Abstract
Background
Fragile X syndrome (FXS) is the most common inherited form of intellectual disability. Prenatal screening of FXS allows for early identification and intervention. The present study explored the feasibility of FXS carrier screening during prenatal diagnosis for those who were not offered screening early in pregnancy or prior to conception.
Methods
Pregnant women to be offered amniotic fluid testing were recruited for the free voluntary carrier screening at a single center between August, 2017 and September, 2019. The number of CGG repeats in the 5’ un‐translated region of the fragile X mental retardation gene 1 (FMR1) was determined.
Results
4286 of 7000 (61.2%) pregnant women volunteered for the screening. Forty (0.93%), five (0.11%), and three (0.07%) carriers for intermediate mutation (45–54 repeats), premutation (55–200 repeats) and full mutation (>200 repeats) of the FMR1 gene were identified respectively. None of the detected premutation alleles were inherited by the fetuses. Of the three full mutation carrier mothers, all had a family history and one transmitted a full mutation allele to her male fetus.
Conclusion
Implementation of FXS carrier screening during prenatal diagnosis may be considered for the need to increase screening for FXS.
Keywords: carrier screening, FMR1, fragile X syndrome, prenatal diagnosis
The present study explored the feasibility of FXS carrier screening during prenatal diagnosis for those who were not offered screening early in pregnancy or prior to conception. Implementation of FXS carrier screening during prenatal diagnosis may be considered for the need to increase screening for FXS.
![]()
1. INTRODUCTION
FXS (OMIM# 300624) is the most common inherited form of intellectual disability and a leading cause of autism spectrum disorder (ASD), with an estimated prevalence of 1 in 4000 males and 1 in 8000 females (Essop & Krause, 2013; Hunter et al., 2014; Razak et al., 2020). About 99% of FXS cases are associated with excessive expansion of the CGG tri‐nucleotide repeats in the 5’ untranslated region of the FMR1 gene (OMIM# 309550)(Monaghan et al., 2013), which results in hypermethylation of the promoter and consequently suppresses the gene transcription, leading to insufficiency or absence of the FMR protein that is required for normal brain development (Esanov et al., 2016). According to the number of CGG repeats, the FMR1 alleles are classified as: (1) normal alleles (6–44 repeats); (2) gray zone or intermediate (IM) alleles (45–54 repeats); (3) premutation (PM) alleles (55–200 repeats), which are associated with fragile X‐associated tremor/ataxia syndrome (FXTAS) in up to 45% of male carriers and 17% of female carriers and FMR1‐related primary ovarian insufficiency (FXPOI) in nearly 20% female carriers (Hagerman & Hagerman, 2016; Hipp et al., 2016); (4) full mutation (FM) alleles (>200 repeats), which cause FXS (Monaghan et al., 2013). A PM may not necessarily result in an affected fetus. But the risk of transition from PM to FM is positively correlated with the size of CGG repeats of the maternal FMR1 allele, being low for less than 60 repeats and almost 100% for over 100 repeats (Hung et al., 2019; Nolin et al., 2003).
Currently, there is no cure or effective treatment for FXS (Hagerman et al., 2017). But early identification of FXS has been suggested considering the potential benefits of early intervention for the affected individuals (Okoniewski et al., 2019). Due to the complex inheritance patterns and wide range of phenotypes associated with FXS, genetic counseling on risk assessment and prognosis prediction for FXS is challenging. The clinical utility of population‐based screening has also been in debate (Arenas et al., 2017; Dimmock, 2017). Therefore, the current guidelines recommend offering carrier screening only to those who have a family history of FXS or intellectual disability suggestive of FXS‐related disorders, and to those who undergo infertility evaluation (Monaghan et al., 2013). However, in light of the high prevalence (~1/150) of PM alleles in Caucasian women and the potential risk conferred by these PM alleles in offspring, many providers advocate universal screening (Archibald et al., 2013). Recently, several studies were carried out to explore wider screening for FXS in populations beyond those recommended by the guidelines (Arenas et al., 2017; Berkenstadt et al., 2010; Johansen Taber et al., 2019). Results from these pilot studies were encouraging, whereas practical difficulties such as ethical considerations and cost‐effectiveness of expanded screening existed.
In China, unlike aenuploidy screening that is routinely carried out at obstetric outpatient, preconceptional or prenatal screening of FXS has not been widely established (Gao et al., 2020). Meanwhile, people lack the knowledge of FXS and the voluntary screening rate is low in the country. In our center, we noticed that pregnant women who were referred for prenatal diagnosis were hardly offered carrier testing early in pregnancy or preconceptionally. Based on this observation, we asked that whether it is feasible to implement FXS carrier screening for pregnant women and the fetuses during prenatal diagnosis.
2. MATERIALS AND METHODS
2.1. Ethical compliance
This study was approved by the Ethics Committee of Hunan Provincial Maternal and Child Health Care Hospital (EC201719).
2.2. Study subjects and samples
This prospective pilot study was conducted at Prenatal Diagnosis Center of Hunan Province between August, 2017 and September, 2019. Pregnant women who would receive amniocentesis because of various indications (Table 1) for prenatal diagnosis were given printed information about FXS carrier screening and invited to participate in the study. The screening was entirely voluntary and free of charge. Pre‐test genetic counseling was provided before the participants signed the informed consent for amniocentesis. Those who reported a family history of intellectual disability (ID) were suggested to take the genetic test with the proband in the family. If the proband was diagnosed as FXS, the pregnant woman was excluded from the PM rate calculation to avoid over‐counting. For each participant, 2 ml of maternal blood and 5 ml of amniotic fluid was collected. All samples were collected with written informed consent for genetic tests.
TABLE 1.
Indications for prenatal diagnosis of the pregnant women enrolled in the study
| Indications | Number (%) |
|---|---|
| Positive screening results for advanced maternal age, serum screening and/or NIPT | 2782 (64.92) |
| Abnormal ultrasound findings | 856 (19.97) |
| Family history of intellectual disability | 36 (0.84) |
| Chromosome abnormality | 76 (1.77) |
| Previous adverse pregnancy outcome | 35 (8.26) |
| Monogenic disease carrier (thalassemia, haemophilia, PKU, etc. except FXS) | 157 (3.66) |
| Others | 25 (0.58) |
| Total | 4286 |
2.3. DNA preparation
Genomic DNA was extracted from peripheral blood or amniotic fluid using DNeasy Blood & Tissue Kit (Qiagen, Hilden, Germany) according to the manufacturer's protocol or using the chemagic Prepito‐D automatic system (PerkinElmer, Turku, Finland) following the manufacturer's protocol.
2.4. Analysis of FMR1 CGG repeats
The FMR1 gene (GenBank accession number: NG_007529.2) was amplified with the primers 5′AAGCCGGAGTCAGTCCGCG AGTCGAG3′ and 5′CACCAGCTCCTCCATCTTCTCTTCAG3′. A fluorescin (FAM)‐labeled primer 5′CACCAGCTCCTCCATCTTCTCTTCAG3′ and a CGG repeats‐containing primer 5′CAGGAAACACGTATGAGGCTGCGC3′ (CGG)7 were employed for amplification of the CGG repeats using DNA polymerase from Expand™ Long Template PCR System (Cat#11681842001, Roche). Thermal cycling was as follows: denaturation at 98°C for 10 min, 35 cycles of 98°C for 35 s, 64°C for 35 s, and 68°C for 4 min, and a final extension at 68°C for 10 min. The PCR products were subjected to sequencing analysis.
2.5. Southern blot analysis
Southern blot analysis was applied when the number of CGG repeats was found more than 55 in PCR sequencing analysis. 5 μg of genomic DNA from blood was digested with EcoR I/Eag I, and hybridized with the digoxigenin‐labeled probe StB12.3 (Cat#11669940910; Roche) as described elsewhere (Gao et al., 2020).
3. RESULTS
3.1. Clinical characteristics of the study cohort
A total of 7000 pregnant women to be offered amniocentesis because of various indications (Table 1) for prenatal diagnosis were invited for the study, and 4286 (61.2%) of them received carrier screening. The average age of the enrolled women was 31.92 years, and 1483 (34.62%) women were at the age of ≥35 years.
3.2. Frequency distribution of FMR1 CGG repeats in the cohort
The most prevalent numbers of FMR1 CGG repeats in the cohort were 29 (35.45%), 30 (28.70%), 31 (11.32%), and 36 (6%). Figure 1 shows the allele frequencies of different CGG repeats in the cohort. Of the 4286 pregnant women, 4238 (98.88%) carried normal alleles of FMR1 (The CGG repeats of both alleles were ≤44, and one woman with a karyotype of 47,XXX possessed three normal alleles). The screening identified forty (0.93%), five (0.11%), and three (0.07%) carriers for IM, PM, and FM of the FMR1 respectively (Table 2). The combined rate of PM and FM in the cohort was 1/857. The prevalence of PM in the population was 1/1071.
FIGURE 1.
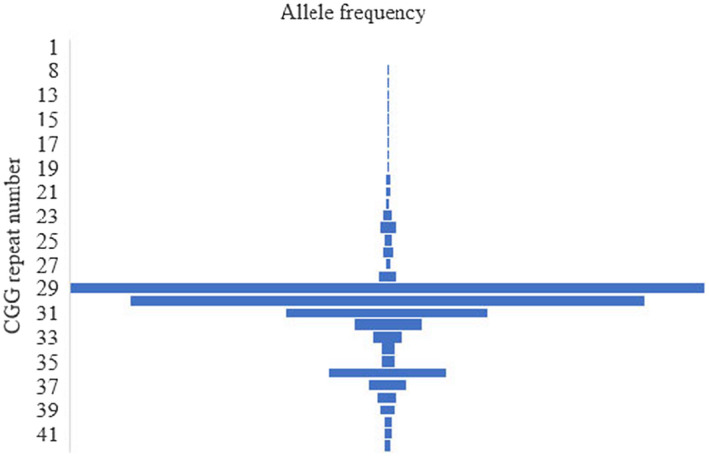
Frequency distribution of FMR1 alleles with various numbers of CGG repeats
TABLE 2.
CGG repeats in the fetuses of the pregnant women with FMR1 IM/PM/FM
| Case | Prenatal diagnostic indications | Repeats in maternal bloodc | Carrier status | Repeats in amniocytes | Fetal karyotype | ||
|---|---|---|---|---|---|---|---|
| X276 | Screening (+)a | 29 | 45 | IMb | 42 | 37 | |
| X287 | Screening (+) | 30 | 45 | 29 | 29 | ||
| X316 | Others | 38 | 48 | 29 | 29 | 46,XX | |
| X811 | Screening (+) | 30 | 46 | 29 | Y | 46,XY | |
| X821 | Screening (+) | 30 | 47 | 29 | Y | 46,XY | |
| X965 | Screening (+) | 29 | 53 | 30 | Y | 46,XY | |
| X797 | Screening (+) | 40 | 71 | PM | 30 | 42 | 47,XX,+18 |
| X823 | Screening (+) | 30 | 58 | 30 | Y | 46,XY | |
| X1190 | Screening (+) | 30 | 66 | 30 | Y | 46,XY | |
| X3607 | Family history | 29 | 92 | 31 | 31 | 46,XX | |
| X3618 | Screening (+) | 32 | 56 | 31 | 34 | 46,XX | |
| X1209 | Family history | 36 | >200 | FM | 29 | 36 | 46,XX |
| X1236 | Family history | 31 | >200 | 29 | 31 | 46,XX | |
| X1216 | Family history | 30 | >200 | >200 | Y | 46,XY | |
Positive screening results for advanced maternal age, serum screening and/or NIPT.
Six cases of IM carrier mothers (n = 40) are representatively shown.
The measurement of CGG repeats in the present study allows for an accuracy of ±3 repeats.
3.3. Results of prenatal diagnosis and follow‐ups
Amniotic fluid testing of the IM carrier mothers revealed no expansion of IM to PM in the fetuses (Table 2). None of the five PM carrier mothers reported menstruation problem or FXTAS in family members during genetic counseling. The woman encoded X3607 previously had a boy of intellectual disability. Amniotic fluid testing showed that none of the PM carriers passed the mutations to the fetuses (Table 2). Karyotyping of amniocytes indicated that the woman encoded X797 carried a fetus with trisomy 18, and she decided to terminate the pregnancy. Follow‐ups confirmed that all other PM carrier mothers delivered healthy babies.
The three FM carriers all reported a family history of intellectual disability or ASD. We thus performed further investigation on these cases. With regard to the pregnant woman encoded X1209, the index patient in her family (her brother) was recalled for genetic testing and confirmed as a FM carrier. Our further analysis revealed that her mother was a PM carrier. Prenatal diagnosis showed that her fetus was female and did not inherit the FM allele (Figure 2, pedigree 1). The woman encoded X1236 previously gave birth to a boy, and later he was suspected with ASD. In the present study, the boy was recalled and the diagnosis of ASD was confirmed. Genetic testing identified that he was a carrier of FM. Prenatal diagnosis showed that the female fetus did not inherit the maternal FM allele (Figure 2, pedigree 2). The woman encoded X1216 had mild intellectual disability and previously gave birth to a boy of intellectual disability. The boy was recalled for genetic testing and confirmed as a FM carrier. Prenatal diagnosis revealed a male fetus carrying a FM allele in the subsequent pregnancy (Figure 2, pedigree 3).
FIGURE 2.
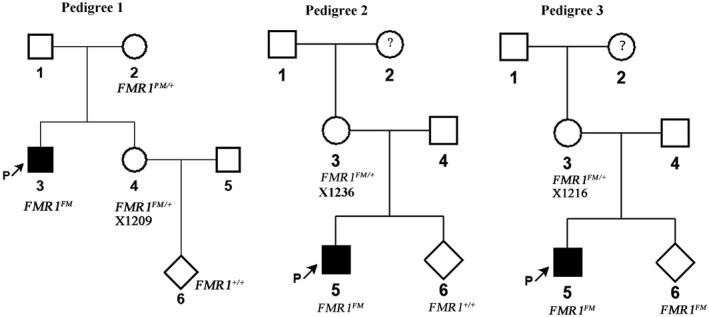
Pedigrees of the pregnant women with a positive result of FMR1 full mutation in FXS carrier screening during prenatal diagnosis. The genotypes of FMR1 in the pregnant women (encoded X1209, X1236 and X1216, respectively) and the fetuses were determined for prenatal diagnosis. The index patients who exhibited intellectual disability (pedigree 1 and 3) or autism spectrum disorders (pedigree 2) in the families were also recalled for genetic testing
4. DISCUSSION
This pilot study explored the feasibility of FXS carrier screening for pregnant women and the fetuses during prenatal diagnosis. The workflow included educating the potential participants the importance of FXS carrier screening, followed by pre‐test counseling, genetic testing, post‐test counseling and follow‐ups. During a period of two years, 4286 out of 7000 pregnant women at our center received carrier testing, showing a high voluntary rate of participation. Knowledge about FXS, free test and easy access to genetic counseling were positive factors that encouraged pregnant women to participate in the study.
A major ethical consideration regarding our screening model is that extra genetic testing may increase anxiety in pregnant women who are already under psychological distress due to the undergoing prenatal diagnostic procedures. Indeed, it would be more appropriate that these women receive screening early in pregnancy or preconceptionally. However, in the context that FXS screening has not been widely established and popularized, our screening strategy may become an option for the pregnant women who are willing to receive testing. The carrier screening in the present study introduced no extra invasive operations to the pregnant women and the fetuses. The amniotic fluid testing itself was warranted by aneuploidy diagnosis. Furthermore, sufficient pre‐test counseling and post‐test counseling were provided to the pregnant women at the prenatal diagnosis center. The potential support from a multidisciplinary team at our center was trusted. Additionally, all FXS related tests in this study were offered free of charge. The screening increased no financial burden to the families. Together, efforts were made to reduce the stress from FXS carrier screening for the pregnant women.
Reportedly, the frequency of carrying a FMR1 PM allele in Caucasian women can be as high as 1 in 150 (Owens et al., 2018). In a study of integared carrier screening for cystic fibrosis, FXS, and spinal muscular atrophy in Australia, results of 12,000 tests(including 8000 pregnant women )showed approxiamtely 1 in 330 individuals to be a carrier of FXS (Archibald et al., 2018). It was regarded that the prevalence of FXS was low in the Chinese population and population‐based screening of FXS was unwarranted (Tzeng et al., 2005). However, recent data from a large cohort of Chinese pregnant women (n = 20,188) showed that the prevalence of PM allele for FXS was as high as 1 in 777, indicating that reproductive FXS carrier screening in this population might be cost‐effective (Hung et al., 2019). The prevalence of PM carrier in our cohort was 1/1071, which is slightly lower than that (1/777) observed in the large cohort aforementioned and that (1/634) recently observed in a cohort consisting of 10,145 Chinese women of childbearing age (Gao et al., 2020). This difference may result from the inherent bias of our study design. Instead of population‐based screening, we focused on pregnant women at a single prenatal diagnosis center. Since our study had a much smaller sample size than did the Hung's and the Gao's studies, this may also introduce bias to the observed frequency of PM. In our cohort, the frequency of IM was 1/107, close to that (1/130) observed by Gao and colleagues in Chinese women of childbearing age (Gao et al., 2020).
Newborn screening (NBS) for FXS is one of the proposed solutions for early identification and intervention (Okoniewski et al., 2019). Our screening model allows for FXS carrier screening for the fetuses, partially playing a role of NBS. Compared with NBS, preconception or prenatal screening enables earlier identification of FXS, which might be more demanded by the consumers (Bailey et al., 2012). However, some challenges faced by the NBS were also encountered by our screening, for example, the difficulty in genetic counseling due to the variable expressivity of FXS and the clinic uncertainty of a PM allele (Okoniewski et al., 2019). Moreover, in the context of prenatal diagnosis, medical advice from clinicians may have great impact on a couple and the fate of a fetus carrying a PM/FM. Thus, the right to know and the right to choose for the individuals must be valued. In our study, we made a careful review on the family history for each of the identified FM carriers before a prenatal diagnosis report was issued.
There are limitations in our preliminary study. Pregnant women who were considered at low risk of fetus aneuploidy based on serum biochemical screening or NIPT were not recommended for amniocentesis and thus not included in our study either. Furthermore, our single center's data may not fully represent the frequency of the FMR1 PM/FM in pregnant women who meet the criteria for prenatal diagnosis. Data from multiple centers’ collaboration are anticipated.
In conclusion, our pilot study proposes implementation of FXS carrier screening during prenatal diagnosis in pregnant women who were not offered or declined screening early in pregnancy or preconceptionally. This approach may give another chance to be offered screening, which will provide medically necessary information for earlier prenatal and postnatal intervention for the affected individuals and inform the risk of FXS for subsequent pregnancies. Furthermore, pedigree investigation and genetic testing in relatives based on the screening results of pregnant women may help other family members to avoid the risk of FXS in reproduction.
CONFLICT OF INTEREST
The authors declared no conflict of interest.
AUTHORS’ CONTRIBUTIONS
H. Xi, Y. Peng, D. Wang, and S. Yang recruited the patients and interpreted the results of prenatal diagnosis. Y. Zhang performed genomic DNA extraction for the samples. J. Chen, W. Tang, X. Deng, and H. Li performed the PCR assay and Southern blot analysis and R. Duan and J. Fang interpreted the data. H. Wang designed the study and reviewed all the data. H. Xi and W. Xie wrote the manuscript, and all authors read and approved the final manuscript.
ACKNOWLEDGMENTS
The authors thank the patients and their families for participation in the present study.
Funding information
Natural Science Foundation of Hunan Province (2017JJ3144); Major Scientific and Technological Projects for Collaborative Prevention and Control of Birth Defects in Hunan Province (2019SK1010); Key Projects of Research and Development of Hunan Provincial Science and Technology Department (2018SK2064).
DATA AVAILABILITY STATEMENT
The data that support the findings of this study are available from the corresponding author upon reasonable request.
REFERENCES
- Archibald, A. D., Hickerton, C. L., Jaques, A. M., Wake, S., Cohen, J., & Metcalfe, S. A. (2013). "It's about having the choice": Stakeholder perceptions of population‐based genetic carrier screening for fragile X syndrome. American Journal of Medical Genetics. Part A, 161(1), 48–58. 10.1002/ajmg.a.35674 [DOI] [PubMed] [Google Scholar]
- Archibald, A. D., Smith, M. J., Burgess, T., Scarff, K. L., Elliott, J., Hunt, C. E., Barns‐Jenkins, C., Holt, C., Sandoval, K., Siva Kumar, V., Ward, L., Allen, E. C., Collis, S. V., Cowie, S., Francis, D., Delatycki, M. B., Yiu, E. M., Massie, R. J., Pertile, M. D., … Amor, D. J. (2018). Reproductive genetic carrier screening for cystic fibrosis, fragile X syndrome, and spinal muscular atrophy in Australia: outcomes of 12,000 tests. Genetics in Medicine, 20(5), 513–523. 10.1038/gim.2017.134 [DOI] [PubMed] [Google Scholar]
- Arenas, R. A., Andreo, J. R., Suner, D. H., Pia, M., Yague, C. F., Cerda, C., & Mariscal, T. (2017). A pilot study of fragile X syndrome screening in pregnant women and women planning pregnancy: Implementation, acceptance, awareness, and geographic factors. Journal of Genetic Counseling, 26(3), 501–510. 10.1007/s10897-016-0005-3 [DOI] [PubMed] [Google Scholar]
- Bailey, D. B., Jr., Bishop, E., Raspa, M., & Skinner, D. (2012). Caregiver opinions about fragile X population screening. Genetics in Medicine, 14(1), 115–121. 10.1038/gim.0b013e31822ebaa6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berkenstadt, M., Ries‐Levavi, L., Cuckle, H., Peleg, L., & Barkai, G. (2010). Preconceptional and prenatal screening for fragile X syndrome: Experience with 40,000 tests. Prenatal Diagnosis, 27(11), 991–994. 10.1002/pd.1815 [DOI] [PubMed] [Google Scholar]
- Dimmock, D. (2017). Should we implement population screening for fragile X. Genetics in Medicine, 19(12), 1295–1299. 10.1038/gim.2017.81 [DOI] [PubMed] [Google Scholar]
- Esanov, R., Andrade, N. S., Bennison, S., Wahlestedt, C., & Zeier, Z. (2016). The FMR1 promoter is selectively hydroxymethylated in primary neurons of fragile X syndrome patients. Human Molecular Genetics, 25(22), 4870–4880. 10.1093/hmg/ddw311 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Essop, F., & Krause, A. (2013). Diagnostic, carrier and prenatal genetic testing for fragile X syndrome and other FMR‐1‐related disorders in Johannesburg, South Africa: A 20‐year review. South African Medical Journal, 103(12 Suppl 1), 994–998. 10.7196/samj.7144 [DOI] [PubMed] [Google Scholar]
- Gao, F., Huang, W., You, Y., Huang, J., Zhao, J., Xue, J., Kang, H., Zhu, Y., Hu, Z., Allen, E. G., Jin, P., Xia, K., & Duan, R. (2020). Development of Chinese genetic reference panel for Fragile X Syndrome and its application to the screen of 10,000 Chinese pregnant women and women planning pregnancy. Molecular Genetics & Genomic Medicine, 8(6), e1236. 10.1002/mgg3.1236 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hagerman, R. J., Berry‐Kravis, E., Hazlett, H. C., Bailey, D. B., Moine, H., Kooy, R. F., Tassone, F., Gantois, I., Sonenberg, N., Mandel, J. L., & Hagerman, P. J. (2017). Fragile X syndrome. Nature Reviews Disease Primers, 3, 17065. 10.1038/nrdp.2017.65 [DOI] [PubMed] [Google Scholar]
- Hagerman, R. J., & Hagerman, P. (2016). Fragile X‐associated tremor/ataxia syndrome – Features, mechanisms and management. Nature Reviews. Neurology, 12(7), 403–412. 10.1038/nrneurol.2016.82 [DOI] [PubMed] [Google Scholar]
- Hipp, H. S., Charen, K. H., Spencer, J. B., Allen, E. G., & Sherman, S. L. (2016). Reproductive and gynecologic care of women with fragile X primary ovarian insufficiency (FXPOI). Menopause, 23(9), 993–999. 10.1097/gme.0000000000000658 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hung, C.‐C., Lee, C.‐N., Wang, Y.‐C., Chen, C.‐L., Lin, T.‐K., Su, Y.‐N., Lin, M.‐W., Kang, J., Tai, Y.‐Y., Hsu, W.‐W., & Lin, S.‐Y. (2019). Fragile X syndrome carrier screening in pregnant women in Chinese Han population. Scientific Reports, 9(1), 15456. 10.1038/s41598-019-51726-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hunter, J. E., Riveroarias, O., Angelov, A., Kim, E., Fotheringham, I., & Leal, J. (2014). Epidemiology of fragile X syndrome: A systematic review and meta‐analysis. American Journal of Medical Genetics. Part A, 164(7), 1648–1658. 10.1002/ajmg.a.36511 [DOI] [PubMed] [Google Scholar]
- Johansen Taber, K., Lim‐Harashima, J., Naemi, H., & Goldberg, J. (2019). Fragile X syndrome carrier screening accompanied by genetic consultation has clinical utility in populations beyond those recommended by guidelines. Molecular Genetics & Genomic Medicine, 7(12), e1024. 10.1002/mgg3.1024 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Monaghan, K. G., Lyon, E., & Spector, E. B. (2013). ACMG Standards and Guidelines for fragile X testing: a revision to the disease‐specific supplements to the Standards and Guidelines for Clinical Genetics Laboratories of the American College of Medical Genetics and Genomics. Genetics in Medicine, 15(7), 575–586. 10.1038/gim.2013.61 [DOI] [PubMed] [Google Scholar]
- Nolin, S. L., Brown, W. T., Glicksman, A., Houck, G. E., Jr., Gargano, A. D., Sullivan, A., Biancalana, V., Bröndum‐Nielsen, K., Hjalgrim, H., Holinski‐Feder, E., Kooy, F., Longshore, J., Macpherson, J., Mandel, J.‐L., Matthijs, G., Rousseau, F., Steinbach, P., Väisänen, M.‐L., von Koskull, H., & Sherman, S. L. (2003). Expansion of the fragile X CGG repeat in females with premutation or intermediate alleles. American Journal of Human Genetics, 72(2), 454–464. 10.1086/367713 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Okoniewski, K. C., Wheeler, A. C., Lee, S., Boyea, B. L., Raspa, M., Taylor, J. L., & Bailey, D. B. (2019). Early identification of fragile X syndrome through expanded newborn screening. Brain Sciences, 9(1), 4. 10.3390/brainsci9010004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Owens, K. M., Dohany, L., Holland, C., Dare, J., Mann, T., Settler, C., & Longman, R. E. (2018). FMR1 premutation frequency in a large, ethnically diverse population referred for carrier testing. American Journal of Medical Genetics. Part A, 176(6), 1304–1308. 10.1002/ajmg.a.38692 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Razak, K. A., Dominick, K. C., & Erickson, C. A. (2020). Developmental studies in fragile X syndrome. Journal of Neurodevelopmental Disorders, 12(1), 13. 10.1186/s11689-020-09310-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tzeng, C.‐C., Tsai, L.‐P., Hwu, W.‐L., Lin, S.‐J., Chao, M.‐C., Jong, Y.‐J., Chu, S.‐Y., Chao, W.‐C., & Lu, C.‐L. (2005). Prevalence of the FMR1 mutation in Taiwan assessed by large‐scale screening of newborn boys and analysis of DXS548‐FRAXAC1 haplotype. American Journal of Medical Genetics. Part A, 133a(1), 37–43. 10.1002/ajmg.a.30528 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The data that support the findings of this study are available from the corresponding author upon reasonable request.


