Abstract
High blood pressure and consequential cardiovascular diseases are among the top causes of death worldwide. The apelinergic (APJ) system has emerged as a promising target for the treatment of cardiovascular issues, especially prevention of ischemia reperfusion (IR) injury after a heart attack or stroke. However, rapid degradation of the endogenous apelin peptides in vivo limits their use as therapeutic agents. Here, we study the effects of simple homologue substitutions, i.e. incorporation of non-canonical amino acids l-cyclohexylalanine (l-Cha) and l-homoarginine (l-hArg), on the proteolytic stability of pyr-1-apelin-13 and apelin-17 analogues. The modified 13-mers display up to 40 times longer plasma half-life than native apelin-13 and in preliminary in vivo assay show moderate blood pressure-lowering effects. The corresponding apelin-17 analogues show pronounced blood pressure-lowering effects and up to a 340-fold increase in plasma half-life compared to the native apelin-17 isoforms, suggesting their potential use in the design of metabolically stable apelin analogues to prevent IR injury.
Cyclohexylalanine- and homoarginine-substituted apelin analogues are demonstrated to be metabolically stable APJR agonistic peptides with hypotensive effect.
Introduction
Cardiovascular diseases are one of the leading causes of mortality in the world, accounting for 31% of deaths, globally.1,2 However, the human body is equipped with the apelinergic system, an endogenous cardioprotective hormone system which depends on the G-protein coupled apelin receptor (APJR) and the natural substrates, apelin and elabela.3–6 Apelin peptides have been shown physiologically as influential regulators of various metabolic functions, including cardiovascular output,7 fluid homeostasis,8 and carbohydrate and fat metabolism.9 In addition, various types of cancers, such as gastroesophageal, glioblastoma, prostate, colon, and oral squamous cell carcinoma, show elevated expression of apelin. Apelin peptides in cancer diseases play a role in tumor neo-angiogenesis through induction of cell proliferation and migration, and the high apelin expression suggests its use as a biomarker in cancerous tissues.10 Recently, researchers have also found that the apelin system is involved in age-associated sarcopenia, where apelin levels were negatively correlated with age in rodents and humans.11
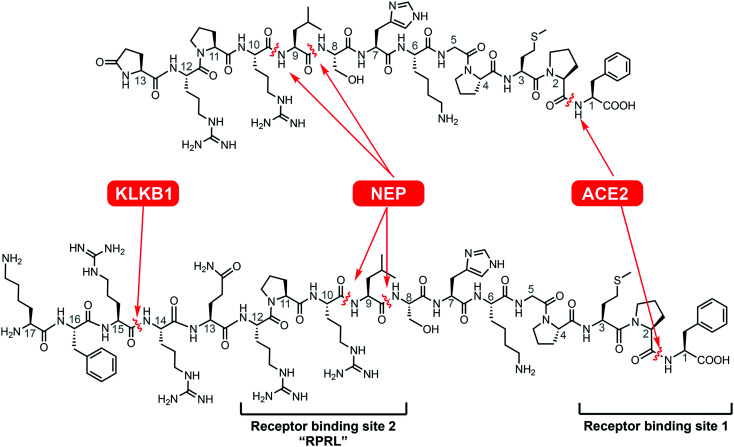
This endogenous hormone is initially expressed as a 77-amino acid long prepropeptide, which is further processed into active apelin-55, apelin-36, apelin-17 and apelin-13/pyr-1-apelin-13 isoforms.12,13 However, in circulation, the biological half-life of the shorter isoforms is usually quite limited (<5 min) due to the activity of various proteases (Scheme 1).14 Previously, we have studied the rapid degradation of apelin by angiotensin-converting enzyme 2 (ACE2) at the C-terminal phenylalanine residue,15 neprilysin (neutral endopeptidase 24.11, NEP) at the “RPRL” region,12,16 and plasma kallikrein (KLKB1).17 The latter protease cleaves apelin-17 between Arg14/Arg15 (Scheme 1). Various strategies have been tested to enhance proteolytic stability of apelin-related peptides, including insertion of non-canonical amino acids,18d-amino acids,19,20 and N- and C- methylation.12 Two apelin analogues incorporating non-canonical amino acids have been reported to be resistant to cleavage by ACE2, while still remaining active against ischemia reperfusion injury both in vivo and ex vivo.21 In addition, internal lactamization has been used as a modification technique which results in conformational restriction and improved stability through prevention of peptidase action.22,23 To improve the pharmaceutical properties – plasma stability, cardiac inotropy, hypotensive effects, and binding affinity – of apelin peptides, acylation24,25 using fatty acids, PEGylation17,26 and more recently the addition of a fluorocarbon chain to the N-terminus of apelin peptides20 have been used. Respectively modified apelin analogues have been shown effective in circulatory diseases including alloimmune-mediated vasculopathy and abdominal aortic aneurysm27,28 and in hyponatremia.29 In addition to peptide-based apelin analogues, non-peptide APJR agonists have also been synthesized. One of the first reported peptidomimetic small molecules was E339-3D6, which showed great selectivity against other GPCRs and reasonable affinity for the APJR.30 Nevertheless, in combination with lower receptor affinity to native apelin peptides and the molecule having a comparatively high molecular weight, E339-3D6 was not classed as drug-like. However, further studies have identified a promising small molecule designated CMF-019.31 This molecule possesses many desirable characteristics, such as low molecular weight (455 Da), high nanomolar APJR affinity, and increased cardiac contractility and vasodilation; allowing for exploration of novel syntheses of new small molecule apelin agonists.32 Combined research efforts led to peptidic and non-peptidic apelin agonists, which have shown increased metabolic stability and improved receptor binding abilities.
Scheme 1. Native apelin-13 and apelin-17 with different protease cleavage sites (KLKB1: plasma kallikrein, NEP: neprilysin, ACE2: angiotensin-converting enzyme 2).
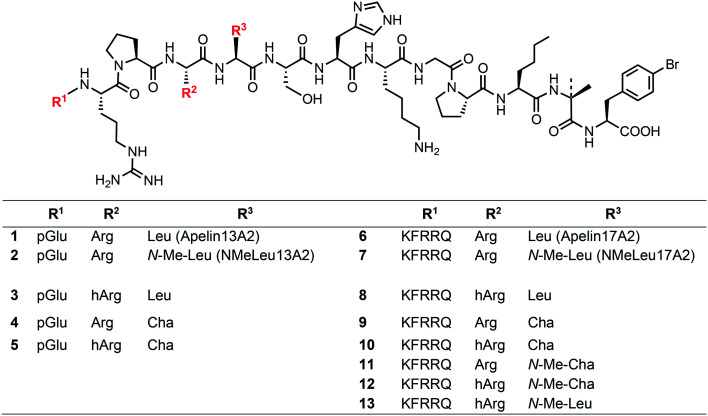
A recently published X-ray crystal structure of an inactive modified APJR in complex with an inactive peptidic derivative (AMG3054) suggests binding interactions of the receptor with substrates at a minimum of two sites.18 Two approaches, amino acid substitution and macrolactamization, were used in combination to rigidify the apelin analogue and enable better fit into the APJ-derived protein (Scheme 1). Among these modifications, l-cyclohexylalanine (l-Cha) and l-homoarginine (l-hArg) were introduced in positions 9 and 10 from the C-terminus within receptor binding site 2 (termed ‘RPRL’ according to the native amino acid sequence).18 Despite an apparent good fit, the impact upon agonistic vs. antagonistic interaction of AMG3054 on the native receptor was not disclosed in this publication. Our current study examines the possibility of metabolic stabilization and receptor activation of such homologue-substituted apelin peptides by dissecting the individual and combined binding contribution of these substitutions. Since these substitutions are within the RPRL binding motif, even minute spatial changes in this region will likely affect receptor binding and overall conformation change that defines the extent of downstream G-protein signaling. Starting from first generation analogues 1 and 6 (Scheme 2), already shown to possess higher proteolytic resistance,15 we synthesized nine derivatives, 3–5 (apelin-13 analogues) and 8–13 (apelin-17 analogues), incorporating methylation and l-Cha and/or l-hArg in positions 9/10. Using enzyme and human plasma assays, we determined the metabolic stability of these compounds and further characterized their receptor activation properties through means of Ca2+-mobilization capacity. In vivo (mouse) blood pressure tests showed significant and prolonged blood pressure-lowering effects of the apelin-17 series (compounds 8–13), suggesting that they can serve as candidates to assist the design of metabolically stable and physiologically active apelin analogues as therapeutic agents.
Scheme 2. Apelin derivatives studied in this paper: ACE2-resistant analogues (1, 6), NEP-stabilized compounds (2, 7), and current title apelin analogues (3–5, 8–13) implementing an ACE2-resistant C-terminus (l-p-bromophenylalanine, aminoisobutyric acid, l-norleucine). Pyroglutamic acid abbreviated as pGlu.
Results and discussion
SPPS synthesis
Starting from the ACE2-stable series (compounds 1 and 6),15 we attempted to achieve higher metabolic stabilization through relatively conservative homologue substitution. Inspired by the literature peptide AMG3054,18 it seemed interesting to determine whether l-hArg and/or l-Cha substitution would lead to increased proteolytic stability and improved receptor activation. This approach has the advantage of affordable and commercially available amino acids that can be readily incorporated in good yield through solid phase peptide synthesis (SPPS). Additionally, there is literature precedent that the use of l-homoarginine and l-cyclohexylalanine may have intrinsic advantages with respect to cardiovascular activity of the resulting analogues. l-Cha has been shown to increase regulatory function in body fluid and blood pressure homeostasis, and vasodilation activity when it was substituted for Phe in an atrial natriuretic peptide.33–35 On the other hand, l-hArg is an independent protective biomarker,36,37 where lower l-hArg plasma levels were correlated with renal failure38 and reduced nitric oxide bioavailability.39 It should also be noted that l-hArg can be formed metabolically through the replacement of ornithine for lysine in liver enzyme-catalyzed reactions in urea cycles40,41 or through l-arginine:glycine amidinotransferase (AGAT) catalysis,42 contributing to its role as a biomarker.
Apelin analogue sets 3–5 and 8–13 were synthesized on trityl-resin using Fmoc-chemistry. First, Fmoc-p-BrPhe-OH was loaded onto 2-chlorotrityl chloride resin and extended by solid phase peptide synthesis until the octapeptide stage, from the C-terminus, was obtained. Incorporation of the appropriate Arg/Leu modifications were completed after this stage, and the 13-mer and 17-mer analogues were extended to the tridecapeptide and heptadecapeptide stage, respectively. Since the peptides are also intended to address stability toward NEP cleavage, we compared their metabolic and physiological features to the most potent NEP-stabilized analogues from a previous series (2 and 7).12 All peptides were analyzed using LC-MS and MALDI-TOF and purified using an analytical HPLC.
In vitro NEP stability
First, the in vitro NEP stability of the Arg/Leu analogues was compared to the ACE2-resistant first generation analogues 1 and 6 and their NEP-stabilized pendants 2 and 7 (Fig. S1 and S2,†Table 1). Analogues were incubated with recombinant human NEP (rhNEP, Sino biological) at 37 °C for up to 24 h, and the extent of degradation was analyzed via LC-MS due to the overlapping elution patterns of peptide fragments. The ACE2-resistant (A2) analogues 1 and 6 showed comparable NEP degradation rates to native apelin isoforms, as previously reported.12 When examining the effects of Arg/Leu modifications, all new analogues (3–5, 8–10) showed improved proteolytic stability to NEP compared to the A2 analogues (Fig. S1 and S2,†Table 1). Overall, Arg/Leu-substituted apelin-17 analogues 8–13 showed increased stability compared to pyr-1-apelin-13 analogues 3–5. Substitutions with l-Cha and l-hArg, for both the pyr-1-apelin-13 and apelin-17 analogues, showed higher stability in the isolated NEP assay, but did not show as much resistance as the NEP-stabilized N-Me-Leu analogues 2 and 7.
Metabolic features of reference apelins and homologue-substituted apelins. Experiments were done in triplicates. Errors represent standard error of the mean (S.E.M.).
| Analogue | t1/2 human NEP (h) | t1/2 human plasma (h) | t1/2 mice plasma (h) | Plasma protein binding efficacy (%) | |
|---|---|---|---|---|---|
| 1 | Apelin13A2 | 10.31 ± 0.01 | 0.81 ± 0.20 | ||
| 2 | NMeLeu13A2 | 40.27 ± 0.01 | 3.54 ± 0.12 | ||
| 3 | hArg13A2 | 19.01 ± 0.01 | 1.31 ± 0.42 | ||
| 4 | Cha13A2 | 22.07 ± 0.01 | 1.77 ± 0.34 | ||
| 5 | hArgCha13A2 | 21.91 ± 0.02 | 2.58 ± 0.19 | ||
| 6 | Apelin17A2 | 10.95 ± 0.03 | 0.90 ± 0.17 | 57 ± 10 | |
| 7 | NMeLeu17A2 | >48 | 8.29 ± 0.21 | 0.99 ± 0.11 | 55 ± 10 |
| 8 | hArg17A2 | 40.59 ± 0.01 | 3.06 ± 0.04 | ||
| 9 | Cha17A2 | 47.03 ± 0.01 | 4.34 ± 0.03 | ||
| 10 | hArgCha17A2 | 46.39 ± 0.01 | 6.28 ± 0.02 | 0.52 ± 0.12 | 65 ± 9 |
| 11 | NMeCha17A2 | 2.90 ± 0.03 | 0.44 ± 0.07 | 68 ± 8 | |
| 12 | hArgNMeCha17A2 | 3.41 ± 0.05 | 0.36 ± 0.25 | 69 ± 8 | |
| 13 | hArgNMeLeu17A2 | 5.63 ± 0.08 | 0.38 ± 0.21 |
In vitro analogue stability – human plasma
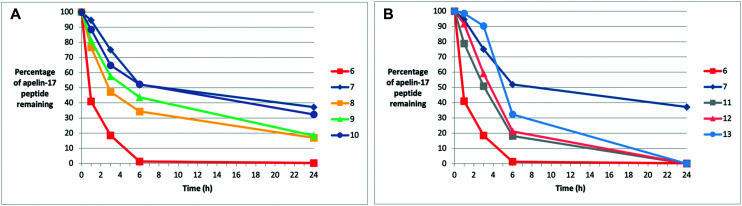
We then examined the in vitro plasma stability of these analogues (Fig. 1, Table 1). Triplicate experiments were performed in human blood plasma and the extent of degradation was analyzed via LC-MS. Arg/Leu substitution had relatively similar impacts on the different apelin isoforms. All pyr-1-apelin-13 (Fig. S3†) and apelin-17 analogues (Fig. 1) showed enhanced stability compared to the ACE2-resistant analogues 1 and 6, with apelin-17 analogues 8–13 showing higher stability compared to the pyr-1-apelin-13 isoforms 3–5. This suggests that NEP proteolysis appears to be more significant in the degradation of pyr-1-apelin-13 analogues than the apelin-17 counterparts. However, compared to the inherently NEP-stabilized N-Me-Leu analogues (2 and 7), all methylated and homologue-substituted pyr-1-apelin-13 and apelin-17 analogues (3–5, 8–13) showed lower levels of plasma stability. This suggests that the modifications of the side-chains, regardless of the bulkiness, had lower ability in stabilizing the peptide against proteases, compared to modifications on the backbone alone. The most stable apelin-17 analogue 10 (hArgCha17A2) still displayed 25% decreased plasma half-life compared to the NEP-stabilized analogue 7 (Table 1).
Fig. 1. In vitro human plasma degradation trends for apelin-17 analogues. (A) Comparison of (6) ACE2-resistant, (7) NMeLeu17A2, and (8–10) novel Arg/Leu substituted analogues. (B) Comparison of (6) ACE2-resistant, (7) NMeLeu17A2, and (11–13) NMeArg/Leu substituted analogues.
In vitro analogue stability – mice plasma
In addition, the in vitro murine plasma stability of four target analogues was tested and compared to the NEP-stabilized analogue 7 (Fig. S4,†Table 1). Triplicate experiments were performed in murine blood plasma and analyzed via LC-MS to determine the extent of degradation. Generally, proteolytic stability is significantly lower due to the higher metabolic rate and renal clearance in rodents compared to humans.43 Compared to the NEP-stabilized NMeLeu17A2 analogue 7 (∼60 min), all the apelin-17 analogues (10–13) showed lower rates of proteolytic stability in murine plasma (≤40 min), with analogue 10 displaying the highest stability of the apelin-17 isoforms. Nevertheless, the enhanced in vitro plasma stabilities of these Arg/Leu-modified analogues to both NEP and non-specific plasma proteolysis, compared to the ACE2-resistant isoforms, were encouraging for exploration of the physiological activities of each analogue in future mouse model studies.
APJR receptor radioligand binding experiments
Next, the newly synthesized pyr-1-apelin-13 (3–5) and apelin-17 (8–13) analogues were compared with the ACE2-resistant (1, 6) and NEP-stabilized (2, 7) to determine their ability to compete with [125I]-pyr-1-apelin-13 (0.2 nM) binding on membrane preparations from CHO cells stably expressing the wild-type rat apelin receptor-EGFP. As shown in Table 2, all pyr-1-apelin-13 (3–5) analogues showed improved competitive binding, in comparison with the radiolabeled ligand from the rat apelin receptor EGFP. These analogues showed lower pKi values compared to the ACE2-resistant (pKi = 8.70 ± 0.06 nM) and the NEP-stabilized analogues (pKi = 10.00 ± 0.17 nM). In addition, the apelin-17 (8–13) analogues showed comparable pKi values at the nanomolar range compared to the ACE2-resistant (pKi = 9.72 ± 0.01 nM) and NEP-stabilized (pKi = 9.35 ± 0.09 nM) isoforms. In conclusion, these data show that all the pyr-1-apelin-13 and apelin-17 analogues were orthosteric ligands as they were able to interact with the rat APJR at the same binding site as the natural ligand.
Receptor binding and physiological response data of reference apelins and homologue-substituted apelins. Experiments were done in triplicates. Errors represent standard error of the mean (S.E.M.).
| Analogue | pKi binding affinity (nM) | pEC50 Ca2+-mobilization (nM) | ΔMABP (mmHg) | Duration of hypotensive effect (min) | |
|---|---|---|---|---|---|
| 1 | Apelin13A2 | 8.70 ± 0.06 | 8.37 ± 0.14 | 23.88 ± 7.60 | 60a |
| 2 | NMeLeu13A2 | 10.00 ± 0.17 | 8.17 ± 0.12 | 9.52 ± 5.60 | 26 |
| 3 | hArg13A2 | 9.17 ± 0.07 | 8.66 ± 0.17 | 26.44 ± 12.60 | 60a |
| 4 | Cha13A2 | 9.24 ± 0.10 | 8.62 ± 0.13 | 0.23 ± 5.30 | 15 |
| 5 | hArgCha13A2 | 9.43 ± 0.01 | 8.43 ± 0.12 | 15.33 ± 7.70 | 57 |
| 6 | Apelin17A2 | 9.72 ± 0.01 | 8.37 ± 0.14 | 10.24 ± 2.60 | 33 |
| 7 | NMeLeu17A2 | 9.35 ± 0.09 | 8.56 ± 0.18 | 31.98 ± 5.90 | 60a |
| 8 | hArg17A2 | 9.08 ± 0.13 | 8.17 ± 0.12 | 40.45 ± 13.30 | 60a |
| 9 | Cha17A2 | 8.80 ± 0.13 | 8.42 ± 0.10 | 19.72 ± 6.00 | 60a |
| 10 | hArgCha17A2 | 8.96 ± 0.15 | 8.36 ± 0.11 | 44.90 ± 12.80 | 60a |
| 11 | NMeCha17A2 | 8.70 ± 0.11 | 7.79 ± 0.42 | 28.83 ± 8.00 | 60a |
| 12 | hArgNMeCha17A2 | 9.19 ± 0.08 | 7.14 ± 0.20 | 47.57 ± 4.60 | 60a |
| 13 | hArgNMeLeu17A2 | 8.85 ± 0.11 | 6.56 ± 0.17 | 34.69 ± 4.90 | 60a |
Hypotensive effect maintained for the duration of the experiment.
Receptor activation-Ca2+-mobilization assay
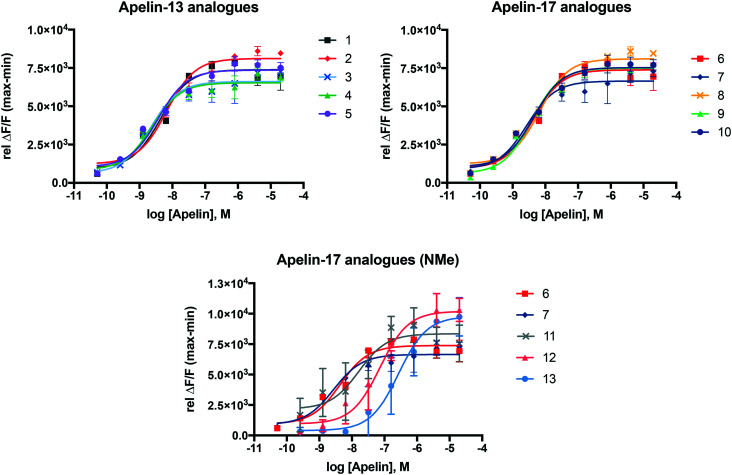
In order to study the extent to which newly derived analogues trigger downstream APJR activation, a fluorescence coupled calcium release assay was used with a recombinant human APJ-Ga16 receptor cell line. All new analogues (3–5, 8–13) showed comparable pEC50 values (Table 2), suggesting a similarly strong APJR-activation potential comparable to the ACE2-resistant (1, 6)15 and NEP-stabilized (2, 7)12 analogues from previous studies. These results were supported by the binding affinity values of these compounds (Table 2). The binding curves (Fig. 2 and S5†) also indicate the agonistic behaviour to the APJR.
Fig. 2. Concentration response (fluorescence) curves of novel analogues 3–5 and 8–13 in comparison to the ACE2-resistant (1, 6) and NEP-stabilized (2, 7) analogues.
Physiological test – blood pressure assay
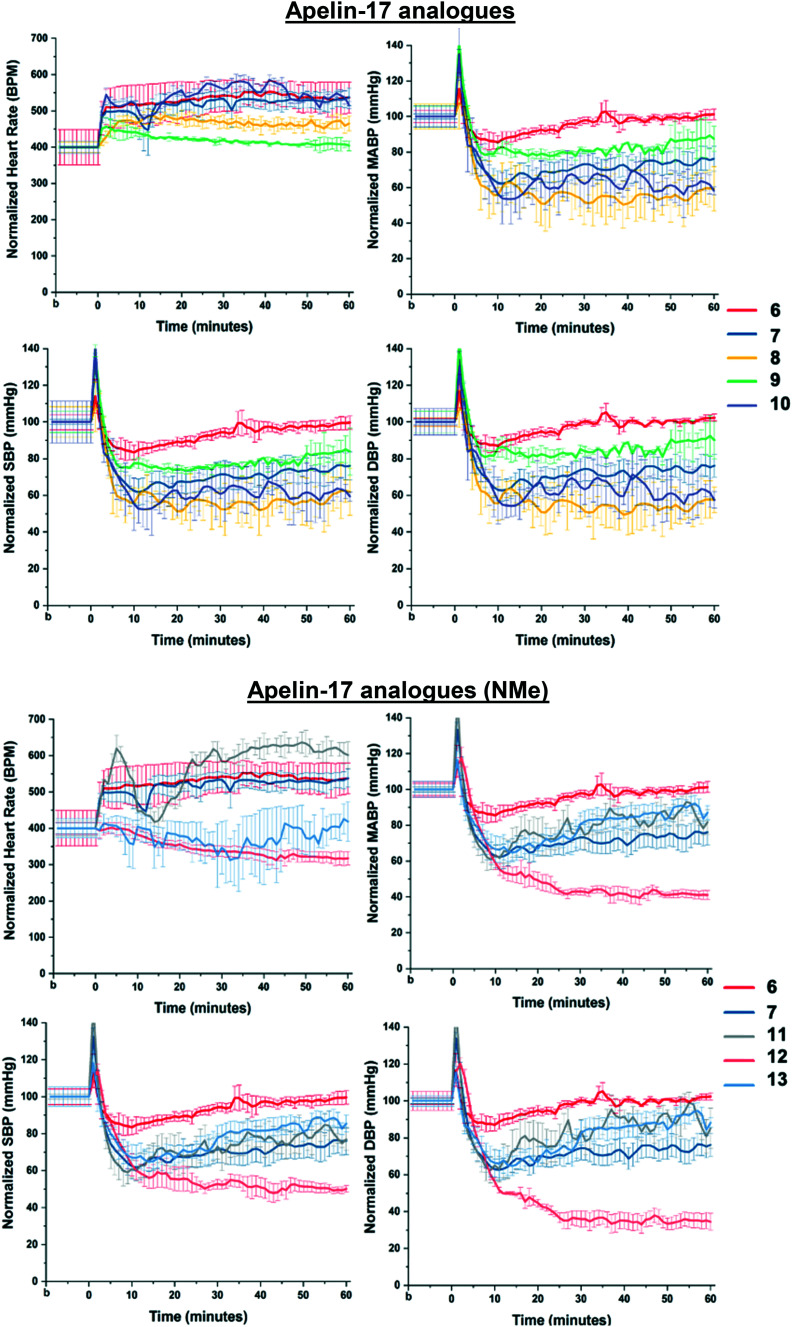
The newly synthesized analogues, 3–5 and 8–13, were tested for blood pressure-lowering abilities in anesthetized mice. Apelin peptide analogues were delivered systematically via the right internal jugular vein, while blood pressure (BP) and heart rate (HR) were continuously monitored in the aorta cannulated via the right carotid artery. The pyr-1-apelin-13 analogues 3–5 displayed marginal cardiovascular effects (Fig. S6†). However, all apelin-17 analogues 8–13 showed more pronounced cardio-physiological effects (Fig. 3). In fact, analogues 10 and 12 showed quick and stable increase in heart rate and prolonged blood pressure-lowering effects, respectively, which are even more profound than the most potent compound from our previous studies, analogue 7 (ref. 12) (Table 2). These results suggests that, in addition to enhanced binding to the APJR, l-hArg/l-Cha substitution is also cardio-physiologically well tolerated and beneficial for the further development of cardiovascular-active apelin analogues.
Fig. 3. In vivo heart rate (HR), mean arterial blood pressure (MABP), systolic blood pressure (SBP) and diastolic blood pressure (DBP) analyses, following intravenous injection of synthesized novel analogues (8–13) compared to the ACE2-resistant (6) and NEP-stabilized (7) isoforms in anesthetized mice (n = 3). Values represent mean ± S.E.M.
Plasma protein-binding assay
Lastly, target analogues, 8, 11, and 13, containing amino acid modifications at the neprilysin proteolytic cleavage site, generally display a slightly higher plasma protein binding (65 ± 8 to 69 ± 8%) than the native (apelin-17),44 the non-modified ACE2-resistant analogue 6 (57 ± 10%) and the NMeLeu17A2 analogue 7 (55 ± 10%) (Table 2). Considering the improved half-life (t1/2) of the apelin-17 analogues (8–13) in human plasma compared to the ACE2-resistant isoform 6, it appears that subtle homologue substitution increases lipophilicity of apelin and proteolytic stability through plasma protein binding.45 These results correlate well with the observed improvement in in vitro plasma stability of these analogues.
Conclusion
In the current study, apelin analogues modified at the NEP cleavage site (Arg9-Leu10 in apelin-17) were studied for their metabolic stability and cardiovascular features. Comparisons of the in vitro NEP and human- and mice- plasma stability between the novel analogues (3–5, 8–13) and previous ACE2-resistant (1, 6)15 and NEP-stabilized (2, 7)12 isoforms revealed that all newly synthesized analogues showed elevated metabolic stability. Blood pressure assays indicate stable and prolonged blood pressure-lowering effects of the apelin-17 analogue series, especially the 17-mer analogues incorporating both homologue-substitutions (analogues 8 and 12). In addition, the synthesized apelin derivatives showed comparable or improved receptor binding and activation based on the results of the Ca2+-mobilization assays and APJR radioligand binding experiments. Hence, these results indicate that relatively conservative homologue-substitution in positions 9/10 contributes to a moderate metabolic peptide stabilization, yielding cardiovascular-active apelin analogues as promising targets for further drug development for cardiovascular diseases.
Experimental section
General information
Solvents, reagents, and purification
Commercially-available biological and chemical reagents were purchased from: Caledon, Chem-Impex International Inc., Fisher Scientific Ltd., R&D Systems Sigma-Aldrich Canada, and VWR International, and used without further purification, unless otherwise stated. All solvents were of American Chemical Society (ACS) grade and used without further purification. Flame-dried glassware, used for anhydrous reactions, were subjected to a positive pressure of argon (Ar). Prior to use, anhydrous reaction solvents were distilled: dichloromethane was distilled over calcium hydride, and methanol was distilled over calcium hydride. ACS grade solvents (>99.0% purity) were used for flash column chromatography with no further purification. All reactions were monitored by thin layer chromatography (TLC) using aluminum plates containing a UV fluorescent indicator (normal SiO2, Merck 60 F254). Analytical, semi-preparative and preparative scale high performance liquid chromatography (HPLC) was performed on a Gilson instrument equipped with 322 pump heads, a model 171 diode array detector, a FC 203B fraction collector, and a Reodyne 7725i injector fitted with a 1000 μL sample loop. Deionized water was filtered through a Milli-Q reagent water filtration system. HPLC grade acetonitrile and deionized water were filtered through a Millipore filtration system under vacuum prior to use. Peptides were purified to ≥95% purity assessed by analytical reinjection.
Characterization
A Perspective Biosystems Voyager Elite MALDI-TOF MS, with a matrix consisting of 4-hydroxy-α-cyanocinnamic acid (HCCA), Kratos AEIMS-50, or Bruker 9.4 T Apex-Qe FTICR (high resolution, HRMS, MS/MS, Fig. S7–S15†) were used to record mass spectra (MS). LC-MS was performed on an Agilent Technologies 6130 LCMS instrument.
1. General apelin analog SPPS elongation method
A suspension of resin in DMF (5 mL) was bubbled under argon gas to swell for 20 min. The N-terminal Fmoc group was taken off by bubbling 20% piperidine in DMF (3 × 5 mL), then extensively washing with DMF (3 × 5 mL) after each individual deprotection. The extent of Fmoc-deprotection was monitored using TLC, where the dibenzofulvene-piperidine adduct appearing as a dark purple spot under UV. The coupling protocol consisted of DIPEA (2.2 equiv) being added to a solution of Fmoc-protected amino acid (1.1 equiv compared to resin loading), PyBOP (1.0 equiv), and HOBt (1.1 equiv) in DMF (5 mL) and stirred for 10 min. The resin was then washed with DMF (3 × 5 mL), and the extent of coupling was assessed by cleaving a small sample of resin with a solution of 95 : 2.5 : 2.5 TFA/TIPS/H2O for 1–2 h, and MALDI-TOF analysis. To end-cap any unreacted amines, a solution of 20% acetic anhydride in DMF (5 mL) was added to the resin and bubbled for 10 min under Ar gas. Thorough washes of the resin with DMG (3 × 5 mL) was done and the resin was subjected to either Fmoc-deprotection to continue elongation of the peptide, or rinsed with CH2Cl2 (3 × 5 mL) and dried thoroughly and stored at −20 °C under Ar gas.
2. General method for apelin resin cleavage
Resin-bound apelin analogue (0.05 mmol) was suspended in 95 : 2.5 : 2.5 TFA/TIPS/H2O with shaking under Ar atmosphere for 2–3 h. The cleaved resin was filtered through glass wool, rinsed thoroughly with TFA, and the solution was concentrated in vacuo. Cold diethyl ether (2 × 5 mL) was added to triturate the crude peptide. The diethyl ether was decanted into a 15 mL Falcon tube and briefly centrifuged to pellet any residual peptide. The diethyl ether pellet and triturated crude residue were pooled together and dissolved in 0.1% aqueous TFA.
3. Apelin analogue synthesis
The synthesis and characterization of the ACE2 resistant (1 and 6) and NEP resistant (2 and 7) analogues have been previously described,5 synthetic compounds were prepared according to literature protocols (ESI†). Through Fmoc-solid phase peptide synthesis (SPPS), three sets of apelin analogues were synthesized for pyr-1-apelin-13 (3–5), apelin-17 (8–10) and apelin-17 NMe (11–13), including l-hAr and/or l-Cha modifications. To ensure that all Arg/Leu modified apelin analogues were resistant to ACE2 enzymatic cleavage, p-bromo-phenylalanine (BrF), 2-aminoisobutyric acid (Aib), and norleucine (Nle), were previously incorporated in exchange for the natural C-terminal Phe, Pro and Met residue, respectively.5 The syntheses of these analogues were carried out using a 2-chlorotritylchloride resin (loading of 0.8 mmol g−1). Prelude X automated peptide synthesizer (Gyros protein technologies) was used to synthesize up to Ser, and subsequent amino acids (1.1 equiv compared to resin loading, 0.672 mmol) were attached in the following order by hand: apelin-13: R3, R2, Fmoc-Pro-OH, Fmoc-Arg(Pbf)-OH, and l-pyroglutamic acid (l-pGlu) and apelin-17: R3, R2, Fmoc-Pro-OH, Fmoc-Arg(Pbf)-OH, Fmoc-Gln(Trt)-OH, Fmoc-Arg(Pbf)-OH, Fmoc-Arg(Pbf)-OH, Fmoc-Phe-OH, and Fmoc-Lys(Boc)-H. Where R2 is Fmoc-Arg(Pbf)-OH or Fmoc-homoArg(Pbf)-OH, and R3 is Fmoc-Leu-OH, Fmoc-Cha-OH, Fmoc-N-Me-Leu-OH or Fmoc-N-Me-Cha-OH. Completeness of each coupling step was checked with matrix assisted laser desorption/ionization (MALDI) coupled with time of flight mass spectrometry (TOF-MS).
Apelin analogue 3 (hArg13A2)
A sample of resin-bound peptide (0.05 mmol) was cleaved as previously described. The resulting precipitate was purified using a C18 RP-HPLC analytical column, eluting at 13.2 min (Fig. S16†). After lyophilization, the desired peptide was isolated as a white solid (10 mg, 12%). Monoisotopic MW calculated for C72H115BrN22O16 811.4017, found high resolution (FTICR-ESI-MS) 811.4019 (M + 2H)2+.
Apelin analogue 4 (Cha13A2)
A sample of resin-bound peptide (0.05 mmol) was cleaved as previously described. The resulting precipitate was purified using a C18 RP-HPLC analytical column, eluting at 13.3 min (Fig. S16†). After lyophilization, the desired peptide was isolated as a white solid (11 mg, 13%). Monoisotopic MW calculated for C70H113BrN22O16 798.3939, found high resolution (FTICR-ESI-MS) 798.3916 (M + 2H)2+.
Apelin analogue 5 (hArgCha13A2)
A sample of resin-bound peptide (0.05 mmol) was cleaved as previously described. The resulting precipitate was purified using a C18 RP-HPLC analytical column, eluting at 13.1 min (Fig. S16†). After lyophilization, the desired peptide was isolated as a white solid (13 mg, 15%). Monoisotopic MW calculated for C73H117BrN22O16 818.4095, found high resolution (FTICR-ESI-MS) 818.4074 (M + 2H)2+.
Apelin analogue 8 (hArg17A2)
A sample of resin-bound peptide (0.05 mmol) was cleaved as previously described. The resulting precipitate was purified using a C18 RP-HPLC analytical column, eluting at 13.2 min (Fig. S17†). After lyophilization, the desired peptide was isolated as a white solid (12 mg, 14%). Monoisotopic MW calculated for C99H164BrN34O20 742.7343, found high resolution (FTICR-ESI-MS) 742.7329 (M + 3H)3+.
Apelin analogue 9 (Cha17A2)
A sample of resin-bound peptide (0.05 mmol) was cleaved as previously described. The resulting precipitate was purified using a C18 RP-HPLC analytical column, eluting at 13.1 min (Fig. S17†). After lyophilization, the desired peptide was isolated as a white solid (15 mg, 18%). Monoisotopic MW calculated for C97H162BrN34O20 734.0624, found high resolution (FTICR-ESI-MS) 734.0605 (M + 3H)3+.
Apelin analogue 10 (hArgCha17A2)
A sample of resin-bound peptide (0.05 mmol) was cleaved as previously described. The resulting precipitate was purified using a C18 RP-HPLC analytical column, eluting at 13.8 min (Fig. S17†). After lyophilization, the desired peptide was isolated as a white solid (12 mg, 14%). Monoisotopic MW calculated for C100H166BrN34O20 747.4062, found high resolution (FTICR-ESI-MS) 747.4050 (M + 3H)3+.
Apelin analogue 11 (NMeCha17A2)
A sample of resin-bound peptide (0.05 mmol) was cleaved as previously described. The resulting precipitate was purified using a C18 RP-HPLC analytical column, eluting at 21.3 min (Fig. S18†). After lyophilization, the desired peptide was isolated as a white solid (14 mg, 17%). Monoisotopic MW calculated for C100H163BrN34O20 747.4076, found high resolution (FTICR-ESI-MS) 747.4062 (M + 3H)3+.
Apelin analogue 12 (hArgNMeCha 17A2)
A sample of resin-bound peptide (0.05 mmol) was cleaved as previously described. The resulting precipitate was purified using a C18 RP-HPLC analytical column, eluting at 21.5 min (Fig. S18†). After lyophilization, the desired peptide was isolated as a white solid (14 mg, 17%). Monoisotopic MW calculated for C100H165BrN34O20 564.3025, found high resolution (FTICR-ESI-MS) 564.3103 (M + 4H)4+.
Apelin analogue 13 (hArgNMeLeu17A2)
A sample of resin-bound peptide (0.05 mmol) was cleaved as previously described. The resulting precipitate was purified using a C18 RP-HPLC analytical column, eluting at 21.2 min (Fig. S18†). After lyophilization, the desired peptide was isolated as a white solid (16 mg, 19%). Monoisotopic MW calculated for C98H161BrN34O20 554.3025, found high resolution (FTICR-ESI-MS) 554.2950 (M + 4H)4+.
4. In vitro protease experiments, neprilysin degradation assay
Neprilysin (SinoBiological, Wayne, PA, USA) was thawed on ice for 10 min. 1 μL of 0.76 mg mL−1 NEP was diluted with 350 μL Buffer NEP-B to 2 ng μL−1, then the solution was incubated at 37 °C for 10 min. Six solutions of each pyr-1-apelin-13 (3–5) and apelin-17 (8–10) analogues were prepared as 1 mM solution in H2O, then were added to NEP/Buffer NEP-B solution and incubated at 37 °C. Aliquots (1 μL) were removed at 0, 2, 10, 20, 48 h and quenched prior to LC-MS quantification. Assays were performed in triplicate (n = 3) for each time point.
5. Isolation and quantification of apelin peptides from plasma
Quantification of peptides in plasma were adapted from a published protocol.46 20 μL of plasma was pre-portioned into microcentrifuge tubes and incubated at 37 °C. 5 μL of apelin peptide (1 mM) was added to each 20 μL plasma vial and then incubated at 37 °C. The plasma solution was then quenched with 25 μL 6 M GuHCl, 300 μL 80% ACN/H2O and 5 μL of internal standard (1 mM Dans-YVG peptide). The plasma sample was then vortexed before centrifuging at 13 200g for 5 min. The supernatant was transferred to a 1 mL microcentrifuge tube and evaporated. Samples were reconstituted with 150 μL 0.1% acetic acid (v/v) in water and loaded onto a pre-equilibrated C18 spin column, which had previously been wet with 2 × 150 μL 30% acetonitrile in 0.1% aqueous TFA and 2 × 150 μL 0.1% aqueous acetic acid (v/v) respectively, centrifuging at 280g for 2 min between each 150 μL aliquot. Quenched plasma assays were centrifuged at 280g until the sample was loaded. The resultant filtrate was reloaded onto the column with 150 μL of 0.1% aqueous acetic acid (v/v) and centrifuged at 280g for another 2 min, then the filtrate was discarded. The desired peptides were washed with 2 × 150 μL 5% MeOH in water with 1% acetic acid (v/v) and eluted from the column using 100 μL 60% MeOH in water with 10% acetic acid (v/v). The eluate was injected and analyzed using LC-MS quantification. To analyze the remaining apelin peptides in plasma, incubations of pyr-1-apelin-13 (1–5) and apelin-17 (6–13) analogues were quenched at different time points (1, 3, 6, 24 h), worked up and analyzed as previously described. The ratio of apelin peptide to internal standard was calculated based on the area under the curve. The 0 h incubation ratio was used to compare the apelin peptide/internal standard ratios for the time experiments.
6. APJR radioligand binding experiments
Membrane preparations from CHO cells, which stably express the wild-type rat APJ receptor-EGFP, were prepared as described from previous literature.47 Crude membrane preparations (1 μg total membrane mass/assay) were incubated at 20 °C for 3 h with 0.2 nM [125I]-pyr-1-apelin-13 (monoiodinated on Lys8 with the Bolton–Hunter reagent, PerkinElmer, Wellesley, MA, USA) in binding buffer (50 mM HEPES, 5 mM MgCl2, pH 7.5, BSA 1%) alone or in the presence of the different analogues at various concentrations. The reaction was quenched with the addition of 4 mL of cold binding buffer, and the product was filtered on Whatman GF/C filters and washed with 5 mL of cold binding buffer. Quantification of radioactivity was determined using a Wizard 1470 Wallac γ counter (PerkinElmer, Turku, Finland). GraphPad Prism 6 was then used to analyze the binding experiment data.
7. Ca2+-mobilization assay
A 96-well assay plate of frozen Chem-5 cells stably expressing the human APJ GPCR (Ready-to-Assay™ APJ assay, EMDMillipore, Burlington, MA) was thawed according to the manufacturer's protocol. Cells were loaded for 1 h with FLIPR Calcium 6-QF fluorescent indicator dye (Molecular Devices, USA) in assay buffer [Hanks balanced salt solution (HBSS), 20 mM HEPES, 0.2% DMSO, 2.5 mM probenecid, pH 7.6], washed three times with assay buffer, then returned to the incubator for 10 min before the assay on a fluorometric imaging plate reader (FLIPR; Molecular Devices, Sunnyvale, CA, USA). Maximum change in fluorescence over baseline was used to determine agonist response. Dose–response curve data were fitted to a four-parameter logistic equation using PRISM (GraphPad, USA) from which the pEC50 values were calculated.
8. Blood pressure assays
All animal studies were conducted according to the Canadian Council for Animal Care guidelines and approved by the Animal Care and Use Committee at the University of Alberta. Male wildtype mice purchased from Jackson Laboratories (Bar Harbor, ME) were anesthetized with 1.5% isoflurane/oxygen. The body temperature was maintained at 36 °C by a heating pad and monitored. A PV loop catheter (model 1.2F from Scisense, Transonic) was used to cannulate the aorta via the right carotid artery, in order to continuously record arterial blood pressure and heart rate (LabScribe 2.0, Scisense). Peptides 1–13 (1.4 μM kg−1 body weight) or the same volume of saline was injected via the right jugular vein (n = 3). Results are reported as heart rate (HR), mean arterial blood pressure (MABP), systolic blood pressure (SBP), and diastolic blood pressure (DBP) ± S.E.M. Blood pressure traces were analyzed with LabScribe2 (iWorx Systems Inc., Dover, NH), and plotted with Origin 2018 (OriginLab, Northampton, MA).
9. Plasma protein binding assay
This assay was ran following a published protocol.44,48 Briefly, 1 mM DMSO stock solutions of each analogue were diluted to a final concentration of 100 μM, with protease inhibitor stabilized (EDTA and MLN-4760) 55% pooled human plasma. 200 μL of this solution was dialyzed in a 96-well equilibrium dialysis apparatus against 200 μL of PBS buffer (pH 7.4) at 37 °C and 120 rpm for 3 h. Thereafter, 25 μL of retentate was diluted with 25 μL of PBS buffer, and 25 μL of dialysate was diluted with 25 μL of pooled plasma, and cooled on ice for 10 min. All samples were quenched with 10% TFA in acetonitrile (200 μL), and 1 mM Dans-YVG peptide (5 μL) was added (internal standard). Samples were vortexed and centrifuged (11 200g) for 5 min. The supernatant was transferred to another microcentrifuge tube and evaporated, yielding a residue which was then reconstituted in 100 μL of 0.1% aqueous TFA. C18 spin columns (pre-equilibrated) were used (see point 5) prior to quantification with LC-MS.
Conflicts of interest
The authors do not claim any immediate conflicts of interest.
Supplementary Material
Acknowledgments
We thank Jing Zheng, Béla Reiz, Dr. Angelina Morales-Izquierdo, and Dr. Randy Whittal (University of Alberta Mass Spectrometry Facility) for assistance with mass spectrometry characterization and analyses of peptides and peptide fragments. We are grateful to Gareth Lambkin for his assistance with the Ca2+-mobilization assays. This research was greatly supported by the Canadian Institutes of Health Research (CIHR Grant 136921), the Natural Sciences and Engineering Council of Canada (NSERC), Alberta Innovates Health Solutions (CF, AIHS), and the French National Institute for Health and Medical Research (INSERM) and the College de France.
Electronic supplementary information (ESI) available. See DOI: 10.1039/d1md00120e
References
- Guo R. Rogers O. Nair S. Targeting apelinergic system in cardiometabolic disease. Curr. Drug Targets. 2017;18:1785–1791. doi: 10.2174/1389450117666160613105152. [DOI] [PubMed] [Google Scholar]
- Finegold J. A. Asaria P. Francis D. sP. Mortality from ischaemic heart disease by country, region, and age: Statistics from World Health Organisation and United Nations. Int. J. Cardiol. 2013;168:934–945. doi: 10.1016/j.ijcard.2012.10.046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O'Dowd B. F. Heiber M. Chan A. Heng H. H. Tsui L.-C. Kennedy J. L. Shi X. Pteronis A. George S. R. Nguyen T. A. A human gene that shows identity with the gene encoding the angiotensin receptor is located on chromosome 11. Gene. 1993;136:355–360. doi: 10.1016/0378-1119(93)90495-o. [DOI] [PubMed] [Google Scholar]
- Yang P. Maguire J. J. Davenport A. P. Apelin, Elabela/Toddler, and biased agonists as novel therapeutic agents in the cardiovascular system. Trends Pharmacol. Sci. 2015;36:355–360. doi: 10.1016/j.tips.2015.06.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhong J. C. Zhang Z. Z. Wang W. McKinnie S. M. K. Vederas J. C. Oudit G. Y. Targeting the apelin pathway as a novel therapeutic approach for cardiovascular diseases. Biochim. Biophys. Acta, Mol. Basis Dis. 2017;1863:1942–1950. doi: 10.1016/j.bbadis.2016.11.007. [DOI] [PubMed] [Google Scholar]
- Pauli A. Norris M. L. Valen E. Chew G. L. Gagnon J. A. Zimmerman S. Mitchell A. Ma J. Dubrulle J. Reyon D. Toddler: an embryonic signal that promotes cell movement via apelin receptors. Science. 2014;343:1248636. doi: 10.1126/science.1248636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bertrand C. Valet P. Castan-Laurell I. Apelin and energy metabolism. Front. Physiol. 2015;6:115. doi: 10.3389/fphys.2015.00115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Galanth C. Hus-Citharel A. Li B. Llorens-Cortès C. Apelin in the control of body fluid homeostasis and cardiovascular functions. Curr. Pharm. Des. 2012;18:789–798. doi: 10.2174/138161212799277770. [DOI] [PubMed] [Google Scholar]
- Folino A. Montarolo P. G. Samaja M. Rastaldo R. Effects of apelin on the cardiovascular system. Heart Failure Rev. 2015;20:505–518. doi: 10.1007/s10741-015-9475-x. [DOI] [PubMed] [Google Scholar]
- Yang Y. Lv S.-Y. Ye W. Zhang L. Apelin/APJ system and cancer. Clin. Chim. Acta. 2016;457:112–116. doi: 10.1016/j.cca.2016.04.001. [DOI] [PubMed] [Google Scholar]
- Vinel C. Lukjanenko L. Batut A. Deleruyelle S. Pradère J. P. Le Gonidec S. Dortignac A. Geoffre N. Pereira O. Karaz S. Lee U. Camus M. Chaoui K. Mouisel E. Bigot A. Mouly V. Vigneau M. Pagano A. F. Chopard A. Pillard F. Guyonnet S. Cesari M. Burlet-Schiltz O. Pahor M. Feige J. N. Vellas B. Valet P. Dray C. The exerkine apelin reverses age-associated sarcopenia. Nat. Med. 2018;24:1360–1371. doi: 10.1038/s41591-018-0131-6. [DOI] [PubMed] [Google Scholar]
- McKinnie S. M. K. Wang W. Fischer C. McDonald T. Kalin R. K. Iturrioz X. Llorens-Cortès C. Oudit G. Y. Vederas J. C. Synthetic modification with the “RPRL” region of apelin peptides: impact on cardiovascular activity and stability to neprilysin and plasma degradation. J. Med. Chem. 2017;60:6408–6427. doi: 10.1021/acs.jmedchem.7b00723. [DOI] [PubMed] [Google Scholar]
- Pitkin S. L. Maguire J. J. Bonner T. I. Davenport A. P. International Union of Basic and Clinical Pharmacology. LXXIV. Apelin receptor nomenclature, distribution, pharmacology, and function. Pharmacol. Rev. 2010;62:331–342. doi: 10.1124/pr.110.002949. [DOI] [PubMed] [Google Scholar]
- Japp A. G. Cruden N. L. Amer D. A. B. Li V. K. Y. Goudie E. B. Johnston N. R. Sharma S. Neilson I. Webb D. J. Megson I. L. Flapan A. D. Newby D. E. Vascular effects of apelin in vivo in man. J. Am. Coll. Cardiol. 2008;52:908–913. doi: 10.1016/j.jacc.2008.06.013. [DOI] [PubMed] [Google Scholar]
- Wang W. McKinnie S. M. K. Farhan M. Paul M. McDonald T. McLean B. Llorens-Cortès C. Hazra S. Murray A. G. Vederas J. C. Oudit G. Y. Angiotensin-converting enzyme 2 metabolizes and partially inactivates pyr-apelin-13 and apelin-17: physiological effects in the cardiovascular system. Hypertension. 2016;68:365–377. doi: 10.1161/HYPERTENSIONAHA.115.06892. [DOI] [PubMed] [Google Scholar]
- McKinnie S. M. K. Fischer C. Tran K. M. H. Wang W. Mosquera F. Oudit G. Y. Vederas J. C. The metalloprotease neprilysin degrades and inactivates apelin peptides. ChemBioChem. 2016;17:1495–1498. doi: 10.1002/cbic.201600244. [DOI] [PubMed] [Google Scholar]
- Fischer C. Lamer T. Wang W. McKinnie S. M. K. Iturrioz X. Llorens-Cortès C. Oudit G. Y. Vederas J. C. Plasma kallikrein cleaves and inactivates apelin-17: Palmitoyl- and PEG-extended apelin-17 analogs as metabolically stable blood pressure-lowering agents. Eur. J. Med. Chem. 2019;166:119–124. doi: 10.1016/j.ejmech.2019.01.040. [DOI] [PubMed] [Google Scholar]
- Ma Y. Yue Y. Ma Y. Zhang Q. Zhou Q. Song Y. Shen Y. Li X. Ma X. Li C. Hanson M. A. Han G. W. Sickmier E. A. Swaminath G. Zhao S. Stevens R. C. Hu L. A. Zhong W. Zhang M. Xu F. Structural basis for apelin control of the human apelin receptor. Structure. 2017;25:858–866. doi: 10.1016/j.str.2017.04.008. [DOI] [PubMed] [Google Scholar]
- Murza A. Parent A. Besserer-Offroy E. Tremblay H. Karadereye F. Beaudet N. Leduc R. Sarret P. Marsault É. Elucidation of the structure-activity relationships of apelin: influence of unnatural amino acids on binding, signaling, and plasma stability. ChemMedChem. 2011;7:318–325. doi: 10.1002/cmdc.201100492. [DOI] [PubMed] [Google Scholar]
- Gerbier R. Alvear-Perez R. Margathe J.-F. Flahault A. Couvineau P. Gao J. De Mota N. Dabire H. Li B. Ceraudo E. Hus-Citharel A. Esteoulle L. Bisoo C. Hibert M. Berdeaux A. Iturrioz X. Bonnet D. Llorens-Cortès C. Development of original metabolically stable apelin-17 analogs with diuretic and cardiovascular effects. FASEB J. 2017;31:687–700. doi: 10.1096/fj.201600784R. [DOI] [PubMed] [Google Scholar]
- Wang W. McKinnie S. M. Patel V. B. Haddad G. Wang Z. Zhabyeyey P. Das S. K. Basu R. McLean B. Kandalam V. Penninger J. M. Kassiri Z. Vederas J. C. Murray A. G. Oudit G. Y. Loss of apelin exacerbates myocardial infarction adverse remodeling and ischemia-reperfusion injury: therapeutic potential of synthetic apelin analogues. J. Am. Heart Assoc. 2013;3:e000249. doi: 10.1161/JAHA.113.000249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamada J. Kimura J. Ishida J. Kohda T. Morishita S. Ichihara S. Fukamizu A. Evaluation of novel cyclic analogues of apelin. Int. J. Mol. Med. 2008;22:547–552. [PubMed] [Google Scholar]
- Trân K. Murza A. Sainsily X. Coquerel D. Côté J. Belleville K. Haroune L. Longpré J.-M. Dumaine R. Salvail D. Lesur O. Auger-Messier M. Sarret P. Marsault É. A systematic exploration of macrocyclization in apelin-13: impact on binding, signaling, stability, and cardiovascular effects. J. Med. Chem. 2018;61:2266–2277. doi: 10.1021/acs.jmedchem.7b01353. [DOI] [PubMed] [Google Scholar]
- Juhl C. Els-Heindl S. Schönauer R. Redlich G. Haaf E. Wunder F. Rjedl B. Burkhardt N. Beck-Sickinger A. G. Bierer D. Development of potent and metabolically stable APJ ligands with high therapeutic potential. ChemMedChem. 2016;11:2378–2384. doi: 10.1002/cmdc.201600307. [DOI] [PubMed] [Google Scholar]
- O'Harte F. P. M. Parthsarathy V. Hogg C. Flatt P. R. Long-term treatment with acylated analogues of apelin-13 amide ameliorates diabetes and improves lipid profile of high-fat fed mice. PLoS One. 2018;13:e0202350. doi: 10.1371/journal.pone.0202350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Turecek P. L. Bossard M. J. Schoetens F. Ivens I. A. PEGylation of bio-pharmaceuticals: a review of chemistry and nonclinical safety information of approved drugs. J. Pharm. Sci. 2016;105:460–475. doi: 10.1016/j.xphs.2015.11.015. [DOI] [PubMed] [Google Scholar]
- Masoud A. G. T. Lin J. X. Farhan M. A. Fischer C. Zhu L. F. Anderson C. C. Sis B. Kassiri Z. Moore R. B. Kim D. Vederas J. C. Adam B. A. Oudit G. Y. Murray A. G. Apelin directs endothelial cell differentiation and vascular repair following immune-mediated injury. J. Clin. Invest. 2020;130:94–107. doi: 10.1172/JCI128469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang W. Shen M. Fischer C. Basu R. Hazra S. Couvineau P. Paul M. Wang F. Toth S. Mix D. S. Poglitsch M. Gerard N. Bouvier M. Vederas J. C. Penninger J. M. Kassiri Z. Oudit G. Y. Apelin protects against abdominal aortic aneurysm: therapeutic role of neutral endopeptidase resistant apelin analogues. Proc. Natl. Acad. Sci. U. S. A. 2019;116:13006–13015. doi: 10.1073/pnas.1900152116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Flahault A. Girault-Sotias P.-E. Keck M. Alvear-Perez R. De Mota N. Estéoulle L. Ramanoudjame S. M. Iturrioz X. Bonnet D. Llorens-Cortès C. A metabolically stable apelin-17 analog decreases AVP-induced antidiuresis and improves hyponatremia. Nat. Commun. 2021;12:1–14. doi: 10.1038/s41467-020-20560-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iturrioz X. Alvear-Perez R. De Mota N. Franchet C. Guillier F. Leroux V. Dabire H. Le Jouan M. Chabane H. Gerbier R. Bonnet D. Berdeaux A. Maigret B. Galzi J.-L. Hibert M. Llorens-Cortès C. Identification and pharmacological properties of E339-3D6, the first nonpeptidic apelin receptor agonist. FASEB J. 2010;24:1505–1517. doi: 10.1096/fj.09-140715. [DOI] [PubMed] [Google Scholar]
- Read C. Fitzpatrick C. M. Yang P. Kuc R. E. Maguire J. J. Glen R. C. Foster R. E. Davenport A. P. Cardiac action of the first G protein biased small molecule apelin agonist. Biochem. Pharmacol. 2016;116:63–72. doi: 10.1016/j.bcp.2016.07.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trifonov L. Afri M. Palczewski K. Korshin E. E. Gruzman A. An expedient synthesis of CMF-019: (S)-5-methyl-3-{1-(pentan-3-yl)-2- (thiophen-2-ylmethyl)-1H- benzo[d]imidazole-5-carboxamido}hexanoic acid, a potent apelin receptor (APJ) agonist. Med. Chem. 2018;14:688–694. doi: 10.2174/1573406414666180412154952. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tatemoto K. Hosoya M. Habata Y. Fujii R. Kakegawa T. Zou M.-X. Kawamat Y. Fukusumi S. Hinuma S. Kitada C. Kurokawa T. Onda H. Fujino M. Isolation and characterization of a novel endogenous peptide ligand for the human APJ receptor. Biochem. Biophys. Res. Commun. 1998;251:471–476. doi: 10.1006/bbrc.1998.9489. [DOI] [PubMed] [Google Scholar]
- Bovy P. R. O'Neal J. M. Olins G. M. Patton D. R. Mehta P. P. McMahon E. G. Palomo M. Schuh J. Blehm D. A synthetic linear decapeptide binds to the atrial natriuretic peptide receptors and demonstrates cyclase activation and vasorelaxant activity. J. Biol. Chem. 1989;264:20309–20313. [PubMed] [Google Scholar]
- Chatenet D. Dubessy C. Boularan C. Scalbert E. Pfeiffer B. Renard P. Lihrmann I. Pacaud P. Tonon M.-C. Vaudry H. Leprince J. Structure-activity relationships of a novel series of urotensin ll analogues: identification of a urotensin ll antagonist. J. Med. Chem. 2006;49:7234–7238. doi: 10.1021/jm0602110. [DOI] [PubMed] [Google Scholar]
- Chafai A. Fromm M. F. König J. Maas R. The prognostic biomarker L-homoarginine is a substrate of the cationic amino acid transporters CAT1, CAT2 and CAT2B. Sci. Rep. 2017;7:1–10. doi: 10.1038/s41598-017-04965-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pilz S. Meinitzer A. Gaksch M. Grübler M. Verheyen N. Drechsler C. Hartaigh B. Ó. Lang F. Alesutan I. Voelkl J. März W. Tomaschitz A. Homoarginine in the renal and cardiovascular systems. Amino Acids. 2015;47:1703–1713. doi: 10.1007/s00726-015-1993-2. [DOI] [PubMed] [Google Scholar]
- Ryan W. L. Wells I. C. Homocitrulline and homoarginine synthesis from lysine. Science. 1964;144:1122–1127. doi: 10.1126/science.144.3622.1122. [DOI] [PubMed] [Google Scholar]
- Ravani P. Maas R. Malberti F. Pecchini P. Mieth M. Quinn R. Tripepi G. Mallamaci F. Zoccali C. Homoarginine and mortality in pre-dialysis chronic kidney disease (CKD) patients. PLoS One. 2013;8:1–6. doi: 10.1371/journal.pone.0072694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ryan W. L. Barak A. J. Johnson R. J. Lysine, homocitrulline, and homoarginine metabolism by the isolated perfused rat liver. Arch. Biochem. Biophys. 1968;123:294–297. doi: 10.1016/0003-9861(68)90137-9. [DOI] [PubMed] [Google Scholar]
- Jaźwińska-Kozuba A. Martens-Lobenhoffer J. Kruszelnicka O. Rycaj J. Chyrchel B. Surdacki A. Bode-Böger S. M. Opposite associations of plasma homoarginine and ornithine with arginine in healthy children and adolescents. Int. J. Mol. Sci. 2013;14:21819–21832. doi: 10.3390/ijms141121819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Atzler D. Cracowski J. L. Cordts K. Böger R. H. Humbert M. Schwedhelm E. Homoarginine predicts mortality in treatment-naive patients with pulmonary arterial hypertension. Int. J. Cardiol. 2016;217:12–15. doi: 10.1016/j.ijcard.2016.04.161. [DOI] [PubMed] [Google Scholar]
- Demetrius L. Of mice and men: when it comes to studying ageing and the means to slow it down, mice are not just small humans. EMBO Rep. 2005;1:S39–S44. doi: 10.1038/sj.embor.7400422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fischer C. Lamer T. Fernandez K. Gheblawi M. Wang W. Pascoe C. Lambkin G. Iturrioz X. Llorens-Cortès C. Oudit G. Y. Vederas J. C. Optimizing PEG-extended apelin analogues as cardioprotective drug leads: importance of the KFRR motif and aromatic head group for improved physiological activity. J. Med. Chem. 2020;63:12073–12082. doi: 10.1021/acs.jmedchem.0c01395. [DOI] [PubMed] [Google Scholar]
- Tucker T. J. Lumma W. C. Lewis S. D. Gardell S. J. Lucas B. J. Baskin E. P. Woltmann R. Lynch J. J. Lyle E. A. Appleby S. D. Chen I. W. Dancheck K. B. Vacca J. P. Potent noncovalent thrombin inhibitors that utilize unique amino acid D-dicyclohexylalanine in the P3 position. Implications on oral bioavailability and antithrombic efficacy. J. Med. Chem. 1997;40:1565–1569. doi: 10.1021/jm970140s. [DOI] [PubMed] [Google Scholar]
- Nyimanu D. Kay R. G. Sulentic P. Kuc R. E. Ambery P. Jermutus L. Reimann F. Gribble F. M. Cheriyan J. Maguire J. J. Davenport A. P. Development and validation of an LC-MS/MS method for detection and quantification of in vivo derived metabolizes of [Pyr1]apelin-13 in humans. Sci. Rep. 2019;9:1–12. doi: 10.1038/s41598-019-56157-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iturrioz X. Vazeux G. Célérier J. Corvol P. Llorens-Cortès C. Histidine 450 plays a critical role in catalysis and, with Ca2+, contributes to the substate specificity of aminopeptidase A. Biochemistry. 2000;39:3061–3068. doi: 10.1021/bi9925726. [DOI] [PubMed] [Google Scholar]
- Riedel J., Distribution-in vitro tests-protein binding, in Drug Discovery and Evaluation: Safety and Pharmacokinetic Assays, ed. G. H. Vogel, F. J. Hock, J. Maas and D. Mayer, Springer, Berlin/Heidelberg, 2nd edn, 2013, pp. 897–913, 10.1007/978-3-642-25240-2_39 [DOI] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.