Abstract
The two major subtypes of diffuse large B cell lymphoma (DLBCL)—activated B cell–like (ABC) and germinal center B cell–like (GCB)—arise by distinct mechanisms, with ABC selectively acquiring mutations that target the B cell receptor (BCR), fostering chronic active BCR signaling1. The ABC subtype has a ∼40% cure rate with currently available therapies, which is worse than the rate for GCB DLBCL, and highlights the need for ABC subtype-specific treatment strategies2. We hypothesized that ABC, but not GCB, DLBCL tumors would respond to ibrutinib, an inhibitor of BCR signaling. In a phase 1/2 clinical trial that involved 80 subjects with relapsed or refractory DLBCL, ibrutinib produced complete or partial responses in 37% (14/38) of those with ABC DLBCL, but in only 5% (1/20) of subjects with GCB DLBCL (P = 0.0106). ABC tumors with BCR mutations responded to ibrutinib frequently (5/9; 55.5%), especially those with concomitant myeloid differentiation primary response 88 (MYD88) mutations (4/5; 80%), a result that is consistent with in vitro cooperation between the BCR and MYD88 pathways. However, the highest number of responses occurred in ABC tumors that lacked BCR mutations (9/29; 31%), suggesting that oncogenic BCR signaling in ABC does not require BCR mutations and might be initiated by non-genetic mechanisms. These results support the selective development of ibrutinib for the treatment of ABC DLBCL.
Nuclear factor kappa B (NF-κB) activity sustains viability of ABC, but not GCB, DLBCL cell lines3 and is constitutively activated by signals from the BCR and MYD88 pathways4,5. Activating mutations that target the genes encoding the BCR subunits CD79a and CD79b, the BCR pathway adaptor caspase recruitment domain family member 11 (CARD11), and MYD88 promote NF-κB activity in ABC DLBCL, as do genetic and epigenetic events that inactivate tumor necrosis factor α–induced protein 3 (TNFAIP3, also known as A20)4–7. Bruton’s tyrosine kinase (BTK) links BCR activity to NF-κB and is essential for the survival of ABC lines with chronic active BCR signaling4. Ibrutinib is a selective, covalent inhibitor of BTK that kills ABC DLBCL lines by reducing NF-κB pathway activity4,8. We hypothesized that ibrutinib would be active in ABC, but not GCB, DLBCL, on the basis of genetic and functional evidence that BCR signaling is central to the pathogenesis of ABC DLBCL.
To assess this hypothesis, we performed a study of ibrutinib (560 mg) given orally on a daily basis until either disease progression or ibrutinib intolerance occurred in 80 subjects with relapsed or refractory de novo DLBCL. Ibrutinib was generally well tolerated, with treatment-emergent adverse events (AE) in line with previous studies (Supplementary Table 1). Overall, responses were observed in 25% (20/80) of subjects, including partial responses (PR; n = 12) and complete responses (CR; n = 8). With a median post-treatment follow-up period of 11.53 months, median progression-free survival (PFS) and overall survival (OS) were 1.64 months and 6.41 months, respectively.
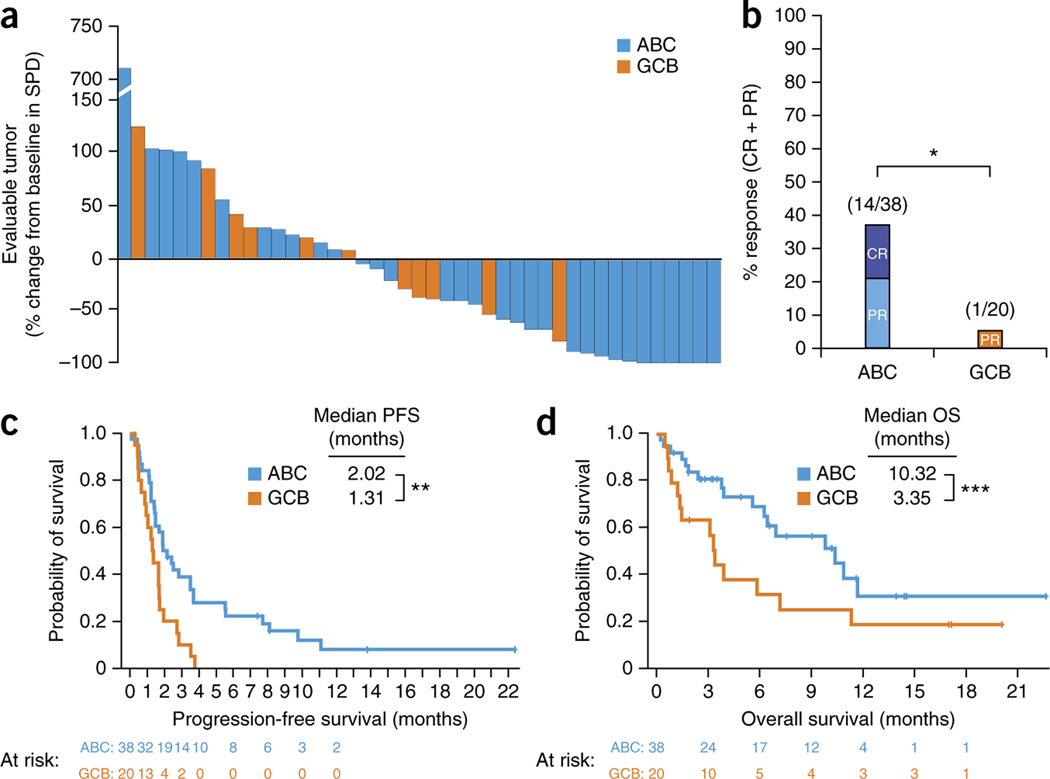
Analysis of tumors by gene expression profiling identified ABC (n = 38), GCB (n = 20), and unclassified (n = 17) cases (Table 1). The ABC and GCB groups were similar in patient median age, poor-risk disease9, number of prior regimens and refractoriness to the most-recent chemotherapy regimen. In the patients with ABC DLBCL, the response rate to ibrutinib was 37% (14/38), with 16% (6/38) CR, whereas the response rate in those with GCB DLBCL was only 5% (1/20) (P = 0.0106; Fig. 1a,b). Median (range) response duration was 4.83 (1.02–9.26) months in ABC DLBCL. Four subjects with ABC DLBCL who achieved CR went into remissions lasting greater than 1 year, with one subject dying at 23 months from the start of treatment and three subjects showing ongoing responses to ibrutinib at 36.3, 32.7 and 52.5 months, respectively. Among subjects with ‘primary refractory’ ABC DLBCL, 22% (4/18) responded to ibrutinib, including three PRs and one CR. With a median follow-up of 10.12 and 17.05 months after start of treatment for subjects with ABC and GCB DLBCL, respectively, median PFS was 2.02 and 1.31 months (P = 0.004), and median OS was 10.32 and 3.35 months (P = 0.056), respectively (Fig. 1c,d). In subjects with ABC DLBCL who achieved either type of response, PFS was longer among those with a CR than with a PR (P = 0.0039; Supplementary Fig. 1).
Table 1.
Baseline characteristics by DLBCL subtype
| Characteristics | ABC (N = 38) | GCB (N = 20) | Unclassified (N = 17) | Unknown (N = 5) |
|---|---|---|---|---|
|
| ||||
| Median age, years (range) | 60 (34–89) | 65 (28–92) | 63 (44–85) | 65 (58–78) |
| Sex (male) | 66% | 70% | 82% | 60% |
| ECOG performance score ≥ 2 | 5% | 20% | 24% | 40% |
| RIPI (poor) | 63% | 59% | 50% | 60% |
| Median time from diagnosis, months (range) | 19 (4–118) | 17 (11–104) | 21 (7–332) | 19 (9–57) |
| Median number of prior regimens (range) | 3 (1–7) | 3.5 (1–7) | 3 (1–4) | 3 (1–3) |
| Prior ASCT | 13% | 30% | 24% | 40% |
| Chemotherapy-refractory disease | 66% | 65% | 59% | 50% |
ABC, activated B cell–like; ASCT, autologous stem cell transplant; DLBCL, diffuse large B cell lymphoma; ECOG, Eastern Cooperative Oncology Group; GCB, germinal center B cell–like; RIPI, revised international prognostic index.
Figure 1.
Tumor response to ibrutinib therapy. (a) Waterfall plot of maximum change from baseline of SPD of lymph nodes for subjects with evaluable tumors (n = 43). (b) Overall response rate by DLBCL subtype. Fisher’s exact test of the overall response rate between the ABC and GCB groups (*P = 0.0106). Fractions above the bars represent the number of subjects showing a complete or partial response (numerator) over the total number of subjects in the group (denominator). (c) Kaplan–Meier analysis of progression-free survival. Log-rank test for analysis between groups (**P = 0.0038). (d) Kaplan–Meier analysis of overall survival. Log-rank test for analysis between groups (***P = 0.056). ABC, activated B cell–like; GCB, germinal center B cell–like; DLBCL, diffuse large B cell lymphoma; CR, complete response; OS, overall survival; PFS, progression-free survival; PR, partial response; SPD, sum of the product of the diameters.
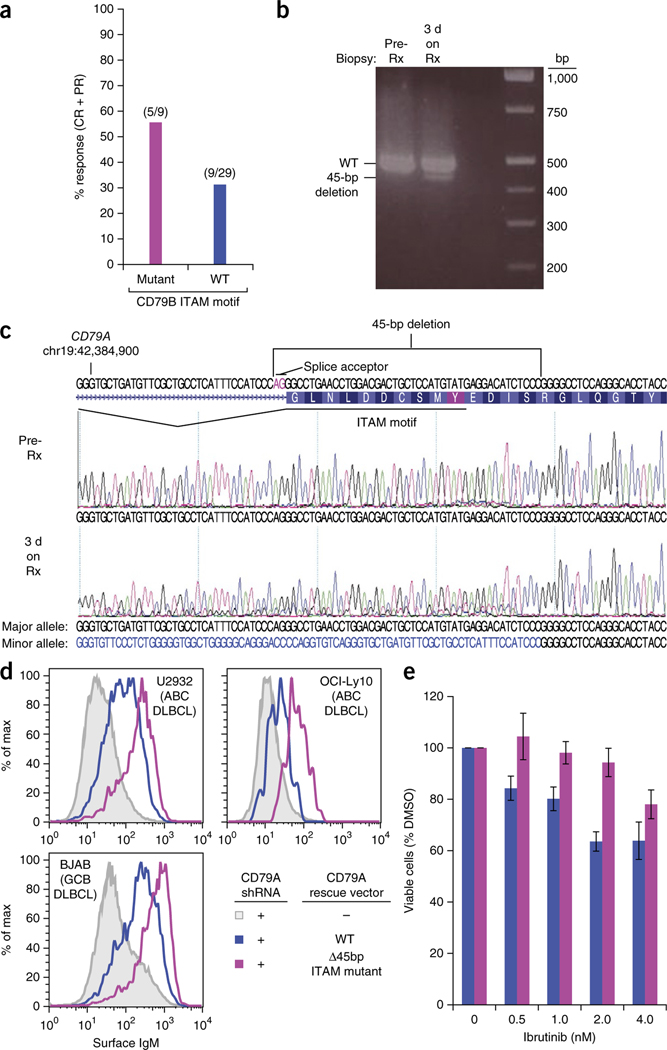
We observed gain-of-function mutations targeting the BCR subunit CD79b in 23% of ABC DLBCL biopsy samples, whereas we detected no CD79A mutations. Among subjects with CD79B mutations, the response rate to ibrutinib was 55.5% (5/9) (Fig. 2a); additionally, two subjects had tumor reductions of 41.3% and 41.2% at the scheduled evaluation after two treatment cycles, and one subject achieved a transient 97% reduction on an unscheduled evaluation after one cycle but had disease progression after two cycles. Thus, tumors with CD79B mutations responded frequently to ibrutinib, albeit to varying degrees and durations. Importantly, 31% (9/29) of tumors with wild-type CD79B also responded, suggesting that a BCR mutation is not required for addiction to chronic active BCR signaling in DLBCL. Of note in this regard was one unclassified DLBCL that responded completely to ibrutinib and had a subclonal, 45-base-pair deletion disrupting the CD79a immunoreceptor tyrosine–based activation signaling motif (ITAM) (Fig. 2b,c). This mutation increased BCR expression on the cell surface (Fig. 2d), as do other mutations in CD79A and CD79B (ref. 4). Upon deep re-sequencing, the mutant allele frequency increased from 5% before treatment to 27% in a biopsy sample of the tumor obtained after 3 d of ibrutinib treatment (Fig. 2b,c), possibly owing to the ability of this mutation to confer relative ibrutinib resistance (Fig. 2e). The fact that ibrutinib produced a CR in this subject implies that most cells in the tumor were dependent on BCR signaling, irrespective of CD79A mutational status, underscoring the view that BCR pathway addiction in DLBCL does not require a BCR mutation.
Figure 2.
Influence of B cell receptor mutations on ibrutinib response in ABC DLBCL. (a) Overall response rate according to CD79B mutational status. Fractions above the bars represent the number of subjects showing a response (complete or partial response; numerator) over the total number of subjects in the group (denominator). (b) PCR analysis of a region of CD79A with a 45-bp deletion in genomic DNA from tumors taken before ibrutinib therapy (pre-Rx) and again after 3 d of ibrutinib therapy (3 d on Rx). (c) Sanger sequencing analysis of tumor DNA as in b. The deletion of a splice acceptor site and part of the ITAM region is indicated. (d) Surface IgM expression in the indicated DLBCL lines in which endogenous CD79A expression was knocked down and cells were reconstituted with the indicated CD79A isoforms. Gating on a co-transduced Lyt2 marker identified the subset of transduced cells with equivalent ectopic CD79A RNA expression. (e) The OCI-Ly10 ABC DLBCL line was transduced with exogenous CD79A isoforms as in d and were treated with the indicated concentrations of ibrutinib for 4 d. Viability of cells relative to DMSO-treated cells is displayed as the mean from three biological repeats. Error bars denote s.e.m. Data from b and c are from single experiments, whereas data in d are representative of three biological repeats. DMSO, dimethyl sulfoxide; PCR, polymerase chain reaction.
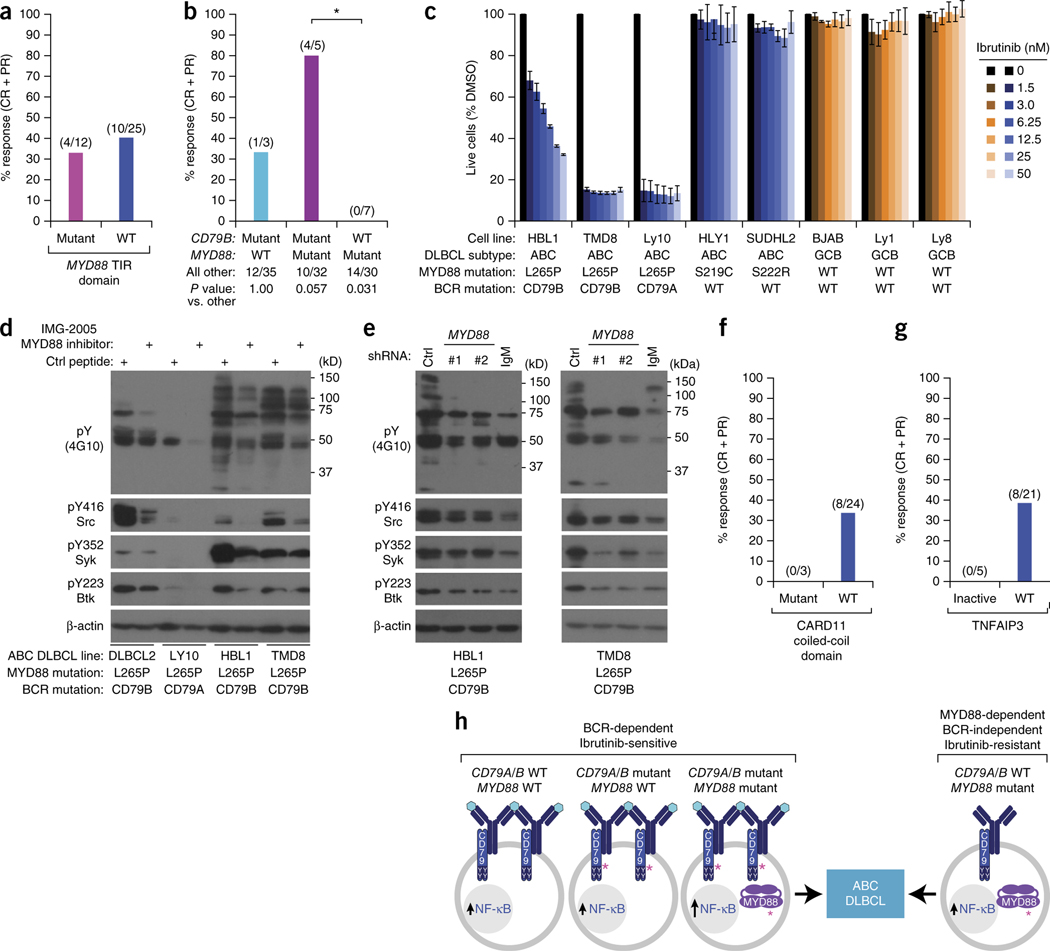
The signaling adaptor MYD88 sustains mutations in 39% of ABC DLBCLs, creating isoforms that spontaneously activate NF-κB5. Conceivably, MYD88 mutations might mitigate the effect of ibrutinib by providing an alternate means by which to activate NF-κB. However, the predominant MYD88 mutation, L265P, co-occurs with CD79B mutations more often than would be expected by chance in ABC tumors5, suggesting cooperation between these pathways. Response rates to ibrutinib were not significantly different (P = 0.493) between tumors with MYD88 mutations (4/12; 33.3%) and those with wild-type MYD88 (10/25; 40%; Fig. 3). However, tumors with both CD79B and MYD88 mutations were more responsive (4/5; 80%) than other tumors (P = 0.057) (Fig. 3b). In ABC lines with MYD88 and CD79A or CD79B mutations, inhibition of MYD88 using an inhibitory peptide10 (Fig. 3d) or small hairpin RNAs5 (Fig. 3e, Supplementary Fig. 2) decreased tyrosine phosphorylation of multiple proteins and reduced phosphorylation of the Src-family kinases, spleen tyrosine kinase (Syk) and Btk, consistent with a functional cooperation in which MYD88 signaling promotes proximal BCR signaling in these ABC lines.
Figure 3.
Influence of recurrent genetic alterations on ibrutinib response in ABC DLBCL. (a) Overall response rates by MYD88 mutation status. Fisher’s exact test of the overall response rate between the MYD88 mutant and non-mutant group (P = 0.493). Fractions above the bars represent the number of subjects with a response (complete or partial response; numerator) over the total number of subjects in the group (denominator). (b) Overall response rates by CD79B and MYD88 mutation status. Fisher’s exact test of the overall response rate between the CD79B mutant/MYD88 mutant group and the CD79B wild type/MYD88 mutant group (*P = 0.01). (c) Toxicity of ibrutinib for cell-line models of ABC and GCB DLBCL harboring genetic lesions in CD79A, CD79B and MYD88, as indicated. Cells were treated for 3 d with ibrutinib at the indicated concentrations and assessed for viability as described5. Error bars denote s.e.m. of triplicates. (d) MYD88 potentiates chronic active BCR signaling in ABC DLBCL. The indicated ABC DLBCL lines were treated with the MYD88 dimerization inhibitor IMG-2005 (100 μM) or a control peptide for 16 h and analyzed by immunoblot for the indicated proteins. Data are representative of three biological repeats. (e) MYD88 knockdown reduces chronic active BCR signaling in ABC DLBCL. TMD8 and HBL1 ABC DLBCL cells were transduced with the indicated shRNAs, induced to express the shRNAs with doxycycline for 48 h, and evaluated by immunoblot analysis for the indicated proteins. Data are representative of independent experiments in HBL1 (n = 7) and TMD8 (n = 10). (f) Overall response rates by CARD11 mutation status. Fractions above the bars represent the number of subjects with a response (complete or partial response; numerator) over the total number of subjects in the group (denominator). (g) Overall response rates by TNFAIP3 status. TNFAIP3 inactivation denotes TNFAIP3 nonsense or frameshift mutation, TNFAIP3 double deletion, or TNFAIP3 mRNA <2 s.d. below the mean of ABC DLBCL samples. TNFAIP3 WT denotes cases without these TNFAIP3 alterations. Fractions above the bars represent the number of subjects with a response (complete or partial response; numerator) over the total number of subjects in the group (denominator). (h) Theoretical model of ABC DLBCL pathogenesis indicating BCR-dependent and BCR-independent genetic pathways. Shown at the left are three genetic scenarios that can be associated with chronic active BCR signaling and ibrutinib sensitivity. Hypothetically, the BCRs in these tumors could be engaged by an antigen (turquoise hexagons) and hence are clustered. The right of this figure illustrates ibrutinib-resistant ABC DLBCL tumors that do not rely on chronic active BCR signaling, but rather use a mutant MYD88 isoform to engage the NF-κB pathway. The BCRs in these tumors are therefore depicted as unclustered and not engaged by an antigen. Pink asterisks (*) indicate activating mutations. ABC, activated B cell–like; BCR, B cell receptor; CR, complete response; DLBCL, diffuse large B cell lymphoma; PR, partial response.
Tumors with MYD88 mutations, but wild-type CD79B, were unresponsive to ibrutinib (0/7, P = 0.031; Fig. 3b). The ibrutinib insensitivity of MYD88-only mutant tumors raises the possibility of a MYD88-dependent, but BCR-independent, genetic pathway to ABC DLBCL. Indeed, although ibrutinib was toxic for lines with CD79A or CD79B mutations and MYD88 L265P, it was ineffective against two lines with wild-type CD79A and CD79B that have other MYD88 mutations, both of which are MYD88 dependent5 (Fig. 3c).
Lastly, we investigated whether other genetic aberrations that activate NF-κB in ABC DLBCL affect ibrutinib responsiveness. Ibrutinib was ineffective in the three subjects with tumor mutations that activated CARD11, which acts downstream of BTK in the BCR pathway6 (Fig. 3f). TNFAIP3, a negative regulator of NF-κB, was inactivated by frameshift or splice site mutations (n = 2), homozygous deletion (n = 1), or transcriptional downregulation consistent with epigenetic silencing or undetected genomic abnormalities (n = 2; mRNA levels <2 s.d. below the ABC DLBCL mean). None of these five subjects responded to ibrutinib, whereas the response rate was 38% (8/21) in cases without TNFAIP3 inactivation (P = 0.13; Fig. 3g).
Our findings establish that the ABC subtype of DLBCL, as defined by gene expression profiling, responds preferentially to pharmacological inhibition of chronic active BCR signaling, which utilizes BTK to activate the downstream NF-κB pathway4. Chronic active BCR signaling is distinct from ‘tonic’ BCR signaling, which stimulates the phosphoinositide-3 kinase pathway but not the NF-κB pathway (reviewed in ref. 11). Although tonic BCR signaling plays a role in other B-cell malignancies, such as Burkitt lymphoma12, the present study provides clinical evidence that chronic active BCR signaling is a feature of ABC, but not GCB, tumors in vivo, and confers ibrutinib sensitivity. On the basis of the observed selectivity of ibrutinib for ABC DLBCL cases in the present trial, a randomized phase 3 study of ibrutinib with R-CHOP treatment (NCT01855750; http://www.clinicaltrials.gov) has begun, and is enrolling only newly diagnosed subjects with non-GCB DLBCL.
Overall, our results are consistent with a theoretical model in which tumors with the ABC DLBCL phenotype arise by two distinct pathogenetic routes, one BCR dependent and the other BCR independent (Fig. 3h). The BCR-dependent route includes tumors with gain-of-function BCR ITAM mutations, which were moderately more frequent in ibrutinib responders, but also some tumors with wild-type BCR and MYD88. Tumors with both CD79B and MYD88 mutations were often sensitive to ibrutinib, suggesting that they arise by a BCR-dependent route, a notion that fits with previous genetic data5 as well as with functional data in the present study showing positive cross-talk between MYD88 and BCR pathways in ABC lines The BCR-independent route to ABC DLBCL might include tumors with MYD88 mutations, but wild-type CD79A and CD79B, because these were typically ibrutinib resistant in the present trial.
An important discovery is that the BCR-dependent route to ABC DLBCL also includes tumors without mutations in the BCR, as 67% of ibrutinib responders had wild-type CD79A and CD79B. Although there could be some hitherto undefined genetic lesions that activate BCR signaling in these tumors, this finding raises the possibility of a non-genetic mechanism of BCR pathway addiction. Consistent with this idea, previous work has shown that CD79B mutants do not initiate BCR signaling de novo when introduced into heterologous cells, but rather increase the amplitude of ongoing BCR signaling4. ABC DLBCL lines and primary tumor biopsy specimens exhibit clusters of BCR in the cell plasma membrane that are reminiscent of BCR clusters formed upon antigen engagement in normal B cells4, suggesting that a similar process might be at play in ABC DLBCL. Other ibrutinib-responsive B-cell malignancies, including chronic lymphocytic leukemia and mantle cell lymphoma13,14, might also depend on similar non-genetic mechanisms; genomic analyses of these malignancies have revealed neither CD79A nor CD79B mutations15–17.
Taken together, these observations suggest that future DLBCL trials involving ibrutinib should not restrict enrollment to subjects with CD79A- or CD79B-mutated tumors, as this approach would probably overlook a large subset of ibrutinib-responsive tumors. Rather, our data support the use of the ABC DLBCL gene-expression signature as a biomarker to enrich for ibrutinib-responsive subjects in such trials. Our molecular analyses provide a foundation for the development of personalized treatment in DLBCL and bode well for the development of a more effective, ibrutinib-based therapy for ABC DLBCL.
METHODS
Methods and any associated references are available in the online version of the paper.
Note: Any Supplementary Information and Source Data files are available in the online version of the paper.
ONLINE METHODS
Study conduct.
This was an open-label, non-randomized, prospective analysis of ibrutinib in relapsed or refractory DLBCL. The study was conducted in two parts. First, a 10-subject pilot ABC DLBCL cohort enrolled at the final dose level of the phase 1 trial of ibrutinib in relapsed or refractory B cell lymphomas (conducted at the National Cancer Institute (NCT00849654; http://www.clinicaltrials.gov)). We enrolled subjects between June 2010 and October 2011. Among these 10 subjects enrolled, 6 (60%) were male and the age range was 40 to 79 years. This study was followed by a phase 2 study of ibrutinib at the same dose and schedule as in phase 1, in 70 subjects with relapsed or refractory de novo DLBCL conducted at 14 sites (NCT01325701). We enrolled subjects between May 2011 and May 2012. Among these 70 subjects enrolled, 50 (71%) were male and the age range was 28 to 92 years. The institutional review boards at each participating center (*full names of institutions provided below) approved both protocols, and subjects provided informed consent. Studies were in compliance with the principles of the Declaration of Helsinki and the International Conference on Harmonisation for Good Clinical Practice. The primary endpoint was response in molecular subtypes of DLBCL, with additional endpoints including progression-free and overall survival, and the association of ibrutinib response with genomic aberrations that alter BCR and NF-κB signaling in ABC DLBCL (CD79B, MYD88, CARD11, and TNFAIP3). We performed gene expression profiling and genomic analyses in a blinded fashion before we had knowledge of the clinical outcomes with ibrutinib treatment. *Institutional review boards: National Cancer Institute National Institutes of Health Protocol Review Office (Bethesda, Maryland); Western Institutional Review Board (Olympia, Washington); University of Texas MD Anderson Cancer Center Office of Protocol Research (Houston, Texas); Stanford University Research Compliance Office, Administrative Panels on Human Subjects Research (Palo Alto, California); Memorial Sloan Kettering Cancer Center Institutional Review Board (New York, New York); Weill Cornell Medical College Institutional Review Board (New York, New York); Mayo Clinic Institutional Review Board (Rochester, Minnesota); University of Nebraska Medical Center Institutional Review Board (Omaha, Nebraska); Biomedical Research Alliance of New York Institutional Review Board (Lake Success, New York); Office of the Human Research Protection Program (Los Angeles, California).
Subject eligibility.
Eligible subjects were ≥18 years old and had pathologically confirmed relapsed or refractory DLBCL, an Eastern Cooperative Oncology Group score of ≤1 (phase 1) or ≤2 (phase 2), adequate tissue for pathology, measurable disease and analysis of molecular subtype by gene expression profiling. Exclusion criteria included primary mediastinal B cell lymphoma or central nervous system disease, history of HIV, active or chronic hepatitis C or B, pregnancy or breast-feeding. In the phase 1 study, exclusion criteria included absolute neutrophil count <1,500 cells/μl, platelet count <75,000 cells/μl, serum aspartate transaminase or alanine transaminase >2.5 times the upper limit of normal (ULN) and serum creatinine >1.5 times ULN. In the phase 2 study, exclusion criteria included absolute neutrophil count <750 cells/μl, platelet count <50,000 cells/μl, serum aspartate transaminase or alanine transaminase ≥3.0 times the upper limit of normal (ULN) and serum creatinine >2.0 times ULN. No subjects were excluded from the study following enrollment in the trial.
Treatment and evaluation.
Evaluation included medical history, physical examination, computed tomography (CT), positron emission tomography (PET), bone marrow biopsy and standard laboratory tests. Subjects received ibrutinib 560 mg PO daily in 4-week cycles until disease progression occurred or unacceptable levels of toxicity were observed. In the event of pre-specified, potentially drug-related toxicity, dosing adjustment was permitted (Supplementary Note 1). Response evaluation employed standard criteria18 based on CT scans of the chest, abdomen and pelvis that were performed every 2 treatment cycles for up to 7 months, followed by every 3 cycles for 2 years and every 6 cycles thereafter until the point of drug discontinuation. PET scans and bone marrow biopsies were required to confirm complete response if results of scans or biopsies were positive at baseline (pre-treatment).
Statistical analyses.
The main analysis was estimation of the response rate within each of the ABC and GCB cohorts. As exploratory analyses for subgroups, the sample sizes of approximately 30 per cohort have the characteristics of α = 0.025 (0.05/2) with power of approximately 90% to test the null hypothesis that overall response rate will be ≤10% (a rate not considered clinically compelling) versus the alternative hypothesis that overall response rate will be ≥35% (considered clinically meaningful for further development of ibrutinib as a single agent in this subject population) within each cohort. Progression-free survival was measured from the first dose until the point of disease progression or death, from any cause whichever came first. Overall survival was measured from the first dose until death from any cause. We performed efficacy analyses on an intent-to-treat basis, including all enrolled subjects. The Kaplan–Meier method was used to provide estimates of median time-to-event, with 95% confidence intervals (CI) in the ABC and GCB cohorts and the log-rank test P values for comparing the endpoints between analysis cohorts. L.M.S., W.H.W., J.M., B.Y.C., M.F., F.C., B. M., D.M. and D.M.B analyzed and reviewed the data.
Molecular analysis and in vitro studies.
We classified tumors as ABC, GCB, or unclassified DLBCL using gene expression profiling of formalin-fixed, paraffin-embedded tumor biopsy samples as described in Supplementary Note 2. We detected CD79B, CD79A, CARD11, MYD88 and TNFAIP3 mutations by Sanger sequencing of polymerase chain reaction–amplified exons from genomic DNA or by next-generation DNA sequencing performed by Foundation Medicine, Cambridge, MA. Methods for molecular analysis, cell-line engineering and analysis, immunoblot analysis, and fluorescence-activated cell sorting analysis are provided in Supplementary Notes 2–5, respectively.
Supplementary Material
ACKNOWLEDGMENTS
This research was supported in part by the Intramural Research Program of the United States National Institutes of Health, National Cancer Institute, Center for Cancer Research. Research grant was provided by the Dr. Mildred Scheel Stiftung für Krebsforschung (Deutsche Krebshilfe; R.S.). Medical writing and editorial support were provided by M. Gersh and funded by Janssen Pharmaceuticals, Titusville, New Jersey. These studies, NCT00849654 and NCT01325701, were sponsored by Pharmacyclics, Inc., Sunnyvale, California.
Footnotes
COMPETING FINANCIAL INTERESTS
The authors declare competing financial interests: details accompany the online version of the paper.
Reprints and permissions information is available online at http://www.nature.com/reprints/index.html.
References
- 1.Shaffer AL III, Young RM & Staudt LM Pathogenesis of human B cell lymphomas. Annu. Rev. Immunol. 30, 565–610 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Lenz G. et al. Stromal gene signatures in large-B-cell lymphomas. N. Engl. J. Med. 359, 2313–2323 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Davis RE, Brown KD, Siebenlist U & Staudt LM Constitutive nuclear factor kappa B activity is required for survival of activated B cell-like diffuse large B cell lymphoma cells. J. Exp. Med. 194, 1861–1874 (2001). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Davis RE et al. Chronic active B-cell-receptor signalling in diffuse large B-cell lymphoma. Nature 463, 88–92 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ngo VN et al. Oncogenically active MYD88 mutations in human lymphoma. Nature 470, 115–119 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Lenz G. et al. Oncogenic CARD11 mutations in human diffuse large B cell lymphoma. Science 319, 1676–1679 (2008). [DOI] [PubMed] [Google Scholar]
- 7.Compagno M. et al. Mutations of multiple genes cause deregulation of NF-kappaB in diffuse large B-cell lymphoma. Nature 459, 717–721 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Yang Y. et al. Exploiting synthetic lethality for the therapy of ABC diffuse large B cell lymphoma. Cancer Cell 21, 723–737 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Sehn LH et al. The revised International Prognostic Index (R-IPI) is a better predictor of outcome than the standard IPI for patients with diffuse large B-cell lymphoma treated with R-CHOP. Blood 109, 1857–1861 (2007). [DOI] [PubMed] [Google Scholar]
- 10.Loiarro M. et al. Peptide-mediated interference of TIR domain dimerization in MyD88 inhibits interleukin-1-dependent activation of NF-κB. J. Biol. Chem. 280, 15809–15814 (2005). [DOI] [PubMed] [Google Scholar]
- 11.Young RM & Staudt LM Targeting pathological B cell receptor signalling in lymphoid malignancies. Nat. Rev. Drug Discov. 12, 229–243 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Schmitz R. et al. Burkitt lymphoma pathogenesis and therapeutic targets from structural and functional genomics. Nature 490, 116–120 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Byrd JC et al. Targeting BTK with ibrutinib in relapsed chronic lymphocytic leukemia. N. Engl. J. Med. 369, 32–42 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Wang ML et al. Targeting BTK with ibrutinib in relapsed or refractory mantle-cell lymphoma. N. Engl. J. Med. 369, 507–516 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Quesada V. et al. Exome sequencing identifies recurrent mutations of the splicing factor SF3B1 gene in chronic lymphocytic leukemia. Nat. Genet. 44, 47–52 (2012). [DOI] [PubMed] [Google Scholar]
- 16.Beà S. et al. Landscape of somatic mutations and clonal evolution in mantle cell lymphoma. Proc. Natl. Acad. Sci. USA 110, 18250–18255 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Wang L. et al. SF3B1 and other novel cancer genes in chronic lymphocytic leukemia. N. Engl. J. Med. 365, 2497–2506 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Cheson BD et al. Revised response criteria for malignant lymphoma. J. Clin. Oncol. 25, 579–586 (2007). [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.