Abstract
Purpose
To detect the risk factors for pulmonary embolism (PE) in patients with COVID-19.
Methods
Studies were searched for in PubMed, Cochrane Library, Web of Science, and EMBASE. Two authors independently screened articles and extracted data. The data were pooled by meta-analysis and three subgroup analyses were performed.
Results
Of the 2210 articles identified, 27 studies were included. Pooled analysis suggested that males (odds ratio (OR) 1.49, 95% confidence interval (CI) 1.26−1.75, P = 0.000), obesity (OR 1.37, 95% CI 1.03−1.82, P = 0.033), mechanical ventilation (OR 3.34, 95% CI 1.90−5.86, P = 0.000), severe parenchymal abnormalities (OR 1.92, 95% CI 1.43−2.58, P = 0.000), ICU admission (OR 2.44, 95% CI 1.48−4.03, P = 0.000), and elevated D-dimer and white blood cell values (at two time points: hospital admission or closest to computed tomography pulmonary angiography) (P = 0.000) correlated with a risk for PE occurrence in COVID-19 patients. However, age and common comorbidities had no association with PE occurrence. Computed tomography pulmonary angiography, unclear-ratio/low-ratio, and hospitalization subgroups had consistent risk factors with all studies; however, other subgroups had fewer risk factors for PE.
Conclusions
Risk factors for PE in COVID-19 were different from the classic risk factors for PE and are likely to differ in diverse study populations.
Keywords: Pulmonary embolism, COVID-19, Risk factor, Venous thrombus embolism
Introduction
Since December 2019, coronavirus disease 2019 (COVID-19) has rapidly spread worldwide and caused more than 1 billion infections and 2 million deaths to date (Ackermann et al., 2020). The pathophysiology of COVID-19 has not yet been fully revealed. However, the direct viral toxicity (Alonso-Fernández et al., 2020), endothelial cell damage, and dysregulation of the immune response (Ameri et al., 2020) are widely believed to participate in the process (Artifoni et al., 2020). Emerging evidence has revealed that pulmonary embolism (PE) is a common complication in patients with COVID-19, with a higher incidence rate of 5−19% (Bavaro et al., 2020, Benito et al., 2020, Bilaloglu et al., 2020) and mortality rate of 8.7−45.1% (Bompard et al., 2020, BujaL et al., 2020, Bunce et al., 2011) than that in patients without COVID-19 (Ceriani et al., 2010, Chen et al., 2020) (incidence: 1.7−7.5%, mortality: 6.8%). Importantly, PE in patients with COVID-19 has been found to be different from classic PE in patients without COVID-19 in demographic, clinical, and laboratory characteristics (Chi et al., 2020, Choi et al., 2020). Even the traditional etiology of PE – venous thrombi dislodging and traveling as emboli to the pulmonary arteries (Connors and Levy, 2020) – has been suspected in COVID-19 patients (Contou et al., 2020, Egger et al., 2011, Fang et al., 2020). Some researchers have proposed a new hypothesis of pulmonary microvascular thrombosis, according to the unusual autopsy finding in COVID-19 that thrombosis and microangiopathy are common in the small vessels and capillaries of the lungs (Contou et al., 2020, Egger et al., 2011, Fang et al., 2020). Therefore, the risk factors for PE in patients with COVID-19 may differ from the classic ones and this is supported by several studies (Bompard et al., 2020, Fauvel et al., 2020, Flumignan et al., 2020, Fox et al., 2020). However, the results on this issue have been inconsistent. One systemic review without sub-analysis of PE recently stated the risk factors of venous thrombus embolism (VTE) in COVID-19, and they were different from the classic ones (Gervaise et al., 2020). Considering that PE in COVID-19 may not only originate from deep vein thrombosis, the detection of risk factors for PE is necessary.
At present, as clinical judgment lacks standardization (such as Wells, the revised Geneva prediction rule, or risk factors), the screening of suspected PE for computed tomography pulmonary angiography (CTPA) in patients with COVID-19 is mostly based on the empirical evaluation of clinicians. The common reasons are unexplained: respiratory deterioration, a rapid increase in D-dimer, or clinical symptoms of PE (Bompard et al., 2020, Choi et al., 2020, Flumignan et al., 2020, Fox et al., 2020). These make a low PE judgment rate with high heterogeneity between studies in COVID-19 (positive CTPA: 8−44%) (Bompard et al., 2020, Fauvel et al., 2020, Grillet et al., 2020) compared with the classic PE judgment using Wells or the revised Geneva prediction rule (confirmed PE expected to be 0−10% in the low-probability category and 65% in the high-probability category) (Ceriani et al., 2010, Gupta and Madhavan, 2020). Also, this rate may be overestimated because of the cautious screening strategy of suspected PE adopted to reduce cross-infection (Hajra et al., 2020, Jalaber et al., 2020). Therefore, it is essential to assess the risk factors for PE in COVID-19 and, aside from improving PE detection, risk factors can also promote the prevention and management of PE.
Therefore, this meta-analysis was conducted to detect the risk factors for PE in patients with COVID-19, along with subgroup analyses, considering the clinical practicability. It is believed that this is the first systematic review to do this and it is hoped that it can help physicians in diagnosing and managing PE. At the same time, it can promote an awareness of the clinical prediction rules for PE in patients with COVID-19, which is similar to the Geneva or Wells score.
Methods
This study was conducted based on the Preferred Reporting Items for Systematic Reviews and Meta-Analyses guidelines, and registered with PROSPERO (CRD42020207652).
Citation search and selection
The PubMed, EMBASE, Web of Science, and Cochrane Library databases were searched from 01 January 2019 to 28 December 2020, with no publication language limited. The following search strategy was used: ("pulmonary embolism" OR "lung embolism" OR "pulmonary thromboembolism" OR "lung thromboembolism") AND ("COVID-19" OR "coronavirus disease 2019” OR "2019-nCoV Disease" OR "2019-nCoV Infection" OR "SARS-CoV-2 Disease" OR "SARS-CoV-2 Infection"). The authors also manually screened the reference lists of reviews to guarantee that all relevant articles were included.
Two authors (YLC, WWC) independently screened out the full-text articles and included studies according to the following criteria. They reached a consensus on inclusion criteria: (1) cohort, case-control, case-series, or cross-sectional study; (2) consecutive COVID-19 patients. The exclusion criteria were: (1) patients aged < 18 years, and pregnant women; (2) a sample size < 10. If an institution published several similar articles, only the one with the largest sample size was included. The differences were resolved by an arbitrator (ZMC).
Data extraction and quality assessment
Data extraction and quality assessment of the included studies were conducted by two authors (DX and YYL), respectively. The data involved study design, publishing location, reasons for CTPA examination, and prophylactic anticoagulation ratio. The demographic, clinical, and laboratory features were also extracted. The study quality was assessed by the Newcastle Ottawa Score checklist (Jevnikar et al., 2020).
Statistical analysis
Weighted mean difference (WMD) with 95% confidence intervals (CI) was chosen as the effect size of a continuous variable, and odds ratio (OR) with 95% CI for a dichotomous variable. All analyses were executed using Stata MP version 14.0 (Stata Corporation, College Station, TX, USA), with heterogeneities assessed by I2 (Jiménez et al., 2020). An I2 of 25%, 50%, and 75% indicates low, moderate, and high heterogeneity, respectively. When I2 < 50%, inverse variance weights (fixed-effect model) were used. If I2 > 50%, the DerSimonian-Laird procedure (random-effect model) was used. At the same time, a further sensitivity analysis was performed with subgroup analyses for every parameter in three categories. According to the different study populations, the included studies were divided into: CTPA vs. COVID-19 subgroup (COVID-19 patients who were suspected of PE and underwent CTPA vs. all COVID-19 patients); unclear-ratio vs. low-ratio (ratio < 80%) vs. high-ratio subgroup (ratio > 80%) (the patients with different ratios of thromboprophylaxis); and the hospitalization vs. ICU-stay subgroup. The publication bias (studies ≥ 10) was evaluated using Egger's test (Higgins et al., 2003). P < 0.05 was considered as statistical significance.
Results
Study selection and quality assessment
The search strategy identified 2210 articles. After the exclusion of duplicates, 1270 articles were screened. Seventy-nine were considered eligible for full-text evaluation. Finally, 27 studies were included according to the inclusion and exclusion criteria (Figure 1 ). The risk of bias was judged as low for all included studies (Table 1 ).
Fig. 1.
Flow chart of article selection.
Table 1.
Characteristics of the included studies.
| Study ID | Region, country | Study design | No. of COVID-19a | Diagnosis of COVID-19 | Reasons for PE screening | Ratiob | No. of CTPAc | No.of PEd | Qualitye |
|---|---|---|---|---|---|---|---|---|---|
| Fauvel C (Bompard et al., 2020) | France | retrospective, multi-center, multi-hospital | 2878 | RT-PCR+, clinical criteria | unexplained respiratory deterioration | 67.5% | 1240 | 103 | 8 |
| Poyiadji N (Fauvel et al., 2020) | Detroit, USA | retrospective, multi-hospital | – | RT-PCR+ | – | – | 328 | 72 | 8 |
| Mestre-Gómez B (Flumignan et al., 2020) | Madrid, Italy | retrospective, single non-critical ward | 452 | RT-PCR +, clinical criteria | unexplained respiratory deterioration, elevation of D-dimer | – | 91 | 29 | 9 |
| Alonso-Fernánde A (Fox et al., 2020) | Palma de Mallorca, Spain | prospective, single hospital | 127 | RT-PCR +, clinical criteria | D-dimer > 1 mg/L | 96.7% | 30 | 15 | 8 |
| Fang C (Grillet et al., 2020) | London, UK | retrospective, single hospital | 1200 | RT-PCR+ | – | – | 93 | 41 | 8 |
| Chen JP (Jalaber et al., 2020) | Wuhan, China | retrospective, single hospital | 1008 | 15 RT-PCR+, 10 clinical criteria | elevated D-dimer, PE symptom(s) | – | 25 | 10 | 9 |
| Leonard-Lorant I (Huisman et al., 2018) | Strasbourg, France | Retr ospective, 2 hospitals | – | 97 RT-PCR+, 9 clinical criteria | – | 46.2% | 106 | 32 | 8 |
| Bompard F (Kim et al., 2007) | Paris, France | retrospective, 2 hospitals | – | – | respiratory deterioration | 100% | 135 | 32 | 8 |
| Ooi MWX (Kirsch et al., 2020) | GreaterManchester, UK | retrospective, 5 hospitals | 974 | RT-PCR+, clinical criteria | respiratory deterioration, elevation of D-dimer | – | 84 | 32 | 9 |
| Ventura-Diaz S (Konstantinides et al., 2019) | Madrid, Spain | retrospective, single hospital | – | RT-PCR+, clinical criteria | – | – | 242 | 73 | 8 |
| Kirsch B (Kosior et al., 2020) | Houston, USA | retrospective, single hospital | 459 | – | – | – | 64 | 12 | 7 |
| Planquette B (Kunutsor and Laukkanen, 2020) | Paris, France | retrospective, 2 hospitals | – | RT-PCR +, clinical criteria | – | 34.6% | 269 | 59 | 8 |
| Mouhat B (Lax et al., 2020) | Besançon, France | retrospective, single hospital | 349 | RT-PCR+ | unexplained respiratory deterioration | 87% | 162 | 44 | 9 |
| Whyte MB (Léonard-Lorant and Delabranche, 2020) | London, UK | retrospective, single hospital | – | 145 RT-PCR, 69 Clinical criteria | unexplained clinical deterioration | 100% | 214 | 80 | 9 |
| Grillet F (Liao et al., 2020) | Besancon Cedex, France | retrospective, single hospital | 280 | RT-PCR+, clinical criteria | – | – | 100 | 23 | 8 |
| Bavaro DF (Llitjos et al., 2020) | Bari, Italy | retrospective, single hospital | – | – | D-dimer > 1 mg/L and clinically suspected PE | 85% | 20 | 8 | 8 |
| Benito N (Mestre-Gómez et al., 2020) | Barcelona, Spain | prospective, single hospital | 1275 | RT-PCR+ | unexplained circulatory/ respiratory deterioration, elevation of D-dimer | 88.2% | 76 | 32 | 9 |
| Gervaise A (Mouhat et al., 2020) | Saint Mandé, France | retrospective, single ED | – | 58 RT-PCR +, 14 clinical criteria | respiratory deterioration, elevation of D-dimer | – | 72 | 13 | 9 |
| Contou D (Mueller-Peltzer et al., 2020) | Argenteuil, France | retrospective, single ICU | 92 | RT-PCR + | unexplained circulatory/ respiratory deterioration | 100% | 26 | 16 | 9 |
| Zotzmann V (Nopp et al., 2020) | Freiburg, Germany | retrospective, single ICU | 113 | RT-PCR + | severe ARDS | – | 20 | 12 | 9 |
| Soumagne T (Ooi et al., 2020) | Besancon, France | retrospective, single ICU | – | RT-PCR + | respiratory deterioration | 81.8% | 44 | 17 | 9 |
| Taccone FS (Pandey and Agarwal, 2020) | Brussels, Belgium | retrospective, single ICU | 82 | RT-PCR + | mechanical ventilation | 100% | 40 | 13 | 9 |
| Jevnikar M (Planquette et al., 2021) | Le Kremlin-Bicêtre, France | prospective, multi-center, multi-hospital | 135 | RT-PCR + | systematic screening | – | 107 | 16 | 9 |
| Jalaber C (Rodriguez-Sevilla et al., 2020) | Saint Priest en Jarez, France | prospective, single ED | 70 | 65 RT-PCR+, 5 clinical criteria | systematic screening | – | 70 | 4 | 9 |
| Ameri P (Poyiadji et al., 2020) | Italy | retrospective, multi-center, 13 cardiology units | 689 | RT-PCR +, clinical criteria | – | – | – | 52 | 8 |
| Lascarrou JB (Salje et al., 2020) | France and Belgium | retrospective, multi-center, 21 ICU | 375 | RT-PCR + | – | 100% | – | 55 | 8 |
| Scudiero F (Scudiero et al., 2021) | Seriate, Italy | retrospective, multi-center, 7 hospitals | 224 | RT-PCR + | – | 18.8% | – | 32 | 8 |
Abbreviations: COVID-19, coronavirus disease 2019; PE, pulmonary embolism; CTPA, computed tomography pulmonary angiography; RT-PCR+, positive reverse transcription-polymerase chain reaction; ICU, intensive care unit; ED, emergency department
No. of COVID-19, number of patients with COVID-19
Ratio, ratio of prophylactic anticoagulation
No. of CTPA, number of patients with CTPA examination or suspicion of PE
No. of PE, number of patients with confirmed PE
Quality, all studies were assessed by the Newcastle Ottawa Score (NOS); –, not available
Characteristics of included studies
All 27 included studies involved 927 PE patients and 3927 non-PE patients (Bompard et al., 2020, Fauvel et al., 2020, Flumignan et al., 2020, Fox et al., 2020, Grillet et al., 2020, Jalaber et al., 2020, Huisman et al., 2018, Kim et al., 2007, Kirsch et al., 2020, Konstantinides et al., 2019, Kosior et al., 2020, Kunutsor and Laukkanen, 2020, Lax et al., 2020, Léonard-Lorant and Delabranche, 2020, Liao et al., 2020, Llitjos et al., 2020, Mestre-Gómez et al., 2020, Mouhat et al., 2020, Mueller-Peltzer et al., 2020, Nopp et al., 2020, Ooi et al., 2020, Pandey and Agarwal, 2020, Planquette et al., 2021, Rodriguez-Sevilla et al., 2020, Poyiadji et al., 2020, Salje et al., 2020, Scudiero et al., 2021), of which 23 were retrospective case-control studies and four were prospective cohort studies (Fox et al., 2020, Mestre-Gómez et al., 2020, Planquette et al., 2021, Rodriguez-Sevilla et al., 2020) (Table 1). Among these studies, 24 were from Europe (833 PEs vs. 3604 non-PEs), two were from America (Fauvel et al., 2020, Kosior et al., 2020), and one was from China (Jalaber et al., 2020). The CTPA subgroup consisted of 22 articles involving 768 PEs and 2621 non-PEs, with 15 articles stating the reason for CTPA examination, of which unexplained respiratory deterioration or a rapid increase in D-dimer counted the most (Bompard et al., 2020, Flumignan et al., 2020, Fox et al., 2020, Jalaber et al., 2020, Kim et al., 2007, Kirsch et al., 2020, Lax et al., 2020, Léonard-Lorant and Delabranche, 2020, Llitjos et al., 2020, Mestre-Gómez et al., 2020, Mouhat et al., 2020, Mueller-Peltzer et al., 2020, Nopp et al., 2020, Ooi et al., 2020, Pandey and Agarwal, 2020) (Table 1). The COVID-19 subgroup consisted of five articles involving 159 PEs and 1306 non-PEs (Planquette et al., 2021, Rodriguez-Sevilla et al., 2020, Poyiadji et al., 2020, Salje et al., 2020, Scudiero et al., 2021). In the subgroup analysis of prophylactic anticoagulation, unclear, low-ratio (18.8−67.5%), and high-ratio (82−100%) subgroups contained 13 (389 PEs vs. 1596 non-PEs) (Fauvel et al., 2020, Flumignan et al., 2020, Grillet et al., 2020, Jalaber et al., 2020, Kirsch et al., 2020, Konstantinides et al., 2019, Kosior et al., 2020, Liao et al., 2020, Mouhat et al., 2020, Nopp et al., 2020, Planquette et al., 2021, Rodriguez-Sevilla et al., 2020, Poyiadji et al., 2020), four (226 PEs vs. 1521 non-PEs) (Bompard et al., 2020, Huisman et al., 2018, Kunutsor and Laukkanen, 2020, Scudiero et al., 2021), and 10 studies (312 PEs vs. 810 non-PEs) (Fox et al., 2020, Kim et al., 2007, Lax et al., 2020, Léonard-Lorant and Delabranche, 2020, Llitjos et al., 2020, Mestre-Gómez et al., 2020, Mueller-Peltzer et al., 2020, Ooi et al., 2020, Pandey and Agarwal, 2020, Salje et al., 2020). Eighteen studies (716 PEs vs. 2710 non-PEs) were carried on the hospitalization of the study population and five studies (113 PEs vs. 393 non-PEs) were carried on the ICU stay (Contou et al., 2020; Zotzmann et al., 2020; Soumagne and Winiszewski, 2020; Taccone et al., 2020; Soumagne and Lascarrou, 2020).
Risk factors
Demographic risk factors
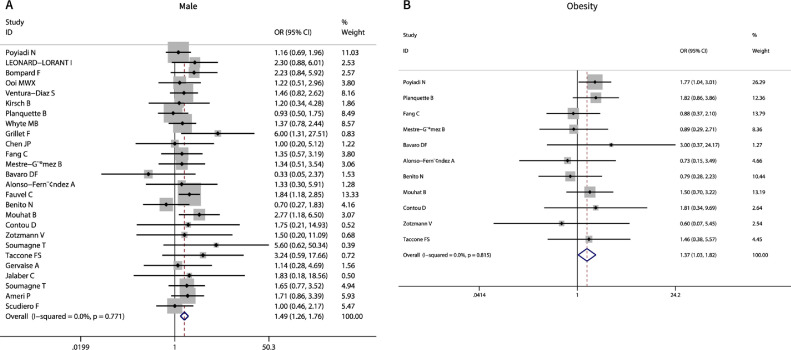
Nearly all included studies reported information about age and sex. The pooled estimates indicated that males developed PE more easily than females (OR 1.49, 95% CI 1.26−1.75, I2 = 0.0%, P = 0.000) (Table 2 , Figure 2 A). Age had no significant influence on the occurrence of PE (WMD 1.57, 95% CI -0.31−3.45, I2 = 64.9%, P = 0.101), excluding one study by sensitivity analysis (Léonard-Lorant and Delabranche, 2020) (Table 2). Eleven studies reported information about BMI, and the pooled data showed that obesity (BMI > 30) was associated with PE occurrence (OR 1.37, 95% CI 1.03−1.82, P = 0.033, I2 = 0%) (Table 2, Figure 2B). Estimates for eight comorbidities were also pooled, including previous VTE, chronic heart failure, cancer, diabetes, hypertension, recent surgery, cardiovascular disease, and chronic obstructive pulmonary disease. All comorbidities were found to have no association with PE occurrence (P > 0.068), with low heterogeneity (I2 = 0−35.3%). Among all the demographic parameters, only age had publication bias (P = 0.002).
Table 2.
Meta-analysis results of the whole studies on PE risk factors in COVID-19.
| Variables | Nstudiesa | PE, n/PE b | non-PE, n/non-PE c | WMD/OR | 95% CI | I2 (%) | P-value | Egger's |
|---|---|---|---|---|---|---|---|---|
| Demographic risk factors | ||||||||
| Age, years (WMD) | 26 | 847 | 3793 | 1.57 | -0.31−3.45 | 64.9% | 0.101 | 0.002 |
| Male, % (OR) | 26 | 627/911 | 2356/3836 | 1.49 | 1.26−1.76 | 0.0% | 0.000 | 0.606 |
| Obesity (BMI > 30%) | 11 | 123/329 | 237/706 | 1.37 | 1.03−1.82 | 0.0% | 0.033 | 0.238 |
| Comorbidities, % (OR) | ||||||||
| Previous VTE | 8 | 47/457 | 168/2160 | 1.37 | 0.96−1.95 | 0.0% | 0.079 | – |
| Chronic heart failure | 7 | 25/358 | 248/2619 | 0.85 | 0.55−1.31 | 35.3% | 0.456 | – |
| Cancer | 13 | 56/530 | 325/2430 | 0.81 | 0.59−1.10 | 32.4% | 0.175 | 0.473 |
| Diabetes | 18 | 146/578 | 746/3073 | 0.98 | 0.78−1.21 | 0.0% | 0.819 | 0.136 |
| Hypertension | 17 | 288/599 | 1615/3099 | 0.84 | 0.70−1.01 | 0.0% | 0.068 | 0.159 |
| Recent surgery | 3 | 4/126 | 10/389 | 0.97 | 0.30−3.12 | 0.0% | 0.955 | – |
| Cardiovascular disease | 12 | 58/381 | 383/2573 | 0.95 | 0.69−1.32 | 0.0% | 0.765 | 0.613 |
| COPD | 8 | 34/381 | 224/2466 | 0.91 | 0.62−1.35 | 0.0% | 0.651 | – |
| Clinical risk factors, % (OR) | ||||||||
| Mechanical ventilation | 5 | 52/119 | 61/266 | 3.34 | 1.90−5.86 | 10.8% | 0.000 | – |
| Severe parenchymal abnormalities on chest CT (> 50%) | 7 | 170/288 | 1183/1555 | 1.92 | 1.43−2.58 | 0.0% | 0.000 | – |
| ICU admission | 7 | 118/272 | 177/676 | 2.65 | 1.48−4.74 | 66.0% | 0.001 | – |
| Laboratory risk factors, (WMD) | ||||||||
| D-dimer, ug/ml (closest to the CTPA) | 10 | 274 | 634 | 6.03 | 5.14−6.92 | 38.0% | 0.000 | 0.872 |
| D-dimer, ug/ml (hospital admission) | 9 | 310 | 2089 | 2.10 | 1.10−3.10 | 73.8% | 0.000 | – |
| WBC, × 109/L (closest to the CTPA) | 5 | 216 | 457 | 1.46 | 0.77−2.15 | 0.0% | 0.000 | – |
| WBC, × 109/L (hospital admission) | 3 | 163 | 1786 | 2.10 | 1.21−3.00 | 0.0% | 0.000 | – |
| Lymphocytes, × 109/L (closest to the CTPA) | 3 | 38 | 57 | -0.09 | -0.62−0.43 | 51.6% | 0.734 | – |
| Lymphocytes, × 109/L (hospital admission) | 4 | 229 | 1907 | 0.009 | -0.09−0.10 | 0.0% | 0.855 | – |
| Fibrinogen, g/L (closest to the CTPA) | 4 | 136 | 268 | -0.10 | -0.77−0.56 | 22.1% | 0.759 | – |
| Fibrinogen, g/L (hospital admission) | 3 | 177 | 1270 | 0.27 | -0.07−0.60 | 0.0% | 0.122 | – |
Abbreviations: PE, pulmonary embolism; COVID-19, coronavirus disease 2019; WMD, weighted mean difference; OR, odds ratio; 95% CI, 95% confidence interval; VTE, venous thrombus embolism; COPD, chronic obstructive pulmonary disease; CT, computed tomography; WBC, white blood cells
Nstudies, number of studies
PE, n/PE, number of PE patients, number of PE patients with variable/number of PE patients
non-PE, n/non-PE, number of non-PE patients, number of non-PE patients with variable/number of non-PE patients; I2, index for the degree of heterogeneity; P value, significant at P < 0.05 and present in bold; Egger's, index for the degree of publication bias; –, not available
Fig. 2.
Meta-analysis of demographical factors associated with PE occurrence in COVID-19. A, Male; B, Obesity.
Clinical risk factors
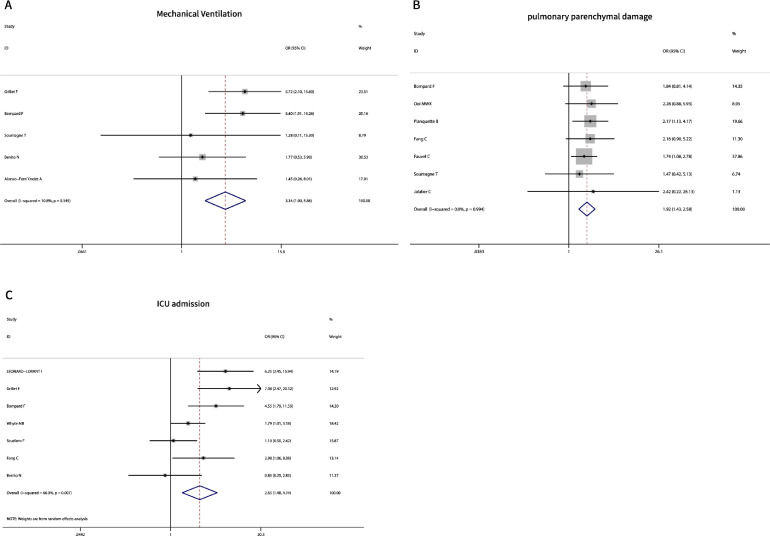
Five studies (119 PEs vs. 266 non-PEs) reported the relationship between mechanical ventilation (MV). The result indicated that patients with MV had a significantly higher rate of PE (OR 3.34, 95% CI 1.90−5.86, P = 0.000) with low heterogeneity (I2 = 10.8%) (Table 2, Figure 3 A). Seven studies (288 PEs vs. 1555 non-PEs) evaluated the extent of parenchymal damage on chest computed tomography (CT) (Bompard et al., 2020, Grillet et al., 2020, Kim et al., 2007, Kirsch et al., 2020, Kunutsor and Laukkanen, 2020, Ooi et al., 2020, Rodriguez-Sevilla et al., 2020). The pooled estimates showed that severe parenchymal damage (> 50% of lung) had a higher PE incidence rate (OR 1.92, 95% CI 1.43−2.58, P = 0.000) with no heterogeneity (I2 = 0%) (Table 2, Figure 3B). The data pooled from seven studies indicated that ICU admission had a higher rate of PE than conventional wards (OR 2.65, 95% CI 1.48−4.74, P = 0.000), with a high heterogeneity (I2 = 66%) (Table 2, Figure 3C) (Fang et al., 2020; Léonard-Lorant et al., 2020; Bompard et al., 2020; Whyte et al., 2020; Grillet et al., 2020; Benito et al., 2020; Scudiero et al., 2021).
Fig. 3.
Meta-analysis of clinical factors associated with PE occurrence in COVID-19. A, Mechanical Ventilation; B, pulmonary parenchymal damage; C, ICU admission.
Laboratory risk factors
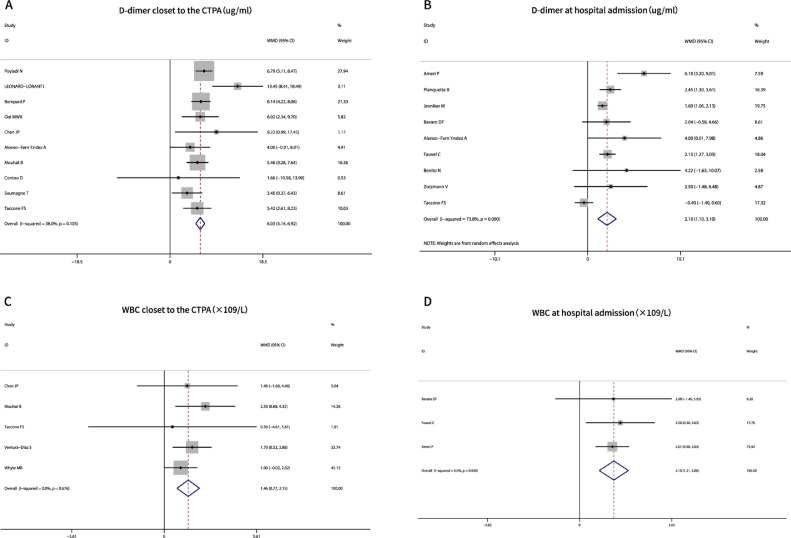
Laboratory parameters were obtained at two time points: hospital admission and closest to the CTPA examination (within 24−48 hours). The D-dimer (P = 0.000, I2 < 73.8%) and white blood cell (WBC) values (P = 0.000, I2 = 0%) in PE patients were significantly higher than that in non-PE patients at both time points (Table 2, Figure 4 A, B, C, D). Lymphocytes (P > 0.73, I2 < 51.6%) and fibrinogen (P > 0.12, I2 < 22.1%) had no significant influence on the occurrence of PEs, no matter at which time point (Table 2).
Fig. 4.
Meta-analysis of laboratory factors associated with PE occurrence in COVID-19. A, D-dimer closet to the CTPA examination; B, D-dimer at hospital admission; C, WBC values closet to the CTPA examination; D, WBC values at hospital admission. Abbreviations:CTPA, computer tomography pulmonary angiography; WBC, white blood cell
Subgroup analysis
There was no significant difference between the CTPA and COVID-19 subgroup (P > 0.073). The CTPA subgroup accounted for the majority of the included studies (22 vs. 27 studies, 768 PEs vs. 927 PEs). It had the same results as whole studies in analyses of each parameter (Supplementary Table 1). However, the COVD-19 subgroup differed from the CTPA and whole studies in males (P = 0.072), previous VTE (P = 0.005), severe parenchymal damage (> 50% of the lung) (P = 0.468), ICU admission (P = 0.816), and D-dimer value at hospital admission (P = 0.107) (Supplementary Table 1, Supplementary Figure 1).
Three subgroups of prophylactic anticoagulation had no difference (P > 0.078). Risk factors of the unclear and low-ratio subgroups were almost consistent with the results of the whole included studies, including male, MV, D-dimer (two time points), WBC (two time points), severe parenchymal damage (> 50% of the lung) (P< 0.035), and ICU admission (P < 0.035) (Supplementary Table 1). However, in the high-ratio subgroup, D-dimer (hospital admission), WBC (hospital admission), severe parenchymal damage, and ICU admission were non-risk factors (P > 0.055) (Supplementary Table 1).
The hospitalization subgroup, which contained 18 studies, had consistent PE risk factors with the overall studies. The ICU-stay subgroup had distinctly different results from them in age, obesity, previous VTE, hypertension, MV, severe parenchymal damage, D-dimer, and WBC (two time points) (Supplementary Table 1).
Discussion
This review analyzed several demographical, clinical, and laboratory indicators of COVID-19 patients for risk factors of PE. The whole included studies revealed that males, obesity, MV, severe parenchymal abnormalities of chest CT, ICU admission, and elevated D-dimer or WBC value (at both hospital admission and closest to CTPA) were risk factors for PE in COVID-19. Age and common comorbidities had no association with PE occurrence. PE risk factors might be different between the subgroups of these three subgroup analyses. The subgroups (the CTPA, unclear-ratio, hospitalization) that accounted for most of the included studies had consistent PE risk factors with the overall studies. Common comorbidities had no significant influence on the occurrence of PEs in all subgroups.
This systemic review revealed that risk factors for PE occurrence in COVID-19 were different from the classic risk factors. First, old age is a weak risk factor for classic PE (OR < 2), and male sex is not (Ceriani et al., 2010). However, in COVID-19, male sex was a weak risk factor (OR 1.49, P = 0.000) for PE occurrence, and age was not associated with PE (OR 1.57, P = 0.101). Second, the classic PE risk factors from comorbidities, including previous VTE with strong risk (OR > 10), chronic heart failure, cancer history with moderate risk (OR 2−9), diabetes, and hypertension with weak risk (OR < 2) (Ceriani et al., 2010) all had no association with PE in COVID-19 patients. A recent meta-analysis of risk factors for VTE in COVID-19 showed similar results to the current ones except in age (Gervaise et al., 2020). Several other studies showed that classic PE and PE in COVID-19 were different in certain characteristics (Chi et al., 2020, Choi et al., 2020, Shi et al., 2020). More interestingly, some studies stated that old age, male, and comorbidities were risk factors for severe COVID-19 (Soumagne et al., 2020, Soumagne et al., 2020) and they partly overlapped with the risk factors for PE or VTE in COVID-19. These indicate that PE or VTE in COVID-19 is affected to a certain extent by the severe acute respiratory syndrome coronavirus 2 (SARS-Cov-2) infection.
Mechanical ventilation (OR 3.72, P = 0.000), severe parenchymal damage (OR 1.92, P = 0.000), and ICU admission (OR 2.44, P = 0.000), which represented the severity of COVID-19 pneumonia, were associated with PE occurrence, with low heterogeneity. One imaging study found that pulmonary thrombi in COVID-19 were located in opacitated lung segments and supported this (Sungnak et al., 2020). It can also be explained by the pathological findings from autopsy: in COVID-19, distinct thrombosis and microangiopathy are common in the small vessels and pulmonary capillaries, along with classic diffuse alveolar damage (Contou et al., 2020, Egger et al., 2011, Fang et al., 2020, Taccone et al., 2020, van Dam et al., 2020). These findings are consistent with the plausible pathophysiological changes of lung lesions in COVID-19: widespread pulmonary endothelial dysfunction associated with direct viral tissue damage (ACE2 as the entry receptor for SARS-CoV-2) or immune-mediated inflammation leads to inflammatory thrombosis and microvascular dysfunction (Artifoni et al., 2020, Varga et al., 2020). Therefore, even if the pulmonary infection is also a moderate risk factor for classic PE (Ceriani et al., 2010), the new hypothesis − the etiology of PE in COVID-19 may be local microthrombosis −cannot be ruled out (Contou et al., 2020, Egger et al., 2011, Fang et al., 2020).
Elevated D-dimer and WBC levels were risk factors for PE occurrence in COVID-19 patients (at both hospital admission and closet to CTPA) (P = 0.000). The reason may be that they are closely related to excessive inflammation and severe COVID-19 (Soumagne et al., 2020). D-dimer has widely been deemed a marker of COVID-19-associated coagulopathy (Artifoni et al., 2020, Ventura-Diaz et al., 2020). Several studies have also proposed that D-dimer level is a good predictor for embolic events in patients with COVID-19. However, more studies are needed to assess the cut-off value, as it is inconsistent among studies (Watchmaker et al., 2020, Whyte et al., 2020, Worldometer COVID-19 Data 2020). Other studies have reported more laboratory indicators related to severe COVID-19 and VTE (Gervaise et al., 2020, Soumagne et al., 2020) than the current review, liking activated partial thromboplastin time, platelets, fibrinogen, C-reactive protein, lower lymphocyte, and so on. The reason may be that the current review separated the collection time of laboratory indicators at hospital admission or closest to the CTPA examination, which was more accurate and less heterogeneous; the small sample size may be another reason. More original articles about the clinical and laboratory characteristics of PE in detail are needed to detect the risk factors.
Although the heterogeneity of most parameters of this systematic review was low, it also performed subgroup analyses according to clinical application. Apparently, both the CTPA vs. COVID-19 and the hospitalization vs. ICU-stay subgroup analyses had distinctly different study populations. The management of thrombus in COVID-19 has always been a hot topic. Studies recommend that the use of preventive anticoagulants above conventional doses may reduce thrombotic events for patients with severe COVID-19 or those at high risk of thrombosis (Wu et al., 2020, Zeng et al., 2015). However, whether prophylactic anticoagulation should apply to all patients with COVID-19 remains controversial (Zheng et al., 2020, Zotzmann et al., 2020). Therefore, this review attempted to conduct an unclear-ratio vs. low-ratio vs. high-ratio subgroup analysis on this issue. The CTPA, unclear-ratio, or hospitalization subgroup was the group with the largest sample size in the three subgroup analyses and they had consistent results with the overall studies in low heterogeneities. This emphasized that the currently reported PE risk factors were calculated based on the population of the CTPA, unclear-ratio, or hospitalization patients. In the remaining subgroups, they had different PE risk factors, with low heterogeneities in most parameters. They had fewer risk factors than the CTPA, unclear-ratio, and hospitalization subgroups. While, given the vast gap in sample size between the subgroups, these differences in PE risk factors between subgroups require more evidence. Most interestingly, the three subgroup analyses only had consistent results in common comorbidities, and these traditional PE risk factors had no significant influence on the occurrence of PEs. This indicated that PE risk factors were different between COVID-19 and non-COVID-19. Moreover, PE risk factors in COVID-19 were more likely to be associated with the severity of illness, for example, MV, severe parenchymal abnormalities, ICU admission, and elevated D-dimer and WBC values.
This review had several limitations. First, due to the limitations of the original studies, several ORs had a small sample size. Also, the number of studies on COVID-19 or low-ratio subgroup was small. Fortunately, the heterogeneities of most results were acceptable. Second, 24 out of 27 included studies were from Europe, and whether the risk factors differ between regions is unclear. Third, risk factors may vary due to different subgroup analyses, and this subgroup analysis was incomplete. Subgroup analysis based on race, country, anticoagulant dose, or severity of illness can provide more comprehensive information about risk factors for PE in COVID-19. Finally, most of the included studies were retrospective case-controls. More accurate relative risks calculated from prospective cohorts are hard to obtain. Therefore, more multicenter, better-designed original studies are needed to ascertain the risk factors for PE in COVID-19 patients.
Conclusion
In conclusion, this review presented different risk factors for PE in COVID-19 from the classic risk factors in non-COVID-19. PE risk factors in COVID-19 were more likely to be associated with the severity of illness. Three subgroup analyses revealed that the currently reported risk factors for PE are mostly based on the population of COVID-19 patients with CTPA, unclear-ratio/low-ratio thromboprophylaxis, or hospitalization; these might be different in other study populations.
Funding support
This study received salary support from "National Key Research and Development Project"(2020YFC2005700) and “High-level Hospital Construction Research Project of Maoming People’s Hospital” during the conduct of the study.
Conflicts of interest
All authors declare that there are no competing interests in the research, authorship, and publication of this article.
Ethical Approval
Not applicable.
Authors' contributions
LYC and WWC were responsible for study design, screening, data extraction, data analysis, and writing the article. And they contributed equally. ZWM, DX, and YYL helped extract and dispose of data. JYL and TWL participated in the data analysis and revision of the article. ZMC designed and revised the article.
Acknowledgements
We are grateful to Jie-ming Shi, who gave us some suggestions in statistical methods.
References
- Ackermann M, Verleden SE, Kuehnel M, et al. Pulmonary vascular endotheliitis, thrombosis, and angiogenesis in COVID-19. The New England journal of medicine. 2020;383(2):120–128. doi: 10.1056/NEJMoa2015432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alonso-Fernández A, Toledo-Pons N, Cosío BG, et al. Prevalence of pulmonary embolism in patients with COVID-19 pneumonia and high D-dimer values: A prospective study. PloS one. 2020;15(8) doi: 10.1371/journal.pone.0238216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ameri P, Inciardi RM, Di Pasquale M, et al. Pulmonary embolism in patients with COVID-19: characteristics and outcomes in the Cardio-COVID Italy multicenter study. Clinical Research in Cardiology. 2020;3:1–9. doi: 10.1007/s00392-020-01766-y. Nov. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Artifoni M, Danic G, Gautier G, et al. Systematic assessment of venous thromboembolism in COVID-19 patients receiving thromboprophylaxis: incidence and role of D-dimer as predictive factors. Journal of Thrombosis and Thrombolysis. 2020;50(1):211–216. doi: 10.1007/s11239-020-02146-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bavaro DF, Poliseno M, Scardapane A, et al. Occurrence of acute pulmonary embolism in COVID-19—a case series. International Journal of Infectious Diseases. 2020;98:225–226. doi: 10.1016/j.ijid.2020.06.066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benito N, Filella D, Mateo J, et al. Pulmonary thrombosis or embolism in a large cohort of hospitalized patients with COVID-19. Frontiers in Medicine. 2020;25(7):557. doi: 10.3389/fmed.2020.00557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bilaloglu S, Aphinyanaphongs Y, Jones S, Iturrate E, Hochman J, Berger JS. Thrombosis in hospitalized patients with COVID-19 in a New York city health system. JAMA. 2020;324(8):799–801. doi: 10.1001/jama.2020.13372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bompard F, Monnier H, Saab I, et al. Pulmonary embolism in patients with COVID-19 pneumonia. The European respiratory journal. 2020;56(1) doi: 10.1183/13993003.01365-2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- BujaL M, Wolf DA, Bihong Z, et al. The emerging spectrum of cardiopulmonary pathology of the coronavirus disease 2019 (COVID-19): Report of 3 autopsies from Houston, Texas, and review of autopsy findings from other United States cities. Cardiovasc Pathol. 2020;48 doi: 10.1016/j.carpath.2020.107233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bunce PE, High SM, Nadjafi M, Stanley K, Liles WC, Christian MD. Pandemic H1N1 influenza infection and vascular thrombosis. Clinical infectious diseases. 2011;52(2):e14–e17. doi: 10.1093/cid/ciq125. [DOI] [PubMed] [Google Scholar]
- Ceriani E, Combescure C, Gal GL, et al. Clinical prediction rules for pulmonary embolism: a systematic review and meta-analysis. J Thromb Haemost. 2010;8(5):957–970. doi: 10.1111/j.1538-7836.2010.03801.x. [DOI] [PubMed] [Google Scholar]
- Chen J, Wang X, Zhang S, et al. Characteristics of acute pulmonary embolism in patients with COVID-19 associated pneumonia from the city of Wuhan. Clinical and applied thrombosis-hemostasis. 2020;26 doi: 10.1177/1076029620936772. 1076029620936772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chi G, Lee JJ, Jamil A, Gunnam V, Najafi H. Venous Thromboembolism among hospitalized patients with COVID-19 undergoing thromboprophylaxis: a systematic review and meta-analysis. Journal of Clinical Medicine. 2020;9(8):e2489. doi: 10.3390/jcm9082489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Choi JJ, Wehmeyer GT, Li HA, et al. D-dimer cut-off points and risk of venous thromboembolism in adult hospitalized patients with COVID-19. Thromb Res. 2020;196:318–321. doi: 10.1016/j.thromres.2020.09.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Connors JM, Levy JH. COVID-19 and its implications for thrombosis and anticoagulation. Blood. 2020;135(23):2033–2040. doi: 10.1182/blood.2020006000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Contou D, Pajot O, Cally R, et al. Pulmonary embolism or thrombosis in ARDS COVID-19 patients: A French monocenter retrospective study. PloS one. 2020;15(8) doi: 10.1371/journal.pone.0238413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Egger M, Davey Smith G, Schneider M, Minder C. Recommendations for examining and interpreting funnel plot asymmetry in meta-analyses of randomised controlled trials. BMJ. 2011;343:d4002. doi: 10.1136/bmj.d4002. [DOI] [PubMed] [Google Scholar]
- Fang C, Garzillo G, Batohi B, et al. Extent of pulmonary thromboembolic disease in patients with COVID-19 on CT: relationship with pulmonary parenchymal disease. Clinical radiology. 2020;75(10):780–788. doi: 10.1016/j.crad.2020.07.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fauvel C, Weizman O, Trimaille A, et al. Pulmonary embolism in COVID-19 patients: a French multicentre cohort study. European heart journal. 2020;41(32):3058–3068. doi: 10.1093/eurheartj/ehaa500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Flumignan RLG, JDDSá Tinôco, Pascoal PIF, et al. Prophylactic anticoagulants for people hospitalised with COVID-19. Cochrane Database of Systematic Reviews. 2020;10 doi: 10.1002/14651858.CD013739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fox SE, Akmatbekov A, Harbert JL, Li G, Quincy Brown J, Vander Heide RS. Pulmonary and cardiac pathology in African American patients with COVID-19: an autopsy series from New Orleans. The Lancet Respiratory medicine. 2020;8(7):681–686. doi: 10.1016/S2213-2600(20)30243-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gervaise A, Bouzad C, Peroux E, Helissey C. Acute pulmonary embolism in non-hospitalized COVID-19 patients referred to CTPA by emergency department. European radiology. 2020;30(11):6170–6177. doi: 10.1007/s00330-020-06977-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grillet F, Behr J, Calame P, Aubry S. Acute pulmonary embolism associated with COVID-19 pneumonia detected with pulmonary CT angiography. Radiology. 2020;296(3):e186–e188. doi: 10.1148/radiol.2020201544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gupta A, Madhavan MV. Extrapulmonary manifestations of COVID-19. Nature Medicine. 2020;26(7):1017–1032. doi: 10.1038/s41591-020-0968-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hajra A, Mathai SV, Ball S, et al. Management of thrombotic complications in COVID-19: an update. Drugs. 2020;80(15):1553–1562. doi: 10.1007/s40265-020-01377-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jalaber C, Revel MP, Chassagnon G, et al. Role of upfront CT pulmonary angiography at admission in COVID-19 patients. Thrombosis Research. 2020;196:138–140. doi: 10.1016/j.thromres.2020.08.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jevnikar M, Sanchez O, Andronikov M, et al. Prevalence of pulmonary embolism in patients at the Time of Hospital Admission for COVID-19. Research and Practice in Thrombosis and Haemostasis. 2020;4(2):11. doi: 10.1002/rth2.12413. SUPPL. [DOI] [Google Scholar]
- Jiménez HS, Lozano PL, Suñen CG, et al. Clinical findings, risk factors, and final outcome in patients diagnosed with pulmonary thromboembolism and COVID-19 in hospital emergency departments. Emergencias. 2020;32(4):253–257. PMID: 32692002. [PubMed] [Google Scholar]
- Higgins JP, Thompson SG, Deeks JJ, Altman DG. Measuring inconsistency in meta-analyses. BMJ. 2003;327(7414):557–560. doi: 10.1136/bmj.327.7414.557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huisman MV, Barco S, Cannegieter SC, et al. Pulmonary embolism. Nature reviews. 2018;4:18028. doi: 10.1038/nrdp.2018.28. [DOI] [PubMed] [Google Scholar]
- Kim KD, Zhao J, Auh S, et al. Adaptive immune cells temper initial innate responses. Nat Med. 2007;13(10):1248–1252. doi: 10.1038/nm1633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kirsch B, Aziz M, Kumar S, et al. Wells score to predict pulmonary embolism in patients with coronavirus disease 2019. Am J Med. 2020;134(5):688–690. doi: 10.1016/j.amjmed.2020.10.044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Konstantinides SV, Meyer G, Becattini C, et al. 2019 ESC Guidelines for the diagnosis and management of acute pulmonary embolism developed in collaboration with the European Respiratory Society (ERS): The Task Force for the diagnosis and management of acute pulmonary embolism of the European Society of Cardiology (ESC) The European respiratory journal. 2019;54(3) doi: 10.1183/13993003.01647-2019. DOI:10.1183 /13993003.01647-2019. [DOI] [PubMed] [Google Scholar]
- Kosior DA, Undas A, Kopec G, et al. Guidance for anticoagulation management in venous thromboembolism during the coronavirus disease 2019 pandemic in Poland an expert opinion of the section on pulmonary circulation of the polish cardiac society. Kardiologia Polska. 2020;78(6):642–646. doi: 10.33963/kp.15425. [DOI] [PubMed] [Google Scholar]
- Kunutsor SK, Laukkanen JA. Incidence of venous and arterial thromboembolic complications in COVID-19: A systematic review and meta-analysis. Thrombosis research. 2020;196:27–30. doi: 10.1016/j.thromres.2020.08.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lax SF, Skok K, Zechner P, et al. Pulmonary arterial thrombosis in COVID-19 with fatal outcome: results from a prospective, single-center. Annals of internal medicine. 2020;173(5):350–361. doi: 10.7326/M20-2566. clinicopathologic case Series. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Léonard-Lorant I, Delabranche X. Acute pulmonary embolism in patients with COVID-19 at CT angiography and relationship to d-dimer levels. Radiology. 2020;296(3):e189–e191. doi: 10.1148/radiol.2020201561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liao SC, Shao SC, Chen YT, Chen YC, Hung MJ. Incidence and mortality of pulmonary embolism in COVID-19: a systematic review and meta-analysis. Critical care. 2020;24(1):464. doi: 10.1186/s13054-020-03175-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Llitjos JF, Leclerc M, Chochois C, et al. High incidence of venous thromboembolic events in anticoagulated severe COVID-19 patients. J Thromb Haemost. 2020;18(7):1743–1746. doi: 10.1111/jth.1486920. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mestre-Gómez B, Lorente-Ramos RM, Rogado J, et al. Incidence of pulmonary embolism in non-critically ill COVID-19 patients. Predicting factors for a challenging diagnosis. Journal of Thrombosis and Thrombolysis. 2020;51(1):40–46. doi: 10.1007/s11239-020-02190-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mouhat B, Besutti M, Bouiller K, et al. Elevated D-dimers and lack of anticoagulation predict PE in severe COVID-19 patients. European Respiratory Journal. 2020;56:4. doi: 10.1183/13993003.01811-2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mueller-Peltzer K, Krauss T, Benndorf M, et al. Pulmonary artery thrombi are co-located with opacifications in SARS-CoV2 induced ARDS. Respir Med. 2020;172:106–135. doi: 10.1016/j.rmed.2020.106135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nopp S, Janata-Schwatczek K, Prosch H, Shulym I, Königsbrügge O. Pulmonary embolism during the COVID-19 pandemic: Decline in diagnostic procedures and incidence at a university hospital. Respirology case reports. 2020;4(5):835–841. doi: 10.1002/rcr2.622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ooi MWX, Rajai A, Patel R, et al. Pulmonary thromboembolic disease in COVID-19 patients on CT pulmonary angiography−Prevalence, pattern of disease and relationship to D-dimer. European Journal of Radiology. 2020;132 doi: 10.1016/j.ejrad.2020.109336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pandey P, Agarwal S. Rajkumar. Lung pathology in COVID-19: A systematic review. Int J Appl Basic Med Res. 2020;10(4):226–233. doi: 10.4103/ijabmr.IJABMR_381_20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Planquette B, Le Berre A, Khider L, et al. Prevalence and characteristics of pulmonary embolism in 1042 COVID-19 patients with respiratory symptoms: A nested case-control study. Thrombosis Research. 2021;197:94–99. doi: 10.1016/j.thromres.2020.11.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rodriguez-Sevilla J, Rodó-Pin A, Espallargas I, et al. Pulmonary embolism in patients with Covid-19 pneumonia: the utility of d-dimer. Arch Bronconeumol. 2020;S0300-2896(20):30216–30217. doi: 10.1016/j.arbres.2020.06.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Poyiadji N, Cormier P, Patel PY, et al. Acute pulmonary embolism and COVID-19. Radiology. 2020;297(3):e335–e338. doi: 10.1148/radiol.2020201955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salje H, Kiem CT, Lefrancq N, et al. Estimating the burden of SARS-CoV-2 in France. Science. 2020;369(6500):208–211. doi: 10.1126/science.abc3517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scudiero F, Silverio A, Di Maio M, et al. Pulmonary embolism in COVID-19 patients: Prevalence, predictors and clinical outcome. Thrombosis Research. 2021;198:34–39. doi: 10.1016/j.thromres.2020.11.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shi L, Jie X, Guang CD, Hai YY, Ya DW. The pooled prevalence of pulmonary embolism in patients with COVID-19. Intensive Care Med. 2020;14:1–3. doi: 10.1007/s00134-020-06235-8. Sep. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Soumagne T, Lascarrou JB, Hraiech S, et al. Factors associated with pulmonary embolism among coronavirus disease 2019 acute respiratory distress syndrome: a multicenter study among 375 patients. Critical care explorations. 2020;2(7):e0166. doi: 10.1097/cce.0000000000000166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Soumagne T, Winiszewski H, Besch G, et al. Pulmonary embolism among critically ill patients with ARDS due to COVID-19. Respiratory Medicine and Research. 2020;78 doi: 10.1016/j.resmer.2020.100789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sungnak W, Huang N, Bécavin C, et al. SARS-CoV-2 entry factors are highly expressed in nasal epithelial cells together with innate immune genes. Nat Med. 2020;26(5):681–687. doi: 10.1038/s41591-020-0868-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taccone FS, Gevenois PA, Peluso L, et al. Higher intensity thromboprophylaxis regimens and pulmonary embolism in critically ill coronavirus disease 2019 patients. Critical care medicine. 2020;48(11):e1087–e1090. doi: 10.1097/CCM.0000000000004548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Dam LF, Kroft LJM, van der Wal LI, et al. Clinical and computed tomography characteristics of COVID-19 associated acute pulmonary embolism: A different phenotype of thrombotic disease? Thrombosis research. 2020;193:86–89. doi: 10.1016/j.thromres.2020.06.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Varga Z, Flammer AJ, Steiger P, et al. Endothelial cell infection and endotheliitis in COVID-19. Lancet. 2020;395(10234):1417–1418. doi: 10.1016/S0140-6736(20)30937-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ventura-Diaz S, Quintana-Perez JV, Gil-Boronat A, et al. A higher D-dimer threshold for predicting pulmonary embolism in patients with COVID-19: a retrospective study. Emergency Radiology. 2020;27(6):679–689. doi: 10.1007/s10140-020-01859-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watchmaker JM, Goldman DT, Lee JY. Increased incidence of acute pulmonary embolism in emergency department patients during the COVID-19 pandemic. J Intensive Care. 2020;27(12):1340–1343. doi: 10.1111/acem.14148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whyte MB, Kelly PA, Gonzalez E, Arya R, Roberts LN. Pulmonary embolism in hospitalised patients with COVID-19. Thrombosis research. 2020;195:95–99. doi: 10.1016/j.thromres.2020.07.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Worldometer COVID-19 Data, Available at https://www.worldometers.info/coronavirus/, [Accessed September 30, 2020].
- Wu T, Zuo Z, Yang D, et al. Venous thromboembolic events in patients with COVID-19: A systematic review and meta-analysis. Age Ageing. 2020;50(2):284–293. doi: 10.1093/ageing/afaa259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zeng X, Zhang Y, Kwong JS, et al. The methodological quality assessment tools for preclinical and clinical studies, systematic review and meta-analysis, and clinical practice guideline: a systematic review. Journal of evidence-based medicine. 2015;8(1):2–10. doi: 10.1111/jebm.12141. [DOI] [PubMed] [Google Scholar]
- Zheng Z, Peng F, Xu B, et al. Risk factors of critical & mortal COVID-19 cases: A systematic literature review and meta-analysis. J Infect. 2020;81(2):e16–e25. doi: 10.1016/j.jinf.2020.04.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zotzmann V, Lang CN, Wengenmayer T, et al. Combining lung ultrasound and Wells score for diagnosing pulmonary embolism in critically ill COVID-19 patients. J Ultrasound Med. 2020;3:1–9. doi: 10.1007/s11239-020-02323-0. Nov. [DOI] [PMC free article] [PubMed] [Google Scholar]