Introduction
To date, the United States Food and Drug Administration has issued emergency use authorization for 3 vaccines for the prevention of COVID-19: Pfizer-BioNTech's COVID-19 vaccine, Moderna's COVID-19 vaccine, and Janssen's COVID-19 vaccine.1,2
The vast majority of COVID-19 vaccinations administered at Loma Linda University Medical Center were messenger RNA (mRNA) vaccines developed by Pfizer and Moderna. The emergency use authorization for the Pfizer vaccine was issued based on a randomized, controlled trial demonstrating a less than 1.2% rate of local side effects rated grade 3 or higher, including redness, swelling, and pain.3 The emergency use authorization for the Moderna vaccine was based on a randomized, controlled trial demonstrating a less than 7.4% rate of local side effects rated grade 3 or higher.4
In October 2020, Wibawa5 explored ethical issues inherent in rapid vaccine development, including the fact that mRNA vaccines have the possibility of inducing a strong type I interferon response contributing to inflammation and autoimmune conditions.
Studies fully elucidating the cutaneous side effects related to the vaccination effort are ongoing. Shimabukuro reported 175 possible cases with 21 confirmed cases of anaphylaxis often with concomitant, diffuse erythematous rash or generalized urticaria after vaccination.6 Castells and Phillips suggest that the Pfizer mRNA vaccine may have higher rates of anaphylaxis when compared with the Moderna vaccine, while another study found similar rates of anaphylaxis with individuals vaccinated for COVID-19- 7 cases with the Pfizer vaccine and 9 cases with the Moderna vaccine.7,8 A now well-documented cutaneous manifestation of COVID-19 vaccination is a delayed local reaction that occurs within a median of 8 days of vaccine administration, presenting with an erythematous, edematous plaque. This reaction was noted by both Wei et al9 and Blumenthal et al,10 whose group theorized that this reaction is a delayed-type or T-cell-mediated hypersensitivity.
The recent publication by McMahon et al11 based on data collected from an international registry outlined delayed large local reactions, local injection site reactions, urticarial eruptions, and morbilliform eruptions as the most common vaccination reactions. The case series that follows outlines unique vaccine reactions that have been observed at the Loma Linda University Department of Dermatology.
Case series
Case 1: Urticaria and angioedema
The patient is a 68-year-old woman with a history of stable multiple sclerosis, not on therapy, who presented with angioedema and an urticarial rash after COVID-19 vaccine administration. The patient experienced an urticarial rash distributed on the trunk and upper and lower extremities (Fig 1), followed by angioedema of the lips and other soft tissues of the face within 48 hours of the first dose of her Moderna vaccine. The angioedema was short-lived, persisting for several hours before resolving with a patient-initiated one-time dose of oral diphenhydramine. The urticarial rash persisted for 3 days and resolved, again, with patient-initiated baking soda baths. The patient did not have a known COVID-19 infection prior to vaccine administration. Additionally, she had no prior history of skin disease, no prior history of cutaneous reactions after other vaccines, nor known allergies to injectable medications.
Fig 1.
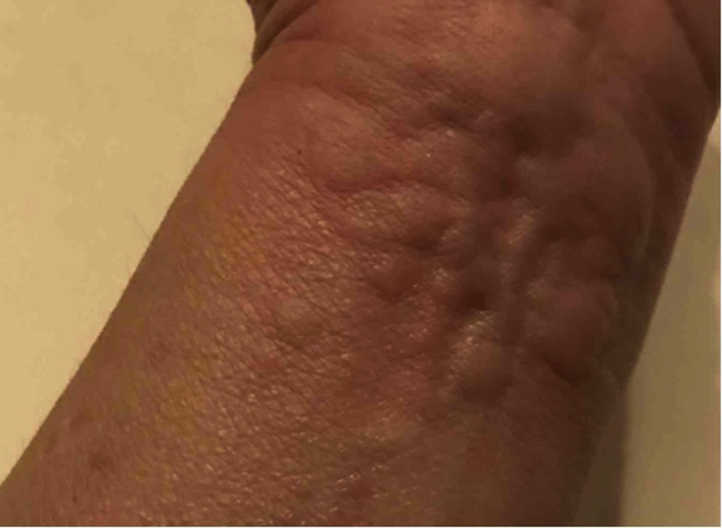
Faintly erythematous, edematous grouped wheals on the volar wrist.
The patient was advised against obtaining the second Moderna vaccine dose due to the risk of airway obstruction with fatal asphyxiation and/or systemic circulatory symptoms.
Case 2: Urticarial vasculitis
An 86-year-old woman with no relevant past medical history, including autoimmune conditions, presented with a rash involving her face, trunk, and extremities after receiving her second Pfizer vaccine. Approximately 5 days after her second Pfizer vaccine dose, she experienced an urticarial rash on her face, trunk, and extremities that coalesced into purpuric, reticulated patches (Fig 2). The rash was intensely pruritic without other symptoms. Review of systems was otherwise negative, including for arthralgias. The patient had no history of dermatologic conditions, nor history of skin reactions following other vaccinations or injectable medication administration. She denied previous COVID-19 infection.
Fig 2.
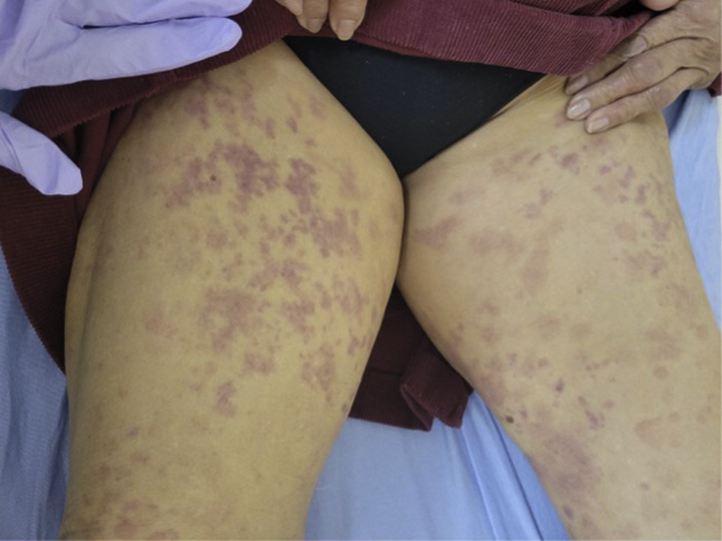
Purpuric macules coalescing into reticulated patches on the bilateral thighs.
Laboratory studies demonstrated a complete blood count and complete metabolic panel within acceptable limits. C1q, C2, C3, C4, C5, and C1 esterase inhibitor were within normal limits. Hepatitis B and hepatitis C serologies were negative. A skin biopsy was performed, revealing superficial perivascular inflammation with rare eosinophils and leukocytoclastic debris, raising suspicion for prior vascular injury. The patient was diagnosed with resolving urticarial vasculitis likely secondary to COVID-19 vaccination.
The patient was treated with oral prednisone 40 mg daily for 5 days, topical triamcinolone ointment, oral fexofenadine, and oral diphenhydramine as needed for itch. She reported progressive improvement with resolution within 2 weeks of treatment initiation.
Case 3: Chilblains-like dermal hypersensitivity reaction
The patient is a 48-year-old woman with a remote history of allergic contact dermatitis to fragrances and positive antinuclear antibodies without rheumatologic disease manifestations, who presented with multiple subtle chilblains-like papules overlying the joint spaces of the hands and feet (Fig 3). The rash started 10 days after the patient received the first dose of her Moderna vaccine. The rash was associated with mild pruritus and tenderness to palpation. The patient had attempted oral diphenhydramine, which did not provide relief, as well as topical hydrocortisone, which improved her pruritus.
Fig 3.
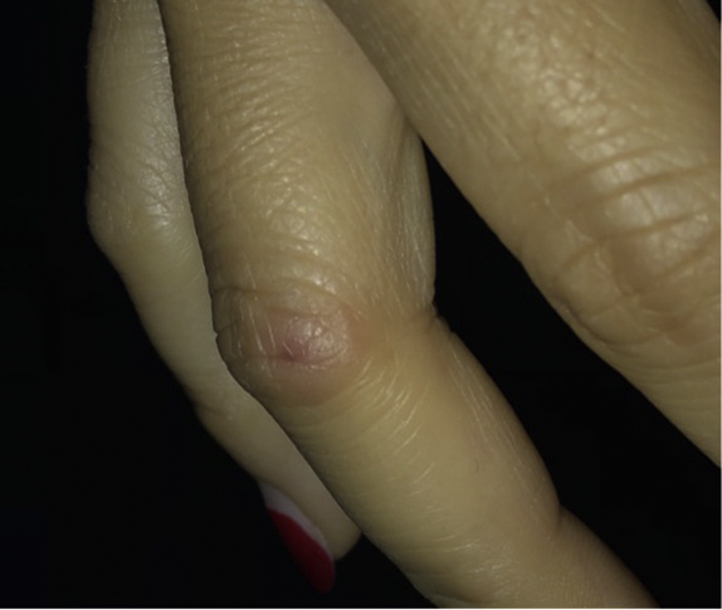
Subtle chilblains-like erythematous-to-blue dermal papules overlying the joint spaces of the hands.
A biopsy was performed at her initial dermatology evaluation, demonstrating a psoriatic and spongiotic dermatitis with superficial and deep perivascular lymphocyte-predominant inflammation as well as numerous perivascular and interstitial eosinophils. There was no evidence of vasculitis. The pathology findings supported a diagnosis of a dermal hypersensitivity reaction.
The rash resolved by postvaccination day 23 without additional treatment. She was advised to proceed with the second Moderna vaccine dose, which she received without side effects or adverse reactions.
Case 4: Morbilliform eruption
The patient is a 33-year-old woman who presented to our dermatology clinic with a morbilliform eruption. The patient received her first dose of the Moderna vaccine and the second dose 4 weeks later. One day after the second dose, she developed a morbilliform rash consisting of erythematous, edematous papules coalescing into plaques on her trunk with eventual spread to her extremities and face (Fig 4). She endorsed pruritus and burning at the areas of involvement. The patient had no history of previous COVID-19 infection, no history of other dermatologic conditions, nor previous allergic reactions to injectable medications or vaccinations.
Fig 4.
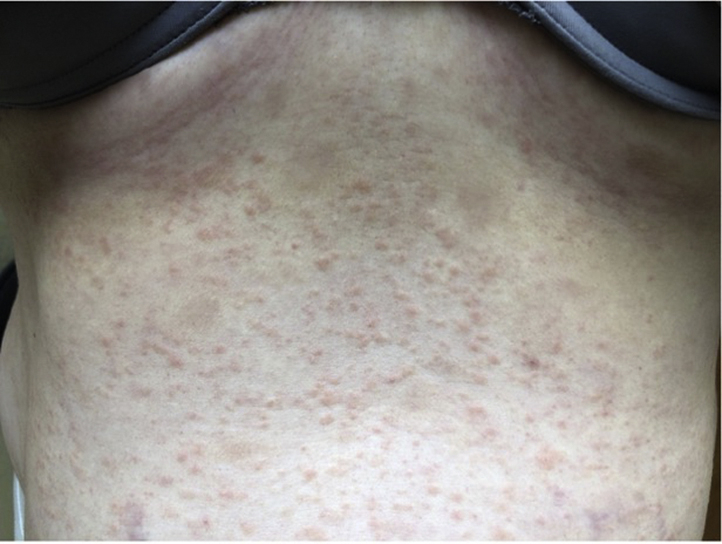
Erythematous, edematous papules scattered across the abdomen.
The patient's primary care physician started the patient on a 7-day prednisone taper (which she had finished 2 weeks prior to her appointment), cetirizine daily, and famotidine daily. A punch biopsy showed a spongiotic/psoriasiform dermatitis with a superficial perivascular lymphocyte-predominant infiltrate and numerous eosinophils without active vasculitis. Since the patient had started no new medications in the months leading up to the vaccine and due to the fact that the prescriptions for famotidine and cetirizine were initiated after the rash had occurred, the patient was diagnosed with a morbilliform vaccine reaction. The patient was instructed to use topical triamcinolone ointment as well as oral fexofenadine and hydroxyzine as needed for pruritus. Her rash resolved within 2 weeks of her clinic visit.
Case 5: Eczematous dermatitis
The patient is a 37-year-old man with no past medical history who presented with an eczematous dermatitis shortly after receiving the COVID-19 vaccine. The patient noticed faint pink, scaly plaques on his eyelids and arms within 7 days of the first dose of his Pfizer vaccine. The patient noticed a similar, more dramatic rash after the second dose of his Pfizer vaccine (Fig 5). The symptoms associated with the rash included burning and stinging. He had used triamcinolone ointment several days prior to his evaluation. The patient had no history of rashes, including atopic dermatitis, denied previous COVID-19 infection, and denied reaction to previous vaccinations.
Fig 5.
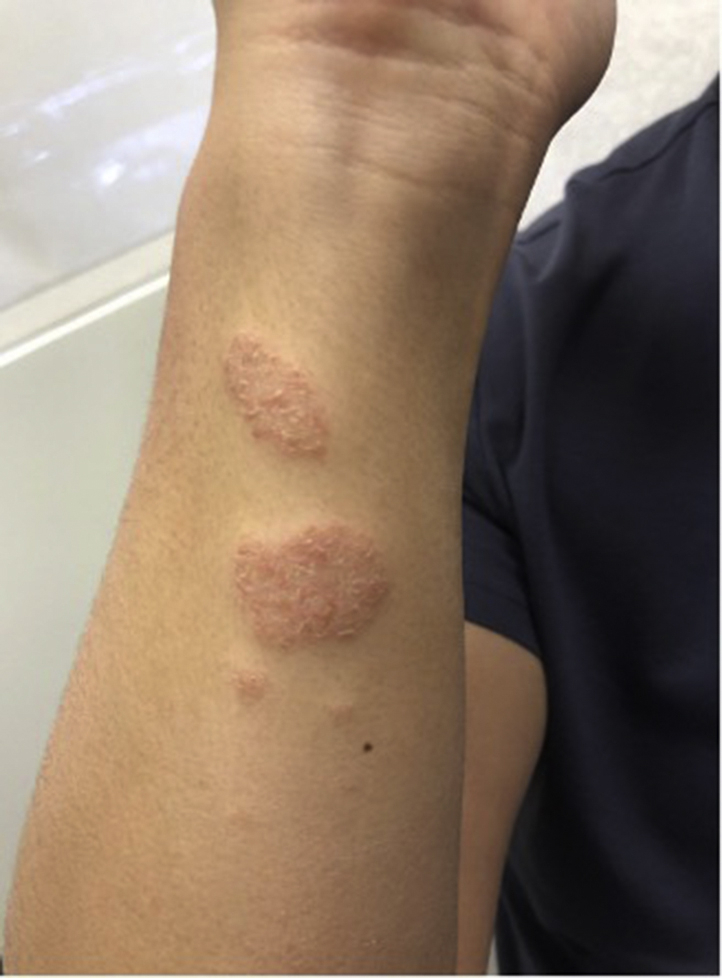
Pink, round plaques with fine overlying scale on the right forearm.
A 4-mm punch biopsy demonstrated a psoriasiform/spongiotic dermatitis with superficial perivascular inflammation and dermal mucin. While not entirely specific, a diagnosis of subacute-to-chronic dermal hypersensitivity reaction producing a nummular dermatitis was made. Tacrolimus ointment was prescribed for his eyelids, and fluocinonide ointment was prescribed for his forearms. The patient noted complete resolution within 2-3 weeks.
Discussion
Cutaneous manifestations of COVID-19 vaccination are variable. All of the vaccine reactions described in this case series tended to resolution. Only one patient with a reaction after the first vaccine dose was advised to forgo completing the vaccine course due to concerns of inciting recurrent angioedema or anaphylaxis.
It is interesting to note that female patients were involved in 4 of the 5 cases and 4 of the 5 individuals had no history of dermatologic conditions. This aligns well with the report by McMahon et al11 on 414 cutaneous reactions to COVID-19 vaccination reported to the international registry between December 2020 and February 2021, finding that 90% of vaccination reactions were noted in female patients and that 84% of vaccination reactions occurred in individuals with no dermatologic history. In contrast, Asian Americans were involved in 3 of our 5 cases, and our patients' ages ranged from 33 to 86 (average, 54.4 years), whereas Asian Americans represented 11% of cutaneous reactions, and vaccine reactions were noted in individuals ranging from 36 to 60 years of age (average, 44 years) in the study by McMahon et al.11
As previously mentioned, mRNA vaccines are associated with important risks, including the possibility of strong type I interferon responses that could result in inflammation and autoimmune conditions. This could potentially explain the reactions observed after vaccination. An alternative explanation could be allergenic components in the vaccine. Both the Pfizer and Moderna vaccines are composed of mRNA and lipids as well as salts, sugars, and buffers (Table I).12,13
Table I.
The ingredients of the Pfizer and Moderna COVID-19 vaccines
| Pfizer vaccine ingredients | Moderna vaccine ingredients |
|---|---|
| mRNA | mRNA |
| ((4-hydroxybutyl)azanediyl)bis(hexane-6,1-diyl)bis(2-hexyldecanoate) | SM-102 |
| 2[(polyethylene glycol)-2000]-N,N-ditetradecylacetamide | Polyethylene glycol 2000 dimyristoyl glycerol |
| 1,2-distearoyl-sn-glycero-3-phosphocholine | 1,2-distearoyl-sn-glycero-3-phosphocholine |
| Cholesterol | Cholesterol |
| Potassium chloride | Tromethamine |
| Monobasic potassium phosphate | Tromethamine hydrochloride |
| Sodium chloride | Acetic acid |
| Dibasic sodium phosphate dihydrate | Sodium acetate |
| Sucrose | Sucrose |
mRNA, Messenger RNA.
2[(polyethylene glycol)-2000]-N,N-ditetradecylacetamide and polyethylene glycol 2000 dimyristoyl glycerol are incorporated as the polyethylene glycol (PEG) products of the polymer-lipid nanoparticle of the Pfizer vaccine and Moderna vaccine, respectively. Topical exposure to products containing a PEG vehicle have been associated with immediate urticarial and delayed eczematous eruptions.14, 15, 16, 17 Paoletti et al18 highlighted the PEG products in the Pfizer and Moderna vaccines as the most likely culprit ingredient resulting in anaphylactic reactions.
Both vaccines also have an aminolipid component of the lipid nanoparticle that aids mRNA compaction and delivery. The aminolipid ((4-hydroxybutyl)azanediyl)bis(hexane-6,1-diyl)bis(2-hexyldecanoate), also known as ALC-0315, is found in the Pfizer vaccine, and SM-102, a proprietary compound, is found in the Moderna vaccine.19 While little is published about the allergenic potential of these compounds, a study on ALC-0315 clearance in rat models demonstrated that maximal concentrations were obtained 3 hours after intravenous injection, and the concentrations dropped 75% over the course of 2 weeks.20 It is notable that the cutaneous reactions to COVID-19 vaccinations all tended to resolution over a similar timeframe.
Finally, the Moderna vaccine contains tromethamine and tromethamine hydrochloride, amino-containing compounds used as buffers. There are reports of eyelid contact allergic eczema in response to topical ketorolac tromethamine ophthalmic drops as well as anaphylaxis in response to ketorolac tromethamine injection in patients without nonsteroidal anti-inflammatory intolerance.21,22
Patch testing or skin prick testing could be performed on this group of patients in an attempt to determine if an allergenic component plays a role in cutaneous reactions to COVID-19 vaccination and potentially confirm the ingredient inciting those reactions.
Conflicts of interest
None disclosed.
Footnotes
Funding sources: None.
IRB approval status: Not applicable.
References
- 1.Oliver S.E., Gargano J.W., Marin M. The Advisory Committee on Immunization Practices' interim recommendation for use of Pfizer-Biontech COVID-19 vaccine - United States, December 2020. MMWR Morb Mortal Wkly Rep. 2021;69(50):1922–1924. doi: 10.15585/mmwr.mm6950e2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Oliver S.E., Gargano J.W., Marin M. The Advisory Committee on Immunization Practices' interim recommendation for use of Moderna COVID-19 vaccine - United States, December 2020. MMWR Morb Mortal Wkly Rep. 2021;69(5152):1653–1656. doi: 10.15585/mmwr.mm695152e1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Centers for Disease Control and Prevention Vaccines & Immunizations. Local reactions, systemic reactions, adverse events, and serious adverse events: Pfizer-BioNTech COVID-19 vaccine. https://www.cdc.gov/vaccines/covid-19/info-by-product/pfizer/reactogenicity.html?CDC_AA_refVal=https%3A%2F%2Fwww.cdc.gov%2Fvaccines%2Fcovid-19%2Finfo-by-manufacturer%2Fpfizer%2Freactogenicity.html
- 4.Centers for Disease Control and Prevention Vaccines & Immunizations. Local reactions, systemic reactions, adverse events, and serious adverse events: Moderna COVID-19 vaccine. https://www.cdc.gov/vaccines/covid-19/info-by-product/moderna/reactogenicity.html
- 5.Wibawa T. COVID-19 vaccine research and development: ethical issues. Trop Med Int Health. 2021;26(1):14–19. doi: 10.1111/tmi.13503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.CDC COVID-19 Response Team, Food and Drug Administration Allergic reactions including anaphylaxis after receipt of the first dose of Pfizer-Biontech COVID-19 vaccine - United States, December 14-23, 2020. MMWR Morb Mortal Wkly Rep. 2021;70(2):46–51. doi: 10.15585/mmwr.mm7002e1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Blumenthal K.G., Freeman E.E., Saff R.R. Delayed large local reactions to mRNA-1273 vaccine against SARS-CoV-2. N Engl J Med. 2021;384(13):1273–1277. doi: 10.1056/NEJMc2102131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Castells M.C., Phillips E.J. Maintaining safety with SARS-CoV-2 vaccines. N Engl J Med. 2021;384(7):643–649. doi: 10.1056/NEJMra2035343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Wei N., Fishman M., Wattenberg D., Gordon M., Lebwohl M. ‘COVID arm’: A reaction to the Moderna vaccine. JAAD Case Rep. 2021;10:92–95. doi: 10.1016/j.jdcr.2021.02.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Blumenthal K.G., Robinson L.B., Camargo C.A. Acute allergic reactions to mRNA COVID-19 vaccines. JAMA. 2021;325(15):1562–1565. doi: 10.1001/jama.2021.3976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.McMahon D.E., Amerson E., Rosenbach M. Cutaneous reactions reported after Moderna and Pfizer COVID-19 vaccination: A registry-based study of 414 cases. J Am Acad Dermatol. 2021;85(1):46–55. doi: 10.1016/j.jaad.2021.03.092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Centers for Disease Control and Prevention Pfizer-BioNTech COVID-19 Vaccine overview and safety. Pfizer-BioNTech COVID-19 EUA fact sheet for recipients and caregivers. https://www.fda.gov/media/144414/download#page=2
- 13.Centers for Disease Control and Prevention Moderna COVID-19 Vaccine overview and safety. Moderna COVID-19 EUA fact sheet for recipients and caregivers. https://www.fda.gov/media/144638/download#page=2
- 14.Fisher A.A. Immediate and delayed allergic contact reactions to polyethylene glycol. Contact Dermatitis. 1978;4(3):135–138. doi: 10.1111/j.1600-0536.1978.tb03759.x. [DOI] [PubMed] [Google Scholar]
- 15.Guijarro S.C., Sánchez-Pérez J., García-Díez A. Allergic contact dermatitis to polyethylene glycol and nitrofurazone. Am J Contact Dermat. 1999;10(4):226–227. doi: 10.1053/AJCD01000226. [DOI] [PubMed] [Google Scholar]
- 16.Antolin-Amerigo D., Sánchez-González M.J., Barbarroja-Escudero J., Rodríguez-Rodríguez M., Álvarez-Perea A., Alvarez-Mon M. Allergic reaction to polyethylene glycol in a painter. Occup Med (Lond) 2015;65(6):502–504. doi: 10.1093/occmed/kqv072. [DOI] [PubMed] [Google Scholar]
- 17.Cox F., Khalib K., Conlon N. Peg that reaction: a case series of allergy to polyethylene glycol. J Clin Pharmacol. 2021;61(6):832–835. doi: 10.1002/jcph.1824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Paoletti G., Racca F., Piona A. Successful SARS-CoV-2 vaccine allergy risk-management: the experience of a large Italian university hospital. World Allergy Organ J. 2021;14(5):100541. doi: 10.1016/j.waojou.2021.100541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Moghimi S.M. Allergic reactions and anaphylaxis to LNP-based COVID-19 vaccines. Mol Ther. 2021;29(3):898–900. doi: 10.1016/j.ymthe.2021.01.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.European Medicines Agency Committee for Medicinal Products for Human Use. COVID-19 mRNA vaccine (nucleosidemodified) assessment report. https://www.ema.europa.eu/en/documents/assessment-report/comirnaty-epar-public-assessment-report_en.pdf
- 21.Scala E., Giani M., Pirrotta L. Selective severe anaphylactic reaction due to ketorolac tromethamine without nonsteroidal anti-inflammatory drug intolerance. J Allergy Clin Immunol. 2001;107(3):557. doi: 10.1067/mai.2001.113241. [DOI] [PubMed] [Google Scholar]
- 22.Rodríguez N.A., Abarzuza R., Cristóbal J.A., Sierra J., Mínguez E., Del Buey M.A. [Eyelid contact allergic eczema caused by topical ketorolac tromethamine 0.5%] Arch Soc Esp Oftalmol. 2006;81(4):213–216. doi: 10.4321/s0365-66912006000400007. [DOI] [PubMed] [Google Scholar]


