Abstract
Background
COVID–19 infection has spread so fast in both low– and high–income countries. In December 2019, an outbreak of a respiratory disease occurred in China, and later, it involved different countries. Acute neurological insults are more likely to occur in severely infected patients.
Methods
We tried to evaluate patients with selective criteria including, the age of participants 18 and older with a confirmed diagnosis of SARS-CoV-2, and developed neurological complications post COVID-19 infection. An overall data of 1500 patients were collected from neurological and primary health care departments. About 970 of them had neurological problems. Patients-related data were gathered and assembled from the patients’ records at participating hospitals from the Ministry of Health and university hospitals.
Results
We presented the results according to several variables including, regional distribution, reasons of presentation, neurological complications, follow-ups, and survival outcome.
Conclusions
To our knowledge, we conducted the first retrospective analysis for neurological problems related to COVID-19 infection in Egypt. COVID-19 patients present with a variety of central and peripheral neurological symptoms, the pathogenic mechanisms of which have not been explained. Robust investigations of the neurological presentations of COVID-19 infection should be recruited for better understanding of the possible association. Moreover, further explaining the pathophysiologic mechanisms will help in designing proper treatment plans.
Keywords: COVID-19, Egypt, Multicenter, Neurological complication, SARS-CoV-2
1. Introduction
COVID-19 infection has spread so fast in both low and high income countries. In December 2019, an outbreak of a respiratory disease occurred in China [1]. The most common presenting symptoms are fever (87.9%), dry cough (67.7%), and fatigue (38.1%) [2]. COVID 19 infection has an incubation period from 1 to 14 days. It is enough time to spread the infection even with being asymptomatic. The most common presentation is usually involving the respiratory system as stipulated. Neurological manifestations can present in early infection or as a late COVID-19 presentation. In three hospitals of Wuhan, About 36.4% of COVID-19 patients had various neurological problems [3]. Acute neurological insults are more likely to occur in severely infected patients [4]. Another case series from France evaluated the neurological problems among COVID-19 patients. 84% of patients suffered from neurological complications [5]. At Beijing Ditan Hospital, researchers detected viral particles in the cerebrospinal fluid of a case with COVID-19 viral encephalitis [6].
SAR-CoV and Middle East respiratory disease (MER) CoV was experimentally inoculated into the brain of transegenic mice and it reached the thalamus and the brain stem [7]. Beside the cells of the respiratory tract, SARS-CoV-2 receptors, the Angiotensin Converting Enzyme (ACE)-2, are expressed also in nervous tissue [8]. Another molecule CD147 which is present in neuronal, myeloid and lymphoid tissues is also suggested to play a role in SARS-CoV-2 invasion [9]. 80–110 nm viral particles have been observed in samples from frontal lobes of patients with COVID-19 infection [10]. Therefore, it is very critical to monitor and investigate COVID-19 infected patients for any neurological problems.
Thus, investigators should analyze the neurological presentations and complications caused by COVID-19. This is the first report on the neurological manifestations of COVID-19 in Egypt. This national retrospective study investigates the neurological manifestations of COVID-19 patients in Egypt.
1.1. Study aim
The study aims to collect information on neurological manifestations of COVID-19 patients. The data will focus on the common neurological presentations in the context of COVID-19.
1.2. Study design
We designed a multicenter observational retrospective study, at the national level. Both university-level and community isolation hospitals participated in this study. In this study, all patients were offered a follow-up over one to three months. Adults diagnosed with COVID-19 according to the WHO guidelines were examined. Many patients were isolated at home until they recover. Physicians advised them to follow up at the outpatient clinic if they developed new symptoms. Data collection and monitoring of patients started from March 2020 to March 2021. Institutional Review Board of Ministry of Health hospitals and university hospitals approved it.
2. Methods
2.1. Participating individuals
All participants are aged 18 or over and have the following criteria:
Positive PCR test for SARS-CoV-2 RNA on a nasopharyngeal swab and/or positive serological test and/or positive chest CT for interstitial pneumonia due to COVID-19.
Presenting symptoms of COVID-19 infection and appearance of neurological symptoms or signs for patients either as a first presentation or on follow-up.
Each participant in the study has written informed consent and was aware of the purpose of the study.
2.2. Defining neurological presentations
We defined neurological problems in the context of COVID-19 as the following: Stroke: We considered stroke in patients with acute onset of a vascular insult. This insult can be diagnosed using CT or MRI. Encephalitis: We defined encephalitis as an altered mental status of more than 24 h. The presence of compatible acute lesions on brain MRI and CSF findings is also diagnostic [11], [12]. All patients with encephalitis should have a CSF examination. Encephalopathy: We defined encephalopathy as an altered mental status lasting ≥ 24 h. Seizures and/or focal neurological signs in the absence of encephalitis are diagnostic [12]. We should rule out other causes as metabolic factors, and drug-induced. Guillain-Barré syndrome: We defined Guillain-Barré syndrome (GBS) according to the established diagnostic criteria
2.3. Data gathering
A primary care physician and a clinical-phase medical student collected the data. We designed a robust data collection that includes epidemiological, clinical, and laboratory data. We collected data in paperwork sheets and saved the excel sheets on a secure system. The main data repository included all the data extracted in Egypt. Two primary health care physicians and a clinical-phase medical student revised the data.
2.4. Baseline characteristics and sample size
Baseline characteristics of the patients are listed in Table 1 .
Table 1.
Baseline characteristics of the patients.
| Males (n = 444) |
Females (n = 533) |
Total (n = 977) |
P-value | ||||
|---|---|---|---|---|---|---|---|
| Mean | SD | Mean | SD | Mean | SD | ||
| Age at admission (years) | 58.81 | 13.8 | 61.27 | 16.7 | 60.15 | 15.47 | 0.05 |
| Hospital stay length (days) | 10.94 | 11.25 | 10.53 | 9.9 | 10.71 | 10.52 | 0.04* |
| Body mass index | 31.49 | 9.7 | 36.72 | 11.21 | 34.35 | 10.87 | 0.1 |
| Heart rate | 81.6 | 6.34 | 90 | 16.6 | 85.9 | 12.8 | 0.044* |
| Systolic blood pressure | 120 | 10.95 | 126.5 | 22.4 | 123.25 | 17.15 | 0.047* |
| Diastolic blood pressure | 72 | 10.48 | 77.66 | 16.46 | 74.83 | 13.48 | 0.051 |
| Oxygen saturation | 92.33 | 2.06 | 90.33 | 5.81 | 91.33 | 4.29 | 0.063 |
| Temperature (Celsius) | 38.1 | 0.75 | 38.66 | 0.9 | 38.38 | 0.83 | 0.03* |
| Respiratory rate | 21.2 | 1.16 | 21.5 | 5.13 | 21.33 | 3.55 | 0.04* |
| Glasgow coma scale | 14 | 1.095 | 14.17 | 1.17 | 14.08 | 1.08 | 0.32 |
| pH | 7.35 | 0.18 | 7.43 | 0.08 | 7.39 | 0.14 | 0.12 |
| Hemoglobin (g/L) | 144.33 | 7.17 | 139.83 | 16.83 | 142.08 | 12.6 | 0.14 |
| Lymphocytes (109/L) | 3.372 | 1.67 | 1.16 | 0.93 | 2.27 | 1.73 | 0.04* |
| Neutrophils (109/L) | 5.43 | 1.8 | 15.98 | 10.57 | 10.7 | 9.1 | 0.047* |
| C-reactive protein test (mg/L) | 49.16 | 10.33 | 91.33 | 20.9 | 70.25 | 15.4 | 0.039* |
| D-Dimer (ng/mL) | 34.9 | 5.1 | 11.76 | 9.13 | 23.33 | 6.8 | 0.046* |
Denotes statistical significance.
2.5. Statistical analysis
Statistical analyses were done using STATA 16.0. Descriptive statistics were used to analyze the collected data according to the variables.
3. Results
3.1. Regional distribution
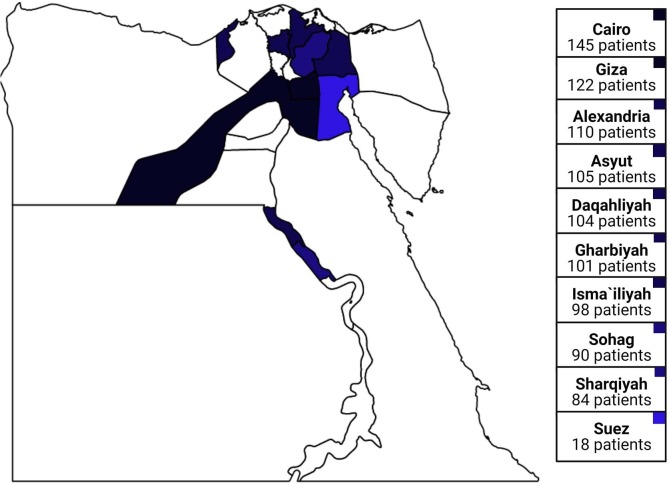
The regional distribution of the data is illustrated in Fig. 1 . Most of the cases were reported in Cairo; while the smallest numbers were reported in Suez.
Fig. 1.
Distribution of the patients across Egypt. (Darker blue refers to more cases). (For interpretation of the references to colour in this figure legend, the reader is referred to the web version of this article.)
3.2. Reason for presentation
Reasons for the presentation of the cases have been listed in Table 2 .
Table 2.
Common presentations.
| Reason of presentation | Number of patients |
|---|---|
| Fatigue | 780 (M = 468, F = 312) |
| Headache | 603 (M = 180, F = 423) |
| Shorting of breath | 588 (M = 235, F = 353) |
| Neuralgia | 503 (M = 250, F = 253) |
| Convulsions | 461 (M = 305, F = 156) |
| Hyperpyrexia | 426 (M = 192, F = 234) |
| Altered mental status | 364 (M = 138, F = 226) |
| Nausea, vomiting and diarrhea | 268 (M = 169, F = 99) |
| Myalgia | 256 (M = 123, F = 133) |
| Chest pain | 240 (M = 77, F = 163) |
| Weakness | 222 (M = 108, F = 114) |
| Memory Problems | 203 (M = 128, F = 75) |
| Cough | 190 (M = 86, F = 104) |
| Hypoxia | 190 (M = 99, F = 91) |
| Syncope | 104 (M = 42, F = 62) |
*M = Male, F = Female.
3.3. Neurological complications
Neurological presentations in the context of COVID-19 infection are listed in Table 3 . The majority of manifestations were optic neuritis, seizures and ataxia. While in rare cases, acute ischemic stroke, and cerebral venous sinus thrombosis developed.
Table 3.
Neurological problems in the context of the COVID-19 infection.
| Neurological complications | Number of patients |
|---|---|
| Optic neuritis | 230 (M = 138, F = 92) |
| Seizures | 201 (M = 129, F = 72) |
| Ataxia | 190 (M = 105, F = 85) |
| Viral encephalitis | 158 (M = 76, F = 82) |
| Acute necrotizing encephalopathy | 134 (M = 80, F = 54) |
| Guillain-barré syndrome | 102 (M = 72, F = 30) |
| Acute ischemic stroke | 88 (M = 50, F = 38) |
| Cerebral venous sinus thrombosis | 53 (M = 41, F = 12) |
*M = Male, F = Female.
3.4. Regression analysis
We used logistic regression to detect the relation of the COVID-19 infection and the neurological presentations. Immunosuppressors, smoking, hypertension and epilepsy were significant factors with a high mortality outcome. Asthma, obesity, diabetes, migraine, cerebrovascular disease, encephalitis and cardiovascular disorders were associated with mortality Table 4 .
Table 4.
Logistic regression of the present medical history and mortality outcome.
| Mean | SE | P-value | 95% CI |
||
|---|---|---|---|---|---|
| Lower limit | Upper limit | ||||
| Immunosuppressors | 2.104956 | 0.6139061 | 0.011 | 1.18848 | 3.728157 |
| Smoking | 0.713269 | 0.119458 | 0.044 | 0.513683 | 0.990404 |
| Asthma | 1.174157 | 0.24098 | 0.434 | 0.785288 | 1.755592 |
| Obesity | 0.830926 | 0.130105 | 0.237 | 0.611339 | 1.129385 |
| Hypertension | 2.019615 | 0.432393 | 0.001 | 1.327484 | 3.072613 |
| Diabetes | 1.120831 | 0.173299 | 0.461 | 0.82781 | 1.517574 |
| Migraine | 1.766509 | 1.090456 | 0.357 | 0.526835 | 5.923218 |
| Epilepsy | 2.75 | 0.964457 | 0.004 | 1.38295 | 5.468384 |
| Cerebrovascular disease | 1.113732 | 0.247249 | 0.628 | 0.720798 | 1.720869 |
| Encephalitis | 1.766509 | 1.090456 | 0.357 | 0.526835 | 5.923218 |
| Cardiovascular disorders | 1.223969 | 0.213188 | 0.246 | 0.869982 | 1.721991 |
3.5. Risk factors correlations
The correlation between age and stroke (r = 0.1) was considered weak. Therefore, age is not a risk factor for patients’ who developed cerebrovascular stroke. We also noticed that there is no evident correlation between cerebrovascular stroke and diabetes (r = 0). Therefore, diabetes is not a risk factor for patients’ who developed cerebrovascular stroke. Table 5
Table 5.
Pearson’s correlation between the variables of patients’ profiles.
| Asthma | BMI | Obesity | Hypertension | Diabetes | Migraine | Epilepsy | Cerebrovascular Stroke | Age | |
|---|---|---|---|---|---|---|---|---|---|
| Asthma | 1 | ||||||||
| BMI | 0.2 | 1 | |||||||
| Obesity | 0.1 | 0.7 | 1 | ||||||
| Hypertension | 0.1 | 0.1 | 0.1 | 1 | |||||
| Diabetes | 0.1 | 0.1 | 0.1 | 0.3 | 1 | ||||
| Migraine | 0.2 | 0 | 0 | 0.1 | 0 | 1 | |||
| Epilepsy | 0.3 | 0.1 | 0 | 0.1 | 0 | 0.2 | 1 | ||
| Cerebrovascular Stroke | 0.1 | −0.1 | 0 | 0.2 | 0 | 0 | 0.1 | 1 | |
| Age | 0.1 | −0.3 | −0.3 | 0.3 | 0 | 0 | −0.1 | 0.1 | 1 |
3.6. Follow-up
Patients had a follow-up over one and three-month period. Results are listed in Table 6 .
Table 6.
Follow-up of neurological manifestations among the surviving patients.
| One month follow-up |
Three month follow-up |
|||
|---|---|---|---|---|
| Male | Female | Male | Female | |
| Optic neuritis | 3 | 2 | 0 | 0 |
| Seizures | 3 | 1 | 0 | 1 |
| Ataxia | 2 | 2 | 0 | 0 |
| Viral encephalitis | 2 | 1 | 0 | 0 |
| Acute necrotizing encephalopathy | 2 | 1 | 0 | 0 |
| Guillain-barré syndrome | 1 | 1 | 0 | 0 |
| Acute ischemic stroke | 2 | 0 | 0 | 1 |
3.7. Survival outcome
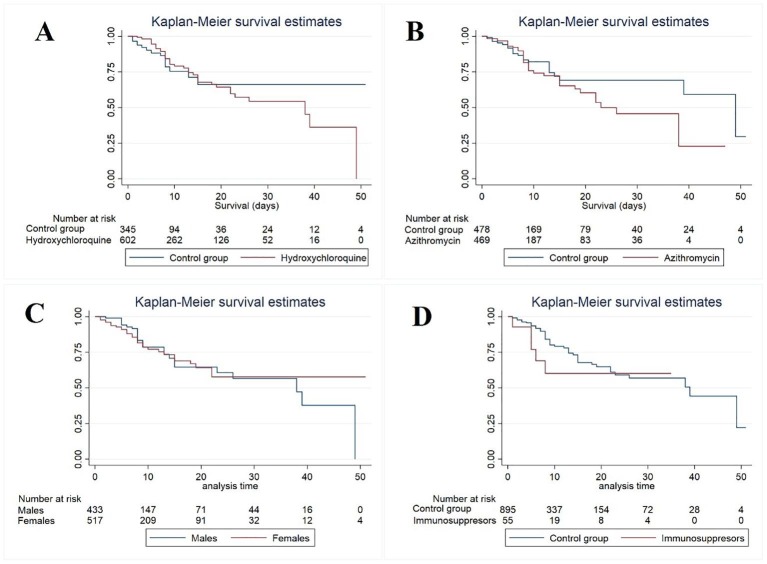
We analyzed mortality among gender, patients who received hydroxychloroquine, azithromycin, and immunosuppressors. Males were more affected with higher mortality than females. Fig. 2 C. Neither hydroxychloroquine nor azithromycin benefited COVID-19 patients with neurological complications. Fig. 2A and B. People using immunosuppressors had a higher mortality rate than people who did not Fig. 2D.
Fig. 2.
Survival analysis among four variables.
4. Discussion
SARS-CoV-2, can cross the blood-brain barrier and cause a direct brain infection [11]. ACE2 is expressed in skeletal muscles and the brain which makes them targets of SARS-CoV-2 [8], [13]. The acute respiratory distress caused by COVID may be caused by CNS inflammation [14]. Moreover, the robust immune response can trigger autoimmune reaction [15]. COVID-19 also promotes the production of antiphospholipid antibodies that induce a hypercoagulable state [16]. The examinations of brain autopsies of COVID-19 patients confirmed its presence [17]. Some studies described also its presence in CNS [10], [18], [19] while others denied its presence at all [20], [21]. Infection of the olfactory neurons can allow the virus to spread from the airways to the brain [13], [16], [22]. In a case report, the penetration of SARS-CoV-2 was possible, and caused viral encephalitis [23]. Yet, cellular reactive changes typical of brain viral infections were not described [24]. We analyzed the demographic, clinical, and mortality data of COVID-19 patients in Egypt. Our study includes a large cross-section of the Egyptian population infected with COVID-19. This observational study aims to detect patients with neurologic manifestations in the context of COVID-19. We were cautious to differentiate between cases where neurological disease is directly caused by COVID-19 from those non-related comorbidities. The separation between true causality and non-etiological coincidence of certain neurological problems is considered a challenge. Positive PCR for COVID-19 and the temporal association of COVID-19 with the presenting problem can add suggest COVID-19 as a cause of the presenting neurological problem. This analysis will familiarize neurologists with the clinical features of NeuroCOVID. We collected these data considering the high infection rate in Egypt. All neurological problems were documented. We tried to follow up with the patients over one to three months.
4.1. Encephalitis
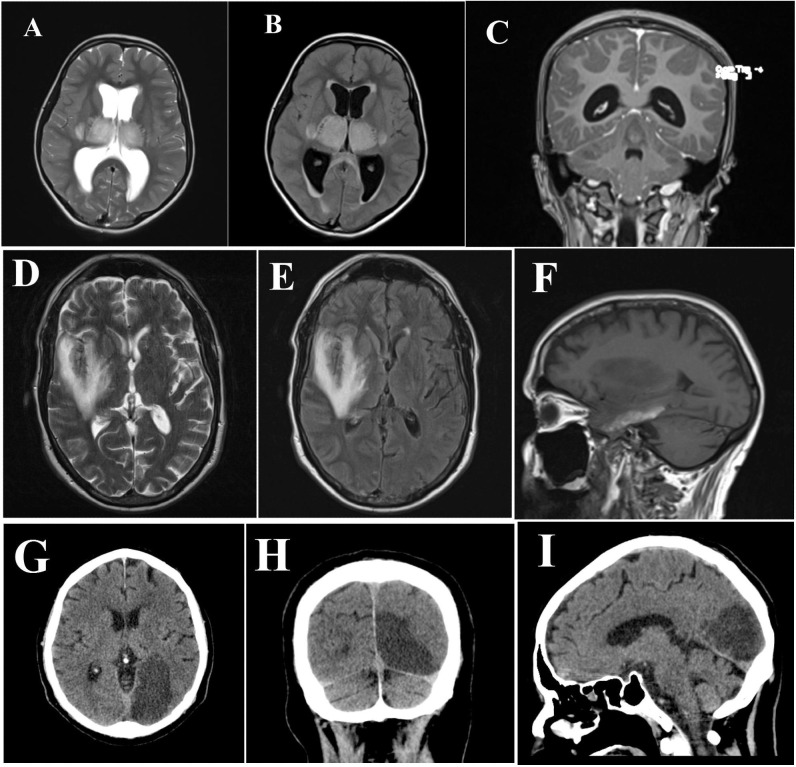
MRI was done only for 34 patients with encephalitis due to the limited availability. The MRI findings of COVID-19 encephalitis included white matter lesions, demyelinating hyper-intensities, leptomeningeal enhancement, and necrotic hemorrhages Fig. 3 . About 21 patients with encephalitis had MRI microvascular brain lesions. This may suggest a COVID-19 vascular-mediated brain injury. Only about 20 patients had a positive CSF PCR test for SARS-CoV-2. This low testing capacity, was due to the limited availability of PCR for CSF analysis. A similar case of COVID-19 induced encephalitis was reported in the literature [25]. Von Weyhern et al. reported lymphocytic panencephalitis and meningitis after examination of 6 postmortem COVID-19 cases [26]. This may suggest another pathogenic mechanism [26]. Another case of encephalitis associated with SARS-CoV-2 was identified by Moriguchi et al. SARS-CoV-2 was detected in the CSF and brain magnetic resonance imaging (MRI) showed hyperintensity in the right mesial temporal lobe [25]. Neo Poyiadji et al. reported a case of acute hemorrhagic necrotizing encephalitis. It showed as a ring enhancement on brain MRI [27]. As the number of cases with COVID-19 rises, neurologists should be alert for patients presenting with altered mental status.
Fig. 3.
First line (A, B and C) MRI for acute necrotizing encephalopathy; second line (D, E and F) MRI for encephalitis; and third line (G, H and I) MRI for posterior cerebral artery ischemic stroke.
4.2. Ischemic stroke
Several studies have reported stroke as the presentation of COVID-19 patients [28], [29], [30], [31], [32]. The role of COVID-19 in inducing a hypercoagulable state is now well-explained [33]. Hospitalized COVID-19 patients are at a risk of thrombotic complications. The prolonged bed rest, hypercoagulable state, inflammation, hypoxia and dehydration are risk factors. A study reported that anticoagulation use resulted in a low COVID-19 patients’ death [34]. We presented this series to alert neurologists that stroke can be an urgent presentation of COVID-19. According to a recent study, acute stroke was reported as a complication or a co-existing problem with COVID-19 infection [35]. We tried to identify if cerebrovascular accidents are caused by COVID-19 or they were just coincident problems caused by other risk factors. We noticed that strokes occurred in many young patients. About 36 (40.9%) patients with stroke were under 40-year-old. Age is a well-known risk factor for stroke and after the age of 55 years the risk of stroke is doubling [36]. That may confirm that COVID-19 is linked to the causation of stroke in young age in our case series.
4.3. Guillain-Barre Syndrome (GBS)
Guillain-Barre Syndrome (GBS) is an acute polyneuropathy usually induced by infections [37]. Campylobacter Jejuni, Epstein-Barr virus, and influenza are frequently involved [37]. The association of GBS with COVID-19 is now extensively studied, however, the mechanism of pathogenesis is still not elucidated [38]. The literature has reported several cases of post-COVID-19 GBS [38], [39], [40], [41], [42], [43]. In our case series, about 102 patients presented with GBS. They are mostly related to COVID as there are no other possible associated reasons. The neurological and CSF examination confirmed the diagnosis of GBS. All the patients presenting with GBS were positive for COVID-19 nasopharyngeal swab PCR. About 55 patients improved and 20 died at the hospital while the others lost their follow-up.
4.4. Optic nerve affection
Optic neuritis is an inflammatory condition that presents with a drop of vision and ocular pain [44]. It is usually unilateral and associated with multiple sclerosis (MS) [44]. Optic neuritis is initiated by demyelination and swelling of optic nerve fibers due to systemic T-cell activation [45]. Several case reports described it in association with COVID-19 infection [46], [47], [48]. Additionally, Miller-Fisher syndrome and polyneuritis cranialis were reported 3 to 5 days after the appearance of COVID-19-related symptoms [49]. In our case series, some patients presented with blurred vision and were diagnosed with optic neuritis. Anterior ischemic optic neuropathy (AION), central retinal artery occlusion (CRAO), and retinal vein occlusion were excluded which may occur as thrombotic complications of COVID-19.
4.5. Seizures
Severe viral respiratory illnesses usually have seizures as a common presentation [50]. Our records show some patients presented with seizures in the context of COVID-19. We cannot judge that COVID-19 is the cause of these seizures. We did not exclude other causes of epilepsy. We did not have electroencephalography done for those patients due to limited facilities. The development of new-onset seizures among 304 hospitalized COVID-19 patients was investigated and no increased risk of acute seizures was documented [51].
4.6. Peripheral nerve sensations
Many patients complained of peripheral sensations within the three months follow-up period. These sensations were paresthesia, buzzing and crawling sensations or even neuralgic pain. Clinical examination and electroneurographic investigations did not reveal any abnormality. Few patients presented with isolated trigeminal neuralgia. It was reported during the three months’ follow-up period. We cannot judge that COVID-19 is the cause of trigeminal neuralgia as it may be isolated or exacerbated by anxiety. A case of isolated trigeminal neuralgia was reported as the presentation of COVID-19 infection [52].
4.7. Limitations
This study has some limitations. First of all, Egypt as an undeveloped country had limited medical facilities. Almost all the medical records are paper sheets. Retrieving patients’ data from the records was extremely tedious. The limited availability of sophisticated investigations disabled us from augmenting the clinical data. Second, the shortage of comprehensive diagnostic work-up to identify the pathogenic mechanisms of cerebrovascular diseases. Third, the medical records were lacking important data as risk factors for diseases. Fourth, most of Egyptian patients are reluctant to visit the clinic for follow-up. This has led to many follow-up skipping and absenteeism. Fifth, the actual cause of death of ICU patients was not documented properly. Thus, we cannot judge that the death cause is related to a neurological problem or not.
5. Conclusions
To the best of our knowledge, we conducted the first analysis of neurological problems related to COVID-19 in Egypt. COVID-19 patients may have a variety of central and peripheral neurological symptoms. The novelty of data and the widening of studies involving COVID make it more challenging for neurologists and encourage them to stay updated. Uncertainty is a feature of brain disorders that neurologists used to deal with and COVID adds more to this uncertainty [53].
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgments
Acknowledgements
We thank the following physicians who contributed in data organization (listed alphabetically): Ahmed M. Ibrahim, Bilal K Ali, Dina Mohammed, Gamal A. Almohamady, Hadeer Abdelraziq, Mohammed A. Ibrahim, Youssry Hesham, and Zayed Nooreddin.
Ethical Approvals
All necessary ethical approvals have been obtained successfully.
References:
- 1.Giwa A.L., Desai A., Duca A. Novel 2019 coronavirus SARS-CoV-2 (COVID-19): an overview for emergency clinicians. Pediatr Emerg Med Pract. 2020;17(5):1–24. [PubMed] [Google Scholar]
- 2.WHO. Report of the WHO-China Joint Mission on Coronavirus Disease 2019 (COVID-19): World Health Organization; 2020 [Available from: https://www.who.int/publications/i/item/report-of-the-who-china-joint-mission-on-coronavirus-disease-2019-(covid-19).
- 3.Mao L., Jin H., Wang M., Hu Y., Chen S., He Q. Neurologic Manifestations of Hospitalized Patients With Coronavirus Disease 2019 in Wuhan. China. JAMA Neurol. 2020;77(6):683–690. doi: 10.1001/jamaneurol.2020.1127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ghannam M., Alshaer Q., Al-Chalabi M., Zakarna L., Robertson J., Manousakis G. Neurological involvement of coronavirus disease 2019: a systematic review. J Neurol. 2020;267(11):3135–3153. doi: 10.1007/s00415-020-09990-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Helms J., Kremer S., Merdji H., Clere-Jehl R., Schenck M., Kummerlen C. Neurologic Features in Severe SARS-CoV-2 Infection. N Engl J Med. 2020;382(23):2268–2270. doi: 10.1056/NEJMc2008597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Wu Y., Xu X., Chen Z., Duan J., Hashimoto K., Yang L. Nervous system involvement after infection with COVID-19 and other coronaviruses. Brain Behav Immun. 2020;87:18–22. doi: 10.1016/j.bbi.2020.03.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Netland J., Meyerholz D.K., Moore S., Cassell M., Perlman S. Severe acute respiratory syndrome coronavirus infection causes neuronal death in the absence of encephalitis in mice transgenic for human ACE2. J Virol. 2008;82(15):7264–7275. doi: 10.1128/JVI.00737-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hamming I., Timens W., Bulthuis M.L., Lely A.T., Navis G., van Goor H. Tissue distribution of ACE2 protein, the functional receptor for SARS coronavirus. A first step in understanding SARS pathogenesis. J Pathol. 2004;203(2):631–637. doi: 10.1002/path.1570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Wang K., Chen W., Zhang Z., Deng Y., Lian J.Q., Du P. CD147-spike protein is a novel route for SARS-CoV-2 infection to host cells. Signal Transduct Target Ther. 2020;5(1):283. doi: 10.1038/s41392-020-00426-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Paniz-Mondolfi A., Bryce C., Grimes Z., Gordon R.E., Reidy J., Lednicky J. Central nervous system involvement by severe acute respiratory syndrome coronavirus-2 (SARS-CoV-2) J Med Virol. 2020;92(7):699–702. doi: 10.1002/jmv.25915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Venkatesan A., Tunkel A.R., Bloch K.C., Lauring A.S., Sejvar J., Bitnun A. Case definitions, diagnostic algorithms, and priorities in encephalitis: consensus statement of the international encephalitis consortium. Clin Infect Dis. 2013;57(8):1114–1128. doi: 10.1093/cid/cit458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Germanò A., Raffa G., Angileri F.F., Cardali S.M., Tomasello F. Coronavirus Disease 2019 (COVID-19) and Neurosurgery: Literature and Neurosurgical Societies Recommendations Update. World Neurosurg. 2020;139:e812–e817. doi: 10.1016/j.wneu.2020.04.181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Baig A.M., Khaleeq A., Ali U., Syeda H. Evidence of the COVID-19 Virus Targeting the CNS: Tissue Distribution, Host-Virus Interaction, and Proposed Neurotropic Mechanisms. ACS Chem Neurosci. 2020;11(7):995–998. doi: 10.1021/acschemneuro.0c00122. [DOI] [PubMed] [Google Scholar]
- 14.Li Y.C., Bai W.Z., Hashikawa T. The neuroinvasive potential of SARS-CoV2 may play a role in the respiratory failure of COVID-19 patients. J Med Virol. 2020;92(6):552–555. doi: 10.1002/jmv.25728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Beyrouti R., Adams M.E., Benjamin L., Cohen H., Farmer S.F., Goh Y.Y. Characteristics of ischaemic stroke associated with COVID-19. J Neurol Neurosurg Psychiatry. 2020;91(8):889–891. doi: 10.1136/jnnp-2020-323586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Zhang Y., Xiao M., Zhang S., Xia P., Cao W., Jiang W. Coagulopathy and Antiphospholipid Antibodies in Patients with Covid-19. N Engl J Med. 2020;382(17) doi: 10.1056/NEJMc2007575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Asbury A.K., Cornblath D.R. Assessment of current diagnostic criteria for Guillain-Barré syndrome. Ann Neurol. 1990;27(Suppl):S21–S24. doi: 10.1002/ana.410270707. [DOI] [PubMed] [Google Scholar]
- 18.Matschke J., Lütgehetmann M., Hagel C., Sperhake J.P., Schröder A.S., Edler C. Neuropathology of patients with COVID-19 in Germany: a post-mortem case series. Lancet Neurol. 2020;19(11):919–929. doi: 10.1016/S1474-4422(20)30308-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Remmelink M., De Mendonça R., D'Haene N., De Clercq S., Verocq C., Lebrun L. Unspecific post-mortem findings despite multiorgan viral spread in COVID-19 patients. Crit Care. 2020;24(1):495. doi: 10.1186/s13054-020-03218-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kantonen J., Mahzabin S., Mäyränpää M.I., Tynninen O., Paetau A., Andersson N. Neuropathologic features of four autopsied COVID-19 patients. Brain Pathol. 2020;30(6):1012–1016. doi: 10.1111/bpa.12889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Schurink B., Roos E., Radonic T., Barbe E., Bouman C.S.C., de Boer H.H. Viral presence and immunopathology in patients with lethal COVID-19: a prospective autopsy cohort study. Lancet Microbe. 2020;1(7):e290–e299. doi: 10.1016/S2666-5247(20)30144-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Manji H., Carr A.S., Brownlee W.J., Lunn M.P. Neurology in the time of COVID-19. J Neurol Neurosurg Psychiatry. 2020;91(6):568–570. doi: 10.1136/jnnp-2020-323414. [DOI] [PubMed] [Google Scholar]
- 23.Azab M.A., Azzam A.Y. SARS-CoV-2 associated viral encephalitis with mortality outcome. Interdiscip Neurosurg. 2021;25 doi: 10.1016/j.inat.2021.101132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Lou J.J., Movassaghi M., Gordy D., Olson M.G., Zhang T., Khurana M.S. Neuropathology of COVID-19 (neuro-COVID): clinicopathological update. Free Neuropathol. 2021;2 doi: 10.17879/freeneuropathology-2021-2993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Moriguchi T., Harii N., Goto J., Harada D., Sugawara H., Takamino J. A first case of meningitis/encephalitis associated with SARS-Coronavirus-2. Int J Infect Dis. 2020;94:55–58. doi: 10.1016/j.ijid.2020.03.062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.von Weyhern C.H., Kaufmann I., Neff F., Kremer M. Early evidence of pronounced brain involvement in fatal COVID-19 outcomes. Lancet. 2020;395(10241) doi: 10.1016/S0140-6736(20)31282-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Poyiadji N., Shahin G., Noujaim D., Stone M., Patel S., Griffith B. COVID-19-associated Acute Hemorrhagic Necrotizing Encephalopathy: Imaging Features. Radiology. 2020;296(2):E119–E120. doi: 10.1148/radiol.2020201187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Yaghi S., Ishida K., Torres J., Mac Grory B., Raz E., Humbert K. SARS-CoV-2 and Stroke in a New York Healthcare System. Stroke. 2020;51(7):2002–2011. doi: 10.1161/STROKEAHA.120.030335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Oxley T.J., Mocco J., Majidi S., Kellner C.P., Shoirah H., Singh I.P. Large-Vessel Stroke as a Presenting Feature of Covid-19 in the Young. N Engl J Med. 2020;382(20) doi: 10.1056/NEJMc2009787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Ashrafi F., Zali A., Ommi D., Salari M., Fatemi A., Arab-Ahmadi M. COVID-19-related strokes in adults below 55 years of age: a case series. Neurol Sci. 2020;41(8):1985–1989. doi: 10.1007/s10072-020-04521-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Fara M.G., Stein L.K., Skliut M., Morgello S., Fifi J.T., Dhamoon M.S. Macrothrombosis and stroke in patients with mild Covid-19 infection. J Thromb Haemost. 2020;18(8):2031–2033. doi: 10.1111/jth.14938. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Franceschi A.M., Arora R., Wilson R., Giliberto L., Libman R.B., Castillo M. Neurovascular Complications in COVID-19 Infection: Case Series. AJNR Am J Neuroradiol. 2020;41(9):1632–1640. doi: 10.3174/ajnr.A6655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Han H., Yang L., Liu R., Liu F., Wu K.L., Li J. Prominent changes in blood coagulation of patients with SARS-CoV-2 infection. Clin Chem Lab Med. 2020;58(7):1116–1120. doi: 10.1515/cclm-2020-0188. [DOI] [PubMed] [Google Scholar]
- 34.Tang N., Bai H., Chen X., Gong J., Li D., Sun Z. Anticoagulant treatment is associated with decreased mortality in severe coronavirus disease 2019 patients with coagulopathy. J Thromb Haemost. 2020;18(5):1094–1099. doi: 10.1111/jth.14817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Li Y., Li M., Wang M., Zhou Y., Chang J., Xian Y. Acute cerebrovascular disease following COVID-19: a single center, retrospective, observational study. Stroke Vasc Neurol. 2020;5(3):279–284. doi: 10.1136/svn-2020-000431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Yousufuddin M., Young N. Aging and ischemic stroke. Aging (Albany NY). 2019;11(9):2542–2544. doi: 10.18632/aging.101931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Jacobs B.C., Rothbarth P.H., van der Meché F.G., Herbrink P., Schmitz P.I., de Klerk M.A. The spectrum of antecedent infections in Guillain-Barré syndrome: a case-control study. Neurology. 1998;51(4):1110–1115. doi: 10.1212/wnl.51.4.1110. [DOI] [PubMed] [Google Scholar]
- 38.Caress J.B., Castoro R.J., Simmons Z., Scelsa S.N., Lewis R.A., Ahlawat A. COVID-19-associated Guillain-Barré syndrome: The early pandemic experience. Muscle Nerve. 2020;62(4):485–491. doi: 10.1002/mus.27024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Rahimi K. Guillain-Barre syndrome during COVID-19 pandemic: an overview of the reports. Neurol Sci. 2020;41(11):3149–3156. doi: 10.1007/s10072-020-04693-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Alberti P., Beretta S., Piatti M., Karantzoulis A., Piatti M.L., Santoro P. Guillain-Barré syndrome related to COVID-19 infection. Neurol Neuroimmunol Neuroinflamm. 2020;7(4) doi: 10.1212/NXI.0000000000000741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Arnaud S., Budowski C., Ng Wing Tin S., Degos B. Post SARS-CoV-2 Guillain-Barré syndrome. Clin Neurophysiol. 2020;131(7):1652–1654. doi: 10.1016/j.clinph.2020.05.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Assini A., Benedetti L., Di Maio S., Schirinzi E., Del Sette M. New clinical manifestation of COVID-19 related Guillain-Barrè syndrome highly responsive to intravenous immunoglobulins: two Italian cases. Neurol Sci. 2020;41(7):1657–1658. doi: 10.1007/s10072-020-04484-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Bigaut K., Mallaret M., Baloglu S., Nemoz B., Morand P., Baicry F. Guillain-Barré syndrome related to SARS-CoV-2 infection. Neurol Neuroimmunol Neuroinflamm. 2020;7(5) doi: 10.1212/NXI.0000000000000785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Balcer L.J. Clinical practice. Optic neuritis. N Engl J Med. 2006;354(12):1273–1280. doi: 10.1056/NEJMcp053247. [DOI] [PubMed] [Google Scholar]
- 45.Roed H., Frederiksen J., Langkilde A., Sørensen T.L., Lauritzen M., Sellebjerg F. Systemic T-cell activation in acute clinically isolated optic neuritis. J Neuroimmunol. 2005;162(1–2):165–172. doi: 10.1016/j.jneuroim.2005.02.002. [DOI] [PubMed] [Google Scholar]
- 46.Zhou S., Jones-Lopez E.C., Soneji D.J., Azevedo C.J., Patel V.R. Myelin Oligodendrocyte Glycoprotein Antibody-Associated Optic Neuritis and Myelitis in COVID-19. J Neuroophthalmol. 2020;40(3):398–402. doi: 10.1097/WNO.0000000000001049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.K. Sawalha S. Adeodokun G.R. Kamoga COVID-19-Induced Acute Bilateral Optic Neuritis J Investig Med High Impact Case Rep. 8 2020 2324709620976018. [DOI] [PMC free article] [PubMed]
- 48.Benito-Pascual B., Gegúndez J.A., Díaz-Valle D., Arriola-Villalobos P., Carreño E., Culebras E. Panuveitis and Optic Neuritis as a Possible Initial Presentation of the Novel Coronavirus Disease 2019 (COVID-19) Ocul Immunol Inflamm. 2020;28(6):922–925. doi: 10.1080/09273948.2020.1792512. [DOI] [PubMed] [Google Scholar]
- 49.Li Z., Li X., Shen J., Chan M.T.V., Wu W.K.K. Miller Fisher syndrome associated with COVID-19: an up-to-date systematic review. Environ Sci Pollut Res Int. 2021;28(17):20939–20944. doi: 10.1007/s11356-021-13233-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Vehapoglu A., Turel O., Uygur Sahin T., Kutlu N.O., Iscan A. Clinical Significance of Human Metapneumovirus in Refractory Status Epilepticus and Encephalitis: Case Report and Review of the Literature. Case Rep Neurol Med. 2015;2015 doi: 10.1155/2015/131780. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Lu L., Xiong W., Liu D., Liu J., Yang D., Li N. New onset acute symptomatic seizure and risk factors in coronavirus disease 2019: A retrospective multicenter study. Epilepsia. 2020;61(6):e49–e53. doi: 10.1111/epi.16524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Molina-Gil J., González-Fernández L., García-Cabo C. Trigeminal neuralgia as the sole neurological manifestation of COVID-19: A case report. Headache. 2021;61(3):560–562. doi: 10.1111/head.14075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Shellhaas R.A. Neurologists and COVID-19: A note on courage in a time of uncertainty. Neurology. 2020;94(20):855–857. doi: 10.1212/WNL.0000000000009496. [DOI] [PubMed] [Google Scholar]