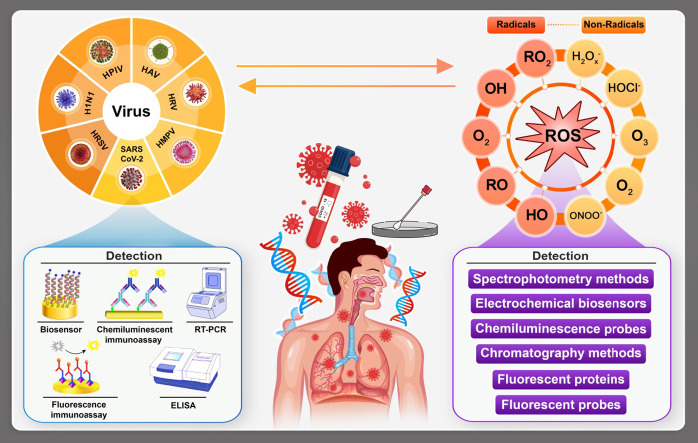
Abstract
COVID-19 disease has put life of people in stress worldwide from many aspects. Since the virus has mutated in absolutely short period of time the challenge to find a suitable vaccine has become harder. Infection to COVID-19, especially at severe life threatening states is highly dependent on the strength of the host immune system. This system is partially dependent on the balance between oxidative stress and antioxidant. Besides, this virus still has unknown mechanism of action companied by a probable commune period. From another hand, some reactive oxygen species (ROS) levels can be helpful on the state determination of the disease. Thus it could be possible to use modern bioanalytical techniques for their detection and determination, which could indicate the disease state at the golden time window since they have the potential to show whether specific DNA, RNA, enzymes and proteins are affected. This also could be used as a preclude study or a reliable pathway to define the best optimized time of cure beside effective medical actions. Herein, some ROS and their relation with SARS-CoV-2 virus have been considered. In addition, modern bioelectroanalytical techniques on this approach from quantitative and qualitative points of view have been reviewed.
Keywords: COVID-19, Oxidative stress, Modern bioelectroanalytical techniques
Graphical abstract
1. Introduction
Nowadays, human and animals are believed to be infected to respiratory viruses, which comprises influenza virus (IV), human respiratory syncytial virus (HRSV), human rhinovirus (HRV), human metapneumovirus (HMPV), vesicular Stomatitis Virus (VSV) and parainfluenza [1], [2], [3]. In this classification, coronaviruses (CoVs), single-stranded RNA viruses, can cause diseases from mild to moderate upper respiratory tract state. Based on historical records, at least three novel human-pathogenic CoV; severe acute respiratory syndrome coronavirus (SARS-CoV), Middle East respiratory syndrome coronavirus (MERS-CoV) and the most recently emerged severe acute respiratory syndrome disease, Coronavirus 2019, have crossed species barriers to cause major epidemics in the past two decades [4], [5].
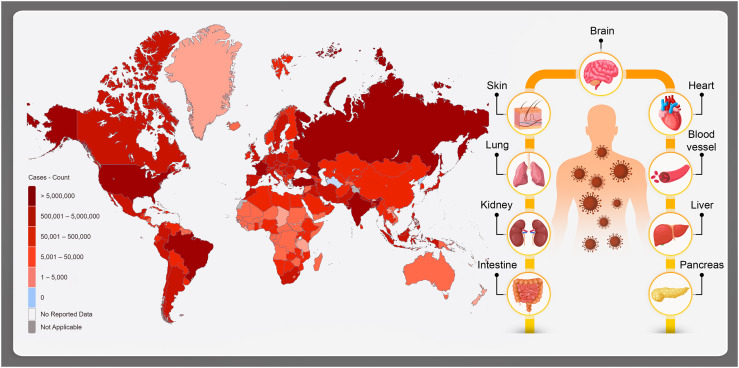
The last type, COVID-19, has lead the world to an outbreak pandemic (Fig. 1 ). This type of CoV source of infection is probably wild animals such as rhinolophus sinicus [6].
Fig. 1.
General map view of COVID-19 infection worldwide (181,521,067 confirmed cases, including 3,937,437 deaths, reported to WHO), and the affected organs; brain, central nervous system, lungs, heart, kidney, liver, pancreas, intestine, skin and the blood vessels.
In the points of diagnosis and also disease state evaluation, imaging has played a very important role. However, the acute diagnosis process is not practical since it takes some days for SARS-CoV-2 to cause obvious cytopathic effects in selected organs (Fig. 1). The organs could include human cells that contain receptor called angiotensin converting enzyme 2 (ACE2) and VeroE6 cells [7], [8]. In fact, if the immune system fails to provide the appropriate response, the virus will propagate and tissues would be endangered by high risk of massive destruction, especially in organs that have high ACE2 expression such as intestine and kidney. In the case of COVID-19, the damaged cells bring natural inflammation in the lungs, which are largely mediated by proinflammatory macrophages and granulocytes. Under this condition, isolation of the virus requires facilities that are not available in most healthcare institutions. Form another aspect, the general health and strength of the immune system of the host toward the virus has significant importance in the infection process. Basically, the immune system response affected by SARS-CoV-2 infection has two phases. The first one is a kind of specific adaptive immune response that would be required to eliminate the virus and to block disease progression. The next one is considering various strategies to boost the immune responses (anti-sera or pegylated IFNα) that is certainly determinative and important [9]. However, for patients who have reached the severe state of the infection, good immune system might not be advantageous and in this case; lung inflammation must be suppressed by medical cures [10]. At that stages, lung inflammation would be the main life-threatening agent in specifically in respiratory disorders [11], [12].
To prevent or reduce the risk of such bad state for the patients, an early detection and screening method for the virus has been used. However, serum antibody and antigen detection tests also have not been easily accessible or validated and there may be cross reactivity with SARS-CoV, which shares a high degree (~82%) of nucleotide identity with COVID- 19. On the other hand, time is a golden factor for the both patient and medical staff since it can effectively help on isolating and treating the infected cases at the right stage in order to reduce mortality risk besides the public contamination [13], [14], [15]. Despite of being time consuming (0.15–6 h for each test), it is being used as the most reliable method for COVID-19 detection in the clinical laboratories worldwide [16]. Also, finding the most appropriate medical treatment has really worth of investigation until the best final cure is founded. In another point of view, most of the respiratory based viral COVID-19 infections can be in strong harmony with inflammation, cytokine production, cell death, and other pathophysiological processes caused by oxidative stress (OS) [17], [18], [19].
Oxidative stress (OS) is induced as an improper distribution between production of reactive oxygen species (ROS) and antioxidant defenses [20], [21], [22]. This simple definition can be counted as a serious life-threating factor since overproduction of ROS and antioxidant mechanisms deprivation are crucial for viral replication and the subsequent virus-associated disease so as COVID-19 [23], [24]. Such stress can occur or be controlled by many agents: the genetic background, biologic enzyme processes, lifestyle and all related issues. Most scientists define OS as vast region of reactions and interactions between ROS and other highly reactive species including free radicals [25]. Mainly, the process consists from chemical chain reactions involving highly reactive ROS species that can be not essentially free radicals. What matters most is that the chain reaction propagation can lead to production of species with higher degree of reactivity that harden the chain reaction to be controlled or stopped and this has the potential to conduct the vast range of diseases from direct irreversible damage of biological tissues to various types of cancers or cardiovascular diseases [26], [27]. Due to close connection between respiratory viral infections and ROS generation and propagation, defining the reactive species, their chemical features and properties can trigger research interest severely. On this pathway, many universal benefits such as cure finding for COVID-19 could be achieved.
In the present review, comprehensive look has been taken on COVID-19 disease and its various possible relations with OS and ROS level in the host body. However, it is hard to determine ROS directly because of their very short time of life and high reactivity. Therefore, using modern bioelectroanalytical methods for detection of ROS from quantitative and qualitative points of view have been considered, compared and evaluated as well. Furthermore, it is aimed to select and discuss only some of the most important and effective bioanalytical/electrochemical methods for detection of COVID-19 and ROS determination.
2. Oxidative stress concept
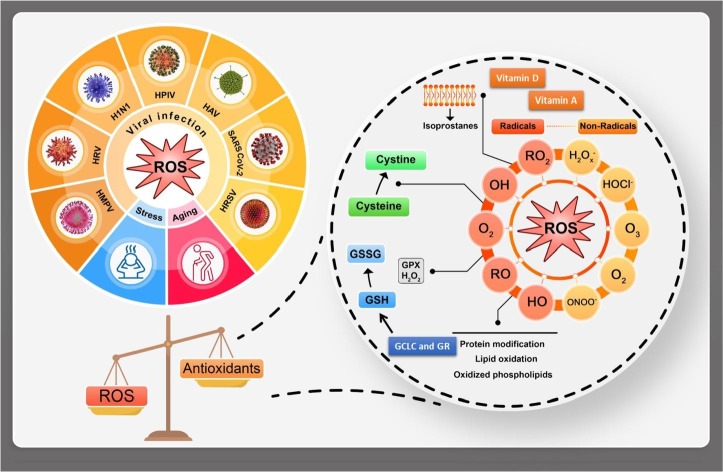
The imbalance between ROS and antioxidant system defines OS (see Fig. 2 ). As mentioned above, it is one of the key factors in the generation and development of many kinds of diseases especially the stress or age-related types: dementia, metabolic syndromes, arthritis, diabetes, vascular diseases, cancer, atherosclerosis, obesity, osteoporosis and virus based diseases [28], [29]. That is important to mention when the ROS/antioxidant balanced, the defense mechanisms are established [22]. Even though ROS could be generated through the biological system inevitably, at the balance state they would have essential roles to modulate cellular activities: cell survival, stressor responses, and inflammations. From another point of view, in the modern life, OS is believed to be generated dependent to many external factors such as various food diet, various kinds of pollutions, stress, level of exercise, climate, pesticides, drugs, smoking and other internal parameters like the age, genetic sequences and disorders. Thus, this concept is known to be controlled strongly through many different effective ways by choosing healthy organic soft lifestyle [30], [31], [32].
Fig. 2.
The viral infection e.g. COVID-19, stress and aging could be happened through imbalance between ROS/antioxidant resulting in OS; the main ROS tracking indicators could be glutamate-cysteine ligase catalytic (GCLC), glutathione reductase (GR), glutathione peroxidases (GPX), oxidized glutathione (GSSG), reduced glutathione (GSH), cysteine and vitamins such as vitamin D and vitamin A.
As mentioned, aging as one of the effective parameters on OS is known to be a universal, multifactorial, intrinsic and progressive process in all kinds of living organisms. Through this process, progressive loss of organ functions could happen, thus the mortality rate would increase as the time passes [33], [34], [35]. Also, even regardless to the age, diseases caused by viruses could target organs that highly consume oxygen with limited respirations like heart or brain that are mainly vulnerable to imbalance (Fig. 2). That is why the high prevalence of cardiovascular diseases (CVDs) and neurological disorders are recorded in the elderly [36], [37].
The free radical theory of aging is the most common accepted definition among the existed theories that defines aging as consequence of several defensive mechanisms that have failed of making the proper response to the induced damage caused by high concentration of ROS species mainly at the mitochondria [38]. All of the stated variations have the potential to be leaded to energy imbalance and mitochondrial failure in the routine functions or tissue damage. Moreover, these perturbations in the cases of being impaired with cellular homeostasis and mitochondrial dysfunction, the risk of being vulnerable to OS would be enhanced as well [39]. Although the correlation between OS markers and the severity level of many viral diseases is observed and accepted for COVID-19, infected cases are required, while clinical data are limited [40], [41]. However, under preclude of COVID-19 condition, lots of evidences indicate that the infection process could be strongly affected by enhancement of the concentration of ROS and reduction antioxidant concentration, so as the progression and level of severity the disease [42]. In one record, experimental set of data collected from animal models of severe acute respiratory SARS-CoV syndrome has shown mutations in ROS levels so as disturbance of antioxidant defense during infection. Some researchers believe that the severe lung injury relies on activation of OS chain reactions coupled with innate immunity and activation of transcription factors; NF-κB, that could lead to exacerbated prionflammatory host response [43].
Age is one of the basic determinative factors in the severity and mortality risk and progress of COVID-19 disease [44]. As current statistics shows, the mean fatality ratio is less than 0.2% for under aged 60 and 9.3% for elderly (over 80). Beside the factor of age, the fatality risk would be magnified at least five times in the cases of comorbidities: diabetes, obesity, and hypertension [35]. Also, it should be considered that aging itself can include imbalance between antioxidant mechanisms and OS. Experimental data have shown that lung lesions occurred more severely in the aged mice than in young-adult ones [45], [46]. In addition, based on the reported data, transcription profile confirmed existence of stronger proinflammatory environment in the aged cases compared with the young ones. Thus, it can be concluded that a very serious disturbance in the redox concentration balance can happen resulted by both of the age related accumulated oxidative injury that can drastically enhance ROS concentration and reducing antioxidant concentration (which are used in the defense system) [47], [48]. As a consequence, despite ROS generation not being vital for aging, but they are more likely to make the age related diseases worse progression through oxidative impairment and its interaction with mitochondria [49]. As a solution, natural compounds can reduce OS and ROS concentration and improve immune functions based on research records [50].
2.1. Viruses-induced diseases and OS
The virus based infections can have the role of start key for ROS production and its concentration inside a living cell undoubtedly would cause irreversible cellular and tissues injuries. The report in details about the possible interaction between viral infection and ROS was presented in 1994 [50]. It was shown that viruses generally use RNA as their genetic material and use DNA intermediates in their replication OS cycle that are known as retroviruses. These viruses have the highest mutation rates among virus based diseases [51]. Consequently, it seems like that the development of effective vaccines or cures against such viruses, in particular COVID-19, are not always easily straight forward. During the infection, the virus is usually detected, surrounded and phagocytosed by macrophages, neutrophils and dendritic cells as inflammatory defense system [52]. From another point of view, OS plays a leading pathogenic role in the virus infections such as COVID-19. In fact, viruses can initiate ROS generation in various ways, but they mostly share a common pathogenic pathway highlighting the important point of ROS production and weaken antioxidant system [53]. More exactly, OS coupled with redox homeostasis has key roles in biological processes that may account for enhancement of susceptibility to various infection agents [17]. Furthermore, growing evidence supports the idea of association of OS and inflammation caused by ROS production at high concentrations. The impressibility of cellular functions inside a cell is highly connected to the ROS concentrations. ROS can act both as friend and enemy here. The opponent role initiates from the point in which ROS accumulation surpasses the oxide removal followed by the imbalance between the oxidation and antioxidant defense system (Fig. 2). This could lead to the neutrophils aggregation, the protease secretion enhancement and oxidation intermediate growth, which certainly can cause inflammation, biological tissue damage, and cell apoptosis [54], [55]. More precisely, as byproducts of mitochondrial metabolism, ROS would be produced not only in the forms of free radicals, but also in a large variety range of compounds containing oxygen that are highly reactive molecules not necessarily free species. However, on the other hand, high concentration of ROS can put the infected cells in a chronic non-acute OS state that would accelerate the diseases spreading in body. Also, reported that a virus could induce OS by increasing the level of ROS [56], [57]. It is described that infection of mouse splenocytes with Sendai virus caused enhancement in chemiluminescence levels due to luminol oxidized by ROS. This result showed that the conformational of the viral structure is important [57], [58].
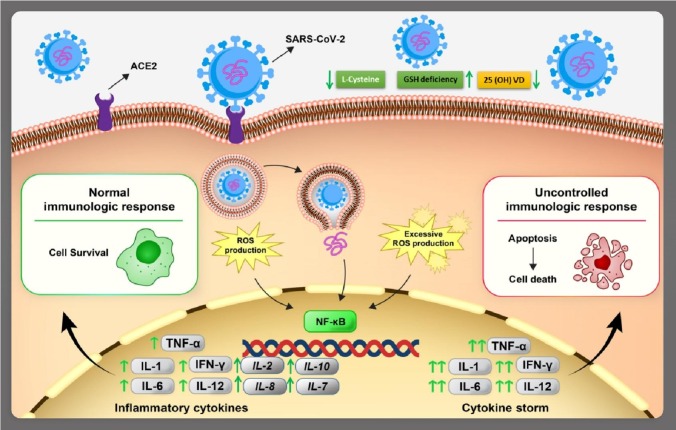
It is also known that the expression of the NADPH oxidase complex and nitric oxide synthase could be activated by the pathogens in phagocytic cells as well, which would result into mutation in ROS production [59]. The OS generated by viral infections can be shown in several pathogenesis aspects: cell death or inflammatory responses. Consequently, this variation in the redox state of the host cell could be the main factor for viral mutations and activate transcription factors: nuclear factor kappa B (NF-kB), which enhances the viral replication itself [60]. Virus infection is known to be responsible for the robust production of cytokine: tumor necrosis factors (TNFs), interferons (IFNs) and Interleukin (ILs) [61]. As indicated in Fig. 3 , ROS concentration at regulated levels helps the immune system function, but at high levels can create pathological inflammatory response [62]. This activation step is connected with OS due to ROS release followed by activated phagocytes that would produce pro-oxidant cytokines; NF and (IL-1) promote iron. At normal stages, ROS can help protection mechanisms for the host cell, which could suppress the apoptosis initiation.
Fig. 3.
The schematic presentation of SARS-CoV-2 connection to angiotensin converting enzyme 2 (ACE2) receptor and inflammation cytokines formation; the cell infection enhances the definition of NFĸB transcription factors that leads to inflammation cytokines production; interleukin IL-1, IL-2, IL-6, IL-7, IL-8, IL-10, IL-12 and interferon (IFN-γ); the balance and imbalance in ROS formation and control causes the normal or severe responses, the proportional ROS production leads to inflammation cytokines production thus the virus could be controlled but the excessive ROS generation could make cytokine storm and the cell finally destroys from apoptosis; moreover reduced L-cysteine as of infection sign would decrease GSH and consequently 25(OH)VD for cellular use.
Up to 1–3% of the pulmonary intake of oxygen by humans is converted into ROS. As of an amazing point, in the cases of which the metabolism is normal, the continuous production of ROS and highly reactive materials have essential roles for regular physiological functions such as ATP generation [42]. As mentioned above, the problem is when the general health system of the body is involved by disease(s). Since under such conditions the balance between oxidation and anti-oxidation would be probably impaired and the whole circumstances would be lifted toward OS and many further consequences would become possible to be happened. ROS could be produced by various biological tissues in the body. They have the ability to level up non-enzymatic and enzymatic antioxidants within the cell direct or indirectly [28]. This turbulence between ROS and antioxidant distributions can cause serious cell damages and endanger health conditions.
To have more detailed view, mitochondria, peroxisomes, various types of oxidases, ascorbic acid oxidation and cytochrome as well as ultraviolet/ionizing radiations, chemotherapeutics, stress, and environmental toxins can be named as sources of production of endogenous ROS [63]. Protective antioxidants enzymes can effectively decompose ROS at their mild states however; the condition can change to toxic levels if the production rate surpasses much more than the antioxidant system capacity [64], [65]. As an example, endogenous and exogenous ROS could affect lens crystallins as of a biological target. On this way, elevated level of OS results in oxidation of Cys/Met residues causes imbalances in the redox state of eye lens. Formation of high molecular weight totals, protein cross-linking by disulfide and non-disulfide bonds and protein insolubilization could be named as the examples [66], [67], [68]. Among all types ROS, species like H2O2, superoxide anion and nitrite radical species are the major factors as chain reaction initiators under many various conditions that are going to be defined and discussed in further subsections.
As mentioned, some records spoke of many retroviruses in which high concentration of ROS caused cell death using DNA and RNA of the viruses [69]. ROS may accelerate the viral replication, which relies on the cell and the virus type involved. At high concentrations of ROS, specifically containing derivatives of superoxide, powerful agents could be made with magnitude potential of lung injury caused by COVID-19 [70], [71]. Prominently, after breaking the host defense system, COVID-19 disease seems to dysfunction the host cell to benefit its replication rate. It can be concluded that the stated imbalance of ROS concentration could be considered as the basis of COVID-19 disease (or vice versa) and its known and unknown consequences. Furthermore, ROS can upsurge the host epithelial cells susceptibility to COVID-19 besides ROS generation promotion could lead to cell-to-cell viral transmission and spread (this could be used for early detection). In addition, COVID-19 invasion may cause OS itself that can make inflammatory injury. Especially, COVID-19 severe infections are often attended by a dysregulation and/or extremely exaggerated immune response introduced as cytokine storm [72], [73]. As mentioned, the most of cases infected by COVID-19 have asymptomatic, mild, or moderate disease and averagely 20% of patients reach the severe critical state. This fact has lightened up serious stress in this vast group of people that makes them more sensitive to environmental stress factors as well as other infectious agents compared with people in other age groups. This connection is mostly companied by weaken antioxidant defense system exposed to the pathogenesis of chronic diseases: diabetes, cardiovascular and respiratory diseases, which they all are now known to enhance the risk of infection to COVID-19 at severe states and death [74], [75]. In another words, COVID-19 especially at severe steps, when lung tissues are involved could have deeper connection with OS since oxygen molecules are vital for keeping ROS and suspending the hypoxia caused by COVID-19. As known, OS not only reduces the free oxygen molecules levels, but also it could form superoxide radicals through ventilation; xanthine oxidase affecting hypoxanthine. Also, in the cases of unappropriated blood circulation and low oxygen in blood, which is known as hypoxia, lipid peroxidation and oxidative damages could occur that have high potential to damage or dysfunction the lung tissues [17], [76], [77]. Moreover, it is essential to group the patients properly base on the disease stage at the time of hospitalization to decide on the most accurate and effective therapy. Regarding to the need, finding reliable, reproducible and selective biomarkers for COVID-19 followed by sensitive determination methods would be crucial to have [78].
2.1.1. COVID-19 general biomarkers
At this stage, particularly for COVID-19, many factors could have the role of biomarkers for disease detection and disease stage determination as well. However, among them the most reliable and reproducible ones that could be more selectively related to COVID-19 would be discussed [79]. Regarding to the published records for COVID-19 patients these indicators could be classified as of homological (lymphocytes counting, neutrophil counting and neutrophil/lymphocyte ratio (NLR)), inflammatory (C-reactive protein (CRP), erythrocyte sedimentation ratio and procalcitonin), immunological (interleukins (ILs)) and biochemical (D-dimer, troponin, keratin kinase and aspartate aminotransferase) biomarkers. In general point of view, there are homological biomarkers for severely infected COVID-19 cases that have lower levels compared to mild infect one [80], [81]. As the records show lymphocytes T and NLR can even precisely determine the hospital fatal rate. Among the stated biomarkers for COVID-19, NLR, platelets counting test, ILs (especially IL-6) and erythrocyte sedimentation rate (ESR) could be considered as reliable biomarkers [82]. It is shown that NLR enhances as the result of scattering of inflammatory cytokines and consequently related genes would be evolved in lymphocytes death, which is the exact mechanism of infection to SARS-Co-V2 [83]. In addition, platelet counting is an available, simple and cheap test for COVID-19 patients, which can independently determine the disease intensity as well its death ratio. Moreover, in more severe cases, lymphocyte and monocyte decrease besides neutrophil and NLR increase and this could be used as complementary data for COVID-19 stage determination [84]. In addition, it is shown that IL-6 increased levels are result of cytokine storm that seriously endangers the lung tissue. This biomarker could effectively conduct the therapy pathway in some cases using anti-IL-6 at the proper golden time. Most of the biomarkers are not reliable individually, while ESR is a sensitive marker that could be used for early prognosis of COVID-19 independently [85]. Regarding to the current records, reliability of other biomarkers for COVID-19 should be examined on vaster statistic societies [86].
2.1.2. OS-COVID-19 joint biomarkers
Fortunately, OS has some biomarkers that could be effectively used for COVID-19 prognosis and monitoring. These biomarkers could be divided into antioxidants (vitamin antioxidants; vitamin A, vitamin C, vitamin D and vitamin E), enzyme antioxidants (endogenous glutathione (GSH), glutathione peroxidase (GPx), superoxide dismutase (SOD)), free elements (Mn, Zn, Cu and chromium) in the roles of antioxidants and lipid oxidative damages [87], [88]. It also should be noted that in COVID-19 disease, the induced virus changes the state of the host antioxidant defense system. Tracking this flow can be used to prevent the progression of the illness. Generally, in COVID-19 patients levels of vitamin C, GSH, PSH, γ-tocopherol and β-carotene is much lower than non-infected cases, which strongly could affect the hospitalization duration. The good point is that not only total antioxidants capacity but also every single of these biomarkers could be quantitatively measured using electroanalytical techniques even in biological matrices based on the reports [89], [90].
Vitamin C in the therapy restrains the cytokine storm and also reduces the immune system suppression. This vitamin level in blood serum can also reduce IL-6 as one of the recovery signs from COVID-19. This vitamin imbalance leads to endogenous glutathione deficiency (which is crucial of ROS scavenging) and it could be leaded to decrease in protein thiolization which enhances the risk of lipid peroxidation as well [91], [92]. This agent could act as the main contributor to the pathogenesis of various diseases including COVID-19 via chemical and biological mechanisms that can involve OS and inflammation as well. Furthermore, N-Acetyl Cysteine (NAC) as GSH precursor could be effectively used since it can enhance level of GSH, amplify T cells and moderate the inflammation through some biological mechanisms. The same pathway could be considered for paroxysmal sympathetic hyperactivity (PSH) as well. It should be also noted that in severely infected cases levels of GPx and SOD are lower than the normal level as well as GSH [93], [94]. On this approach, this protecting system response against the initiated OS triggers much interest in the concept of understanding the mechanisms explaining the nonspecific sensitivity or resistance to COVID-19 infections. The OS level related to the patient sex is one other factor effective on the mortality caused by COVID-19 universally. This fact is consistent on the sexual dimorphism, which is highly possible to be effective on the susceptibility to COVID-19 at severe levels. On this basis, due to OS level (as well as the level of ROS), men considerably suffer more severely from consequences of COVID-19 infection and compared with women have shown higher mortality rates. In addition, men are known to have lower plasma levels of GSH than women and that makes them more vulnerable to OS and other related inflammations [95]. Besides of all stated factors at different levels of ROS, dietary factors may also contribute to endogenous glutathione deficiency in cases COVID-19 illness at sever states [96]. Specifically, lack of fresh vegetables and fruits as natural sources of glutathione in the daily diet, seems to have essential role as another risk factor for glutathione deficiency in infected cases severely by COVID-19 illness. Thus, as discussed above, the association between the risk factors and serious manifestations and death in COVID-19 cases could highly be attributed to serious obstacles in the antioxidant defense system such as the common stated cause; GSH deficiency.
Another crucial determinative biomarker both for COVID-19 and OS is vitamin D, which is in deep relation with GSH. Glutamate cysteine ligase (GCLC) and glutamine reductase (GR) are believed to adjust and moderate the biosynthesis of GSH [97], [98]. Based on the reports, vitamin D itself has the ability to adjust GCLC and GR. GSH deficiency enhances OS and increases the risk of protein carbonylation in large scale which in turn worsens the condition. In such situation, L-cysteine (LC) has a direct effect on modification of protein expression that leads to protection of the proteins from OS destructive consequences [99], [100]. In the cases of OS enhancement, blood sugar would increase and LC consumption would be augmented and consequently the risk for other infections whether severe COVID-19 infections would occur [101], [102]. From another hand, vitamin D deficiency is a vital factor for some chronic diseases such as insulin resistance, obesity, diabetes and etc. As OS levels up in such cases, GSH comes down as well as the antioxidant defense system get weakened and this cycle goes on and the negative point is that in such cases if infection to COVID-19 happens, it could lead to tragedy [100]. Regarding to the related research, losing GSH and high levels of OS could change vitamin D genes and vitamin D receptors, thus the usable form of vitamin D for cells would be decreased. This stated chain cycle that includes roles of LC and GSH could be effectively adjusted taking proper doses of vitamin D at the appropriate time [103], [104]. This modification has the potential to inhibit a wide range of diseases such as renal inflammations, myogenic disorders, chronic diseases and particularly COVID-19. In addition, recent researches support the role of free elements such as Cu, Zn and Se, as antioxidants (scavengers) since they can cut the chain reactions of lipid peroxidation in companion of GSH through Fenton cycle or boosting the antioxidant system through their selective interactions [105]. These free elements are usually used in the therapy of COVID-19 patients especially at mild stages of infections. It is proved that they have the ability to recover the patients sooner since they can boost the immune system particularly within the golden time window and prevent the disease to get intensified [106]. Furthermore, since these elements ratio (Cu/Zn) is in direct connection with CRP level and indirect connection with lipid peroxidation as well, taking appropriate doses of them could effectively help the health system to overcome the disease and recover sooner.
3. COVID-19 diagnosis and point of care testing
In fact, COVID-19 pandemic has shown its major impact on clinical laboratories time of response duration, which is challenging task for the laboratory diagnosis of COVID-19 infections. Due to crucial importance of COVID-19 detection with adequate level of reliability and safety and also to be able to control the chain of infection, it is essential to have the required laboratory tools for COVID-19 diagnosis, identification, contact tracing, source of infection finding and rationalization. Therefore, in the analytical point of view, collection of suitable respiratory tract specimens at the right time from the right source could be very indispensable for obtaining an accurate molecular COVID-19 detection. Also, since the recorded symptoms from COVID-19 patients are neither specific none constant, thus they cannot be used for an accurate defined diagnosis protocol. For instance, in Guan et al. work, less than half of the infected population had fever at the time of entrance to the hospital, while 89% developed fever during the hospitalization [107], [108]. Moreover, patients with other symptoms including shortness of breath, fatigue, cough and sputum production were observed in the same population case [109]. The point is that many of the stated symptoms could be appeared as the result of other respiratory infections not only as of COVID-19 and this issue makes the diagnosis step harder and more complicated. Thus, in so many cases only nucleic acid testing and computed tomography (CT) scans have been reported as the most reliable techniques for diagnosing and monitoring of COVID-19 [8].
Because of cytopathic effects in selected cell lines (VeroE6 cells) and also being time consuming at this time, the viral culture for COVID-19 diagnosis with high precision could not be applicable. Furthermore, virus isolation at the desired biosafety level demands its own facilities that are not accessible in most healthcare institutions especially in developing countries [110]. Serum antibody and antigen detection tests are now being validated while there could be cross reactivity with some other infection agents at high degree of similarities (~82%) that makes it hard to identify the virus individually. According to these current limitations, reverse transcription-polymerase chain reaction (RT-PCR) is being used as a complementary technique with high level of certainty beside swab and/or antibody test for laboratory COVID-19 diagnosis worldwide [9], [111].
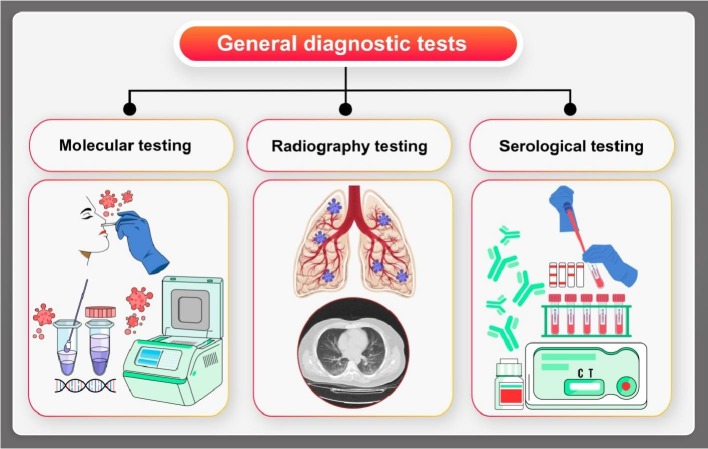
As shown in Fig. 4 , there are three general applied protocols for COVID-19 diagnosis. One of the standardized laboratory/clinical protocols for COVID-19 is now performed on the basis of the accessibility of the complete genome of the virus [112], which includes real-time RT-PCR assays, which uses the RNA-dependent RNA polymerase (RdRp), envelope, and nucleocapsid genes of COVID-19. Between these classifications, the RdRp assay presented the maximum analytical sensitivity, copies of RNA with reactivity that makes the detection process work correctly with high probability (95%), in which the first probe was a “pan Sarbeco-Probe” which would detect COVID 19, SARS-CoV, bat-SARS-related coronaviruses [13], [18], [69], [113]. These successful reported assays had been employed in more than thirty laboratories in Europe. In another study, a novel, highly sensitive and specific real-time RT-PCR assays for COVID-19 was designed. The data collection of the work for both synthetic samples and clinical specimens from patients with laboratory-confirmed COVID-19 established that the novel assay that used a different region of 109 the RdRp/Hel as the target had meaningfully more sensitivity and specificity than the reported RdRp-P2 types of assays [110]. However, with help of electrochemical methods, the speed of this testing process has been significantly increased for using in Point Of Care (POC) devices, which would allow testing to be carried out at home [114]. Pathogens in molecular based techniques, thus they are more appropriate than clinical assays such as CT and syndromic testing to have a precise diagnosis process [115]. Two factors can be effectively used for development of a molecular based technique: the genomic and proteomic composition of the pathogen as the monitored target and the occurred variants in the proteins/genes definitions in the host during compared to after infection. The stated compositions for SARS-CoV-2 virus have been freshly recognized but since the host response is not still clear, it is currently under study. In addition, metagenomic RNA sequencing as a high throughput method for multiple genomes sequencing was the first used technique for SARS-CoV-2 sequencing. The findings have been collected in to the GenBank sequence repository [116], [117]. Now there are many added sequences that are accessible on this and other relative data bases for researchers globally. Moreover, Table 1 shows some general applied methods for COVID-19 detection in susceptible patients.
Fig. 4.
Schematic presentation of COVID-19 diagnostic methods using nasopharynx and serum samples demonstrating the clinical procedure; including molecular testing based on RT-PCR, radiography (CT scan and MRI) and serological testing (see Table 1).
Table 1.
Some successful applied methods for COVID-19 diagnosis.
| Applied detection method | Ref. |
|---|---|
| Dual-function plasmonic photothermal biosensor | [93] |
| Field effect transistor based biosensor | [94] |
| Lateral flow immunoassay | [95] |
| Dynamic variance of Antibody in the blood serum | [96] |
| Reverse transcription polymerase chain reaction | [97] |
| Reverse Transcription Loop-Mediated Isothermal Amplification | [98] |
| Nucleic acid detection | [99], [100] |
| Clustered regularly interspaced short palindromic repeats | [101], [102] |
| Electrochemical immunosensor | [103] |
| Real time qRT-PCR; Antibody based | [104] |
| Capture enzyme-linked immunosorbent assay | [105] |
| Loop mediated isothermal amplification | [106] |
| rRT-PCR; First-line screening tool: E gene assay; Confirmatory testing: RdRp gene assay | [107] |
| Genome subtraction | [87] |
| Nucleoprotein antigen detection test | [108] |
| Enzyme-linked immunosorbent assay | [108] |
| Chemiluminescence immunoassay | [108] |
| Nested Reverse transcription polymerase chain reaction | [109] |
Point-of-care (POC) tests are employed for patient diagnosis without the need of complex facilities, thus qualifying societies deprived of laboratory infrastructures would be employed to detect the infected cases as well. On this approach, lateral flow of SARS-CoV-2 antigen detection is one POC pathways under progress and growth for COVID-19 diagnosis [110], [111], [112]. In the commercial form of the assays, a membrane test strip is coated with two lines; one for gold nanoparticle-antibody conjugates, which are responsible for capturing the antibodies in the other line. The patient's sample that could be blood, urine or other biological fluid that would be casted on the membrane and capillary action would conduct the proteins across the strip. As the first line is covered by the fluid, the gold nanoparticle-antibody conjugates bind to the antigens and the complicated streams together over the membrane. Reaching to the second line would be synchronizing with immobilization of the complex that captures the antibodies (if existed) and a red or blue line would show up [113]. It should be noted that individual gold nanoparticles present red color but this color of solution would change to blue in the presence of clustered gold nanoparticles in the same solution. This lateral flow assay has shown accepted clinical accuracy, sensitivity and specificity. Nucleic acid testing can also be combined into the lateral flow assay in order to have more sensitivity and accuracy. Since previously, a RT-LAMP test was successfully coupled with this assay for MERS-CoV detection [114]. Besides all the efforts to make the results more reliable, most of these tests are single-use and lack of adequate analytical sensitivity compared to RT-PCR is their main problem. In order to overcome the drawbacks and enhance the signal to noise ratio, wide range of various signal amplifying techniques such as thermal imaging, assembly of multiple gold nanoparticles have been developed. Another solution could be using microfluidic devices point-of-cares. These devices usually have chip (in palm-sized) that has micrometer-sized etched channels and reaction chambers. The chip as the main body is responsible for mixing and/or separating the liquid samples via different ways: vacuums, electrokinetic and capillary effect. These chips can be made out of glass, poly-dimethylsulfoxide, paper or other probable polymer materials. Using microfluidics could provide general rapid detection, small sample volume, miniaturization and portability as advantages [115].
3.1. The nucleic acid testing
Nucleic acid testing is being used as the current initial method for COVID-19 detection. On this approach, PCR involves the reverse transcription of SARS-CoV-2 RNA with the complementary DNA strands and then some specific regions of the complementary would be amplified in order to generate the signal. This general design process consists of two pathways: 1) primer design and sequence alignment and 2) assay parameters optimization and result recording [116]. A number of SARS-related viral genome sequences were analyzed for designing a set of primers and probes. Three regions with preserved sequences were found among the SARS-related viral genomes which were 1) the RdRP gene (RNA dependent RNA polymerase gene) in the open reading frame ORF1ab region, 2) the E gene (envelope protein gene), and 3) the N gene (nucleocapsid protein gene). Based on the obtained data of the RdRP and E genes had high analytical sensitivity, thus they could be effectively used for detection however, and the N gene had shown less analytical sensitivity [117]. To be more exact, the assay has the possibility to be designed as a system, which can detect numerous coronaviruses including SARS-CoV-2 and specifically only COVID-19 simultaneously using two separate primers. The next step was to optimize the parameters such as reagent conditions, incubation times, temperatures and at last and at last PCR testing was done [118]. Also, RT-PCR is being used as the most reliable technique for COVID-19 as a complementary method beside CT scans, but the point is that each of these techniques has their own obstacles. For instance, about RT-PCR; the reagent kits availability is not in agreement with the global demand for them [119], [120], [121]. In addition, there are so many hospitals outside of urban cities, which suffer from absence of the PCR facilities so it would not be possible to handle high sample throughput. Most important of all, RT-PCR detectable SARS-CoV-2 presence in the sample is crucial for the detection step, thus PCR would not identify the surpassed infection and the measurement result is not correct if the case was infected with SARS-CoV-2 but has been recovered [119].
Reverse transcription PCR is known to be carried out whether in one or two-step assay, however in the first form, the amplification steps are compressed into one reaction for having quick and reproducible results for high-throughput analysis. About this type of method, the main challenge refers to the parameters optimizing section as well as the amplification step since they should be done at the same time. However, in the two-step type of the assay, separate tubes are responsible for the reaction sequentially. In some points of view, this assay type can be considered more sensitive than the first one, while being more time-consuming due to having additional parameters to be optimized [122].
3.2. The enzyme based assays and protein testing
While RT-PCR is a time consuming complex detection method, the human antibody response could be effectively used to recognize virus infection at initial steps and it has been globally used [123]. This detection assay has its unique advantages such as being quicker, cost effective, user friendly and it is also more available and does not require expert operators as well [68], [124]. Moreover, dynamic characteristics and magnitude of antibody response in cases infected by COVID-19 have been studied. The recorded data have been validated via serodiagnostic value of ELISA-based IgM, IgG tests for COVID-19 detection results. The sensitivity and specificity of antibody tests for detection of IgM and IgG were recoded and the clinical application of the assays showed adequate satisfactory for serodiagnosis of COVID-19 [125], [126]. In another research reported IgG and IgM from human serum of COVID-19 infected cases via the classic method of enzyme-linked immunosorbent assay (ELISA) [127]. For this process, SARS-CoV-2 Rp3 nucleocapsid protein was used, which has high similarity of amino acid sequence to other SARS-related viruses (90%). The recombinant proteins were adsorbed onto the surface of the plates, while other excess proteins were washed away. In another record, the IgM test had a similar result, while using of an anti-human IgM adsorbed to the plate and an anti-Rp3 nucleocapsid probe. Also, SARS-CoV-2 IgG and IgM antibodies were successfully recognized in some suspected cases [128]. Considering the fresh records, other protein or cellular markers have the potential to be used for detection. Like it was observed that in infected cases C-reactive protein and D-dimer were in high levels, while lymphocytes, leukocytes, and blood platelets were in low levels [129]. But the point is that these biomarkers levels could also be abnormal in other illnesses and that makes the detection process more complex. However, multiplex tests with both biomarkers and antibodies could hopefully modify the specificity. On the other hand, antigens and antibodies as of viral proteins shaped in response to infection to COVID-19 can also be used for this disease diagnosis [127]. In the research of Lung et al. it was found out that after onset of symptoms, high salivary viral loads of the first week tend to decline versus time. This is while the generated antibodies as the response to viral proteins have the ability to prepare more time for SARS-CoV-2 indirect detection [130]. On this pathway, antibody tests can be effectively taken for COVID-19 investigation. However, the point that serological tests may confront cross-reactivity between SARS-CoV-2 and other CoVs antibodies should not be underestimated. It has been shown that examined 15 COVID-19 infected cases plasma samples against S protein of SARS-CoV and SARS-CoV-2 had high degree of cross-reactivity [131], [132]. Regarding to this, serological tests like blood assessments for particular antibodies are still in progress currently.
4. The level of OS and related compounds
Due to high reactivity, short time of life and also being involved in chemical chain reactions, complex antioxidant defense system would be responsible for preventing catastrophic tissue damages [41], [133]. As shown in Fig. 5 , these processes and their related consequences can include many types of diseases direct and/or indirectly. But the main approved potential that can happen with degree of possibility by free radical attack on essential cellular components is lipid peroxidation process [134]. Moreover, numerous disorders in childhood with genetic backgrounds have also been associated with OS as of the pathogenesis and progression of these diseases [135]. Also, oxidative conditions have been proposed to be significant factors for developmental and maturational diseases that could be effectively interpreted in adulthood. Similarly, defined optimum levels of antioxidants in the life diet as well as the vitamins and minerals would be triggering subjects in adults, in the cases of making age groups since childhood of the considered cases [135].
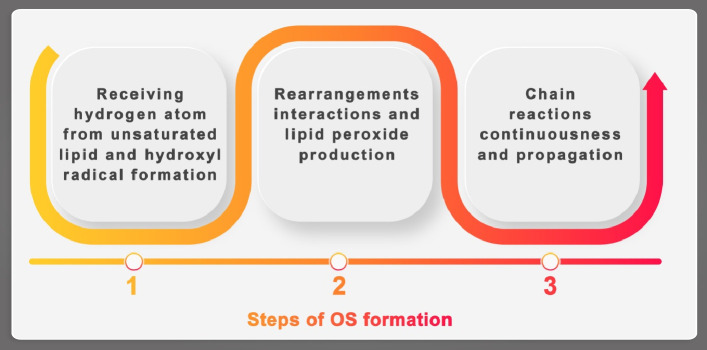
Fig. 5.
The states of initiation (1), continuation lipid and hydroxyl radical, (2) rearrangements and chain reactions propagation, (3) in the case of lipid peroxidation, in which degradation of lipids converts to free radicals resulting in cell damage; the process goes on through free radical chain reactions mechanism involving fatty acids containing several double bonds.
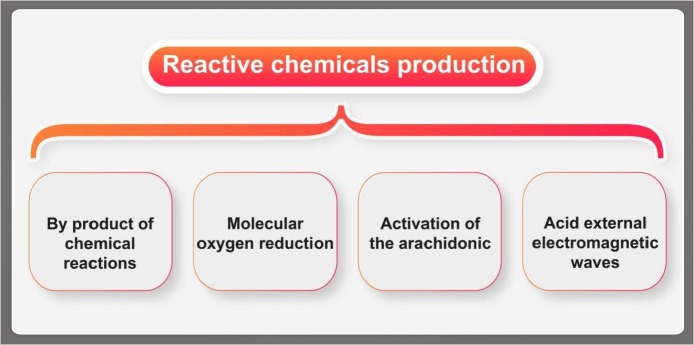
The highly reactive OS species have very short time of life (even less than 1 microsecond). Thus the damage or the start key of the chain reaction would be taken almost instantaneously right at the time of their formation [136]. This fact makes the direct measurement of ROS level a severely challenging task. The proper way to overcome could be considering methods that concentrate mainly on the detection of the consequences of attacks by reactive materials or determination of some related compounds that are formed at their presence with more time of life and stability [137]. For example, there is a close interaction between glycation and OS, which has been used in the etiology of diabetic tissue damage [138]. Also, there are many other reactive chemicals that can be used as indications of specific diseases such as anion (Oz-), nitric oxide (NO), transition metals such as iron and copper or peroxynitrite (ONOO-) (Fig. 2). Chemicals could be produced within a cell or the body (summarized in Fig. 6 ).
Fig. 6.
Some of the main agents for production of OS in cell: 1- by chemical reactions, 2-by molecular oxygen reduction, 3 - by activation of arachidonic acid (e.g. the metabolic precursor of several groups of biologically active) and the most notable initiators in living cells and 4 - via external electromagnetic wave in the environment, which could produce ROS inside of the living cell.
It is known that main targets of OS could be proteins, DNA and unsaturated lipids. On this pathway, DNA can be affected by reactions under effects of ROS including double and/or single DNA breaks, base modifications that can lead to genetic mutations and cell death (Fig. 3) and DNA repair enzymes damages [65], [139]. The lipid tissue of biological membranes is particularly susceptible to oxidation and can be exposed to many chain chemical reactions even multiple peroxidation processes. The occurred damage could be generalized as three major steps (Fig. 5) in the case of lipid peroxidation [140], [141].
4.1. The analytical importance of antioxidants and ROS
Due to this fact that the concentrations of ROS and antioxidants have very important role in viral infections, their measurements have been intensely noticed by researchers. Direct measurements of ROS and other highly reactive species may not be simply possible in the scale of normal experimental conditions, thus attempts have been conducted to use second reactant agents, which are in close association with the reactive unstable compounds. This secondary materials could be simply and directly measured on behalf of the main considered targets [142], [143].
4.1.1. The role of antioxidants concentration
One of the desired materials is the antioxidants, which are in balance with ROS under normal conditions. Antioxidants can be classified from various points of view such as endogenous and exogenous types, which is a common well-known taxonomy. In fact, they are biological molecules that neutralize free radicals and suppress their reactivity in order to break their chain reactions and more ROS production (as well as the tissue damage would be prevented) [144], [145]. Also, regarding to the availability and diversity of natural products present in the environment as well as the eating habits, the use of natural or synthetic supplements is vital to provide the daily doses of such compounds for the general health system [146]. Fresh fruits and vegetables comprise many antioxidants that can strongly help the immune system against many types of diseases such as vitamin C that has the power to suppress chronic and degenerative diseases or vitamin E as the major membrane protectants toward ROS and lipid peroxidation [147], [148]. Moreover, polyphenols which contain one vast group of antioxidants provided by the diet are active agents that prohibit cardiovascular diseases due to their anti-inflammatory and anti-viral activity [149]. Thus, antioxidants trigger intense interest for the researchers in the fields of food industry, biology, drug design and medicine [150].
The balance for ROS and antioxidant concentration is essential to be at adequate states for preventing wide range of the viral infection and diseases including neurodegenerative ones, cancers, cardiovascular types, inflammation and more specifically aging dependent diseases [151]. It should be noted that the nature of the antioxidant as the indicator in such studies is the main factor that determines the experimental conditions as well as the type of the product, which would be generated through the process and could be in direct connection with the target [152]. Generally, OS can be evaluated via oxidative damage reaction products determinations. The monitored reaction could be DNA break or oxidation, lipid peroxidation, and protein oxidation, which are well-known processes. Among stated reactions, lipid peroxidation is the most monitored on by many biological assays: thiobarbituric acid-reactive material assays, conjugated dienes, hydroperoxides, and nitroxides [153], [154], [155]. However, levels of such compounds may quickly change as the function of ROS production propagations, when the balance has been impaired, they have the potential to be appropriate markers of oxidative reactions often in in vitro tests [156]. Analytical structures coupled with classic detection systems such as high pressure liquid chromatography (HPLC), gas chromatography, micellar electrokinetic capillary chromatography and etc. have been frequent techniques for identification and quantification of various antioxidants [157], [158]. Based on the records, modern bio-electroanalytical techniques could be considered as acceptable determination methods due to their unique advantages compared to the classic detections [159]. The point is that considering modern advanced electroanalytical techniques beside the biological assays can provide the researchers too many unique advantages that can enable them for design and fabrication of applicable detection kits for pre-early-detection of even COVID-19.
4.1.2. Advanced electrochemical determination methods
Electroanalytical techniques have been the center of attention for many interdisciplinary researchers since they have excellent remarkable advantages such as, low cost instrumentations, simple application procedures, suitability for real-time detection, notable quickness, high throughput and portability [160]. For bioanalytical measurements, modern electroanalytical methods such as fast Fourier transform (FFT) coupled voltammetric techniques have shown to be appropriate replacements of the classic conventional electrochemical techniques for monitoring the kinetics and determinations of the unstable species including drugs and ROS [161]. Thus, in these cases using stated advantages specifically their short needed time for response providing even direct determination of these targets could be possible [162]. In these techniques, voltammetric and background signals are split in to the frequency domain by a separate FFT, and then the systematic inevitable noise could easily be filtered [163], [164], [165], and all analog filters could be removed from the electrochemical system for reach to a small potentiostat that can have effective role for miniaturizing the systems and their relative applications in medical devices [166], [167]. Also, low concentrations of the analyte in complicated matrix specifically in the cases of biological samples such as blood serum, urine and plasma is one of the most serious challenges in these kinds of research studies [189]. Thus, on the approach, it would be very crucial to design and fabricate efficient sample preconcentration and cleanup systems prior to the detection steps [162]. Among many possible sample preparation techniques, membrane technology is one example of a kind of modern approach and can be effectively exchanged with current classic separation and refinement procedures [161], [169].
These systems for analyzing biological complex samples, are specific, reliable, sensitive and cost effective for chemical and biological warfare agents analyses, drug design and discovery, genomics and proteomics and medical diagnostics such as COVID-19 [170]. In order to cover as many as possible of the probable advantages miniaturization of the employed study set ups is the most notable driving force in the bioanalytical laboratories. Miniaturization could be the effective correct solution for many of the current challenges. Moreover, using miniature set ups would provide possibility of high throughput or the ability of analyzing large numbers of assays. These assays would not only be much quicker than the classic setups, but also would be very cost efficient since much smaller amounts of reagents are used on these purposes [171]. As an example, 25,000 or even more gene sequences could now be analyzed simultaneously using just one DNA microarray chip, so more sequence per cost and time could be adequately analyzed [172]. Regarding to this concept, biosensors have been initially defined as a self-contained integrated device that has the ability to produce specific qualitative, quantitative or semi-quantitative analytical information via biological target interaction/reaction directly or indirectly with the transducer [173]. Besides, biosensors have shown their potential that they can not only detect analytes directly in with no any sample pretreatment steps, but also they can perform the process continuously and/or with reversibility [174]. It is true that there might be very few biosensors that have fulfilled all of stated requirements; however, current century as of the age of miniaturization and the development of micro-kits to cover more and more of these advantages would become practical. Likewise, rapid analyses of thousands of other biologic samples per day could be possible to successfully fabricate detection kits for pre-early detection and state monitoring of the newly unknown hardly curable diseases including COVID-19. Also, beside of having high throughput screening scale; the miniaturization of biosensors would provide multi-analyte parallels assays. So numerous pathogens as biological indicator agents could be accurately detected using one or a cassette of transducers, while the size of the biosensor was kept in the range of 2 cm × 2 cm [175]. For example, if all steps are followed by satisfactory results, COVID-19 disease could be detected quickly by analyzing important determinative pathogens in one assay. In the other words, in the case of COVID-19 detection, all known biological markers in one assay and one blood sample could be analyzed in one step and the positive or negative result could be reached with high accuracy. Moreover, besides having throughput ability, miniaturized biosensors can open up new aspect of portability property, which is again a very valuable characteristic since it could greatly improve the simplicity and process pace [176]. Again, this feature highest importance would be observed in medical diagnosis purposes. Besides it has many other applications in food safety and environmental monitoring for numerous analyses of complicated analytes and sample matrices [177], [178]. The aspect of signal amplification would also be a challenging task since in miniaturized biosensors the signal can be severely weaken due to using small sample volumes and low concentration of the analyte [179]. Many of the currently employed biosensors (in micro/nano dimensions) have failed these criteria. DNA microarrays are forced to use confocal microscopes and CCD cameras for data acquisition. On the same purpose, cantilever based biosensors use atomic force microscope (AFM). Thus, although these biosensing set ups have the potential to successfully improve the molecular performance of micro/nano-bioassays, but they currently cannot be considered as portable, inexpensive and friendly systems to be used.
4.1.3. Optical approaches for determination of ROS related compounds
Biochemical interactions could be also interpreted to suitable output signals by other kinds of biosensors, which work on the basis of optical principles and are called optical biosensors [180]. Due to the occurred biomolecular interactions on the active surface, the transducer light characteristics such as intensity, phase and polarization could be diverse compared with before the interaction, thus on this basis, the target can be detected or monitored through this change regarding to numerous techniques [181].
Optical biosensors have been noticed from analytical point of view on the purpose of biological and chemical species qualification and quantification [182]. In addition, this novel technology can be employed as effective complementary technique besides classic analytical systems due to being simple, cost effective and effectively time saving [183]. Also, this sensing system offers many other advantages; the very low risk of electrical shocks or explosions, higher sensitivity, a wider bandwidth and the immunity to electromagnetic interferences. Moreover, since optical fibers have the potential to be used in these systems to guide light within the device, remote sensing would also be applicable [184]. Interestingly, optical transducers have the ability of parallel detection that provides the array or imaging kind of detection possible.
The optical waveguide has the key role in almost any kind of optical sensor for ROS. On the other hand, their unique properties like flexible geometry, mechanical stability, efficient light-conducting over long distances and noise immunity have made them potential of appropriate candidates in sensor fabrication field. However, about using optical fibers their disability of being designed for specific place or role is somehow a disadvantage since it limits the user to the available market samples. To overcome this problem, planar or channel optical waveguides for sensors are developing even maybe superior than optical fiber based sensors [178]. On all application purposes, having the sensor with fast response time, high sensitivity, which is able to perform real-time experiments, would be essential. Regarding to the intrinsic nature of optical measurements systems, these sensors can cover up great number of different physico-chemical techniques that works on the basis of absorption, emission, polarimetry, fluorescence and refractometry and etc. As of this property, detection of both passive and active optical components could be possible even at the same time on to miniaturized designed sensing devices whether with one or more sensors in one set up. Besides, accuracy, miniaturization, signal amplification, potential of cost effective, low energy usage, and ease in the alignment of the high throughput could be named as other advantages [182]. However, the class of micro-optical biosensors sensing are seriously comparable to complicated analytical laboratory systems and this excellent feature would be more highlighted in medical detection kits fabrication.
The most optical biosensors are an evanescent application at the detection step. This principle permits the direct observing of minor variations in the optical features that are mainly helpful in the straight detection affinity of the target biomolecular interaction. This point should be noted that the indirect detection method is much more sensitive than the direct ones. However, it mostly would need no sample preparation, which is good for real-time evaluations and makes the biomolecule determination, binding specificity and its kinetic properties [185], [186].
4.2. Reactive oxygen species (ROS) in COVID-19 infection
Reactive oxygen species (ROS) is mainly produced by the pulmonary intake of oxygen conversion (up to 1–3%). As of an amazing point, in the cases of which the metabolism is normal, the continuous production of ROS and highly reactive materials have essential roles for regular physiological functions such as ATP generation, several catabolic and anabolic procedures and the complementary cellular redox cycles. Inevitably, body cells are frequently exposed to different endogenous and exogenous highly reactive agents including ROS. These conditions are not considered as a threatening necessarily since ROS can be just possible candidates by products of occurring reactions due to the exposure [42]. Moreover, besides of inflammatory, there are other pathobiology mechanisms such as angiotensin inhibition from conversion (ACE-2), hemoglobin denaturation, intravascular spread thrombus and endothelial function disorder that could help COVID-19 prognosis using OS enhancement.
4.2.1. The role of hydrogen peroxide level
Considering the critical role and also due to its short time of life of hydrogen peroxide, it is considered as the most important reactive oxidant agent among ROS. Its most notable property is that it freely can diffuse across biological membranes. Furthermore, it has been shown that H2O2 is chiefly captivating applicant as both intercellular and intracellular signaling molecule due to the biological membrane permeability toward it [187], [188]. Its concentration is related to OS state and it can be produced and decomposed through enzymatic processes. It also can participate in many other reactions during its short life time; thiol oxidation that is responsible for the formation of sulfonic acid derivatives or disulfides as well as the thermodynamic and kinetic supports for oxidation states of cysteine [148]. To be more specific, H2O2 can easily oxidize cysteine thiol group and produce sulfonic acid that can form a sulfonyl amide with amides or shape a disulfide bond with adjacent thiols by being glutathionylated. Under Fenton theory, in the presence iron or copper as transition metals, H2O2 easily initiate hydroxyl radical (HO∙) production. That product is indiscriminately reactive, toxic and can participate in a wide range of reactions itself [189].
Each of the probable ways can alternate the reactivity degree of the products and determine the most susceptible biologic target(s), which makes the conditions more complicated [190]. For example, phosphatases can be regulated in a biologic cycle by ROS on this pathway, as their catalytic domain having a reactive cysteine moiety that can be reversibly oxidized as the preventer of probable dephosphorylation activity [191]. Moreover, enhanced H2O2 concentration has been considered as the main etiological agent in viral infections (e.g. COVID-19 [168]) including swine flu, rubella, rabies, and others [169], [170], [171] and the most important of all OS-related diseases [133]. Thus, having access to quantitative and qualitative tools are essential for detection and determination of hydrogen peroxide with adequate level of accuracy and precision [192], [193], [194], [195].
The detection of H2O2 can now be done through many analytical techniques and commercial test kits including fluorimetry [196], colorimetry [197], chemiluminescence [198], spectrophotometry [199] methods. The conventional classic techniques have confronted serious weaknesses since they mostly are time-consuming due to having complicated sample preparation procedures, expensive instrumentals and reagents and requiring expert users. However, among stated approaches, electrochemical advanced methods have the potential to prepare more appropriate ways for H2O2 determination. Unique advantages of them could be named as their ease of fabrication, inherent sensitivity, low cost of instrumentation and high selectivity [200]. H2O2 is possible to be reduced/oxidized directly on solid electrode surfaces. In spite of this, H2O2 detection through conventional noble metal electrodes is limited due to the required high existed over voltage values that lead to slow electrode kinetics and biofouling including other coexisting electroactive species that might be interfering with the analytes. In another words, using advanced modern bioanalytical techniques on the purpose of H2O2 detection or determination has also the advantage of using electrodes that have been chemically modified in order to overcome the challenges of such problems [201].
4.2.2. The role of superoxide anion level
The superoxide anion radical (O2 −•) is another highly reactive compound among ROS [202]. It usually is the byproduct of aerobic respiration in the biological systems, which could damage the cell seriously. Besides, it is one of the main factors that accelerate the aging process as well as several deteriorating viral infection (COVID-19) [203] and other illnesses [55], [204]. One of the elements of the enzymatic defense system is superoxide dismutase (SOD), which catalyzes the dismutation of superoxide anions (O2 .-) as the initially defense against ROS. They could reduce O2 −• to neutral oxygen and H2O2, which, as mentioned above, then is converted to water through enzymatic reactions by catalase or glutathione peroxidase. It should be noted that biological systems often use the endogenous antioxidant system as one of the defenses lines against superoxide damaging effects [205]. Such enzymatic processes are needed in the cell to maintain balance ROS/antioxidant ratio. These defense mechanisms are strongly linked with OS and exogenous antioxidants provided by the dietary sources that mainly could be found in fresh fruit and vegetables.
For experimental study, numerous sources can be used for its generation. As of these sources, non-enzymatic options using phenazine methosulphate [206], NADH and molecular oxygen or enzymatic reaction by xanthine dehydrogenase [207] could be named. After the superoxide anion radical is generated, wide range of detection techniques could be used for its detection, such as colorimetric, fluorescence, spectrophotometric and chemiluminescence [208], [209], [210], [211], [212]. As the chemical agent, 5,5-dimethyl-1-pyrroline-N-oxide (DMPO) has the ability to trap superoxide anion and ESR can detect the resultant DMPO-OH adducts [213]. Despite hydrogen peroxide, O2 −• has the potential to be detected (since it has longer time of life). However, most of its detection methods are considered as indirect extents since most of them focus on measuring a product generated by superoxide consumption or production as the criteria of superoxide presence or its concertation [214]. The most notable point about the indirect measurements is that it is better to validate the results with other methods in order to have reliable recorded data. Also, dissolving potassium superoxide can produce superoxide anion in a defined anhydrous solution or strongly basic water environment as it can be affected by protons [215]. Compared with indirect measurements, electrochemical methods could be the most reliable options for superoxide detection because of low instrument cost, simplicity and feasibility for in-situ screening [216]. On this approach, nanomaterials have been used for fabrication of novel interfaces due to significant superoxide dismutase activity and high conductivity [189], [217], [218]. However, their synthesis processes are expensive, complex and time consuming. Thus these are some of current drawbacks, which are essential to be solved.
The easy way to provide superoxide radicals, using a voltaic cell through advanced modern designed bio-electroanalytical technique in combination with cyclic voltammetry. On this pathway, the current intensity would be considered as a function of superoxide anion concentration. This process is again an indirect way of detection since the relevant added antioxidant concentration varies the superoxide concentration [219], [220].
4.2.3. The role of nitrogen reactive species levels
NOx reactive species could trigger intense processes in live cells, since it is the marker of various pathological and physiological cells conditions. But the point is that NOx reactive species quantifications are restricted since they are regularly located in sub-cellular areas at trace or even ultra-trace levels [221]. Reactive nitrogen species (RNS) molecules can be produced in living cells through some specific protein activities or undesirable proceedings under effect of pathological circumstances. Their very short life time besides their extraordinary degree of reactivity makes them significant in the literature records. Moreover, RNS unbalanced levels can have dominant roles in human viral infection or diseases [222], [223]. Therefore, its concentration accurate monitoring is essential. As NOx can affect the oxygen transport system in the body, thus irreversible conversion of hemoglobin to methemoglobin in the blood stream could be occurred and through this disaster the ability of hemoglobin to exchange oxygen would be severely conceded [224]. Also, RNS concentration in the body could be influenced by nitrite and nitrate as other forms of nitrogen reactive species in many other various forms exist in food products as well as the environment. Regarding irreparable side toxic effects and their related consequences, many countries have set severe restrictions on the applied amount of nitrite/nitrate in their food industries. As the major concern of food safety, it would be essential to have exceedingly selective, sensitive, quick, simple and accurate method for determination of the excessive amounts of nitrite, nitrate and their derivatives in the products textures [225]. However, the exact role of nitrogen reactive species on this purpose is to postpone the product decomposition by conquering the propagation of food poisoning microorganisms, remarkably C. botulinum [211]. This threat and its relative consequences could be even more important in the cases of pregnant women and infants [226]. Moreover, in case of viral infection to COVID-19, carcinogenic N-nitrosamines could be formed out of nitrite and its derivatives reactions with secondary amines and amides [227]. Abundant methods have been successfully employed for NOx determination and among them spectrophotometric [228], chemiluminescent [229], chromatographic [230], capillary electrophoresis [231] and spectrofluorimetry [232] can be named as conventional classic ones. Table 2 shows some applied electrochemical methods for NOx determination.
Table 2.
Some employed methods for electrochemical determination of nitrogen reactive species.
| The active surface | The target | Limit of detection | Linear range of response | Ref. |
|---|---|---|---|---|
| Platinum-black coated platinum electrode | Nitrite | 10 nM | 15-200 nM | [233] |
| Platinum-black coated electrode | Nitric oxide | 30 nM | ~1-10 μM | [234] |
| Reduced graphene oxide coated screen printed carbon electrode | Nitrite | 830 nM | 20–100 μM | [235] |
| Fe2O3 nanoparticles decorated reduced graphene oxide nanosheets | Nitrite | 1.5 × 10−8 M | 5.0 × 10−8-7.8 × 10−4 M | [236] |
| Carbon paste electrode/Ru(III) | Nitrite | 1.39 × 10−6 M | 0–1.38 × 10−2 M | [237] |
| Urchin-like palladium nanostructures on carbon nanotube thin film electrode | Nitrite | 0.25 μM | 2-238 μM | [238] |
| Porphyrin-based metal–organic framework thin films | Nitrite | 2.1 μM | 20–800 μM | [239] |
| gold nanoparticles decorated flower-like graphene | Nitrite | 0.01 μM | 20.375 nM-0.125 μM | [240] |
| Cu metal nanoparticles-multiwall carbon nanotubes-reduced graphene oxide | Nitrite Nitrate |
30 nM 40 nM |
0.1-75 μM | [241] |
However, electrochemical based techniques can be proper options since they have the potential to use electrical conductive properties combined with high chemical stable materials at the active detector surface [242]. Thus, these detection or determination techniques seem to have the adequate flexibility to be used in design and fabrication of biosensors for nitrite/nitrate detection in food or biological samples. Moreover, modified solid state surfaces on this approach were applied because of catalytic efficiency beside enhancing the surface-to-volume ratios [243]. In a nut shell, using chemically modified electrodes can lead to satisfactory results of electrocatalysis, high effective surface area, improvement of mass transport, and good biocompatibility [243], [244]. All of these achievements could be used for development of portable medical devices or kits for detection and controlling viral infections.
5. Conclusion
Herein, COVID-19 disease the whole world threatening issue was focused. Since there is no fully certain cure yet, the most reliable way is to be safe and alert by monitoring or detection the concentration of various related species in the body including ROS. On this approach, the possible connections between the disease and OS or ROS were considered and studied from biomolecular and bioanalytical points of view. In addition, since COVID-19 infection mechanism has not become clarified yet, having the accurate information about the level of ROS species by various methods would be essential for pre-detection of the disease. Based on the records, bio-electroanalytical techniques have the standard potentials to be appropriately used for design and fabrication of detection kits or devices for medical purposes; finding the optimized safe cure for the whole world via controlling the OS level or balancing the ROS/antioxidant ratio. In this direction, reliability, sensitivity and having cost effective devices to be applicable are the most serious obsessions especially for proteomics and medical diagnostics of COVID-19 disease.
Funding
This work was not supported by any grant.
Declaration of competing interest
The authors declare no conflict of interests.
Acknowledgment
The support of University of Tehran, Iran National Science Foundation (INSF), UNESCO Chair on Interdisciplinary Research in Diabetes is gratefully acknowledged.
References
- 1.South A.M., Diz D.I., Chappell M.C. COVID-19, ACE2, and the cardiovascular consequences. Am. J. Phys. 2020;318:H1084–H1090. doi: 10.1152/ajpheart.00217.2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Sikora A., Irby S.M., Hall B.L., Mills Stephen A., Koeppe J.R., Pikaart M.J., Wilner S.E., Craig P.A., Roberts R. Responses to the covid-19 pandemic by the biochemistry authentic scientific inquiry lab (basil) cure consortium: reflections and a case study on the switch to remote learning. J. Chem. Educ. 2020;97:3455–3462. doi: 10.1021/acs.jchemed.0c00729. [DOI] [Google Scholar]
- 3.Lau S.K., Che X.-Y., Woo P.C., Wong B.H., Cheng V.C., Woo G.K., Hung I.F., Poon R.W., Chan K.-H., Peiris J.M. SARS coronavirus detection methods. Emerg. Infect. Dis. 2005;11:1108–1111. doi: 10.3201/eid1107.041045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Zhai P., Ding Y., Wu X., Long J., Zhong Y., Li Y. The epidemiology, diagnosis and treatment of covid-19. Int. J. Antimicrob. Agents. 2020;55:105955–105968. doi: 10.1016/j.ijantimicag.2020.105955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Butowt R., Bilinska K. SARS-CoV-2: olfaction, brain infection, and the urgent need for clinical samples allowing earlier virus detection. ACS Chem. Neurosci. 2020;11:1200–1209. doi: 10.1021/acschemneuro.0c00172. [DOI] [PubMed] [Google Scholar]
- 6.Rabiee N., Bagherzadeh M., Ghasemi A., Zare H., Ahmadi S., Fatahi Y., Dinarvand R., Rabiee M., Ramakrishna S., Shokouhimehr M. Point-of-use rapid detection of sars-cov-2: nanotechnology-enabled solutions for the covid-19 pandemic. Int. J. Mol. Sci. 2020;21:5126–5150. doi: 10.3390/ijms21145126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Parlakpinar H., Gunata M. SARS-COV-2 (COVID-19): cellular and biochemical properties and pharmacological insights into new therapeutic developments. Cell Biochem. Funct. 2021;39:10–28. doi: 10.1002/cbf.3591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Dashraath P., Jeslyn W.J.L., Karen L.M.X., Min L.L., Sarah L., Biswas A., Choolani M.A., Mattar C., Lin S.L. Coronavirus disease 2019 (COVID-19) pandemic and pregnancy. Am. J. Obst. Gyn. 2020;222:521–531. doi: 10.1016/j.ajog.2020.03.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Yelin I., Aharony N., Shaer Tamar E., Argoetti A., Messer E., Berenbaum D., Shafran E., Kuzli A., Gandali N., Shkedi O., Hashimshony T., Mandel-Gutfreund Y., Halberthal M., Geffen Y., Szwarcwort-Cohen M., Kishony R. Evaluation of COVID-19 RT-qPCR test in multi sample pools. Clin. Inf. Dis. 2020;71:2073–2078. doi: 10.1093/cid/ciaa531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Du Z., Zhu F., Guo F., Yang B., Wang T. Detection of antibodies against SARS-CoV-2 in patients with COVID-19. J. Med. Vir. 2020;92:1735–1738. doi: 10.1002/jmv.25820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Li G., He X., Zhang L., Ran Q., Wang J., Xiong A., Wu D., Chen F., Sun J., Chang C. Assessing ACE2 expression patterns in lung tissues in the pathogenesis of COVID-19. J. Autoimmun. 2020;112:102463–102470. doi: 10.1016/j.jaut.2020.102463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Prasad K., Khatoon F., Rashid S., Abdullah N.A., AlAsmari F., AhmeddAli M.Z., Alqahtani S., Alqahtani M.S., Kumara V. Targeting hub genes and pathways of innate immune response in COVID-19: a network biology perspective. Int. J. Biol. Macromol. 2020;163:1–8. doi: 10.1016/j.ijbiomac.2020.06.228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Yang N., Shen H.-M. Targeting the endocytic pathway and autophagy process as a novel therapeutic strategy in COVID-19. Int. J. Biol. Sci. 2020;16:1724–1731. doi: 10.7150/ijbs.45498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Lu Q., Shi Y. Coronavirus disease (COVID-19) and neonate: what neonatologists need to know. J. Med. Virol. 2020;92:546–547. doi: 10.1002/jmv.25740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Sun J., Zhu A., Li H., Zheng K., Zhuang Z., Chen Z., Shi Y., Zhang Z., Chen S.-B., Liu X. Isolation of infectious SARS-CoV-2 from urine of a COVID-19 patient. Emerg. Micr. Infect. 2020;9:991–993. doi: 10.1080/22221751.2020.1760144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Liu R., Han H., Liu F., Lv Z., Wu K., Liu Y., Feng Y., Zhu C. Positive rate of RT-PCR detection of SARS-CoV-2 infection in 4880 cases from one hospital in Wuhan, China, from Jan to Feb 2020. Clin. Chim. Acta. 2020;505:172–175. doi: 10.1016/j.cca.2020.03.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Delgado-Roche L., Mesta F. Oxidative stress as key player in severe acute respiratory syndrome coronavirus (SARS-CoV) infection. Arch. Med. Res. 2020;51:384–387. doi: 10.1016/j.arcmed.2020.04.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Sies H., Parnham M.J. Potential therapeutic use of ebselen for COVID-19 and other respiratory viral infections. Free Radic. Biol. Med. 2020;156:107–122. doi: 10.1016/j.freeradbiomed.2020.06.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Garrido J., Gaspar A., Garrido E.M., Miri R., Tavakkoli M., Pourali S., Saso L., Borges F., Firuzi O. Alkyl esters of hydroxycinnamic acids with improved antioxidant activity and lipophilicity protect PC12 cells against oxidative stress. Biochimie. 2012;94:961–967. doi: 10.1016/j.biochi.2011.12.015. [DOI] [PubMed] [Google Scholar]
- 20.Betteridge D.J. What is oxidative stress? Metab. Clin. Exp. 2000;49:3–8. doi: 10.1016/S0026-0495(00)80077-3. [DOI] [PubMed] [Google Scholar]
- 21.Møller P., Wallin H., Knudsen L.E. Oxidative stress associated with exercise, psychological stress and life-style factors. Chem. Biol. Inter. 1996;102:17–36. doi: 10.1016/0009-2797(96)03729-5. [DOI] [PubMed] [Google Scholar]
- 22.Yoshikawa T., Naito Y. What is oxidative stress? J. Am. Med. Ass. 2002;45:271–276. [Google Scholar]
- 23.Dai J., Gu L., Su Y., Wang Q., Zhao Y., Chen X., Deng H., Li W., Wang G., Li K. Inhibition of curcumin on influenza a virus infection and influenzal pneumonia via oxidative stress, TLR2/4, p38/JNK MAPK and NF-?B pathways. Int. Immun. 2018;54:177–187. doi: 10.1016/j.intimp.2017.11.009. [DOI] [PubMed] [Google Scholar]
- 24.Hashemi H., Niakousari G.M. Antioxidant, antimicrobial, cell viability and enzymatic inhibitory of antioxidant polymers as biological macromolecules. Int. J. Biol. Macromol. 2017;104:606–617. doi: 10.1016/j.ijbiomac.2017.06.021. [DOI] [PubMed] [Google Scholar]
- 25.Barsan M.M., Diculescu V.C. New electrochemical sensor based on CoQ10 and cyclodextrin complexes for the detection of oxidative stress initiators. Electrochim. Acta. 2019;302:441–448. doi: 10.1016/j.electacta.2019.02.060. [DOI] [Google Scholar]
- 26.Camini F.C., da Silva Caetano C.C., Almeida L.T., de Brito Magalhães C.L. Implications of oxidative stress on viral pathogenesis. Arch. Virol. 2016;162:907–917. doi: 10.1007/s00705-016-3187-y. [DOI] [PubMed] [Google Scholar]
- 27.Camini F.C., da Silva T.F., da Silva Caetano C.C., Almeida L.T., Ferraz A.C., Vitoreti V.M.Alves, de Mello Silva B., de Queiroz Silva S., de Magalhães J.C., de Brito Magalhães C.L. Antiviral activity of silymarin against Mayaro virus and protective effect in virus-induced oxidative stress. Antivir. Res. 2018;158:8–12. doi: 10.1016/j.antiviral.2019.05.008. [DOI] [PubMed] [Google Scholar]
- 28.Barzegar A., Moosavi-Movahedi A.A. Intracellular ROS protection efficiency and free radical-scavenging activity of curcumin. PLoS One. 2011;6(10) doi: 10.1371/journal.pone.0026012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Taghavi F., Moosavi-Movahedi A.A. In: Plant and Human Health. Ozturk M., Hakeem K., editors. vol. 2. Springer; Cham: 2019. Free radicals, diabetes, and its complexities. [DOI] [Google Scholar]
- 30.Carraro E., Schilirò T., Biorci F., Romanazzi V., Degan R., Buonocore D., Verri M., Dossena M., Bonetta S., Gilli G. Physical activity, lifestyle factors and oxidative stress in middle age healthy subjects. Int. J. Environ. Res. Pub. Health. 2018;15:1152–1163. doi: 10.3390/ijerph15061152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Srivastava R.A.K. Life-style-induced metabolic derangement and epigenetic changes promote diabetes and oxidative stress leading to NASH and atherosclerosis severity. J. Diabetes Metab. Disord. 2018;17:381–389. doi: 10.1007/s40200-018-0378-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Moosavi-Movahedi A.A. 1st Ed. Springer; 2021. Book: Rationality and Scientific Lifestyle for Health. [DOI] [Google Scholar]
- 33.Trist B.G., Hare D.J., Double K.L. Oxidative stress in the aging substantia nigra and the etiology of Parkinson's disease. Aging Cell. 2019;18:e10331–e10354. doi: 10.1111/acel.13031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Castelli V., Benedetti E., Antonosante A., Catanesi M., Pitari G., Ippoliti R., Cimini A., d’Angelo M. Neuronal cells rearrangement during aging and neurodegenerative disease: metabolism, oxidative stress and organelles dynamic. Front. Mol. Neurosci. 2019;12:1–13. doi: 10.3389/fnmol.2019.00132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Koff W.C., Williams M.A. COVID-19 and immunity in aging populations—a new research agenda. N. Engl. J. Med. 2020;383:804–805. doi: 10.1056/NEJMp2006761. [DOI] [PubMed] [Google Scholar]
- 36.Papaconstantinou J. The role of signaling pathways of inflammation and oxidative stress in development of senescence and aging phenotypes in cardiovascular disease. Cells. 2019;8:1383–1407. doi: 10.3390/cells8111383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Incalza M.A., D'Oria R., Natalicchio A., Perrini S., Laviola L., Giorgino F. Oxidative stress and reactive oxygen species in endothelial dysfunction associated with cardiovascular and metabolic diseases. Vasc. Pharmacol. 2018;100:1–19. doi: 10.1016/j.vph.2017.05.005. [DOI] [PubMed] [Google Scholar]
- 38.M. Panel B.Ghaleh., D. Morin Mitochondria and aging: a role for the mitochondrial transition pore? Aging Cell. 2018;17:e12793–e12838. doi: 10.1111/acel.12793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Pishkari N., Habibi-Rezaei M., Taghavi F., Amanlou M., Sheibani N., Saso L., Moosavi-Movahedi A.A. The correlation between ROS generation and LPO process as the function of methylparaben concentrations during hemoglobin fructation. J. Iran. Chem. Soc. 2020;17:1249–1255. doi: 10.1007/s13738-020-01852-y. [DOI] [Google Scholar]
- 40.Ríos-Ocampo W.A., Daemen T., Buist-Homan M., Faber K.N., Navas M.-C., Moshage H. Hepatitis C virus core or NS3/4A protein expression preconditions hepatocytes against oxidative stress and endoplasmic reticulum stress. Redox Rep. 2019;24:17–26. doi: 10.1080/13510002.2019.1596431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.de Almeida J.P.S., Liberatti L.S., Barros F.E.N., Kallaur A.P., Lozovoy M.A.B., Scavuzzi B.M., Panis C., Reiche E.M.V., Dichi I., Simão A.N.C. Profile of oxidative stress markers is dependent on vitamin D levels in patients with chronic hepatitis C. Nutrition J. 2016;32:362–367. doi: 10.1016/j.nut.2015.09.016. [DOI] [PubMed] [Google Scholar]
- 42.Laforge M., Elbim C., Frère C., Hémadi M., Massaad C., Nuss P., Benoliel J.-J., Becker C. Tissue damage from neutrophil-induced oxidative stress in COVID-19. Nat. Rev. Immun. 2020;20:515–516. doi: 10.1038/s41577-020-0407-1. s41577-020-0407-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Cheng R.Z. Can early and high intravenous dose of vitamin C prevent and treat coronavirus disease 2019 (COVID-19)? Med. Drug Dis. 2020;5:10028–10030. doi: 10.1016/j.medidd.2020.100028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Liu K., Chen Y., Lin R., Han K. Clinical features of COVID-19 in elderly patients: a comparison with young and middle-aged patients. J. Infect. 2020;80:e14–e18. doi: 10.1016/j.jinf.2020.03.005. 1016/j.jinf.2020.03.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Dong J., Ma Q. Suppression of basal and carbon nanotube-induced oxidative stress, inflammation and fibrosis in mouse lungs by Nrf2. Nanotox. 2016;10:699–709. doi: 10.3109/17435390.2015.1110758. [DOI] [PubMed] [Google Scholar]
- 46.Wang Q., Wei S., Zhou H., Shen G., Gan X., Zhou S., Qiu J., Shi C., Lu L. Hyperglycemia exacerbates acetaminophen-induced acute liver injury by promoting liver-resident macrophage proinflammatory response via AMPK/PI3K/AKT-mediated oxidative stress. Cell Death Dis. 2019;5:119–131. doi: 10.1038/s41420-019-0198-y. s41420-019-0198-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Khan M., Liu X., Tang Y., Liu X. Ultra-sensitive electrochemical detection of oxidative stress biomarker 8-hydroxy-2'-deoxyguanosine with poly (L-arginine)/graphene wrapped au nanoparticles modified electrode. Biosens. Bioelectron. 2018;117:508–514. doi: 10.1016/j.bios.2018.06.048. [DOI] [PubMed] [Google Scholar]
- 48.Goyal R.N. Determination of 8-hydroxydeoxyguanosine: a potential biomarker of oxidative stress, using carbon-allotropic nanomaterials modified glassy carbon sensor. Talanta. 2016;161:735–742. doi: 10.1016/j.talanta.2016.09.038. [DOI] [PubMed] [Google Scholar]
- 49.Guo Q., Li F., Duan Y., Wen C., Wang W., Zhang L., Huang R., Yin Y. Oxidative stress, nutritional antioxidants and beyond. Sci. China Life Sci. 2020;63:866–874. doi: 10.1007/s11427-019-9591-5. [DOI] [PubMed] [Google Scholar]
- 50.Elfawy H.A., Das B. Crosstalk between mitochondrial dysfunction, oxidative stress, and age related neurodegenerative disease: etiologies and therapeutic strategies. Life Sci. 2019;218:65–184. doi: 10.1016/j.lfs.2018.12.029. [DOI] [PubMed] [Google Scholar]
- 51.Altable M., Serna J.M. Neuropathogenesis in COVID-19. J. Neuropathol. Exp. 2020;79:1247–1249. doi: 10.1093/jnen/nlaa116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Zhang J., Fu Y., Yang P., Liu X., Li Y., Gu Z. ROS scavenging biopolymers for anti-inflammatory diseases: classification and formulation. Adv. Mater. Inter. 2020;7:2000632–2000645. doi: 10.1002/admi.202000632. [DOI] [Google Scholar]
- 53.El-Kenawi A., Ruffell B. Inflammation, ROS, and mutagenesis. Cancer Cell. 2017;32:727–729. doi: 10.1016/j.ccell.2017.11.015. [DOI] [PubMed] [Google Scholar]
- 54.Chakraborti S., Parinandi N., Ghosh R., Ganguly N., Chakraborti T. Oxidative Stress in Lung Diseases. Springer; Singapore: 2020. pp. 297–330. [DOI] [Google Scholar]
- 55.Poprac P., Jomova K., Simunkova M., Kollar V., Rhodes C.J., Valko M. Targeting free radicals in oxidative stress-related human diseases. Trends Pharm. Sci. 2017;38:592–607. doi: 10.1016/j.tips.2017.04.005. [DOI] [PubMed] [Google Scholar]
- 56.Gebicki J.M. Oxidative stress, free radicals and protein peroxides. Arch. Biochem. Biophys. 2016;595:33–39. doi: 10.1016/j.abb.2015.10.021. [DOI] [PubMed] [Google Scholar]
- 57.Camini F.C., da Silva Caetano C.C., Almeida L.T., de Brito Magalhaes C.L. Implications of oxidative stress on viral pathogenesis. Arch. Virol. 2017;162:907–917. doi: 10.1007/s00705-016-3187-y. [DOI] [PubMed] [Google Scholar]
- 58.Peterhans E. Reactive oxygen species and nitric oxide in viral diseases. Biol. Trace Elem. Res. 1997;56:107–116. doi: 10.1007/BF02778986. [DOI] [PubMed] [Google Scholar]
- 59.Aviello G., Knaus U.G. NADPH oxidases and ROS signaling in the gastrointestinal tract. Mucosal Immun. 2018;11:1011–1023. doi: 10.1038/s41385-018-0021-8. s41385-018-0021-8. [DOI] [PubMed] [Google Scholar]
- 60.He J., Wei W., Yang Q., Wang Y. Phillygenin exerts in vitro and in vivo antitumor effects in drug-resistant human esophageal cancer cells by inducing mitochondrial-mediated apoptosis, ROS generation, and inhibition of the nuclear factor kappa B NF-?B signaling pathway. Med. Sci. Monit. 2019;25:725–739. doi: 10.12659/MSM.913138. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 61.Schett G., Sticherling M., Neurath M.F. COVID-19: risk for cytokine targeting in chronic inflammatory diseases? Nat. Rev. Immun. 2020;20:271–272. doi: 10.1038/s41577-020-0312-7. s41577-020-0312-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Choi J.S. Development of surface curcumin nanoparticles modified with biological macromolecules for anti-tumor effects. Int. J. Biol. Macromol. 2016;92:850–859. doi: 10.1016/j.ijbiomac.2016.07.101. [DOI] [PubMed] [Google Scholar]
- 63.Barzegar A., Davari M.D., Chaparzadeh N., Zarghami N., Pedersen J.Z., Incerpi S., Saso L., Moosavi-Movahedi A.A. Theoretical and experimental studies on the structure-antioxidant activity relationship of synthetic 4-methylcoumarins. J. Iran. Chem. Soc. 2011;8:973–982. doi: 10.1007/BF03246553. [DOI] [Google Scholar]
- 64.Biswal S., Rizwan H., Pal S., Sabnam S., Parida P., Pal A. Oxidative stress, antioxidant capacity, biomolecule damage, and inflammation symptoms of sickle cell disease in children. Hematology. 2019;24:1–9. doi: 10.1080/10245332.2018.1498441. [DOI] [PubMed] [Google Scholar]
- 65.Mohammadi H., Djalali M., Daneshpazhooh M., Honarvar N., Chams-Davatchi C., Sepandar F., Fakhri Z., Yaghubi E., Zarei M., Javanbakht M. Effects of L-carnitine supplementation on biomarkers of oxidative stress, antioxidant capacity and lipid profile, in patients with pemphigus vulgaris: a randomized, double-blind, placebo-controlled trial. Eur. J. Clin. Nutr. 2018;72:99–104. doi: 10.1038/ejcn.2017.131. [DOI] [PubMed] [Google Scholar]
- 66.Bhagat S., Thakur A.S. Influence of ß-globin haplotypes on oxidative stress, antioxidant capacity and inflammation in sickle cell patients of Chhattisgarh. Ind. J. Clin. Biochem. 2019;34:201–206. doi: 10.1007/s12291-017-0729-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Yousefi M., Vatnikov Y.A., Kulikov E.V., Ghelichpour M. Change in blood stress and antioxidant markers and hydromineral balance of common carp (Cyprinus carpio) anaesthetized with citronellal and linalool: comparison with eugenol. Aquac. Res. 2019;50:1313–1320. doi: 10.1111/are.14007. [DOI] [Google Scholar]
- 68.Mazaheri M., Moosavi-Movahedi A.A., Saboury A.A., Habibi Rezaei M., Shourian M., Farhadi M., Sheibani N. Curcumin mitigates the fibrillation of human serum albumin and diminishes the formation of reactive oxygen species. Protein Pept. Lett. 2015;22:348–353. doi: 10.2174/0929866522666150209150004. [DOI] [PubMed] [Google Scholar]
- 69.Czarny P., Wigner P., Galecki P., Sliwinski T. The interplay between inflammation, oxidative stress, DNA damage, DNA repair and mitochondrial dysfunction in depression. Prog. Neuro-Psychopharmacol. Biol. Psychiatry. 2018;80:309–321. doi: 10.1016/j.pnpbp.2017.06.036. [DOI] [PubMed] [Google Scholar]
- 70.Barnes R.P., Fouquerel E., Opresko P.L. The impact of oxidative DNA damage and stress on telomere homeostasis. Mech. Ageing Dev. 2019;177:37–45. doi: 10.1016/j.mad.2018.03.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Nemmar A., Karaca T., Beegam S., Yuvaraju P., Yasin J., Ali B.H. Lung oxidative stress, DNA damage, apoptosis, and fibrosis in adenine-induced chronic kidney disease in mice. Front. Physiol. 2017;8:1–10. doi: 10.3389/fphys.2017.00896. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Pan Y., Li X., Yang G., Fan J., Tang Y., Zhao J., Long X., Guo S., Zhao Z., Liu Y. Serological immunochromatographic approach in diagnosis with SARS-CoV-2 infected COVID-19 patients. J. Infect. 2020;81 doi: 10.1016/j.jinf.2020.03.051. e-28-e32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Liu B., Li M., Zhou Z., Guan X., Xiang Y. Can we use interleukin-6 (IL-6) blockade for coronavirus disease 2019 (COVID-19)-induced cytokine release syndrome (CRS)? J. Autoimmun. 2020;111:102452–102550. doi: 10.1016/j.jaut.2020.102452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Lipsitch M., Swerdlow D.L., Finelli L. Defining the epidemiology of Covid-19—studies needed. N. Engl. J. Med. 2020;382:1194–1196. doi: 10.1056/NEJMp2002125. [DOI] [PubMed] [Google Scholar]
- 75.Wright A., Salazar A., Mirica M., Volk M.A., Schiff G.D. The invisible epidemic: neglected chronic disease management during covid-19. J. Gen. Int. Med. 2020;35:2816–2817. doi: 10.1007/s11606-020-06025-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Derouiche S. Oxidative stress associated with SARS-Cov-2 (COVID-19) increases the severity of the lung disease-a systematic review. J. Infect. Dis. Epidemiol. 2020;6:121–127. doi: 10.23937/2474-3658/1510121. [DOI] [Google Scholar]
- 77.Scigliano G., Scigliano G.A. Acute respiratory distress syndrome from Covid-19: a perfect storm from free radicals? Proposal for a new treatment. Med. Hypotheses. 2020;144:110120–110122. doi: 10.1016/j.mehy.2020.110120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Yang Z., Shi J., He Z., Lü Y., Xu Q., Ye C., Chen S., Tang B., Yin K., Lu Y. Predictors for imaging progression on chest CT from coronavirus disease 2019 (COVID-19) patients. Aging. 2020;12:6037–6048. doi: 10.18632/aging.102999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Li S., Tang Z., Li Z., Liu X. Searching therapeutic strategy of new coronavirus pneumonia from angiotensin-converting enzyme 2: the target of COVID-19 and SARSCoV. Eur. J. Clin. Microbiol. Infect. Dis. 2020;39:1021–1026. doi: 10.1007/s10096-020-03883-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Yang X., Yu Y., Xu J., Huaqing Shu P., Xia J., Liu H., Wu Y., Zhang L., Yu Z., Fang M., Yu T., Wang Y., Pan S., Zou X., Yuan S., Shang Y. Patients with SARS-CoV-2 pneumonia in Wuhan, China: a single-centered, retrospective, observational study. Lancet Res. Med. 2020;8:475–481. doi: 10.1016/S2213-2600(20)30079-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Zhang B., Zhou X., Zhu C., Song Y., Feng F., Qiu Y., Feng J., Jia Q., Song Q., Zhu B., Wang J. Immune phenotyping based on the neutrophil-to-lymphocyte ratio and igg level predicts disease severity and outcome for patients with COVID-19. Front. Mol. Biosci. 2020;7:1–7. doi: 10.3389/fmolb.2020.00157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Ponti G., Maccaferri M., Ruini C., Tomasi A., Ozben T. Biomarkers associated with COVID-19 disease progression. Crit. Rev. Clin. Lab. Sci. 2020;57:389–399. doi: 10.1080/10408363.2020.1770685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Lagunas-Rangel F.A. Neutrophil-to-lymphocyte ratio and lymphocyte-to-C-reactive protein ratio in patients with severe coronavirus disease 2019 (COVID-19): a meta-analysis. J. Med. Virol. 2020;92:1733–1734. doi: 10.1002/jmv.25819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Metha P., McAuley D.F., Brown M., Sanchez E., Tattersall R.S., Manson J.J. COVID-19: consider cytokine storm syndromes and immunosuppression. Lancet. 2020;395:1033–1034. doi: 10.1016/S0140-6736(20)30628-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Tan C., Huang Y., Shi F., Tan K., Ma Q., Chen Y., Jiang X., Li X. C-reactive protein correlates with computed tomographic findings and predicts severe COVID-19 early. J. Med. Virol. 2020;92:856–862. doi: 10.1002/jmv.25871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Zhang G., Hub C., Luoc L., Fang F., Chene Y., Li J., Peng Z., Pan H. Clinical features and short-term outcomes of 221 patients with COVID-19 in Wuhan, China. J. Clin. Virol. 2020;127 doi: 10.1016/j.jcv.2020.104364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Pincemail J., Cavalier E., Charlier C., Cheramy-Bien J.P., Brevers E., Courtois A., Fadeur M., Meziane S., Le Goff C., Misset B., Albert A., Defraigne J.O., Rousseau A.F. Oxidative stress status in covid-19 patients hospitalized in intensive care unit for severe pneumonia. A pilot study. Antioxidants. 2021;10:257–269. doi: 10.3390/antiox10020257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Munster V.J., Koopmans M., van Doremalen N., Riel D., Wit E. A novel coronavirus emerging in China-key questions for impact assessment. N. Engl. J. Med. 2020;382:692–694. doi: 10.1056/NEJMP2000929. [DOI] [PubMed] [Google Scholar]
- 89.Coperchinia F., Chiovatoa L., Crocea L., Magria F., Rotondia M. The cytokine storm in COVID-19: an overview of the involvement of the chemokine/chemokine-receptor system. Cytokine Growth Factor Rev. 2020;53:25–32. doi: 10.1016/j.cytogfr.2020.05.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Iddir M., Brito A., Dingeo G., Sosa S., Campo F.D., Samouda H., La Frano M.R., Bohn T. Strengthening the immune system and reducing inflammation and oxidative stress through diet and nutrition: considerations during the COVID-19 crisis. Nutrients. 2020;12:1562–1601. doi: 10.3390/nu12061562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Carpagnano G.E., Di Lecce V., Quaranta V.N., Zito A., Buonamico E., Capozza E., Palumbo A., Di Gioia G., Valerio V.N., Resta O. Vitamin D deficiency as a predictor of poor prognosis in patients with acute respiratory failure due to COVID-19. J. Endocrinol. Investig. 2021;44:765–771. doi: 10.1007/s40618-020-01370-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Wong S.H., Lui R.N.S., Sung J.J.Y. Covid-19 and the digestive system. J. Gaster. Hept. 2020;35:744–748. doi: 10.1111/jgh.15047. [DOI] [PubMed] [Google Scholar]
- 93.Zhou F., Yu T., Du R., Fan G., Liu Y., Liu Z., Xiang J., Wang Y., Song B., Gu X. Clinical course and risk factors for mortality of adult inpatients with COVID-19 in Wuhan, China: a retrospective cohort study. Lancet. 2020;395:1054–1062. doi: 10.1016/S0140-6736(20)30566-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Rothan H.A., Byrareddy S.N. The epidemiology and pathogenesis of coronavirus disease (COVID-19) outbreak. Lancet. 2020;109 doi: 10.1016/j.jaut.2020.102433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Dietz W., Santos-Burgoa C. Obesity and its implications for COVID-19 mortality. Obesity. 2020;28:1005-1005. doi: 10.1002/oby.22818. [DOI] [PubMed] [Google Scholar]
- 96.Polonikov A. Endogenous deficiency of glutathione as the most likely cause of serious manifestations and death in covid-19 patients. ACS Infect. Dis. 2020;6:1558–1562. doi: 10.1021/acsinfecdis.0c00288. [DOI] [PubMed] [Google Scholar]
- 97.Jain S.K., Micinski D. Vitamin D upregulates glutamate cysteine ligase and glutathione reductase, and GSH formation, and decreases ROS and MCP-1 and IL-8 secretion in high-glucose exposed U937 monocytes. Biochem. Biophys. Res. Comm. 2013;437:7–11. doi: 10.1016/j.bbrc.2013.06.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Jain S.K., Parsanathan R., Achari A.E., Kanikarla-Marie P., Bocchini J.A. Glutathione stimulates vitamin D regulatory genes, lowers oxidative stress and inflammation, and increases 25-hydroxyvitamin D levels in blood: a novel approach to treat 25-hydroxyvitamin D deficiency. Antioxid. Redox Signal. 2017:1–42. doi: 10.1089/ars.2017.7462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Jai S.K., Micinski D., Parsanathan R. L-cysteine stimulates the effect of vitamin d on inhibition of oxidative stress, il-8, and mcp-1 secretion in high glucose treated monocytes. J. Am. Coll. Nutr. 2021;40:327–332. doi: 10.1080/07315724.2020.1850371. [DOI] [PubMed] [Google Scholar]
- 100.Parsanathan R., Jain S.K. Glutathione deficiency induces epigenetic alterations of vitamin D metabolism genes in the livers of high-fat diet-fed obese mice. Sci. Rep. 2019;9:14784. doi: 10.1038/s41598-019-51377-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Radujkovic A., Hippchen T., Tiwari-Heckler S., Dreher S., Boxberger M., Merle U. Vitamin D deficiency and outcome of covid-19 patients. Nutrients. 2020;12:2757. doi: 10.3390/nu12092757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Parsanathan R., Achari A.E., Manna P., Jain S.K. L-cysteine and vitamin D co-supplementation alleviates markers of musculoskeletal disorders in vitamin D-deficient high-fat diet-fed mice. Nutrients. 2020;12:3406. doi: 10.3390/nu12113406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Parsanathan R., Jain S.K. Glutathione deficiency alters the vitamin D metabolizing enzymes CYP27B1 and CYP24A1 in human renal proximal tubule epithelial cells and kidney of HFD-fed mice. Free Rad. Biol. Med. 2019;131:376–381. doi: 10.1016/j.freeradbiomed.2018.12.017. [DOI] [PubMed] [Google Scholar]
- 104.Perchetti G.A., Nalla A.K., Huang M.L., Jerome K.R., Greninge A.L. Multiplexing primer/probe sets for detection of SARS-CoV-2 by qRT-PCR. J. Clin. Virol. 2020;129 doi: 10.1016/j.jcv.2020.104499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Ebadi M., Montano-Loza A.J. Perspective: improving vitamin D status in the management of COVID-19. Eur. J. Clin. Med. 2020;74:856–859. doi: 10.1038/s41430-020-0661-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Cheng R.Z. Can early and high intravenous dose of vitamin C prevent and treat coronavirus disease 2019 (COVID-19)? Med. Drug Discov. 2020;5 doi: 10.1016/j.medidd.2020.100028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Roche L.D., Mesta F. Oxidative stress as key player in severe acute respiratory syndrome coronavirus (SARS-COV) infection. Microsyst. Nanoeng. 2020;51:384–387. doi: 10.1016/j.arcmed.2020.04.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Lan L., Xu D., Ye G., Xia C., Wang S., Li Y., Xu H. Positive RT-PCR test results in patients recovered from COVID-19. JAMA. 2020;323:1502–1503. doi: 10.1001/jama.2020.2783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Bachanova V., Bishop M.R., Dahi P., Dholaria B., Grupp S.A., Hayes-Lattin B., Janakiram M., Maziarz R.T., McGuirk J.P., Nastoupil L.J. Car T cell therapy during the covid-19 pandemic. Biol. Blood Marr. Transplant. 2020;26:1239–1246. doi: 10.1016/j.bbmt.2020.04.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Ravi N., Cortade D.L., Ng E., Wang S.X. Diagnostics for SARS-CoV-2 detection: a comprehensive review of the FDA-EUA COVID-19 testing landscape. Biosens. Bioelectron. 2020;165 doi: 10.1016/j.bios.2020.112454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Nguyen T., Duong Bang D., Wolff A. Novel coronavirus disease (COVID-19): paving the road for rapid detection and point-of-care diagnostics. Micromachines. 2019;11(2020):306–313. doi: 10.3390/mi11030306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Smith M., Hayward S., Innes S., Miller A. Point-of-care lung ultrasound in patients with COVID-19–a narrative review. Anaesthesia. 2020;75:1096–1104. doi: 10.1111/anae.15082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Matsuda E.M., Campos I.B., Oliveira I.P., Colpas D.R., Santos Carmo A.M., Macedo Brígido L.F. Field evaluation of COVID-19 antigen tests versus RNA based detection: potential lower sensitivity compensated by immediate results, technical simplicity, and low cost. J. Med. Virol. 2021;93:4405–4410. doi: 10.1002/jmv.26985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Teengam P., Siangproh W., Tuantranont A., Vilaivan T., Chailapakul O., Henry C.S. Multiplex paper-based colorimetric DNA sensor using pyrrolidinyl peptide nucleic acid-induced AgNPs aggregation for detecting MERS-CoV, MTB, and HPV oligonucleotides. Anal. Chem. 2017;89:5428–5435. doi: 10.1021/acs.analchem.7b00255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Choi J.R. Development of point-of-care biosensors for COVID-19. Front. Chem. 2020;8:517. doi: 10.3389/fchem.2020.00517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Wu J., Liu J., Li S., Peng Z., Xiao Z., Wang X., Yan R., Luo J. Detection and analysis of nucleic acid in various biological samples of COVID-19 patients. Travel Med. Infect. Dis. 2020;37:101673–101675. doi: 10.1016/j.tmaid.2020.101673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Xiao A.T., Tong Y.X., Zhang S. False-negative of RT-PCR and prolonged nucleic acid conversion in COVID-19: rather than recurrence. J. Med. Virol. 2020;92:1755–1756. doi: 10.1002/jmv.25855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Lu S., Lin J., Zhang Z., Xiao L., Jiang Z., Chen J., Hu C., Luo S. Alert for non-respiratory symptoms of coronavirus disease 2019 (COVID-19) patients in epidemic period: a case report of familial cluster with three asymptomatic COVID-19 patients. J. Med. Virol. 2020;93:518–521. doi: 10.1002/jmv.25776. [DOI] [PubMed] [Google Scholar]
- 119.Li L., Qin L., Xu Z., Yin Y., Wang X., Kong B., Bai J., Lu Y., Fang Z., Song Q. Artificial intelligence distinguishes COVID-19 from community acquired pneumonia on chest CT. Radiology. 2020;25:100–116. doi: 10.1148/radiol.2020200905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Udugama B., Kadhiresan P., Kozlowski H.N., Malekjahani A., Osborne M., Li V.Y., Chen H., Mubareka S., Gubbay J.B., Chan W.C. Diagnosing COVID-19: the disease and tools for detection. ACS Nano. 2020;566:3822–3835. doi: 10.1021/acsnano.0c02624. [DOI] [PubMed] [Google Scholar]
- 121.Yin K.K., Wu M., Wu Z. Evaluation of deep learning-based approaches for COVID-19 classification based on chest X-ray images. SIViP. 2021;15:959–966. doi: 10.1007/s11760-020-01820-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Xie C., Jiang L., Huang G., Pu H., Gong B., Lin H., Ma S., Chen X., Long B., Si G. Comparison of different samples for 2019 novel coronavirus detection by nucleic acid amplification tests. Int. J. Infect. Dis. 2020;93:264–267. doi: 10.1016/j.ijid.2020.02.050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Yong S.E.F., Anderson D.E., Wei W.E., Pang J., Chia W.N., Tan C.W., Teoh Y.L., Rajendram P., Toh M.P.H.S., Poh C. Connecting clusters of COVID-19: an epidemiological and serological investigation. Lancet Infect. Dis. 2020;20:809–815. doi: 10.1016/S1473-3099(20)30273-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Zhang J., Zhang X., Liu J., Ban Y., Li N., Wu Y., Liu Y., Ye R., Liu J., Li X. Serological detection of 2019-nCoV respond to the epidemic: a useful complement to nucleic acid testing. Int. Immun. 2020;88:106861–106868. doi: 10.1016/j.intimp.2020.106861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Espejo A.P., Akgun Y., Al Mana A.F., Tjendra Y., Millan N.C., Gomez-Fernandez C., Cray C. Review of current advances in serologic testing for COVID-19. Am. J. Clin. Pathol. 2020;154:293–304. doi: 10.1093/ajcp/aqaa112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Zhong L., Chuan J., Gong B., Shuai P., Zhou Y., Zhang Y., Jiang Z., Zhang D., Liu X., Ma S. Detection of serum IgM and IgG for COVID-19 diagnosis. Sci. Chin. Life Sci. 2020;63:777–780. doi: 10.1007/s11427-020-1688-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Liu W., Liu L., Kou G., Zheng Y., Ding Y., Ni W., Wang Q., Tan L., Wu W., Tang S. Evaluation of nucleocapsid and spike protein-based enzyme-linked immunosorbent assays for detecting antibodies against SARS-CoV-2. J. Clin. Microbiol. 2020;58:e00461–e00481. doi: 10.1128/JCM.00461-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Carter L.J., Garner L.V., Smoot J.W., Li Y., Zhou Q., Saveson C.J., Sasso J.M., Gregg A.C., Soares D.J., Beskid T.R. Assay techniques and test development for COVID-19 diagnosis. ACS Cent. Sci. 2020;6:591–605. doi: 10.1021/acscentsci.0c00501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Ibarrondo F.J., Fulcher J.A., Goodman-Meza D., Elliott J., Hofmann C., Hausner M.A., Ferbas K.G., Tobin N.H., Aldrovandi G.M., Yang O.O. Rapid decay of anti–SARS-CoV-2 antibodies in persons with mild Covid-19. N. Engl. J. Med. 2020;353:2158–2168. doi: 10.1056/NEJMc2025179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Lipsitch M., Kahn R., Mina M.J. Antibody testing will enhance the power and accuracy of COVID-19-prevention trials. Nat. Med. 2020;26:816–818. doi: 10.1038/s41591-020-0887-3. s41591-020-0887-3. [DOI] [PubMed] [Google Scholar]
- 131.Long Q.-X., Liu B.-Z., Deng H.-J., Wu G.-C., Deng K., Chen Y.-K., Liao P., Qiu J.-F., Lin Y., Cai X.-F. Antibody responses to SARS-CoV-2 in patients with COVID-19. Nat. Med. 2020;26:845–848. doi: 10.1038/s41591-020-0897-1. s41591-020-0897-1. [DOI] [PubMed] [Google Scholar]
- 132.Ni L., Ye F., Cheng M.-L., Feng Y., Deng Y.-Q., Zhao H., Wei P., Ge J., Gou M., Li X. Detection of SARS-CoV-2-specific humoral and cellular immunity in COVID-19 convalescent individuals. Immunity. 2020;52:971–977. doi: 10.1016/j.immuni.2020.04.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Valipour M., Maghami P., Habibi-Rezaei M., Sadeghpour M., Khademian M.A., Mosavi K., Sheibani N., Moosavi-Movahedi A.A. Interaction of insulin with methyl tert-butyl ether promotes molten globule-like state and production of reactive oxygen species. Int. J. Biol. Macromol. 2015;80:610–614. doi: 10.1016/j.ijbiomac.2015.07.030. [DOI] [PubMed] [Google Scholar]
- 134.Fotouhi L., Moosavi-Movahedi A.A., Yousefinejad S., Shourian M., Sheibani N., Habibi-Rezaei M., Saboury A.A. Hydrophobic behavior, ROS production, and heme degradation of hemoglobin upon interaction with n-alkyl sulfates. J. Iran. Chem. Soc. 2016;13:2103–2111. doi: 10.1007/s13738-016-0928-5. [DOI] [Google Scholar]
- 135.Granot E., Kohen R. Oxidative stress in childhood—in health and disease states. Clin. Nutr. 2004;23(23):3–11. doi: 10.1016/S0261-5614(03)00097-9. [DOI] [PubMed] [Google Scholar]
- 136.Gallelli C.A., Calcagnini S., Romano A., Koczwara J.B., De Ceglia M., Dante D., Villani R., Giudetti A.M., Cassano T., Gaetani S. Modulation of the oxidative stress and lipid peroxidation by endocannabinoids and their lipid analogues. Antioxidants. 2018;7:93–137. doi: 10.3390/antiox7070093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Ni H., Peng L., Gao X., Ji H., Ma J., Li Y., Jiang S. Effects of maduramicin on adult zebrafish (Danio rerio): acute toxicity, tissue damage and oxidative stress. Ecotoxicol. Environ. Saf. 2019;168:249–259. doi: 10.1016/j.ecoenv.2018.10.040. [DOI] [PubMed] [Google Scholar]
- 138.Yigitturk G., Acara A.C., Erbas O., Oltulu F., Yavasoglu N.U.K., Uysal A., Yavasoglu A. The antioxidant role of agomelatine and gallic acid on oxidative stress in STZ induced type I diabetic rat testes. Biomed. Pharm. 2017;87:240–246. doi: 10.1016/j.biopha.2016.12.102. [DOI] [PubMed] [Google Scholar]
- 139.Afsar T., Razak S., Almajwal A. Acacia hydaspica ethyl acetate extract protects against cisplatin-induced DNA damage, oxidative stress and testicular injuries in adult male rats. BMC Cancer. 2017;17:883–897. doi: 10.1186/s12885-017-3898-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Ito F., Sono Y., Ito T. Measurement and clinical significance of lipid peroxidation as a biomarker of oxidative stress: oxidative stress in diabetes, atherosclerosis, and chronic inflammation. Antioxidants. 2019;8:72–100. doi: 10.3390/antiox8030072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Yuan P., Zhou Q., Hu X. The phases of WS2 nanosheets influence uptake, oxidative stress, lipid peroxidation, membrane damage, and metabolism in algae. Environ. Sci. Technol. 2018;52:13543–13552. doi: 10.1021/acs.est.8b04444. [DOI] [PubMed] [Google Scholar]
- 142.Kalaiyarasan G., Joseph J. Efficient dual-mode colorimetric/fluorometric sensor for the detection of copper ions and vitamin C based on pH-sensitive amino-terminated nitrogen-doped carbon quantum dots: effect of reactive oxygen species and antioxidants. Anal. Bioanal. Chem. 2019;411:2619–2633. doi: 10.1007/s00216-019-01710-8. [DOI] [PubMed] [Google Scholar]
- 143.Hosseini A., Pandey R., Osman E., Victorious A., Li F., Didar T., Soleymani L. Roadmap to the bioanalytical testing of COVID-19: from sample collection to disease surveillance. ACS Sens. 2020;11:3328–3345. doi: 10.1021/acssensors.0c01377. [DOI] [PubMed] [Google Scholar]
- 144.Salami M., Moosavi-Movahedi A.A., Ehsani M.R., Yousefi R., Haertlé T., Chobert J.-M., Razavi S.H., Henrich R., Balalaie S., Ebadi S.A., Pourtakdoost S., Niasari-Naslaji A. Improvement of the antimicrobial and antioxidant activities of camel and bovine whey proteins by limited proteolysis. J. Agric. Food Chem. 2010;58:3297–3302. doi: 10.1021/jf9033283. [DOI] [PubMed] [Google Scholar]
- 145.Moslehishad M., Ehsani M.R., Salami M., Mirdamadi S., Ezzatpanah H., Naslaji A.N., Moosavi-Movahedi A.A. The comparative assessment of ACE-inhibitory and antioxidant activities of peptide fractions obtained from fermented camel and bovine milk by lactobacillus rhamnosus PTCC 1637. Int. Dairy J. 2013;29:82–87. doi: 10.1016/j.idairyj.2012.10.015. [DOI] [Google Scholar]
- 146.Fakruddin M., Hossain M.N., Ahmed M.M. Antimicrobial and antioxidant activities of Saccharomyces cerevisiae IFST062013, a potential probiotic. BMC Complement. Altern. Med. 2017;26:1142–1153. doi: 10.1186/s12906-017-1591-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Mohd Mutalip S.S., Ab-Rahim S., Rajikin M.H. Vitamin E as an antioxidant in female reproductive health. Antioxidants. 2018;7:22–37. doi: 10.3390/antiox7020022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Jung B., Darvin M.E., Jung S., Albrecht S., Schanzer S., Meinke M.C., Thiede G., Lademann J. Kinetics of the carotenoid concentration degradation of smoothies and their influence on the antioxidant status of the human skin in vivo during 8 weeks daily consumption. Nutr. Res. 2020;81:38–46. doi: 10.1016/j.nutres.2020.06.011. [DOI] [PubMed] [Google Scholar]
- 149.da Costa E., Amaro H.M., Melo T., Guedes A.C., Domingues M.R. Screening for polar lipids, antioxidant, and anti-inflammatory activities of gloeothece sp. lipid extracts pursuing new phytochemicals from cyanobacteria. J. Appl. Phycol. 2020;32:3030–3105. doi: 10.1007/s10811-020-02173-6. [DOI] [Google Scholar]
- 150.Jahanbani R., Ghaffari S.M., Salami M., Vahdati K., Sepehri H., Sarvestani N.N., Sheibani N., Moosavi-Movahedi A.A. Antioxidant and anticancer activities of walnut (Juglans regia L.) protein hydrolysates using different proteases. Plant Foods Hum. Nutr. 2016;71 doi: 10.1007/s11130-016-0576-z. 402-109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Kelishomi Z.H., Goliaei B., Mahdavi H., Nikoofar A., Rahimi M., Moosavi-Movahedi A.A., Mamashli F., Bigdeli B. Antioxidant activity of low molecular weight alginate produced by thermal treatment. Food Chem. 2016;196:897–902. doi: 10.1016/j.foodchem.2015.09.091. [DOI] [PubMed] [Google Scholar]
- 152.Fetoni A.R., Paciello F., Rolesi R., Paludetti G., Troiani D. Targeting dysregulation of redox homeostasis in noise-induced hearing loss: oxidative stress and ROS signaling. Free Rad. Biol. Med. 2019;135:46–59. doi: 10.1016/j.freeradbiomed.2019.02.022. [DOI] [PubMed] [Google Scholar]
- 153.Lammi C., Arnoldi A. Food-derived antioxidants and COVID-19. J. Food Biochem. 2020;45:e13557–e13563. doi: 10.1111/jfbc.13557. [DOI] [PubMed] [Google Scholar]
- 154.Kellett M.E., Greenspan P., Pegg R.B. Modification of the cellular antioxidant activity (CAA) assay to study phenolic antioxidants in a Caco-2 cell line. Food Chem. 2018;244:359–363. doi: 10.1016/j.foodchem.2017.10.035. [DOI] [PubMed] [Google Scholar]
- 155.Guo F., Zhang S., Yan X., Dan Y., Wang J., Zhao Y., Yu Z. Bioassay-guided isolation of antioxidant and a-glucosidase inhibitory constituents from stem of vigna angularis. Bioorgan. Chem. 2019;87:312–320. doi: 10.1016/j.bioorg.2019.03.041. [DOI] [PubMed] [Google Scholar]
- 156.Oddone N., Boury F., Garcion E., Grabrucker A.M., Martinez M.C., Da Ros F., Janaszewska A., Forni F., Vandelli M.A., Tosi G. Dynthesis, characterization, and in vitro studies of an reactive oxygen species (ROS)-responsive methoxy polyethylene glycol-thioketal-melphalan prodrug for glioblastoma treatment. Front. Pharmacol. 2020;11:1–15. doi: 10.3389/fphar.2020.00574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Apak R., Capanoglu E., Shahidi F. John Wiley & Sons; 2018. Measurement of Antioxidant Activity and Capacity: Recent Trends and Applications. [DOI] [Google Scholar]
- 158.Hoyos-Arbeláez J., Vázquez M., Contreras-Calderón J. Electrochemical methods as a tool for determining the antioxidant capacity of food and beverages: a review. Food Chem. 2017;221:1371–1381. doi: 10.1016/j.foodchem.2016.11.017. [DOI] [PubMed] [Google Scholar]
- 159.Li J., Peng Z., Wang E. Tackling grand challenges of the 21st century with electroanalytical chemistry. J. Am. Chem. Soc. 2018;140:10629–10638. doi: 10.1021/jacs.8b01302. [DOI] [PubMed] [Google Scholar]
- 160.Haji-Hashemi H., Norouzi P., Safarnejad M.R., Larijani B., Habibi M.M., Raeisi H., Ganjali M.R. Sensitive electrochemical immunosensor for citrus bacterial canker disease detection using fast fourier transformation square-wave voltammetry method. J. Electroanal. Chem. 2018;820:111–117. doi: 10.1016/j.jelechem.2018.04.062. [DOI] [Google Scholar]
- 161.Mofidi Z., Norouzi P., Sajadian M., Ganjali M.R. Simultaneous extraction and determination of trace amounts of diclofenac from whole blood using supported liquid membrane microextraction and fast fourier transform voltammetry. J. Sep. Sci. 2018;47:1644–1650. doi: 10.1002/jssc.201701119. [DOI] [PubMed] [Google Scholar]
- 162.Norouzi P., Larijani B., Alizadeh T., Pourbasheer E., Aghazadeh M., Ganjali M.R. Application of advanced electrochemical methods with nanomaterial-based electrodes as powerful tools for trace analysis of drugs and toxic compounds. Curr. Anal. Chem. 2019;15:143–151. doi: 10.2174/1573411014666180316170607. [DOI] [Google Scholar]
- 163.Norouzi P., Larijani B., Ganjali M.R., Faridbod F. Admittometric electrochemical determination of atrazine by nano-composite immune-biosensor using FFT-square wave voltammetry. Int. J. Electrochem. Sci. 2012;7:10414–10426. [Google Scholar]
- 164.Esmaeili C., Norouzi P., Zar M.S., Eskandari M., Faridbod F., Ganjali M.R. A FFT square wave voltammetry sensing method for highly sensitive detection of phytic acid using a cerium oxide nanoparticles decorated graphene oxide. J. Electrochem. Soc. 2019;166(15):1630–1637. doi: 10.1149/2.1191914jes/meta. [DOI] [Google Scholar]
- 165.Moghaddam M.R., Ghasemi J.B., Norouzi P., Salehnia F. Simultaneous determination of dihydroxybenzene isomers at nitrogen-doped graphene surface using fast fourier transform square wave voltammetry and multivariate calibration. Microchem. J. 2019;145:596–605. doi: 10.1016/j.microc.2018.11.009. [DOI] [Google Scholar]
- 166.Mohamed M.A. Wearable miniaturized electrochemical sensors: benefits and challenges. Electrochemistry. 2019;15:147–185. doi: 10.1039./9781788013985-00147. [Google Scholar]
- 167.M. Tokeshi (Ed.) Applications of microfluidic systems in biology and medicine. Bioanalysis (Advanced Materials, Methods, and Devices), vol. 7, Springer, Singapore, pp. 71-98, 10.1007/978-981-13-6229-3_4.
- 168.Norouzi P., Haji-Hashemi H., Larijani B., Aghazadeh M., Pourbasheer E., Ganjali M.R. Application of new advanced electrochemical methods combine with nano-based materials sensor in drugs analysis. Curr. Anal. Chem. 2017;13:70–80. [Google Scholar]
- 169.Mofidi Z., Norouzi P., Larijani B., Seidi S., Ganjali M.R., Morshedi M. Simultaneous determination and extraction of ultra-trace amounts of estradiol valerate from whole blood using FFT square wave voltammetry and low-voltage electrically enhanced microextraction techniques. J. Electroanal. Chem. 2018;813:83–91. doi: 10.1016/j.jelechem.2018.01.048. [DOI] [Google Scholar]
- 170.Heiat M., Hashemi-Aghdam M.-R., Heiat F., Panahi M.Rastegar Shariat, Aghamollei H., Moghaddam M.Moosazadeh, Sathyapalan T., Ranjbar R., Sahebkar A. Integrative role of traditional and modern technologies to combat COVID-19. Expert Rev. Anti-Infect. Ther. 2020;19:23–33. doi: 10.1080/14787210.2020.1799784. [DOI] [PubMed] [Google Scholar]
- 171.Long C., Xu H., Shend Q., Zhang X., Fana B., Wang C., Zeng B., Lia Z., Lia X., Li H. Diagnosis of the coronavirus disease (covid-19): RRT-PCR OR CT? Eur. J. Radiol. 2020;126:108961–108966. doi: 10.1016/j.ejrad.2020.108961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 172.Saka C. Analytical methods on determination in pharmaceuticals and biological materials of chloroquine as available for the treatment of COVID-19. Crit. Rev. Anal. Chem. 2020;6:1–16. doi: 10.1080/10408347.2020.1781592. [DOI] [PubMed] [Google Scholar]
- 173.Kumar A., Purohit B., Maurya P.K., Pandey L.M., Chandra P. Engineered nanomaterial assisted signal-amplification strategies for enhancing analytical performance of electrochemical biosensors. Electroanalysis. 2019;31:1615–1629. doi: 10.1002/elan.201900216. [DOI] [Google Scholar]
- 174.Pruna R., Palacio F., Baraket A., Zine N., Streklas A., Bausells J., Errachid A., López M. A low-cost and miniaturized potentiostat for sensing of biomolecular species such as TNF-a by electrochemical impedance spectroscopy. Biosens. Bioelectron. 2018;100:533–540. doi: 10.1016/j.bios.2017.09.049. [DOI] [PubMed] [Google Scholar]
- 175.Samson R., Navale G.R., Dharne M.S. Biosensors: frontiers in rapid detection of COVID-19. Biotech. 2020;10:358–394. doi: 10.1007/s13205-020-02369-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 176.Silveira C.M., Monteiro T., Almeida M.G. Biosensing with paper-based miniaturized printed electrodes–a modern trend. Biosensors. 2016;6:51–60. doi: 10.3390/bios6040051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 177.Li H., Liu X., Li L., Mu X., Genov R., Mason A.J. CMOS electrochemical instrumentation for biosensor microsystems: a review. Sensors. 2017;17:74–100. doi: 10.3390/s17010074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 178.Ünlüer Ö.B., Ghorbani-Bidkorbeh F., Keçili R., Hussain C.M. Handbook on Miniaturization in Analytical Chemistry. Elsevier; 2020. Future of the modern age of analytical chemistry: nanominiaturization; pp. 277–296. [DOI] [Google Scholar]
- 179.Campuzano S., Pedrero M., González-Cortés A., Yáñez-Sedeño P., Pingarrón J.M. Electrochemical biosensors for autoantibodies in autoimmune and cancer diseases. Anal. Methods. 2019;11:871–887. doi: 10.1039/C8AY02742K. [DOI] [Google Scholar]
- 180.Ünlü M.S., Chiari M., Özcan A. Introduction to the special issue of optical biosensors. Nanophoton. 2017;6:623–625. doi: 10.1515/nanoph-2017-0053. [DOI] [Google Scholar]
- 181.Shao B., Xiao Z. Recent achievements in exosomal biomarkers detection by nanomaterials-based optical biosensors a review. Anal. Chim. Acta. 2020;1114:74–84. doi: 10.1016/j.aca.2020.02.041. [DOI] [PubMed] [Google Scholar]
- 182.Liao Z., Zhang Y., Li Y., Miao Y., Gao S., Lin F., Deng Y., Geng L. Microfluidic chip coupled with optical biosensors for simultaneous detection of multiple analytes: a review. Biosens. Bioelectron. 2019;126:697–706. doi: 10.1016/j.bios.2018.11.032. [DOI] [PubMed] [Google Scholar]
- 183.Kim Y., Kim Y., Choi J., Kang T., Choi I. Determination of nanomolar levels of reactive oxygen species in microorganisms and aquatic environments using a single nanoparticle-based optical sensor. Anal. Chim. Acta. 2017;967:85–92. doi: 10.1016/j.aca.2017.03.012. [DOI] [PubMed] [Google Scholar]
- 184.Munzert P., Danz N., Sinibaldi A., Michelotti F. Multilayer coatings for Bloch surface wave optical biosensors. Surf. Coat. Technol. 2017;314:79–84. doi: 10.1016/j.surfcoat.2016.08.029. [DOI] [Google Scholar]
- 185.Chen C., Wang J. Optical biosensors: an exhaustive and comprehensive review. Analyst. 2020;145:1605–1628. doi: 10.1039/C9AN01998G. [DOI] [PubMed] [Google Scholar]
- 186.Li Z., Zhang W., Xing F. Graphene optical biosensors. Int. J. Mol. Sci. 2019;20:2461–2483. doi: 10.3390/ijms20102461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 187.Hong J., Yang W.-Y., Zhao Y.-X., Xiao B.-L., Gao Y.-F., Yang T., Ghourchian H., Moosavi-Movahedi Z., Sheibani N., Li J.-G., Moosavi-Movahedi A.A. Catalase immobilized on a functionalized multi-walled carbon nanotubes–gold nanocomposite as a highly sensitive bio-sensing system for detection of hydrogen peroxide. Electrochim. Acta. 2013;89:317–325. doi: 10.1016/j.electacta.2012.11.054. [DOI] [Google Scholar]
- 188.Khoshaman K., Yousefi R., Tamaddon A.M., Saso L., Moosavi-Movahedi A.A. The impact of hydrogen peroxide on structure, stability and functional properties of human R12C mutant aA-crystallin: the imperative insights into pathomechanism of the associated congenital cataract incidence. Free Rad. Biol. Med. 2015;89:819–830. doi: 10.1016/j.freeradbiomed.2015.09.013. [DOI] [PubMed] [Google Scholar]
- 189.Molaabasi F., Hosseinkhani S., Moosavi-Movahedi A.A., Shamsipur M. Hydrogen peroxide sensitive hemoglobin-capped gold nanoclusters as a fluorescence enhancing sensor for the label-free detection of glucose. RSC Adv. 2015;5:33123–33135. doi: 10.1039/C5RA00335K. [DOI] [Google Scholar]
- 190.Salehi N., Moosavi-Movahedi A.A., Fotouhi L., Yousefinejad S., Shourian M., Hosseinzadeh R., Sheibani N., Habibi-Rezaei M. Heme degradation upon production of endogenous hydrogen peroxide via interaction of hemoglobin with sodium dodecyl sulfate. J. Photochem. Photobiol. B Biol. 2014;133:11–17. doi: 10.1016/j.jphotobiol.2014.02.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 191.Shamsipur M., Asgari M., Maragheh M.G., Moosavi-Movahedi A.A. A novel impedimetric nanobiosensor for low level determination of hydrogen peroxide based on biocatalysis of catalase. Bioelectrochem. 2012;83:31–37. doi: 10.1016/j.bioelechem.2011.08.003. [DOI] [PubMed] [Google Scholar]
- 192.Schönrich G., Raftery M.J., Samstag Y. Devilishly radical network in COVID-19: oxidative stress, neutrophil extracellular traps (NETs), and T cell suppression. Adv. Biol. Reg. 2020;77:100741–100759. doi: 10.1016/j.jbior.2020.100741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 193.Zobel S., Lorenz M., Frascaroli G., Böhnke J., Bilz N.C., Stanifer M.L., Boulant S., Bergs S., Liebert U.G., Claus C. Rubella virus strain-associated differences in the induction of oxidative stress are independent of their interferon activation. Viruses. 2018;10:540–559. doi: 10.3390/v10100540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 194.Kalmar I., Cay A., Tignon M. Sensitivity of african swine fever virus (ASFV) to heat, alkalinity and peroxide treatment in presence or absence of porcine plasma. Vet. Microbiol. 2018;219:144–149. doi: 10.1016/j.vetmic.2018.04.02. [DOI] [PubMed] [Google Scholar]
- 195.Baker K.L., Thomas P.R., Karriker L.A., Ramirez A., Zhang J., Wang C., Holtkamp D.J. Evaluation of an accelerated hydrogen peroxide disinfectant to inactivate porcine epidemic diarrhea virus in swine feces on aluminum surfaces under freezing conditions. BMC Vet. Res. 2017;13:372–383. doi: 10.1186/s12917-017-1300-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 196.Qu Z., Na W., Nie Y., Su X. A novel fluorimetric sensing strategy for highly sensitive detection of phytic acid and hydrogen peroxide. Anal. Chim. Acta. 2018;1039:74–81. doi: 10.1016/j.aca.2018.07.030. [DOI] [PubMed] [Google Scholar]
- 197.Lima M.J.A., Sasaki M.K., Marinho O.R., Freitas T.A., Faria R.C., Reis B.F., Rochaa F.R.P. Spot test for fast determination of hydrogen peroxide as a milk adulterant by smartphone-based digital image colorimetry. Microchem. J. 2020;157:105042–105072. doi: 10.1016/j.microc.2020.105042. [DOI] [Google Scholar]
- 198.Ye S., Hananya N., Green O., Chen H., Zhao A.Q., Shen J., Shabat D., Yang D. A highly selective and sensitive chemiluminescent probe for real-time monitoring of hydrogen peroxide in cells and animals. Angew. Chem. Int. Ed. Engl. 2020;59:14326–14330. doi: 10.1002/anie.202005429. [DOI] [PubMed] [Google Scholar]
- 199.Zou J., Cai H., Wang D., Xiao J., Zhou Z., Yuan B. Spectrophotometric determination of trace hydrogen peroxide via the oxidative coloration of DPD using a Fenton system. Chemosphere. 2019;224:646–652. doi: 10.1016/j.chemosphere.2019.03.005. [DOI] [PubMed] [Google Scholar]
- 200.Zhao C., Wu X., Li P., Zhao C., Qian X. Hydrothermal deposition of CuO/rGO/Cu2O nanocomposite on copper foil for sensitive nonenzymatic voltammetric determination of glucose and hydrogen peroxide. Microchim. Acta. 2017;184:2341–2348. doi: 10.1007/s00604-017-2229-9. [DOI] [Google Scholar]
- 201.Roberts J.G., Voinov M.A., Schmidt A.C., Smirnova T.I., Sombers L.A. The hydroxyl radical is a critical intermediate in the voltammetric detection of hydrogen peroxide. J. Am. Chem. Soc. 2016;138:2516–2519. doi: 10.1021/jacs.5b13376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 203.Laforge M., Elbim C., Frère C., Hémadi M., Massaad C., Nuss P., Benoliel J.-J., Becker C. Tissue damage from neutrophil-induced oxidative stress in COVID-19. Nat. Rev. Immunol. 2020;20:515–516. doi: 10.1038/s41577-020-0407-1. s41577-020-0407-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 204.Younus H. Therapeutic potentials of superoxide dismutase. Int. J. Health Sci. 2018;12:88–93. [PMC free article] [PubMed] [Google Scholar]
- 205.Cai X., Chen H., Wang Z., Sun W., Shi L., Zhao H., Lan M. 3D graphene-based foam induced by phytic acid: an effective enzyme-mimic catalyst for electrochemical detection of cell-released superoxide anion. Biosens. Bioelectron. 2019;123:101–109. doi: 10.1016/j.bios.2018.06.043. [DOI] [PubMed] [Google Scholar]
- 206.Hua A.B., Justiniano R., Perer J., Park S.L., Li H., Cabello C.M., Wondrak G.T. Repurposing the electron transfer reactant phenazine methosulfate (PMS) for the apoptotic elimination of malignant melanoma cells through induction of lethal oxidative and mitochondriotoxic stress. Cancers. 2019;11:590–607. doi: 10.3390/cancers11050590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 207.Zeng N., Zhang G., Hu X., Pan J., Gong D. Mechanism of fisetin suppressing superoxide anion and xanthine oxidase activity. J. Funct. Foods. 2019;58:1–10. doi: 10.1016/j.jff.2019.04.044. [DOI] [Google Scholar]
- 208.Su Y., Song H., Lv Y. Recent advances in chemiluminescence for reactive oxygen species sensing and imaging analysis. Microchem. J. 2019;146:83–97. doi: 10.1016/j.microc.2018.12.056. [DOI] [Google Scholar]
- 209.Lu S., Zhang X., Chen L., Yang P. Colorimetric visualization of superoxide dismutase in serum via etching of au nanorods from superoxide radical. Sensors Actuators B Chem. 2018;259:1066–1072. doi: 10.1016/j.snb.2017.12.164. [DOI] [Google Scholar]
- 210.Hong S.C., Murale D.P., Jang S.Y., Haque M.M., Seo M., Lee S., Woo D.H., Kwon J., Song C.S., Kim Y.K. Discrimination of avian influenza virus subtypes using host-cell infection fingerprinting by a sulfinate-based fluorescence superoxide probe. Angew. Chem. Int. Ed. Engl. 2018;57:9716–9721. doi: 10.1002/anie.201804412. [DOI] [PubMed] [Google Scholar]
- 211.Cortés-Ríos J., Torres M., Campos-Bustamante M., Romero-Parra J., Letelier M., Pessoa-Mahana D., Chung H., Faúndez M. NADPH oxidase activity: spectrophotometric determination of superoxide using pyrogallol red. Anal. Biochem. 2017;536:96–100. doi: 10.1016/j.ab.2017.08.013. [DOI] [PubMed] [Google Scholar]
- 212.Saqib M., Bashir S., Li H., Wang S., Jin Y. Lucigenin-tris (2-carboxyethyl) phosphine chemiluminescence for selective and sensitive detection of TCEP, superoxide dismutase, mercury (II), and dopamine. Anal. Chem. 2019;91:3070–3077. doi: 10.1021/acs.analchem.8b05486. [DOI] [PubMed] [Google Scholar]
- 213.Pirozzi D., Imparato C., D’Errico G., Vitiello G., Aronne A., Sannino F. Three-year lifetime and regeneration of superoxide radicals on the surface of hybrid TiO2 materials exposed to air. J. Hazard. Mater. 2020;387:121716–121725. doi: 10.1016/j.jhazmat.2019.121716. [DOI] [PubMed] [Google Scholar]
- 214.Belli S., Rossi M., Molasky N., Middleton L., Caldwell C., Bartow-McKenney C., Duong M., Chiu J., Gibbs E., Caldwell A. Effective and novel application of hydrodynamic voltammetry to the study of superoxide radical scavenging by natural phenolic antioxidants. Antioxidants. 2019;8:14–34. doi: 10.3390/antiox8010014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 215.Juárez-Gómez J., Ramírez-Silva M.T., Guzmán-Hernández D.S., Romero-Romo M., Palomar-Pardavé M. Novel electrochemical method to evaluate the antioxidant capacity of infusions and beverages, based on in situ formation of free superoxide radicals. Food Chem. 2020;332:127409–127416. doi: 10.1016/j.foodchem.2020.127409. [DOI] [PubMed] [Google Scholar]
- 216.Wang Y., Wang D., Sun L.-H., Xue P., Wang M.-Q., Lu Z., Wang F., Xia Q., Xu M.-W., Bao S.-J. Constructing high effective nano-Mn3(PO4) 2-chitosan in situ electrochemical detection interface for superoxide anions released from living cell. Biosens. Bioelectron. 2019;133:133–140. doi: 10.1016/j.bios.2019.03.029. [DOI] [PubMed] [Google Scholar]
- 217.Braik M., Barsan M.M., Dridi C., Ali M.B., Brett C.M. Highly sensitive amperometric enzyme biosensor for detection of superoxide based on conducting polymer/CNT modified electrodes and superoxide dismutase. Sens. Actuators B Chem. 2016;236:574–582. doi: 10.1016/j.snb.2016.06.032. [DOI] [Google Scholar]
- 218.Singh S. Nanomaterials exhibiting enzyme-like properties (nanozymes): current advances and future perspectives. Front. Chem. 2019;7:46. doi: 10.3389/fchem.2019.00046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 219.Holubowitch N.E., Crabtree C., Budimir Z. Electroanalysis and spectroelectrochemistry of non-aromatic explosives in acetonitrile containing dissolved oxygen. Anal. Chem. 2020;92:11617–11626. doi: 10.1021/acs.analchem.0c01174. [DOI] [PubMed] [Google Scholar]
- 220.Seenivasan R., Kolodziej C., Karunakaran C., Burda C. Nanotechnology for electroanalytical biosensors of reactive oxygen and nitrogen species. Chem. Rec. 2017;17:886–901. doi: 10.1002/tcr.201600143. [DOI] [PubMed] [Google Scholar]
- 221.Malferrari M., Becconi M., Rapino S. Electrochemical monitoring of reactive oxygen/nitrogen species and redox balance in living cells. Anal. Bioanal. Chem. 2019;411:4365–4374. doi: 10.1007/s00216-019-01734-0. [DOI] [PubMed] [Google Scholar]
- 222.Borgmann S. Electrochemical quantification of reactive oxygen and nitrogen: challenges and opportunities. Anal. Bioanal. Chem. 2009;394:95–105. doi: 10.1007/s00216-009-2692-1. [DOI] [PubMed] [Google Scholar]
- 223.Calas-Blanchard C., Catanante G., Noguer T. Electrochemical sensor and biosensor strategies for ROS/RNS detection in biological systems. Electroanal. 2014;26:1277–1286. doi: 10.1002/elan.201400083. [DOI] [Google Scholar]
- 224.Jancinová V., Nosál R., Lojek A., Cíž M., Ambrožová G., Mihalová D., Bauerová K., Harmatha J., Perecko T. Formation of reactive oxygen and nitrogen species in the presence of pinosylvin—an analogue of resveratrol. Neur. Lett. 2010;31:79–83. [PubMed] [Google Scholar]
- 225.Suzuki T., Mower H.F., Friesen M.D., Gilibert I., Sawa T., Ohshima H. Nitration and nitrosation of N-acetyl-L-tryptophan and tryptophan residues in proteins by various reactive nitrogen species. Free Rad. Biol. Med. 2004;37:671–681. doi: 10.1016/j.freeradbiomed.2004.05.030. [DOI] [PubMed] [Google Scholar]
- 226.Zhang X.W., Oleinick A., Jiang H., Liao Q.L., Qiu Q.F., Svir I., Liu Y.L., Amatore C., Huang W.H. Electrochemical monitoring of ROS/RNS homeostasis within individual phagolysosomes inside single macrophages. Angew. Chem. Int. Ed. Engl. 2019;131:7835–7838. doi: 10.1002/ange.201902734. [DOI] [PubMed] [Google Scholar]
- 227.Lu Q.-B. Reaction cycles of halogen species in the immune defense: implications for human health and diseases and the pathology and treatment of COVID-19. Cells. 2020;9:1461–1479. doi: 10.3390/cells9061461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 228.Wang J., Hassan M.M., Ahmad W., Jiao T., Xu Y., Li H., Ouyang Q., Guo Z., Chen Q. A highly structured hollow ZnO@Ag nanosphere SERS substrate for sensing traces of nitrate and nitrite species in pickled food. Sensors Actuators B Chem. 2019;285:302–309. doi: 10.1016/j.snb.2019.01.052. [DOI] [Google Scholar]
- 229.Gill A., Zajda J., Meyerhoff M.E. Comparison of electrochemical nitric oxide detection methods with chemiluminescence for measuring nitrite concentration in food samples. Anal. Chim. Acta. 2019;1077:167–173. doi: 10.1016/j.aca.2019.05.065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 230.Lin S.-L., Hsu J.-W., Fuh M.-R. Simultaneous determination of nitrate and nitrite in vegetables by poly (vinylimidazole-co-ethylene dimethacrylate) monolithic capillary liquid chromatography with UV detection. Talanta. 2019;205:120082–120089. doi: 10.1016/j.talanta.2019.06.082. [DOI] [PubMed] [Google Scholar]
- 231.Moravský L., Troška P., Klas M., Masár M., Matejcík Š. Determination of nitrites and nitrates in plasma-activated deionized water by microchip capillary electrophoresis. Contr. Plasma Phys. 2020;60:e202000014–e202000019. doi: 10.1002/ctpp.202000014. [DOI] [Google Scholar]
- 232.Sharma D., Singh K., Raj P. Validation of a spectrofluorimetric method for the determination of thiram and thiophanate methyl fungicides in environmental samples. Anal. Bioanal. Chem. Res. 2020;7:483–495. doi: 10.22036/ABCR.2020.203999.1405. [DOI] [Google Scholar]
- 233.Li Y., Sella C., Lemaître F., Guille Collignon M., Thouin L., Amatore C. Highly sensitive platinum-black coated platinum electrodes for electrochemical detection of hydrogen peroxide and nitrite in microchannel. Electroanal. 2013;25:895–902. doi: 10.1002/elan.201200456. [DOI] [Google Scholar]
- 234.Li Y., Sella C., Lemaître F., Guille-Collignon M., Thouin L., Amatore C. Electrochemical detection of nitric oxide and peroxynitrite anion in microchannels at highly sensitive platinum-black coated electrodes. application to ROS and RNS mixtures prior to biological investigations. Electrochim. Acta 144. 2014:111–118. doi: 10.1016/j.electacta.2014.08.046. [DOI] [Google Scholar]
- 235.Gholizadeh A., Voiry D., Weisel C., Gow A., Laumbach R., Kipen H., Chhowalla M., Javanmard M. Toward point-of-care management of chronic respiratory conditions: electrochemical sensing of nitrite content in exhaled breath condensate using reduced graphene oxide. Microsyst. Nanoeng. 2017;3:17022. doi: 10.1038/micronano.2017.22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 236.Radhakrishnan S., Krishnamoorthy K., Sekar C., Wilson J., Kim S.J. A highly sensitive electrochemical sensor for nitrite detection based on Fe2O3 nanoparticles decorated reduced graphene oxide nanosheets. App. Catal. B Environ. 2014;148:22–28. doi: 10.1016/j.apcatb.2013.10.044. [DOI] [Google Scholar]
- 237.Terbouche A., Lameche S., Ait-Ramdane-Terbouche C., Guerniche D., Lerari D., Bachari K., Hauchard D. A new electrochemical sensor based on carbon paste electrode/Ru(III) complex for determination of nitrite: electrochemical impedance and cyclic voltammetry measurements. Measurement. 2016;92:524–533. doi: 10.1016/j.measurement.2016.06.034. [DOI] [Google Scholar]
- 238.Pham X.-H., Li C.A., Han K.N., Huynh-Nguyen B.-C., Le T.-H., Ko E., Kim J.H., Seong G.H. Electrochemical detection of nitrite using urchin-like palladium nanostructures on carbon nanotube thin film electrodes. Sensors Actuators B Chem. 2014;193:815–822. doi: 10.1016/j.snb.2013.12.034. [DOI] [Google Scholar]
- 239.Kung C.-W., Chang T.-H., Chou L.-Y., Hupp J.T., Farha O.K., Ho K.-C. Porphyrin-based metal–organic framework thin films for electrochemical nitrite detection. Electrochem. Comm. 2015;58:51–56. doi: 10.1016/j.elecom.2015.06.003. [DOI] [Google Scholar]
- 240.Zou C.E., Yang B., Bin D., Wang J., Li S., Yang P., Wang C., Shiraishi Y., Du Y. Electrochemical synthesis of gold nanoparticles decorated flower-like graphene for high sensitivity detection of nitrite. J. Colloid Interface Sci. 2017;488:135–141. doi: 10.1016/j.jcis.2016.10.088. [DOI] [PubMed] [Google Scholar]
- 241.Bagheri H., Hajian A., Rezaei M., Shirzadmehr A. Composite of cu metal nanoparticles-multiwall carbon nanotubes-reduced graphene oxide as a novel and high performance platform of the electrochemical sensor for simultaneous determination of nitrite and nitrate. J. Hazard. Mater. 2017;324:762–772. doi: 10.1016/j.jhazmat.2016.11.055. [DOI] [PubMed] [Google Scholar]
- 242.Shinde R.B., Veerapandian M., Kaushik A., Manickam P. State-of-art bio-assay systems and electrochemical approaches for nanotoxicity assessment. Front. Bioeng. Biotechnol. 2020;8:325–340. doi: 10.3389/fbioe.2020.00325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 243.Morais A.L., Rijo P., Hernán B., Nicolai M. Biomolecules and electrochemical tools in chronic non-communicable disease surveillance: a systematic review. Biosensors (Basel) 2020;10:121–168. doi: 10.3390/bios10090121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 244.Valentini F. Smart electrochemical portable tools for cultural heritage analysis: a review. Sensors. 2019;19:4303–4336. doi: 10.3390/s19194303. [DOI] [PMC free article] [PubMed] [Google Scholar]