Abstract
Introduction:
A biomarker is a substance, structure, or process that indicates the presence of a disease, infection, or environmental exposure. Clinically useful biomarkers are measurable, improve diagnostic or prognostic performance, and ultimately aid clinicians in determining the initiation, duration, or magnitude of therapy.
Areas Covered:
The purpose of this review is to explore the roles of various blood biomarkers of atherosclerotic cardiovascular disease (ASCVD) and how their use may improve the precision with which clinicians can identify, treat, and ultimately prevent ASCVD. Our review will include lipid biomarkers, markers of cardiac injury and wall stress, markers of inflammation, and a few others.
Expert Opinion:
Several biomarkers have recently been highlighted as “risk-enhancing factors” in the 2019 American College of Cardiology/American Heart Association Guideline for the Primary Prevention of ASCVD, which can help guide shared decision-making. These included elevated low-density lipoprotein cholesterol, triglycerides, lipoprotein(a), apolipoprotein B, or high-sensitivity C-reactive protein. However, some other biomarkers mentioned in this review are not commonly used despite showing initial promise as prognostic of ASCVD risk, as it is not clear how treatment decisions should be changed after their measurement among asymptomatic individuals. Future studies should focus on whether biomarker-directed management strategies can improve clinical outcomes.
Keywords: biomarkers, atherosclerotic cardiovascular disease, precision medicine, lipids, inflammation, cardiac injury, cardiac wall stress
1. Introduction
1.1. Burden of ASCVD
Over the last several decades, there have been major advances in the treatment and prevention of atherosclerotic cardiovascular disease (ASCVD), evidenced by dramatic reductions in overall mortality rates [1]. While progress had been made in prior decades to improve survival of cardiovascular disease [2], CVD has remained the number one cause of death in the United States (U.S.) and worldwide, at least prior to the COVID-19 pandemic in 2020 [3]. As the U.S. population ages and grows, the absolute number of deaths due to ASCVD has increased in spite of these improvements in mortality rates [4]. Furthermore, evidence suggests that the rate of improvement in ASCVD mortality is slowing and even reversing in some groups, particularly women, racial and ethnic minorities, and those lacking health insurance [1,2,5,6].
The exact reason for the stagnation in ASCVD mortality rates among specific cohorts is not fully understood and is likely affected by complex social, political, and environmental factors. What is clear is that disparities in ASCVD morbidity and mortality underscore the shortcomings of current risk estimation practices and their reliance on broad, population-level disease markers rather than patient-specific metrics. Precision medicine, the practice of integrating an individual’s genetics, social environment, and specific disease phenotype to guide management [7], is gaining increased attention as a way to improve ASCVD morbidity and mortality.
The purpose of this review is to explore the roles of various novel biomarkers of ASCVD and how their use may improve the precision with which clinicians, in partnership with patients, can identify, treat, and ultimately prevent ASCVD. Our focus will be on lipoprotein biomarkers, cardiac biomarkers, and biomarkers of inflammation, as well as a few select others.
1.2. ASCVD Risk Estimation Tools
At present, ASCVD risk prediction is largely derived from the Pooled Cohort Equations (PCE), in which a patient’s age, total and HDL-cholesterol levels, systolic blood pressure, smoking history, diabetes history, and antihypertensive therapy status are used to predict 10-year ASCVD risk according to sex- and race-specific formulae [8]. The calculated risk, reported as a percentage likelihood of having a fatal or non-fatal coronary heart disease (CHD) or stroke event in the next 10 years, is then used to decide whether or not to initiate preventive therapy (e.g. statins) and how intensive that therapy should be. However, the PCE and other similar ASCVD Risk Scores are not without their shortcomings [9,10]. For one, these equations are heavily driven by age. Furthermore, there is a complex pathophysiology that underpins ASCVD and many factors linked to ASCVD are not consistently captured by population-level models. It can be problematic applying results from population-based estimators (which estimate the average risk in a group of individuals who have similar risk profiles) to clinical decision-making at the individual level [11]. Risk may be overestimated in some groups (such as those with higher socioeconomic status) and underestimated in others (such as those with more social deprivation or among those with unique health factors such as human immunodeficiency virus (HIV)-infection or inflammatory diseases). The PCE are best calibrated for non-Hispanic White and non-Hispanic Black adults, aged 40 to 79 years, living in the U.S. The risk equations may perform poorly for other race/ethnicities and in other global populations; thus in response, other regional risk score tools have been developed as well [12–14].
The recent 2019 guideline put forth by the American College of Cardiology (ACC) and American Heart Association (AHA) for the primary prevention of CVD recognized some of the limitations of the PCE [8]. The guideline indicates that 10-year ASCVD risk estimation is just the start of the clinician-patient risk discussion but not the end of the conversation. After estimating 10-year risk, the guideline encourages the consideration of “risk-enhancing” factors that would modify ASCVD risk estimation upward, as well as the selective use of coronary artery calcium (CAC) scores if risk-based decisions for preventive pharmacotherapies such as statins remained uncertain, as part of shared decision-making with the patient (Figure 1) [8].
Figure 1:
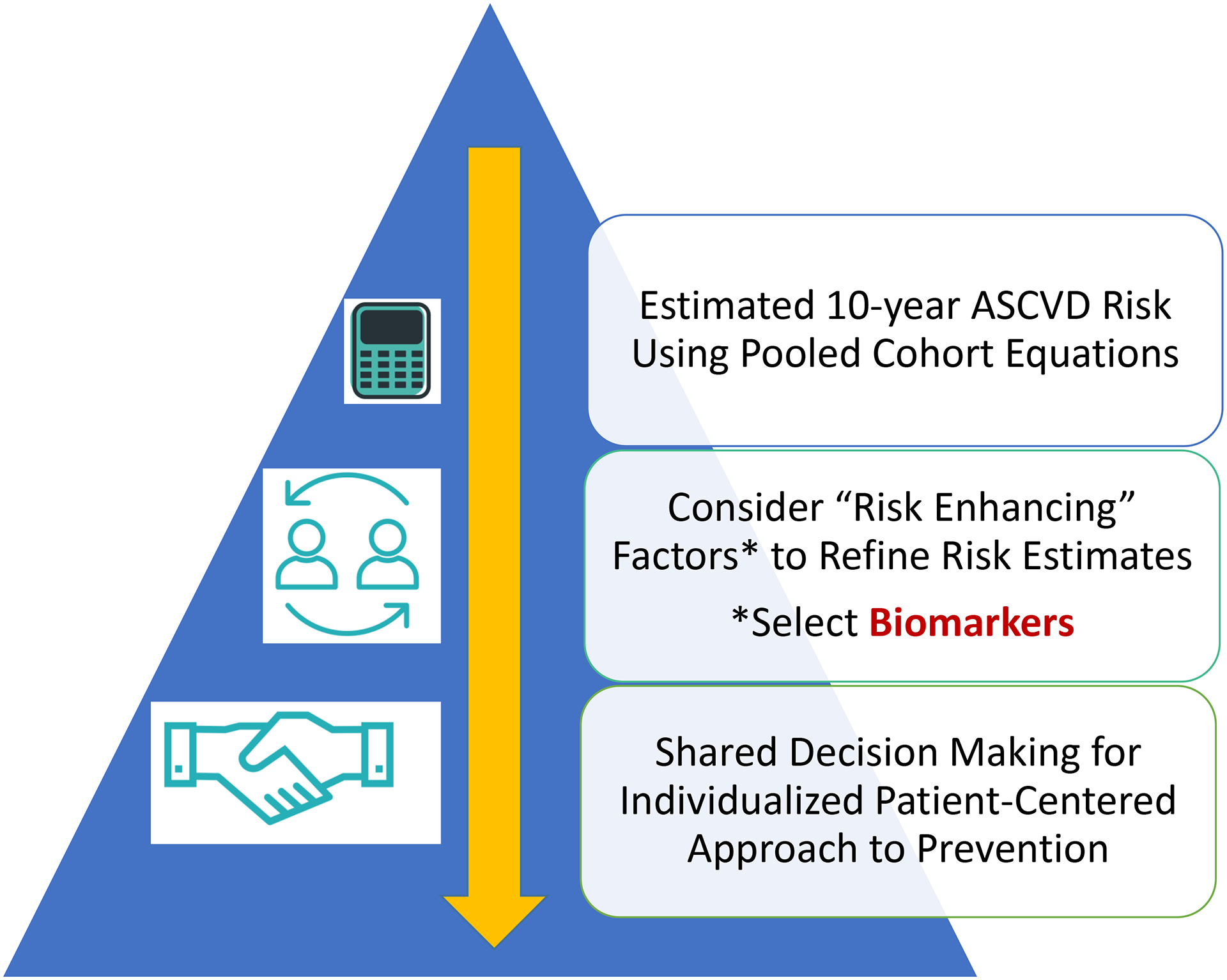
Current model of preventive cardiology incorporating the Pooled Cohort Equations to determine a rough estimation of risk that is then further refined with the use of validated biomarkers and other risk enhancers. The result of this refined estimation is then used to inform shared decision-making discussions with patients before selecting appropriate therapies.
1.3. Role of Biomarkers
A biomarker is a substance, structure, or process that indicates the presence of a disease, infection, or environmental exposure [15]. Clinically useful biomarkers are those that are measurable, improve diagnostic or prognostic performance, and ultimately aid clinicians in determining the initiation, duration, or magnitude of therapy. The identification of a putative biomarker typically begins when large epidemiological studies identify a relationship between a marker (e.g. serum cholesterol) and an outcome of interest (e.g. incident myocardial infarction). Subsequent studies then establish population-level reference ranges to define abnormal levels of said biomarker. Ideally, studies will then seek to identify interventions (e.g. statin therapy) that modify levels of the proposed biomarker in vivo, and determine whether there is a resultant change in the original outcome of interest. It should be noted that a biomarker need not be part of the causal chain leading to a particular disease state. As such, modulation of biomarker levels may not necessarily affect clinical outcomes, but this does not disqualify said molecule from being classified as a biomarker. When sufficient lines of evidence implicate the new molecule as a predictor of disease, it is incorporated into prediction models for validation.
Since the introduction of the term in 1989, interest in biomarkers has grown dramatically; a PubMed search for the MESH term “biomarker” produces over 59,000 results from 2020 alone. However, not all proposed biomarkers are clinically useful. Increasing efforts are being made to identify clinical biomarkers that improve the precision of risk prediction tools, extending the reach of recent advances in preventive medicine. Several biomarkers have already been highlighted as “risk-enhancing factors” in the 2019 ACC/AHA prevention guideline. These included elevated low-density lipoprotein cholesterol (LDL-C), persistently elevated triglycerides (TG), elevated lipoprotein (a) [Lp(a)], apolipoprotein B (apoB), and high-sensitivity C-reactive protein (hsCRP) (Figure 2). These biomarkers, along with other promising candidates, are further discussed with below.
Figure 2:
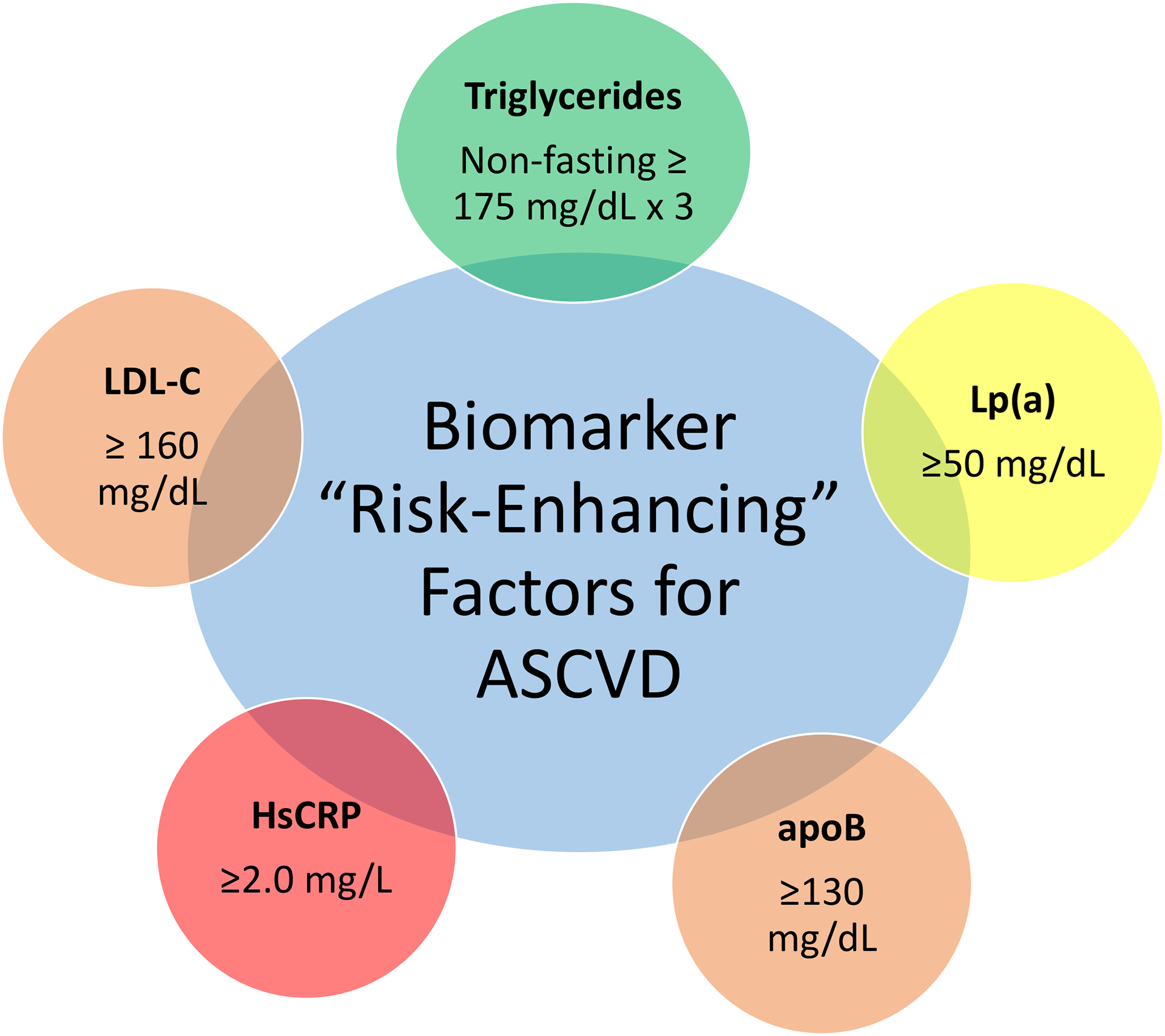
Serum biomarkers included in the 2019 ACC/AHA Guideline on the Primary Prevention of Cardiovascular Disease as risk enhancers.
2. Lipid Biomarkers
2.1. Low-density Lipoproteins
The first and most widely recognized ASCVD biomarker is serum cholesterol, implicated in the development of heart disease in 1961 by investigators of the Framingham Heart Study [16]. While most of the body’s cholesterol mass is located in cell membranes, cholesterol found within the circulation is carried by soluble transport protein complexes called lipoproteins. These lipoproteins interact with various receptors in the body and ultimately determine the fate of the cholesterol mass they carry. LDL-C, the cholesterol mass carried by low-density lipoproteins (LDL) has been shown to be the most important driver of atherosclerosis in circulation [17–19].
As such, LDL-C is the primary target for therapies aimed at reducing ASCVD risk [8,20–22]. Although it can be measured directly, LDL-C is typically estimated on a lipid panel by the Friedewald equation [23]. A novel equation for estimating LDL-C (the Martin/Hopkins method) has been shown to better correlate with directly measured LDL-C, particularly among patients with elevated TG or very low LDL-C concentrations [24–26]. The 2019 ACC/AHA primary prevention guideline considers a moderately elevated LDL-C of 160–189 mg/dL as a “risk enhancer” that would favor statin treatment among individuals otherwise at borderline- or intermediate- estimated ASCVD [8]; of note, individuals with severe primary hypercholesterolemia (LDL-C ≥190 mg/dL) are already strongly recommended for statin therapy in the guidelines [8,20].
It is worth noting that the establishment of biomarker reference ranges typically involves the calculation of statistics as they apply to defined populations, e.g. the catchment area of a hospital laboratory. This should not be confused with what are considered to be clinically relevant serum biomarker ranges. For example, in patients at elevated ASCVD risk receiving lipid lowering therapy, target LDL-C levels are generally recommended to be 50% of initial values or <70 mg/dL, even though the typical laboratory reference range sets the upper limit of “normal” (i.e. the 99th percentile for the general population of “healthy” adults) at around 100 mg/dL [20]. In seeking to establish clinically useful biomarkers, one should recognize that reference ranges for use in laboratories may not necessarily coincide with ranges considered to be clinically meaningful.
While LDL-C has long been the most widely available and measured biomarker for clinical use, it is not a perfect predictor of ASCVD and in certain circumstances can falsely under- or over-estimate risk [27]. Many individuals with moderate elevation in LDL-C never have a clinical ASCVD event, and approximately 40% of individuals who do experience an event have a total cholesterol level less than 200 mg/dL [27]. Therefore, measuring cholesterol mass alone is insufficient to predict adverse cardiac outcomes. Other non-lipid drivers such as genetic risk, insulin resistance, and inflammatory states may fuel atherogenesis even in absence of very elevated LDL-C.
Some patients at elevated ASCVD risk actually have a “discordance” between the mass of cholesterol contained within LDL (LDL-C) and the number of individual LDL particles carrying that cholesterol mass (LDL-P) [28–30]. LDL particles that are relatively cholesterol-depleted (and therefore smaller and denser) are more likely to enter the vascular intima to cause atherosclerosis [31]. In other words, a fixed mass of cholesterol trafficked by smaller, more numerous particles produces a more atherogenic milieu than if that same mass were carried by fewer, larger LDL particles. The patients at highest risk of atherosclerosis, then, are those with many small and dense LDL particles.
Indeed, LDL-P may predict ASCVD risk better than LDL-C [29,32–34]. Patients with metabolic syndrome, diabetes, and hypertriglyceridemia tend to exhibit the small-dense LDL particle phenotype. Studies have also shown that patients adhering to a ketogenic diet may demonstrate changes in particle morphology (increase in LDL particle size), though it is unclear what clinical relevance these changes have [35,36]. Current methods of risk prediction based on LDL-C do not take LDL particle morphology or quantity into account and therefore may not accurately estimate risk for patients with identical LDL-C levels but dissimilar particle characteristics. However, LDL particle number and size are measured by nuclear magnetic resonance (NMR) or other techniques, which, while commercially available, are not readily accessible in clinical practice, are not well standardized, lack well-established clinical cut-points, and are of unclear cost-effectiveness, which limits their routine use.
2.2. Apolipoprotein B
At present, it is not practical to collect LDL particle number, size, and density data for each patient encountered in routine clinical practice, nor is there sufficient evidence to advise such an approach. Furthermore, LDL is not the only atherogenic particle in circulation: chylomicrons, chylomicron remnants, very low-density lipoproteins (VLDL), intermediate-density lipoproteins (IDL), and Lp(a) particles all may enter the arterial wall and contribute to atherosclerotic disease. (Of note, IDL-C and Lp(a)-C are included in LDL-C, as defined clinically.) Given that all of these atherogenic particles contain one apoB molecule, measurement of apoB accurately quantifies the total number of atherogenic particles in plasma [37].
As with comparisons between LDL-P and LDL-C, several studies have shown apoB to outperform Friedewald-estimated LDL-C for ASCVD risk estimation. An analysis from the Women’s Health Study compared the predictive value of these two markers among lipoprotein discordant individuals, with discordance defined as an LDL-C level above the median and an apoB level below the median, or vice versa. Among participants with discordant lipid parameters, apoB was found to more accurately predict coronary events than LDL-C [29]. Similar findings have been obtained from Mendelian randomization analyses [38,39].
Sniderman et al have argued for apoB to replace LDL-C as the main lipoprotein marker for ASCVD risk [40]. As mentioned in the Introduction, at present, elevated apoB is included in the guidelines as a “risk-enhancer”—a binary indicator that, when present, signals ASCVD risk above and beyond the numeric value obtained from the PCE [8]. While apoB has not yet seen its day as the primary lipoprotein biomarker for ASCVD, this designation is a step towards a more precise paradigm for ASCVD risk assessment, particularly if apoB is used to make more specific diagnoses of Fredrickson-Levy-Lees lipid disorders [41,42]. This transition may enable clinicians to use a widely available test to provide counseling and treatment options that are better tailored to the specific individual. On the other hand, a contrasting argument can be made that apoB, as well as non-HDL-C, may be considered less precise because they lump all atherogenic lipoproteins together, and LDL-C is more specific to the lipid-lowering drugs which upregulate the LDL receptor and have been proven to have efficacy for ASCVD reduction. It is important to acknowledge that a key barrier to increasing the utilization of apoB is the lack of standardized methods and the lack of clinical trials showing that incorporating apoB into decision making improves outcomes [43].
2.3. Lipoprotein (a)
Lp(a) is an LDL-like particle with a single apoB-100 moiety bound to one of multiple isoforms of apolipoprotein(a) [44]. In addition, Lp(a) has structural homology with plasminogen, which means it competes for binding of fibrin and inhibits tissue plasminogen activator, creating the potential for a hypercoagulable state [45,46]. Thus, elevated Lp(a) can increase the risk for both atherosclerosis and thrombosis [47].
Lp(a) levels have been shown to vary with certain interventions but are most significantly governed by genetics, in particular by the LPA gene [48,49]. In several observational studies, Lp(a) was found to be associated with an increased incidence of CHD and stroke [50–55]. Lp(a) has also been associated with valvular calcification and aortic stenosis [56,57].
The clinical relevance of Lp(a) was illustrated by the results of the Atherothrombosis Intervention in Metabolic Syndrome with Low HDL/High Triglyceride and Impact on Global Health Outcomes (AIM-HIGH) trial, which identified Lp(a) as a significant contributor to residual ASCVD risk in patients receiving statin therapy [58]. Randomized clinical trials investigating proprotein convertase subtilisin/kexin type 9 (PCSK9) inhibitor therapy in secondary prevention populations similarly noted that Lp(a) was a marker of elevated residual ASCVD risk [59], as well as for peripheral arterial disease (PAD) and venous thromboembolism (VTE) [60]. Mendelian randomization studies have also independently linked elevations in Lp(a) with increased ASCVD risk [61,62]. Lp(a), unsurprisingly, has improved risk discrimination for ASCVD when added to traditional risk prediction tools [63].
The cholesterol mass contained within Lp(a) particles is counted as LDL-C by conventional methods, causing appreciation for the residual atherogenicity risk it confers to be lost when LDL-C is used in isolation for risk prediction. This issue highlights the problem with imprecise ASCVD risk methods: two patients with equal LDL-C but with different contributions from Lp(a) will have similar estimated risk using conventional calculations, even though the patient with higher Lp(a) likely has higher “true” risk. This imprecision is partially addressed by recent primary prevention guidelines through the inclusion of Lp(a) as a risk-enhancer [8,22]. While the U.S. guidelines have endorsed measuring Lp(a) selectively, such as in those with a personal or family history of premature CHD [8], the European guidelines actually recommended measuring Lp(a) at least once in each adult’s lifetime since it is highly heritable, to identify those with very high inherited Lp(a) levels who might have an ASCVD risk similar to those with familial hypercholesterolemia [22]. This designation holds promise to refine risk estimation beyond the PCE.
2.4. Triglycerides
TG are the primary energy storage molecules of the body and are found predominantly in adipose tissue. In circulation, TG are trafficked alongside cholesterol in the largest of the lipoprotein subclasses, namely VLDL and chylomicrons. These structures are responsible for carrying TG from endogenous (hepatic) and exogenous (dietary) sources, respectively, throughout the body [64]. As these structures are depleted of their TG contents, the remnants left behind are able to penetrate the arterial wall and begin the atherosclerosis cascade. TG are easily measured on routine lipid panels and, though they are not included in the PCE, have been shown to predict ASCVD risk independent of LDL-C [65]. While the role of chylomicron and VLDL remnants in ASCVD has been established, the mechanisms by which TGs themselves affect atherosclerosis are still under investigation. Several cross-sectional studies have identified an association between elevated TG levels and endothelial dysfunction, an important step in atherosclerosis. Increased expression of cellular adhesion molecules and inflammation have also been postulated as mechanisms by which hypertriglyceridemia influences ASCVD [66].
The 2019 ACC/AHA Guideline on the Primary Prevention of CVD includes persistently elevated TG >175 mg/dL (non-fasting, on ≥3 occasions) as a risk-enhancer for those at borderline or intermediate ASCVD risk [8]. Exercise, limitation of simple dietary carbohydrates, reduction in alcohol consumption, and weight loss have all been shown to decrease TG levels in the blood [20,67].
There are several pharmaceuticals that reduce serum TG levels. While the primary effect of statins is to reduce LDL-C, this class of medications has also demonstrated a modest TG-lowering effect [20]. Of note, patients receiving statin therapy who continue to have elevated TG levels have residual ASCVD risk beyond what would be expected based on achieved LDL-C alone [68]. The mechanism of this residual risk is under continuing investigation, though several attempts have been made to reduce this risk through pharmacologic intervention.
Several non-statin agents are available that reduce TG levels further, including niacin, fibrates, and omega-3 fatty acids. Unfortunately, neither niacin nor fibrate therapy has been shown in large randomized clinical trials to significantly reduce adverse cardiovascular outcomes for patients already treated with a statin [69,70] Conversely, certain formulations of omega-3 fatty acids have produced impressive reductions in CVD mortality when used in conjunction with statins. In the Reduction of Cardiovascular Events with Icosapent Ethyl–Intervention Trial (REDUCE-IT), participants randomized to daily use of icosapent ethyl, a highly purified form of eicosapentaenoic acid (EPA), at 4 grams per day in addition to a statin experience significantly fewer adverse cardiovascular outcomes than counterparts treated with statin alone [71]. While the results of the REDUCE-IT trial are certainly exciting, it should be noted that the mortality benefits seen were independent of achieved TG level, signaling the possibility of effects not limited to changes in lipid profile.
Other studies testing the effect of omega-3 fatty acid supplements on ASCVD outcomes have been unable to produce similar findings. A Study of Cardiovascular Events in Diabetes (ASCEND) used a factorial design to randomize over 15,000 participants to receive a combination of aspirin, omega-3 fatty acids, or placebo [72]. Patients randomized to receive 1 gram of omega-3 fatty acid did not show significant improvements in cardiovascular events compared to those receiving placebo. The Outcomes Study to Assess Statin Residual Risk Reduction With Epanova in High CV Risk Patients With Hypertriglyceridemia (STRENGTH) trial randomized participants to receive either 4 grams per day of a high bioavailability mixture of EPA and docosahexaenoic acid or a corn oil placebo in addition to maximally tolerated statin therapy. The trial was halted early due to early data showing low probability of clinically significant results [73]. The negative findings of ASCEND and STRENGTH underscore the importance of both omega-3 dose and formulation for reducing cardiovascular events. It also highlights the issue that improvement of a biomarker alone (in this case, TGs) is not necessarily sufficient to translate to meaningful ASCVD risk reduction. While high dose icosapent ethyl is a proven and available therapy for patients with hypertriglyceridemia-related ASCVD risk, further investigation is warranted to determine through what mechanisms this drug acts [74].
3. Cardiac Markers of Injury and Wall Stress
3.1. High-sensitivity Cardiac Troponin I and T
Cardiac troponin is a regulatory protein found in cardiac myocytes and is released in the setting of myocardial injury [75]. As such, troponin assays have found use primarily in the diagnosis of active clinical events such as acute coronary syndrome [76]. However, there has been increasing recognition of their role in prognosticating future ASCVD risk even among asymptomatic individuals [77]. With the development of high-sensitivity cardiac troponin (hs-cTn) assays that can detect elevated circulating troponin on the order of tens of nanograms per liter, investigators have begun to explore the importance of previously undetectable troponin elevations as markers of subclinical myocardial injury and, by extension, risk of progression to clinical ASCVD events [78]. A hs-cTn assay is one that can measure a value above the limit of detection in >50% of healthy men and women, and there are high sensitivity assays for both cardiac troponin T and cardiac troponin I.
Hs-cTn may have both positive and negative predictive value with respect to ASCVD. One analysis of high-risk patients in a cohort of high-risk but asymptomatic participants found that elevated baseline hs-cTn predicted future major adverse cardiac events [79]. Similar results were found in other large cohort studies that included participants free from ASCVD at baseline. In the Atherosclerosis in Communities (ARIC) study, which included 8,121 participants free from ASCVD at baseline, those in the highest quintile of hs-cTn were found to have increased risk of global CVD (ASCVD plus heart failure) compared to those in the lowest quintile, even after adjusting for the traditional risk factors in the PCE [80]. Similarly, the West of Scotland Coronary Prevention Study randomized 3,318 men with elevated LDL-C (but without established ASCVD) to receive pravastatin or placebo and followed them for an average duration of five years. Elevated hs-cTn was again associated with increased risk of ASCVD [81]. Additionally, statin therapy was shown to significantly reduce hs-cTn over five years compared to placebo and participants who sustained the greatest reduction in hs-cTn enjoyed a five-fold reduction in CHD events compared to those whose hs-cTn increased by the study’s close [81].
The addition of hs-cTn has been shown to improve risk prediction when considered alongside results from the PCE and the Framingham Risk Score [80,82]. In spite of this, hs-cTn is not included in major society guidelines for use in ASCVD risk prediction. The reason for hs-cTn’s omission may be related to the significant variations in baseline troponin among different populations. Patients with chronic kidney disease, for example, have reduced troponin clearance and thus higher baseline levels in circulation [83]; nevertheless, troponin still predicts ASCVD risk even among patients with chronic kidney disease [84]. While this poses a challenge when attempting to describe population level statistics, it is less problematic for use in the care of an individual patient. Periodic measurement of hs-cTn over time may provide insight into subtle changes in a patient’s physiology before any overt manifestations are clinically appreciable [85,86]. However, which preventive therapies should be implemented (statin therapy vs. more intensive blood pressure control vs. other) is not exactly certain. Further study is required before such practices can become commonplace.
3.2. Natriuretic peptides
B-type natriuretic peptides (BNP) are secreted from cardiomyocytes in response to myocardial wall stress from volume expansion and pressure overload [87]. BNP plays an important role in volume homeostasis by promoting vasodilation, natriuresis, and ventricular relaxation, and by inhibiting renin secretion [87]. BNP is synthesized first as a pre-hormone (proBNP) which is cleaved to the active hormone BNP and also to the N-terminal pro-B type natriuretic peptide (NT-proBNP). NT-proBNP and BNP levels are used clinically for of heart failure, as well as to indicate left ventricular hypertrophy, silent cardiac dysfunction, and myocardial ischemia [88–91]. However, even among asymptomatic individuals free of clinical ASCVD or heart failure, elevated NT-proBNP levels are associated with an increased risk of incident future CVD [92], heart failure hospitalizations [93], and cardiovascular mortality events [94,95]. While BNP levels are incorporated into specific disease management guidelines such as those for heart failure and valvular dysfunction, BNP levels were not considered one of the risk enhancing factors in the ACC/AHA primary prevention guideline. Further work is needed about how to best incorporate this biomarker in the evaluation of the asymptomatic individual to guide risk-based decisions for preventive therapies.
4. Inflammatory Biomarkers
4.1. High-sensitivity C-reactive Protein (hsCRP)
Inflammation is fundamental to the development of atherosclerosis and has received significant attention as both a diagnostic and therapeutic target [96]. Interleukin-1β (IL-1β) is a cytokine that is thought to be causally related to atherosclerosis by increasing production of interleukin-6 (IL-6), which stimulates liver production of C-reactive protein (CRP) and further amplifies the inflammatory cascade [97]. An inhibitor of IL-1β (canakinumab) has been shown to reduce levels of hsCRP and IL-6 and notably reduce ASCVD events without lowering LDL-C [98], which provided proof of concept of the role of inflammation in atherogenesis independent of lipids.
Inflammatory biomarkers like CRP and hsCRP are measured as part of the workup for a variety of pathologies, including rheumatological conditions and infectious processes. CRP is a pattern recognition molecule whose plasma levels increase markedly in the setting of infection or tissue damage [99]. Circulating levels of CRP have been shown to have utility in predicting cardiac events. In a prospective case-control study of 28,263 women, hsCRP was a strong independent predictor of future cardiovascular events and improved risk estimation when added to standard lipid-based risk prediction models [100]. These findings were corroborated in an ARIC study involving 9,784 participants that found hsCRP to predict ASCVD risk independently of the lipid profile [101]. Cardiovascular risk prediction tools that incorporate CRP measurements have been developed and improve risk discrimination over traditional methods [102,103]. Given the association of elevated inflammation with ASCVD risk, hsCRP has been included as a “risk enhancer” in the most recent ACC/AHA primary prevention guidelines [8].
Although hsCRP is now used to inform the decision to initiate or escalate lipid lowering treatment in patients at borderline or intermediate risk by PCE, further study is required to identify those who might benefit from targeted anti-inflammatory therapy. Post-hoc “responders” analyses from randomized clinical trials such as the Justification for the Use of Statins in Prevention: An Intervention Trial Evaluating Rosuvastatin (JUPITER) [104], Improved Reduction of Outcomes: Vytorin Efficacy International Trial (IMPROVE IT) [105] and the Pravastatin or Atorvastatin Evaluation and Infection Therapy Thrombolysis in Myocardial Infarction 22 (PROVE IT-TIMI 22) [106] suggested that those patients who achieved the dual targets of LDL-C <70 mg/dL and hsCRP <2 mg/L while on statin treatment experienced the lowest rates of ASCVD events. Similarly, the Canakinumab Anti-Inflammatory Thrombosis Outcome Study (CANTOS) also showed that an on-treatment hsCRP level <2 mg/L was associated with reduction in cardiovascular and all-cause mortality [107]. Nevertheless, it remains controversial whether a “treat to target” approach should be used for hsCRP. While participants who achieved lower levels of hsCRP on treatment in these trials had better outcomes, those reaching the <2 mg/L benchmark were more likely to have had lower hsCRP levels at baseline and concern for residual confounding remains [108–110]. Further investigation is warranted to confirm whether baseline inflammation or on-treatment reduction is more closely related to improvement in outcomes. This would allow clinicians to better identify which patients stand to derive the maximal benefit from inflammation-directed therapies.
4.2. GlycA
GlycA, a quantitative measurement of glycan N-acetylglucosamine residues on enzymatically glycosylated acute-phase proteins using nuclear magnetic resonance, correlates with other established markers of inflammation including CRP and IL-6 [111,112]. GlycA has been shown to have superior intra-individual precision when compared to hsCRP, making it a compelling target for ASCVD risk prediction.
Several investigations have validated the relationship between GlycA and ASCVD risk. Analyses from the Women’s Health Study involving over 25,000 participants free from CVD at baseline have shown GlycA to independently predict future CVD events, even after adjustment for traditional ASCVD risk factors [113,114]. A similar study of 6,523 men and women in the Multi-ethnic Study of Atherosclerosis (MESA) cohort corroborated these findings and showed that GlycA retained its predictive value with adjustment for other inflammatory biomarkers including CRP [115]. GlycA has been further associated with specific markers of subclinical atherosclerosis, including carotid plaque, CAC, valvular calcium, and thoracic aortic calcification [116–118]. GlycA has also been linked to incident PAD events, as well as heart failure with preserved ejection fraction [116,119].
Although GlycA appears to hold promise as a useful biomarker for CVD risk determination, additional investigation is needed before it is recommended for clinical use. Further work is needed for GlycA to be considered a risk enhancer, particularly the characterization of formal reference ranges. Furthermore, although a plasma GlycA test is commercially available, it is measured via NMR, and so may not be as readily accessible as other markers, like CRP.
Finally, it is worth noting that biomarkers that track with a process as dynamic as inflammation are vulnerable to misinterpretation if only observed at a single moment in time. One can imagine testing hsCRP or GlycA in a patient recently getting over the common cold would see these markers substantially elevated. If these stand-alone values were used to predict ASCVD risk, it would lead to overestimation of risk. Certainly, any interpretation of a biomarker should be in the clinical context of which it was drawn. However, the true utility in these biomarkers may be in the context of serial measurements to identify concerning trends, rather than as snapshot tests.
4.3. Lipoprotein-associated phospholipase A2
Lipoprotein-associated phospholipase A2 (Lp-PLA2) is an enzyme involved in the generation of pro-atherogenic and pro-inflammatory molecules that has also been associated with increased ASCVD risk [120,121]. A large case-control cohort study involving 12,819 asymptomatic men and women identified higher baseline activity of Lp-PLA2 in those who went on to suffer cardiac events than in those who did not, and prognostic information was complementary to CRP [122]. A separate analysis assessing the relationship of smoking on both Lp-PLA2 and CRP showed that both measures correlated with smoking intensity and with risk of major cardiac events, though CRP outperformed Lp-PLA2 as a risk predictor [123]. An inhibitor of Lp-PLA2, darapladib, has been developed and was tested in a randomized controlled trial for secondary prevention of CHD. As darapladib was not associated with reductions in ASCVD in this study [124], it is unlikely to play a role in primary prevention, though this has not specifically been assessed.
5. Other biomarkers
5.1. Adipokines
Fat tissue is now widely understood to be an endocrine organ, responsible for the regulation of energy partitioning and utilization. Hormones secreted from adipose tissue, known as adipokines, participate in myriad biochemical pathways [125–127]. Generally, a greater burden of adipose tissue is associated with unfavorable levels of adipokines [128]. Leptin regulates satiety and lowers blood glucose, and leptin levels have been shown to be higher among individuals with obesity, suggesting a leptin-resistant phenotype [129,130]. Adiponectin reduces inflammation and improves insulin sensitivity, and levels are decreased in patients who are obese [125]. Resistin promotes endothelial dysfunction and foam cell formation, and levels are higher in states of chronic inflammation like obesity [131]. Thus, it comes as no surprise that multiple studies have identified a link between adipokine dysregulation with incident diabetes [132] and atherosclerotic disease [129,133–135], and these associations have been independent of body mass index.
However, at present, the measurement of adipokines has been predominantly conducted in the research setting and is not widely used in clinical practice. While lifestyle modification and other interventions can affect the levels of various adipokines in circulation, the benefit of changes in these hormone levels is unclear and difficult to parse out from the other benefits these interventions confer (such as weight loss). Further study is required before adipokines are ready for use in ASCVD risk prediction or treatment monitoring.
6. Conclusions
Serum biomarkers have long been central to estimating ASCVD risk. Since the discovery of a link between circulating cholesterol levels and cardiac events, huge progress has been made in terms of refining risk predictions models and developing new treatment and prevention strategies. With the development of high sensitivity assays and the elucidation of pathophysiologic mechanisms leading to atherosclerosis, the field has more risk prediction tools at its disposal than ever before. The challenge now is to identify which biomarkers have a place in common clinical practice. As our understanding of the complex physiology of heart disease grows, so too should our understanding that no two patients are alike. Here, we have touched upon several promising biomarkers that we believe are poised to advance the practice of precision medicine in ASCVD prevention. We expect these tools will add to current population-based risk prediction models such that each patient may receive the optimal care to live longer and healthier.
7. Expert Opinion
Population-level studies have been fundamental to the massive improvements in cardiovascular disease mortality experienced throughout the world. Since the seminal work of the Framingham Heart Study, the analysis of large repositories of data continues to identify new therapeutic targets against ASCVD. While progress to-date has been impressive, we are at risk of reaching an asymptote. The next several decades of CVD prevention will hinge upon the implementation of extant and novel biomarkers of CVD risk to personalize therapy at the patient (rather than population) level.
The measurement of blood biomarkers allows clinicians to capture many different biological processes that lead to development of atherosclerosis and to detect subclinical cardiac dysfunction. Thus, these biomarkers offer much promise of personalizing risk-based decisions, with the opportunity to intervene with preventive strategies early and to deliver medicine with more precision at the individual level. However, it should be noted that many of these biomarkers discussed in this review have not been used in routine CVD risk assessment as they have not been shown to sufficiently change the area under the curve (C-statistic) compared to traditional risk factors alone.
Additionally, while the epidemiology behind many of these biomarkers is well-established (i.e., their associations with incident CVD events), there are other gaps missing which limit moving some of these markers forward in clinical practice. Some of these missing pieces include: (1) population reference ranges and their association with outcomes; (2) effects of treatments (i.e., statins, blood pressure reduction, etc.) on these biomarkers; (3) change in these biomarkers over time in relation to long-term events; (4) studies formally evaluating predictive models with the addition of the new markers – such as does risk prediction improve beyond PCE; and (5) clinical trials where markers are incorporated to assess impact of therapies on CVD risk.
Consideration of serum biomarkers is but one component of the clinical decision-making process. Not included in most risk models are social determinants of health (SDOH) other than self-reported skin color, which SDOH also need to be considered when personalizing preventive cardiology interventions [8,136]. Additionally, while serum biomarkers offer insight into the risk of atherosclerotic disease, the identification of other markers, like CAC, provides useful information on atherosclerotic disease already in progress. An advantage of blood biomarkers is the potential to capture dynamic changes/fluctuations in risk, which may not be possible with CAC. The combination of these data is fundamental to patient-centered management. Studies have already demonstrated the improvement in risk prediction when a multimodal approach is taken, and as computational power continues to improve, so too will our ability to identify previously unknown phenotypic patterns at a higher resolution [11,137–139]. Finally, as more clinically relevant variables are described, one can imagine a time when a suite of different predictive models are available, each tailored to populations with different socioeconomic statuses, different comorbid disease burdens, and different CVD histories.
A “one size fits all” approach has limitations and guidelines are just that… guides, not rules. Ultimately decisions regarding initiation and intensification of preventive therapies need to be patient-centered, with shared decision making not only discussing risks and benefits but understanding a patient’s own preferences and values in regards to their views on taking long-term preventive therapies [140]. This review has been an attempt to collate some emerging serum-based biomarkers that we believe are most useful in achieving precision medicine as it relates to atherosclerotic disease and the clinician-patient risk discussion. These molecules, when considered alongside other clinical data such as CAC scoring and sociodemographic information, will be key to maximizing preventive efforts.
Funding statement:
Dr. Michos is supported by the Amato Fund in Women’s Cardiovascular Health Research at the Johns Hopkins School of Medicine and by grants from the American Heart Association (20SFRN35120152 and 20SFRN35380046). Dr. Juraschek is supported by National Heart, Lung, and Blood Institute grants K23HL135273 and R21HL144876. Dr. Quispe is supported by an NIH T32 training grant (5T32HL007227). Dr. Martin is supported by the American Heart Association (20SFRN35380046 and COVID19-811000), PCORI (ME-2019C1-15328), NIH (P01 HL108800), and the David and June Trone Family Foundation.
Footnotes
Declaration of interest: Dr. Martin has served on scientific advisory boards and received consulting fees in the last 36 months from AstraZeneca, Amgen, Esperion, Kaneka, Novo Nordisk, Regeneron, REGENXBIO, Sanofi, and 89bio. He is a co-inventor on a system to estimate LDL cholesterol levels, patent application pending. He is a founder of and holds equity in Corrie Health, which intends to further develop the platform. This arrangement has been reviewed and approved by the Johns Hopkins University in accordance with its conflict of interest policies. None of the other authors report any disclosures.
References
*Reference of interest
**Reference of considerable interest
- 1.Mensah GA, Wei GS, Sorlie PD, et al. Decline in Cardiovascular Mortality: Possible Causes and Implications. Circ Res. 2017;120(2):366–380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Virani SS, Alonso A, Benjamin EJ, et al. Heart Disease and Stroke Statistics-2020 Update: A Report From the American Heart Association. Circulation. 2020;141(9):e139–e596. [DOI] [PubMed] [Google Scholar]
- 3.Woolf SH, Chapman DA, Lee JH. COVID-19 as the Leading Cause of Death in the United States. JAMA 2021;325(2):123–124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Roth GA, Forouzanfar MH, Moran AE, et al. Demographic and epidemiologic drivers of global cardiovascular mortality. N Engl J Med. 2015;372(14):1333–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Sidney S, Quesenberry CP Jr., Jaffe MG, et al. Recent Trends in Cardiovascular Mortality in the United States and Public Health Goals. JAMA Cardiol. 2016;1(5):594–9. [DOI] [PubMed] [Google Scholar]
- 6.Curtin SC. Trends in Cancer and Heart Disease Death Rates Among Adults Aged 45–64: United States, 1999–2017. Natl Vital Stat Rep 2019;68(6):1–8. [PubMed] [Google Scholar]
- 7.Jameson JL, Longo DL. Precision medicine--personalized, problematic, and promising. N Engl J Med. 2015;372(23):2229–34. [DOI] [PubMed] [Google Scholar]
- 8.Arnett DK, Blumenthal RS, Albert MA, et al. 2019 ACC/AHA Guideline on the Primary Prevention of Cardiovascular Disease: Executive Summary: A Report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines. Circulation. 2019;140(11):e563–e595. [DOI] [PMC free article] [PubMed] [Google Scholar]; ** Most recent U.S. professional society guideline detailing best practices for the primary prevention of CVD
- 9.Amin NP, Martin SS, Blaha MJ, et al. Headed in the right direction but at risk for miscalculation: a critical appraisal of the 2013 ACC/AHA risk assessment guidelines. J Am Coll Cardiol 2014;63:2789–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Lloyd-Jones DM, Braun LT, Ndumele CE, et al. Use of Risk Assessment Tools to Guide Decision-Making in the Primary Prevention of Atherosclerotic Cardiovascular Disease: A Special Report From the American Heart Association and American College of Cardiology. J Am Coll Cardiol 2019;73:3153–3167. [DOI] [PubMed] [Google Scholar]
- 11.McEvoy JW, Diamond GA, Detrano RC, et al. Risk and the physics of clinical prediction. Am J Cardiol 2014;113:1429–35. [DOI] [PubMed] [Google Scholar]
- 12.Conroy RM, Pyorala K, Fitzgerald AP, et al. Estimation of ten-year risk of fatal cardiovascular disease in Europe: the SCORE project. Eur Heart J. 2003;24(11):987–1003. [DOI] [PubMed] [Google Scholar]
- 13.Hippisley-Cox J, Coupland C, Vinogradova Y, et al. Predicting cardiovascular risk in England and Wales: prospective derivation and validation of QRISK2. BMJ. 2008;336(7659):1475–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Yang X, Li J, Hu D, et al. Predicting the 10-Year Risks of Atherosclerotic Cardiovascular Disease in Chinese Population: The China-PAR Project (Prediction for ASCVD Risk in China). Circulation. 2016;134(19):1430–1440. [DOI] [PubMed] [Google Scholar]
- 15.Strimbu K, Tavel JA. What are biomarkers? Curr Opin HIV AIDS. 2010November;5(6):463–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kannel WB, Dawber TR, Kagan A, et al. Factors of risk in the development of coronary heart disease--six year follow-up experience. The Framingham Study. Ann Intern Med. 1961;55:33–50. [DOI] [PubMed] [Google Scholar]; ** First study identifying a relationship between serum cholesterol and risk of heart disease. Coined the term “risk factor.”
- 17.Cholesterol Treatment Trialists C, Baigent C, Blackwell L, et al. Efficacy and safety of more intensive lowering of LDL cholesterol: a meta-analysis of data from 170,000 participants in 26 randomised trials. Lancet. 2010;376(9753):1670–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Cholesterol Treatment Trialists C, Mihaylova B, Emberson J, et al. The effects of lowering LDL cholesterol with statin therapy in people at low risk of vascular disease: meta-analysis of individual data from 27 randomised trials. Lancet. 2012;380(9841):581–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ference BA, Ginsberg HN, Graham I, et al. Low-density lipoproteins cause atherosclerotic cardiovascular disease. 1. Evidence from genetic, epidemiologic, and clinical studies. A consensus statement from the European Atherosclerosis Society Consensus Panel. Eur Heart J. 2017;38(32):2459–2472. [DOI] [PMC free article] [PubMed] [Google Scholar]; ** Large review of data identifying a relationship between low-density lipoproteins and atherosclerotic disease risk. Part of a two part series clearly delineating the role of LDL in development of heart disease.
- 20.Grundy SM, Stone NJ, Bailey AL, et al. 2018 AHA/ACC/AACVPR/AAPA/ABC/ACPM/ADA/AGS/APhA/ASPC/NLA/PCNA Guideline on the Management of Blood Cholesterol: Executive Summary: A Report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines. Circulation. 2019;139(25):e1046–e1081. [DOI] [PubMed] [Google Scholar]; ** Recent U.S. professional society guideline on the management of elevated blood cholesterol
- 21.Michos ED, McEvoy JW, Blumenthal RS. Lipid Management for the Prevention of Atherosclerotic Cardiovascular Disease. N Engl J Med. 2019;381(16):1557–1567. [DOI] [PubMed] [Google Scholar]
- 22.Mach F, Baigent C, Catapano AL, et al. 2019 ESC/EAS Guidelines for the management of dyslipidaemias: lipid modification to reduce cardiovascular risk. Eur Heart J. 2020;41(1):111–188. [DOI] [PubMed] [Google Scholar]
- 23.Friedewald WT, Levy RI, Fredrickson DS. Estimation of the concentration of low-density lipoprotein cholesterol in plasma, without use of the preparative ultracentrifuge. Clin Chem. 1972;18(6):499–502. [PubMed] [Google Scholar]; * Development of equation to estimate LDL-C from basic lipid panel, currently employed by most laboratories.
- 24.Martin SS, Blaha MJ, Elshazly MB, et al. Comparison of a novel method vs the Friedewald equation for estimating low-density lipoprotein cholesterol levels from the standard lipid profile. JAMA. 2013;310(19):2061–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Martin SS, Giugliano RP, Murphy SA, et al. Comparison of Low-Density Lipoprotein Cholesterol Assessment by Martin/Hopkins Estimation, Friedewald Estimation, and Preparative Ultracentrifugation: Insights From the FOURIER Trial. JAMA Cardiol. 2018;3(8):749–753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Quispe R, Hendrani A, Elshazly MB, et al. Accuracy of low-density lipoprotein cholesterol estimation at very low levels. BMC Med. 2017;15(1):83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Castelli WP. Lipids, risk factors and ischaemic heart disease. Atherosclerosis. 1996;124 Suppl:S1–9. [DOI] [PubMed] [Google Scholar]
- 28.Kathiresan S, Otvos JD, Sullivan LM, et al. Increased small low-density lipoprotein particle number: a prominent feature of the metabolic syndrome in the Framingham Heart Study. Circulation. 2006;113(1):20–9. [DOI] [PubMed] [Google Scholar]; * Important paper describing the phenomenon of LDL-C/LDL-P discordance, a key way in which traditional ASCVD risk estimation may fall short for some patients.
- 29.Mora S, Buring JE, Ridker PM. Discordance of low-density lipoprotein (LDL) cholesterol with alternative LDL-related measures and future coronary events. Circulation. 2014;129(5):553–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Martin SS, Michos ED. Are we moving towards concordance on the principle that lipid discordance matters? Circulation. 2014;129(5):539–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Packard CJ. Small dense low-density lipoprotein and its role as an independent predictor of cardiovascular disease. Curr Opin Lipidol. 2006;17(4):412–7. [DOI] [PubMed] [Google Scholar]
- 32.Cromwell WC, Otvos JD, Keyes MJ, et al. LDL Particle Number and Risk of Future Cardiovascular Disease in the Framingham Offspring Study - Implications for LDL Management. J Clin Lipidol. 2007;1(6):583–92. [DOI] [PMC free article] [PubMed] [Google Scholar]; * Research implicating LDL particle number as an independent risk factor for ASCVD, building off earlier work in LDL-C/LDL-P discordance
- 33.Sniderman AD. Differential response of cholesterol and particle measures of atherogenic lipoproteins to LDL-lowering therapy: implications for clinical practice. J Clin Lipidol. 2008;2(1):36–42. [DOI] [PubMed] [Google Scholar]
- 34.Otvos JD, Mora S, Shalaurova I, et al. Clinical implications of discordance between low-density lipoprotein cholesterol and particle number. J Clin Lipidol. 2011;5(2):105–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Sharman MJ, Kraemer WJ, Love DM, et al. A ketogenic diet favorably affects serum biomarkers for cardiovascular disease in normal-weight men. J Nutr. 2002;132(7):1879–85. [DOI] [PubMed] [Google Scholar]
- 36.Westman EC, Yancy WS Jr., Olsen MK, et al. Effect of a low-carbohydrate, ketogenic diet program compared to a low-fat diet on fasting lipoprotein subclasses. Int J Cardiol. 2006;110(2):212–6. [DOI] [PubMed] [Google Scholar]
- 37.Shapiro MD, Fazio S. Apolipoprotein B-containing lipoproteins and atherosclerotic cardiovascular disease. F1000Res. 2017;6:134. [DOI] [PMC free article] [PubMed] [Google Scholar]; * Important review of the role of apolipoprotein B-containing particles in ASCVD
- 38.Ference BA, Kastelein JJP, Ginsberg HN, et al. Association of Genetic Variants Related to CETP Inhibitors and Statins With Lipoprotein Levels and Cardiovascular Risk. JAMA. 2017;318(10):947–956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Ference BA, Kastelein JJP, Ray KK, et al. Association of Triglyceride-Lowering LPL Variants and LDL-C-Lowering LDLR Variants With Risk of Coronary Heart Disease. JAMA. 2019;321(4):364–373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Sniderman AD, Pencina M, Thanassoulis G. ApoB. Circ Res. 2019;124(10):1425–1427. [DOI] [PubMed] [Google Scholar]
- 41.Pallazola VA, Sathiyakumar V, Park J, et al. Modern prevalence of dysbetalipoproteinemia (Fredrickson-Levy-Lees type III hyperlipoproteinemia). Arch Med Sci 2020;16(5):993–1003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Sathiyakumar V, Pallazola VA, Park J, et al. Modern prevalence of the Fredrickson-Levy-Lees dyslipidemias: findings from the Very Large Database of Lipids and National Health and Nutrition Examination Survey. Arch Med Sci 2020;16(6):1279–1287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Contois JH, Delatour V. Apolipoprotein B measurement: Need for standardization. J Clin Lipidol. 2018;12(2):264–265. [DOI] [PubMed] [Google Scholar]
- 44.Nordestgaard BG, Chapman MJ, Ray K, et al. Lipoprotein (a) as a cardiovascular risk factor: current status. Eur Heart J. 2010;31(23):2844–2853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Caplice NM, Panetta C, Peterson TE, et al. Lipoprotein (a) binds and inactivates tissue factor pathway inhibitor: a novel link between lipoproteins and thrombosis. Blood. 2001;98(10):2980–7. [DOI] [PubMed] [Google Scholar]
- 46.von Depka M, Nowak-Gottl U, Eisert R, et al. Increased lipoprotein (a) levels as an independent risk factor for venous thromboembolism. Blood. 2000;96(10):3364–8. [PubMed] [Google Scholar]
- 47.Moriarty PM, Gorby LK, Stroes ES, et al. Lipoprotein(a) and Its Potential Association with Thrombosis and Inflammation in COVID-19: a Testable Hypothesis. Curr Atheroscler Rep. 2020;22(9):48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Schmidt K, Noureen A, Kronenberg F, et al. Structure, function, and genetics of lipoprotein (a). J Lipid Res. 2016;57(8):1339–59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Tsimikas S, Gordts P, Nora C, et al. Statin therapy increases lipoprotein(a) levels. Eur Heart J. 2020;41(24):2275–2284. [DOI] [PubMed] [Google Scholar]
- 50.Danesh J, Collins R, Peto R. Lipoprotein(a) and coronary heart disease. Meta-analysis of prospective studies. Circulation. 2000;102(10):1082–5. [DOI] [PubMed] [Google Scholar]
- 51.Erqou S, Kaptoge S, Perry PL, et al. Lipoprotein(a) concentration and the risk of coronary heart disease, stroke, and nonvascular mortality. JAMA. 2009;302(4):412–23. [DOI] [PMC free article] [PubMed] [Google Scholar]; * Meta-analysis showing a relationship between Lp(a) and vascular outcomes
- 52.Smolders B, Lemmens R, Thijs V. Lipoprotein (a) and stroke: a meta-analysis of observational studies. Stroke. 2007;38(6):1959–66. [DOI] [PubMed] [Google Scholar]
- 53.Nave AH, Lange KS, Leonards CO, et al. Lipoprotein (a) as a risk factor for ischemic stroke: a meta-analysis. Atherosclerosis. 2015;242(2):496–503. [DOI] [PubMed] [Google Scholar]
- 54.Virani SS, Brautbar A, Davis BC, et al. Associations between lipoprotein(a) levels and cardiovascular outcomes in black and white subjects: the Atherosclerosis Risk in Communities (ARIC) Study. Circulation. 2012;125(2):241–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Aronis KN, Zhao D, Hoogeveen RC, et al. Associations of Lipoprotein(a) Levels With Incident Atrial Fibrillation and Ischemic Stroke: The ARIC (Atherosclerosis Risk in Communities) Study. J Am Heart Assoc. 2017;6(12). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Thanassoulis G, Campbell CY, Owens DS, et al. Genetic associations with valvular calcification and aortic stenosis. N Engl J Med. 2013;368(6):503–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Makshood M, Joshi PH, Kanaya AM, et al. Lipoprotein (a) and aortic valve calcium in South Asians compared to other race/ethnic groups. Atherosclerosis. 2020;313:14–19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Albers JJ, Slee A, O’Brien KD, et al. Relationship of apolipoproteins A-1 and B, and lipoprotein(a) to cardiovascular outcomes: the AIM-HIGH trial (Atherothrombosis Intervention in Metabolic Syndrome with Low HDL/High Triglyceride and Impact on Global Health Outcomes). J Am Coll Cardiol 2013;62:1575–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.O’Donoghue ML, Fazio S, Giugliano RP, et al. Lipoprotein(a), PCSK9 Inhibition, and Cardiovascular Risk. Circulation. 2019;139(12):1483–1492. [DOI] [PubMed] [Google Scholar]
- 60.Schwartz GG, Steg PG, Szarek M, et al. Peripheral Artery Disease and Venous Thromboembolic Events After Acute Coronary Syndrome: Role of Lipoprotein(a) and Modification by Alirocumab: Prespecified Analysis of the ODYSSEY OUTCOMES Randomized Clinical Trial. Circulation. 2020;141(20):1608–1617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Sandholzer C, Saha N, Kark JD, et al. Apo(a) isoforms predict risk for coronary heart disease. A study in six populations. Arterioscler Thromb. 1992;12(10):1214–26. [DOI] [PubMed] [Google Scholar]
- 62.Sandholzer C, Boerwinkle E, Saha N, et al. Apolipoprotein(a) phenotypes, Lp(a) concentration and plasma lipid levels in relation to coronary heart disease in a Chinese population: evidence for the role of the apo(a) gene in coronary heart disease. J Clin Invest. 1992;89(3):1040–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Willeit P, Kiechl S, Kronenberg F, et al. Discrimination and net reclassification of cardiovascular risk with lipoprotein(a): prospective 15-year outcomes in the Bruneck Study. J Am Coll Cardiol 2014;64:851–60. [DOI] [PubMed] [Google Scholar]; * Research showing improved risk discrimination when Lp(a) is added to conventional risk prediction tools
- 64.Budoff M Triglycerides and Triglyceride-Rich Lipoproteins in the Causal Pathway of Cardiovascular Disease. Am J Cardiol. 2016;118(1):138–45. [DOI] [PubMed] [Google Scholar]
- 65.Boullart AC, de Graaf J, Stalenhoef AF. Serum triglycerides and risk of cardiovascular disease. Biochim Biophys Acta. 2012;1821(5):867–75. [DOI] [PubMed] [Google Scholar]
- 66.Hypertriglyceridaemia Reiner Ž. and risk of coronary artery disease. Nat Rev Cardiol. 2017;14(7):401. [DOI] [PubMed] [Google Scholar]; * Important review detailing the role of hypertriglyceridemia in coronary artery disease
- 67.Catapano AL, Graham I, De Backer G, et al. 2016 ESC/EAS Guidelines for the Management of Dyslipidaemias. Eur Heart J. 2016;37(39):2999–3058. [DOI] [PubMed] [Google Scholar]
- 68.Miller M, Cannon CP, Murphy SA, et al. Impact of triglyceride levels beyond low-density lipoprotein cholesterol after acute coronary syndrome in the PROVE IT-TIMI 22 trial. J Am Coll Cardiol. 2008;51(7):724–30. [DOI] [PubMed] [Google Scholar]
- 69.Garg A, Sharma A, Krishnamoorthy P, et al. Role of Niacin in Current Clinical Practice: A Systematic Review. Am J Med. 2017;130(2):173–187. [DOI] [PubMed] [Google Scholar]
- 70.Jakob T, Nordmann AJ, Schandelmaier S, et al. Fibrates for primary prevention of cardiovascular disease events. Cochrane Database Syst Rev. 2016;11:CD009753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Bhatt DL, Steg PG, Miller M, et al. Cardiovascular Risk Reduction with Icosapent Ethyl for Hypertriglyceridemia. N Engl J Med. 2019January3;380(1):11–22. [DOI] [PubMed] [Google Scholar]; ** The REDUCE-IT trial was one of the first large studies to show improved cardiovascular outcomes with omega-3 fatty acid supplementation (specifically high dose EPA) in patients already receiving statin therapy
- 72.ASCEND Group, Bowman L, Mafham M, et al. Effects of n-3 Fatty Acid Supplements in Diabetes Mellitus. N Engl J Med. 2018;379(16):1540–1550. [DOI] [PubMed] [Google Scholar]
- 73.Nicholls SJ, Lincoff AM, Garcia M, et al. Effect of High-Dose Omega-3 Fatty Acids vs Corn Oil on Major Adverse Cardiovascular Events in Patients at High Cardiovascular Risk: The STRENGTH Randomized Clinical Trial. JAMA. 2020;324(22):2268–2280. [DOI] [PMC free article] [PubMed] [Google Scholar]; * A recent negative trial of high dose combined DHA+EPA omega 3 and cardiovascular outcomes in contrast with REDUCE-IT. This suggests the type of omega 3 preparation and achieved blood levels of EPA matters for cardiovascular disease prevention.
- 74.Orringer CE, Jacobson TA, Maki KC. National Lipid Association Scientific Statement on the use of icosapent ethyl in statin-treated patients with elevated triglycerides and high or very-high ASCVD risk. J Clin Lipidol. 2019;13(6):860–872. [DOI] [PubMed] [Google Scholar]
- 75.Gaze DC, Collinson PO. Multiple molecular forms of circulating cardiac troponin: analytical and clinical significance. Ann Clin Biochem. 2008;45(Pt 4):349–55. [DOI] [PubMed] [Google Scholar]
- 76.Amsterdam EA, Wenger NK, Brindis RG, et al. 2014 AHA/ACC Guideline for the Management of Patients with Non-ST-Elevation Acute Coronary Syndromes: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines. J Am Coll Cardiol. 2014;64(24):e139–e228. [DOI] [PubMed] [Google Scholar]
- 77.Park KC, Gaze DC, Collinson PO, et al. Cardiac troponins: from myocardial infarction to chronic disease. Cardiovasc Res 2017;113:1708–1718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Apple FS, Collinson PO, IFCC Task Force on Clinical Applications of Cardiac Bio-Markers. Analytical characteristics of high-sensitivity cardiac troponin assays. Clin Chem. 2012;58(1):54–61. [DOI] [PubMed] [Google Scholar]
- 79.Martin Raymondi D, Garcia H, Alvarez I, et al. TUSARC: Prognostic Value of High-Sensitivity Cardiac Troponin T Assay in Asymptomatic Patients with High Cardiovascular Risk. Am J Med. 2019;132(5):631–638. [DOI] [PubMed] [Google Scholar]
- 80.Jia X, Sun W, Hoogeveen RC, et al. High-Sensitivity Troponin I and Incident Coronary Events, Stroke, Heart Failure Hospitalization, and Mortality in the ARIC Study. Circulation. 2019;139(23):2642–2653. [DOI] [PMC free article] [PubMed] [Google Scholar]; ** Large study implicating hs-cTn as a useful predictor of future CVD in patients without symptoms even after adjustment for traditional risk factors
- 81.Ford I, Shah AS, Zhang R, et al. High-Sensitivity Cardiac Troponin, Statin Therapy, and Risk of Coronary Heart Disease. J Am Coll Cardiol. 2016;68(25):2719–2728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Lan NSR, Bell DA, McCaul KA, et al. High-Sensitivity Cardiac Troponin I Improves Cardiovascular Risk Prediction in Older Men: HIMS (The Health in Men Study). J Am Heart Assoc 2019;8:e011818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Diris JH, Hackeng CM, Kooman JP, et al. Impaired renal clearance explains elevated troponin T fragments in hemodialysis patients. Circulation. 2004;109(1):23–5. [DOI] [PubMed] [Google Scholar]
- 84.Michos ED, Wilson LM, Yeh HC, et al. Prognostic value of cardiac troponin in patients with chronic kidney disease without suspected acute coronary syndrome: a systematic review and meta-analysis. Ann Intern Med. 2014October7;161(7):491–501. [DOI] [PubMed] [Google Scholar]; *Meta-analysis showing that troponin is still prognostic of future CVD in patients with chronic kidney disease.
- 85.Ebong IA, Wilson MD, Bertoni AG, et al. High-sensitivity cardiac troponin T and the risk of heart failure in postmenopausal women of the ARIC Study. Menopause. 2021January4;28(3):284–291 [DOI] [PubMed] [Google Scholar]
- 86.Sandoval Y, Bielinski SJ, Daniels LB, et al. Atherosclerotic Cardiovascular Disease Risk Stratification Based on Measurements of Troponin and Coronary Artery Calcium. J Am Coll Cardiol. 2020;76(4):357–370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Daniels LB, Maisel AS. Natriuretic peptides. J Am Coll Cardiol. 2007;50(25):2357–68. [DOI] [PubMed] [Google Scholar]
- 88.Goetze JP, Mogelvang R, Maage L, et al. Plasma pro-B-type natriuretic peptide in the general population: screening for left ventricular hypertrophy and systolic dysfunction. Eur Heart J. 2006;27(24):3004–10. [DOI] [PubMed] [Google Scholar]
- 89.Nadir MA, Rekhraj S, Wei L, et al. Improving the primary prevention of cardiovascular events by using biomarkers to identify individuals with silent heart disease. J Am Coll Cardiol 2012;60:960–8. [DOI] [PubMed] [Google Scholar]
- 90.Rana BS, Davies JI, Band MM, et al. B-type natriuretic peptide can detect silent myocardial ischaemia in asymptomatic type 2 diabetes. Heart. 2006;92(7):916–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Vasan RS, Benjamin EJ, Larson MG, et al. Plasma natriuretic peptides for community screening for left ventricular hypertrophy and systolic dysfunction: the Framingham heart study. JAMA. 2002;288(10):1252–9. [DOI] [PubMed] [Google Scholar]
- 92.Wang TJ, Larson MG, Levy D, et al. Plasma natriuretic peptide levels and the risk of cardiovascular events and death. N Engl J Med. 2004;350(7):655–63. [DOI] [PubMed] [Google Scholar]
- 93.Nambi V, Liu X, Chambless LE, et al. Troponin T and N-terminal pro-B-type natriuretic peptide: a biomarker approach to predict heart failure risk--the atherosclerosis risk in communities study. Clin Chem. 2013;59(12):1802–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Linssen GC, Bakker SJ, Voors AA, et al. N-terminal pro-B-type natriuretic peptide is an independent predictor of cardiovascular morbidity and mortality in the general population. Eur Heart J. 2010;31(1):120–7. [DOI] [PubMed] [Google Scholar]
- 95.Marz W, Tiran B, Seelhorst U, et al. N-terminal pro-B-type natriuretic peptide predicts total and cardiovascular mortality in individuals with or without stable coronary artery disease: the Ludwigshafen Risk and Cardiovascular Health Study. Clin Chem 2007;53:1075–83. [DOI] [PubMed] [Google Scholar]
- 96.Alfaddagh A, Martin SS, Leucker TM, et al. Inflammation and cardiovascular disease: From mechanisms to therapeutics. Am J Prev Cardiol 2020;4:100130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Libby P Interleukin-1 Beta as a Target for Atherosclerosis Therapy: Biological Basis of CANTOS and Beyond. J Am Coll Cardiol. 2017;70(18):2278–2289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Ridker PM, Everett BM, Thuren T, et al. Antiinflammatory Therapy with Canakinumab for Atherosclerotic Disease. N Engl J Med. 2017;377(12):1119–1131. [DOI] [PubMed] [Google Scholar]; ** Important clinical trial that proved the concept that modulation of the inflammatory cascade can improve cardiovascular outcomes independent of lipid lowering
- 99.Black S, Kushner I, Samols D. C-reactive Protein. J Biol Chem. 2004;279(47):48487–90. [DOI] [PubMed] [Google Scholar]
- 100.Ridker PM, Hennekens CH, Buring JE, et al. C-reactive protein and other markers of inflammation in the prediction of cardiovascular disease in women. N Engl J Med 2000;342:836–43. [DOI] [PubMed] [Google Scholar]
- 101.Quispe R, Michos ED, Martin SS, et al. High-Sensitivity C-Reactive Protein Discordance With Atherogenic Lipid Measures and Incidence of Atherosclerotic Cardiovascular Disease in Primary Prevention: The ARIC Study. J Am Heart Assoc. 2020;9(3):e013600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Ridker PM, Buring JE, Rifai N, et al. Development and validation of improved algorithms for the assessment of global cardiovascular risk in women: the Reynolds Risk Score. JAMA. 2007;297(6):611–9. [DOI] [PubMed] [Google Scholar]; * Derivation of a risk predicton model that included hsCRP in addition to traditional risk factors.
- 103.Ridker PM, Paynter NP, Rifai N, et al. C-reactive protein and parental history improve global cardiovascular risk prediction: the Reynolds Risk Score for men. Circulation. 2008;118(22):2243–51, 4p following 2251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Ridker PM, Danielson E, Fonseca FA, et al. Reduction in C-reactive protein and LDL cholesterol and cardiovascular event rates after initiation of rosuvastatin: a prospective study of the JUPITER trial. Lancet. 2009;373(9670):1175–82. [DOI] [PubMed] [Google Scholar]; **Landmark primary prevention clinical trial showing that statins can reduce CVD events among adults who have elevated hsCRP but without hyperlipidemia. This study shaped future guidelines that now list elevated hsCRP as a “risk-enhancer” that might favor initiation of statins among borderline or intermediate risk adults.
- 105.Bohula EA, Giugliano RP, Cannon CP, et al. Achievement of dual low-density lipoprotein cholesterol and high-sensitivity C-reactive protein targets more frequent with the addition of ezetimibe to simvastatin and associated with better outcomes in IMPROVE-IT. Circulation. 2015;132(13):1224–33. [DOI] [PubMed] [Google Scholar]
- 106.Ridker PM, Cannon CP, Morrow D, et al. C-reactive protein levels and outcomes after statin therapy. N Engl J Med. 2005;352(1):20–8. [DOI] [PubMed] [Google Scholar]
- 107.Ridker PM, MacFadyen JG, Everett BM, et al. Relationship of C-reactive protein reduction to cardiovascular event reduction following treatment with canakinumab: a secondary analysis from the CANTOS randomised controlled trial. Lancet. 2018;391(10118):319–328. [DOI] [PubMed] [Google Scholar]
- 108.Michos ED, Martin SS, Blumenthal RS. Bringing back targets to “IMPROVE” atherosclerotic cardiovascular disease outcomes: the duel for dual goals; are two targets better than one? Circulation. 2015;132(13):1218–20. [DOI] [PubMed] [Google Scholar]
- 109.Michos ED, Blumenthal RS. Treatment concentration of high-sensitivity C-reactive protein. Lancet. 2018;391(10118):287–289. [DOI] [PubMed] [Google Scholar]
- 110.Cardoso R, Kaul S, Okada DR, et al. A Deeper Dive Into the CANTOS “Responders” Substudy. Mayo Clin Proc. 2018;93(7):830–833. [DOI] [PubMed] [Google Scholar]
- 111.Otvos JD, Shalaurova I, Wolak-Dinsmore J, et al. GlycA: A Composite Nuclear Magnetic Resonance Biomarker of Systemic Inflammation. Clin Chem. 2015;61(5):714–23. [DOI] [PubMed] [Google Scholar]
- 112.Benson EA, Tibuakuu M, Zhao D, et al. Associations of ideal cardiovascular health with GlycA, a novel inflammatory marker: The Multi-Ethnic Study of Atherosclerosis. Clin Cardiol. 2018;41(11):1439–1445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Akinkuolie AO, Buring JE, Ridker PM, et al. A novel protein glycan biomarker and future cardiovascular disease events. J Am Heart Assoc 2014;3:e001221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Lawler PR, Akinkuolie AO, Chandler PD, et al. Circulating N-Linked Glycoprotein Acetyls and Longitudinal Mortality Risk. Circ Res. 2016;118(7):1106–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Duprez DA, Otvos J, Sanchez OA, et al. Comparison of the Predictive Value of GlycA and Other Biomarkers of Inflammation for Total Death, Incident Cardiovascular Events, Noncardiovascular and Noncancer Inflammatory-Related Events, and Total Cancer Events. Clin Chem. 2016;62(7):1020–31. [DOI] [PubMed] [Google Scholar]
- 116.Fashanu OE, Oyenuga AO, Zhao D, et al. GlycA, a Novel Inflammatory Marker and Its Association With Peripheral Arterial Disease and Carotid Plaque: The Multi-Ethnic Study of Atherosclerosis. Angiology. 2019;70(8):737–746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Tibuakuu M, Fashanu OE, Zhao D, et al. GlycA, a novel inflammatory marker, is associated with subclinical coronary disease. AIDS. 2019;33(3):547–557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Ezeigwe A, Fashanu OE, Zhao D, et al. The novel inflammatory marker GlycA and the prevalence and progression of valvular and thoracic aortic calcification: The Multi-Ethnic Study of Atherosclerosis. Atherosclerosis. 2019;282:91–99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Jang S, Ogunmoroti O, Ndumele CE, et al. Association of the Novel Inflammatory Marker GlycA and Incident Heart Failure and Its Subtypes of Preserved and Reduced Ejection Fraction: The Multi-Ethnic Study of Atherosclerosis. Circ Heart Fail. 2020;13(8):e007067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Burke JE, Dennis EA. Phospholipase A2 biochemistry. Cardiovasc Drugs Ther. 2009;23(1):49–59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.MacPhee CH, Moores KE, Boyd HF, et al. Lipoprotein-associated phospholipase A2, platelet-activating factor acetylhydrolase, generates two bioactive products during the oxidation of low-density lipoprotein: use of a novel inhibitor. Biochem J. 1999;338 (Pt 2):479–87. [PMC free article] [PubMed] [Google Scholar]
- 122.Ballantyne CM, Hoogeveen RC, Bang H, et al. Lipoprotein-associated phospholipase A2, high-sensitivity C-reactive protein, and risk for incident coronary heart disease in middle-aged men and women in the Atherosclerosis Risk in Communities (ARIC) study. Circulation. 2004;109(7):837–42. [DOI] [PubMed] [Google Scholar]
- 123.Tibuakuu M, Kianoush S, DeFilippis AP, et al. Usefulness of Lipoprotein-Associated Phospholipase A2 Activity and C-Reactive Protein in Identifying High-Risk Smokers for Atherosclerotic Cardiovascular Disease (from the Atherosclerosis Risk in Communities Study). Am J Cardiol 2018;121:1056–1064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.STABILITY Investigators, White HD, Held C, et al. Darapladib for preventing ischemic events in stable coronary heart disease. N Engl J Med. 2014;370(18):1702–11. [DOI] [PubMed] [Google Scholar]; * Trial showing lack of benefit of Lp-PLA2 modulator darapladib for secondary prevention of coronary heart disease
- 125.Li S, Shin HJ, Ding EL, et al. Adiponectin levels and risk of type 2 diabetes: a systematic review and meta-analysis. JAMA. 2009;302(2):179–88. [DOI] [PubMed] [Google Scholar]
- 126.D’Souza AM, Neumann UH, Glavas MM, et al. The glucoregulatory actions of leptin. Mol Metab. 2017;6(9):1052–1065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Hsu WY, Chao YW, Tsai YL, et al. Resistin induces monocyte-endothelial cell adhesion by increasing ICAM-1 and VCAM-1 expression in endothelial cells via p38MAPK-dependent pathway. J Cell Physiol. 2011;226(8):2181–8. [DOI] [PubMed] [Google Scholar]
- 128.Unamuno X, Gomez-Ambrosi J, Rodriguez A, et al. Adipokine dysregulation and adipose tissue inflammation in human obesity. Eur J Clin Invest. 2018;48(9):e12997. [DOI] [PubMed] [Google Scholar]
- 129.Landecho MF, Tuero C, Valenti V, et al. Relevance of Leptin and Other Adipokines in Obesity-Associated Cardiovascular Risk. Nutrients. 2019;11(11). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Martin SS, Qasim A, Reilly MP. Leptin resistance: a possible interface of inflammation and metabolism in obesity-related cardiovascular disease. J Am Coll Cardiol. 2008;52(15):1201–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Acquarone E, Monacelli F, Borghi R, et al. Resistin: A reappraisal. Mech Ageing Dev. 2019;178:46–63. [DOI] [PubMed] [Google Scholar]
- 132.Wannamethee SG, Lowe GD, Rumley A, et al. Adipokines and risk of type 2 diabetes in older men. Diabetes Care. 2007;30(5):1200–5. [DOI] [PubMed] [Google Scholar]
- 133.Muse ED, Feldman DI, Blaha MJ, et al. The association of resistin with cardiovascular disease in the Multi-Ethnic Study of Atherosclerosis. Atherosclerosis. 2015;239(1):101–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Pischon T, Girman CJ, Hotamisligil GS, et al. Plasma adiponectin levels and risk of myocardial infarction in men. JAMA. 2004;291(14):1730–7. [DOI] [PubMed] [Google Scholar]
- 135.Martin SS, Blaha MJ, Muse ED, et al. Leptin and incident cardiovascular disease: the Multi-ethnic Study of Atherosclerosis (MESA). Atherosclerosis. 2015;239(1):67–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Havranek EP, Mujahid MS, Barr DA, et al. Social Determinants of Risk and Outcomes for Cardiovascular Disease: A Scientific Statement From the American Heart Association. Circulation. 2015;132(9):873–98. [DOI] [PubMed] [Google Scholar]; ** Important statement from the American Heart Association regarding the continued importance of social determinants of health and their influence on CVD risk
- 137.Yeboah J, McClelland RL, Polonsky TS, et al. Comparison of novel risk markers for improvement in cardiovascular risk assessment in intermediate-risk individuals. JAMA. 2012;308(8):788–95. [DOI] [PMC free article] [PubMed] [Google Scholar]; * Study evaluating the improvement in risk stratification that comes with the addition of multiple risk markers such as biomarkers to traditional risk calculations
- 138.Yeboah J, Young R, McClelland RL, et al. Utility of Nontraditional Risk Markers in Atherosclerotic Cardiovascular Disease Risk Assessment. J Am Coll Cardiol. 2016;67(2):139–147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Pender A, Lloyd-Jones DM, Stone NJ, et al. Refining Statin Prescribing in Lower-Risk Individuals: Informing Risk/Benefit Decisions. J Am Coll Cardiol 2016;68:1690–1697. [DOI] [PubMed] [Google Scholar]
- 140.Kambhampati S, Ashvetiya T, Stone NJ, et al. Shared Decision-Making and Patient Empowerment in Preventive Cardiology. Curr Cardiol Rep. 2016;18(5):49. [DOI] [PubMed] [Google Scholar]; ** Important article detailing the importance of shared decision making, particularly in the field of preventive cardiology


