Graphical abstract
Lipid squalene nanoparticles (SQ@NPs) play a crucial role in the immunogenicity of the SARS-CoV-2 spike subunit protein against COVID-19. In a mouse model, we found that one dose of the S-protein with SQ@NPs works as well as two doses of the S-protein alone without compromising safety aspects following i.m. injection. These results pave the way for optimal vaccine formulations against COVID-19.
Keywords: COVID-19 vaccine, Immunomodulation, Lipid squalene nanoparticles, Single injection, Vaccine adjuvant
Abstract
Vaccination is regarded as the most effective intervention for controlling the coronavirus disease 2019 (COVID-19) pandemic. The objective of this study is to provide comprehensive information on lipid squalene nanoparticle (SQ@NP)-adjuvanted COVID-19 vaccines regarding modulating immune response and enhancing vaccine efficacy. After being adjuvanted with SQ@NP, the SARS-CoV-2 spike (S) subunit protein was intramuscularly (i.m.) administered to mice. Serum samples investigated by ELISA and virus neutralizing assay showed that a single-dose SQ@NP-adjuvanted S-protein vaccine can induce antigen-specific IgG and protective antibodies comparable with those induced by two doses of nonadjuvanted protein vaccine. When the mice received a boosting vaccine injection, anamnestic response was observed in the groups of adjuvanted vaccine. Furthermore, the secretion of cytokines in splenocytes, such as interferon (IFN)-γ, interleukin (IL)-5 and IL-10, was significantly enhanced after adjuvantation of S-protein vaccine with SQ@NP; however, this was not the case for the vaccine adjuvanted with conventional aluminum mineral salts. Histological examination of injection sites showed that the SQ@NP-adjuvanted vaccine was considerably well tolerated following i.m. injection in mice. These results pave the way for the performance tuning of optimal vaccine formulations against COVID-19.
1. Introduction
COVID-19 is a highly infectious respiratory disease caused by a novel Coronaviridae family (SARS-CoV-2) that has resulted in a global pandemic (Dai and Gao, 2021). Vaccination is regarded as the most effective intervention in controlling disease pandemics and to prevent SARS-CoV-2 infection and its complications (Dai and Gao, 2021). To implement government policy for developing vaccine self-manufacturing capability, the National Health Research Institutes (NHRI) of Taiwan is currently developing cutting edge platform technologies as alternatives to conventional processes to quickly produce vaccines against COVID-19.
Vaccine-induced humoral and cellular immunity play a role in SARS-CoV-2 infection (Dai and Gao, 2021, Jeyanathan et al., 2020). It is well known that SARS-CoV-2 virus neutralizing antibodies could block viral entry and prevent virus infection. If the titer of neutralizing antibodies is not sufficient to confer protection against virus infection, T cell-mediated immunity will be important in host defense and viral clearance towards association with milder disease severity (Jeyanathan et al., 2020). Both targets can be accomplished by using the adjuvantation strategy to stimulate the innate immune system and provide sufficient signals such that a robust and broadened immune response can be generated (Gupta and Gupta, 2020). Moreover, mass vaccination with a reduced number of injections can simplify the logistics and time schedule. However, FDA-approved aluminum-based mineral salts are poor adjuvants to induce cellular immunity (Gupta and Gupta, 2020). For feasibility studies on the development of COVID-19 vaccines, it will be important to investigate the possibility of enhancing vaccine immunogenicity with non-aluminum-based adjuvants that allow antigen recognition by the immune system to integrate appropriate immune responses.
Squalene is an ingredient of some oily adjuvants that are applied to FDA-approved influenza vaccines to enhance vaccine efficacy (Gupta and Gupta, 2020). Squalene plays a key role as a precursor of cholesterol in the body and a natural antioxidant extracted from shark liver and olive oil (Narayan Bhilwade et al., 2019). It should be noted that squalene by itself is not an adjuvant; however, emulsions comprising squalene with emulsifiers do enhance immune response (Gupta and Gupta, 2020). We have previously demonstrated that unsaturated squalene content in emulsion adjuvant induced reactive oxidative species (ROS) production and resulted in cellular apoptosis and necrosis at the local injection tissues. Subsequently, the presence of cell debris facilitated antigen uptake into antigen-presenting cells so as to enhance cellular immunity (Huang et al., 2018). It will therefore be very interesting to investigate the potential of lipid squalene nanoparticles as adjuvants in enhancing the immunogenicity of COVID-19 vaccine candidates.
In this study, we aimed to investigate the impact of squalene nanoparticle (SQ@NP) on the immunogenicity of a SARS-CoV-2 subunit spike (S) protein against COVID-19, leading to optimal vaccine formulations. First, we tuned process parameters on SQ@NP production at pilot scale issued from high-shear fluid process. Then, we investigated the adjuvantation of SQ@NP on the generation of antigen-specific protective antibodies against the S-protein following a single-dose injection. We also investigated the modulation of spleen and histological examination in local vaccination tissues. The results were compared with those obtained from vaccine adjuvanted with conventional aluminum phosphate mineral suspensions and those obtained following repeated doses of nonadjuvanted spike protein.
2. Materials and methods
2.1. Adjuvant preparation
Aluminum phosphate wet gel suspensions (AlPO4, Adju-Phos®) were obtained from Brenntag AG (Frederikssund, Danish). Squalene nanoparticle production was accomplished by homogenization with NanoLyzer™, a high-pressure microfluidizer developed by a local company (Gogene Corporation, Hsinchu, Taiwan). The ingredients of SQ@NPs were based on laboratory scale squalene nanoemulsion preparations as previously described (Huang et al., 2020). Briefly, a 30-ml mixture containing aqueous solution comprising 180 mg of poly(ethylene glycol)-block-poly(lactide-co-ε-caprolactone) amphiphilic bioresorbable polymer in 26 ml of phosphate buffer saline (PBS, HyClone, Utah, USA), oily solution comprising 1.377 ml of squalene (Sigma-Aldrich, Steinheim, Germany) and 0.243 ml of Span®85 (sorbitan trioleate, Sigma-Aldrich, Steinheim, Germany) was homogenized using a microfluidization process at 20,000 psi. The hydrodynamic size was detected by a dynamic light scattering (DLS) instrument (90Plus with ZetaPALS, Brookhaven Instruments Limited, NY, USA). Nanometer-scale images were recorded using a transmission electron microscope (H-7650, Hitachi, Japan).
2.2. Mice and ethics statement
Pathogen-free female BALB/c mice (5 weeks old) were obtained from the National Laboratory Animal Center of Taiwan and were housed for one week at the laboratory animal facility of the NHRI in Miaoli County, Taiwan. All experiments were conducted according to the guidelines of the Laboratory Animal Center of NHRI. The animal use protocol was approved by the NHRI Institutional Animal Care and Use Committee under the agreement of NHRI-IACUC-109060-A.
2.3. Vaccination and immunoassays
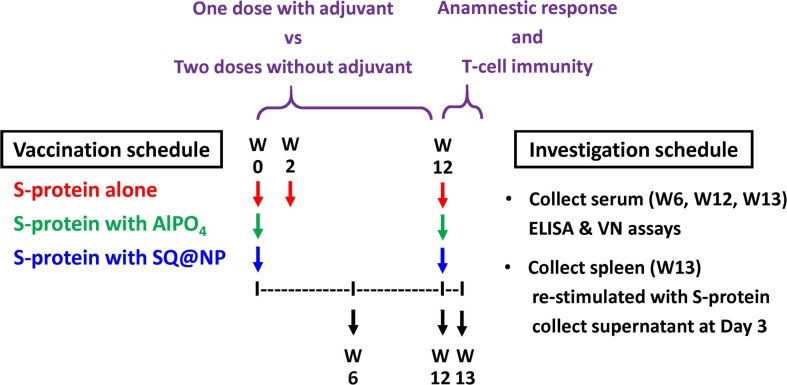
Six mice per group were injected intramuscularly (i.m.) with a total of 20 μg of SARS-CoV-2 spike subunit protein that contains the ectodomain of SARS-CoV-2 S-protein (amino acid residues 16–1213, Acro Biosystems, China). The recombinant protein was expressed from human embryonic kidney 293 (HEK293) cells with glycosylation and a polyhistidine tag at the C-terminus. Injections were performed in the quadriceps muscle of both hind limbs (50 μL per hind limb) to administer the antigen in PBS, adsorbed with AlPO4 (given as 150 µg per mouse) or adjuvanted with lipid squalene nanoparticles (given as 2 mg of squalene per mouse). At Week 2, the mice that received antigen in PBS were repeated i.m. with the same vaccine formulations. To investigate the anamnestic response and T-cell immunity, all mice were boosted i.m. at Week 12 with the same vaccine formulations as the priming injection. Sera and spleen were collected to assay B- and T-cell immunity. Tissues of injection site were prepared for hematoxylin and eosin (H&E)-stained sections by the NHRI Pathology Core Laboratory for histological examination. The vaccination schedule is shown in Fig. 1 .
Fig. 1.
Study design of immunogenicity of a SARS-CoV-2 spike subunit protein against COVID-19. Six mice per group were injected with either two intramuscular doses of SARS-CoV-2 S-protein alone or one intramuscular dose of AlPO4- or SQ@NP-adjuvanted SARS-CoV-2 S-protein. At Week 12, all mice were boosted i.m. with the same vaccine formulations as priming injection. Sera and spleen collection were performed to determine the B- and T-cell responses.
2.4. B-cell immunity
Anti-serum samples were first separated by centrifugation of clotted whole blood and heat inactivation at 56 °C for 30 min. The antigen-specific antibody responses were determined by virus neutralizing (VN) activity and enzyme-linked immunosorbent assay (ELISA). The VN assay was conducted in a space specified as P3 facility. VN antibody titers were expressed as the reciprocal of the highest dilution of serum sample that gave 50% neutralized infectivity of 100 TCID50 (50% tissue culture infective dose) of the SARS-CoV-2 virus in Vero cells. The seroconversion rate (SCR) was designed as the ratio of mice with a minimum 4-fold increase between prevaccination and postvaccination antibody titers. The presence of antigen-specific IgG1 and IgG2a antibodies in the sera was determined by ELISA, as described previously (Huang et al., 2020). Dilute SARS-CoV-2 S-proteins (0.1 μg/well) were coated in 96-well microtiter plates with 0.05 M carbonate buffer (pH 9.6). Diluted sera from vaccinated mice were assayed, and titers that gave a 450 nm OD reading at least two times greater than that of the prevaccination sera were read as the highest dilution. The data will be represented as the geometric mean titer (GMT) and 95% confidence interval (CI). Log-transformed values were compared by performing Student's t-test, and a p-value < 0.05 was considered an indication of significance.
2.5. T-cell Immunity and histological examination
To investigate SARS-CoV-2-specific T-cell responses, mouse spleen was removed aseptically 7 days after the boosting vaccination. Spleen organs from vaccinated mice (n = 6) were isolated, and cell suspensions were harvested as a pooled sample. Single-cell suspensions (5 × 106 cells/ml) were restimulated in the absence or the presence of 1 μg/ml recombinant SARS-CoV-2 S-protein. Interferon (IFN)-γ, interleukin (IL)-5 and IL-10 concentrations were analyzed by ELISA immunoassay (InvitroGen, Thermo Fisher Scientific Inc., Vienna, Austria) following manufacturer instructions. Student's t-test was used to assess significance among the treatment and control groups. The level of significance was set at p < 0.05. The muscle tissues at the injection sites were excised, fixed and embedded in paraffin. Then, 4-μm sections were stained with H&E and examined using an optical microscope.
3. Results
3.1. Lipid squalene nanoparticles (SQ@NPs) issued from a microfluidizer
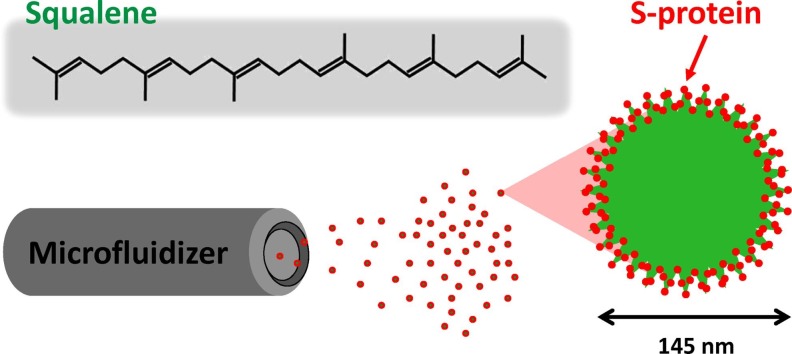
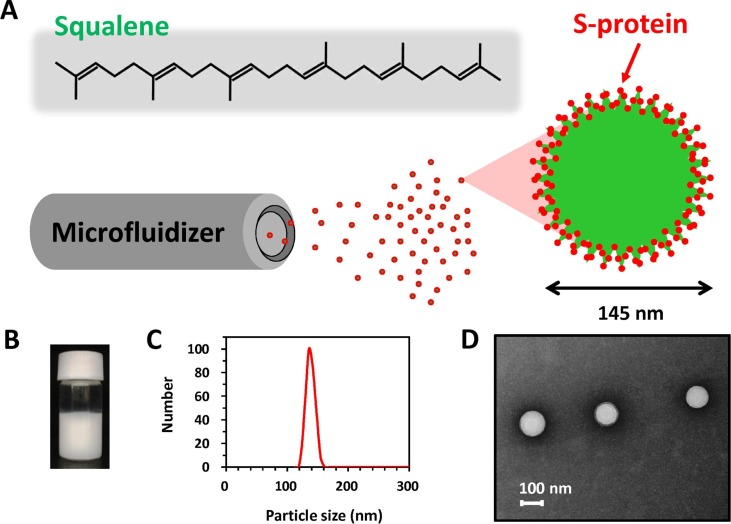
Lipid squalene nanoparticles contain lipid squalene, the lipophilic emulsifier Span®85 and a bioresorbable polymer suspended in PBS buffer. In a small pilot study (30 ml per batch), we demonstrated that milky white stable squalene lipid nanoparticles, named SQ@NPs, could be prepared by a high-shear microfluidized process operating at 20,000 psi (Fig. 2 A,B). This apparatus equipped with interaction chamber which with fixed geometry orifice and micro-channels drives the formulation into homogenization chambers at high-shear fluid process, thus providing particles with a narrow size distribution. The process is consistent and reproducible, and the emulsified products can be easily scaled to small pilot scale. DLS showed emulsified SQ@NP particles with a narrow distribution and an average diameter of 145 ± 30 nm (mean ± SEM, n = 3) (Fig. 2C), which is comparable with the dimensions of coronaviruses (Araf et al., 2021). Transmission electron microscopy (TEM) results showed that SQ@NPs were spherical in shape (Fig. 2D). It is worth noting that the sizes measured from DLS were higher than those from TEM images. This finding could be assigned to changes of hydrodynamic volume of SQ@NP in the aqueous (DLS) as compared with the dry samples in high-vacuum chambers (TEM). Prior to vaccination, SQ@NPs were further mixed 1:1 v/v with SARS-CoV-2 antigen, which yielded SQ@NP-adjuvanted vaccine formulations with 2 wt-% squalene.
Fig. 2.
(A) Schematic representation, (B) Visual aspects, (C) particle size distribution, (D) TEM images of lipid squalene nanoparticles issued from the high-shear microfluidization process. The particle size was measured by DLS.
3.2. Single-dose vaccination of SQ@NP-adjuvanted SARS-CoV-2 S-protein
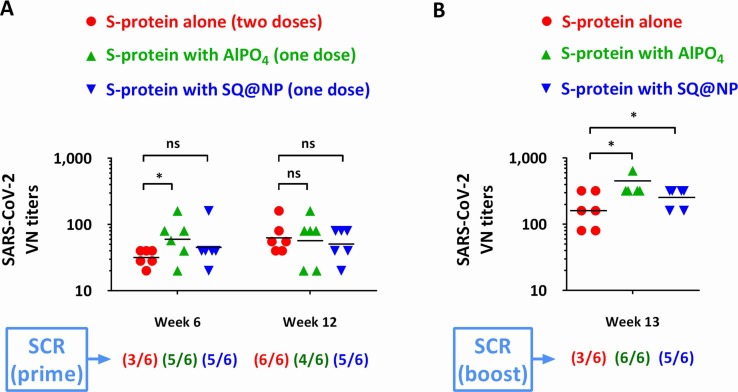
To verify whether antigen-specific antibodies can be driven by a single-dose vaccination, mice were vaccinated i.m. with 20 μg of SARS-CoV-2 S-protein adjuvanted with SQ@NP or AlPO4. The results were compared with mice vaccinated with protein alone following a schedule of repeated doses at Week 0 and Week 2. The elicited S-protein-specific antibodies are shown in Fig. 3 A. Following repeated vaccination, sera from mice that received nonadjuvanted SARS-CoV-2 S-protein elicited an antigen-specific VN titer of less than 40 at Week 6. This finding revealed that a two-dose vaccination schedule of the nonadjuvanted SARS-CoV-2 S-protein subunit likely would not provide sufficient serological protection against homologous SARS-CoV-2 virus challenge at this dosage.
Fig. 3.
Antigen-specific antibody responses to VN elicited in BALB/c mice. (A) Adjuvantation effect, (B) anamnestic response. Mice (n = 6) were injected with either two intramuscular doses of SARS-CoV-2 S-protein alone or one intramuscular dose of AlPO4- or SQ@NP-adjuvanted SARS-CoV-2 S-protein. At Week 12, all mice were boosted i.m. with the same vaccine formulations. Data are presented as GMT with individual mice. The seroconversion rate (SCR) was calculated from the proportion of mice with a minimum 4-fold increase between prevaccination and postvaccination antibody titers. The data are expressed as the individual values plus the GMT. The Log-transformed values of VN titers were compared by performing Student's t-test. *p < 0.05: Comparison with the group of protein alone. ns represents no significant difference.
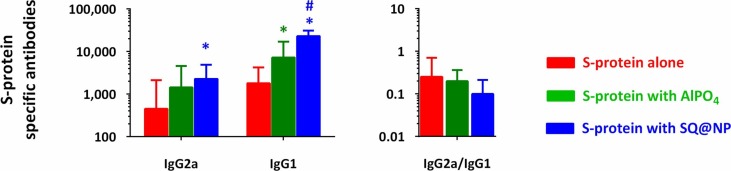
When the same amount of vaccine protein was adjuvanted with AlPO4 or SQ@NP, a protective level of SARS-CoV-2 VN antibody was generated following a single injection. GMTs of 60 and 45 were detected at Week 6 in the AlPO4 and SQ@NP groups, respectively. At Week 12, SARS-CoV-2 VN antibody responses were elicited at high level of protective antibodies in the three groups. GMTs of 63, 57 and 50 were detected in the antigen alone, AlPO4 and SQ@NP groups, respectively (Fig. 3A). It should be noted that the titers were induced by two doses of nonadjuvanted proteins compared with one dose of AlPO4 or SQ@NP-adjuvanted proteins. The SCR was calculated from the proportion of mice with a postvaccination titer ≥ 40 compared to a prevaccination VN antibody titer of 10. The results demonstrated that S-protein-based vaccines triggered a high serum seroconversion after vaccination. Interestingly, we found that the SQ@NP-adjuvanted vaccine generated elevated levels of antigen-specific IgG1 and IgG2a antibodies (Fig. 4 ). Accordingly, both adjuvanted vaccines were able to trigger the immune system following single-dose injections.
Fig. 4.
Antigen-specific IgG subtype response at Week 12 elicited in BALB/c mice. Mice (n = 6) were injected with either two intramuscular doses of SARS-CoV-2 S-protein alone or one intramuscular dose of AlPO4- or SQ@NP-adjuvanted SARS-CoV-2 S-protein. Data are presented as GMT with 95% CI of six mice per group. *p < 0.05: Comparison with the group of protein alone. #p < 0.05: Comparison with the AlPO4-adjuvanted protein group.
3.3. Anamnestic response and T-cell investigation
To investigate the anamnestic response and T-cell immunity elicited by the vaccine candidates, all mice were injected with the same vaccine formulations at Week 12. After the boosting injection, the titer rapidly increased to a GMT of 160 at Week 13 in the antigen alone group (Fig. 3B). A high level of anamnestic response was found when the spike protein vaccine was adjuvanted with AlPO4 or SQ@NP, and GMTs of 453 and 254 were detected, respectively. Based on these values, SCR can be calculated as the ratio of antibody titers with a minimum 4-fold increase between prevaccination (Week 12) and postvaccination (Week 13). Overall, AlPO4- or SQ@NP-adjuvanted vaccine easily reached a high seroconversion after boosting the dose, indicating the merit of this platform technology in the development of COVID-19 vaccines.
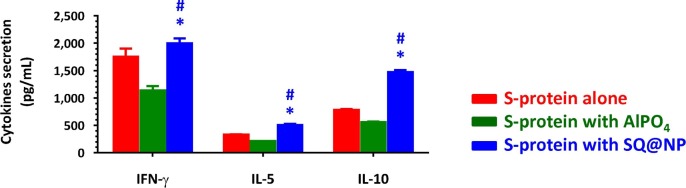
T-cell responses were assessed in splenocyte suspensions following restimulation of cells in vitro with SARS-CoV-2 S-protein. Fig. 5 shows that sufficiently elevated IFN-γ, IL-5- and IL-10 cytokine secretions were detected in cell supernatants collected from mice treated with SQ@NP-adjuvanted vaccine compared with the PBS control group, whereas these cytokines were measured at reduced levels in AlPO4-adjuvanted vaccine. These findings suggested that SQ@NP, but not AlPO4, may be a potential tool for reinforcing T-cell immunity. In fact, adjuvantation of a subunit COVID-19 vaccine with aluminum-based mineral suspensions in the absence of immunomodulatory agents does not elicit protective levels of immunity was described in the literature (Arunachalam et al., 2021).
Fig. 5.
T-cell investigation. BALB/c mice were injected with either two intramuscular doses of SARS-CoV-2 S-protein alone or one intramuscular dose of AlPO4- or SQ@NP-adjuvanted SARS-CoV-2 S-protein. At Week 12, all mice were boosted i.m. with the same vaccine formulations. One week after the boosting vaccination, splenocyte suspensions (5 × 106 cells/ml) were harvested and incubated in the absence or the presence of 1 μg/ml SARS-CoV-2 S-protein. Supernatants collected from cell cultures at Day 3 were assessed by IFN-γ, IL-5, and IL-10 ELISA. The data are presented as cytokine release in the presence of S-protein minus release in the presence of medium control and expressed as the mean ± standard deviation of triplicate cultures. *p < 0.05: Comparison with the group of protein alone. #p < 0.05: Comparison with the AlPO4-adjuvanted protein group.
3.4. Histological examination of the injection site
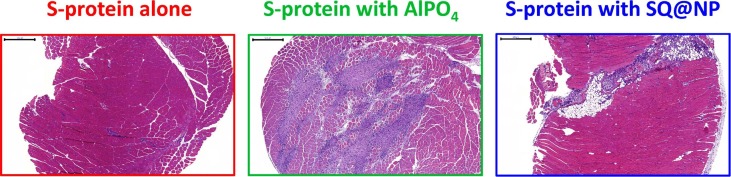
To investigate whether SQ@NPs were harmful to the host, histological examinations of injection site tissues were conducted (Fig. 6 ). No cellular infiltration was observed in the antigen alone group one week after injection, whereas cellular infiltration was widespread and necrosis of the muscular tissue and calcification were visible at local injection sites in the AlPO4 group. Beyond, cell necrosis were rarely observed around the injected mass. It is worth mentioning that vaccination of mice with antigen plus aluminum salts induced local tissue and cellular damage and the subsequent release of host cell DNA that acts as a damage-associated molecular pattern (DAMP) in the recruitment of neutrophils and formation of neutrophil extracellular traps (NETs), which triggered immune responses (Pulendran et al., 2021). Recruitment of infiltrated cells to the injection sites was also observed in the SQ@NP group; however, cell necrosis was negligible around the injected tissues. These findings indicate that injection with SQ@NP induced less tissue damage than injection with AlPO4. The low squalene oil content (2 wt%) in the SQ@NP-adjuvanted vaccine caused only mild inflammatory reactions and can prospectively be used as an adjuvant for massive vaccination. In addition, it has been well documented that conventional AlPO4 adjuvants, which are highly heterogeneous and difficult to elaborate in a consistent manner, are obstacles that are limited in pandemic vaccine preparedness. Furthermore, there were no obvious clinical signs of allergic disorders observed in the mice following SQ@NP injection. The SQ@NP-adjuvanted vaccine was considerably well tolerated following i.m. injection in mice.
Fig. 6.
Representative images of hematoxylin & eosin-stained tissue sections at the injection site 1 week following the boosting injection of mice with SARS-CoV-2 S-protein alone or AlPO4- or SQ@NP-adjuvanted SARS-CoV-2 S-protein for histological examination. Scale bar: 500 μm.
4. Discussion
Many vaccine candidates against COVID-19 are being developed as the genome sequencing of SARS-CoV-2 viruses is completed. These include whole-virus vaccines, nucleic-acid vaccines, viral-vector vaccines, and protein-based vaccines (Callaway, 2020). Whole-virus vaccines use weakened or inactivated viruses to provoke an immune response but require extensive safety testing (Dai and Gao, 2021). The first two vaccines being used in the U.S. under Emergency Use Authorization (EUA), BNT162b2 and mRNA-1273 are genetic material (mRNA) vaccines (Koff et al., 2021). This mRNA signal, after vaccination, causes a person's own cells to make parts of the SARS-CoV-2 viral particles against which immunity is generated (Livingston, 2021). However, mRNA is highly unstable and requires protection by lipid nanoparticles (Jeyanathan et al., 2020, Schoenmaker et al., 2021). There are two major challenges for vaccines based on mRNA molecules: the necessity of two intramuscular injections to confer adequate immunity and ultralow temperatures during storage and transportation (Meo et al., 2021). The latter represents a logistical obstacle and may not be feasible for communities lacking cold chain facilities. The Janssen vaccine is the first viral-vector vaccine authorized in the U.S. This vaccine uses a genetically engineered, replication-incompetent adenovirus that delivers SARS-CoV-2 DNA (Mercado et al., 2020). Because DNA molecules are stable, they do not require ultracold storage and distribution. Preclinical and clinical data suggest that one dose of vaccine meets the expectations for safety and effectiveness in preventing COVID-19-related hospitalization and death, which could simplify the logistics of mass vaccination (Mercado et al., 2020). However, existing immunity to the vector could blunt the effectiveness of viral-vector vaccines (Callaway, 2020).
In so far as safety is concerned, the currently authorized COVID-19 vaccines use new techniques and lack long-term safety and immunogenicity data in humans. One adverse event common to clinical trials for these vaccines is Bell's palsy, a form of temporary facial paralysis on one side of the face; an analysis of the combined Phase 3 data of mRNA vaccine trials from Pfizer and Moderna concluded that the rate of Bell's palsy across the two trials was between 3.5 and 7 times higher than the expected rate in the general population (Ozonoff et al., 2021). Another adverse event common to clinical trials is complement activation-related pseudoallergy (CARPA) to lipid nanoparticles (Szebeni, 2014, Garvey and Nasser, 2021). Indeed, the best and safest vaccine may not necessarily be the first developed vaccines. Further studies are warranted to determine whether people with a history of allergies are at high risk for systemic allergic reaction to an innovative structure-based vaccine.
Protein-based vaccines comprise only key viral proteins or protein fragments that can be manufactured in vitro in bacteria, yeast, insects or mammalian cells (Callaway, 2020). Different from nucleic acid and viral vector vaccines, the production of protein-based vaccines comprises sequential expression and purification, resulting in the extension of manufacturing costs and time consumption (Dai and Gao, 2021). These vaccines are highly purified and not immunogenic; indeed, they typically require high dosages or adjuvants to boost immunity (Dai and Gao, 2021, Jeyanathan et al., 2020, Gupta and Gupta, 2020). Fortunately, this platform technology is well established, and the safety and immunogenicity data of candidate proteins are understood and ready for large-scale production to Good Manufacturing Practice (GMP) standards (Jeyanathan et al., 2020). Similar to DNA vaccines, protein-based vaccines are stable and do not require ultracold storage and distribution. The largest numbers of SARS-CoV-2 vaccine candidates in clinical trials are based on this strategy (Dai and Gao, 2021). Herein, we proposed/demonstrated that one dose of S-protein with SQ@NPs works as well as repeated doses of S-protein alone. In addition, SQ@NP activates T cells without compromising safety following i.m. injection in mice. Therefore, the spike subunit protein accompanied by lipid squalene nanoparticles is a potential adjuvantation strategy for enhancing the immunogenicity of vaccine candidates against COVID-19.
5. Conclusions
In this study, we proposed/demonstrated that the COVID-19 vaccine S-protein antigens could be loaded within lipid squalene nanoparticles and transported alongside the colloidal matrix in a desired profile in vivo; hence, a single injection can achieve repeated vaccination. Subsequently, high levels of antigen-loaded immune cells in peripheral tissues migrate to local draining lymph nodes, where they trigger immune responses. It should be kept in mind that the induction of cell-mediated immunity is a critical parameter in effective vaccination by clearance of virus-infected cells. It was concluded that both AlPO4 and SQ@NP are suitable to be a vaccine adjuvant for eliciting humoral immune responses against SARS-CoV-2; on the other hand, SQ@NP may be a more efficient vaccine adjuvant for the induction of T-cell immunity. These results pave the way for optimal vaccination formulations against COVID-19 disease. This study addresses NHRI policies for developing vaccine self-manufacturing capability and responding to pandemic outbreaks. The presented results will also allow us to foster the production of biotech companies in vaccine-related biotechnology.
CRediT authorship contribution statement
Hui-Min Ho: Methodology, Data curation, Investigation, Visualization, Writing–original draft. Chiung-Yi Huang: Methodology, Data curation, Investigation, Visualization, Writing–original draft. Yu-Jhen Cheng: Resources, Methodology. Kuan-Yin Shen: Methodology. Tsai-Teng Tzeng: Methodology. Shih-Jen Liu: Methodology, Conceptualization. Hsin-Wei Chen: Methodology, Conceptualization. Chung-Hsiung Huang: Methodology, Conceptualization. Ming-Hsi Huang: Conceptualization, Investigation, Supervision, Project administration, Funding acquisition.
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgment
This work was supported by the National Health Research Institutes (NHRI) of Taiwan (110A1-IVPP18-014 and 110A1-BNSP12-014) and by grants from the Ministry of Science and Technology of Taiwan (MOST 109-2314-B-400-018-MY3). The authors are grateful to the team members of the P3 facility, Bioproduction Plant of NHRI, for conducting virus neutralizing assays.
References
- Araf Y., Faruqui N.A., Anwar S., Hosen M.J. SARS-CoV-2: a new dimension to our understanding of coronaviruses. Int. Microbiol. 2021;24(1):19–24. doi: 10.1007/s10123-020-00152-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arunachalam P.S., Walls A.C., Golden N., Atyeo C., Fischinger S., Li C., Aye P., Navarro M.J., Lai L., Edara V.V., Röltgen K., Rogers K., Shirreff L., Ferrell D.E., Wrenn S., Pettie D., Kraft J.C., Miranda M.C., Kepl E., Sydeman C., Brunette N., Murphy M., Fiala B., Carter L., White A.G., Trisal M., Hsieh C.-L., Russell-Lodrigue K., Monjure C., Dufour J., Spencer S., Doyle-Meyers L., Bohm R.P., Maness N.J., Roy C., Plante J.A., Plante K.S., Zhu A., Gorman M.J., Shin S., Shen X., Fontenot J., Gupta S., O’Hagan D.T., Van Der Most R., Rappuoli R., Coffman R.L., Novack D., McLellan J.S., Subramaniam S., Montefiori D., Boyd S.D., Flynn J.L., Alter G., Villinger F., Kleanthous H., Rappaport J., Suthar M.S., King N.P., Veesler D., Pulendran B. Adjuvanting a subunit COVID-19 vaccine to induce protective immunity. Nature. 2021;594(7862):253–258. doi: 10.1038/s41586-021-03530-2. [DOI] [PubMed] [Google Scholar]
- Callaway E. The race for coronavirus vaccines: a graphical guide. Nature. 2020;580(7805):576–577. doi: 10.1038/d41586-020-01221-y. [DOI] [PubMed] [Google Scholar]
- Dai L., Gao G.F. Viral targets for vaccines against COVID-19. Nat. Rev. Immunol. 2021;21(2):73–82. doi: 10.1038/s41577-020-00480-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garvey L.H., Nasser S. Anaphylaxis to the first COVID-19 vaccine: is polyethylene glycol (PEG) the culprit? Br. J. Anaesth. 2021;126(3):e106–e108. doi: 10.1016/j.bja.2020.12.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gupta T., Gupta S.K. Potential adjuvants for the development of a SARS-CoV-2 vaccine based on experimental results from similar coronaviruses. Int Immunopharmacol. 2020;86:106717. doi: 10.1016/j.intimp.2020.106717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang C.-H., Huang C.-Y., Ho H.-M., Lee C.-H., Lai P.-T., Wu S.-C., Liu S.-J., Huang M.-H. Nanoemulsion adjuvantation strategy of tumor-associated antigen therapy rephrases mucosal and immunotherapeutic signatures following intranasal vaccination. J. Immunother. Cancer. 2020;8(2):e001022. doi: 10.1136/jitc-2020-001022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang C.-H., Huang C.-Y., Huang M.-H. Unsaturated squalene content in emulsion vaccine adjuvants plays a crucial role in ROS-mediated antigen uptake and cellular immunity. Mol. Pharm. 2018;15(2):420–429. doi: 10.1021/acs.molpharmaceut.7b0080010.1021/acs.molpharmaceut.7b00800.s001. [DOI] [PubMed] [Google Scholar]
- Jeyanathan M., Afkhami S., Smaill F., Miller M.S., Lichty B.D., Xing Z. Immunological considerations for COVID-19 vaccine strategies. Nat. Rev. Immunol. 2020;20(10):615–632. doi: 10.1038/s41577-020-00434-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koff W.C., Schenkelberg T., Williams T., Baric R.S., McDermott A., Cameron C.M., Cameron M.J., Friemann M.B., Neumann G., Kawaoka Y., Kelvin A.A., Ross T.M., Schultz-Cherry S., Mastro T.D., Priddy F.H., Moore K.A., Ostrowsky J.T., Osterholm M.T., Goudsmit J. Development and deployment of COVID-19 vaccines for those most vulnerable. Sci. Transl. Med. 2021;13:eabd1525. doi: 10.1126/scitranslmed.abd1525. [DOI] [PubMed] [Google Scholar]
- Livingston E.H. Necessity of 2 doses of the Pfizer and Moderna COVID-19 vaccines. JAMA. 2021;325(9):898. doi: 10.1001/jama.2021.1375. [DOI] [PubMed] [Google Scholar]
- Meo S.A., Bukhari I.A., Akram J., Meo A.S., Klonoff D.C. COVID-19 vaccines: comparison of biological, pharmacological characteristics and adverse effects of Pfizer/BioNTech and Moderna Vaccines. Eur. Rev. Med. Pharmacol. Sci. 2021;25:1663–1669. doi: 10.26355/eurrev_202102_24877. [DOI] [PubMed] [Google Scholar]
- Mercado N.B., Zahn R., Wegmann F., Loos C., Chandrashekar A., Yu J., Liu J., Peter L., McMahan K., Tostanoski L.H., He X., Martinez D.R., Rutten L., Bos R., van Manen D., Vellinga J., Custers J., Langedijk J.P., Kwaks T., Bakkers M.J.G., Zuijdgeest D., Rosendahl Huber S.K., Atyeo C., Fischinger S., Burke J.S., Feldman J., Hauser B.M., Caradonna T.M., Bondzie E.A., Dagotto G., Gebre M.S., Hoffman E., Jacob-Dolan C., Kirilova M., Li Z., Lin Z., Mahrokhian S.H., Maxfield L.F., Nampanya F., Nityanandam R., Nkolola J.P., Patel S., Ventura J.D., Verrington K., Wan H., Pessaint L., Van Ry A., Blade K., Strasbaugh A., Cabus M., Brown R., Cook A., Zouantchangadou S., Teow E., Andersen H., Lewis M.G., Cai Y., Chen B., Schmidt A.G., Reeves R.K., Baric R.S., Lauffenburger D.A., Alter G., Stoffels P., Mammen M., Van Hoof J., Schuitemaker H., Barouch D.H. Single-shot Ad26 vaccine protects against SARS-CoV-2 in rhesus macaques. Nature. 2020;586(7830):583–588. doi: 10.1038/s41586-020-2607-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Narayan Bhilwade H., Tatewaki N., Konishi T., Nishida M., Eitsuka T., Yasui H., Inanami O., Handa O., Naito Y., Ikekawa N., Nishida H. The adjuvant effect of squalene, an active ingredient of functional foods, on doxorubicin-treated allograft mice. Nutr. Cancer. 2019;71(7):1153–1164. doi: 10.1080/01635581.2019.1597900. [DOI] [PubMed] [Google Scholar]
- Ozonoff A.l., Nanishi E., Levy O. Bell's palsy and SARS-CoV-2 vaccines. Lancet Infect. Dis. 2021;21(4):450–452. doi: 10.1016/S1473-3099(21)00076-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pulendran B., S. Arunachalam P., O’Hagan D.T. Emerging concepts in the science of vaccine adjuvants. Nat. Rev. Drug Discov. 2021;20(6):454–475. doi: 10.1038/s41573-021-00163-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schoenmaker L., Witzigmann D., Kulkarni J.A., Verbeke R., Kersten G., Jiskoot W., Crommelin D.J.A. mRNA-lipid nanoparticle COVID-19 vaccines: Structure and stability. Int. J. Pharm. 2021;601:120586. doi: 10.1016/j.ijpharm.2021.120586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Szebeni J. Complement activation-related pseudoallergy: a stress reaction in blood triggered by nanomedicines and biologicals. Mol. Immunol. 2014;61(2):163–173. doi: 10.1016/j.molimm.2014.06.038. [DOI] [PubMed] [Google Scholar]