interact with LIRE1, thereby restricting mobilization of LINE‐1 retrotransposons in the developing germline (MacLennan et al., 2017). Unlike mice, little is known regarding the interaction between TEX19 with LIRE1 and the effect of missense polymorphisms on this interaction. The main protein interacting partner of TEX19.1 in vivo is Ubr2 (MacLennan et al., 2017; Reichmann et al., 2020; Yang et al., 2010) and the human TEX19 also interacts with UBR2 (Reichmann et al., 2020), and Ubr2 also physically interacts with LINE‐1 ORF1p (MacLennan et al., 2017). Accordingly, there is potentially a trimeric complex between TEX19.1, Ubr2 and LINE‐1 ORF1p.
Previously, we reported several SNPs that associated with cancer (Alzahrani et al., 2020; Hawsawi et al., 2019; Semlali, Almutairi, et al., 2018; Semlali, Parine, et al., 2018). This in silico study aimed to predict the interaction of TEX19 with LIRE1 and the role of TEX19 gene polymorphisms in the stability of produced protein and the interaction with LIRE1.
2. MATERIALS AND METHODS
2.1. Protein structures and homology modeling
The protein sequence of the TEX19 was downloaded from NCBI (ID: NP_997342.1). The 3D structure of TEX19 protein was predicted by using the Iterative‐Threading ASSEmbly Refinement (I‐TASSER) server (Yang & Zhang, 2015). This method generated five models, and the best one was selected based on the C‐Score, which is a measure to observe the quality of resulting models showed the correlation quality of the model prediction results. C‐score is typically in the range of (−5, 2). A C‐score of higher value signifies a model with high confidence and vice versa. The model selected with C‐score (−5.0) was further subjected to molecular dynamic simulation to remove any steric clashes and get a stable structure (Yang et al., 2015). To validate the TEX19 model, a PROSA statistic was used. PROSA is a web‐based interactive software application which shows the energy plots and scores. It aids in identifying the potential problems spotted model structure of the protein. It has a full application in evaluating errors in 3D models of protein. The crystal structure of LINE‐1 protein (PDB ID: 2W7A) was obtained RCSB PDB database (Berman et al., 2002).
2.2. Docking
Protein–protein interaction study was performed by High Ambiguity Driven protein‐protein DOCKing (HADDOCK) software (Dominguez et al., 2003). Proteins were uploaded for docking at HDDOCK server and all parameters were kept as default.
2.3. Molecular dynamic (MD) simulation
MD simulation of the interested TEX19 and LIRE1 complex was carried out using GROMACS package (Hess et al., 2008), CHARMM 36 force‐field (Huang et al., 2017), and the TIP3P water model (Price & Brooks, 2004). The system charges were then neutralized by addition of ions. Energy minimization was performed using the steepest descent method of 10,000 steps, followed by the conjugate gradient method for 10,000 steps. NVT equilibration was done at 300 K and 100 ps of the run, followed by NPT equilibration of 100 ps. Finally, the production MD run was performed for 20 ns, whereas for TEX19 model, MD simulation was carried out at 30 ns.
2.4. Prediction of the pathogenic effects and disease‐related of SNPs
Different software were used for prediction of the effect of missense single nucleotide polymorphisms (SNPs) on the structure and function of the TEX19 gene. A total of 102 missense TEX19 SNPs obtained from dbSNP and screened by Polymorphism Phenotyping 2 (PolyPhen‐2) for possible damaging effect on the protein. Sorting Tolerant From the Intolerant (SIFT) server was used for the prediction of the deleterious effect of mutations. For the prediction of disease‐related SNPs, we used Predictor of human Deleterious Single‐Nucleotide Polymorphisms (PhD‐SNP), and SNPs&GO servers. Project HOPE webserver was used to analyze the effect of single point mutation on protein structure.
2.5. Prediction of amino acid conservation
Amino acid conservation among different related proteins was predicted by ConSerf server. BioEdit version 2.7.5 (Hall, 1999) was used for multiple sequence alignment and prediction of conserved sequences.
3. RESULTS AND DISCUSSION
3.1. Validation of the model
The generated model of TEX19 by I‐TASSER was done according to the template of Streptomyces castaneoglobisporus tyrosinase (1WX2) and was then validated by PROSA statistic (Figure 1). The model had an averaged Z score of −4.7, Z‐score provides an estimate of the absolute quality of a model by relating it to reference structures solved by X‐ray crystallography (Gupta et al., 2017). All these results indicated that the helicase protein model was valid.
FIGURE 1.
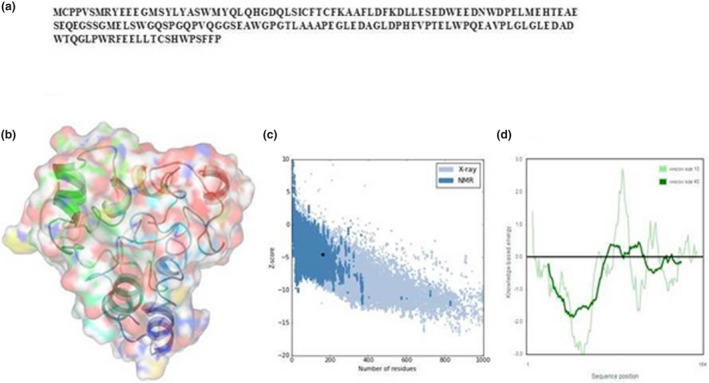
3D structure of TEX19 protein. (a) The protein sequence of TEX19. (b) 3D structure. (c) Z‐scores of all protein chains in PDB, which are determined by X‐ray crystallography (light blue) or NMR spectroscopy (dark blue). (d) Validation of the developed model
3.2. Molecular docking
Figure 2 shows the interaction pattern between TEX19 and LIRE1 proteins. Ten amino acids of TEX19 were found to stabilize the complex through the hydrogen bonding (Table 1). Hydrogen bonding is an interaction between ligand and its protein, which results in specificity and directionality to the interaction that is a fundamental aspect of molecular recognition (Itoh et al., 2019; Pace et al., 2014). Strong interaction was formed with very low binding free energy (−129.22 ± 4.5) for LIRE1 and TEX 19 proteins. Binding leads to the formation of complexes which are formed and broken depending upon several environment or external factors (Gohlke et al., 2003). The protein interactions have great significance in biology, mainly governed by the van der Waals interactions, electrostatic interactions, hydrogen bonding. A direct correlation has been reported between binding affinity and the buried surface area between a protein interface (Chen et al., 2013).
FIGURE 2.
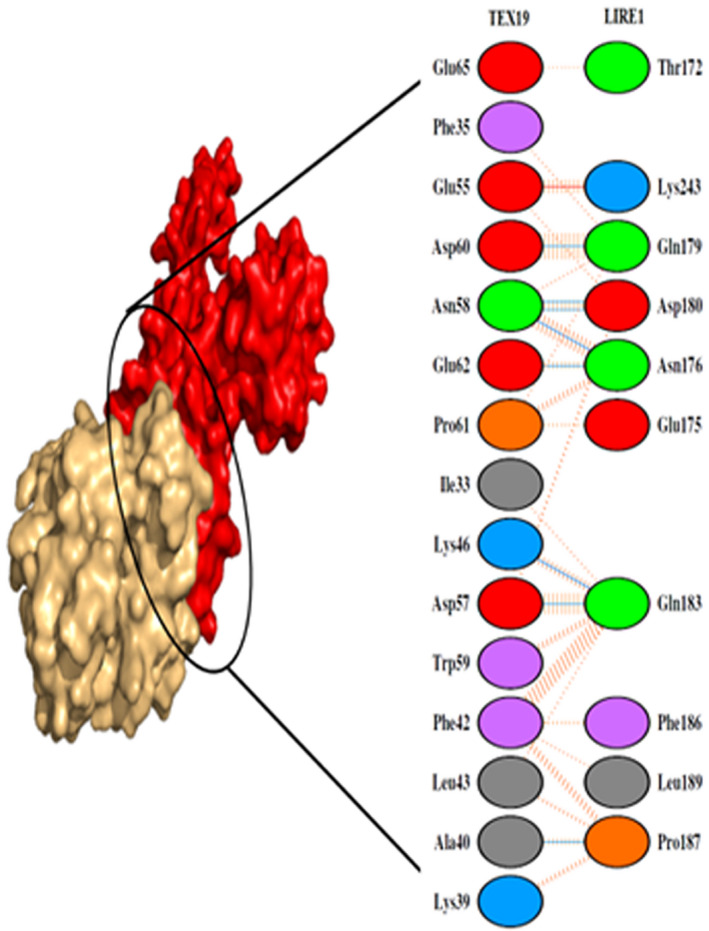
Dimplot showing the interaction between the amino acids of TEX19 and LIRE1 proteins (PDB ID: 2W7A)
TABLE 1.
Hydrogen bonding between the TEX19 and LIRE1 protein
| S. No | TEX19 | LIRE1 | Distance (Å) |
| 1 | LYS 39 | LEU189 | 2.73 |
| 2 | ALA 40 | PRO187 | 2.82 |
| 3 | LYS 46 | ASP 180 | 2.69 |
| 4 | LYS 46 | GLN183 | 2.80 |
| 5 | GLU55 | LYS243 | 2.63 |
| 6 | ASP 57 | GLN183 | 2.86 |
| 7 | ALA 58 | ASP180 | 2.97 |
| 8 | ASP 60 | GLN179 | 2.78 |
| 9 | GLU 62 | ASN176 | 2.82 |
| 10 | GLU 65 | ASN176 | 3.05 |
3.3. Molecular dynamics simulation
The stability and properties of the docking formed complex was studied by explicit solvent MD simulation. The root means square deviation (RMSD) analysis not only reflects the change of protein backbone versus simulation time but also indicates the divergence of the structure. The RMSD of the complex became stable at 15 ns. The RMSD value of modeled helicase was 0.45 nm (Figure 3a). The values of RMSD also indicate the identification of appropriate interaction sites for both proteins. The root means square fluctuation (RMSF) reflects the mobility of a certain residue around its mean position, which is another tool for studying the dynamic stability of the system. Although there were some deviations among the trajectories (Especially in loop region), the present data suggested that fewer fluctuations, which further highlighted the reliability of the model structure (Figure 3b). The simulation results showed that TEX19 could bind to LIRE1 protein. This could also help in preventing the mobilization of LINE‐1 retrotransposons as was experimentally proved in mice (MacLennan et al., 2017). This could predict TEX19 potential in maintaining the trans‐generational stability of the human genome similar to the role played by TEX19.1 in mice (MacLennan et al., 2017).
FIGURE 3.
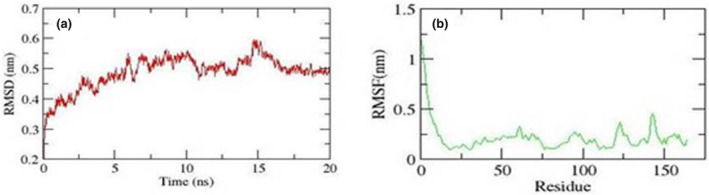
Molecular Dynamic Simulation of TEX19 and TIRE1 complex
3.4. Prediction of SNPs affect the TEX19 structure and function
Table 2 shows the predicted effect of missense SNPs on TEX19 protein structure and function. From 102‐screened missense SNPs, 37 were probably damaging, 18 were possible damaging, and 47 were benign. Out of 102 SNPs only six (S15C, C34Y, W147R, R8 W, C37S, and W141R) were predicted by PhD server to be disease‐related, while only two (C34Y, C37S) predicted by SNPs and GO to be disease‐related. From 37 probably damaging SNPs, 29 predicted deleterious to TEX19 protein. Three probably damaging polymorphisms (F35S, P61R, and E55L) with Minor Allele Frequency (MAF) 0.000004/1, located at the binding site of LIRE1 were observed, the presence of these variants at the binding site of this TEX19 could affect its activity and binding affinity. The substitution of Phenylalanine (F) into a Serine (S) at position 35, could disturb this binding site, because the mutant residue is smaller than the wild‐type residue, and the wild‐type residue is more hydrophobic than the mutant residue, in addition to Phe35 located at highly conserved region so the differences in amino acid properties can disturb this region and thus disturb its function. The substitution of proline (P) into arginine (R) at position 61 could disrupt the structure and binding cavity of protein, due to the fact that the mutant residue is bigger than the wild‐type residue and the wild‐type residue charge was neutral. In contrast, the mutant residue charge is positive, and the wild‐type residue is more hydrophobic than the mutant. Besides, prolines are known to be very rigid and therefore induce a special backbone conformation which might be required at this position, thereby the loss of proline at this point could disturbing the local structure. The mutation of a Glutamic Acid (E) into a leucine (L) at position 55 could disrupt the pocket used for binding of the LIRE1. Due to difference in charge, size, and hydrophobicity, these variations can result in loss of hydrogen bonds and/or disturb correct protein folding.
TABLE 2.
Predicted effect of missense SNPs on TEX19 protein structure and function
| SNP ID | AA | PolyPhen−2 (score) | PhD | SNPs and GO | SIFT |
|---|---|---|---|---|---|
| rs377629628 | M1T | Probably damaging (0.998) | Neutral | Neutral | Deleterious |
| rs371327683 | P4L | Probably damaging (1.000) | Neutral | Neutral | Deleterious |
| rs761018318 | P4A | Probably damaging (1.000) | Neutral | Neutral | Deleterious |
| rs900962762 | S6G | Probably damaging (0.969) | Neutral | Neutral | Deleterious |
| rs1245990387 | S6R | Probably damaging (0.997) | Neutral | Neutral | Deleterious |
| rs868177850 | R8W | Probably damaging (0.999) | Disease | Neutral | Deleterious |
| rs1318138374 | H27Q | Probably damaging (0.998) | Neutral | Neutral | Deleterious |
| rs150740969 | C34Y | Probably damaging (0.993) | Disease | Disease | Deleterious |
| rs367711836 | F35S | Probably damaging (0.999) | Neutral | Neutral | Deleterious |
| rs1257967420 | C37S | Probably damaging (0.999) | Disease | Disease | Deleterious |
| rs1185108733 | A41V | Probably damaging (1) | Neutral | Neutral | Deleterious |
| rs1259349893 | A41S | Probably damaging (1) | Neutral | Neutral | Deleterious |
| rs116114329 | E50A | Probably damaging (1.000) | Neutral | Neutral | Deleterious |
| rs1156477833 | E50D | Probably damaging (1) | Neutral | Neutral | Deleterious |
| rs1599669625 | E55L | Probably damaging (0.997) | Neutral | Neutral | Deleterious |
| rs1385130699 | D60N | Probably damaging (0.998) | Neutral | Neutral | Deleterious |
| rs1331784175 | P61R | Probably damaging (0.962) | Neutral | Neutral | Tolerated |
| rs1266481991 | L63M | Probably damaging (0.987) | Neutral | Neutral | Tolerated |
| rs761225499 | W83R | Probably damaging (0.991) | Neutral | Neutral | Tolerated |
| rs1390892071 | W83C | Probably damaging (0.998) | Neutral | Neutral | Tolerated |
| rs1319739886 | P90H | Probably damaging (0.99) | Neutral | Neutral | Tolerated |
| rs777468964 | Q92H | Probably damaging (0.965) | Neutral | Neutral | Tolerated |
| rs147220016 | G93W | Probably damaging (0.997) | Neutral | Neutral | Deleterious |
| rs140570015 | G99R | Probably damaging (0.979) | Neutral | Neutral | Deleterious |
| rs1372613314 | A104E | Probably damaging (0.997) | Neutral | Neutral | Tolerated |
| rs1184019063 | P121S | Probably damaging (1) | Neutral | Neutral | Deleterious |
| rs1403014449 | P126S | Probably damaging (1) | Neutral | Neutral | Deleterious |
| rs1599669625 | Q127L | Probably damaging (0.979) | Neutral | Neutral | Deleterious |
| rs1479032497 | P131L | Probably damaging (0.99) | Neutral | Neutral | Deleterious |
| rs200970555 | G135S | Probably damaging (0.998) | Neutral | Neutral | Deleterious |
| rs1439503805 | W141R | Probably damaging (1) | Disease | Neutral | Deleterious |
| rs1276048234 | Q143H | Probably damaging (1) | Neutral | Neutral | Deleterious |
| rs771614199 | G144C | Probably damaging (0.997) | Neutral | Neutral | Deleterious |
| rs759919288 | W147R | Probably damaging (1) | Disease | Neutral | Deleterious |
| rs933781675 | W158C | Probably damaging (1) | Disease | Neutral | Deleterious |
| rs767149161 | P159S | Probably damaging (0.979) | Neutral | Neutral | Tolerated |
3.5. Conservation score
Residues of TEX19 (LYS 39, GLU 62) showing hydrogen bonds with LIRE1 were among conserved sequences. Phe35 of TEX19 which have an interaction with LIRE1 predicted among highly conserved buried residues (Figures 4 and 5).
FIGURE 4.
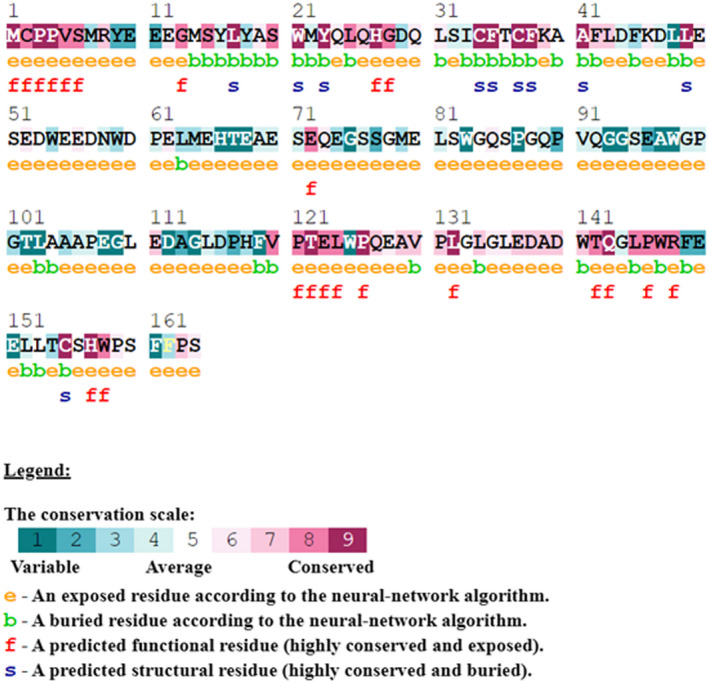
TEX19 amino acid sequences conservation score among different species
FIGURE 5.
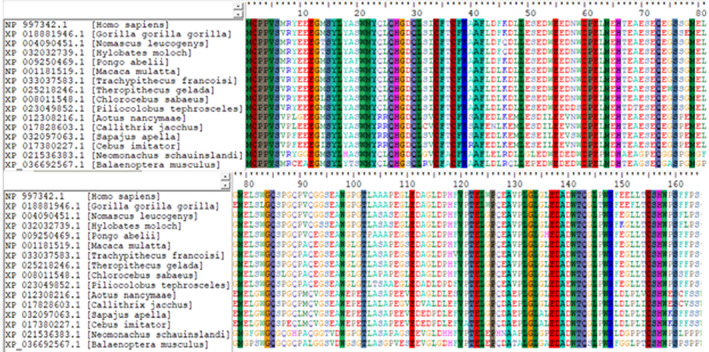
Multiple sequence alignment of TEX19 gene sequences of different species showing conserved sequences in vertical lines
4. CONCLUSIONS
In silico methods such as docking and molecular dynamic (MD) simulations are used to find the correct conformation of a ligand and its receptor and have been used previously in drug design (Bissaro et al., 2020; Garofalo et al., 2020; Maximov et al., 2020; Salmaso & Moro, 2018). In this study we performed docking and MD simulations methods was to evaluate the interaction between TEX19 and LIRE1 proteins and identified an alternative binding pocket in the TEX19 protein based on the consensus binding site. For this protein, 10 amino acids of TEX19 were found to stabilize the complex through the hydrogen bonding. A total of 37 missense variants were predicted to play a probably damaging role for the protein, three of them (F35S, P61R, and E55L) located at the binding site of LIRE1 and could disturb this binding affinity. The F35S located at highly conserved region, mutations at highly conserved region could severely affect protein function and structure (Liu et al., 2014; Stefancsik et al., 1998).
CONFLICTS OF INTEREST
The authors have declared no conflict of interest.
AUTHOR CONTRIBUTIONS
Conceptualization, O.R.A, F.A., and Y.H.; methodology, F.A., Y.H., H.A., and N.A.; software, Y.H., H.A., and N.A.; validation, F.A., Y.H., H.A., and N.A.; formal analysis, Y.H., H.E.A., and N.A.; investigation, Y.H., H.A., L.M., S.A., and N.A.; resources, F.A., Y.H., H.A., and N.A.; data curation, F.A., Y.H., H.A., L.M., and N.A.; writing—original draft preparation, O.M.A., F.A., and Y.H.; writing—review and editing, F.A., Y.H., H.A., S.A., L.M., N.A.,O.R.A., H.E.A., and O.M.A.; funding acquisition, F.A., N.A., S.A., and O.R.A.
ACKNOWLEDGMENTS
This work was supported by the Deanship of Scientific Research (DSR), King Abdulaziz University, Jeddah, under grant No. (D‐055‐662‐40). The authors, therefore, gratefully acknowledge the DSR for technical and financial support. YH thanks Saudi Human Genome Program and King Faisal Specialist Hospital and Research Center for their support (IRB approval no: 2018‐36). The authors also extend their appreciation to the Deanship of Scientific Research (DSR), University of Tabuk for funding this work through Grant number: S‐1441‐0080. We are also grateful to the Deanship of Scientific Research, King Saud University for funding through Vice Deanship of Scientific Research Chairs.
Contributor Information
Suliman Alomar, Email: syalomar@ksu.edu.sa.
Lamjed Mansour, Email: lmansour@ksu.edu.sa.
DATA AVAILABILITY STATEMENT
The data that support the findings of this study are available on request from the corresponding author.
REFERENCES
- Al‐Amer, O., Hawasawi, Y., Oyouni, A. A. A., Alshehri, M., Alasmari, A., Alzahrani, O., & Aljohani, S. A. S. (2020). Study the association of transmembrane serine protease 6 gene polymorphisms with iron deficiency status in Saudi Arabia. Gene, 751, 144767. [DOI] [PubMed] [Google Scholar]
- Alsohime, F., Almaghamsi, T., Basha, T. A., Alardati, H., Alghamdi, M., & Hawsawi, Y. M. (2021). Unusual prominent pulmonary involvement in a homozygous PRF1 gene variant in a female patient. Journal of Clinical Immunology, 41, 217–220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alzahrani, F. A., Ahmed, F., Sharma, M., Rehan, M., Mahfuz, M., Baeshen, M. N., Hawsawi, Y., Almatrafi, A., Alsagaby, S. A., Kamal, M. A., Warsi, M. K., Choudhry, H., & Jamal, M. S. (2020). Investigating the pathogenic SNPs in BLM helicase and their biological consequences by computational approach. Scientific Reports, 10, 12377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beck, C. R., Garcia‐Perez, J. L., Badge, R. M., & Moran, J. V. (2011). LINE‐1 elements in structural variation and disease. Annual Review of Genomics and Human Genetics, 12, 187–215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berman, H. M., Battistuz, T., Bhat, T. N., Bluhm, W. F., Bourne, P. E., Burkhardt, K., Feng, Z., Gilliland, G. L., Iype, L., & Jain, S. (2002). The protein data bank. Acta Crystallographica Section D: Biological Crystallography, 58, 899–907. [DOI] [PubMed] [Google Scholar]
- Bissaro, M., Sturlese, M., & Moro, S. (2020). The rise of molecular simulations in fragment‐based drug design (FBDD): an overview. Drug Discovery Today, 25, 1693–1701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen, J., Sawyer, N., & Regan, L. (2013). Protein‐protein interactions: general trends in the relationship between binding affinity and interfacial buried surface area. Protein Science, 22, 510–515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dominguez, C., Boelens, R., & Bonvin, A. M. (2003). HADDOCK: a protein‐protein docking approach based on biochemical or biophysical information. Journal of the American Chemical Society, 125, 1731–1737. [DOI] [PubMed] [Google Scholar]
- Feichtinger, J., Aldeailej, I., Anderson, R., Almutairi, M., Almatrafi, A., Alsiwiehri, N., Griffiths, K., Stuart, N., Wakeman, J. A., Larcombe, L., & McFarlane, R. J. (2012). Meta‐analysis of clinical data using human meiotic genes identifies a novel cohort of highly restricted cancer‐specific marker genes. Oncotarget, 3, 843–853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garcia‐Perez, J. L., Widmann, T. J., & Adams, I. R. (2016). The impact of transposable elements on mammalian development. Development, 143, 4101–4114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garofalo, M., Grazioso, G., Cavalli, A., & Sgrignani, J. (2020). How computational chemistry and drug delivery techniques can support the development of new anticancer drugs. Molecules, 25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gohlke, H., Kiel, C., & Case, D. A. (2003). Insights into protein‐protein binding by binding free energy calculation and free energy decomposition for the Ras‐Raf and Ras‐RaIGDS complexes. Journal of Molecular Biology, 330, 891–913. [DOI] [PubMed] [Google Scholar]
- Gupta, R., Dey, A., Vijan, A., & Gartia, B. (2017). In Silico structure modeling and characterization of hypothetical protein YP_004590319. 1 present in enterobacter aerogens. Journal of Proteomics & Bioinformatics, 10, 152–170. [Google Scholar]
- Hall, T. A.BioEdit: a user‐friendly biological sequence alignment editor and analysis program for Windows 95/98/NT. Nucleic acids symposium series, 1999. [London]: Information Retrieval Ltd., c1979‐c2000. 95‐98.
- Hawsawi, Y. M., Al‐Numair, N. S., Sobahy, T. M., Al‐Ajmi, A. M., Al‐Harbi, R. M., Baghdadi, M. A., Oyouni, A. A., & Alamer, O. M. (2019). The role of BRCA1/2 in hereditary and familial breast and ovarian cancers. Molecular Genetics & Genomic Medicine, 7, e879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hawsawi, Y. M., Al‐Zahrani, F., Mavromatis, C. H., Baghdadi, M. A., Saggu, S., & Oyouni, A. A. A. (2018). Stem cell applications for treatment of cancer and autoimmune diseases: its promises, obstacles, and future perspectives. Technol Cancer Res Treat, 17, 1533033818806910. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hawsawi, Y. M., Zailaie, S. A., Oyouni, A. A. A., Alzahrani, O. R., Alamer, O. M., & Aljohani, S. A. S. (2020). Prostate cancer and therapeutic challenges. J Biol Res (Thessalon), 27, 20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hess, B., Kutzner, C., van der Spoel, D., & Lindahl, E. (2008). GROMACS 4: Algorithms for highly efficient, load‐balanced, and scalable molecular simulation. Journal of Chemical Theory and Computation, 4, 435–447. [DOI] [PubMed] [Google Scholar]
- Huang, J., Rauscher, S., Nawrocki, G., Ran, T., Feig, M., de Groot, B. L., Grubmuller, H., & Mackerell, A. D. (2017). CHARMM36m: an improved force field for folded and intrinsically disordered proteins. Nature Methods, 14, 71–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Itoh, Y., Nakashima, Y., Tsukamoto, S., Kurohara, T., Suzuki, M., Sakae, Y., Oda, M., Okamoto, Y., & Suzuki, T. (2019). N(+)‐C‐H…O Hydrogen bonds in protein‐ligand complexes. Scientific Reports, 9, 767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kotb, A., el Fakih, R., Hanbali, A., Hawsawi, Y., Alfraih, F., Hashmi, S., & Aljurf, M. (2018). Philadelphia‐like acute lymphoblastic leukemia: diagnostic dilemma and management perspectives. Experimental Hematology, 67, 1–9. [DOI] [PubMed] [Google Scholar]
- Kuntz, S., Kieffer, E., Bianchetti, L., Lamoureux, N., Fuhrmann, G., & Viville, S. (2008). Tex19, a mammalian‐specific protein with a restricted expression in pluripotent stem cells and germ line. Stem Cells, 26, 734–744. [DOI] [PubMed] [Google Scholar]
- Liu, Y. I., Rao, U., McClure, J., Konopa, P., Manocheewa, S., Kim, M., Chen, L., Troyer, R. M., Tebit, D. M., Holte, S., Arts, E. J., & Mullins, J. I. (2014). Impact of mutations in highly conserved amino acids of the HIV‐1 Gag‐p24 and Env‐gp120 proteins on viral replication in different genetic backgrounds. PLoS One, 9, e94240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maclennan, M., Garcia‐Canadas, M., Reichmann, J., Khazina, E., Wagner, G., Playfoot, C. J., Salvador‐Palomeque, C., Mann, A. R., Peressini, P., Sanchez, L., Dobie, K., Read, D., Hung, C. C., Eskeland, R., Meehan, R. R., Weichenrieder, O., Garcia‐Perez, J. L., & Adams, I. R. (2017). Mobilization of LINE‐1 retrotransposons is restricted by Tex19.1 in mouse embryonic stem cells. Elife, 6, 1–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martin, S. L., & Bushman, F. D. (2001). Nucleic acid chaperone activity of the ORF1 protein from the mouse LINE‐1 retrotransposon. Molecular and Cellular Biology, 21, 467–475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maximov, P. Y., Abderrahman, B., Hawsawi, Y. M., Chen, Y., Foulds, C. E., Jain, A., Malovannaya, A., Fan, P., Curpan, R. F., Han, R., Fanning, S. W., Broom, B. M., Quintana Rincon, D. M., Greenland, J. A., Greene, G. L., & Jordan, V. C. (2020). The structure‐function relationship of angular estrogens and estrogen receptor alpha to initiate estrogen‐induced apoptosis in breast cancer cells. Molecular Pharmacology, 98, 24–37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ollinger, R., Childs, A. J., Burgess, H. M., Speed, R. M., Lundegaard, P. R., Reynolds, N., Gray, N. K., Cooke, H. J., & Adams, I. R. (2008). Deletion of the pluripotency‐associated Tex19.1 gene causes activation of endogenous retroviruses and defective spermatogenesis in mice. PLoS Genetics, 4, e1000199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pace, C. N., Fu, H., Lee Fryar, K., Landua, J., Trevino, S. R., Schell, D., Thurlkill, R. L., Imura, S., Scholtz, J. M., Gajiwala, K., Sevcik, J., Urbanikova, L., Myers, J. K., Takano, K., Hebert, E. J., Shirley, B. A., & Grimsley, G. R. (2014). Contribution of hydrogen bonds to protein stability. Protein Science, 23, 652–661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Planells‐Palop, V., Hazazi, A., Feichtinger, J., Jezkova, J., Thallinger, G., Alsiwiehri, N. O., Almutairi, M., Parry, L., Wakeman, J. A., & McFarlane, R. J. (2017). Human germ/stem cell‐specific gene TEX19 influences cancer cell proliferation and cancer prognosis. Molecular Cancer, 16, 84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Price, D. J., & Brooks, C. L.III (2004). A modified TIP3P water potential for simulation with Ewald summation. The Journal of Chemical Physics, 121, 10096–10103. [DOI] [PubMed] [Google Scholar]
- Reichmann, J., Crichton, J. H., Madej, M. J., Taggart, M., Gautier, P., Garcia‐Perez, J. L., Meehan, R. R., & Adams, I. R. (2012). Microarray analysis of LTR retrotransposon silencing identifies Hdac1 as a regulator of retrotransposon expression in mouse embryonic stem cells. PLoS Computational Biology, 8, e1002486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reichmann, J., Dobie, K., Lister, L. M., Crichton, J. H., Best, D., Maclennan, M., Read, D., Raymond, E. S., Hung, C. C., Boyle, S., Shirahige, K., Cooke, H. J., Herbert, M., & Adams, I. R. (2020). Tex19.1 inhibits the N‐end rule pathway and maintains acetylated SMC3 cohesin and sister chromatid cohesion in oocytes. Journal of Cell Biology, 219, 1–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reichmann, J., Reddington, J. P., Best, D., Read, D., Ollinger, R., Meehan, R. R., & Adams, I. R. (2013). The genome‐defence gene Tex19.1 suppresses LINE‐1 retrotransposons in the placenta and prevents intra‐uterine growth retardation in mice. Human Molecular Genetics, 22, 1791–1806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salmaso, V., & Moro, S. (2018). Bridging molecular docking to molecular dynamics in exploring ligand‐protein recognition process: An overview. Frontiers in Pharmacology, 9, 1–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Semlali, A., Almutairi, M., Rouabhia, M., Reddy Parine, N., Al Amri, A., & Saud Alanazi, M. (2018). Novel sequence variants in the TLR6 gene associated with advanced breast cancer risk in the Saudi Arabian population. PLoS One, 13, e0203376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Semlali, A., Parine, N. R., Al‐Numair, N. S., Almutairi, M., Hawsawi, Y. M., Amri, A. A., Aljebreen, A. M., Arafah, M., Almadi, M. A., Azzam, N. A., Alharbi, O., & Alanazi, M. S. (2018). Potential role of Toll‐like receptor 2 expression and polymorphisms in colon cancer susceptibility in the Saudi Arabian population. OncoTargets and Therapy, 11, 8127–8141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stefancsik, R., Jha, P. K., & Sarkar, S. (1998). Identification and mutagenesis of a highly conserved domain in troponin T responsible for troponin I binding: potential role for coiled coil interaction. Proceedings of the National Academy of Sciences, 95, 957–962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tarabay, Y., Achour, M., Teletin, M., Ye, T., Teissandier, A., Mark, M., Bourc'His, D., & Viville, S. (2017). Tex19 paralogs are new members of the piRNA pathway controlling retrotransposon suppression. Journal of Cell Science, 130, 1463–1474. [DOI] [PubMed] [Google Scholar]
- Tarabay, Y., Kieffer, E., Teletin, M., Celebi, C., van Montfoort, A., Zamudio, N., Achour, M., el Ramy, R., Gazdag, E., Tropel, P., Mark, M., Bourc'His, D., & Viville, S. (2013). The mammalian‐specific Tex19.1 gene plays an essential role in spermatogenesis and placenta‐supported development. Human Reproduction, 28, 2201–2214. [DOI] [PubMed] [Google Scholar]
- Wang, C., Gu, Y., Zhang, K., Xie, K., Zhu, M., Dai, N., Jiang, Y., Guo, X., Liu, M., Dai, J., Wu, L., Jin, G., Ma, H., Jiang, T., Yin, R., Xia, Y., Liu, L., Wang, S., Shen, B., … Hu, Z. (2016). Systematic identification of genes with a cancer‐testis expression pattern in 19 cancer types. Nature Communications, 7, 10499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang, P. J., McCarrey, J. R., Yang, F., & Page, D. C. (2001). An abundance of X‐linked genes expressed in spermatogonia. Nature Genetics, 27, 422–426. [DOI] [PubMed] [Google Scholar]
- Xu, Z., Tang, H., Zhang, T., Sun, M., Han, Q., Xu, J., Wei, M., & Yu, Z. (2020). TEX19 promotes ovarian carcinoma progression and is a potential target for epitope vaccine immunotherapy. Life Sciences, 241, 117171. [DOI] [PubMed] [Google Scholar]
- Yang, F., Cheng, Y., An, J. Y., Kwon, Y. T., Eckardt, S., Leu, N. A., McLaughlin, K. J., & Wang, P. J. (2010). The ubiquitin ligase Ubr2, a recognition E3 component of the N‐end rule pathway, stabilizes Tex19.1 during spermatogenesis. PLoS One, 5, e14017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang, J., Yan, R., Roy, A., Xu, D., Poisson, J., & Zhang, Y. (2015). The I‐TASSER Suite: protein structure and function prediction. Nature Methods, 12, 7–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang, J., & Zhang, Y. (2015). Protein structure and function prediction using I‐TASSER. Curr Protoc Bioinformatics, 52, 1–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhong, J., Chen, Y., Liao, X., Li, J., Wang, H., Wu, C., Zou, X., Yang, G., Shi, J., Luo, L., Liu, L., Deng, J., & Tang, A. (2016). Testis expressed 19 is a novel cancer‐testis antigen expressed in bladder cancer. Tumour Biology, 37, 7757–7765. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The data that support the findings of this study are available on request from the corresponding author.


