Abstract
Cross-sectional studies of the prevalence of anti-SARS-CoV-2 in representative groups are routinely used for surveillance of public health in Norway. The group of blood donors is easily accessible to provide an estimate over the infection prevalence. Repeated testing of returning donors also generates data about the duration of the antibody response following infection and vaccination.
The aim of the current study was to provide updated information about the development of the pandemic in the blood donor population, and to estimate the number of asymptomatic donors visiting the blood center, in an effort to evaluate the measures to prevent virus spreading between donors and staff.
In the two main blood banks in the Oslo area, all blood donors were offered antibody testing for a period of three months. Almost 12,000 donors were tested, and the mean weekly prevalence of antibody positive donors due to infection was 2.7 % (varied from 2.1 to 4.0 %). The number of donors presenting following vaccination was 810 (6.9 %). An average of 38 % of the infections had been asymptomatic, and 31 % of the antibody-positive donors were unaware of having been infected.
In conclusion, the proportion of blood donors seropositive for anti-SARS-CoV-2 in our blood centers was stable whereas the number of vaccinated blood donors rapidly increased. This indicates that the virus spreading in the third wave of infection in the Oslo area mainly happened in groups underrepresented as blood donors. Health care workers prioritized for early vaccination may be overrepresented in the study period.
Keywords: Blood donor, Coronavirus, SARS-CoV-2 antibody, Prevalence, Norway
1. Introduction
The SARS-CoV-2 pandemic has exhibited diverse characteristics in different communities, and such epidemiological aspects of different countries and population groups have necessitated a plethora of infection surveillance studies. In Norway, the Institute of Public Health performed infection surveillance through weekly testing of randomly selected individuals belonging to previously established study groups in the Oslo area from week 18-50/2020 [1]. Blood donors constitute a selection of healthy adults aged 18 – >70 years, more or less representative of the general population. Antibody seroprevalence studies in blood donors contribute naturally to the data collection, and have been used in a number of countries [[2], [3], [4], [5]]. In Denmark, continuous antibody screening of all donors is being used to estimate the number of asymptomatic and undiagnosed cases [6].
Pandemic spread of SARS-CoV-2 has introduced a number of problems, also for the transfusion services, some of which have been described [7]. Luckily, the infection dynamics in Norway were never of a magnitude to threaten the provision of donated blood; both because the need for blood was reduced, whereas blood donors, most of the time, faithfully kept coming to donate. Because undiagnosed or asymptomatic infected blood donors may introduce the virus in the blood center without being aware of it [3,8,9], strict safety measures are being followed. Practical procedures to protect both donors and staff implies increased work load and psychosocial stress in the personnel group, and have to be balanced against the risk of infection at a given time [7].
This prevalence study was initiated in the same period as Norway was hit by the 3rd wave of COVID-19. The Oslo area had been most affected by positive cases of the infection so far, and in response, lockdown was implemented in an attempt to flatten the COVID-19 curve. There were restrictions of the population's movements, work, gatherings, and general activities. Despite this, kindergartens and primary schools remained open, and the risk of more infection among the younger children was feared as a result of more infectious variants of coronavirus gaining a foothold in Norway [[10], [11], [12]]. Further transmission from children to parents and siblings became a major concern. Children are reported to have mild or no symptoms and this raised the question of whether it could lead to more silent disease transmission. Many of the repeat donors have small children in kindergarten or school. Therefore, one focus of this study was to determine the prevalence of blood donors who had undergone COVID-19, and to establish whether there were hidden cases of SARS-CoV-2 among our blood donors [3,13]. It was therefore interesting to test the blood donor population and ask them whether they had been diagnosed and/or had noticed symptoms possibly being due to SARS-CoV-2 infection. The purpose was to evaluate the risk of infection posed by a donor at the time of donation, based on the prevalence of asymptomatic infections in the blood donor group. In addition, we wanted to collect data on convalescence time, symptoms and antibody levels, to learn more about the immune response to the virus. Also, since a number of blood donors experienced symptoms in the first wave when PCR testing was not generally available, many of them had requested the test.
At the time the project started, vaccination of the elderly was well advanced and the first vaccines had been given to health workers. Considering that a number of health workers are blood donors, we added to the questionnaire a question whether they had received the vaccine or not.
This project therefore aimed to provide updated information about the virus spread in the blood donor group, with some relevance to the general population, and to calculate the number of asymptomatic donors coming to the blood center. We also wanted to learn more about the immunity to SARS-CoV-2 following natural infection and vaccination.
2. Materials and methods
2.1. Participants
The study period was 3 months from Jan. 12th to April 9th in Oslo Blood Center (BiO), and from February 11th to May 14th in Akershus University Hospital (Ahus) Blood Center.
All regular blood donors scheduled for donation in the study period were invited to participate in the study, both whole blood and apheresis donors. Project information and consent forms were posted on the blood bank websites, and as part of the appointment reminder, donors received a link to this information and were encouraged to be informed before arrival.
Upon arrival, donors had to sign the consent form which also included information about previous PCR-testing (date and result), previous antibody testing (date and result), presence of symptoms since February 2020 and vaccination (date and type). In Oslo Blood Center, an extra serum sample was drawn together with routine tests, whereas in Ahus, SARS-CoV-2 antibodies were analyzed in the same tube as routinely performed virus testing.
The studies were approved by the Regional Ethical Committee (REK, numbers: 204104 and 203926).
2.2. Antibody testing
Serum drawn from donors of Oslo Blood Center was analyzed at the Department of Immunology, Oslo University Hospital, using a semi-quantitative microtiterplate assay (Microsphere Affinity Proteomics) developed in-house [7]. The test was positive if both antibodies to RBD (Receptor Binding Domain of the Spike protein) and nucleocapsid were found [14]. Inconclusive tests were reanalyzed by the Department of Microbiology, OUH, where a commercial assay is performed (Roche).
Samples from the blood donors of Ahus Blood Center were analyzed by the Department of Multidisciplinary Laboratory Medicine and Medical Biochemistry, Ahus. Screening investigation was performed with Roche Anti-SARS-CoV-2 nucleocapsid total antibody assays, and positive and inconclusive results were reanalyzed with an alternative method, SARS-CoV-2 S1/S2 IgG, from DiaSorin. This last test was additionally used to detect anti-spike antibodies in samples from blood donors who had received one or two doses of a COVID-19 vaccine.
2.3. Data analysis
Results from antibody testing and donor data from the blood bank information system were used to identify the numbers of donors who had positive antibody tests, and whether they had previously reported symptoms or positive antigen or antibody tests in contact with the blood centers. Data analysis and graphics were performed with Microsoft Excel.
3. Results
In 2020, approximately 31,000 whole blood donations were performed at the Oslo Blood Center, and almost 12,000 at Ahus. In the current study, 8,203 donors from Oslo and 3,589 donors from Ahus consented to participate.
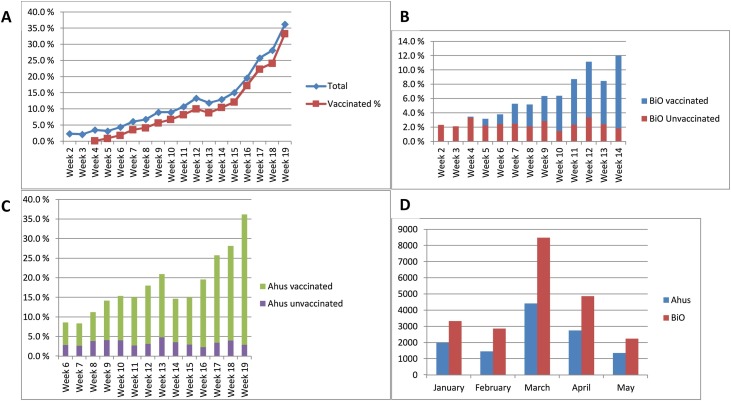
In total, 1,131 donors had a positive test or reported to be vaccinated (9.6 %). The percentage rose rapidly from 2.3 to 36 % in the study period (Fig. 1 A), mostly due to the increased number of vaccinated donors. The prevalence of natural infection varied between 2.1 and 4.0 %, with a mean of 2.7 %. The distribution of positive samples was slightly different in the two blood centers. At BiO, on average 2.4 % (1.5–3.4) were positive due to infection and 3.3 % were vaccinated with one or two doses (Fig. 1B). In Ahus, 3.3 % (2.3–4.8) had been infected whereas 15 % had received one or two vaccine doses (Fig. 1C). Fig. 1D shows the background distribution of positive antigen tests in the geographic area these two blood banks cover, in the study period; The third wave of infection peaked clearly in the month of March.
Fig. 1.
A: Percentage of donors with positive antibody tests by week, both blood centers combined. Blue diamonds: total numbers, red squares: vaccinated donors. The first vaccinated donors were seen in week 4.
B: Percentage of donors with positive antibody tests by week in Oslo Blood Center (BiO). Red columns: unvaccinated donors with antibody profile indicating natural infection with SARS-CoV-2 in the past. Blue columns: vaccinated donors.
C: Percentage of donors from the Ahus Blood Center by week. Purple columns: unvaccinated donors who have recovered from natural SARS-CoV-2 infection. Green columns: vaccinated donors.
D: Number of positive SARS-CoV-2 antigen tests in the general population in the areas relevant to the blood banks. Ahus: Districts around Oslo, including four heavily populated areas within Oslo. BiO: The rest of the Oslo districts. Data from the Norwegian Surveillance System for Communicable Diseases (MSIS), at the Norwegian Institute of Public Health.
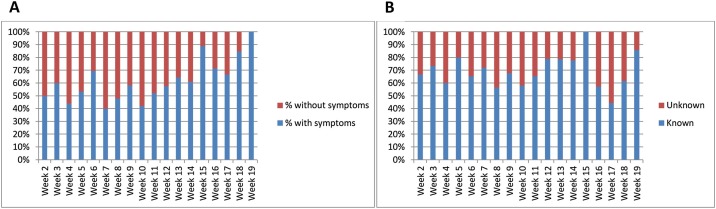
Based on reports from the donors and information in the blood bank systems, we found that 62 % of the donors who had positive antibody tests due to natural SARS-CoV-2 infection, had experienced symptoms of COVID-19 to some degree, whereas 38 % had not reported symptoms. The fraction of asymptomatic infections varied per week, from 0 to 60 percent (Fig. 2 A). Similarly, the fraction of donors who presented with antibodies without knowledge of having been infected, was 31 %, varying between 0 and 56 % (Fig. 2B).
Fig. 2.
A: Percentage of donors who have reported symptoms indicating SARS-CoV-2 infection, per week. Data from both blood centers combined.
B: Percentage of donors with previously established SARS-CoV-2 infection by way of antigen or antibody tests. Data from both blood centers combined.
4. Discussion
In a study of 1,912 residual sera from Norwegian microbiology laboratories collected in January 2021, the sero-prevalence in the Norwegian population was estimated to 3.2 % [15]. This reflects the status before the third wave of infection hit Norway. Although the sample size is small compared to the current study, the results are similar, both studies measuring antibodies produced following the infections caused by the first two waves of virus spread. In spite of an increased number of infected persons in the larger Oslo area following the import of the “UK” virus variant (B.1.1.7) [10], the prevalence of SARS-CoV-2 antibodies in blood donors in the Oslo area did not increase notably during the study period. This may have several explanations.
The typical blood donor is a middle-aged, established person without risk behavior [16,17]. We expect this population to take seriously the advice of precaution issued by health authorities, and successfully protect themselves from virus spread. In contrast, in the population groups where the virus has spread fast this spring, e.g., ethnic minorities and youngsters, blood donation is less common.
Secondly, the timing of the study may have failed to include the persons contracting the virus in the third wave of infection, since they are deferred from blood donation for 28 days following infection, mostly to ensure that they met the health requirements for donation [18]. The closing of the study coincided roughly with the peak of the third wave, as shown in Fig. 1D, and it is clear in retrospect that continued screening could have revealed different results when sufferers of the third wave return to donation. However, at this time the extra logistics of this sampling had become a resource challenge, at least for the Oslo Blood Center, and the project was not prolonged beyond the original plan.
Also, COVID-19 leads to protracted symptoms in a subset of sufferers [19], preventing them from blood donation for several months. Data from COVID-19 convalescent donors of plasma indicate that reduced hemoglobin levels compared to pre-infection values are a general feature of COVID-19 [Nissen-Meyer et al., unpublished] and this was a major source of donor deferral in a study from New York [20]. Additionally, the antibody screening will fail to identify donors with a weak antibody response or whose antibody levels over time have decreased below the detection limit. Although the test is sensitive, a number of persons respond to infection with a low, transient antibody response [21], often waning within 4 months [22]. Several convalescents from the first waves of infection may have therefore gone undetected by this project.
Both blood centers display similarly stable fractions of antibody-positive donors, but slightly higher in the Ahus Blood Center. A possible explanation for this is the higher distribution of new virus cases in districts geographically located in Oslo, but belonging to Ahus Blood Center. In these parts of the city, where a somewhat larger proportion of immigrants and youngsters can be found, virus spread has been most extensive (Fig. 1D).
There are few studies on the prevalence of anti-SARS-CoV-2 in blood donors. Two of them reported 1.9 % sero-prevalence in blood donors in Denmark, and 2.7 % in the Netherlands [4,5]. Apart from the fact that these studies were performed at a very early stage in the pandemic, the lower prevalence may also be due to the selection bias known as the “healthy donor effect” [23], reminding us that these data must be interpreted with caution, and in adequate sample sizes [24].
A relatively high proportion of donors reported having had asymptomatic infection with SARS-CoV-2 (Fig. 2A), and together with the number of previously unknown infections detected in this study (Fig. 2B) it seems reasonable to conclude that the security measures taken in the blood bank have been warranted [7]. The statistical risk for an asymptomatic donor to bring the virus into the blood center without knowing it is small, but considering the number of donors visiting the blood banks, there is certainly a need for protective measures. We believe the combination of pre-visit information given to donors and personal protection equipment has been vital in preventing virus spread on our sites. Blood donation, being non-remunerable and voluntary, depends heavily on the degree to which donors can feel safe when they visit the donation site.
As expected, we found an increasing linear trend in the prevalence of vaccinated donors along the study period. In fact, this increase was a lot higher than expected. Vaccination of health care workers in Norway was given priority in January 2021 when forecasts of the third SARS-CoV-2 wave indicated large numbers of COVID-19 patients to occupy intensive care beds and possibly exceed hospital capacity. Starting with intensive care workers and key personnel critical for operation both in hospitals and in primary care health services, vaccines were distributed to prevent the coming crisis. Although we did expect an increased number of blood donors to request testing based on the large estimates of non-diagnosed SARS-CoV-2 infection from 2020, the number of vaccinated donors arriving was surprising. Vaccinated health workers obviously took the opportunity to rejuvenate their blood donor status, in return for the antibody testing to prove the effect of the vaccine and support their status of immunity. This widespread interest in vaccine efficacy may also have been aggravated by the reports of serious cases of atypical thrombocytopenia and thrombosis in younger people vaccinated with the Astra Zeneca vaccine [25]. For blood banks, always struggling to keep the donor numbers high, this is a welcome side effect, although it remains to be seen if these donors will return to repeated donations in the future.
The participants of the current project have consented to repeated testing and follow-up of their immune status at later donations, and this will provide valuable material for research. We look forward to presenting a more detailed characterisation of these donors and their immune responses to both infection and vaccination in a future paper.
Acknowledgments
We thank all the blood donors for participation and everyone involved in this project in the blood centers. Special thanks to Jerard Seghatchian for inspiration and encouragement. The projects have been run in the routine service in the respective blood centers and have not received any additional funding.
References
- 1.https://www.fhi.no/studier/prevalensundersokelser-korona/resultat---moba/ [Accessed June 14th, 2021].
- 2.Lieshout-Krikke R., O’Brien S., Saeed S., Lewin A. COVID-19: blood centers rise to the occasion and lead seroprevalence studies. Transfusion Today. 2020;125(December):10–11. [Google Scholar]
- 3.Lewin A., Therrien R., De Serres G., Grégoire Y., Perreault J., Drouin M., et al. SARS-CoV-2 seroprevalence among blood donors in Québec, and analysis of symptoms associated with seropositivity: a nested case-control study. Can J Public Health. 2021 doi: 10.17269/s41997-021-00531-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Erikstrup C., Hother C.E., Pedersen O.B.V., Mølbak K., Skov R.L., Holm D.K., et al. Estimation of SARS-CoV-2 infection fatality rate by real-time antibody screening of blood donors. Clin Infect Dis. 2021;72(Jan. (2)):249–253. doi: 10.1093/cid/ciaa849. PMID: 33501969; PMCID: PMC7337681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Slot E., Hogema B.M., Reusken C.B.E.M., Reimerink J.H., Molier M., Karregat J.H.M., et al. Low SARS-CoV-2 seroprevalence in blood donors in the early COVID-19 epidemic in the Netherlands. Nat Commun. 2020;11(Nov. (1)):5744. doi: 10.1038/s41467-020-19481-7. PMID: 33184284; PMCID: PMC7665189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.https://bloddonor.dk/coronavirus/ [accessed June 14th, 2021].
- 7.Nissen-Meyer L.S.H., Brantsæter A.B. The story of an extraordinary year: challenges and opportunities in responding to Covid-19. Transfus Apher Sci. 2021;60(Apr. (2)) doi: 10.1016/j.transci.2021.103092. Epub 2021 Feb 16. PMID: 33612447; PMCID: PMC7885672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Jung C.Y., Park H., Kim D.W., Choi Y.J., Kim S.W., Chang T.I. Clinical characteristics of asymptomatic patients with COVID-19: a nationwide cohort study in South Korea. Int J Infect Dis. 2020;99 doi: 10.1016/j.ijid.2020.08.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Rothe C., Schunk M., Sothmann P., Bretzel G., Groeschl G., Wallrauch C., et al. Transmission of 2019-nCoV infection from an asymptomatic contact in Germany. N Engl J Med. 2020;382:970–971. doi: 10.1056/NEJMc2001468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Davies N.G., Abbott S., Barnard R.C., Jarvis C.I., Kucharski A.J., Munday J.D., et al. Estimated transmissibility and impact of SARS-CoV-2 lineage B.1.1.7 in England. Science. 2021;372(Apr. (6538)) doi: 10.1126/science.abg3055. Epub 2021 Mar 3. PMID: 33658326; PMCID: PMC8128288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ratmann O., Bhatt S., Flaxman S. Implications of a highly transmissible variant of SARS-CoV-2 for children. Arch Dis Child. 2021 doi: 10.1136/archdischild-2021-321903. Published Online First: 30 March. [DOI] [PubMed] [Google Scholar]
- 12.Ulyte A., Radtke T., Abela I.A., Haile S.R., Berger C., Huber M., et al. Clustering and longitudinal change in SARS-CoV-2 seroprevalence in school children in the canton of Zurich, Switzerland: prospective cohort study of 55 schools. BMJ. 2021;372:n616. doi: 10.1136/bmj.n616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Corman V.M., Rabenau H.F., Adams O., Oberle D., Funk M.B., Keller-Stanislawski B., et al. SARS-CoV-2 asymptomatic and symptomatic patients and risk for transfusion transmission. Transfusion. 2020;60(Jun. (6)):1119–1122. doi: 10.1111/trf.15841. Epub 2020 May 27. PMID: 32361996; PMCID: PMC7267331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Holter J.C., Pischke S.E., de Boer E., Lind A., Jenum S., Holten A.R., et al. Systemic complement activation is associated with respiratory failure in COVID-19 hospitalized patients. Proc Natl Acad Sci U S A. 2020;117(40):25018–25025. doi: 10.1073/pnas.2010540117. PubMed 32943538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Tunheim G., Kran A.B., Rø G., Hungnes O., Lund-Johansen F., Vaage E.B., et al. Norwegian Institute of Public Health; Oslo: 2021. Seroprevalence of SARS-CoV-2 in the Norwegian population measured in residual sera collected in January 2021. Report 2021.https://www.fhi.no/globalassets/dokumenterfiler/rapporter/2021/seroprevalence-of-sars-cov-2.pdf [Accessed June 14th, 2021] [Google Scholar]
- 16.Ullum H., Rostgaard K., Kamper-Jørgensen M., Reilly M., Melbye M., Nyrén O., et al. Blood donation and blood donor mortality after adjustment for a healthy donor effect. Transfusion. 2015;55(Oct. (10)):2479–2485. doi: 10.1111/trf.13205. Epub 2015 Jun 22. PMID: 26098293. [DOI] [PubMed] [Google Scholar]
- 17.Mousavi S.A., Hermundstad B., Saether P.C., Nybruket M.J., Knutsen T.R., Llohn A.H. Health behavior and lifestyle trends among platelet donors: results from a questionnaire-based survey in Norway. Biomed Res Int. 2021;2021(Mar) doi: 10.1155/2021/8891885. PMID: 33860057; PMCID: PMC8009726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Nissen-Meyer L.S.H., Seghatchian J. Donor health assessment - when is blood donation safe? Transfus Apher Sci. 2019;58(Feb. (1)):113–116. doi: 10.1016/j.transci.2018.12.016. Epub 2018 Dec 31. PMID: 30630765. [DOI] [PubMed] [Google Scholar]
- 19.Yong S.J. Long COVID or post-COVID-19 syndrome: putative pathophysiology, risk factors, and treatments. Infect Dis. 2021 doi: 10.1080/23744235.2021.1924397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Jain S., Garg K., Tran S.M., Rask I.L., Tarczon M., Nandi V., et al. Characteristics of coronavirus disease 19 convalescent plasma donors and donations in the New York metropolitan area. Transfusion. 2021:1–10. doi: 10.1111/trf.16421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Figueiredo-Campos P., Blankenhaus B., Mota C., Gomes A., Serrano M., Ariotti S., et al. Seroprevalence of anti-SARS-CoV-2 antibodies in COVID-19 patients and healthy volunteers up to 6 months post disease onset. Eur J Immunol. 2020;50(Dec. (12)):2025–2040. doi: 10.1002/eji.202048970. Epub 2020 Nov 10. PMID: 33084029; PMCID: PMC7756220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Perreault J., Tremblay T., Fournier M.J., Drouin M., Beaudoin-Bussières G., Prévost J., et al. Waning of SARS-CoV-2 RBD antibodies in longitudinal convalescent plasma samples within 4 months after symptom onset. Blood. 2020;136(Nov. (22)):2588–2591. doi: 10.1182/blood.2020008367. PMID: 33001206; PMCID: PMC7714093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Shehu E., Hofmann A., Clement M., Langmaack A.C. Healthy donor effect and satisfaction with health: the role of selection effects related to blood donation behavior. Eur J Health Econ. 2015;16(Sep. (7)):733–745. doi: 10.1007/s10198-014-0625-1. Epub 2014 Aug 29. PMID: 25168291. [DOI] [PubMed] [Google Scholar]
- 24.Sughayer M.A., Mansour A., Al Nuirat A., Souan L., Ghanem M., Siag M. Dramatic rise in seroprevalence rates of SARS-CoV-2 antibodies among healthy blood donors: the evolution of a pandemic. Int J Infect Dis. 2021;107 doi: 10.1016/j.ijid.2021.04.059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Schultz N.H., Sørvoll I.H., Michelsen A.E., Munthe L.A., Lund-Johansen F., Ahlen M.T., et al. Thrombosis and thrombocytopenia after ChAdOx1 nCoV-19 vaccination. N Engl J Med. 2021;384(Jun. (22)):2124–2130. doi: 10.1056/NEJMoa2104882. Epub 2021 Apr 9. PMID: 33835768; PMCID: PMC8112568. [DOI] [PMC free article] [PubMed] [Google Scholar]