Introduction
Coronavirus infectious disease 2019 (COVID-19), caused by severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2), has caused significant global morbidity and mortality since first emerging as a pathogen at the end of 2019. Acute kidney injury, most commonly due to acute tubular injury, is a frequent finding in COVID-19, occurring in 17% to 37% of patients.1, 2, 3 De novo glomerular disease is much less common with COVID-19, although cases of new-onset collapsing glomerulopathy, immune-complex glomerulonephritis, and antineutrophil cytoplasmic autoantibody (ANCA)–associated glomerulonephritis have been reported.4, 5, 6 The development and rapid use of several effective vaccines against SARS-CoV-2 has helped reduce spread of the virus in countries able to implement a vaccination program. Although the vaccines are well tolerated and major side effects are uncommon, case reports have emerged of rare side effects, including vaccine-induced immune thrombotic thrombocytopenia and minimal change disease.7,8 We report here a case of pauci-immune crescentic glomerulonephritis associated with de novo development of ANCAs following vaccination with the Pfizer-BioNTech COVID-19 vaccine.
Case Report
A 29-year-old woman was referred for evaluation of acute kidney injury. She had a history significant for congenital diffuse cystic lung disease, attributed to a mutation in the surfactant SFTPC gene. She had progressive lung failure complicated by pulmonary hypertension and was on the waitlist for a lung transplant. Her medications included tadalafil, macitentan, and escitalopram. She had chronic dyspnea and dry cough, which were stable over the preceding 6 months. Her review of systems was otherwise negative, and she reported no symptoms suggestive of infection in the past year or recent change in medications. On examination, her blood pressure was 117/81 mm Hg, her lungs had bilateral crackles (chronic), and there was no edema and no neurologic or skin findings. Her baseline creatinine was 0.8 mg/dl 1 week prior to receiving her first dose of the Pfizer-BioNTech COVID-19 vaccine. Sixteen days after receiving her second vaccine dose, her creatinine had increased to 1.25 on routine laboratory tests. Routine laboratory tests 7 weeks after her second dose of vaccine showed a creatinine of 1.91 mg/dl. Further evaluation showed the following: urine with 12 red blood cells / high-power field; urine albumin-creatinine ratio 633 μg/mg; serum albumin 4.4 g/dl; C3 108 mg/dl; C4 29 mg/dl; negative serologic test results for hepatitis B, hepatitis C, and HIV; negative test results for antiglomerular basement antibody and proteinase 3 (PR3)-ANCA; and a positive test result for myeloperoxidase (MPO)-ANCA at 71 AU (arbitrary units)/ml. Test results for ANCA 2 years earlier was negative. Screening test results for SARS-CoV-2 were negative 2 months before the first dose of vaccine and 3 weeks after the second dose of vaccine.
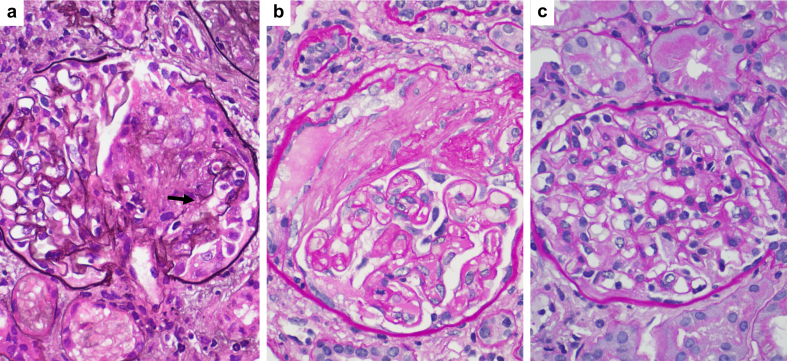
A percutaneous kidney biopsy was performed 9 weeks after the second vaccine dose. The light microscopy sample contained 44 glomeruli, of which 8 were globally sclerotic. Of the 36 nonglobally sclerotic glomeruli, 9 showed cellular crescents (Figure 1a), 5 displayed fibrocellular crescents, and 7 exhibited fibrous crescents (Figure 1b). The remaining glomeruli appeared of normal size without significant mesangial proliferation, mesangial sclerosis, or endocapillary proliferation (Figure 1c). These findings were accompanied by mild diffuse mononuclear interstitial inflammation, foci of moderate tubulitis, and scattered red blood cell casts. These changes were superimposed on 40% tubulointerstitial scarring and mild vascular sclerosis. Except for fibrin staining in Bowman space in 4 of the 7 sampled glomeruli, which was consistent with crescents, the remaining immunofluorescence findings were essentially unremarkable. Electron microscopic evaluation revealed focal foot process effacement without discrete electron-dense deposits. Taken together, the above findings were consistent with pauci-immune crescentic glomerulonephritis, correlating with her positive MPO-ANCA serologies.
Figure 1.
Kidney biopsy findings. (a) A glomerulus with a segmental cellular crescent and a focus of glomerular basement membrane rupture (arrow) (Jones methenamine silver, original magnification ×600). (b) A glomerulus with a segmental cellular crescent (Periodic acid–Schiff, original magnification ×600). (c) A noninvolved glomerulus showing no significant mesangial hypercellularity or sclerosis, open capillaries without endocapillary proliferation, and lack of extracapillary lesions (Periodic acid–Schiff, original magnification ×600).
Based on the results of her biopsy, she was started on treatment for her ANCA-associated glomerulonephritis with steroids (methylprednisolone 500 mg daily for 3 doses, followed by prednisone 1 mg/kg daily), rituximab (1000 mg for 2 doses, spaced 2 weeks apart), and intravenous cyclophosphamide (500 mg for 2 doses, spaced 2 weeks apart). At last follow-up, 10 weeks after her biopsy, her creatinine was 1.01 mg/dl, her albumin-creatinine ratio was 1393 μg/mg, her urine showed 0 to 2 red blood cells / high-power field, and her MPO-ANCA titer was negative.
Discussion
Several vaccines have been associated with the development of postvaccination autoimmune phenomena, including multiple case reports of de novo ANCA-associated vasculitis following seasonal influenza vaccination.9,S1 To date, there are a few reports of ANCA-associated glomerulonephritis following both the Moderna and Pfizer COVID-19 vaccines.S2–S4 Although there is no definitive test to prove causality, the development of MPO-ANCA in a patient with previous negative testing, acute kidney injury, and crescentic glomerulonephritis occurring shortly after vaccination raises suspicion that the 2 events are more than coincidence.
Events leading to the development of ANCA and ANCA-associated vasculitis are not well understood, although infectious agents and other environmental exposures may play a role. Several groups have found an increased prevalence of autoantibodies in patients with COVID-19 infection. Vlachoyiannopoulos et al. examined 29 patients with severe COVID-19 and found p-ANCA in 2 patients and c-ANCA in 2 patients. However, using enzyme-linked immunosorbent assay, they were unable to identify any known ANCA specificities in these 4 patients.S5 Similarly, Sacchi et al. found ANCA in 10 of 40 patients (25%) with COVID-19, although they were only able to identify a known ANCA specificity in 1 patient (PR3-ANCA).S6 There have been several case reports of de novo ANCA-associated glomerulonephritis occurring in patients with COVID-19,6 lending support to the hypothesis that SARS-CoV-2, or the immune response to the virus, could trigger the development of ANCA, leading to ANCA-associated vasculitis.
There are at least 6 case reports of new-onset ANCA occurring within 1 month of patients receiving the seasonal influenza vaccine. Four cases were associated with MPO-ANCA and 2 cases with PR3-ANCA.9 Two patients developed end-stage kidney disease, suggesting that ANCA-associated vasculitis occurring after influenza vaccination may have a similar clinical course as ANCA-associated vasculitis occurring in other settings. Several mechanisms have been suggested to explain the association of de novo ANCA and influenza vaccine, including an immune response to either the vaccine antigen or one of the excipients in the vaccine. In support of the former, in 1 patient who developed ANCA-associated vasculitis following influenza vaccination, stimulation of the peripheral blood mononuclear cells with 6 of 8 different influenza vaccines was able to cause production of PR3-ANCA in vitro.S7
Hundreds of millions of doses of COVID-19 vaccines have been administered worldwide, with rare autoimmune side effects reported to date. In this case, ANCA and ANCA-associated glomerulonephritis developed shortly after receiving the COVID-19 vaccine, in the absence of any recent infection or medication use known to trigger ANCA development (Table 1). Although causality can be difficult to establish, the development of ANCA and ANCA-associated vasculitis after both natural SARS-CoV-2 infection and following other vaccinations lends support to our hypothesis that the ANCA-associated vasculitis in this case is related to the recent Pfizer-BioNTech COVID-19 vaccine.
Table 1.
Teaching points
| 1. Vaccines rarely are associated with the development of postvaccination autoimmune phenomena, including de novo ANCA vasculitis. |
| 2. Although the SARS-CoV-2 vaccines are exceptionally safe and effective, de novo ANCA-associated glomerulonephritis can be associated with recent COVID-19 vaccination. |
| 3. ANCA-associated vasculitis should be considered in the differential diagnosis of acute kidney injury following COVID-19 vaccination. |
ANCA, antineutrophil cytoplasmic autoantibody; COVID-19, coronavirus disease 2019; SARS-CoV-2, severe acute respiratory syndrome coronavirus 2.
Disclosure
All the authors declared no competing interests.
Patient Consent
The authors declare that they have obtained consent from the patient discussed in the report.
Footnotes
Supplementary References.
Supplementary Material
Supplementary References.
References
- 1.Robbins-Juarez S.Y., Qian L., King K.L., et al. Outcomes for patients with COVID-19 and acute kidney injury: a systematic review and meta-analysis. Kidney Int Rep. 2020;5(8):1149–1160. doi: 10.1016/j.ekir.2020.06.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Hirsch J.S., Ng J.H., Ross D.W., et al. Acute kidney injury in patients hospitalized with COVID-19. Kidney Int. 2020;98(1):209–218. doi: 10.1016/j.kint.2020.05.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ferlicot S, Jamme M, Gaillard F, et al. The spectrum of kidney biopsies in hospitalized patients with COVID-19, acute kidney injury, and/or proteinuria. Nephrol Dial Transplant. Published online February 12, 2021. https://doi.org/10.1093/ndt/gfab042. [DOI] [PMC free article] [PubMed]
- 4.Kudose S., Batal I., Santoriello D., et al. Kidney biopsy findings in patients with COVID-19. J Am Soc Nephrol. 2020;31(9):1959–1968. doi: 10.1681/ASN.2020060802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Sethi S., D'Costa M.R., Hermann S.M., et al. Immune-complex glomerulonephritis after COVID-19 infection. Kidney Int Rep. 2021;6(4):1170–1173. doi: 10.1016/j.ekir.2021.02.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Uppal N.N., Kello N., Shah H.H., et al. De novo ANCA-associated vasculitis with glomerulonephritis in COVID-19. Kidney Int Rep. 2020;5(11):2079–2083. doi: 10.1016/j.ekir.2020.08.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Scully M., Singh D., Lown R., et al. Pathologic antibodies to platelet factor 4 after ChAdOx1 nCoV-19 vaccination. N Engl J Med. 2021;384(23):2202–2211. doi: 10.1056/NEJMoa2105385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Lebedev L., Sapojnikov M., Wechsler A., et al. Minimal change disease following the Pfizer-BioNTech COVID-19 vaccine. Am J Kidney Dis. 2021;78(1):142–145. doi: 10.1053/j.ajkd.2021.03.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Duggal T., Segal P., Shah M., et al. Antineutrophil cytoplasmic antibody vasculitis associated with influenza vaccination. Am J Nephrol. 2013;38(2):174–178. doi: 10.1159/000354084. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.