Abstract
Immune-mediated myocardial injury following Severe Acute Respiratory Syndrome Coronavirys-2 (SARS-CoV2) infection has been described in adults and children. Cases of myocarditis following immunization for SARS-CoV2 have recently been documented, mostly associated with mild severity and spontaneous recovery. We herein report two cases of fulminant myocarditis following BNT162b2 mRNA Covid-19 vaccination associated with systemic hyperinflammatory syndrome and refractory shock requiring support with veno-arterial extracorporeal membrane oxygenation.
Keywords: Vaccine, Myocarditis, Coronavirus, Covid-19
Immune-mediated myocardial injury following Severe Acute Respiratory Syndrome Coronavirus-2 (SARS-CoV2) infection has been described in adults and children [1,2]. Cases of myocarditis following immunization for SARS-CoV2 have recently been documented [[3], [4], [5], [6], [7], [8]]. We herein report two cases of fulminant myocarditis following BNT162b2 mRNA Covid-19 vaccination the show unique features of systemic hyperinflammation, already described in young males affected by Covid-19 [9]. The cases were reported to the Vaccine Adverse Event Reporting System of the Food & Drug Administration of the United States of America. Informed consent was provided by the next of kin.
A 27-year-old male with trisomy 21 complicated by speech impairment without history of cardiovascular disease presented in cardiogenic shock 2 days after his second vaccine dose. He had received the first dose without adverse effects. Approximately 36 h after the second dose, he developed nausea and vomiting. He presented to another hospital in shock (blood pressure 77/54 mmHg and heart rate 133/min) and found to have diffuse ST segment elevation in electrocardiogram (Fig. 1 ). Cardiac catheterization showed no coronary obstructions. Initially, creatine kinase myocardial band level (CK-MB) was 252 ng/mL (normal value [NV] < 5). Transthoracic echocardiogram showed severe left ventricular systolic dysfunction (LVEF 20%) and a small circumferential pericardial effusion without tamponade. A diagnosis of presumed fulminant pericarditis was made and methylprednisolone 1000 mg and human immunoglobulin (IVIG) 60 g were given. The course was complicated by hemodynamically unstable ventricular tachycardia refractory to electrical cardioversion followed by pulseless electrical activity. He was resuscitated with veno-arterial extracorporeal mechanical oxygenation (VA-ECMO). After return of circulation, he was supported by multiple vasopressors, mechanical ventilation, and renal replacement therapy (RRT). Despite these interventions, multiorgan failure and refractory shock persisted. Lactic acid increased from 6 to 28 mmol/L (NV <2), D-dimer increased from 4.21 to >20 μg/mL (critically high >5.0), INR increased from 2.0 to 10.0, and fibrinogen dropped to 100 mg/dL. C-reactive protein (CRP) and ferritin were highly elevated on admission at 13.1 mg/dL (NV <0.5) and 23,000 ng/mL, respectively, leading to a decision to administer anakinra (Kineret®). Interleukin-6 level eventually came back highly elevated at 333 pg/mL (NV <13). Thrombocytopenia developed progressively from 223 × 109/L to 21 × 109/L, while hemoglobin decreased from 11.5 to 8.7 g/dL. The patient demonstrated acute liver injury (ALI) with alanine transferase (ALT) and aspartate transferase (AST) at 7995 and 9003 u/L. Polymerase chain reaction (PCR) for SARS-CoV2 and other common respiratory viruses more frequently associated with myocarditis was negative. SARS-CoV2 spike protein IgG antibody was positive (62.8 arbitrary units/ml [NV <15.0]), and anti-nucleocapsid IgG was negative, consistent with immunization status. Approximately 21 h after admission, patient died due to recurrent cardiac arrest and refractory shock. Family declined request for autopsy.
Fig. 1.
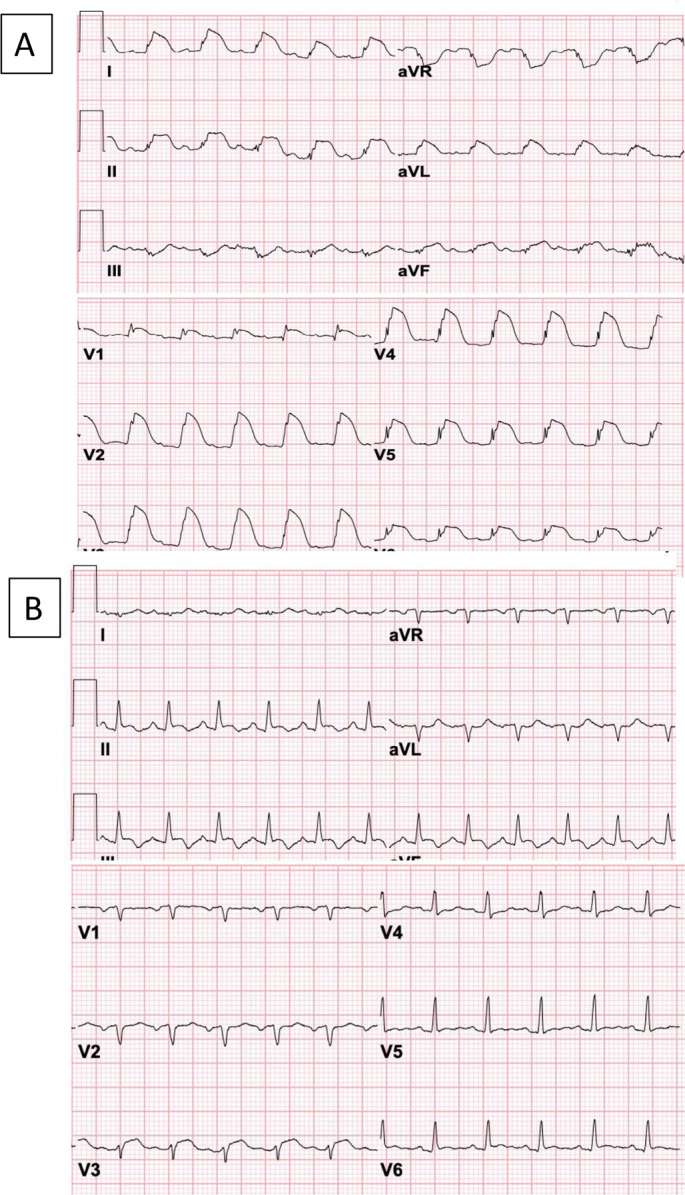
Electrocardiogram (ECG) of two patients with fulminant myocarditis and hyperinflammatory syndrome: (A) The ECG of patient #1 shows diffuse ST segment elevations in a ‘shark fin’ pattern that is classically associated with unfavorable prognosis. (B) The ECG of patient #2 shows sinus tachycardia and non-specific abnormalities of the repolarization.
A 34-year-old female without prior medical history presented 9 days after her first vaccine dose. On day 4 after vaccine, she developed fevers, cough, chest pain, nausea, and vomiting. She presented to another institution with hypotension and sinus tachycardia, an echocardiogram showed severely reduced LVEF of 15%. Cardiac catheterization showed no obstructive coronary artery disease. She was transferred for initiation of VA-ECMO. On admission, she was in severe shock requiring multiple vasopressors. CRP, ferritin and CK-MB were elevated at 5.6 mg/dL, >30,000 ng/mL, and 42.4 ng/mL, respectively. VA-ECMO and RRT were initiated. Treatment was initiated with methylprednisolone 1000 mg daily and continued for 3 days followed by a slow taper over, with IVIG 30 mg daily for 4 days, and anakinra 100 mg daily. Lactic acid 13 mmol/L on admission normalized within 24 h of ECMO onset, INR improved from 4 to 1.7. Interleukin-6 level was mildly elevated at 17 pg/mL and interleukin-2 soluble receptor alpha was 1067 U/mL (NV <710). Thrombocytopenia was noted at 106 × 109/L which persisted for several days. ALI was noted with ALT and AST at 14,477 and 6086 u/L which steadily improved thereafter. PCR for SARS-CoV2 and other common respiratory viruses was negative. SARS-CoV2 spike protein IgG antibody was positive (64 arbitrary units/ml), and IgG anti-nucleocapsid was negative, consistent with immunization due to vaccine without prior infection. Vasopressors were weaned off, LVEF was documented at 50% by transthoracic echocardiography, VA-ECMO was discontinued. She was treated with prednisone 1 mg/kg and anakinra with prednisone tapered first (by reducing 0.1 mg every week) and tapering anakinra planned after prednisone is discontinued. An endomyocardial biopsy performed on day 13 of hospital admission, after recovery of cardiac function and near resolution of the systemic hyperinflammation, showed cardiomyocytes with minute foci of cytoplasmic vacuolization and rare interstitial lymphocytic infiltrate, which is consistent with healing myocarditis, but did not meet conventional diagnostic criteria for acute myocarditis. A cardiac catheterization at the same time showed preservation of cardiac output and normal filling pressures. A cardiac magnetic resonance, performed 45 days after the initial presentation, showed LVEF of 35%, diffuse elevation of native T1 values, small pericardial effusion with enhancement of the pericardium anterior and anterolaterally and small patchy areas of enhancement following delayed imaging after gadolinium in the mid-wall of the anterior wall, consistent with myopericarditis. Genetic testing for 121 genes (Invitae, San Francisco, CA, USA) showed no variants associated with genetic disorders. Guideline-directed heart failure treatment was also initiated. She was discharged from the hospital after 73 days.
In summary, both cases presented features of fulminant myocarditis with a temporal association with the BNT162b2 mRNA Covid-19 vaccination, in absence of other apparent causes, and with unique features of systemic hyperinflammation associated with refractory shock.
Several reports in the past months had suggested a possible association between the BNT162b2 mRNA Covid-19 and myocarditis [[3], [4], [5], [6], [7], [8]], and the Center for the Disease Control (CDC) in the United States of America has now acknowledged an increased risk of myocarditis, especially in young adults [10]. An excess in number of cases after the second injection, as compared with the first injection, in young adults has been noted [10]. The severity of the cases reported so far have been, for the most part, rather mild [8,10]. The CDC report of 12 July 2021 identified 633 reports of myocarditis and pericarditis with a very small number being critical requiring intensive care [10].
The CDC report also recommends that despite a higher risk of vaccine associated myocarditis, Covid-19 vaccination appears to be overall associated with significantly less adverse outcomes than Covid-19 infection among all age groups [10]. The rapid presentation of the two cases presented herein and the evolution into multiorgan failure and refractory shock are therefore highly unusual and reflects the fact the fulminant myocarditis can present as part of a systemic hyperinflammatory syndrome, as seen in patients with Covid-19 [9], and it is associated with grave prognosis and death. These cases identify the need for awareness of a potential, albeit extremely rare, link of BNT162b2 mRNA Covid-19 vaccination associated with fulminant myocarditis as part of a severe systemic hyperinflammatory syndrome, requiring mechanical cardiac support and, most importantly, immunosuppressive therapy. The degree of the inflammatory biomarkers and multiorgan dysfunction seen in the two cases described, as in some patients with Covid-19, is indeed out of proportion to with hemodynamic failure and shock and it is not rapidly resolved by cardiac mechanical support, reflecting a significant degree of vasoplegia. The optimal immunosuppressive treatment for systemic hyperinflammation associated with fulminant myocarditis is unknown [11]. We chose a combination of high dose methylprednisolone, IVIG and anakinra (Table 1 ) as a strategy used across a variety of immunologic and rheumatologic diseases characterized with inappropriate macrophage activation.
Table 1.
Key biomarkers at admission and immunomodulating therapy.
| Patient #1 | Patient #2 | |
|---|---|---|
| Presentation | ||
| Symptoms | Nausea and vomiting | Fever, cough, chest pain, nausea and vomiting |
| Vaccine type | BNT162b2 mRNA Covid-19 | BNT162b2 mRNA Covid-19 |
| Timing | 2 days after 2nd dose | 9 days after 1st dose |
| Hemodynamics | Hypotension and tachycardia | Hypotension and tachycardia |
| ECG | ST segment elevation | Non-specific changes |
| Echocardiogram | LVEF 20% | LVEF 15% |
| Biomarkers | ||
| CRP | 13.1 mg/dl | 5.6 mg/dl |
| Interleukin-6 | 333 pg/ml | 17.2 pg/ml [12 h after therapy] |
| Ferritin | 23,000 ng/ml | >30,000 ng/ml |
| INR | 2.0 | 4.5 |
| D-dimer | 4.21 μg/ml | >20 μg/ml |
| Lactic acid | 6 mmol/l | 13 mmol/l |
| CK-MB | 252 ng/ml | 42 ng/ml |
| SARS-CoV2 spike IgG | 62.8 a.u./ml | 64 a.u./ml |
| SARS-CoV2 NC IgG | negative | negative |
| Immunosuppressive Therapy | ||
| Methylprednisolone | 1000 mg | 1000 mg daily for 3 days, followed by a slow prednisone taper over several weeks |
| Immunoglobulins | 60 mg | 30 mg daily for 4 days |
| Anakinra | 100 mg (2 doses) | 100 mg daily |
| Outcome | ||
| Deceased 21 h after admission | Recovering, minimal symptoms, LVEF 35%, delayed enhancement after gadolinium at cardiac magnetic resonance; discharged from the hospital after 73 days | |
Abbreviations: a.u. = arbitrary units; CRP=C reactive protein; CK-MB = creatine kinase myocardial band; IgG = Immunoglobulin G; INR = International normalized ratio; SARS-CoV2 = Severe Acute Respiratory Distress Syndrome Coronavirus-2.
Author statement
Conceptualization:
Antonio Abbate, MD, PhD1,⁎; Josh Gavin, MD2,⁎; Nima Madanchi, MD2; Seema Patel, MD2; Stamatina Danielides, MD2.
Data recovery:
Antonio Abbate, MD, PhD1,⁎; Josh Gavin, MD2,⁎; Nima Madanchi, MD2; Stamatina Danielides, MD2.
Critical discussion of the data:
Antonio Abbate, MD, PhD1,⁎; Josh Gavin, MD2,⁎; Nima Madanchi, MD2; Christin Kim, MD3; Pranav R. Shah, MD3; Katherine Klein, MD4; Julie Boatman, DO5; Charlotte Roberts, NP1; Seema Patel, MD2; Stamatina Danielides, MD2.
Initial drafting:
Antonio Abbate, MD, PhD1,⁎; Josh Gavin, MD2,⁎; Stamatina Danielides, MD2.
Revision of the draft:
Antonio Abbate, MD, PhD1,⁎; Josh Gavin, MD2,⁎; Nima Madanchi, MD2; Seema Patel, MD2; Stamatina Danielides, MD2.
Final approval of the manuscript:
Antonio Abbate, MD, PhD1,⁎; Josh Gavin, MD2,⁎; Nima Madanchi, MD2; Christin Kim, MD3; Pranav R. Shah, MD3; Katherine Klein, MD4; Julie Boatman, DO5; Charlotte Roberts, NP1; Seema Patel, MD2; Stamatina Danielides, MD2.
Funding
None.
Acknowledgements
None.
References
- 1.Inciardi R.M., Lupi L., Zaccone G., et al. Cardiac involvement in a patient with coronavirus disease 2019 (COVID-19) JAMA Cardiol. 2020;5:819–824. doi: 10.1001/jamacardio.2020.1096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Blondiaux E., Parisot P., Redheuil A., et al. Cardiac MRI in children with multisystem inflammatory syndrome associated with COVID-19. Radiology. 2020;297:E283–E288. doi: 10.1148/radiol.2020202288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ammirati E., Cavalotti C., Milazzo A., et al. Temporal relation between second dose BNT162b2 mRNA Covid-19 vaccine and cardiac involvement in a patient with previous SARS-COV-2 infection. Int. J. Cardiol. Heart Vasc. 2021;34:100774. doi: 10.1016/j.ijcha.2021.100774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Uwaydah A.K., Hassan N.M.M., Abu Ghoush M.S., Shahin K.M.M. Adult multisystem inflammatory syndrome in a patient who recovered from COVID-19 postvaccination. BMJ Case Rep. 2021;14 doi: 10.1136/bcr-2021-242060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Larson K.L., Ammirati E., Adler E.D., et al. Myocarditis after BNT162b2 and mRNA-1273 vaccination. Circulation. 2021;144:506–508. doi: 10.1161/CIRCULATIONAHA.121.055913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Montgomery J., Ryan M., Engler R., et al. Myocarditis following immunization with mRNA COVID-19 vaccines in members of the US Military. JAMA Cardiol. 2021 Jun 29 doi: 10.1001/jamacardio.2021.2833. e212833. Epub ahead of print. PMID: 34185045; PMCID: PMC8243257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kim H.W., Jenista E.R., Wendell D.C., et al. Patients with acute myocarditis following mRNA COVID-19 vaccination. JAMA Cardiol. 2021 Jun 29:e212828. doi: 10.1001/jamacardio.2021.2828. Epub ahead of print. PMID: 34185046; PMCID: PMC8243258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Diaz G.A., Parsons G.T., Gering S.K., Meier A.R., Hutchinson I.V., Robicsek A. Myocarditis and pericarditis after vaccination for COVID-19. JAMA. 2021 Aug 4 doi: 10.1001/jama.2021.13443. 34347001 Epub ahead of print. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Chau V.Q., Giustino G., Mahmood K., et al. Cardiogenic shock and hyperinflammatory syndrome in young males with COVID-19. Circ. Heart Fail. 2020 Oct;13(10) doi: 10.1161/CIRCHEARTFAILURE.120.007485. 32844662 Epub 2020 Aug 26. [DOI] [PubMed] [Google Scholar]
- 10.https://www.cdc.gov/coronavirus/2019-ncov/vaccines/safety/myocarditis.html (accessed on July 17, 2021)
- 11.Veronese G., Ammirati E., Chen C., et al. Management perspectives from the 2019 Wuhan international workshop on fulminant myocarditis. Int. J. Cardiol. 2021 Feb 1;324:131–138. doi: 10.1016/j.ijcard.2020.10.063. 33122017 Epub 2020 Oct 26. [DOI] [PubMed] [Google Scholar]


