ABSTRACT
Up to 80% of people who experience a right-hemisphere stroke suffer from hemispatial neglect. This syndrome is debilitating and impedes rehabilitation. We carried out a clinical feasibility trial of transcranial direct current stimulation (tDCS) and a behavioural rehabilitation programme, alone or in combination, in patients with neglect. Patients >4 weeks post right hemisphere stroke were randomized to 10 sessions of tDCS, 10 sessions of a behavioural intervention, combined intervention, or a control task. Primary outcomes were recruitment and retention rates, with secondary outcomes effect sizes on measures of neglect and quality of life, assessed directly after the interventions, and at 6 months follow up. Of 288 confirmed stroke cases referred (representing 7% of confirmed strokes), we randomized 8% (0.6% of stroke cases overall). The largest number of exclusions (91/288 (34%)) were due to medical comorbidities that prevented patients from undergoing 10 intervention sessions. We recruited 24 patients over 29 months, with 87% completing immediate post-intervention and 67% 6 month evaluations. We established poor feasibility of a clinical trial requiring repeated hospital-based tDCS within a UK hospital healthcare setting, either with or without behavioural training, over a sustained time period. Future trials should consider intensity, duration and location of tDCS neglect interventions.
Trial registration:ClinicalTrials.gov identifier: NCT02401724.
KEYWORDS: Non-invasive brain stimulation, Hemispatial neglect, Stroke, Prospective randomized open blinded end-point (PROBE) trial, Transcranial direct current stimulation (tDCS)
Introduction
Hemispatial neglect is a disorder resulting in an inability to respond to events in the left half of subjective space and up to 85% of patients with right hemisphere stroke can experience it acutely after stroke (see Stone et al., 1991 for overview). Recovery tends to be most rapid over the first 10 days (Stone et al., 1992) with some further improvement within 12–14 weeks, and neglect still present after this period in over 50% of cases (Nijboer et al., 2013). Manifestations include visual or sensory inattention and anosognosia (impaired awareness of the affected side), both of which impede participation in rehabilitation therapies. The presence of neglect is further associated with increased length of hospital stay (Nys et al., 2005), increased physical and occupational therapy requirements, and reduced likelihood of regaining independence (Kalra et al., 1997; Katz et al., 1999). Evidence for the benefit of any intervention for neglect is currently insufficient (Bowen et al., 2013; Ten Brink et al., 2017).
Theoretical background and rationale
A model by Corbetta et al. (2005) proposed that spatial attention involves two distinct networks of fronto-parietal brain regions. The dorsal system mediates response selection and biases visual cortex activity towards one side of space, and encompasses the superior parietal lobe and the intraparietal sulcus as well as the dorsal frontal cortex including the frontal eye fields. The ventral fronto-parietal network mediates reorienting towards novel, behaviourally relevant stimuli and includes the temporo-parietal junction, inferior parietal lobe and ventral frontal cortex. Neglect is more often observed following lesions to the ventral system. Functional magnetic resonance imaging (fMRI) studies have reported that ventral temporo-parietal junction lesions lead to abnormal patterns of activity in the intact dorsal system, with over-activity in the left versus right hemisphere. Such hemispheric functional imbalance correlates with the severity of the symptoms and normalizes with recovery at > 6 months post stroke (Corbetta et al., 2005; He et al., 2007).
Hyperexcitability of the intact hemisphere has therefore been hypothesized to cause the spatial bias that is characteristic of the neglect syndrome, and non-invasive brain stimulation techniques have been investigated as a means of modulating this excitability (Koch et al., 2008; Sparing et al., 2009; Song et al., 2009). Sparing et al. (2009) reported that the inhibitory effect of cathodal transcranial direct current stimulation (tDCS), applied over the intact posterior parietal cortex, reduced symptoms of neglect in 10 patients.
We have previously reported that behavioural training (based on a rod-lifting exercise delivered over 12 sessions) ameliorated neglect symptoms and improved daily functioning and quality of life (on average) in 10 chronic neglect patients (>3months to 2 years post stroke onset) at one and four months post-intervention, when compared to 10 controls (Harvey et al., 2003, 2010; Rossit et al., 2019). We hypothesize that behavioural training that is designed to engage the ipsilesional dorsal system might counteract interhemispheric imbalance and drive a reduction of neglect symptoms (Harvey et al., 2003; Robertson et al., 1995; Rossetti et al., 1998).
Although tDCS has been used experimentally to rehabilitate a range of physical and cognitive functions after stroke (Schlaug et al., 2008), at present, the National Institute for Health and Care Excellence (NICE) guidelines provide no recommendations for the use of tDCS post-stroke. In addition, a recent Cochrane review concluded that it remains uncertain whether tDCS is able to generate improvements in arm and leg function, muscle strength or cognitive abilities (including neglect) after stroke, but it may effect an improvement in activities of daily living (Elsner et al., 2016). However, the quality of the evidence was deemed very low to moderate, and larger randomized trials were recommended. For the present study, we reason that there is tentative evidence from experimental studies (e.g., Allman et al., 2016; Nowak et al., 2009; O’Shea et al., 2017) to suggest that administering tDCS and behavioural training in combination could be more effective than administering either intervention alone. We aimed to test this hypothesis in a larger, randomized controlled trial in patients with hemispatial neglect.
Feasibility and efficacy of tDCS interventions in neglect patients
It is claimed that tDCS elicits polarity dependent excitability changes in the cortical area under the electrodes, with early studies in the motor cortex showing that the anodal electrode increases motor excitability, while the cathode effects a decrease (Antal et al., 2004; Nitsche & Paulus, 2000).
Regarding neglect rehabilitation, in a cross-over design, Ko et al. (2008) were the first to apply single session, right parietal anodal tDCS to neglect patients, documenting subsequent improvements in figure cancellation and line bisection (when compared to sham (i.e., placebo) stimulation). Sparing et al. (2009) then applied (in separate sessions) right anodal, left anodal and left cathodal tDCS stimulation, plus a sham protocol, to stroke patients with hemispatial neglect. They found the greatest neglect improvements (measured as a reduction in rightward line bisection bias) when 1 mA cathodal tDCS was applied to the intact (left) hemisphere. Subsequently, a number of small scale studies have been conducted to investigate the effects of both unilateral cathodal tDCS over the intact hemisphere, unilateral anodal over the lesioned hemisphere and bilateral stimulation (simultaneous left cathodal and right anodal), in both single- and multi-session designs, in order to assess efficacy for neglect rehabilitation. Table 1 gives a brief overview of these studies (this table is for illustrative purposes only and not meant to be exhaustive).
Table 1. Examples of tDCS neglect studies listing patient numbers, stimulation type, sessions and type of intervention as well as the main findings.
| Study | N of Patients | Type of Stimulation | Number of Sessions | Main Findings |
|---|---|---|---|---|
| Ko et al. (2008) | 15 | R Anodal; Sham | Crossover, 1 session each | Line bisection and Figure cancellation improvements |
| Sparing et al. (2009) | 10 | R Anodal; L Cathodal; L Anodal; Sham | Crossover, 1 session each | Line bisection improvement with R Anodal; L Cathodal |
| Sunwoo et al. (2013) | 10 | Bilateral R Anodal & L Cathodal; R anodal; Sham | Crossover, 1 session each | Line bisection improvements greatest for combined stimulation |
| Brem et al. (2014) | 1 | Bilateral R Anodal & L Cathodal; Sham; Cognitive Therapy | Crossover, 5 sessions each, various combinations | Covert attention and qualitative Line bisection and Copying improvements |
| Ladavas et al. (2015) | 30 | R Anodal; L Cathodal; Sham (combined with prism adaptation) | Between groups, 10 sessions | Greatest BIT improvement for R Anodal |
| Smit et al. (2015) | 5 | Bilateral R Anodal & L Cathodal; Sham | Crossover, 5 sessions each | No effects |
| Bang and Bong (2015) | 12 | Bilateral R Anodal & L Cathodal; Control group (with feedback training) | Between groups, 15 sessions | Greatest changes for Line bisection, Perception test |
| Yi et al. (2016) | 30 | R Anodal; L Cathodal; Sham | Between groups, 15 sessions | Greatest changes for Line bisection, Cancellation for both anodal, cathodal conditions |
| O’Shea et al. (2017) | 3 | L Anodal with prism adaptation; Sham | Crossover, 4 sessions each, various combinations | Long term gains in neglect scores mainly in cancellation tasks |
| Turgut et al. (2018) | 32 | Bilateral R Anodal & L Cathodal; Standard treatment control group (with optokinetic training) | Between groups, 8 sessions | Greatest improvement for Clock Drawing, Body orientation |
| Bornheim et al. (2018) | 4 | R Anodal; Sham | Crossover, 2 sessions each | Star cancellation, line bisection and Catherine Bergego Scale. “During the weeks of real tDCS, improvements were much greater than in the weeks with sham” (no statistics reported). |
| Chieffo et al. (2019) | 15 | R Anodal, L Cathodal, Sham (with prism adaptation) | Crossover, 1 session each | R anodal reduced leftward pointing shift after prism adaptation. |
Four of these studies (Bang & Bong, 2015; Ko et al., 2008; Sunwoo et al., 2013; Yi et al., 2016) are also cited in a recent meta-analysis by Salazar et al. (2018) that evaluated the effects of non-invasive brain stimulation on neglect more generally, including repetitive transcranial magnetic stimulation (rTMS) as well as tDCS.
Apart from Smit et al. (2015), all studies report some improvements on neglect symptoms, yet patient numbers are low, tDCS current strength, electrode size and placement, and session numbers vary and none of the studies were designed as prospective randomized double-blind, or open blinded end-point (PROBE) trials. In addition, it is surprising that considering the novelty of the approach and overall scarcity of the data, feasibility has as yet not been investigated: none of the studies describe and/or break down the recruitment base, provide a Consort Chart or list compliance/dropout rates. The only exception to this is Smit et al. (2015) who described the numbers identified and a detailed breakdown of reasons for exclusion. Ladavas et al. (2015) mention that from a sample of 92 stroke patients, 30 met the inclusion criteria.
So we aimed to investigate the feasibility of a clinical trial comparing behavioural training, tDCS, and a combination of both interventions, compared to a control group, in patients with post-stroke hemispatial neglect. We hypothesized that cathodal stimulation to the left hemisphere might interact with the behavioural intervention (Harvey et al., 2003, 2010) and aimed to gather exploratory data on potential effect sizes, both immediately after the interventions and at a delayed 6 months’ time point.
Methods
Study design and participants
NIBS was a prospective randomized open blinded end-point (PROBE) trial conducted at 4 hospitals across Greater Glasgow & Clyde and Lanarkshire with recruitment from October 2015 to June 2017.
Participants were eligible if they were aged 18 years or older, had a clinical diagnosis of stroke with brain imaging compatible with right hemisphere intracerebral haemorrhage or ischaemic stroke; had a modified Rankin score estimated as 0-3; and had persistent hemispatial neglect ≥4 weeks post-stroke, confirmed by a Behavioural Inattention Test (BIT) score of <129 (Wilson et al., 1987) and/or a line bisection bias of >6 mm (Rossit et al., 2009) and/or a Balloons test overall score of <17 or left Balloons test cancellations totalling <44% of total (Edgeworth et al., 1998), and/or an overall score of <42 on the Broken Hearts subtest of the Oxford Cognitive Screen (Demeyere et al., 2015).
Participants were excluded if they were unable to consent or unable to understand spoken or written English; had a Montreal Cognitive Assessment score <26 (Nasreddine et al., 2005); had other comorbidities that would limit or make participation unlikely, had a history of alcohol excess (>50 units per week for men and >40 for women); had a history of seizures; had metallic implants such as a pacemaker; were taking imipramine, amitriptyline, doxepin, nortriptyline, maprotiline, chlorpromazine, clozapine, foscarnet, ganciclovir, ritonavir, theophylline or had a history of use of amphetamine, cocaine, MDMA, ecstasy, phencyclidine, ketamine, gamma-hydroxybutyrate; had withdrawal from alcohol, barbiturates, benzodiazepines, meprobamate, or chloral hydrate, were pregnant.
Patients were identified for potential inclusion by the research nurses from the Scottish Stroke Research Network. The research nurses were based in the recruiting hospitals. They had access to the admission and medical notes of all patients admitted with right hemisphere stroke. They were given the inclusion/exclusion criteria, assessed for eligibility on this basis and were informed of any protocol-amended eligibility changes throughout the study as per below. Patients were approached face to face by the research nurses. Once we expanded recruitment to include outpatients (see below) this first approach was done via letter (sent by the research assistants). If the patients asked for more detailed information, the research assistants assisted in this. All patients provided the researchers with written informed consent.
The trial was approved by the NHS West of Scotland Research Ethics Committee (15/WS/005) and registered on ClinicalTrials.gov (NCT02401724).
We monitored the quality and integrity of the trial according to a protocol agreed with the study sponsors (NHS Greater Glasgow and Clyde) with a Trial Steering Committee (TSC) that met 6 times and an independent Data Monitoring Committee (iDMC) that met 4 times throughout the 3 years of the trial. In November 2015, in response to the low recruitment rate, the inclusion criteria were widened to include bilateral infarcts, increased modified Rankin Score, haemorrhagic strokes and no upper age limit. In September 2016, a number of further recommendations were made to tackle the low recruitment rate: include a pre-screen of patients with right hemisphere lesions for neglect symptoms (using the conventional subtests of the Behavioural Inattention Test (BIT) (Wilson et al., 1987)) to identify the disorder more accurately, and thus increase recruitment of patients whose diagnosis might have been missed previously; investigate recruiting patients that had finished participating in acute trials; investigate the feasibility of including other NHS sites; approach the community stroke team (CST) to identify patients in the community still suffering from neglect. All these recommendations were implemented in a 2nd protocol amendment. The multi-centre approval was given on 23/12/16.
As a result, the default patient pool was from inpatients, as our original intention was to treat incidence based (post/sub-acute neglect with recruitment done from within each hospital). Yet due the emerging recruitment issues we then also added prevalence and amended our original protocol to add outpatients (patients who had exited other clinical trials in particular).
Although recruitment improved significantly after these implementations, the trial was stopped in June 2017 due to low recruitment.
Sample size
The sample size was pragmatic and intended to inform the design of a larger definitive trial. A sample size of 60 (n = 15 per each of the 4 groups) was planned to give the study 90% power to detect a difference in means of 1.5 standard deviations, with a 5% level of significance for secondary outcome measures, allowing for a drop-out rate of 33%.
Randomization and masking
Participants were randomly allocated to 1 of 4 groups (tDCS, behavioural training, combined, control; see below) in a 1:1:1:1 ratio by a computerized programme, with stratification to balance the groups for neglect severity (measured with the conventional measures of the Behavioural Inattention Test (BIT), with participant’s scores below 115 indexed as high severity). Participant’s group was revealed to the research assistant delivering the treatment via pre-prepared envelopes completed by the Robertson Centre for Biostatistics (who conducted this randomization in advance of recruitment). In view of the nature of the interventions, participants and the Research Assistant delivering the treatment were aware of the group allocation, but allocations were concealed from outcome assessors. This concealment was maintained throughout the trial.
Procedures
Group allocation was achieved by opening the pre-prepared envelopes (that balanced the groups for severity of neglect symptoms) after the Behavioural Inattention Test (1 of the 7 outcome measures, see below) was administered and scored. The 4 groups were: (1) Transcranial direct current stimulation (tDCS); (2) Behavioural Training; (3) Combined Intervention and (4) Control Training.
(1). Transcranial direct current stimulation (tDCS)
A 1 mA direct current was applied for 15 min using a battery-driven constant current stimulator (NeuroConn GmbH, Germany), with additional 30s ramp-up and ramp-down periods. A 5 × 5 cm cathode was centred over the left parietal cortex (P3 of the 10–20 International EEG system) and a 5 × 7 cm anode (reference) was situated just anterior to the vertex, with the 5 cm edge aligned with electrode position Cz. Both carbon rubber electrodes were encased in 0.9% NaCl saline-soaked sponges and were held in place using rubber headbands. Participants sat passively during the stimulation period.
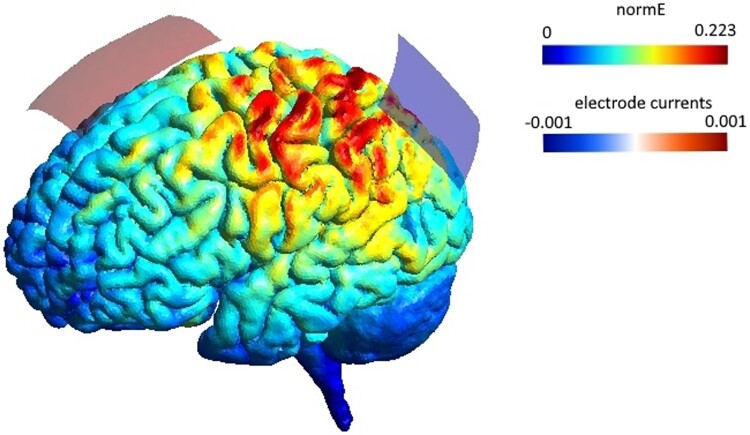
At the time of trial inception only Sparing et al. (2009) had compared right anodal, left cathodal and sham stimulation in a single session, and left cathodal stimulation had the greater effect on alleviating neglect symptoms. As a result, we wrote to Sparing to recreate their stimulation procedure exactly. The flow diagram (Figure 1) demonstrates which anatomic structures were most likely affected by the electrode montage, bearing in mind that this would be the assumed flow in a healthy brain. Modelling of this kind was not standard procedure at the time of trial inception.
(2). Behavioural Training
Figure 1.
Electrode montage and simulated current flow, performed with SimNIBS 3.1.0 (Thielscher et al., 2015). The 5 × 7 cm anode was positioned just anterior to the vertex (Cz) and the 5 × 5 cm cathode positioned over the left parietal cortex (P3). The normalized induced electric field (normE) is shown in V/m and the current induced by each electrode in mA.
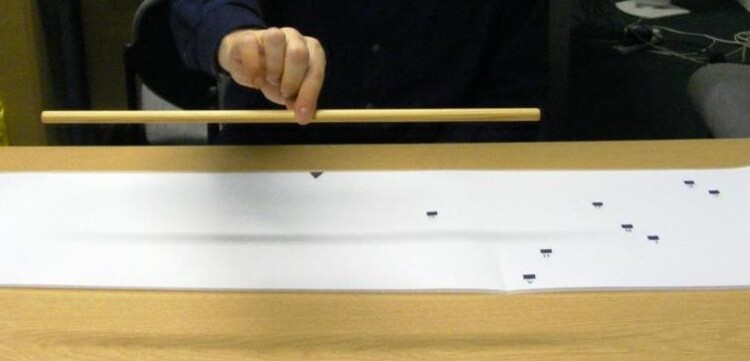
Based on Harvey et al. (2003, 2010) and Robertson et al. (1997), this intervention involved picking up and balancing wooden rods at their midpoint (Figure 2). Participants were seated at a table in front of a mat measuring 140 × 30 cm. Nine black squares labelled A-I were positioned on the left side of the mat, and were used as starting positions for each trial. Three wooden rods of different lengths (50, 75 and 100 cm, all 1.1 cm in diameter) were placed in front of the participant. At the start of each trial, participants were asked to pick up one of the rods (short, medium or long) and place the left end of the rod on one of the 9 starting positions on the mat. They were then instructed to pick up the rod at its midpoint with their right hand using a pincer grip, and to assess whether the rod was balanced at its midpoint. If they felt that it was unbalanced, they were instructed to place the rod back down on its starting position and to adjust their grip (usually leftwards) until the rod was balanced. The training was intended for 15 min and involved roughly 54 trials.
(3). tDCS and Behavioural Training (Combined Intervention)
Figure 2.
Example of the Behavioural Training.
Both the tDCS and behavioural training were administered simultaneously. The behavioural training began as soon as the tDCS equipment had fully ramped up to 1 mA.
(4). Control Training
The control training was identical to the behavioural training, but participants were instructed to lift up the rod at its rightmost end rather than at its midpoint. They thus performed a motor task, yet did not receive corrective proprioceptive nor visual feedback on their actions.
The majority of participants were outpatients by the time they were tested, however, some remained inpatients throughout (see Table 3). Participants travelled (or if they were inpatients, were transported) either to a Clinical Research Facility or simply to a local hospital testing room, where the outcome assessments and the delivery of the 10 intervention sessions were performed (a single session lasted no longer than 1 h). On 3 separate occasions throughout the study period, secondary outcome measures were given by researchers blinded to the group allocation: before the interventions, after the 10 interventions (with a target period of 3 weeks overall), and at a late time point (6 months after the interventions (follow-up)).
Table 3. Characteristics of the randomized cohort.
| Intervention | ID | Age | Sex | Neglect severity | Days from stroke to T1 | Days to complete interventions (T2-T1) | Days to follow-up (T3-T2) | Aetiology | Lesion location | Visual field deficit | Inpatient/Outpatient at time of intervention | SAEs |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| tDCS | 1 | 78 | M | High | 34 | 23 | Died | I | Right temporo-occipital + thalamus | Y | IP | Unrelated: DVT + PTE, died |
| 2 | 56 | M | High | 495 | 16 | 264 | I | Right PCA (temporo-occipito-parietal + basal ganglia) | Y | IP | – | |
| 3 | 61 | F | Low | 39 | 15 | 179 | I | Right MCA | N | OP | – | |
| 4 | 67 | F | Low | 1394 | 34 | 192 | H | Right fronto-parietal | N | OP | – | |
| 5 | 64 | M | Low | 786 | 66 | 181 | H | Right parietal | N | OP | – | |
| 6 | 70 | F | Low | 68 | 47 | 178 | I | Right MCA | N | OP | – | |
| Median | 66 | 282 | 28.5 | 181 | ||||||||
| Behavioural training | 7 | 54 | M | High | 38 | 19 | Lost Contact | I | Right MCA | Not testable | IP | – |
| 8 | 79 | M | High | 74 | 46 | 193 | I | Right PCA | Y | OP | – | |
| 9 | 75 | M | Low | 32 | 42 | 217 | I | Right MCA + temporal | N | OP | – | |
| 10 | 72 | F | Low | 37 | 15 | Died | I | Right fronto-parietal | Y | IP | Unrelated: died | |
| 11 | 59 | F | Low | 2020 | 43 | Lost Contact | H | Right fronto-parietal | N | OP | – | |
| 12 | 62 | M | Low | 55 | Withdrew | Withdrew | H | Right basal ganglia | N/A tested | OP | – | |
| Median | 67 | 47 | 42 | 205 | ||||||||
| Combined intervention | 13 | 84 | F | High | 36 | Unable | Withdrew | I | Right uncus, hippocampus, internal capsule, pons and cerebellum | Y | IP | – |
| 14 | 73 | F | High | 34 | 60 (6/10 sessions) | Lost Contact | I | Right MCA | Not testable | IP | – | |
| 15 | 65 | M | Low | 45 | 21 | 193 | I | Right fronto-parietal + temporo-occipital + thalamus | Y | OP | Unrelated: new onset seizures | |
| 16 | 62 | M | Low | 1727 | 28 | 192 | I | Right MCA | N | OP | – | |
| 17 | 73 | F | Low | 75 | 51 | 189 | I | Nil new, old right frontal | N | OP | – | |
| 18 | 66 | F | Low | 426 | 44 | Lost Contact | I | Right MCA | N | OP | – | |
| Median | 70 | 60 | 44 | 192 | ||||||||
| Control training | 19 | 45 | F | High | 38 | 36 | Lost Contact | I | Right MCA | N | IP | – |
| 20 | 60 | M | High | 78 | 11 | 229 | I | Right MCA + internal capsule | Y | IP | Unrelated: PTE | |
| 21 | 76 | M | Low | 39 | 25 | 199 | I | Right MCA | Y | OP | – | |
| 22 | 67 | F | Low | 1859 | Withdrew | Withdrew | I | Right MCA | N/A | OP | – | |
| 23 | 63 | M | Low | 1028 | 29 | 210 | I | Right parietal | N | OP | – | |
| 24 | 52 | M | Low | 458 | 38 | 227 | H | Right fronto-parietal | Y | OP | – | |
| Median | 62 | 268 | 29 | 218.5 |
Note: Neglect Severity: high = BIT score of <115. Visual Field Deficit: not testable: 2 patients could not be assessed due to their inability to sustain attention sufficiently to complete the test, N/A: 2 patients withdrew before the in-house testing could be performed. Unable: the patient could not tolerate the tDCS montage as experienced severe itching and was unable to carry out behavioural training. T1: time of baseline secondary outcome measure testing, T2: time of post-intervention testing, T3: time of follow-up testing. SAE: Serious Adverse Event. DVT: Deep Vein Thrombosis. PTE: Pulmonary Thromboembolism. IP: inpatient. OP: outpatient.
As the primary outcome of the study aim was feasibility of repeated stimulation sessions in patients with neglect, rather than efficacy, the extra time and cost of adding either a Sham condition (and see issues with this in Greinacher et al., 2019) or a control site was not considered to be warranted at this early trial stage (but see discussion for an elaboration of this).
Primary and secondary outcome measures/planned statistical analyses
Our primary outcome measures were rates of participants recruited into the study out of the number of confirmed strokes/estimated right hemisphere strokes (separately for the different recruitment sites), inclusion and retention at primary endpoint and late (6 months) follow up, and compliance rates (as measured by adherence to task instructions during the interventions). Secondary outcome measures focussed on effect size and variance scores of the neglect, activities of daily living and quality of life measures taken directly after the interventions and at 6 months follow-up.
The secondary outcome measures involved the following assessments:
(1). Behavioural Intervention Test (BIT) conventional sub-tests (line cancellation, letter cancellation, star cancellation, line bisection, figure & shape copying and representational drawing) (Wilson et al., 1987). Total scores were calculated for each participant, with a maximum possible score of 146. A score of <129 was considered to be indicative of neglect.
(2). Line bisection (Schenkenberg et al., 1980). Nine horizontal lines measuring 20.7 × 1 cm were presented individually on A4 sheets of paper. Each line was presented at the vertical midpoint of the page, and was jittered by 1.3 or 2.6 cm to the left or right of the horizontal midpoint. Participants were asked to mark the point at which they perceived the horizontal midpoint of the line to be using a pen in their right hand. Separately for each line, the distance of this mark from the veridical midpoint was calculated (in mm) and then averaged across all 9 trials. A mean deviation of >6 mm from the veridical midpoint is indicative of neglect (Rossit et al., 2009).
(3). The Balloons test (Edgeworth et al., 1998). Subtests A and B were administered on separate A3 sheets of paper. In both subtests, participants were asked to cross out 20 targets which were randomly interspersed amongst distractors within 2 min (10 targets on the left of the page and 10 on the right). Subtest A involved parallel, automatic processing, in which 20 target “balloons” were hidden within an array of distractor circles. Subtest B involved a more challenging, serial search process, where 20 target circles were located within an array of distractor balloons. A lateralized score was calculated for each subtest, representing the percentage of cancellations that were located on the left side of space (a score of <44% was indicative of left neglect, as was a total cancellation score of <17/20).
(4). The Broken Hearts test is a subtest of the tablet-based version of the Oxford Cognitive Screen (Demeyere et al., 2015). Subtests A and B were administered on a Microsoft SurfacePro 3 tablet using an electronic pen. Participants were presented with an array of hearts on the screen. Some hearts had “gaps” cut out of either their left or their right side, and a further 50 hearts were complete with no gaps. In subtest A, participants were asked to cancel the complete hearts on the screen in a maximum of 3 min. In subtest B, participants were instructed to do the same as per subtest A, however their cancellation mark immediately disappeared from the screen, thus requiring participants to remember which hearts they had already scored out. In both subtests, a score of <42 (maximum = 50) was considered indicative of neglect. Egocentric and allocentric neglect scores were then calculated separately. For egocentric neglect (i.e., space asymmetry), the total number of cancellations on the left side of the screen was subtracted from the total number of cancellations on the right side (a score of >2 was indicative of egocentric neglect). Allocentric neglect (i.e., object asymmetry) was assessed by identifying the number of hearts with a gap that the participant had erroneously cancelled. The number of cancelled right-gap hearts was subtracted from the number of cancelled left-gap hearts (a score of >1 was indicative of allocentric neglect).
(5). Visual field testing. An in-house test lasting 3–4 min was administered on a PC (Learmonth et al., 2017a; Rossit et al., 2009). Small black square targets (10 × 10 pixels) were presented individually for 150 ms at one of 36 spatial locations on the screen (6 vertical and 6 horizontal positions). Participants were asked to fixate on a cross in the centre of the screen and to press the space bar on a keyboard when they detected a target. Each target was presented twice in a random order, interspersed with 24 additional “catch” trials in which no target was presented. The spatial locations in which the participant had missed targets were identified, and each participant was classified as either showing presence or absence of visual field impairment, in combination with neuro-ophthalmologist review letters, where available.
(6). Stroke Impact Scale (SIS, Duncan et al., 1999). The SIS (version 3.0) is a 59-item questionnaire measuring 8 health-related quality of life domains: strength, hand function, mobility, activities of daily living, memory, communication, emotion, and participation. Each domain is scored on a scale from 0 (highly impaired) to 100 (unimpaired), and a final question assesses the participants’ own perceived percentage recovery from their stroke, from 0-100%. The SIS was completed by the participants’ carer when possible, although in a number of cases no carer was available and the test was completed by the participant themselves.
(7). Beck Depression Inventory (BDI-II, Beck et al., 1996). The BDI-II is a 21-item, self-report questionnaire used to evaluate depression. The scale has a maximum score of 63, where mild depression >14, moderate >20, and severe depression >29.
Lesion mapping
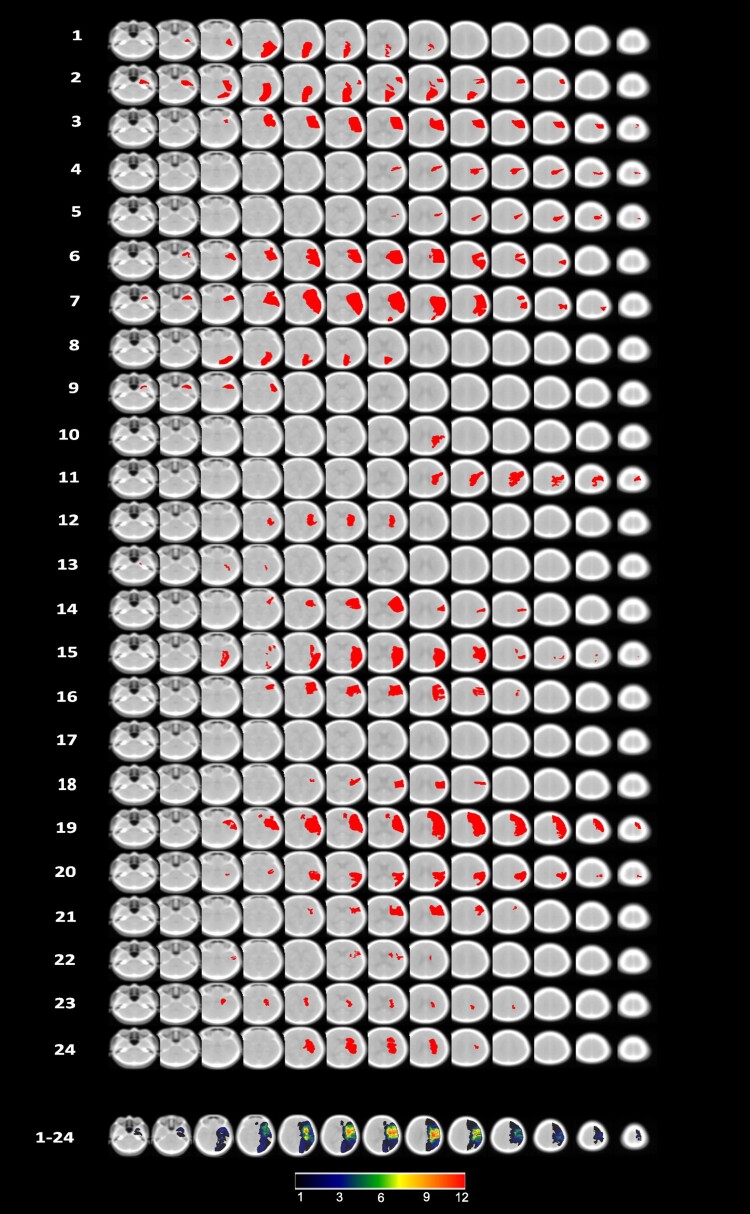
Lesion mapping was performed using CT and MRI scans that had been obtained as part of routine stroke care. Where an individual had both CT and MRI scans available, the MRI was selected in the majority of cases. Where participants had their stroke many months or years previously and a subsequent scan was available that more accurately visualized their lesion at the time of intervention, this later scan was used (the clinical neuro-radiologist drove this decision). First, the raw DICOM data were converted to NIfTI format using the dcm2nii function from the MRIcro toolbox (www.mricro.com). They were then spatially normalized to a standardized older adult template (scct.nii) using the clinical toolbox for SPM12 (Rorden et al., 2012). Slices that corresponded to, or were closest to the MNI z-coordinates of −44, −34, −24, −14, −4, 6, 16, 26, 36, 46, 56, 66 and 76 were selected for each scan. Comparisons of the identified lesions with the patients’ clinical scans were then performed by a clinical neuro-radiologist, who was blinded to the outcome measures and intervention arm to which the participant was allocated, in a final cross-check. Lesions were mapped onto the standard older adult template developed by Karnath and colleagues (Rorden et al., 2012) and a cumulative overlap map was derived for all 24 participants.
Results
Primary outcome
Recruitment
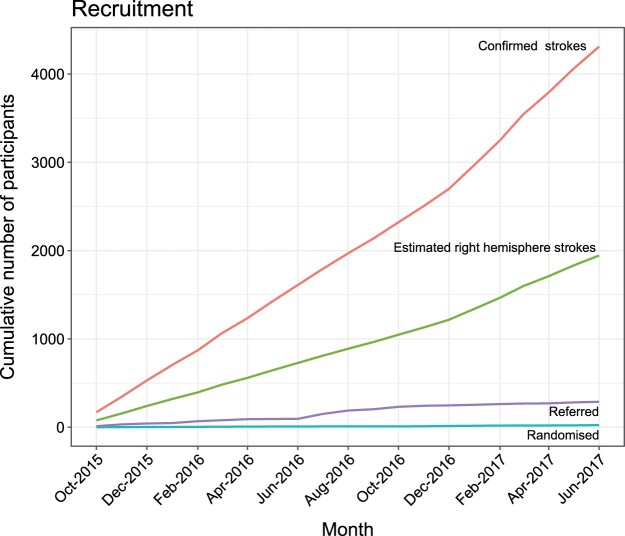
Twenty-four participants (11 female) were randomized between October 2015 and June 2017. The overall number of patients referred for screening across the recruitment sites was 288 out of a total of 4311 confirmed stokes (and an estimated total right hemisphere strokes of 1941) admitted during the recruitment period. The percentage referral was thus 6.7% out of all confirmed strokes (and 14.8% out of estimated right hemisphere strokes). The percentage randomized was 0.6% (and 1.2% respectively). Figure 3 gives a detailed breakdown of the cumulative confirmed and estimated strokes, as well as referral and randomization rates over time. Table 2 lists overall numbers separately for the 4 recruitment sites (Queen Elizabeth University Hospital (QEUH), Glasgow Royal Infirmary (GRI), Royal Alexandra Hospital (RAH) and Lanarkshire).
Figure 3.
Number of confirmed (red line), estimated right hemisphere (green line), referred (purple line) and randomized strokes (blue line) presented cumulatively over the recruitment period.
Table 2. Number of patients referred and randomized out of all patients admitted with confirmed stroke (Scottish Stroke Care Unit (SSCA) records) and out of estimated (estimated at 45% of the total confirmed strokes) right hemisphere strokes.
| Recruitment Site | N of confirmed strokes | N of estimated right hemisphere strokes (estimated at 45% of total confirmed) | N referred to trial | N randomized into trial |
|---|---|---|---|---|
| QEUH | 2038 | 919 | 161 | 19 |
| GRI | 1100 | 495 | 113 | 1 |
| RAH | 678 | 305 | 11 | 2 |
| Lanarkshire | 495 | 223 | 3 | 2 |
Note: Data listed separately for the 4 recruitment sites: QEUH: Queen Elizabeth University Hospital; GRI: Glasgow Royal Infirmary; RAH: Royal Alexandra Hospital.
As is apparent from Table 1, the 4 recruitment sites differed in the size of their confirmed strokes as well as their referral rates, and the conversions achieved from these referrals.
Inclusion, retention & compliance, cohort characteristics
Overall inclusion/retention/compliance rates
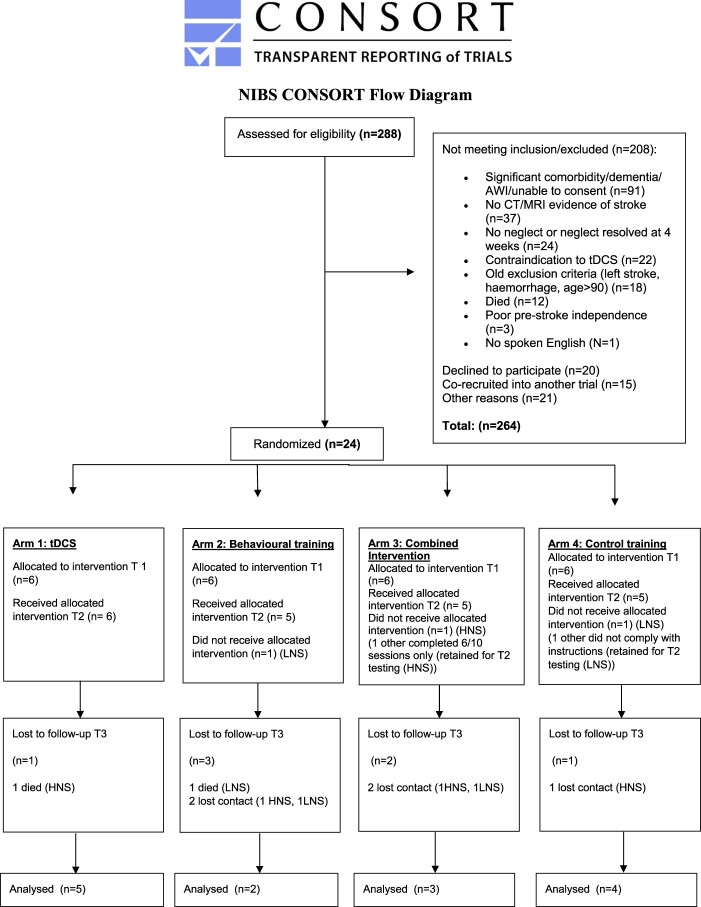
Of the 288 referrals, 8.3% (24) of participants were randomized into the trial. The Consort Chart below (Figure 4) gives a detailed breakdown of the reasons for exclusion. It is possible that both the referral and randomization rates reflect a bias towards excluding more severely impaired patients as it was clear from the protocol that patients had to undergo 10 sessions and that travel was involved for some of them too.
Figure 4.
Consort Flow Diagram. T1: time of baseline secondary outcome measure testing, T2: time of post-intervention testing, T3: time of follow-up testing. LNS: low neglect severity (BIT score of >115). HNS: high neglect severity (BIT score of <115).
Two participants (with low neglect severity (LNS)) withdrew after randomization (1 Behavioural, 1 Control Training). One participant (Combined Intervention, with high neglect severity (HNS)) could not tolerate the tDCS, and was unable to carry out the behavioural training, and another high severity participant managed only 6/10 sessions. One low neglect severity participant (Control Training) did not follow the control training instructions (observation of patient by researcher giving intervention). These last 2 participants still performed the secondary outcome measures post intervention. Overall retention at the primary endpoint after the intervention was thus still high with 87.5% (21/24) of participants remaining. Of these participants, 67% (14) remained at the 6 months follow up point, while 2 participants had died and 5 did not respond to follow up communication.
Characteristics of the randomized cohort
Twenty-four patients (13 males, 11 females) with a mean age of 65 years were recruited. The time interval between stroke to pre-intervention testing varied greatly among participants (Table 3) due to some of these having been recruited from previously completed trials.
Serious adverse events (SAEs further details)
Two participants died of unrelated causes prior to the 6 months follow-up testing and 1 participant with low neglect severity, who had received the combined intervention, experienced new onset seizures approximately 2 months after receiving the 10 sessions. This was deemed as possibly intervention related by the neurologist all SAEs were reported to, and the iDMC were alerted. The iDMC judged the seizures to be unrelated to the intervention, so no further action was taken. All other serious adverse events were deemed to be unrelated to the interventions and are listed in Table 3.
Lesion mapping
Figure 5 shows individual lesion maps of each randomized patient, and a combined map of lesion frequency. The final dataset involved 16 CT and 8 MRI scans. Participant 17 showed no abnormality.
Figure 5.
Lesion maps for each of the 24 individuals, projected onto 13 axial slices of the standardized older adult MNI template ((scct.nii) developed by Rorden et al., 2012), plus the cumulative overlap map. Orientation is per neuroscience standard with the right hemisphere on the right side in each slice.
Secondary outcome measures
Raw data
Due to the low recruitment rate we were unable to perform robust statistical tests on the secondary outcome measures. Individual participant results are detailed in Tables 4–6.
Table 5. Scores for each participant, separated by secondary outcome measures.
| Group | Hearts A egocentric4 | Hearts A allocentric5 | Hearts B egocentric4 | Hearts B allocentric5 | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| ID | T1 | T2 | T3 | T1 | T1 | T2 | T1 | T1 | T2 | T1 | T1 | T2 | |
| tDCS | 1 | 7 | 2 | – | −2 | 1 | 4 | 3 | 3 | – | 1 | 4 | – |
| 2 | 17 | 10 | 11 | 0 | 1 | 4 | 9 | 5 | 7 | 1 | 4 | 2 | |
| 3 | 0 | 0 | 4 | 1 | 3 | 2 | 1 | −2 | −2 | 3 | 2 | 0 | |
| 4 | 4 | 3 | −6 | 2 | 4 | −5 | 0 | −3 | 5 | 4 | −5 | −3 | |
| 5 | 1 | 1 | −3 | 2 | −2 | 1 | −4 | 3 | 4 | −2 | 1 | 2 | |
| 6 | 6 | 4 | 1 | 0 | 0 | 3 | 5 | −1 | 4 | 0 | 3 | 2 | |
| Mean | 5.83 | 3.33 | 1.40 | 0.50 | 1.17 | 1.50 | 2.33 | 0.83 | 3.60 | 1.17 | 1.50 | 0.60 | |
| Median | 5.00 | 2.50 | 1.00 | 0.50 | 1.00 | 2.50 | 2.00 | 1.00 | 4.00 | 1.00 | 2.50 | 2.00 | |
| Behavioural training | 7 | 12 | 9 | – | 16 | 15 | 16 | 8 | 14 | – | 15 | 16 | – |
| 8 | 10 | 10 | 11 | 6 | 6 | 5 | 7 | 11 | 6 | 6 | 5 | 1 | |
| 9 | 5 | 6 | 3 | 10 | 10 | 5 | 4 | −5 | −1 | 10 | 5 | 2 | |
| 10 | 2 | −1 | – | 16 | 18 | 17 | −7 | −6 | – | 18 | 17 | – | |
| 11 | −2 | −3 | – | 0 | 4 | −2 | −6 | 0 | – | 4 | −2 | – | |
| 12 | 1 | – | – | 3 | 1 | – | 3 | – | – | 1 | – | – | |
| Mean | 4.67 | 4.20 | 7.00 | 8.50 | 9.00 | 8.20 | 1.50 | 2.80 | 2.50 | 9.00 | 8.20 | 1.50 | |
| Median | 3.50 | 6.00 | 7.00 | 8.00 | 8.00 | 5.00 | 3.50 | 0.00 | 2.50 | 8.00 | 5.00 | 1.50 | |
| Combined intervention | 13 | 6 | – | – | 3 | 1 | – | 3 | – | – | 1 | – | – |
| 14 | 19 | 17 | – | 24 | 5 | 3 | 19 | 1 | – | 5 | 3 | – | |
| 15 | 3 | – | 0 | 0 | 5 | 10 | 8 | 11 | 2 | 5 | 10 | 9 | |
| 16 | 0 | 1 | −1 | 0 | 1 | 5 | 0 | −1 | 6 | 1 | 5 | 10 | |
| 17 | −1 | 1 | −6 | −1 | 0 | 2 | −5 | −2 | −10 | 0 | 2 | −2 | |
| 18 | −2 | 0 | – | 2 | 1 | 1 | −4 | 4 | – | 1 | 1 | – | |
| Mean | 4.17 | 4.40 | −2.33 | 4.67 | 2.17 | 4.20 | 3.50 | 2.60 | −0.67 | 2.17 | 4.20 | 5.67 | |
| Median | 1.50 | 1.00 | −1.00 | 1.00 | 1.00 | 3.00 | 1.50 | 1.00 | 2.00 | 1.00 | 3.00 | 9.00 | |
| Control training | 19 | 15 | 18 | – | 1 | 4 | 1 | 13 | 8 | – | 4 | 1 | – |
| 20 | 5 | 10 | 9 | 1 | 2 | 0 | 6 | 4 | 9 | 2 | 0 | 0 | |
| 21 | 14 | 8 | 1 | 0 | −2 | 0 | 2 | 5 | −1 | −2 | 0 | 0 | |
| 22 | 1 | – | – | 2 | 4 | – | −1 | – | – | 4 | – | – | |
| 23 | −5 | −5 | −5 | 0 | 2 | 0 | 5 | 4 | −2 | 2 | 0 | −3 | |
| 24 | 5 | 2 | 2 | 1 | 0 | 0 | 8 | 1 | 2 | 0 | 0 | 0 | |
| Mean | 5.83 | 6.60 | 1.75 | 0.83 | 1.67 | 0.20 | 5.50 | 4.40 | 2.00 | 1.67 | 0.20 | −0.75 | |
| Median | 5.00 | 8.00 | 1.50 | 1.00 | 2.00 | 0.00 | 5.50 | 4.00 | 0.50 | 2.00 | 0.00 | 0.00 | |
Notes: 4. Hearts (egocentric). Negative change scores indicate a reduction of egocentric neglect (i.e., more cancellations on the left in T2 relative to T1). 5. Hearts (allocentric). Negative change scores indicate a reduction of allocentric neglect (i.e., fewer errors on the left in T2 relative to T1). T1: time of baseline secondary outcome measure testing, T2: time of post-intervention testing, T3: time of follow-up testing.
Table 4. Scores for each participant, separated by secondary outcome measures.
| Group | BIT1 | Line bisection2 | Balloons A3 | Balloons B3 | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| ID | T1 | T2 | T3 | T1 | T2 | T3 | T1 | T2 | T3 | T1 | T2 | T3 | |
| tDCS | 1 | 45 | 58 | – | 7.69 | 5.94 | – | 25.00 | 18.18 | – | – | 100.0 | – |
| 2 | 56 | 121 | 131 | 0.74 | −1.67 | 0.90 | 0.00 | 45.00 | 50.00 | 0.00 | 61.54 | 43.75 | |
| 3 | 134 | 142 | 144 | −1.01 | −0.43 | −0.01 | 52.63 | 50.00 | 50.00 | 47.06 | 35.71 | 50.00 | |
| 4 | 137 | 140 | 136 | 0.85 | 0.42 | 0.48 | 50.00 | 50.00 | 50.00 | 62.50 | 63.60 | 52.94 | |
| 5 | 138 | 142 | 143 | 0.62 | 0.53 | 0.02 | 50.00 | 47.37 | 50.00 | 50.00 | 56.25 | 50.00 | |
| 6 | 121 | 113 | 127 | −0.31 | −0.73 | −0.37 | 41.18 | 50.00 | 50.00 | 14.29 | 12.50 | 25.00 | |
| Mean | 105.17 | 119.33 | 136.20 | 1.43 | 0.68 | 0.20 | 36.47 | 43.43 | 50.00 | 34.77 | 54.93 | 44.34 | |
| Median | 127.50 | 130.50 | 136.00 | 0.68 | −0.01 | 0.02 | 45.59 | 48.69 | 50.00 | 47.06 | 58.90 | 50.00 | |
| Behavioural training | 7 | 89 | 122 | – | 3.88 | 2.36 | – | 0.00 | 40.00 | – | 14.29 | 42.86 | – |
| 8 | 75 | 117 | 104 | 8.56 | 0.65 | 6.77 | 35.71 | 52.63 | 0.00 | 25.00 | 54.55 | 0.00 | |
| 9 | 142 | 145 | 142 | 0.96 | −0.02 | 0.01 | 52.63 | 50.00 | 55.56 | 42.86 | 53.85 | 56.25 | |
| 10 | 117 | 132 | – | 0.66 | 0.29 | – | 50.00 | 50.00 | – | 46.67 | 58.30 | – | |
| 11 | 135 | 136 | – | −0.63 | −0.10 | – | 50.00 | 50.00 | – | 52.90 | 56.25 | – | |
| 12 | 132 | – | – | 0.51 | – | – | 47.37 | – | – | 27.27 | – | – | |
| Mean | 115 | 130.4 | 123 | 2.32 | 0.64 | 3.39 | 39.29 | 48.53 | 27.78 | 34.83 | 53.16 | 28.13 | |
| Median | 124.5 | 132 | 123 | 0.81 | 0.29 | 3.39 | 48.69 | 50.00 | 27.78 | 35.07 | 54.55 | 28.13 | |
| Combined intervention | 13 | 30 | – | – | 8.47 | – | – | 0.00 | – | – | 0.00 | – | – |
| 14 | 81 | 109 | – | 0.57 | 1.63 | – | 23.08 | 25.00 | – | 0.00 | 0.00 | – | |
| 15 | 123 | 136 | 137 | 0.01 | 0.06 | 0.15 | 52.63 | 50.00 | 50.00 | 50.00 | 50.00 | 70.00 | |
| 16 | 124 | 135 | 137 | 0.27 | −0.20 | −0.44 | 50.00 | 50.00 | 50.00 | 66.67 | 50.00 | 53.33 | |
| 17 | 122 | 125 | 117 | −0.17 | −0.20 | −0.32 | 55.56 | 52.63 | 50.00 | 52.94 | 66.67 | 57.14 | |
| 18 | 138 | 126 | – | −0.15 | −0.69 | – | 50.00 | 50.00 | – | 56.25 | 44.44 | – | |
| Mean | 103.00 | 126.20 | 130.33 | 1.50 | 0.12 | −0.20 | 38.55 | 45.53 | 50.00 | 37.64 | 42.22 | 60.16 | |
| Median | 122.50 | 126.00 | 137.00 | 0.14 | −0.20 | −0.32 | 50.00 | 50.00 | 50.00 | 51.47 | 50.00 | 57.14 | |
| Control training | 19 | 108 | 124 | – | −0.12 | −0.57 | – | 23.08 | 50.0 | – | 0.00 | 30.00 | – |
| 20 | 98 | 114 | 126 | −0.76 | 0.36 | 0.30 | 0.00 | 0.00 | 44.44 | 0.00 | 11.11 | 28.57 | |
| 21 | 125 | 137 | 135 | −0.30 | −0.34 | −0.12 | 58.82 | 52.63 | 50.00 | 57.14 | 55.56 | 43.75 | |
| 22 | 134 | – | – | −0.68 | – | – | 52.63 | – | – | 27.27 | – | – | |
| 23 | 142 | 132 | 138 | 0.39 | 0.08 | 0.42 | 50.00 | 50.00 | 50.00 | 47.06 | 50.00 | 52.94 | |
| 24 | 135 | 144 | 141 | 0.18 | −1.08 | −0.55 | 50.00 | 50.00 | 50.00 | 50.00 | 33.30 | 47.06 | |
| Mean | 123.67 | 130.20 | 135.00 | −0.22 | −0.31 | 0.01 | 39.09 | 40.53 | 48.61 | 30.25 | 35.99 | 43.08 | |
| Median | 129.50 | 132.00 | 136.50 | −0.21 | −0.34 | 0.09 | 50.00 | 50.00 | 50.00 | 37.17 | 33.30 | 45.41 | |
Notes: 1. Behavioural Inattention Test (BIT). Positive change scores indicate a reduction of neglect (i.e., higher score in T2 relative to T1). 2. Line bisection. Negative change scores indicate a reduction of neglect (i.e., line bisected further to the left in T2 relative to T1). 3. Balloons (A&B). Positive change scores indicate a reduction of neglect (i.e., higher percentage of cancellations on the left in T2 relative to T1). T1: time of baseline secondary outcome measure testing, T2: time of post-intervention testing, T3: time of follow-up testing.
Table 6. Scores for each participant, separated by secondary outcome measures.
| Group | BDI6 | SIS ADL/IADL7 | SIS Percentage recovery8 | |||||||
|---|---|---|---|---|---|---|---|---|---|---|
| ID | T1 | T2 | T3 | T1 | T2 | T3 | T1 | T2 | T3 | |
| tDCS | 1 | 10 | 4 | – | 50 | 95.8 | – | 20 | 15 | – |
| 2 | 3 | 7 | 2 | 90.0 | 70.0 | 100.0 | – | 15 | 70 | |
| 3 | 6 | 3 | 21 | 60.0 | 90.0 | 97.5 | 60 | 80 | 90 | |
| 4 | 3 | 3 | 1 | 75.0 | 80.0 | 90.0 | 80 | 80 | 80 | |
| 5 | 25 | 11 | 9 | 55.0 | 55.0 | 55.0 | 30 | 40 | 40 | |
| 6 | – | – | – | – | – | – | – | – | – | |
| Mean | 9.40 | 5.60 | 8.25 | 66 | 78.16 | 85.63 | 47.5 | 46 | 70 | |
| Median | 6.00 | 4.00 | 5.50 | 60 | 80 | 93.75 | 45 | 40 | 75 | |
| Behavioural training | 7 | 30 | 19 | – | 5 | 15 | – | 20 | 40 | – |
| 8 | 5 | 3 | 1 | 62.5 | 90 | 90 | 50 | 50 | 80 | |
| 9 | 5 | 4 | 3 | 33.3 | 82.5 | 90 | 60 | 80 | 80 | |
| 10 | 8 | 14 | – | 57.5 | 41.7 | – | 45 | 35 | – | |
| 11 | 30 | 28 | – | 35 | 32.5 | – | – | 35 | – | |
| 12 | – | – | – | – | – | – | – | – | – | |
| Mean | 15.60 | 13.60 | 2.00 | 38.66 | 52.34 | 90 | 43.75 | 48 | 80 | |
| Median | 8.00 | 14.00 | 2.00 | 35 | 41.7 | 90 | 47.5 | 40 | 80 | |
| Combined intervention | 13 | – | – | – | – | – | – | – | – | – |
| 14 | 13 | 19 | – | – | – | – | – | – | ||
| 15 | 9 | 8 | 6 | 62.5 | 87.5 | 86.1 | 70 | 80 | 70 | |
| 16 | 27 | 44 | 33 | 35 | 2.5 | 37.5 | 70 | 40 | 70 | |
| 17 | 12 | 6 | – | 85 | 86.1 | 83.3 | 60 | 80 | 60 | |
| 18 | 6 | 4 | – | 97.5 | 97.5 | – | 60 | 60 | – | |
| Mean | 13.40 | 16.20 | 19.50 | 70 | 68.4 | 68.97 | 65 | 65 | 66.67 | |
| Median | 12.00 | 8.00 | 19.50 | 73.75 | 86.8 | 83.3 | 65 | 70 | 70 | |
| Control training | 19 | 24 | 23 | – | 36.1 | 37.5 | – | 20 | 50 | – |
| 20 | 3 | 0 | 11 | 82.5 | 92.5 | 70.0 | 75 | 80 | – | |
| 21 | 6 | 5 | 7 | – | – | – | – | – | – | |
| 22 | – | – | – | 47.5 | 44.4 | 58.3 | 60 | 60 | 30 | |
| 23 | 13 | 16 | 34 | – | 86.1 | 2.5 | – | 70 | 40 | |
| 24 | 12 | 9 | 37.5 | 82.5 | – | 70 | 75 | – | ||
| Mean | 11.60 | 10.60 | 17.33 | 50.9 | 68.6 | 43.6 | 56.25 | 67 | 35 | |
| Median | 12.00 | 9.00 | 11.00 | 42.5 | 82.5 | 58.3 | 65 | 70 | 35 | |
Notes: 6. Beck Depression Inventory (BDI). Negative change scores indicate an improvement (i.e., lower score in T2 relative to T1). 7. Stroke Impact Scale (ADL/IADL). Positive change scores indicate an improvement in activities of daily living (i.e., higher score in T2 relative to T1). 8. Stroke Impact Scale (Stroke recovery). Positive change scores indicate an improvement in the patient’s subjective total recovery (i.e., higher score in T2 relative to T1). T1: time of baseline secondary outcome measure testing, T2: time of post-intervention testing, T3: time of follow-up testing.
Discussion
Feasibility
This study has generated important feasibility data (recruitment, inclusion, retention, compliance rates (our primary outcome measures)) for future neglect trials intending to use tDCS as an intervention method. We found that 7% of confirmed strokes admitted during the study period were screened as potentially eligible. For the estimated right hemisphere strokes (local audit records did not list lesion side yet this is important to inform a tDCS protocol) this number was 15%, and 8% were randomized. The most common reason for exclusion was significant co-morbidity (including cognitive impairment) judged to prevent participants from undergoing 10 sessions of intervention (34.4%). This pattern was further reflected in the randomized sample, with 2/3rds of patients classified as low, compared to only 1/3rd categorized as high neglect severity. Unfortunately, it is well-known that neglect is associated with different cognitive and emotional disorders and the coincidence of persisting neglect and severe additional cognitive impairment tends to be high, due to the underlying large brain lesion. This was very likely the pattern for the patients excluded here (and large lesions were also present in over 50% of the randomized cases (see Figure 5)). So barriers to recruitment and retention were the perceived burden of the number of interventions and the requirement for hospital attendance.
Future trials may consider reduced sessions (although studies published since have applied very similar, if not more, tDCS sessions (Bang & Bong, 2015; Brem et al., 2014; Ladavas et al., 2015; Turgut et al., 2018; Yi et al., 2016)). Unfortunately, as none of these studies give feasibility data it is not clear how this was achieved: maybe researchers were recruiting from a greater number of centres and/or a different population base with different patient groups. The latter seems likely, and it is important to note that these studies will have almost certainly been undertaken within specialist rehabilitation hospitals that are common throughout the US and continental Europe. The UK generally does not admit people for long-term rehabilitation but provides a continuation of acute hospital care for those who are not fit to go home. Individuals who have been admitted to rehabilitation units tend not to have the same clinical issues as the unwell people with big strokes that characterized our inpatient sample. As such, some of the difficulties we encountered in this study may be specific to the UK stroke pathway. Nevertheless, providing this type of patient sampling information is essential for informing future definitive tDCS neglect trials.
When we designed the study we had safety concerns with respect to running the treatments as a home intervention. It is worth noting that there were no intervention-related SAEs in this trial, and that only 8.3% of referred patients had to be excluded due to tDCS contraindications. This number was lower than anticipated, although referral staff may have preselected on this criterion more closely than on others. Nonetheless we cannot advocate such an approach, as one of our patients had a seizure about 2 months after the combined intervention and even though this event was judged to be unrelated to the tDCS stimulation received, we would caution against unsupervised home intervention.
The recruitment data we summarize here are unique at present. No other phase I neglect trial that has investigated the effects of tDCS on neglect recovery has so far given such summative information (see Table 1), although Smit et al. (2015) list the exclusion criteria for their 89 identified patients that led to an inclusion of only 7 neglect patients into the tDCS trial. It is interesting that this trial (the only other trial who detailed exclusion reasons) had poor conversion rates just as we report here. None of the trials were PROBE or fully blinded prospective randomized trials either, so our data speaks to future trial designs.
Unfortunately, the small sample size limits severely the precision of the effect sizes of our secondary outcome measures. The challenge for designing future informative clinical tDCS trials is that the patient pool and sample size required are large, and researchers will have to make decisions about the number of sessions administered, stimulation duration and intensity, electrode montage and type of stimulation (anodal/cathodal/combined), balanced against practicality. Trials need to be large enough to show a credible effect, and the design needs to be based on realistic estimates of recruitment rates and effect size, which this study informs.
From this particular study, due to the low recruitment rates, we were unable to perform the necessary statistical analyses to determine whether tDCS had any effect on behavioural measures of neglect. Prior positive (and negative) evidence rests, as yet, on small scale studies lacking stringent randomization, appropriate control conditions and/or parallel designs. In addition, tDCS experiments more broadly have been limited by small effect sizes and often poor replicability (see Greinacher et al., 2019; Horvath et al., 2015; Learmonth et al., 2017b). A lot of future work must be undertaken to understand the basic mechanisms of tDCS action in healthy adults, particularly the influence of methodological design choices (e.g., electrode placement, intensity, stimulation duration, number of stimulation sessions) and how these factors might interact (perhaps even non-linearly) with a range of cognitive and physical states (Benwell et al., 2015; Fertonani & Miniussi, 2017). These issues are likely to be even greater in complexity when combined with the cortical heterogeneity observed in individuals after stroke (perhaps necessitating an individualized approach), and further question the overall viability of tDCS interventions for the treatment of hemispatial neglect.
Study limitations
Two patients (1 allocated to the control, the other to the behavioural arm) withdrew after randomization and anecdotally participants expressed a wish to undergo the tDCS intervention over the other allocations. Adding a control stimulation condition (e.g., a different electrode montage) would be important for future efficacy trials, although the majority of recent clinical and healthy participant studies include a fade-in fade-out sham protocol (but see Greinacher et al., 2019; Turi et al., 2019 for failure of placebo blinding during low-intensity tDCS fade-in fade-out sham stimulation in healthy adults).
Despite success in other trials (see Harvey et al., 2003, 2010; Rossit et al., 2019), 2 patients in our cohort struggled with the SIS and the BDI at some stage in the assessments, although they engaged with all the other outcome assessments. Future trials might want to consider using shorter and/or more sensitive scales (such as the HADS (Zigmond & Snaith, 1983) or the PRECiS (Patchick et al., 2016)) for an assessment of life quality and mood. In addition, we did not always manage to involve carers in the SIS assessment. This is problematic as neglect patients often lack insight into their impairment and scores thus lack validity.
Although we tried to keep each intervention session across all 4 groups constant at 15 min, for arms 2–4 this time varied between participants and sessions depending on how slow/fast individual rod lifts were performed. In future rather than trying to achieve a set number of lifts, it might be better to simply keep the time and thus the dose constant across the different interventions.
Finally, 1 patient was randomized as she showed strong clinical neglect acutely and also failed the BIT, but imaging data showed no new lesion.
Conclusions
This study investigated the feasibility of a trial in patients with post-stroke neglect, comparing tDCS with behavioural training, applied separately and in combination, to a control task, pre/post intervention and at a late time point. We aimed to assess if the proposed interventions could feasibly be applied in a clinical setting, and translate into a definitive, pragmatic multicentre trial.
Despite being conducted at a high-volume stroke centre, <10% of referred patients were eligible for the trial protocol, precluding us from achieving the envisaged recruitment rate of 60 patients, and further preventing the gathering of definitive data on our secondary outcomes measures (calculation of effects sizes). We thus established poor feasibility for a larger trial, with the primary reason for this being that, despite good referral rates, too many patients had to be excluded due to significant co-morbidity, that was judged to prevent them from undergoing 10 intervention sessions. Future hemispatial neglect studies of tDCS, either with or without behavioural training, should carefully consider the recruitment base and eligibility criteria, together with the duration and location of the planned interventions.
Acknowledgements
We would like to thank the Scottish Stroke Research Network Nurses for their assistance with patient recruitment (NHS Greater Glasgow & Clyde: Angela Welch, Nicola Day, Wilma Smith, Lesley McDonald & Belinda Manak and NHS Lanarkshire: Derek Esson & Lyndsey Forsyth). We thank Louise Richardson and Joanna Ashby for their support in identifying and screening patients in the wards, and Ozzy Dincarslan for referrals from the RATULS study. We further thank Occupational Therapists Lisa Slowey, Gillian Sweeney & Chloe Gordon who also referred patients to the study. Special thanks to Prof Valerie Pomeroy for her advice and guidance as Chair of the Trial Steering Committee (TSC) and similarly to Prof Thomas Schenk and Dr Harinath Chandrashekar for their roles as advisors of the independent Data Monitoring Committee (iDMC). We would also like to thank Dr Merle Ahrens for assisting the iDMC committee.
Funding Statement
This worked was funded by the Chief Scientist Office (ETM353).
Disclosure statement
No potential conflict of interest was reported by the author(s).
References
- Allman, C., Amadi, U., Winkler, A. M., Wilkins, L., Filippini, N., Kischka, U., Stagg, C., & Johansen-Berg, H. (2016). Ipsilesional anodal tDCS enhances the functional benefits of rehabilitation in patients after stroke. Science Translational Medicine, 8(330), 330re1–330re1. 10.1126/scitranslmed.aad5651 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Antal, A., Kincses, T. Z., Nitsche, M. A., Bartfai, O., & Paulus, W. (2004). Excitability changes induced in the human primary visual cortex by transcranial direct current stimulation: Direct electrophysiological evidence. Investigative Ophthalmology & Visual Science, 45(2), 702–707. 10.1167/iovs.03-0688 [DOI] [PubMed] [Google Scholar]
- Bang, D.-H., & Bong, S.-Y. (2015). Effect of combination of transcranial direct current stimulation and feedback training on visuospatial neglect in patients with sub-acute stroke: A pilot randomized controlled trial. Journal of Physical Therapy Science, 27(9), 2759–2761. 10.1589/jpts.27.2759 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beck, A. T., Steer, R. A., & Brown, G. K. (1996). Beck depression inventory-second edition manual. The Psychological Corporation. [Google Scholar]
- Benwell, C. S. Y., Learmonth, G., Miniussi, C., Harvey, M., & Thut, G. (2015). Non-linear effects of transcranial direct current stimulation as a function of individual baseline performance: Evidence from biparietal tDCS influence on lateralized attention bias. Cortex, 69, 152–165. 10.1016/j.cortex.2015.05.007 [DOI] [PubMed] [Google Scholar]
- Bornheim, S., Maquet, P., Croisier, J. L., Crielaard, J. M., & Kaux, J. F. (2018). Motor cortex transcranial direct current stimulation (tDCS) improves acute stroke visuo-spatial neglect: A series of four case reports. Brain Stimulation, 11(2), 459–461. 10.1016/j.brs.2017.11.018 [DOI] [PubMed] [Google Scholar]
- Bowen, A., Hazelton, C., Pollock, A., & Lincoln, N. B. (2013). Cognitive rehabilitation for spatial neglect following stroke. Cochrane Database Systematic Review, 2013(7), CD003586. 10.1002/14651858.CD003586.pub3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brem, A.-K., Unterburger, E., Speight, I., & Jaencke, L. (2014). Treatment of visuospatial neglect with biparietal tDCS and cognitive training: A single-case study. Frontiers in Systems Neuroscience, 8, 180. 10.3389/fnsys.2014.00180 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chieffo, R., Arcari, C., Comi, G., Comola, M., & Leocani, L. (2019). Effects of tDCS in modulating the after-effect of prismatic lenses training in stroke patients with neglect. Clinical Neurophysiology, 130(1), e15. 10.1016/j.clinph.2018.09.088 [DOI] [Google Scholar]
- Corbetta, M., Kincade, M. J., Lewis, C., Snyder, A. Z., & Sapir, A. (2005). Neural basis and recovery of spatial attention deficits in spatial neglect. Nature Neuroscience, 8(11), 1603–1610. 10.1038/nn1574 [DOI] [PubMed] [Google Scholar]
- Demeyere, N., Riddoch, M. J., Slavkova, E. D., Bickerton, W.-L., & Humphreys, G. W. (2015). The Oxford cognitive screen (OCS): Validation of a stroke-specific short cognitive screening tool. Psychological Assessment, 27(3), 883–894. 10.1037/pas0000082 [DOI] [PubMed] [Google Scholar]
- Duncan, P. W., Wallace, D., Lai, S. M., Johnson, D., Embretson, S., & Laster, L. J. (1999). The stroke impact scale version 2.0. Stroke, 30(10), 2131–2140. 10.1161/01.STR.30.10.2131 [DOI] [PubMed] [Google Scholar]
- Edgeworth, J. A., Robertson, I. H., & McMillan, T. M. (1998). The balloons test. Thames Valley Test Company. [Google Scholar]
- Elsner, B., Kugler, J., Pohl, M., & Mehrholz, J. (2016). Transcranial direct current stimulation (tDCS) for improving activities of daily living, and physical and cognitive functioning, in people after stroke. Cochrane Database Systematic Review, 3(3), CD009645. 10.1002/14651858.CD009645.pub3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fertonani, A., & Miniussi, C. (2017). Transcranial electrical stimulation. The Neuroscientist, 23(2), 109–123. 10.1177/1073858416631966 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greinacher, R., Buhôt, L., Möller, L., & Learmonth, G. (2019). The time course of ineffective sham blinding during low-intensity (1 mA) transcranial direct current stimulation. European Journal of Neuroscience, 50(8), 3380–3388. 10.1111/ejn.14497 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harvey, M., Hood, B. M., North, A., & Robertson, I. H. (2003). The effects of visuomotor feedback training on the recovery of hemispatial neglect symptoms: Assessment of a 2-week and follow-up intervention. Neuropsychologia, 41(8), 886–893. 10.1016/S0028-3932(03)00003-4 [DOI] [PubMed] [Google Scholar]
- Harvey, M., Muir, K., Reeves, I., Duncan, G., Birschel, P., Roberts, M., Livingstone, K., Jackson, H., Hogg, C., Castle, P., Learmonth, G., & Rossit, S. (2010). Long term improvements in activities of daily living in patients with hemispatial neglect. Behavioural Neurology, 23(4), 237–239. 10.1155/2010/253161 [DOI] [PMC free article] [PubMed] [Google Scholar]
- He, B. J., Snyder, A. Z., Vincent, J. L., Epstein, A., Shulman, G. L., & Corbetta, M. (2007). Breakdown of functional connectivity in frontoparietal networks underlies behavioral deficits in spatial neglect. Neuron, 53(6), 905–918. 10.1016/j.neuron.2007.02.013 [DOI] [PubMed] [Google Scholar]
- Horvath, J. C., Forte, J. D., & Carter, O. (2015). Transcranial direct current stimulation demonstrates little-to-no reliable or significant effect on any cognitive/behavioral or neurophysiologic outcome measure: A comprehensive meta-analysis. Brain Stimulation, 2, 318–319. [Google Scholar]
- Kalra, L., Perez, I., Gupta, S., & Wittink, M. (1997). The influence of visual neglect on stroke rehabilitation. Stroke, 28(7), 1386–1391. 10.1161/01.STR.28.7.1386 [DOI] [PubMed] [Google Scholar]
- Katz, N., Hartman-Maeir, A., Ring, H., & Soroker, N. (1999). Functional disability and rehabilitation outcome in right hemisphere damaged patients with and without unilateral spatial neglect. Archives of Physical Medicine and Rehabilitation, 80(4), 379–384. 10.1016/S0003-9993(99)90273-3 [DOI] [PubMed] [Google Scholar]
- Ko, M.-H., Han, S.-H., Park, S.-H., Seo, J.-H., & Kim, Y.-H. (2008). Improvement of visual scanning after DC brain polarization of parietal cortex in stroke patients with spatial neglect. Neuroscience Letters, 448(2), 171–174. 10.1016/j.neulet.2008.10.050 [DOI] [PubMed] [Google Scholar]
- Koch, G., Oliveri, M., & Caltagirone, C. (2008). Hyperexcitability of parietal-motor functional connections in the intact left-hemisphere of patients with neglect. Brain, 131, 3147–3155. 10.1093/brain/awn273 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ladavas, E., Giulietti, S., Avenanti, A., Bertini, C., Lorenzini, E., Quinquinio, C., & Serino, A. (2015). A-tDCS on the ispilesional parietal cortex boots the effects of prism adaptation treatment in neglect. Restorative Neurology and Neuroscience, 33(5), 647–662. 10.3233/RNN-140464 [DOI] [PubMed] [Google Scholar]
- Learmonth, G., Benwell, C. S. Y., Thut, G., & Harvey, M. (2017a). Age-related reduction of hemispheric lateralization for spatial attention: An EEG study. Neuro-Image, 153, 139–151. [DOI] [PubMed] [Google Scholar]
- Learmonth, G., Felisatti, F., Siriwardena, N., Checketts, M., Benwell, C. S. Y., Märker, G., Thut, G., & Harvey, M. (2017b). No interaction between tDCS current strength and baseline performance: A conceptual replication. Frontiers in Neuroscience, 11, 664. 10.3389/fnins.2017.00664 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nasreddine, S. Z., Phillips, N. A., Bedirian, V., Charbonneau, S., Whitehead, V., Collin, I., Cummings, J. L., & Chertkow, H. (2005). The montreal cognitive assessment, MoCA: A brief screening tool for mild cognitive impairment. Journal of the American Geriatrics Society, 53(4), 695–699. 10.1111/j.1532-5415.2005.53221.x [DOI] [PubMed] [Google Scholar]
- Nijboer, T. C. W., Kollen, B. J., & Kwakkel, G. (2013). Time course of visuospatial neglect early after stroke: A longitudinal cohort study. Cortex, 49(8), 2021–2027. 10.1016/j.cortex.2012.11.006 [DOI] [PubMed] [Google Scholar]
- Nitsche, M. A., & Paulus, W. (2000). Excitability changes induced in the human motor cortex by weak transcranial direct current stimulation. The Journal of Physiology, 527(3), 633–639. 10.1111/j.1469-7793.2000.t01-1-00633.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nowak, D. A., Grefkes, C., Ameli, M., & Fink, G. R. (2009). Interhemispheric competition after stroke: Brain stimulation to enhance recovery of function of the affected hand. Neurorehabilitation and Neural Repair, 23(7), 641–656. 10.1177/1545968309336661 [DOI] [PubMed] [Google Scholar]
- Nys, G. M. S., van Zandvoort, M. J. E., de Kort, P. L. M., van der Worp, H. B., Jansen, B. P. W., Algra, A., de Haan, E. H. F., & Kappelle, L. J. (2005). The prognostic value of domain-specific cognitive abilities in acute first-ever stroke. Neurology, 64(5), 821–827. 10.1212/01.WNL.0000152984.28420.5A [DOI] [PubMed] [Google Scholar]
- O’Shea, J., Revol, P., Cousijn, H., Near, J., Petitet, P., Jacquin-Courtois, S., Johansen-Berg, H., Rode, G., & Rossetti, Y. (2017). Induced sensorimotor cortex plasticity remediates chronic treatment-resistant visual neglect. eLIFE, 6, 1–38. doi: 10.7554/eLife.26602 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patchick, E., Vail, A., Wood, A., & Bowen, A. (2016). PRECis (patient reportd evaluation of cognitive state): Psychometric evaluation of a new patient reported outcome measure of the impact of stroke. Clinical Rehabilitation, 30(12), 1229–1241. 10.1177/0269215515624480 [DOI] [PubMed] [Google Scholar]
- Robertson, I. H., Nico, D., & Hood, B. M. (1995). The intention to act improves unilateral left neglect: Two demonstrations. NeuroReport, 7, 246–248. [PubMed] [Google Scholar]
- Robertson, I. H., Nico, D., & Hood, B. M. (1997). Believing what you feel: Using proprioceptive feedback to reduce unilateral neglect. Neuropsychology, 11(1), 53–58. 10.1037/0894-4105.11.1.53 [DOI] [PubMed] [Google Scholar]
- Rorden, C., Bonilha, L., Fridriksson, J., Bender, B., & Karnath, H. O. (2012). Age-specific CT and MRI templates for spatial normalization. NeuroImage, 61(4), 957–965. 10.1016/j.neuroimage.2012.03.020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rossetti, Y., Rode, G., Pisella, L., Farné, A., Li, L., Boisson, D., & Perenin, M. T. (1998). Prism adaptation to a rightward optical deviation rehabilitates left hemispatial neglect. Nature, 395(6698), 166–169. 10.1038/25988 [DOI] [PubMed] [Google Scholar]
- Rossit, S., Benwell, C. S. Y., Szymanek, L., Learmonth, G., McKernan-Ward, L., Corrigan, E., Muir, K., Reeves, I., Duncan, G., Birschel, P., Roberts, M., Livingstone, K., Jackson, H., Hogg, C., Castle, P., & Harvey, M. (2019). Efficacy of home-based visuomotor feedback training in stroke patients with chronic hemispatial neglect. Neuropsychological Rehabilitation, 29(2), 251–272. 10.1080/09602011.2016.1273119 [DOI] [PubMed] [Google Scholar]
- Rossit, S., Malhotra, P., Muir, K., Reeves, I., Duncan, G., Livingstone, K., Jackson, H., Hogg, C., Castle, P., Learmonth, G., & Harvey, M. (2009). No neglect-specific deficits in reaching tasks. Cerebral Cortex, 19(11), 2616–2624. 10.1093/cercor/bhp016 [DOI] [PubMed] [Google Scholar]
- Salazar, A. P. S., Vaz, P., Marchese, R. R., Stein, C., Pinto, C., & Pagnussat, A. S. (2018). Noninvasive brain stimulation improves hemispatial neglect after stroke: A systematic review and meta-analysis. Archives of Physical Medicine and Rehabilitation, 99(2), 355–366.e1. 10.1016/j.apmr.2017.07.009 [DOI] [PubMed] [Google Scholar]
- Schenkenberg, T., Bradford, D., & Ajax, E. (1980). Line bisection and unilateral visual neglect in patients with neurologic impairment. Neurology, 30(5), 509–509. 10.1212/WNL.30.5.509 [DOI] [PubMed] [Google Scholar]
- Schlaug, G., Renga, V., & Nair, D. (2008). Transcranial direct current stimulation in stroke recovery. Archives of Neurology, 65(12), 1571–1576. 10.1001/archneur.65.12.1571 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smit, M., Schutter, D. J., Nijober, T. C., Visser-Meily, J. M., Kappelle, L. J., Kant, N., Pennix, J., & Dijkerman, H. C. (2015). Transcranial direct current stimulation to the parietal cortex in hemispatial neglect: A feasibility study. Neuropsychologia, 74, 152–161. 10.1016/j.neuropsychologia.2015.04.014 [DOI] [PubMed] [Google Scholar]
- Song, W., Du, B., Xu, Q., Hu, J., Wang, M., & Luo, Y. (2009). Low-frequency transcranial magnetic stimulation for visual spatial neglect: A pilot study. Journal of Rehabilitation Medicine, 41(3), 162–165. 10.2340/16501977-0302 [DOI] [PubMed] [Google Scholar]
- Sparing, R., Thimm, M., Hesse, M. D., Küst, J., Karbe, H., & Fink, G. R. (2009). Bidirectional alterations of interhemispheric parietal balance by non-invasive cortical stimulation. Brain, 132(11), 3011–3020. 10.1093/brain/awp154 [DOI] [PubMed] [Google Scholar]
- Stone, S. P., Patel, P., Greenwood, R. G., & Halligan, P. W. (1992). Measuring visual neglect in acute stroke and predicting its recovery: The visual neglect recovery index. Journal of Neurology, Neurosurgery, and Psychiatry, 55(6), 431–436. 10.1136/jnnp.55.6.431 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stone, S. P., Wilson, B., Wroot, A., Halligan, P. W., Lange, L. S., Marshall, J. C., & Greenwood, R. G. (1991). The assessment of visuo-spatial neglect after acute stroke. Journal of Neurology, Neurosurgery, and Psychiatry, 54(4), 345–350. 10.1136/jnnp.54.4.345 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sunwoo, H., Kim, Y.-H., Chang, W. H., Noh, S., Kim, E.-J., & Ko, M.-H. (2013). Effects of dual transcranial direct current stimulation on post-stroke unilateral visuospatial neglect. Neuroscience Letters, 554, 94–98. 10.1016/j.neulet.2013.08.064 [DOI] [PubMed] [Google Scholar]
- Ten Brink, A. F., Visser-Meily, J. M. A., Schut, M. J., Kouwenhoven, M., Eijsackers, A. L. H., & Nijboer, T. C. W. (2017). Prism adaptation in rehabilitation? No additional effects of prism adaptation on neglect recovery in the subacute phase poststroke: A randomized controlled trial. Neurorehabilitation and Neural Repair, 31(12), 1017–1028. 10.1177/1545968317744277 [DOI] [PubMed] [Google Scholar]
- Thielscher, A., Antunes, A., & Saturnino, G. B. (2015). FEield modeling for transcranial magnetic stimulation: A useful tool to understand the physiological effects of TMS? IEEE EMBS 2015, Milano, Italy. [DOI] [PubMed] [Google Scholar]
- Turgut, N., Miranda, M., Kastrup, A., Eling, P., & Hildebrandt, H. (2018). tDCS combined with optokinetic drift reduces egocentric neglect in severely impaired post-acute patients. Neuropsychological Rehabilitation, 28(4), 515–526. 10.1080/09602011.2016.1202120 [DOI] [PubMed] [Google Scholar]
- Turi, Z., Csifcsák, G., Boayue, N. M., Aslaksen, P., Antal, A., Paulus, W., Groot, J., Hawkins, G. E., Forstmann, B., Opitz, A., Thielscher, A., & Mittner, M. (2019). Blinding is compromised for transcranial direct current stimulation at 1 mA for 20 minutes in young healthy adults. European Journal of Neuroscience, 50, 3261–3268. 10.1111/ejn.14403 [DOI] [PubMed] [Google Scholar]
- Wilson, B., Cockburn, J., & Halligan, P. W. (1987). Behavioural inattention test. Thames Valley Test Company. [Google Scholar]
- Yi, Y. G., Chun, M. H., Do, K. H., Sung, E. J., Kwon, Y. G., & Kim, D. Y. (2016). The effect of transcranial direct current stimulation on neglect syndrome in stroke patients. Annals of Rehabilitation Medicine, 40(2), 223–229. 10.5535/arm.2016.40.2.223 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zigmond, A. S., & Snaith, R. P. (1983). The hospital anxiety and depression scale. Acta Psychiatrica Scandinavica, 67(6), 361–370. 10.1111/j.1600-0447.1983.tb09716.x [DOI] [PubMed] [Google Scholar]