Abstract
Regeneration is widespread across the animal kingdom but varies vastly across phylogeny and even ontogeny. Adult mammalian regeneration in most organs and appendages is limited, while vertebrates such as zebrafish and salamanders are able to regenerate various organs and body parts. Here, we focus on the regeneration of appendages, spinal cord, and heart—organs and body parts that are highly regenerative among fish and amphibian species but limited in adult mammals. We then describe potential genetic, epigenetic, and post‐transcriptional similarities among these different forms of regeneration across vertebrates and discuss several theories for diminished regenerative capacity throughout evolution.
Keywords: appendage, heart, regeneration, spinal cord
Regeneration is widespread across the animal kingdom but varies vastly across phylogeny and even ontogeny. Adult mammalian regeneration in most organs and appendages is limited, while vertebrates such as zebrafish and salamanders are able to regenerate various organs and body parts. In this review, we describe potential genetic, epigenetic, and post‐transcriptional similarities among heart, appendage, and spinal cord regeneration across vertebrates and discuss several theories for diminished regenerative capacity throughout evolution.
1. INTRODUCTION TO REGENERATION IN THE ANIMAL KINGDOM
Regenerative capacity varies widely in the animal kingdom. Invertebrate species such as planarians are champions of regeneration, capable of regenerating the entire organism. 1 , 2 Within vertebrate species, those most closely resembling their fossil ancestors—“phylogenetically primitive” organisms such as fish (with zebrafish being a prominent example) and urodele amphibians—are capable of regenerating the limb, fin, spinal cord, heart, and other organs. Meanwhile, adult mammalian species are largely limited to regenerating specific tissues such as the skin, liver, and skeletal muscle and not body parts such as the heart, spinal cord, and limbs. 3 , 4 , 5 Strikingly, neonatal mammals retain a lot of the regenerative capacity observed in fish and amphibian species. Whether this regenerative trait is a unique regeneration response, a modified injury response, or a combination of both remains unclear. The subsequent critical question is whether regeneration is an ancestral trait lost through evolution or a novel trait gained. Despite evidence supporting both theories, the ancestral trait theory has been gaining popularity with increasing evidence of conserved genetic and molecular pathways governing regeneration across phylogeny. 6
This review will first briefly introduce regeneration in invertebrate species, then focus on examining the diverse regenerative capacities of appendage, spinal cord, and heart in key model organisms including zebrafish (Danio rerio), axolotl salamanders (Ambystoma mexicanum), frogs (Rana spp., Xenopus spp.), and mice (laboratory strains of Mus musculus unless otherwise identified). We hope to highlight some genetic and epigenetic commonalities of regeneration across vertebrate species and address a few theories seeking to explain the general decline in regenerative capacity accompanying evolutionary development (Figures 1 and 2).
FIGURE 1.
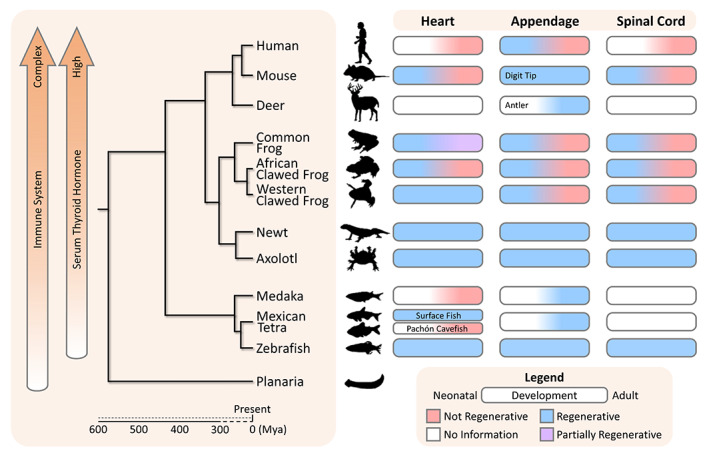
Phylogenetic distribution of species demonstrating varied heart, appendage, and spinal cord regenerative capacities across development
FIGURE 2.
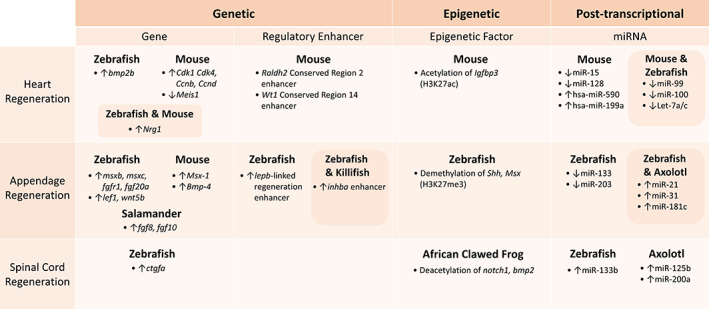
Table of essential genetic, epigenetic, and post‐transcriptional regulators of regeneration categorized by heart, appendage, or spinal cord regeneration. Arrows indicate whether overexpression or inhibition promotes regeneration
1.1. Regeneration in invertebrates
Unlike complex vertebrates, most invertebrates demonstrate a robust ability to regenerate. 7 For instance, the planarian flatworm (Schmidtea mediterranea) is prized for its astonishing regenerative potential. 1 Planaria maculata fully restore their bodies when left with a mere 1/279th fraction of the original body. 8 Throughout development, planaria rely on neoblast stem cells to regenerate tissues; upon injury, positional control genes, such as notum and wnt1, are activated to re‐establish positional information and serve as guides for progenitor cell differentiation. 9 On the other hand, insects demonstrate limited regenerative capacity—possibly due to their complex, derived morphogenesis—and are an anomaly amongst invertebrates. 10 For example, Drosophila melanogaster imaginal discs, which develop into limbs, appendages, and other parts, are highly regenerative during early larval stages but lose the ability to regenerate in larval development as they differentiate exoskeletal cuticle. 11 Still, many invertebrates of both the protostome and deuterostome lineages, such as flatworms and echinoderms, retain high regenerative potential. 7
1.2. Appendage regeneration in vertebrates
Contemporary vertebrate appendage regeneration models follow a similar order of events: epithelial closure of the wound site, establishment of a wound epithelium, dedifferentiation or activation of cells leading to epimorphic blastema formation, and proliferation and redifferentiation of progenitor cells to regenerate various appendage cell types in a lineage‐restricted manner (or what appears to be lineage‐restricted in species thoroughly investigated thus far). 12 The model is not all‐encompassing: morphologically simpler vertebrates can fully regenerate appendages, while more complex vertebrates generally possess extremely limited appendage regeneration.
Regeneration of the teleost fin was first documented by French naturalist Broussonet, then further explored by Thomas Hunt Morgan. 13 , 14 , 15 , 16 To date, the signaling pathways essential for zebrafish fin regeneration include many that are necessary for vertebrate embryonic development. Specifically, Wnt/β‐catenin, RA, IGF, and FGF signaling pathways drive blastema formation and expression of fgf20a, essential for initiating fin regeneration and subsequent proliferative outgrowth. 14 , 17 , 18
Axolotl salamanders (Ambystoma mexicanum) are among the oldest studied regenerative models with the first evidence of whole adult tail and limb regeneration documented by Spallanzani. 19 Molecular pathways similar to those in zebrafish fin regeneration also drive salamander limb regeneration. For instance, FGF signaling is required for blastema formation, with fgf8 and fgf10 expression essential for successful completion. 20 In addition, Wnt/β‐catenin signaling is necessary during early stages of limb regeneration but not after blastema formation. 21 , 22
Although complete limb regeneration is not observed in mammals, neonatal mice, adult mice, and humans can regenerate digit tips amputated through the distal region of the terminal phalanx. 23 , 24 , 25 Amputations transecting more proximal regions, however, result in regenerative failure and scarring instead. 26 Mammalian digit tip regeneration correlates with regions that express Msx‐1 and Msx‐1‐mutant mice cannot regenerate the digit tip. 27 , 28 However, BMP‐4 can rescue this phenotype, suggesting the importance of the BMP/Msx signaling pathway in digit tip regeneration. 28
Antlers in all Cervidae and female reindeer (Rangifer tarandus) are the only mammalian appendages that can fully regenerate, not only once, but many times throughout the host's lifetime. Scholars still debate the classification of this form of annual tissue replacement since seasonal hormonal changes, rather than injuries, prompt antler regeneration. Regardless, deer antlers are used as a model to understand complete mammalian appendage regeneration being a unique example of stem cell‐based epimorphic regeneration that also depends on blastema‐based appendage regeneration pathways. 29 , 30 , 31 , 32
1.3. Spinal cord regeneration in vertebrates
Spinal cord injuries in adult mammals generate permanent glial scars and lead to motor and sensory impairment. 33 , 34 Studying organisms capable of spinal cord regeneration can provide insights into various injury responses permitting pro‐regenerative environments. Here, we will discuss zebrafish and salamanders, species regenerative throughout their lifetimes, as well as frogs and mice, species only regenerative soon after birth.
Upon injury, the adult zebrafish spinal cord triggers proliferation and differentiation of resident neural progenitor cells called ependymoradial glial cells. 35 Spinal cord regeneration reuses major developmental pathways including Notch, Hh, Wnt, and FGF signaling. 36 , 37 FGF signaling in particular induces glial cell bridge formation, which provides a scaffold for regenerating axonal growth. Moreover, connective tissue growth factor a (ctgfa) is necessary and sufficient to initiate glial cell bridging and regeneration. 38 , 39
Spallanzani first described axolotl salamander spinal cord regeneration in 1768; subsequent studies of the field appeared in the mid‐20th century. 40 , 41 , 42 , 43 , 44 After tail amputation, salamanders can regenerate their spinal cord through ependymoradial glial cell proliferation and differentiation, similar to zebrafish. 45 , 46 , 47 Radial glial cells in axolotl tail regeneration can switch lineages to generate surrounding muscle and cartilage tissue in addition to regenerating a functional spinal cord. 48 This phenomenon contrasts the emerging theme that transdifferentiation is not a major mechanism governing limb and fin regeneration.
Xenopus, more phylogenetically complex than fish and salamanders, are able to regenerate their spinal cords as larvae but not post‐metamorphosis. 49 Unlike the axolotl, each main tissue group in the regenerated tail—notochord, spinal cord, and myofibers—arises from its respective remaining tissue in a lineage‐restricted fashion (again, given current limitations on genetic tools). 50 , 51 Regeneration‐organizing cells are present and essential during tail development and regeneration. 52 These cells express multiple regeneration‐supportive genes (Wnt5a, Fgf10, Fgf20, and Msx) and simultaneously upregulate ligand expression for common pro‐regenerative signaling pathways. 52 Although similar cell types have yet to be discovered in other regeneration models, regeneration‐organizing cells offer a fresh perspective to current known regenerative mechanisms.
Recent studies have revealed the capability of neonatal mice to heal, scar‐free, after spinal cord injuries. This discovery dispels the long‐standing theory that mammals are incapable of such regeneration. 53 Following injury, microglia transiently secrete fibronectin and binding proteins to form bridges across the severed spinal cord (like that in zebrafish) and express peptidase inhibitors to help resolve inflammation. 53 Future studies can help identify key genetic and molecular factors regulating pro‐regenerative traits of neonatal, but not adult, microglia.
1.4. Cardiac regeneration in vertebrates
Similar to spinal cord regeneration, adult mammalian hearts have limited regenerative potential compared to certain fish and amphibians. The general consensus within available models across phylogeny is that cardiac regeneration depends on the dedifferentiation and proliferation of pre‐existing cardiomyocytes. 54 , 55 , 56 , 57 , 58 , 59
Zebrafish—and other teleost fish including Giant Danio (Devario aequipinnatus) 60 and goldfish (Carassius auratus) 61 but not medaka (Oryzias latipes) 62 —retain robust regenerative capacity throughout life; adult zebrafish can regenerate their heart without scarring within 2 months post‐injury after 20% ventricular amputation. 63 Although zebrafish reuse many developmental signaling pathways seen in other models of regeneration, Jak/Stat, BMP, and Notch pathways are regeneration‐specific and not essential for cardiac development. 64 All three signaling pathways regulate cardiomyocyte proliferation after injury; bmp2b overexpression, in particular, is sufficient to enhance cardiomyocyte dedifferentiation and proliferation. 65
Mexican tetra (Astyanax mexicanus) are teleost fish whose surface populations can regenerate the heart. Groups of Mexican tetra diverged into caves and evolved independently 1.5 million years ago, gaining traits not seen in surface fish and losing traits such as their eyes, pigment, and, at least in the Pachón cavefish population, cardiac tissue regeneration. 66 , 67 Intra‐species breeding and genetic analyses between these distinct populations provide genomic explanations for differences in cardiac regenerative capacity. Lrrc10, a highly conserved gene unique to the heart, is differentially expressed and positively regulates cardiac regeneration in surface fish. Pachón cavefish, which exhibit low endogenous levels of lrrc10, and mutant zebrafish with lrrc10 knocked out, both demonstrate impaired cardiac regeneration. 67
Initial studies on adult newt (Notophthalmus viridescens) cardiac regeneration report incomplete regenerative capacity 30 days post‐injury. 68 , 69 Newer studies suggest that adult newts can fully regenerate their hearts 60 days post‐injury, with damaged tissue completely restored structurally and functionally. 57 , 58 Molecular pathways governing adult newt cardiac regeneration are not well established due to difficulties in sequencing their complex genome. Transcriptional approaches, however, show strong gene expression changes associated with the extracellular matrix post‐injury, including matrix metalloproteinases, collagen, keratin, and tenascin‐C. 70 , 71 , 72 The extracellular matrix may be important in signaling to the regenerating myocardium. For example, tenascin‐C can promote cell cycle re‐entry of newt cardiomyocytes in vitro. 73
The adult mammalian heart is mainly thought to be a post‐mitotic organ incapable of regeneration. In contrast, neonatal mice can fully regenerate their hearts without scarring within the first week after birth. 74 Significant molecular pathways that mediate cardiac regeneration in neonatal mice include Hippo, neuregulin, FGF, and thyroid hormone signaling, each altering cardiomyocyte cell cycle activity. 72 , 75 , 76 Positive cell cycle regulators can promote dedifferentiation and proliferation of post‐mitotic cardiomyocytes, with the combination of cyclin‐dependent kinase 1 (CDK1), CDK4, cyclin B1 (CCNB), and cyclin D1 (CCND) inducing post‐mitotic cell proliferation. 77 Deletion of Meis1—normally required for transcriptional activation of CDK inhibitors—is also sufficient to extend the perinatal cardiomyocyte proliferative window. 78
1.5. Liver Regeneration in Vertebrates
Although more complex vertebrates exhibit limited regenerative potential overall, most, if not all, are capable of liver regeneration. 79 Upon partial hepatectomy, the liver undergoes both hyperplasia and hypertrophy to regenerate and restore homeostasis. 80 , 81 Once signaling pathways targeting hepatocyte growth factor are activated, hepatocytes enter the cell cycle and restore the liver to its normal size. 82 Rats can regenerate the liver even after 70% partial hepatectomy, while zebrafish are able to after 30% partial hepatectomy. 80 , 83 Also, much like other examples of regeneration, zebrafish, rats, and mice utilize BMP, FGF, and Wnt signaling pathways to regenerate the liver. 83 , 84
2. SIMILARITIES IN REGENERATION ACROSS VERTEBRATES
Despite divergent tissue regeneration capacities observed across the animal kingdom, commonalities exist among appendage, heart, and spinal cord regeneration in fish, amphibians, and mammals. Here, we first highlight how regeneration is generally dependent on nerves, and proceed to discuss conserved genetic, epigenetic, and post‐transcriptional machinery governing regenerative potential.
2.1. Nerve dependence
Various organ regeneration in diverse species is nerve‐dependent. 85 Marcus Singer's neurotrophic hypothesis of regeneration suggests that nerves influence regeneration by producing factors promoting progenitor cell proliferation and differentiation. 86 These factors are still not clearly characterized today but suggest an evolutionarily conserved role and function of nerves in regeneration across vertebrates.
Seminal work by Tweedy John Todd demonstrates salamander limb regeneration's nerve‐dependent nature. 87 Limb and fin regeneration occur through blastema formation adjacent to the wound epithelium. If the nerve is severed at the base of the limb prior to or shortly after amputation, the wound epithelium still forms but the apical epithelial cap does not, and limb regeneration fails due to loss of blastema cell proliferation. 85 , 88 Proliferation is mediated by the apical epithelial cap dependence on nerves and its interaction with the blastema. In the newt, regenerating axons in the apical epithelial cap synthesize and secrete anterior gradient proteins, which then bind to its receptor Prod1 in the blastema to promote cell cycling. 86 Local expression of the anterior gradient protein after electroporation into a denervated newt limb blastema is sufficient to induce distal structure regeneration. Moreover, in vitro studies show mesenchymal blastema cells can re‐enter S phase when exposed to recombinant anterior gradient protein. 89
There are around 100 differentially expressed genes between innervated and denervated Mexican axolotl limbs, half of which are annotated to cell cycle and mitosis‐related ontologies. 90 Genes encoding cell cycle regulators such as ccna2 (a positive regulator of cardiac regeneration in mice and pigs 91 , 92 ) and those associated with cell proliferation and differentiation are enriched and only affected during the formation of the blastema following denervation. 90
In zebrafish fin regeneration, denervation affects mesenchymal cell proliferation, apical epithelial cap formation and signaling, and blastema formation and outgrowth. Genes essential for blastema formation, including msxb, msxc, fgfr1, and fgf20a, are significantly altered in denervated fins, suggesting the role of nerves in FGF signal regulation. 88 In addition, lef1 and wnt5b expression levels are significantly altered in denervated fins, suggesting the nerves' importance in regulating Wnt signaling during regeneration as well. 88
Although less understood mechanistically, mouse digit tip regeneration is also dependent on innervation. Following amputation, Schwann cell‐derived precursor cells from intact peripheral nerves secrete pro‐proliferative factors that are necessary for digit tip regeneration. These mitogenic factors include oncostatin M and platelet derived growth factor AA, which are sufficient to rescue digit tip regeneration following denervation. 93
Despite the general consensus that appendage regeneration is dependent on nerves, antler regeneration is in fact nerve independent. Denervation of antlerogenic regions does not affect the pedicles' or first antlers' formation and growth, and denervation of pedicles is not essential for antler regeneration. 94 , 95 , 96 In both cases, however, denervation affects antler size and shape, meaning nerves may help determine antler growth specifically. 94 , 95 This may be due to the stem cell‐based, rather than blastema‐based, nature of antler regeneration. Overall, current literature suggests that nerves are crucial for blastema formation in appendage regeneration.
The heart is extensively innervated. Multiple studies show that nerve function is essential for complete cardiac regeneration in adult zebrafish and neonatal mice. 97 , 98 Loss of cholinergic signaling in adult zebrafish and neonatal mice leads to reduced cardiomyocyte proliferation, evidenced by reduced expression of cell cycle genes such as Ccnd2 and Cdk4 and reduced expression of growth factors Nrg1 and Ngf 98. Nrg1 expression in particular is necessary and sufficient to induce cardiomyocyte proliferation in zebrafish, while administration of Nrg1 and Ngf recombinant proteins can rescue direct mechanical denervation in neonatal mice. 98 , 99
2.2. Regulatory enhancers
One of the major hypotheses regarding the evolution of regeneration is that regeneration is an innate ability and crucial regenerative genes already exist in most animal genomes; mechanisms to activate them after injury are simply lost. Low regenerative capacity of more complex vertebrates may result from differences in or loss of such regulatory mechanisms.
Enhancer elements are cis‐acting DNA regulatory elements that control gene transcription. They can be activated by specific developmental, injury‐related, or regeneration‐specific cues, or a combination thereof. Epicardial enhancer sequences Raldh2 conserved region 2 and Wt1 conserved region 14 are activated during development. The transcription factor C/EBP mediates injury‐induced reactivation of these enhancers following myocardial infarction and ischemia reperfusion in adult mice. 100 This suggests that developmental enhancers can be reused as injury‐responsive enhancers in adult tissue, warranting further investigation of transcriptional mechanisms controlling enhancer element activation as a means of promoting regeneration.
Recently, more regeneration‐associated enhancers have been discovered. 101 , 102 , 103 Transcriptome analysis in the regenerating zebrafish fin and heart reveal leptin b (lepb) transcription is significantly upregulated during both forms of regeneration. A sequence upstream of lepb, described as lepb‐linked regeneration enhancer (LEN), directs regeneration‐specific gene expression without developmental activity. This enhancer sequence is also separable into regulatory elements with distinct tissue preferences—heart or fin—which are activated by the respective injuries. 103
An evolutionary emergence or loss of regeneration enhancers through natural selection may explain the wide variance in regenerative capacity across phylogeny. Limited DNA sequence conservation of zebrafish LEN is found upstream of the leptin gene in mice. Despite this, zebrafish LEN is able to direct injury‐dependent gene expression following cardiac injury and digit tip amputation. 103 , 104 This suggests that although the regeneration‐specific enhancer was lost in mammals during evolution, regulatory mechanisms to activate zebrafish LEN still exist and can be reactivated in the context of an injury. There thus exists the possibility that LEN acts more like an injury‐responsive enhancer in mammals.
An outstanding question is whether an enhancer element that is activated following an injury is an injury‐responsive enhancer or a regeneration‐specific enhancer. Moreover, is the regeneration‐specific enhancer function important for tissue regeneration? A recent comprehensive study addresses these questions by comparing zebrafish and killifish fin and heart regeneration. 105 A set of evolutionarily conserved enhancer regulatory sequences is activated in both species and during both processes. Deletion of a conserved inhibin beta A (inhba) enhancer element in killifish delays fin regeneration and abrogates heart regeneration. While the predicted zebrafish inhba enhancer recapitulates killifish inhba enhancer activity and can functionally replace it for effective fin and heart regeneration, the predicted human inhba enhancer neither shows the same spatiotemporal activity nor rescues defective fin regeneration in killifish. 105 Thus, these findings present an example in which both of the following occur simultaneously: enhancer element conservation leading to regenerative competency in simpler vertebrates and enhancer sequence variation resulting in regenerative failure in more complex vertebrates.
2.3. Epigenetic modifications
Regardless of the cellular mode of regeneration—self‐renewing stem cells, cell dedifferentiation, or proliferation of pre‐existing cells—extensive epigenetic reprogramming occurs to generate an appropriate regenerative response. 106 Gene expression is regulated through chromatin structure modifications, cis‐regulatory DNA elements, and transcriptional machinery. Understanding evolutionarily conserved gene regulatory mechanisms may, in the future, allow researchers to reactivate dormant regeneration‐associated genes in mammals.
Regenerative processes often reuse genes and pathways involved in development. To ensure that these genes are only activated in a particular spatiotemporal context, epigenetic modifications are required to alter gene accessibility. To illustrate, caudal fin amputation in zebrafish leads to the reactivation of developmental regulators such as Shh and Msx. 107 , 108 Histone demethylases can activate these developmental regulatory genes by removing H3K27me3 marks. 109 Similarly, Shh enhancer methylation status dictates limb regenerative capacity in the Xenopus; here, hypomethylation contributes positively to regeneration in tadpoles, but negatively in adults. 110
Epigenetic modifications also regulate spinal cord regeneration. Histone deacetylases are necessary for both axolotl and Xenopus tail regeneration. 111 , 112 Histone deacetylase inhibition in the Xenopus tail leads to aberrant expression of notch1 and bmp2 and regenerative failure. 111 Likewise, multiple Notch and BMP pathway targets are also dysregulated when a histone deacetylase inhibitor is added to the axolotl tail regeneration model. 112
Igf2bp3, a regulator of IGF2 signaling, is highly expressed in the regenerative neonatal mouse heart and significantly downregulated in the nonregenerating adult heart. 113 This positive regulator of cardiomyocyte proliferation and cardiac regeneration is initially modified with H3K27ac activators but is then replaced with H3K27me3 silencers during postnatal development. 113 In zebrafish, H3K27me3 deposition is necessary for silencing structural genes in the heart to complete regeneration. 114 These findings suggest the critical role epigenetic modifications play in regenerative processes; modifications may both stimulate and stymie regeneration.
2.4. miRNA
MicroRNAs (miRNAs) are small noncoding mRNAs that post‐transcriptionally regulate gene expression by degrading their target mRNA and/or inhibiting their translation. They are required for development, stem cell maintenance, injury response, and multiple forms of regeneration. 115 , 116 , 117
miRNAs play a crucial role in zebrafish caudal fin regeneration. FGF‐dependent miR‐133 depletion enhances regeneration by increasing proliferation within the blastema, and miR‐203 downregulation allows for essential lef1 (a Wnt signaling transcription factor) expression. 118 , 119 Furthermore, a cross‐species analysis of miRNA expression accompanying blastema formation in zebrafish caudal fins, bichir pectoral fins, and axolotl forelimbs reveals a shared set of differentially regulated miRNAs. Upregulation of miR‐21, miR‐31 and miR‐181c leads to the downregulation of anti‐proliferative genes such as pdcd4, tgfbr2, bcl2l13, rgs5 and chka, in turn increasing proliferation necessary for blastema formation. 120
In zebrafish spinal cord regeneration, miR‐133b is upregulated following a complete spinal cord transection and positively regulates regeneration and functional recovery. 121 In axolotl glial cells, miR‐200a is upregulated and necessary for axonal regrowth and regeneration. 122 Another cross‐species study identifies miR‐125b differential expression in the axolotl and the rat following spinal cord transections. 123 Inhibiting miR‐125b in axolotls to a level similar to that in rats leads to axonal regenerative failure and deposition of fibrin (resembling glial scars observed in nonregenerative organisms). 123
miRNAs are also pivotal in cardiac regeneration. The miR‐15 family contributes to the postnatal mitotic arrest responsible for regenerative decline throughout development. 124 , 125 Overexpressing miR‐195 (a member of the miR‐15 family) in neonatal mice hearts causes cell cycle arrest and prevents cardiac regeneration post‐myocardial infarction. Inhibiting miR‐195 in adult hearts, on the other hand, increases cardiomyocyte proliferation and improves cardiac function. In both cases, miR‐195 may directly target the mRNA Chek1, which encodes for a protein kinase promoting the G2‐M phase cell cycle transition. 124
A more recent study shows that miR‐128 also plays a role in regulating cell cycle arrest in mice postnatally. 126 Cardiac‐specific miR‐128 overexpression impairs cardiomyocyte proliferation and regeneration following apical resection in neonatal mice. miR‐128 deletion instead extends the cardiomyocyte proliferative window by increasing Suz12 expression, which then suppresses p27 expression and activates positive cell cycle regulators. 126
miRNAs not only temper cardiomyocyte cell cycle activity, but also stimulate cell cycle re‐entry and promote cardiac regeneration. A screen for miRNAs upregulating DNA synthesis and cytokinesis in neonatal mice and rat cardiomyocytes reveals two miRNAs: hsa‐miR‐590 and hsa‐miR‐199a. These miRNAs increase both neonatal and adult cardiomyocyte proliferation in vivo and can also reduce infarct size in adult mice. 127
Another cross‐species study characterizes two miRNA families as critical regulators of adult zebrafish heart regeneration. 128 Cardiomyocytes within uninjured zebrafish hearts demonstrate high levels of miR‐99/100 and Let‐7a/c expression, but significantly downregulate them post‐injury. This allows fntβ and smarca5 upregulation and consequent cardiomyocyte dedifferentiation and proliferation. This miRNA program is present but dormant in mammals. The inability to endogenously downregulate these miRNAs following an injury correlates with low mammalian cardiac regenerative capacity. Guided manipulation with anti‐miRs can lead to significantly reduced fibrotic scarring and enhanced cardiomyocyte dedifferentiation and proliferation in mice. 128
3. POTENTIAL EXPLANATIONS FOR DIFFERENCES IN REGENERATIVE CAPACITIES AMONG VERTEBRATES
There are several potential explanations for the widely varied regenerative capacities observed across phylogeny and among vertebrates. Some of these theories include the evolutionary gain or loss of regeneration‐specific genes and molecular machinery, oxygenation and increase in oxidative damage, and the development of a more advanced immune system. Here, we will focus on the last two theories.
3.1. Acquisition of endothermy
Simpler vertebrates, such as fish and amphibians, are generally ectotherms, while more complex vertebrates, such as mammals and birds, are endotherms. Thyroid hormones are key regulators of energy metabolism and thermogenesis and also drive the evolutionary ectotherm‐to‐endotherm transition. 129 , 130 Regarding ontogenic development, a sharp postnatal increase in serum thyroid hormone levels coincides with the loss of cardiac regenerative capacity in mice. 74 , 131 Neonatal mice and other ectotherms—including zebrafish, axolotls and newts—have higher diploid cardiomyocyte content (indicative of higher cardiac regenerative potential) than their adult or endothermic counterparts. 131 This trend also inversely correlates with standard metabolic rate, serum thyroid hormone levels, and body temperature. In other words, ectothermic animals demonstrate higher cardiac regenerative potential. 131
Genetic inhibition of functional thyroid hormone receptor alpha in the heart directly shows how thyroid hormones affect mammalian cardiac regenerative potential. In this model, postnatal and adult mice demonstrate enhanced cardiomyocyte proliferation, a higher percentage of diploid cardiomyocytes, and regeneration after injury. 131 In contrast to this, when exposed to exogenous thyroid hormones, zebrafish, which typically demonstrate robust regeneration, experience pronounced scarring, a 45% reduction in cardiomyocyte proliferation, and increased polyploidization (more reminiscent of the nonregenerative mammalian heart). These results recapitulate an evolutionarily conserved function of thyroid hormone in regulating cardiac regeneration and suggest that the decline in cardiac regenerative potential may have been a trade‐off for endothermy acquisition.
Xenopus laevis (African clawed frog) serves as an excellent model for exploring the relationship between thyroid hormone and ectotherm regeneration. In this species, a transient surge in thyroid hormone drives the complete metamorphosis from a highly regenerative larva to a minimally regenerative adult. 132 , 133 Xenopus tadpoles can regenerate their hindlimbs and tail but lose this capability as metamorphosis proceeds. 134 , 135 In respect to spinal cord axonal regeneration, accelerating metamorphosis through pharmacological thyroid hormone treatment leads to axonal growth inhibition and paralysis. 136 Xenopus laevis tadpoles can also regenerate the heart but again, not after thyroid hormone‐regulated metamorphosis. 137 , 138 Both thyroid hormone excess and deprivation, however, lead to impaired cardiac regeneration in the tadpole, suggesting that modulation, rather than deprivation, of thyroid hormone is required for Xenopus regeneration. 137
Analogous to the regenerative dichotomy that exists between zebrafish and medaka, adult Xenopus tropicalis (Western clawed frog) can regenerate hearts without scarring. 139 In addition, adult Rana temporaria (common frog) can partially regenerate their hearts, inducing cardiomyocyte proliferation post‐injury and demonstrating almost complete functional recovery. 140 , 141 Regarding the two closer Xenopus species, a major difference in their genomes may explain the stark difference in cardiac regenerative potential: Xenopus laevis and all other Xenopus species are polyploid, while Xenopus tropicalis is the single exception with a diploid genome. 142 Although more precise analysis of their respective cardiomyocyte ploidies is needed, this intrinsic genomic difference may grant Xenopus tropicalis greater cardiac regenerative potential over close relatives.
Another manifestation of thyroid hormone's regeneration‐conducive effect is its blastema cell proliferation enhancement and subsequent accelerated hindlimb regeneration in Xenopus tadpoles. 143 This effect, however, depends on the degree of limb differentiation when amputation occurs. If the limb is amputated through an undifferentiated or differentiating region, thyroid hormone improves regeneration. Conversely, if the limb is amputated through an already differentiated region, thyroid hormone inhibits blastema formation and promotes further differentiation instead. 144 Altogether, these findings highlight thyroid hormone's multifaceted role in regeneration and suggest that ontogenetic regenerative decline in Xenopus is a much more nuanced issue. However, that regenerative potential diminishes after thyroid hormone‐induced metamorphosis remains consistent. Decoupling thyroid hormone's effects from thyroid hormone‐induced metamorphic events will help us better understand if and how a transient surge in thyroid hormone levels can directly cause regenerative loss in ectothermic animals like the Xenopus.
3.2. Development of an advanced immune response
Similar to changes in thyroid hormone levels across phylogeny and ontogeny, the development of a more complex and advanced immune system parallels the regenerative decline observed across vertebrate species and within an animal's development. 145 , 146 Xenopus have simpler immune systems during highly regenerative larval stages but develop a more advanced immune system akin to that of mammals after metamorphosis. 147 As adults, Xenopus are protected from viruses that kill axolotls (Ambystoma mexicanum). In turn, Xenopus lose regenerative capacity that even sexually mature but morphologically larval axolotls retain. 148 , 149 This may be in part due to the increase in pro‐inflammatory immune cells and cytokines that accompany an adaptive immune response. Although unclear mechanistically, this different immune response is observed during the refractory period when Xenopus larval tails fail to regenerate. At this time, there is a chronic immune response accompanied by a delay in local inflammation resolution and T cell maturation. 150 Immunosuppressants can restore regenerative capacity during this refractory period, implying a correlation between a subdued or underdeveloped immune system and regeneration. More detailed studies are needed to show this relationship is causal.
That being said, an innate immune response is necessary for blastema formation in zebrafish fin and urodele limb regeneration. 151 Successful axolotl limb regeneration requires proper inflammation modulation and macrophage recruitment. 152 Ablating these macrophages reduces MMP9 and MMP3 activation and ultimately results in scarring. 153 , 154 Besides salamander limb regeneration, macrophages are also necessary for zebrafish fin 155 and spinal cord 156 regeneration as well as neonatal mouse digit tip 157 and cardiac 158 regeneration.
Within mammalian regeneration, neonatal mice lose their high regenerative capacity throughout development with concomitant immune system maturation. 146 , 159 Although pro‐regenerative macrophages with properties similar to those present in zebrafish and urodeles are found in neonatal mice hearts, they are progressively replaced with bone marrow‐derived macrophages which hinder regeneration. 160 , 161 As such, immunosuppression in neonatal mice inhibits cardiac regeneration, with macrophage‐secreted IL‐6 essential to initiate cardiomyocyte proliferation. 162 To further illustrate, IL‐10 is another anti‐inflammatory cytokine which regulates the neonatal inflammatory response. After dermal injury, IL‐10 deletion in neonates results in scar tissue formation, and overexpression in postnatal mice allows for scarless wound healing. 163 , 164 , 165 Besides skin wounds, IL‐10 can also improve cardiac function after myocardial infarction. 166 , 167 , 168 Although present in adult mice, IL‐10 may not be upregulated enough following an injury, yielding a more pro‐inflammatory response that accelerates wound healing via fibrosis instead.
In addition to innate immunity, jawed fish possess adaptive immunity, albeit less evolved and diversified than mammals. 169 , 170 , 171 Having a conserved adaptive immune system coupled with genetic tractability, zebrafish are an increasingly popular model to uncover ancestral pro‐regenerative immune signals and pathways that may have been lost or modified in mammals. In established models of spinal cord, heart, and retina regeneration, zebrafish regulatory T (Treg) cells modulate inflammation and promote precursor cell proliferation by secreting organ‐specific regenerative factors. 172 Mature mouse Treg cells can also decrease inflammation and promote cell differentiation and proliferation but only in regeneration‐competent tissues. 173 , 174 , 175 The critical role of zebrafish Treg cells in tissues normally regeneration‐incompetent in mammals has alluded to possible evolutionary pressures that limited mammalian Treg cell pro‐regenerative capacity and/or altered the microenvironments in nonregenerative tissues so that they no longer stimulate Treg cell pro‐regenerative activity.
Experiments in T cell‐deficient neonatal mice show that the latter hypothesis is more likely. Treg cells are necessary for neonatal cardiac regeneration. 176 Not only are Treg cells from neonates pro‐regenerative, those from adult mice can also induce cardiomyocyte proliferation and regeneration in nonregenerative T cell‐deficient neonatal mice. 176 Adoptive Treg cell transfer to adult mice hearts can improve cardiac function and ameliorate adverse ventricular remodeling after injury. 177 This transfer, however, is not sufficient for regeneration like in mutant neonates. These results indicate that the regenerative decline that accompanies mammalian immune system development is at least not due to Treg cell maturation. The decline may partially be explained by an increasing pro‐fibrotic CD4+ T cell population but more studies are needed to annotate the changes in immune cell populations that negatively regulate regeneration. 178
In addition to neonates, the African spiny mouse (Acomys kempi and Acomys percivali) is a unique and exceptional mammalian model capable of scarless wound healing. Acomys can regenerate full‐thickness skin injuries and ear holes even as adults. 179 This trait, likely to have evolved as a defense mechanism to escape predation, may be possible due to differences in immune profiles following injuries. For instance, pro‐inflammatory factors are downregulated and pro‐reparative factors are upregulated in the Acomys wound bed compared to Mus (standard laboratory mouse strains). 180 , 181 Moreover, inflammatory M1 macrophages are largely absent in healing Acomys tissue but abundant in scarring Mus tissue. 181 Although many details have yet to be teased apart, Acomys provides a promising platform to study robust scarless wound healing in adult mammals. Future comparative studies with regeneration in simpler vertebrates may narrow down components essential for a pro‐regenerative immune landscape.
4. CONCLUDING REMARKS AND FUTURE PERSPECTIVES
Are regenerative traits gained or lost throughout evolution? Are these traits positively, negatively, or neutrally selected by natural selection? Lastly, what is the major driving force that results in divergent tissue regenerative capacities across phylogeny and ontogeny? Among many theories that attempt to address these questions, ones suggesting regeneration is an ancestral trait have garnered increasing support with recent scientific discoveries. 6 Seeing how regenerative traits are conserved throughout phylogeny, one can argue that regeneration is positively selected for if this trait correlates with increased survival. In some species, the ability to recover from physical injuries via regeneration can help individuals successfully reach maturity and pass down genes to next generations. Evidently, most simpler vertebrates retain the ability to regenerate various whole organs and body parts throughout their lifetime. At the same time, the only whole complex organ most adult mammals can regenerate is the liver. Yet, natural selection may favor traits that improve fitness to a greater extent in some species. Thus, it is possible that other traits may be selected over regenerative traits and that regeneration is lost neutrally throughout evolution.
Across evolution, regenerative potential declines with increasing phylogenetic complexity. However, while invertebrates are considered more primitive than vertebrates, insects—arguably more evolutionarily complex than flatworms but still invertebrates—do not demonstrate the same robust regeneration. There are thus parallel trends in regeneration even within invertebrate and vertebrate species, respectively.
Furthermore, this evolutionary trend also manifests as developmental changes in some vertebrates: losing regenerative traits and simultaneously gaining other evolutionary traits like endothermy and more advanced immune systems with maturation. The inverse correlation between heart regenerative potential and endothermic adequacy is an apt example. 131 In an evolutionary perspective, the ability to regulate body temperature likely permitted early mammals to occupy new ecological niches, allowing them to thrive in colder climates and also nocturnally. 182 Cardiac regeneration may not have been negatively selected for in this case, but rather neutrally lost as endothermy posed greater advantages in evolution. Still, why cardiac regeneration potential had to wane alongside the acquisition of endothermy across phylogeny, and why it continues to wane throughout mammalian ontogeny, requires further study.
For deeper understanding of regenerative processes or the lack thereof, it is crucial to harness nature's diversity and conduct more comparative analyses across the animal kingdom to identify shared regenerative tropes. Although zebrafish and salamanders are excellent regeneration models, they are quite distant from mammals in the phylogenetic tree. Genomic and transcriptomic divergences make it difficult, if not impossible, to pinpoint key molecular pathways underlying regenerative potential loss in most mammalian organs and appendages. Are there mammals that retain significant regenerative potential in organs and appendages that mice and humans cannot regenerate? Such mammals would enable direct tissue gene expression and physiology comparisons to help uncover molecules and pathways controlling regeneration.
Moreover, with the rapid development of next‐generation sequencing technologies and genomic editing tools such as CRISPR/Cas9, we are experiencing the emergence of many exciting genetic models in nontraditional organisms. Rigorous genetic lineage‐tracing experiments will define the cellular origins of new tissues better than traditional cell transplant assays. In addition, genetic gain‐ and loss‐of‐function studies will help elucidate candidate genes and pathways more convincingly than some current studies relying on pharmacological reagents. Armed with recent developments in single‐cell and nucleus RNA sequencing, DNA sequencing, and chromatin state analysis technologies, scientists can continue to dissect the genetic, epigenetic, and post‐transcriptional basis of regeneration across evolution.
AUTHOR CONTRIBUTIONS
Guo Huang: Conceptualization; writing‐review & editing. Sheamin Khyeam: Conceptualization; writing‐original draft; writing‐review & editing. Sukjun Lee: Writing‐review.
CONFLICT OF INTEREST
The authors declare no conflicts of interest.
PEER REVIEW
The peer review history for this article is available at https://publons.com/publon/10.1002/ggn2.10042.
Supporting information
Supplementary TPR File
ACKNOWLEDGEMENTS
This research was made possible by NIH (R01HL138456), Department of Defense (W81XWH1910206), Program for Breakthrough Biomedical Research, and UCSF Eli and Edythe Broad Center of Regeneration Medicine and Stem Cell Research Seed Grant (G.N.H.).
Khyeam S, Lee S, Huang GN. Genetic, epigenetic, and post‐transcriptional basis of divergent tissue regenerative capacities among vertebrates. Advanced Genetics. 2021;2(2):e10042. 10.1002/ggn2.10042
Funding information National Heart, Lung, and Blood Institute, Grant/Award Number: R01HL138456; U.S. Department of Defense, Grant/Award Number: W81XWH1910206
REFERENCES
- 1. Sánchez AA. Planarian regeneration: its end is its beginning. Cell. 2006;124:241‐245. 10.1016/j.cell.2006.01.012. [DOI] [PubMed] [Google Scholar]
- 2. Rink JC. Stem cell systems and regeneration in planaria. Dev Genes Evol. 2013;223:67‐84. 10.1007/s00427-012-0426-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Iismaa SE, Kaidonis X, Nicks AM, et al. Comparative regenerative mechanisms across different mammalian tissues. NPJ Regen Med. 2018;3:6. 10.1038/s41536-018-0044-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Mokalled MH, Poss KD. A regeneration toolkit. Dev Cell. 2018;47:267‐280. 10.1016/j.devcel.2018.10.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Yun MH. Changes in regenerative capacity through lifespan. Int J Mol Sci. 2015;16:25392‐25432. 10.3390/ijms161025392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Bely AE, Nyberg KG. Evolution of animal regeneration: re‐emergence of a field. Trends Ecol Evol. 2010;25(3):161–170. 10.1016/j.tree.2009.08.005. [DOI] [PubMed] [Google Scholar]
- 7. Allen JM, Ross KG, Zayas RM. Regeneration in invertebrates: model systems. eLS, Chichester, UK: John Wiley & Sons, Ltd; 2016, p. 1–9. 10.1002/9780470015902.a0001095.pub2. [DOI] [Google Scholar]
- 8. Morgan TH. Experimental studies of the regeneration of Planaria maculata . Arch Für Entwickelungsmechanik Der Org. 1898;7:364‐397. 10.1007/BF02161491. [DOI] [Google Scholar]
- 9. Reddien PW. The cellular and molecular basis for planarian regeneration. Cell. 2018;175:327‐345. 10.1016/j.cell.2018.09.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Maruzzo D, Bortolin F. Arthropod Regeneration. Arthropod Biology and Evolution: Molecules, Development, Morphology. Berlin, Heidelberg: Springer; 2013;149‐169. 10.1007/978-3-642-36160-9_7. [DOI] [Google Scholar]
- 11. Smith‐Bolton RK, Worley MI, Kanda H, Hariharan IK. Regenerative growth in drosophila Imaginal discs is regulated by wingless and Myc. Dev Cell. 2009;16:797‐809. 10.1016/j.devcel.2009.04.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Miller BM, Johnson K, Whited JL. Common themes in tetrapod appendage regeneration: a cellular perspective. Evodevo. 2019;10:11. 10.1186/s13227-019-0124-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Broussonet PMA. Memoir on the regeneration of certain parts of the bodies of fishes. 3. London: The Literary magazine and British review; 1789;111–113. 10.5962/bhl.title.5761. [DOI] [Google Scholar]
- 14. Gemberling M, Bailey TJ, Hyde DR, Poss KD. The zebrafish as a model for complex tissue regeneration. Trends Genet. 2013;29(11):611–620. 10.1016/j.tig.2013.07.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Morgan TH. Regeneration in teleosts. Arch Für Entwicklungsmechanik Der Org. 1900;10:120–134. 10.1007/BF02156348. [DOI] [Google Scholar]
- 16. Pfefferli C, Jaźwińska A. The art of fin regeneration in zebrafish. Regeneration. 2015;2:72–83. 10.1002/reg2.33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Whitehead GG, Makino S, Lien CL, Keating MT. fgf20 is essential for initiating zebrafish fin regeneration. Science. 2005;310(5756):1957–1960. 10.1126/science.1117637. [DOI] [PubMed] [Google Scholar]
- 18. Wehner D, Weidinger G. Signaling networks organizing regenerative growth of the zebrafish fin. Trends Genet. 2015;31(6):336–343. 10.1016/j.tig.2015.03.012. [DOI] [PubMed] [Google Scholar]
- 19. Tsonis PA, Fox TP. Regeneration according to spallanzani. Dev Dyn. 2009;238:2357–2363. 10.1002/dvdy.22057. [DOI] [PubMed] [Google Scholar]
- 20. Yokoyama H. Initiation of limb regeneration: the critical steps for regenerative capacity. Dev Growth Differ. 2008;50:13‐22. 10.1111/j.1440-169X.2007.00973.x. [DOI] [PubMed] [Google Scholar]
- 21. Kawakami Y, Esteban CR, Raya M, et al. Wnt/β‐catenin signaling regulates vertebrate limb regeneration. Genes Dev. 2006;20:3232‐3237. 10.1101/gad.1475106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Yokoyama H, Ogino H, Stoick‐Cooper CL, Grainger RM, Moon RT. Wnt/β‐catenin signaling has an essential role in the initiation of limb regeneration. Dev Biol. 2007;306(1):170–178. 10.1016/j.ydbio.2007.03.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. McKim L. Regeneration of the distal phalanx. Can Med Assoc J. 1932;26:549‐550. [PMC free article] [PubMed] [Google Scholar]
- 24. Illingworth CM. Trapped fingers and amputated finger tips in children. J Pediatr Surg. 1974;9(6):853–858. 10.1016/S0022-3468(74)80220-4. [DOI] [PubMed] [Google Scholar]
- 25. Douglas BS. Conservative management of guillotine amputation of the finger in children. J Paediatr Child Health. 1972;8:86–89. 10.1111/j.1440-1754.1972.tb01793.x. [DOI] [PubMed] [Google Scholar]
- 26. Borgens RB. Mice regrow the tips of their foretoes. Science. 1982;217(4561):747–750. 10.1126/science.7100922. [DOI] [PubMed] [Google Scholar]
- 27. Reginelli AD, Wang YQ, Sassoon D, Muneoka K. Digit tip regeneration correlates with regions of Msx1 (Hox 7) expression in fetal and newborn mice. Development. 1995;121(4):1065–1076. 10.1242/dev.121.4.1065. [DOI] [PubMed] [Google Scholar]
- 28. Han M, Yang X, Farrington JE, Muneoka K. Digit regeneration is regulated by Msx1 and BMP4 in fetal mice. Development. 2003;130(21):5123–5132. 10.1242/dev.00710. [DOI] [PubMed] [Google Scholar]
- 29. GOSS RJ. Experimental investigation of morphogenesis in the growing antler. J Embryol Exp Morphol. 1961;9(2):342–354. 10.1242/dev.9.2.342. [DOI] [PubMed] [Google Scholar]
- 30. Li C, Yang F, Sheppard A. Adult stem cells and mammalian epimorphic regeneration‐insights from studying annual renewal of deer antlers. Curr Stem Cell Res Ther. 2009;4(3):237–251. 10.2174/157488809789057446. [DOI] [PubMed] [Google Scholar]
- 31. Kierdorf U, Li C, Price JS. Improbable appendages: deer antler renewal as a unique case of mammalian regeneration. Semin Cell Dev Biol. 2009;20(5):535–542. 10.1016/j.semcdb.2008.11.011. [DOI] [PubMed] [Google Scholar]
- 32. Kierdorf U, Kierdorf H. Deer antlers – a model of mammalian appendage regeneration: an extensive review. Gerontology. 2010;57:53‐65. 10.1159/000300565. [DOI] [PubMed] [Google Scholar]
- 33. Thuret S, Moon LDF, Gage FH. Therapeutic interventions after spinal cord injury. Nat Rev Neurosci. 2006;7:628‐643. 10.1038/nrn1955. [DOI] [PubMed] [Google Scholar]
- 34. Ahuja CS, Wilson JR, Nori S, et al. Traumatic spinal cord injury. Nat Rev Dis Prim. 2017;3:1‐21. 10.1038/nrdp.2017.18. [DOI] [PubMed] [Google Scholar]
- 35. Ghosh S, Hui SP. Regeneration of zebrafish CNS: adult neurogenesis. Neural Plast. 2016;2016:1‐21. 10.1155/2016/5815439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Cardozo MJ, Mysiak KS, Becker T, Becker CG. Reduce, reuse, recycle – developmental signals in spinal cord regeneration. Dev Biol. 2017;432(1):53–62. 10.1016/j.ydbio.2017.05.011. [DOI] [PubMed] [Google Scholar]
- 37. Kizil C, Kaslin J, Kroehne V, Brand M. Adult neurogenesis and brain regeneration in zebrafish. Dev Neurobiol. 2011;72:429‐461. 10.1002/dneu.20918. [DOI] [PubMed] [Google Scholar]
- 38. Goldshmit Y, Sztal TE, Jusuf PR, Hall TE, Nguyen‐Chi M, Currie PD. Fgf‐dependent glial cell bridges facilitate spinal cord regeneration in Zebrafish. J Neurosci. 2012;32:7477‐7492. 10.1523/JNEUROSCI.0758-12.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Mokalled MH, Patra C, Dickson AL, Endo T, Stainier DYR, Poss KD. Injury‐induced ctgfa directs glial bridging and spinal cord regeneration in zebrafish. Science. 2016;354:630‐634. 10.1126/science.aaf2679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Butler EG, Ward MB. Reconstitution of the spinal cord following ablation in urodele larvae. J Exp Zool. 1965;160(1):47–65. 10.1002/jez.1401600106. [DOI] [PubMed] [Google Scholar]
- 41. Butler EG, Ward MB. Reconstitution of the spinal cord after ablation in adult Triturus. Dev Biol. 1967;15(5):464–486. 10.1016/0012-1606(67)90038-3. [DOI] [PubMed] [Google Scholar]
- 42. Clarke JDW, Alexander R, Holder N. Regeneration of descending axons in the spinal cord of the axolotl. Neurosci Lett. 1988;89(1):1–6. 10.1016/0304-3940(88)90471-5. [DOI] [PubMed] [Google Scholar]
- 43. Clemente CD. Regeneration in the vertebrate central nervous system. Int Rev Neurobiol. 1964;6:257–301. 10.1016/S0074-7742(08)60771-0. [DOI] [PubMed] [Google Scholar]
- 44. Piatt J. Regeneration of the spinal cord in the salamander. J Exp Zool. 1955;129:177‐207. 10.1002/jez.1401290109. [DOI] [Google Scholar]
- 45. O'Hara CM, Egar MW, Chernoff EAG. Reorganization of the ependyma during axolotl spinal cord regeneration: changes in intermediate filament and fibronectin expression. Dev Dyn. 1992;193:103‐115. 10.1002/aja.1001930202. [DOI] [PubMed] [Google Scholar]
- 46. Nordlander RH, Singer M. The role of ependyma in regeneration of the spinal cord in the urodele amphibian tail. J Comp Neurol. 1978;180(2):349–373. 10.1002/cne.901800211. [DOI] [PubMed] [Google Scholar]
- 47. Tazaki A, Tanaka EM, Fei JF. Salamander spinal cord regeneration: the ultimate positive control in vertebrate spinal cord regeneration. Dev Biol. 2017;432(1):63–71. 10.1016/j.ydbio.2017.09.034. [DOI] [PubMed] [Google Scholar]
- 48. Echeverri K, Tanaka EM. Ectoderm to mesoderm lineage switching during axolotl tail regeneration. Science. 2002;298:1993‐1996. 10.1126/science.1077804. [DOI] [PubMed] [Google Scholar]
- 49. Freitas PD, Yandulskaya AS, Monaghan JR. Spinal cord regeneration in amphibians: a historical perspective. Dev Neurobiol. 2019;79(5):437–452. 10.1002/dneu.22669. [DOI] [PubMed] [Google Scholar]
- 50. Gargioli C, Slack JMW. Cell lineage tracing during Xenopus tail regeneration. Development. 2004;131:2669‐2679. 10.1242/dev.01155. [DOI] [PubMed] [Google Scholar]
- 51. Lee‐Liu D, Méndez‐Olivos EE, Muñoz R, Larraín J. The African clawed frog Xenopus laevis: a model organism to study regeneration of the central nervous system. Neurosci Lett. 2017;652:82–93. 10.1016/j.neulet.2016.09.054. [DOI] [PubMed] [Google Scholar]
- 52. Aztekin C, Hiscock TW, Marioni JC, Gurdon JB, Simons BD, Jullien J. Identification of a regeneration‐organizing cell in the Xenopus tail. Science. 2019;364:653‐658. 10.1126/science.aav9996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Li Y, He X, Kawaguchi R, et al. Microglia‐organized scar‐free spinal cord repair in neonatal mice. Nature. 2020;587:613‐618. 10.1038/s41586-020-2795-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Vivien CJ, Hudson JE, Porrello ER. Evolution, comparative biology and ontogeny of vertebrate heart regeneration. NPJ Regen Med. 2016;1:1. 10.1038/npjregenmed.2016.12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Jopling C, Sleep E, Raya M, Martí M, Raya A, Belmonte JCI. Zebrafish heart regeneration occurs by cardiomyocyte dedifferentiation and proliferation. Nature. 2010;464:606–609. 10.1038/nature08899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Kikuchi K, Holdway JE, Werdich AA, et al. Primary contribution to zebrafish heart regeneration by gata4+ cardiomyocytes. Nature. 2010;464:601–605. 10.1038/nature08804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Witman N, Murtuza B, Davis B, Arner A, Morrison JI. Recapitulation of developmental cardiogenesis governs the morphological and functional regeneration of adult newt hearts following injury. Dev Biol. 2011;354(1):67–76. 10.1016/j.ydbio.2011.03.021. [DOI] [PubMed] [Google Scholar]
- 58. Laube F, Heister M, Scholz C, Borchardt T, Braun T. Re‐programming of newt cardiomyocytes is induced by tissue regeneration. J Cell Sci. 2006;119:4719‐4729. 10.1242/jcs.03252. [DOI] [PubMed] [Google Scholar]
- 59. Senyo SE, Steinhauser ML, Pizzimenti CL, et al. Mammalian heart renewal by pre‐existing cardiomyocytes. Nature. 2013;493:433–436. 10.1038/nature11682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Lafontant PJ, Burns AR, Grivas JA, et al. The Giant Danio (D. aequipinnatus) as a model of cardiac remodeling and regeneration. Anat Rec Adv Integr Anat Evol Biol. 2012;295:234‐248. 10.1002/ar.21492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Grivas J, Haag M, Johnson A, et al. Cardiac repair and regenerative potential in the goldfish (Carassius auratus) heart. Comp Biochem Physiol C Toxicol Pharmacol. 2014;163:14‐23. 10.1016/j.cbpc.2014.02.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Ito K, Morioka M, Kimura S, Tasaki M, Inohaya K, Kudo A. Differential reparative phenotypes between zebrafish and medaka after cardiac injury. Dev Dyn. 2014;243:1106‐1115. 10.1002/dvdy.24154. [DOI] [PubMed] [Google Scholar]
- 63. Poss KD, Wilson LG, Keating MT. Heart regeneration in zebrafish. Science. 2002;298(5601):2188–2190. 10.1126/science.1077857. [DOI] [PubMed] [Google Scholar]
- 64. Sehring IM, Jahn C, Weidinger G. Zebrafish fin and heart: what's special about regeneration? Curr Opin Genet Dev. 2016;40:48–56. 10.1016/j.gde.2016.05.011. [DOI] [PubMed] [Google Scholar]
- 65. Wu CC, Kruse F, Vasudevarao MD, et al. Spatially resolved genome‐wide transcriptional profiling identifies BMP signaling as essential regulator of Zebrafish cardiomyocyte regeneration. Dev Cell. 2016;36:36‐49. 10.1016/j.devcel.2015.12.010. [DOI] [PubMed] [Google Scholar]
- 66. Jeffery WR. Regressive evolution in Astyanax cavefish. Annu Rev Genet. 2009;43:25‐47. 10.1146/annurev-genet-102108-134216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. Stockdale WT, Lemieux ME, Killen AC, et al. Heart regeneration in the Mexican cavefish. Cell Rep. 2018;25(8):1997–2007. 10.1016/j.celrep.2018.10.072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68. Becker RO, Chapin S, Sherry R. Regeneration of the ventricular myocardium in amphibians. Nature. 1974;248:145‐147. 10.1038/248145a0. [DOI] [PubMed] [Google Scholar]
- 69. Oberpriller JO, Oberpriller JC. Response of the adult newt ventricle to injury. J Exp Zool. 1974;187(2):249–259. 10.1002/jez.1401870208. [DOI] [PubMed] [Google Scholar]
- 70. Gamba L, Harrison M, Lien C‐L. Cardiac regeneration in model organisms. Curr Treat Options Cardiovasc Med. 2014;16:288. 10.1007/s11936-013-0288-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71. Mercer SE, Cheng CH, Atkinson DL, et al. Multi‐tissue microarray analysis identifies a molecular signature of regeneration. PLoS One. 2012;7(12):e52375. 10.1371/journal.pone.0052375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72. Uygur A, Lee RT. Mechanisms of cardiac regeneration. Dev Cell. 2016;36(4):362–374. 10.1016/j.devcel.2016.01.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73. Mercer SE, Odelberg SJ, Simon HG. A dynamic spatiotemporal extracellular matrix facilitates epicardial‐mediated vertebrate heart regeneration. Dev Biol. 2013;382(2):457–469. 10.1016/j.ydbio.2013.08.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74. Porrello ER, Mahmoud AI, Simpson E, et al. Transient regenerative potential of the neonatal mouse heart. Science. 2011;331(6020):1078–1080. 10.1126/science.1200708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75. Xiang MSW, Kikuchi K. Endogenous mechanisms of cardiac regeneration. Int Rev Cell Mol Biol. 2016;326:67–131. 10.1016/bs.ircmb.2016.04.002. [DOI] [PubMed] [Google Scholar]
- 76. Xin M, Olson EN, Bassel‐Duby R. Mending broken hearts: cardiac development as a basis for adult heart regeneration and repair. Nat Rev Mol Cell Biol. 2013;14:529–541. 10.1038/nrm3619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77. Mohamed TMA, Ang YS, Radzinsky E, et al. Regulation of cell cycle to stimulate adult cardiomyocyte proliferation and cardiac regeneration. Cell. 2018;173:104‐116.e12. 10.1016/j.cell.2018.02.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78. Mahmoud AI, Kocabas F, Muralidhar SA, et al. Meis1 regulates postnatal cardiomyocyte cell cycle arrest. Nature. 2013;497:249‐253. 10.1038/nature12054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79. Delgado‐Coello B. Liver regeneration observed across the different classes of vertebrates from an evolutionary perspective. Heliyon. 2021;7:e06449. 10.1016/j.heliyon.2021.e06449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80. Higgins G, Anderson RM. Experimental pathology of the liver. I. Restoration of the liver of the white rat following partial surgical removal. Arch Pathol. 1931;12:186‐202. [Google Scholar]
- 81. Columbano A, Shinozuka H. Liver regeneration versus direct hyperplasia. FASEB J. 1996;10:1118‐1128. 10.1096/fasebj.10.10.8751714. [DOI] [PubMed] [Google Scholar]
- 82. Michalopoulos GK. Hepatostat: liver regeneration and normal liver tissue maintenance. Hepatology. 2017;65:1384‐1392. 10.1002/hep.28988. [DOI] [PubMed] [Google Scholar]
- 83. Kan NG, Junghans D, Belmonte JCI. Compensatory growth mechanisms regulated by BMP and FGF signaling mediate liver regeneration in zebrafish after partial hepatectomy. FASEB J. 2009;23:3516‐3525. 10.1096/fj.09-131730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84. Gilgenkrantz H, Collin de l'Hortet A. Understanding liver regeneration: from mechanisms to regenerative medicine. Am J Pathol. 2018;188:1316‐1327. 10.1016/j.ajpath.2018.03.008. [DOI] [PubMed] [Google Scholar]
- 85. Kumar A, Brockes JP. Nerve dependence in tissue, organ, and appendage regeneration. Trends Neurosci. 2012;35(11):691–699. 10.1016/j.tins.2012.08.003. [DOI] [PubMed] [Google Scholar]
- 86. Stocum DL. The role of peripheral nerves in urodele limb regeneration. Eur J Neurosci. 2011;34(6):908–916. 10.1111/j.1460-9568.2011.07827.x. [DOI] [PubMed] [Google Scholar]
- 87. Farkas JE, Monaghan JR. A brief history of the study of nerve dependent regeneration. Neurogenesis. 2017;4:e1302216. 10.1080/23262133.2017.1302216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88. Simões MG, Bensimon‐Brito A, Fonseca M, et al. Denervation impairs regeneration of amputated zebrafish fins. BMC Dev Biol. 2014;14:49. 10.1186/s12861-014-0049-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89. Kumar A, Godwin JW, Gates PB, Garza‐Garcia AA, Brockes JP. Molecular basis for the nerve dependence of limb regeneration in an adult vertebrate. Science. 2007;318(5851):772–777. 10.1126/science.1147710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90. Monaghan JR, Athippozhy A, Seifert AW, et al. Gene expression patterns specific to the regenerating limb of the Mexican axolotl. Biol Open. 2012;1(10):937–948. 10.1242/bio.20121594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91. Cheng RK, Asai T, Tang H, et al. Cyclin A2 induces cardiac regeneration after myocardial infarction and prevents heart failure. Circ Res. 2007;100:1741‐1748. 10.1161/CIRCRESAHA.107.153544. [DOI] [PubMed] [Google Scholar]
- 92. Shapiro SD, Ranjan AK, Kawase Y, et al. Cyclin A2 induces cardiac regeneration after myocardial infarction through cytokinesis of adult cardiomyocytes. Sci Transl Med. 2014;6(224):224ra27. 10.1126/scitranslmed.3007668. [DOI] [PubMed] [Google Scholar]
- 93. Johnston APW, Yuzwa SA, Carr MJ, et al. Dedifferentiated Schwann cell precursors secreting paracrine factors are required for regeneration of the mammalian digit tip. Cell Stem Cell. 2016;19(4):433–448. 10.1016/j.stem.2016.06.002. [DOI] [PubMed] [Google Scholar]
- 94. Li C, Sheard PW, Corson ID, Suttie JM. Pedicle and antler development following sectioning of the sensory nerves to the antlerogenic region of red deer (Cervus elaphus). J Exp Zool. 1993;267:188‐197. 10.1002/jez.1402670212. [DOI] [PubMed] [Google Scholar]
- 95. Suttie JM, Fennessy PF. Regrowth of amputated velvet antlers with and without innervation. J Exp Zool. 1985;234(3):359–366. 10.1002/jez.1402340305. [DOI] [PubMed] [Google Scholar]
- 96. Wislocki GB, Singer M. The occurrence and function of nerves in the growing antlers of deer. J Comp Neurol. 1946;85(1):1–19. 10.1002/cne.900850102. [DOI] [PubMed] [Google Scholar]
- 97. White IA, Gordon J, Balkan W, Hare JM. Sympathetic reinnervation is required for mammalian cardiac regeneration. Circ Res. 2015;117(12):990–994. 10.1161/CIRCRESAHA.115.307465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98. Mahmoud AI, O'Meara CC, Gemberling M, et al. Nerves regulate cardiomyocyte proliferation and heart regeneration. Dev Cell. 2015;34(4):387–399. 10.1016/j.devcel.2015.06.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99. Gemberling M, Karra R, Dickson AL, Poss KD. Nrg1 is an injury‐induced cardiomyocyte mitogen for the endogenous heart regeneration program in zebrafish. Elife. 2015;2015(4):e05871. 10.7554/eLife.05871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100. Huang GN, Thatcher JE, McAnally J, et al. C/EBP transcription factors mediate epicardial activation during heart development and injury. Science. 2012;338(6114):1599–1603. 10.1126/science.1229765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101. Goldman JA, Kuzu G, Lee N, et al. Resolving heart regeneration by replacement histone profiling. Dev Cell. 2017;40(4):392–404.E5. 10.1016/j.devcel.2017.01.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102. Harris RE, Setiawan L, Saul J, Hariharan IK. Localized epigenetic silencing of a damage‐activated WNT enhancer limits regeneration in mature Drosophila imaginal discs. Elife. 2016;5:e11588. 10.7554/eLife.11588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103. Kang J, Hu J, Karra R, et al. Modulation of tissue repair by regeneration enhancer elements. Nature. 2016;532:201–206. 10.1038/nature17644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104. Dolan CP, Dawson LA, Muneoka K. Digit tip regeneration: merging regeneration biology with regenerative medicine. Stem Cells Transl Med. 2018;7:262‐270. 10.1002/sctm.17-0236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105. Wang W, Hu CK, Zeng A, et al. Changes in regeneration‐responsive enhancers shape regenerative capacities in vertebrates. Science. 2020;369(6508):eaaz3090. 10.1126/SCIENCE.AAZ3090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106. Barrero MJ, Izpisua Belmonte JC. Regenerating the epigenome. EMBO Rep. 2011;12(3):208–215. 10.1038/embor.2011.10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107. Akimenko MA, Johnson SL, Westerfield M, Ekker M. Differential induction of four msx homeobox genes during fin development and regeneration in zebrafish. Development. 1995;121(2):347–357. 10.1242/dev.121.2.347. [DOI] [PubMed] [Google Scholar]
- 108. Laforest L, Brown CW, Poleo G, et al. Involvement of the sonic hedgehog, patched 1 and bmp2 genes in patterning of the zebrafish dermal fin rays. Development. 1998;125(21):4175–4184. 10.1242/dev.125.21.4175. [DOI] [PubMed] [Google Scholar]
- 109. Stewart S, Tsun ZY, Belmonte JCI. A histone demethylase is necessary for regeneration in zebrafish. Proc Natl Acad Sci U S A. 2009;106:19889‐19894. 10.1073/pnas.0904132106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110. Yakushiji N, Suzuki M, Satoh A, et al. Correlation between Shh expression and DNA methylation status of the limb‐specific Shh enhancer region during limb regeneration in amphibians. Dev Biol. 2007;312(1):171–182. 10.1016/j.ydbio.2007.09.022. [DOI] [PubMed] [Google Scholar]
- 111. Tseng AS, Carneiro K, Lemire JM, Levin M. HDAC activity is required during Xenopus tail regeneration. PLoS One. 2011;6(10):e26382. 10.1371/journal.pone.0026382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112. Voss SR, Ponomareva LV, Dwaraka VB, et al. HDAC regulates transcription at the outset of axolotl tail regeneration. Sci Rep. 2019;9:6751. 10.1038/s41598-019-43230-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113. Wang Z, Cui M, Shah AM, et al. Mechanistic basis of neonatal heart regeneration revealed by transcriptome and histone modification profiling. Proc Natl Acad Sci U S A. 2019;116:18455‐18465. 10.1073/pnas.1905824116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114. Ben‐Yair R, Butty VL, Busby M, et al. H3K27me3‐mediated silencing of structural genes is required for zebrafish heart regeneration. Development. 2019;146(19):dev178632. 10.1242/dev.178632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115. Alvarez‐Garcia I, Miska EA. MicroRNA functions in animal development and human disease. Development. 2005;132:4653‐4662. 10.1242/dev.02073. [DOI] [PubMed] [Google Scholar]
- 116. Gangaraju VK, Lin H. MicroRNAs: key regulators of stem cells. Nat Rev Mol Cell Biol. 2009;10:116‐125. 10.1038/nrm2621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117. Sen CK, Ghatak S. miRNA control of tissue repair and regeneration. Am J Pathol. 2015;185(10):2629–2640. 10.1016/j.ajpath.2015.04.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118. Thatcher EJ, Paydar I, Anderson KK, Patton JG. Regulation of zebrafish fin regeneration by microRNAs. Proc Natl Acad Sci U S A. 2008;105:18384‐18389. 10.1073/pnas.0803713105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119. Yin VP, Thomson JM, Thummel R, Hyde DR, Hammond SM, Poss KD. Fgf‐dependent depletion of microRNA‐133 promotes appendage regeneration in zebrafish. Genes Dev. 2008;22:728–733. 10.1101/gad.1641808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120. King BL, Yin VP. A conserved microRNA regulatory circuit is differentially controlled during limb/appendage regeneration. PLoS One. 2016;11(6):e0157106. 10.1371/journal.pone.0157106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121. Yu YM, Gibbs KM, Davila J, et al. MicroRNA miR‐133b is essential for functional recovery after spinal cord injury in adult zebrafish. Eur J Neurosci. 2011;33(9):1587–1597. 10.1111/j.1460-9568.2011.07643.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122. Sabin KZ, Jiang P, Gearhart MD, Stewart R, Echeverri K. AP‐1cFos/JunB/miR‐200a regulate the pro‐regenerative glial cell response during axolotl spinal cord regeneration. Commun Biol. 2019;2:1‐13. 10.1038/s42003-019-0335-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123. Diaz Quiroz JF, Tsai E, Coyle M, Sehm T, Echeverri K. Precise control of miR‐125b levels is required to create a regeneration‐permissive environment after spinal cord injury: a cross‐species comparison between salamander and rat. DMM Dis Model Mech. 2014;7:601‐611. 10.1242/dmm.014837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124. Porrello ER, Johnson BA, Aurora AB, et al. MiR‐15 family regulates postnatal mitotic arrest of cardiomyocytes. Circ Res. 2011;109(6):670–679. 10.1161/CIRCRESAHA.111.248880. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125. Porrello ER, Mahmoud AI, Simpson E, et al. Regulation of neonatal and adult mammalian heart regeneration by the miR‐15 family. Proc Natl Acad Sci U S A. 2013;110:187‐192. 10.1073/pnas.1208863110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126. Huang W, Feng Y, Liang J, et al. Loss of microRNA‐128 promotes cardiomyocyte proliferation and heart regeneration. Nat Commun. 2018;9:700. 10.1038/s41467-018-03019-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127. Eulalio A, Mano M, Ferro MD, et al. Functional screening identifies miRNAs inducing cardiac regeneration. Nature. 2012;492:376‐381. 10.1038/nature11739. [DOI] [PubMed] [Google Scholar]
- 128. Aguirre A, Montserrat N, Zacchigna S, et al. In vivo activation of a conserved microRNA program induces mammalian heart regeneration. Cell Stem Cell. 2014;15:589‐604. 10.1016/j.stem.2014.10.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129. Little AG, Seebacher F. The evolution of endothermy is explained by thyroid hormonemediated responses to cold in early vertebrates. J Exp Biol. 2014;217:1642‐1648. 10.1242/jeb.088880. [DOI] [PubMed] [Google Scholar]
- 130. Mullur R, Liu YY, Brent GA. Thyroid hormone regulation of metabolism. Physiol Rev. 2014;94(2):355–382. 10.1152/physrev.00030.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131. Hirose K, Payumo AY, Cutie S, et al. Evidence for hormonal control of heart regenerative capacity during endothermy acquisition. Science. 2019;364(6436):184–188. 10.1126/science.aar2038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132. Beck CW, Belmonte JCI, Christen B. Beyond early development: Xenopus as an emerging model for the study of regenerative mechanisms. Dev Dyn. 2009;238(6):1226–1248. 10.1002/dvdy.21890. [DOI] [PubMed] [Google Scholar]
- 133. Furlow JD, Neff ES. A developmental switch induced by thyroid hormone: Xenopus laevis metamorphosis. Trends Endocrinol Metab. 2006;17:40‐47. 10.1016/j.tem.2006.01.007. [DOI] [PubMed] [Google Scholar]
- 134. Dent JN. Limb regeneration in larvae and metamorphosing individuals of the South African clawed toad. J Morphol. 1962;110:61‐77. 10.1002/jmor.1051100105. [DOI] [PubMed] [Google Scholar]
- 135. Bosco L. Expression of regenerative capacity of caudal spinal cord during the larval development of Xenopus laevis. Acta Embryol Exp (Palermo). 1979;(3):275–285. [PubMed] [Google Scholar]
- 136. Gibbs KM, Chittur SV, Szaro BG. Metamorphosis and the regenerative capacity of spinal cord axons in Xenopus laevis . Eur J Neurosci. 2011;33(1):9–25. 10.1111/j.1460-9568.2010.07477.x. [DOI] [PubMed] [Google Scholar]
- 137. Marshall LN, Vivien CJ, Girardot F, et al. Stage‐dependent cardiac regeneration in Xenopus is regulated by thyroid hormone availability. Proc Natl Acad Sci U S A. 2019;116:3614‐3623. 10.1073/pnas.1803794116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138. Marshall L, Vivien C, Girardot F, et al. Persistent fibrosis, hypertrophy and sarcomere disorganisation after endoscopy‐guided heart resection in adult Xenopus. PLoS One. 2017;12:e0173418. 10.1371/journal.pone.0173418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139. Liao S, Dong W, Lv L, et al. Heart regeneration in adult Xenopus tropicalis after apical resection. Cell Biosci. 2017;7:70. 10.1186/s13578-017-0199-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140. Rumyantsev P. Evidence of the regeneration of the considerable part of the frog myocardial fibers following traumatization. Arkh Anat Histol Embriol. 1961;40:65‐74. [PubMed] [Google Scholar]
- 141. Rumyantsev P. Post‐injury DNA synthesis, mitosis and ultrastructural reorganization of adult frog cardiac myocytes – an electron microscopic‐autoradiographic study. Z Zellforsch Und Mikroskopische Anat. 1973;139:431‐450. 10.1007/BF00306596. [DOI] [PubMed] [Google Scholar]
- 142. Evans BJ, Carter TF, Greenbaum E, et al. Genetics, morphology, advertisement calls, and historical records distinguish six new polyploid species of African clawed frog (Xenopus, Pipidae) from West and Central Africa. PLoS One. 2015;10:e0142823. 10.1371/journal.pone.0142823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143. La Mesa G, Bernardini S, Cannata SM, Filoni S. Effects of thyroxine and propyl‐thiouracil on hindlimb regeneration of larval Xenopus laevis . Rouxs Arch Dev Biol. 1994;203:205–214. 10.1007/BF00636336. [DOI] [PubMed] [Google Scholar]
- 144. La Mesa G, Bernardini S, Cannata SM, Filoni S. Xenopus laevis tadpole limb regeneration in vivo and in vitro: thyroxine directly promotes blastemal cell proliferation and morphogenesis. Rouxs Arch Dev Biol. 1995;204:223–228. 10.1007/BF00208489. [DOI] [PubMed] [Google Scholar]
- 145. Aurora AB, Olson EN. Immune modulation of stem cells and regeneration. Cell Stem Cell. 2014;15:14‐25. 10.1016/j.stem.2014.06.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146. Mescher AL, Neff AW. Regenerative Medicine I. Vol. 93. Berlin, Heidelberg: Springer; 2005:39–66. 10.1007/b99966. [DOI] [Google Scholar]
- 147. Robert J, Ohta Y. Comparative and developmental study of the immune system in Xenopus. Dev Dyn. 2009;238:1249‐1270. 10.1002/dvdy.21891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148. Cotter JD, Storfer A, Page RB, Beachy CK, Voss SR. Transcriptional response of Mexican axolotls to Ambystoma tigrinum virus (ATV) infection. BMC Genomics. 2008;9:493. 10.1186/1471-2164-9-493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149. Godwin JW, Rosenthal N. Scar‐free wound healing and regeneration in amphibians: immunological influences on regenerative success. Differentiation. 2014;87(1‐2):66–75. 10.1016/j.diff.2014.02.002. [DOI] [PubMed] [Google Scholar]
- 150. Fukazawa T, Naora Y, Kunieda T, Kubo T. Suppression of the immune response potentiates tadpole tail regeneration during the refractory period. Development. 2009;136(14):2323–2327. 10.1242/dev.033985. [DOI] [PubMed] [Google Scholar]
- 151. Mescher AL, Neff AW, King MW. Inflammation and immunity in organ regeneration. Dev Comp Immunol. 2017;66:98–110. 10.1016/j.dci.2016.02.015. [DOI] [PubMed] [Google Scholar]
- 152. Godwin JW, Pinto AR, Rosenthal NA. Macrophages are required for adult salamander limb regeneration. Proc Natl Acad Sci U S A. 2013;110:9415‐9420. 10.1073/pnas.1300290110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153. Vinarsky V, Atkinson DL, Stevenson TJ, Keating MT, Odelberg SJ. Normal newt limb regeneration requires matrix metalloproteinase function. Dev Biol. 2005;279(1):86–98. 10.1016/j.ydbio.2004.12.003. [DOI] [PubMed] [Google Scholar]
- 154. Yang EV, Gardiner DM, Carlson MRJ, Nugas CA, Bryant SV. Expression of Mmp‐9 and related matrix metalloproteinase genes during axolotl limb regeneration. Dev Dyn. 1999;216(1):2–9. . [DOI] [PubMed] [Google Scholar]
- 155. Petrie TA, Strand NS, Tsung‐Yang C, Rabinowitz JS, Moon RT. Macrophages modulate adult zebrafish tail fin regeneration. Development. 2014;141:2581‐2591. 10.1242/dev.098459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156. Tsarouchas TM, Wehner D, Cavone L, et al. Dynamic control of proinflammatory cytokines Il‐1β and Tnf‐α by macrophages in zebrafish spinal cord regeneration. Nat Commun. 2018;9:4670. 10.1038/s41467-018-07036-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157. Simkin J, Sammarco MC, Marrero L, et al. Macrophages are required to coordinate mouse digit tip regeneration. Development. 2017;144(21):3907–3916. 10.1242/dev.150086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158. Aurora AB, Porrello ER, Tan W, et al. Macrophages are required for neonatal heart regeneration. J Clin Invest. 2014;124:1382‐1392. 10.1172/JCI72181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159. Sattler S, Rosenthal N. The neonate versus adult mammalian immune system in cardiac repair and regeneration. Biochim Biophys Acta – Mol Cell Res. 2016;1863(7, Part B):1813–1821. 10.1016/j.bbamcr.2016.01.011. [DOI] [PubMed] [Google Scholar]
- 160. Molawi K, Wolf Y, Kandalla PK, et al. Progressive replacement of embryo‐derived cardiac macrophages with age. J Exp Med. 2014;211(11):2151–2158. 10.1084/jem.20140639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161. Pinto AR, Godwin JW, Chandran A, et al. Age‐related changes in tissue macrophages precede cardiac functional impairment. Aging (Albany NY). 2014;6(5):399–413. 10.18632/aging.100669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162. Han C, Nie Y, Lian H, et al. Acute inflammation stimulates a regenerative response in the neonatal mouse heart. Cell Res. 2015;25:1137–1151. 10.1038/cr.2015.110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163. Liechty KW, Kim HB, Adzick NS, Crombleholme TM. Fetal wound repair results in scar formation in interleukin‐10‐deficient mice in a syngeneic murine model of scarless fetal wound repair. J Pediatr Surg. 2000;35(6):866–873. 10.1053/jpsu.2000.6868. [DOI] [PubMed] [Google Scholar]
- 164. Peranteau WH, Zhang L, Muvarak N, et al. IL‐10 overexpression decreases inflammatory mediators and promotes regenerative healing in an adult model of scar formation. J Invest Dermatol. 2008;128(7):1852–1860. 10.1038/sj.jid.5701232. [DOI] [PubMed] [Google Scholar]
- 165. Gordon A, Kozin ED, Keswani SG, et al. Permissive environment in postnatal wounds induced by adenoviral‐mediated overexpression of the anti‐inflammatory cytokine interleukin‐10 prevents scar formation. Wound Repair Regen. 2008;16(1):70–79. 10.1111/j.1524-475X.2007.00326.x. [DOI] [PubMed] [Google Scholar]
- 166. Kaur K, Sharma AK, Singal PK. Significance of changes in TNF‐α and IL‐10 levels in the progression of heart failure subsequent to myocardial infarction. Am J Physiol – Hear Circ Physiol. 2006;291(1):H106–H113. 10.1152/ajpheart.01327.2005. [DOI] [PubMed] [Google Scholar]
- 167. Jung M, Ma Y, Iyer RP, et al. IL‐10 improves cardiac remodeling after myocardial infarction by stimulating M2 macrophage polarization and fibroblast activation. Basic Res Cardiol. 2017;112:33. 10.1007/s00395-017-0622-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168. Wu L, Dalal R, Cao CD, et al. IL‐10‐producing B cells are enriched in murine pericardial adipose tissues and ameliorate the outcome of acute myocardial infarction. Proc Natl Acad Sci U S A. 2019;116:21673‐21684. 10.1073/pnas.1911464116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169. Lieschke GJ, Trede NS. Fish immunology. Curr Biol. 2009;19(16):PR678–R682. 10.1016/j.cub.2009.06.068. [DOI] [PubMed] [Google Scholar]
- 170. Trede NS, Langenau DM, Traver D, Look AT, Zon LI. The use of zebrafish to understand immunity. Immunity. 2004;20(4):367–379. 10.1016/S1074-7613(04)00084-6. [DOI] [PubMed] [Google Scholar]
- 171. Secombes CJ, Belmonte R. Overview of the fish adaptive immune system. Fish Vaccines. Birkhäuser Advances in Infectious Diseases Basel: Springer; 2016;35–52. 10.1007/978-3-0348-0980-1_2. [DOI] [Google Scholar]
- 172. Hui SP, Sheng DZ, Sugimoto K, et al. Zebrafish regulatory T cells mediate organ‐specific regenerative programs. Dev Cell. 2017;43(6):659–672.E5. 10.1016/j.devcel.2017.11.010. [DOI] [PubMed] [Google Scholar]
- 173. Castiglioni A, Corna G, Rigamonti E, et al. FOXP3+ T cells recruited to sites of sterile skeletal muscle injury regulate the fate of satellite cells and guide effective tissue regeneration. PLoS One. 2015;10(6):e0128094. 10.1371/journal.pone.0128094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 174. Dombrowski Y, O'Hagan T, DIttmer M, et al. Regulatory T cells promote myelin regeneration in the central nervous system. Nat Neurosci. 2017;20:674–680. 10.1038/nn.4528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 175. Li J, Tan J, Martino MM, Lui KO. Regulatory T‐cells: potential regulator of tissue repair and regeneration. Front Immunol. 2018;9:585. 10.3389/fimmu.2018.00585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 176. Li J, Yang KY, Tam RCY, et al. Regulatory T‐cells regulate neonatal heart regeneration by potentiating cardiomyocyte proliferation in a paracrine manner. Theranostics. 2019;9:4324‐4341. 10.7150/thno.32734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 177. Tang TT, Yuan J, Zhu ZF, et al. Regulatory T cells ameliorate cardiac remodeling after myocardial infarction. Basic Res Cardiol. 2012;107:232. 10.1007/s00395-011-0232-6. [DOI] [PubMed] [Google Scholar]
- 178. Li J, Liang C, Yang KY, et al. Specific ablation of CD4+ T‐cells promotes heart regeneration in juvenile mice. Theranostics. 2020;10:8018‐8035. 10.7150/thno.42943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 179. Seifert AW, Kiama SG, Seifert MG, Goheen JR, Palmer TM, Maden M. Skin shedding and tissue regeneration in African spiny mice (Acomys). Nature. 2012;489:561–565. 10.1038/nature11499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 180. Brant JO, Lopez M‐C, Baker HV, Barbazuk WB, Maden M. A comparative analysis of gene expression profiles during skin regeneration in Mus and Acomys. PLoS One. 2015;10:e0142931. 10.1371/journal.pone.0142931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 181. Simkin J, Gawriluk TR, Gensel JC, Seifert AW. Macrophages are necessary for epimorphic regeneration in African spiny mice. Elife. 2017;6:e24623. 10.7554/eLife.24623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 182. Clarke A, Pörtner H‐O. Temperature, metabolic power and the evolution of endothermy. Biol Rev. 2010;85(4):703–727. 10.1111/j.1469-185X.2010.00122.x. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary TPR File


