Highlights
-
•
Myotonic dystrophy, a neuromuscular disease, affects at least around half a million people worldwide.
-
•
Close to two dozen preclinical and clinical drug development programs active.
-
•
Drugs encompass new chemical entities, repurposing, oligonucleotide, and gene therapy.
-
•
Tideglusib, mexiletine, and metformin are close to reaching marketing authorization.
Keywords: Myotonic dystrophy, Drug development, Repurposing drug, Antisense oligonucleotide, Gene therapy, Clinical trial
Abstract
Myotonic dystrophy type 1 (DM1) is a multisystemic neuromuscular genetic disease with an estimated prevalence of approximately at least half a million individuals based on its vast ethnic variation. Building upon a well-known physiopathology and several proof-of-concept therapeutic approaches, herein we compile a comprehensive overview of the most recent drug development programs under preclinical and clinical evaluation. Specifically, close to two dozen drug developments, eight of which are already in clinical trials, explore a diversity of new chemical entities, drug repurposing, oligonucleotide, and gene therapy-based approaches. Of these, repurposing of tideglusib, mexiletine, or metformin appear to be therapies with the most potential to receive marketing authorization for DM1.
Introduction
The past two decades have witnessed the generation of breakthrough knowledge regarding the molecular causes and conceptual approaches to treating neuromuscular disorders. The consequence has been the approval of the first oligonucleotide-based drug treatments for Duchenne muscular dystrophy (DMD; eteplirsen and golodirsen) and spinal muscular atrophy (SMA; nusinersen) [1], and the first gene therapy (Zolgensma; SMA), which complement more traditional small-molecule therapeutic options (e.g., risdiplam; SMA) [2]. Next in line with at least one therapeutic option might be Myotonic dystrophy type 1 (DM1). DM1 is a life-threatening chronic disease with symptoms in the neuromuscular, cardiac, and central nervous systems, and compared with most neuromuscular diseases, its clinical presentation is very variable [3], [4]. DM1 can affect newborns (congenital DM1) to older adults, with around at least half a million patients worldwide based on the reported prevalence estimation of 1 in 3,000–8,000 people [3], [5].
A repeat length >50 units of the repetitive trinucleotide sequence (CTG)n in the 3′-untranslated region (UTR) of the DM1 protein kinase (DMPK) gene causes DM1. Mutant DMPK transcripts in skeletal muscle, heart, and brain tissue are retained in the cell nucleus in microscopically visible ribonuclear foci, which are the most prominent histopathological hallmark of the disease. CUG expansions fold into stable double-stranded stem-loop structures with U-U mismatches with a strong affinity for proteins of the Muscleblind-like (MBNL) family, which are sequestered and become depleted and unable to perform their normal function. MBNL loss of function contributes significantly to DM1 phenotypes [6]. Likewise, stress responses triggered by the toxic DMPK RNA cause the stabilization/activation of MBNL antagonists CELF1 and hnRNPA1 [6]. Together, these proteins function as developmental switches, and their imbalance results in the abnormal persistence of fetal patterns of alternative splicing in adult tissues, termed ‘spliceopathy’, which affects hundreds of genes, some of which explain specific symptoms in DM1. Various signaling pathways also have a role in DM1 muscle pathogenesis, including impaired protein kinase B (AKT), activated autophagy and ubiquitin–proteasome activity, AMP-activated protein kinase (AMPK) downregulation, and activated glycogen synthase kinase 3 beta (GSK3β) [7]. Taken together, this reasonably detailed knowledge of the molecular pathophysiology of DM1, availability of cell and mouse preclinical models, and the exploration of several proof-of-concept therapeutic strategies have culminated in a promising drug development pipeline for DM1.
Drug development programs
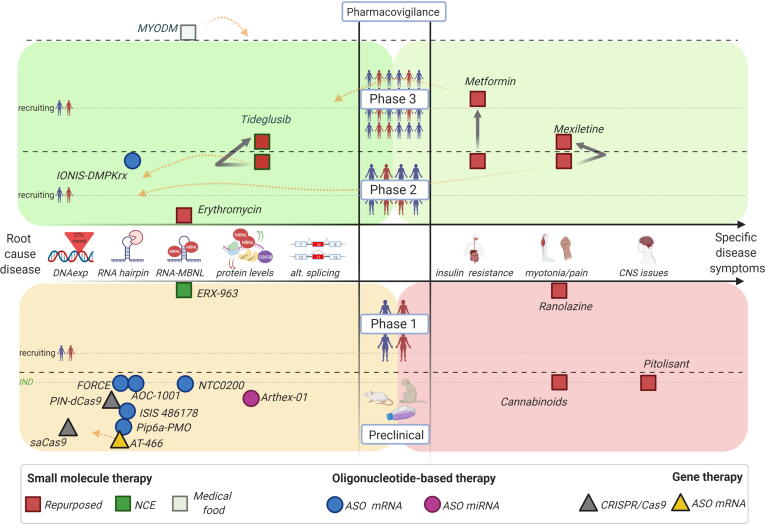
Herein, we summarize existing drug development programs in DM1 encompassing advanced candidates from the preclinical stage to human clinical trials. Therapies are further classified into three broad categories of small molecules, oligonucleotide-based therapies, and gene therapies, encompassing mechanisms of action targeting from root cause disease events up to specific clinical symptoms (Fig. 1).
Figure 1.
Graphical representation of the preclinical and clinical DM1 drug candidate pipeline. The middle X-axis indicates the step targeted within the DM1 physiopathological model (left side: the root causes of disease; right side: specific clinical disease symptoms), while the middle Y-axis shows the level of drug evaluation from preclinical to market authorization. Black discontinued lines separate clinical phases, while grey discontinued lines indicate human recruiting timing for each clinical phase. The green discontinued line denotes the IND milestone at the end of the preclinical evaluation phase. Drug candidates are represented in different shapes according to the type of therapy and colours for specific types of molecules. Black solid arrows display progression of those drug candidates evaluated in more than one clinical trial. Orange discontinued arrows indicate the description of additional mechanisms of action proposed for the drug candidate (see text and tables for detailed description and references) (Created with BioRender.com).
Small-molecule drugs
In this category, we have mainly identified repurposed pre- and investigational new drugs (INDs), which were initially developed to treat a different condition, compared with only one new chemical entity (NCE) IND with first therapeutic use in DM1. Given that drug-repurposing candidates have already demonstrated safety in humans, they can be tested in patients more quickly and at less cost and lower risk than for entirely new drugs, which explains why they are closest to reaching market authorization (Table 1).
Table 1.
Myotonic dystrophy drug candidates currently in clinical trials.
| Phase | Clinical trial | Status | Clinical trial information | Results |
|---|---|---|---|---|
| Mexiletine (repurposed small molecule): Orphan Drug Designation by FDA and EMA | ||||
| III | NCT04624750 | Not yet recruiting: 2020–2024 | Safety, efficacy, and steady-state PK of mexiletine in paediatric patients with myotonic disorders. Sponsor: Lupin, Ltd | Not posted |
| II | NCT01406873 | Completed: 2011–2018 | Effects of mexiletine on ambulation, myotonia, muscle function, strength, pain, gastrointestinal functioning, cardiac conduction, and quality of life in DM1. Sponsor: University of Rochester | [24] |
| Metformin (repurposed small molecule) | ||||
| III | 2018–000692-32 | Ongoing: 2019–2021 | Efficacy of metformin on motility and strength in DM1. Sponsor: Tor Vergata | Not posted |
| II | 2013–001732-21 | Completed: 2013–2017 | Randomized, double-blind, placebo-controlled Phase II study of metformin in patients with DM1. Sponsor: Centre d’Etude des Cellules Souches/Istem | [21] |
| Tideglusib (repurposed small molecule): Orphan Drug Designation by FDA | ||||
| II/III | NCT03692312 | Not yet recruiting: 2020–2022 | Randomized, multicenter, double-blind, placebo-controlled, Phase II/III study of patients (aged 6–16 years) with congenital DM1. Sponsor: AMO Pharma Ltd | Not posted |
| II | NCT02858908 | Completed: 2016–2018 | Safety, efficacy and PK of tideglusib in treatment of adolescents and adults with congenital and juvenile-onset DM1. Sponsor: AMO Pharma Ltd | [12] |
| Erythromycin (repurposed small molecule) | ||||
| II | jRCT2051190069 | Recruiting: 2019–ongoing | Blinded, placebo-controlled study to assess the safety, tolerability, and efficacy of MYD-0124 in adult patients with DM1. Sponsor: Hospital Osaka University | Not posted |
| IONIS-DMPKRx (ISIS 598769) (ASO) | ||||
| I/II | NCT02312011 | Completed: 2014–2016 | Safety, tolerability, and PK of multiple escalating doses of ISIS 598769 administered subcutaneously to adult patients with DM1. Sponsor: IONIS-Biogen | Not posted |
| ERX-963 (new chemical small molecule) | ||||
| I | NCT03959189 | Completed: 2019–2020 | Safety, tolerability, and potential reduction of excessive daytime sleepiness/hypersomnia and improvement of cognitive function in patients with DM1. Sponsor: Expansion Therapeutics, Inc. | Not posted |
| Ranolazine Ralexa™ (repurposed small molecule) | ||||
| I | NCT02251457 | Completed: 2014–2017 | Preliminary data to determine safety and efficacy of ranolazine on symptoms of DM1. Sponsor: Ohio State University/Gilead Sciences | [28] |
| Caffeine and theobromine formulation MYODM™ (natural compounds) | ||||
| N/A | NCT04634682 | Recruiting: 2020–2021 | Effect of food supplement MYODM™ on excessive daytime sleepiness and quality of life in adults with DM1. Sponsor: Myogem Health Company, S.L. | Not posted |
aN/A, not applicable.
Levels of GSK3β are elevated in DM1 skeletal muscles [8], leading to the proposal that resulted in a new therapeutic target to prevent myopathy in DM1 [9]. Tideglusib is a marine-derived GSK3β inhibitor initially developed to treat Alzheimer’s disease [10]; it causes a significant correction of GSK3β and CELF1 levels and reduces expression of toxic DMPK RNA in normal and congenital DM1 (CMD) myoblasts [11]. GSK3β is abnormally high in the HSALR mouse model (Box 1), and tideglusib was found to improve functional parameters, such as muscle weakness and myotonia; in the muscles and brain of the DMSXL mouse (Box 1), as a model of CMD, the drug improved postnatal survival, weight, and neuromotor activity [8], [11]. These results supported the potential of tideglusib to target early events of the disease in congenital and adult forms of DM1 and on skeletal muscle and brain defects via multiple pathways. Orally administered tideglusib, developed by AMO-Pharma, has already completed Phase II trials on patients with CMD and childhood-onset DM1, where most patients reported improved CNS and clinical neuromuscular symptoms [12]. Given the favorable pharmacokinetic (PK) and clinical risk/benefit profiles, an extended Phase II/III clinical trial has been announced.
Box 1. Mouse preclinical models.
HSALR mouse model
The HSALR mouse model is a homozygous transgenic mouse that includes a genomic fragment of the human skeletal actin gene to express 250 CTG repeats in the 3′-UTR of the gene. It shows myotonia and muscle weakness, centrally located nuclei in muscle fibers, and alternative splicing defects [64].
DM200 mouse model
DM200 mice overexpress a GFP-DMPK 3′-UTR (CTG)200 transgene carrying a DMPK 3′-UTR with 200 CTG repeats under the control of a doxycycline-responsive human DMPK promoter. The model reproduces myotonic dystrophy cardinal features, including myotonia, cardiac conduction abnormalities, histopathology, and RNA splicing defects [65].
DMSXL mouse model
The DMSXL mouse model is a homozygous mouse line carrying >700 CTG repeats, derived from the DM300–328 transgenic mouse line, which carries 45 kb of human genomic sequence from the DM1 locus [66]. The model shows mild splicing defects but ribonuclear foci, growth retardation, histology alterations, muscle weakness and atrophy, and myotonia in skeletal muscles [67], [68]. It has been proposed as a study model for brain function alterations typical of DM1 [69].
Inhibition of the interaction between the toxic CUG hairpin and the MBNL1 splicing factor is one of the most common ongoing therapeutic avenues in DM1 [13]. The most-advanced lead compounds, MYD-0124 (erythromycin) and ERX-963, can bind to the hairpin RNA structure with high selectivity, decreasing foci formation and rescuing mis-splicing in DM1 cell and mouse models. A Phase II clinical trial sponsored by Osaka University Hospital is currently testing the oral administration of erythromycin in adult patients with DM1 [14], uncovered as an anti-DM1 candidate after a targeted screen for RNA-binding potential of US Food and Drug Administration (FDA)-approved antibiotics [15]. ERX-963 is a rationally designed NCE that can strongly bind pathogenic expanded CUG repeats (CUGexp) but not normal repeats, limiting off-target effects [16] (Expansion Therapeutics, www.expansionrx.com/dm1/#research). ERX-963 recently finished a Phase I clinical trial in adult patients with DM1, according to the sponsor Expansion Therapeutics.
Among candidates targeting specific disease symptoms, the most advanced is metformin, a first-line agent for type 2 diabetes mellitus. The drug has been suggested to treat the insulin resistance phenotype in patients with DM1 [17] and promote alternative splicing correction via AMPK-dependent and independent mechanisms [18]. More recently, metformin was also reported to improve mitochondrial dysfunction and impaired metabolism in DM1 fibroblasts and prevent cancer risk, thus suggesting ample therapeutic benefits in DM1 [19], [20]. A completed Phase II clinical trial assessed oral metformin, revealing a significant improvement in mobility in a 6-min walk test (6MWT) associated with an increase in total mechanical power and a suggestive effect on gait parameters [21]. These encouraging results supported a replication study in a multicenter Phase III clinical trial sponsored by the Tor Vergata University of Rome.
Additional molecules in development address the symptomatic management of DM1 features, such as myotonia, chronic pain, or daytime sleepiness, namely mexiletine, ranolazine, cannabinoids and pitolisant.
Mexiletine is an antiarrhythmic medicine used to reduce or prevent myotonia through blocking sodium channels involved in the contraction and relaxation of muscles [22]. Mexiletine was orally administered to evaluate the managing of myotonia symptoms in adult patients with DM1 in two Phase II clinical trials [23], [24] that reached similar results indicating a positive effect on handgrip myotonia in ambulatory patients but no significant benefit on 6MWT. Given that mexiletine was safe over a 9-year follow-up study [25], Lupin Ltd is evaluating this drug in a Phase III clinical trial in children and adolescent patients with DM1 under the name NaMuscla, already approved for nondystrophic myotonic disorders [26]. Interestingly, mexiletine has been reported to downregulate DMPK mRNA levels, pointing to additional activity through the DM1 disease pathway [27].
In addition to mexiletine, ranolazine, which acts by enhancing the slow inactivation of sodium channels, is a therapeutic alternative targeting similar DM1 molecular defects, as concluded from a completed Phase I clinical trial [28].
Similar to myotonia, attempts to treat myalgia and other muscular complaints led to the evaluation of cannabinoids (cannabidiol and tetrahydrocannabinol or CBD/THC, respectively) in DM1, driven by the presence of cannabinoid receptors in muscles [29] and by modulation of both central and peripheral pain pathways [30], [31]. In this regard, Nexien Biopharma recently filed a patent covering methods and compositions for treating patients with myotonic dystrophy (DM1 and DM2) with oral formulations of CBD and THC [32].
On a similar theme, pitolisant, a stimulant drug that antagonizes histamine H3 receptors to treat excessive daytime sleepiness in patients with narcolepsy, will be evaluated by Harmony Biosciences to treat the same symptom in DM1. This is a clinical hallmark in most patients and one of the most frequent non-muscular symptoms contributing to a reduced quality of life [33]. A repurposing program with a Phase II clinical trial in adult patients with DM1 was announced for the first half of 2021.
Finally, a complementary therapeutic strategy for DM1 comes from evaluating methylxanthines, natural alkaloid products commonly used as mild stimulants and bronchodilators [34]. A synergistic combination of theobromine and caffeine (commercialized by Myogem Health Company as MYODM™) showed the ability to reduce the number of foci aggregates per cell and increase MBNL1 at the transcript and protein level [35], [36]. Data on Drosophila DM1 flies rendered a recovery of survival and cardiac and locomotor dysfunction (M. Pascual-Gilabert et al., unpublished 2021). The clinical trial NCT04634682 is evaluating the effect of MYODM™ on the quality of life, fatigue, and hypersomnia in adult patients with DM1 as a novel nutritional management strategy. MYODM™ is expected to be upgraded into a food for special medical purposes or medical food [European Food Safety Authority (EFSA) and FDA classification, respectively, for foods specifically intended for the nutritional management of patients, under medical supervision].
Oligonucleotide-based therapeutics
RNA-based therapies can target any gene in the genome and, thus, are particularly well suited to address the diversity among rare genetic diseases. The promise of oligonucleotide-based drugs is starting to be realized with the increasing pace at which these drugs reach authorization, with at least ten already approved for clinical use [2]. In DM1, chief approaches have been designing antisense oligonucleotides (ASOs) able to degrade DMPK transcripts via the activation of RNase-H machinery in the nucleus (i.e., gapmers) or able to prevent MBNL1 sequestration by CUG repeats (or displace prebound MBNL1 proteins) by an occupancy-based mechanism (i.e., mixmers) (reviewed in [37]). In contrast to their high specificity, oligonucleotide-based therapies suffer from poor delivery to the muscle tissues, which, in the case of DM1, is further aggravated because cells do not display compromised membrane integrity that could facilitate cell uptake [38].
Screening of >3000 ASOs led IONIS to identify the IONIS-DMPKRx (ISIS 598769) gapmer-type candidate; the first and still single ASO evaluated in a clinical trial for the treatment of DM1 [39], [40]. IONIS-DMPKRx produces significant DMPK mRNA degradation through base pairing with a specific 3′-UTR gene sequence outside the repeat tract. The drug did not generate safety concerns, being well tolerated even at the highest dose tested (600 mg) (Myotonic Dystrophy Foundation Annual Conference; www.myotonic.org/digital-academy/ionis-pharmaceuticals-industry-updates-drug-development-2018-mdf-annual-conference). Still, IONIS concluded that insufficient drug reached the muscles (Muscular Dystrophy Association, Ionis Reports Setback on DMPKRx Program for Myotonic Dystrophy, https://strongly.mda.org/ionis-reports-setback-dmpkrx-program-myotonic-dystrophy) to elicit a significant therapeutic response on the functional and biological end-points set up in the trial (Table 1). The company announced a program to enhance the potency of future DM1 candidates in the skeletal and heart muscle through Ligand-Conjugated Antisense (LICA) technology [41].
IONIS also reported preclinical data for ISIS 486178 (Table 2), a different gapmer ASO designed to target the 3′-UTR region of the hDMPK transcript [42]. In cynomolgus monkeys, ISIS 486178 reduced DMPK expression by 70% in several muscles and 50% in cardiac tissue, with no skeletal muscle or cardiac toxicity after 13 weeks [42]. Importantly, this was also the first ASO reported to reach the heart with demonstrated benefit on molecular and functional alterations in the cardiac conduction defects of the DM200 mouse model (Box 1), thus suggesting ASOs as a viable option for treating cardiac pathology in DM1 [43]. This molecule also showed relevant activity in the DMSXL mouse model in terms of body-weight increase, muscle strength, and muscle histology, whereas no overt toxicity was detected [44].
Table 2.
Myotonic dystrophy drug candidates in preclinical stages of development.
| Preclinical status | Preclinical information | Refs |
|---|---|---|
| Cannabinoids CBD/THC (small molecule/repurposing) | ||
| IND-enabling phase | Management of chronic neuropathic pain, myotonia, and myalgia. Pilot survey in DM1, DM2, and congenital myotonia, with two patients per disease. Sponsor: Nexien Biopharma (https://nexienbiopharma.com/news) | [30], [31], [32] |
| Pitolisant; Wakix (small molecule/repurposing) | ||
| IND-enabling phase: Phase II by 2021 | Orally available to and able to cross the blood–brain barrier in animal models. Sponsor: Harmony Biosciences (www.harmonybiosciences.com/science) | [57] |
| ISIS 486178 (oligonucleotide-based therapy) | ||
| Lead optimization | Systemic treatment resulting in myotonia and cardiac conduction improvement, correction of splicing defects, reduction in RNA foci, and redistribution of MBNL1. DMSXL and DM200 mice models. Sponsor: Ionis Pharmaceuticals | [43], [44], [58], [59] |
| AOC1001 (oligonucleotide-based therapy) | ||
| IND enabling phase: Phase I/II by 2021 | Delivered to muscle cells reduces levels of DMPK mRNA in a durable, dose-dependent manner. AOC™ platform. Sponsor: Avidity Biosciences (www.aviditybiosciences.com/) | [60] |
| FORCE-DMPK candidate (oligonucleotide-based therapy) | ||
| IND enabling phase | In vivo dose-dependent correction of splicing and myotonia in the HSALR model after single low dose. DMPK mRNA reduction in mouse and nonhuman primates. FORCE™ platform. Sponsor: Dyne Therapeutics (www.dyne-tx.com/pipeline/) | [61] |
| Pip6a-PMO (oligonucleotide-based therapy) | ||
| Lead optimization | Intravenously injected in HSALR. Nuclear foci reduction, MBNL1 redistribution, splicing, and myotonia correction. Sponsor: Oxford University | [45] |
| NTC0200 (oligonucleotide-based therapy) | ||
| IND enabling phase | In vitro ability to target and open up aberrant DM1-linked secondary RNA structure in mutant transcript, thereby displacing sequestered splice proteins. PATrOL™ platform. Sponsor: Neubase (www.neubasetherapeutics.com/pipeline/) | [46] |
| Arthex-01 (oligonucleotide-based therapy) | ||
| Lead optimization: start of Phase I/II by 2022 | Subcutaneous injection in HSALR. Enhanced MBNL1/2 protein levels, recovered missplicing, muscle strength, and myotonia. Long-lasting activity of antagomiR-23b for up to 45 days. Sponsor: Arthex Biotech (https://arthexbiotech.com/pipeline/) | [49], [50] |
| AAV-PIN-dCas9 (gene therapy) | ||
| Lead optimization: start of IND-enabling studies by 2021 | Intramuscular/systemic delivery in adult and neonatal HSALR. RNA-targeting Cas9 lasted for up to 3 months. Elimination of RNA foci, reversal of splicing biomarkers, and myotonia. Sponsor: Locanabio (https://locanabio.com/pipeline/) | [56], [62] |
| AT-466 (gene therapy) | ||
| Lead optimization | AAV delivery to overcome the biodistribution limitations of ASO-based therapies. Sponsor: Audentes (www.audentestx.com/our-approach/) | [63] |
| AAV-CRISPR-Cas9 (gene therapy) | ||
| Preclinical proof of concept | In vivo genome editing for DM1: deletion of CTG repeat tract leading to reduction in nuclear foci in muscle fibres after intramuscular injection of SaCas 9 and sgRNA rAAV9 vectors in DMSXL mice. Sponsor: Genethon-INSERM | [55] |
Conjugation of therapeutic ASOs to carrier delivery systems, such as peptides or antibodies, is an active field to enhance their skeletal muscles and heart uptake. In this regard, AOC1001 is a conjugate of a small interfering (si)RNA degrading DMPK with a proprietary monoclonal antibody against the transferrin receptor 1 (TfR1) protein. Developed by Avidity Biosciences, the company recently stated that preclinical results confirmed the ability of AOC1001 to deliver siRNAs to muscle cells, boosting the reduction in DMPK mRNA levels in a durable and dose-dependent manner (Avidity Biosciences, www.aviditybiosciences.com/programs). Although details regarding the level of DMPK reduction, in vivo drug administration route, doses, or drug safety for AOC1001 have not been disclosed, Avidity has announced a Phase I/II clinical trial by the end of 2021 (Table 2).
Using a similar approach, Dyne Therapeutics is developing the FORCE-DMPK ASO candidate, which involves the conjugation of a proprietary ASO designed to reduce DMPK RNA levels in the nucleus by RNase H cleavage with a TfR1-binding Fab for effective delivery to muscles. According to Dyne’s preclinical results in mouse models, the FORCE-DMPK candidate reached potent Dmpk RNA knockdown in skeletal muscles (70–78%) and durable ASO muscle distribution on its proprietary wild-type hTfR1 mouse (up to 12 weeks) as well as in nonhuman primates. Dmpk knockdown was also observed in the heart, with an acceptable toxicity profile for blood, kidney, and liver function parameters. For therapeutic parameters, the FORCE candidate rescued mis-splicing and myotonia to near-normal levels after a single low dose in HSALR mice within 14 days after administering a single low dose (R. Subramanian et al., unpublished 2021).
An alternative to enhance muscle and heart uptake of ASOs is conjugation with cell-penetrating peptides (CPPs). The Pip6a-PMO-CAG7, a conjugate that combines a CPP moiety (Pip6a) and an ASO with morpholino chemistry-targeting repeats, promotes an occupancy-based mechanism for MBNL1 displacement from the toxic CUG repeat. In vivo, CPP-conjugated CAG7 PMO displayed significant improvement of oligonucleotide delivery into the striated muscles of HSALR mice following systemic administration, which promoted reversion of myotonia and splicing defects that were long-lasting and involved most of the altered transcriptome [45].
The NTC0200 (or Compound A) preclinical candidate developed by NeuBase Therapeutics explores a peptide-nucleic acid ASO identified through the proprietary platform PATrOL™. The resultant compound is a short, flexible, and selective peptide-nucleic acid sequence proved in vitro to discriminate the pathogenic CUG expansions from the wild-type transcripts, opening up CUG RNA secondary structures in the mutant transcript and displacing sequestered MBNL proteins [46]. Recent communication from the company on social networks announced long-lasting correction of global levels of mis-spliced transcripts in the HSALR transgenic mouse after a well-tolerated single intravenous injection.
There is ample evidence that limited MBNL protein function contributes to DM1. Given that the endogenous genes remain normal in patients with DM1, a therapeutic gene modulation approach to compensate for their depletion appears feasible. Building upon previous proof-of-concept reports [47], [48], this therapeutic avenue is being tested based on the identification of miR-23b and miR-218 as natural endogenous translational repressors of MBNL1/2 genes. Treatment with antimiRs, a class of chemically engineered ASOs complementary to their cognate target miRNA, significantly upregulated MBNL1/2 protein levels and improved molecular defects in cell models and functional muscle defects in the HSALR mouse model. Additionally, no safety concerns were observed in treatments in vivo [49], [50]. ARTHEx Biotech is working on the preclinical evaluation of the antimiR lead candidate (Arthex-01) against miR-23b or miR-218 with planned clinical trials by the beginning of 2022 (Table 2).
Gene therapy approaches
The most straightforward approach to overcome the limited biodistribution of AONs is to promote its endogenous expression using gene therapy vectors. Adeno-associated viruses (AAVs) are being evaluated for drug development in several muscular dystrophies [51], and with recent success with Zolgensma gene replacement approach in SMA. Benefits of this avenue in DM1 were initially disclosed with a proof-of-concept approach for an AAV-delivered RNAi in the HSALR mice [52]. Audentes is taking a similar approach with the AT466 candidate, a vectorized ASO-like design targeting the reduction of toxic DMPK RNA levels in cells from patients with DM1 by RNA degradation or exon skipping (or both simultaneously) mechanisms of action, both clinically already validated in studies with ASOs (Audentes, www.audentestx.com/innovative-therapies). Preclinical studies are underway to determine the optimal construct for AT466 (Table 2).
CRISPR/Cas9 genetic scissors have also emerged as innovative approaches to treat DM1 by targeting the removal of the expansions at the DNA level, preventing their transcription, or targeting degradation of the toxic RNA, therefore effectively targeting the underlying DM1 etiology [53], [54]. The fundamental requirement for genome editing by CRISPR is a purified Cas9 nuclease and a guide RNA (gRNA) targeting the desired genome sequence with an adjacent protospacer motif (PAM sequence). The endonuclease cuts both strands of the chromosomal DNA, making genome modifications possible through nonhomologous end joining or homologous-driven repair.
More recently, CRISPR-Cas9 has emerged as a multifunctional platform for sequence-specific gene expression regulation. Engineered nuclease-deficient Cas9, termed dCas9, when fused to effector domains with distinct regulatory functions, enables the CRISPR-Cas9 system to be repurposed as a general platform for RNA-guided DNA targeting without cleavage activity. Two approaches (Table 2) are moving through preclinical stages, making use of vectorized AAV administration.
The approach reported by Lo Scrudato et al. [55] at Généthon demonstrated the excision of long CTG repeats and reduced pathological RNA foci within tibialis anterior muscle in the DMSXL mouse model after a single intramuscular injection of recombinant AAV vectors expressing CRISPR-SaCas9 components.
On a related approach, but targeting the toxic RNA molecules, Locanabio is developing AAV-vectors encoding PIN-dCas9 (a dCas9 fused to the PIN RNA endonuclease) and a single-gRNA targeting CUG repeats, which, in intramuscular and systemic administration in adult and neonatal HSALR mice, showed up to 3 months of expression of the RNA-targeting Cas9. The treatment eliminated RNA foci, redistributed the MBNL1 factor, reversed dysregulated splicing biomarkers and transcriptional profiles, and ameliorated myotonia in skeletal muscle without significant toxicity signs, thus demonstrating potential clinical utility [56]. Future studies in large animals will be needed to assess further the safety, dose levels, and immunosuppression regimens required for safe and long-term treatments.
Concluding remarks and future challenges
We anticipate that patients with DM1 will have at least one therapeutic option targeting the genetic cause of the disease within the next few years. To reach this goal, it is imperative to maintain ongoing close collaboration between the academic and pharmaceutical sectors and to keep developing new and better preclinical models for hypothesis testing and drug evaluation. In this regard, it is vital to develop human 3D skeletal muscle models using tissue engineering to overcome the numerous limitations of 2D cultured myoblasts. These bioengineered microtissues would better recapitulate structural and molecular DM1 phenotypes as the gene expression changes associated with the CUG toxic RNA, and provide platforms to test drug activity and uptake under conditions closer to in vivo. Additional mammal models outside the murine paradigm would also significantly speed up preclinical validation in support for clinical programs. Overall, over the past decade, the DM1 community has smoothly transitioned from basic to translational research with the common goal of improving the lives of patients.
Note added in proofs
Since the information described in this paper was completed and accepted for publication, Expansion Therapeutics has disclosed the name of the ERX-963 lead compound as flumazenil (online talk: https://youtu.be/Nf7AUdTvC9k). Instead of a new chemical entity able to bind the CUG expanded repeat, as described through the paper and suggested on the previous limited available data, ERX-963 is a repurposed drug targeting the GABA receptor in the brain that showed no significant improvement in DM1 daytime sleepiness through the NCT03959189 clinical trial.
Declaration of interest
The authors declare the following financial interests/personal relationships which may be considered as potential competing interests: R.A. is an inventor in patent PCT/EP2017/073685, currently licensed to Arthex Biotech, of which he is a co-founder and scientific consultant. R.A. and M.P-G. are inventors in patents WO2016075285A1 and WO2016075288A1, currently licensed to Myogem Health Company. M.P-G. is a former member of the Board of Directors and shareholder of Myogem Health Company. A.L-C. is a member of the Arthex Board of Directors.
Acknowledgments
We thank the continued support on DM1 research from the Generalitat Valenciana (PROMETEO/2020/081), ‘la Caixa’ Banking Foundation (ID 100010434) under agreement HR17-00268, and Ministerio de Ciencia e Innovación (RTI2018-094599-B-100, which includes funds from the European Regional Development Fund) to R.A. Myogem thanks the European Union for supporting the nutritional management clinical trial through the H2020 research and innovation program under the Grant Agreement 875615. We thank the Marigold Foundation for support toward open access publishing of this paper.
References
- 1.Aartsma-Rus A., Corey D.R. The 10th oligonucleotide therapy approved: Golodirsen for Duchenne muscular dystrophy. Nucleic Acid Ther. 2020;30:67–70. doi: 10.1089/nat.2020.0845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Iftikhar M. Current and emerging therapies for Duchenne muscular dystrophy and spinal muscular atrophy. Pharmacol. Ther. 2020 doi: 10.1016/j.pharmthera.2020.107719. [DOI] [PubMed] [Google Scholar]
- 3.Ashizawa T. Consensus-based care recommendations for adults with myotonic dystrophy type 1. Neurology. Clinical Practice. 2018;8:507–520. doi: 10.1212/CPJ.0000000000000531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Johnson N.E. Myotonic muscular dystrophies. Continuum. 2019;25:1682–1695. doi: 10.1212/CON.0000000000000793. [DOI] [PubMed] [Google Scholar]
- 5.Kumar A. Variable ethnic frequency and risk ratio of DMPK gene: a meta-analysis survey. J. Steroids Hormonal Sci. 2015;6:1–4. [Google Scholar]
- 6.Chau A., Kalsotra A. Developmental insights into the pathology of and therapeutic strategies for DM1: back to the basics. Dev. Dyn. 2015;244:377–390. doi: 10.1002/dvdy.24240. [DOI] [PubMed] [Google Scholar]
- 7.Ozimski L.L. The hallmarks of myotonic dystrophy type 1 muscle dysfunction. Biol. Rev. Camb. Philos. Soc. 2020;96:716–730. doi: 10.1111/brv.12674. [DOI] [PubMed] [Google Scholar]
- 8.Jones K. GSK3β mediates muscle pathology in myotonic dystrophy. Journal of Clinical Investigation. 2012;122:4461–4472. doi: 10.1172/JCI64081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Wei C. GSK3β is a new therapeutic target for myotonic dystrophy type 1. Rare Diseases. 2013;1 doi: 10.4161/rdis.26555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Serenó L. A novel GSK-3beta inhibitor reduces Alzheimer’s pathology and rescues neuronal loss in vivo. Neurobiology of Disease. 2009;35:359–367. doi: 10.1016/j.nbd.2009.05.025. [DOI] [PubMed] [Google Scholar]
- 11.Wang M. Correction of glycogen synthase kinase 3β in Myotonic Dystrophy 1 reduces the mutant RNA and improves postnatal survival of DMSXL mice. Mol. Cell. Biol. 2019;39:E00155–E219. doi: 10.1128/MCB.00155-19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Horrigan J. A Phase 2 study of AMO-02 (tideglusib) in congenital and childhood-onset Myotonic Dystrophy Type 1 (DM1) Pediatr. Neurol. 2020;112:84–93. doi: 10.1016/j.pediatrneurol.2020.08.001. [DOI] [PubMed] [Google Scholar]
- 13.Konieczny P. Myotonic dystrophy: candidate small molecule therapeutics. Drug Discovery Today. 2017;22:1740–1748. doi: 10.1016/j.drudis.2017.07.011. [DOI] [PubMed] [Google Scholar]
- 14.Jenquin J.R. Combination treatment of erythromycin and furamidine provides additive and synergistic rescue of mis-splicing in Myotonic Dystrophy Type 1 models. ACS Pharmacol. Translat. Sci. 2019;2:247–263. doi: 10.1021/acsptsci.9b00020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Nakamori M. Oral administration of erythromycin decreases RNA toxicity in myotonic dystrophy. Ann. Clin. Transl. Neurol. 2016;3:42–54. doi: 10.1002/acn3.271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Rzuczek S.G. Precise small-molecule recognition of a toxic CUG RNA repeat expansion. Nat. Chem. Biol. 2017;13:188–193. doi: 10.1038/nchembio.2251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kouki T. Low-dose metformin improves hyperglycaemia related to myotonic dystrophy. Diabet. Med. 2005;22:346–347. doi: 10.1111/j.1464-5491.2005.01432.x. [DOI] [PubMed] [Google Scholar]
- 18.Laustriat D. In vitro and in vivo modulation of alternative splicing by the biguanide metformin. Mol. Therapy Nucleic Acids. 2015;4 doi: 10.1038/mtna.2015.35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.García-Puga M. Myotonic Dystrophy type 1 cells display impaired metabolism and mitochondrial dysfunction that are reversed by metformin. Aging. 2020;12:6260–6275. doi: 10.18632/aging.103022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Alsaggaf R. Diabetes, metformin and cancer risk in myotonic dystrophy type I. Int. J. Cancer. 2020;147:785–792. doi: 10.1002/ijc.32801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Bassez G. Improved mobility with metformin in patients with myotonic dystrophy type 1: a randomized controlled trial. Brain. 2018;141:2855–2865. doi: 10.1093/brain/awy231. [DOI] [PubMed] [Google Scholar]
- 22.Takahashi M.P., Cannon S.C. Mexiletine block of disease-associated mutations in S6 segments of the human skeletal muscle Na(+) channel. J. Physiol. 2001;537:701–714. doi: 10.1111/j.1469-7793.2001.00701.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Logigian E.L. Mexiletine is an effective antimyotonia treatment in myotonic dystrophy type 1. Neurology. 2010;74:1441–1448. doi: 10.1212/WNL.0b013e3181dc1a3a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Heatwole C. Mexiletine in myotonic dystrophy type-1: a randomized, double-blind, placebo-controlled trial. Neurology. 2020;96:e228–e240. doi: 10.1212/WNL.0000000000011002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Vio R. Evaluation of mexiletine effect on conduction delay and bradyarrhythmic complications in patients with myotonic dystrophy type 1 over long-term follow-up. Heart Rhythm. 2020;17:1944–1950. doi: 10.1016/j.hrthm.2020.05.043. [DOI] [PubMed] [Google Scholar]
- 26.Suetterlin K.J. Mexiletine (NaMuscla) for the treatment of myotonia in non-dystrophic myotonic disorders. Expert Opin. Orphan Drugs. 2020;8:43–49. [Google Scholar]
- 27.Witherspoon L. Sodium channel inhibitors reduce DMPK mRNA and protein. Clin. Transl. Sci. 2015;8:298–304. doi: 10.1111/cts.12275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Lawless M. Investigation of ranolazine as an anti-myotonia treatment in Myotonic Dystrophy Type 1. Neurology. 2018;90 P5.443. [Google Scholar]
- 29.Eckardt K. Cannabinoid type 1 receptors in human skeletal muscle cells participate in the negative crosstalk between fat and muscle. Diabetologia. 2009;52:664–674. doi: 10.1007/s00125-008-1240-4. [DOI] [PubMed] [Google Scholar]
- 30.Montagnese F. Cannabis use in myotonic dystrophy patients in Germany and USA: a pilot survey. J. Neurol. 2019;266:530–532. doi: 10.1007/s00415-018-9159-2. [DOI] [PubMed] [Google Scholar]
- 31.Montagnese F. A role for cannabinoids in the treatment of myotonia? Report of compassionate use in a small cohort of patients. J. Neurol. 2020;267:415–421. doi: 10.1007/s00415-019-09593-6. [DOI] [PubMed] [Google Scholar]
- 32.Klumpers, L. et al. Method and compositions for treating dystrophies and myotonia. Nexien Biopharma, Inc. US10702495B2.
- 33.Subramony S.H. Sleep disorders in myotonic dystrophies. Muscle Nerve. 2020;62:309–320. doi: 10.1002/mus.26866. [DOI] [PubMed] [Google Scholar]
- 34.Fredholm B.B. Springer; 2010. Methylxanthines. [Google Scholar]
- 35.Artero, R. et al. Compounds for the treatment of Myotonic Dystrophy. Myogem Health Company S.L. WO2016075285A1.
- 36.Artero, R. et al. Caffeine for the treatment of Myotonic Dystrophy Type 1 and Type 2. Myogem Health Company S.L. WO2016075288A1.
- 37.Overby S.J. RNA-mediated therapies in myotonic dystrophy. Drug Discovery Today. 2018;23:2013–2022. doi: 10.1016/j.drudis.2018.08.004. [DOI] [PubMed] [Google Scholar]
- 38.González-Barriga A. Cell membrane integrity in myotonic dystrophy type 1: implications for therapy. PLoS ONE. 2015;10 doi: 10.1371/journal.pone.0121556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Thornton C. Study design of a Phase 1/2a trial with ISIS-DMPKRx for the treatment of Myotonic Dystrophy Type 1. Neurology. 2016;86 P3.167. [Google Scholar]
- 40.Mignon L. ISIS-DMPKRx in healthy volunteers: a placebo-controlled, randomized, single ascending-dose Phase 1 study. Neurology. 2016;86 P3.166. [Google Scholar]
- 41.Hu N. Non-invasive monitoring of alternative splicing outcomes to identify candidate therapies for myotonic dystrophy type 1. Nat. Commun. 2018;9:5227. doi: 10.1038/s41467-018-07517-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Pandey S.K. Identification and characterization of modified antisense oligonucleotides targeting DMPK in mice and nonhuman primates for the treatment of myotonic dystrophy type 1. J. Pharmacol. Exp. Therap. 2015;355:329–340. doi: 10.1124/jpet.115.226969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Yadava R.S. Systemic therapy in an RNA toxicity mouse model with an antisense oligonucleotide therapy targeting a non-CUG sequence within the DMPK 3’UTR RNA. Hum. Mol. Genet. 2020;29:1440–1453. doi: 10.1093/hmg/ddaa060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Jauvin D. Targeting DMPK with antisense oligonucleotide improves muscle strength in Myotonic Dystrophy Type 1 mice. Mol. Therapy Nucleic Acids. 2017;7:465–474. doi: 10.1016/j.omtn.2017.05.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Klein A.F. Peptide-conjugated oligonucleotides evoke long- lasting myotonic dystrophy correction in patient- derived cells and mice. J. Clin. Investig. 2011;129:4739–4744. doi: 10.1172/JCI128205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Hsieh W.C. Design of a ‘mini’ nucleic acid probe for cooperative binding of an RNA-repeated transcript associated with Myotonic Dystrophy Type 1. Biochemistry. 2018;57:907–911. doi: 10.1021/acs.biochem.7b01239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Kanadia R.N. Reversal of RNA missplicing and myotonia after muscleblind overexpression in a mouse poly (CUG) model for myotonic dystrophy. Proc. Natl. Acad. Sci. USA. 2006;103:11748–11753. doi: 10.1073/pnas.0604970103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Chamberlain C.M., Ranum L.P.W. Mouse model of muscleblind-like 1 overexpression: skeletal muscle effects and therapeutic promise. Hum. Mol. Genet. 2012;21:4645–4654. doi: 10.1093/hmg/dds306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Cerro-Herreros E. miR-23b and miR-218 silencing increase Muscleblind-like expression and alleviate myotonic dystrophy phenotypes in mammalian models. Nat. Commun. 2018;9:2482. doi: 10.1038/s41467-018-04892-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Cerro-Herreros E. Therapeutic potential of Antagomir-23b for treating Myotonic Dystrophy. Mol. Therapy Nucleic Acids. 2020;21:837–849. doi: 10.1016/j.omtn.2020.07.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Crudele J.M., Chamberlain J.S. AAV-based gene therapies for the muscular dystrophies. Hum. Mol. Genet. 2019;28:R102–R107. doi: 10.1093/hmg/ddz128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Bisset D.R. Therapeutic impact of systemic AAV-mediated RNA interference in a mouse model of myotonic dystrophy. Hum. Mol. Genet. 2015;24:4971–4983. doi: 10.1093/hmg/ddv219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Raaijmakers R.H.L. CRISPR/Cas applications in myotonic dystrophy: expanding opportunities. Int. J. Mol. Sci. 2019;20:3689. doi: 10.3390/ijms20153689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Marsh S. Application of CRISPR-Cas9-mediated genome editing for the treatment of Myotonic Dystrophy Type 1. Mol. Ther. 2020;28:2527–2539. doi: 10.1016/j.ymthe.2020.10.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Lo Scrudato M. Genome editing of expanded CTG repeats within the human DMPK gene reduces nuclear RNA foci in the muscle of DM1 mice. Mol. Ther. 2019;27:1372–1388. doi: 10.1016/j.ymthe.2019.05.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Batra R. The sustained expression of Cas9 targeting toxic RNAs reverses disease phenotypes in mouse models of myotonic dystrophy type 1. Nat. Biomed. Eng. 2021;5:157–168. doi: 10.1038/s41551-020-00607-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Lamb Y.N. Pitolisant: a review in narcolepsy with or without cataplexy. CNS Drugs. 2020;34:207–218. doi: 10.1007/s40263-020-00703-x. [DOI] [PubMed] [Google Scholar]
- 58.Carrell S.T. Dmpk gene deletion or antisense knockdown does not compromise cardiac or skeletal muscle function in mice. Hum. Mol. Genet. 2016;25:4328–4338. doi: 10.1093/hmg/ddw266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Swayse, E.E. and Freier S.M. Compounds and methods for modulation of Dystrophia Myotonica-Protein Kinase (DMPK) expression. Ionis Pharmaceuticals, Inc. US2019276832A1.
- 60.Geall, A.J. et al. Compositions and methods of treating muscle atrophy and myotonic dystrophy. Avidity Biosciences LLC. US2019298847A1.
- 61.Weeden, T. and Spring, S. Methods of preparing protein-oligonucleotide complexes. Dyne Therapeutics, Inc. WO2020247738A1.
- 62.Nelles, D.A. et al. RNA-targeting fusion protein compositions and methods for use. Locana, Inc. US10822617B2.
- 63.Gray, J.T. Nucleic acid molecules containing spacers and methods of use thereof. Audentes Therapeutics, Inc. US2018305715A1.
- 64.Mankodi A. Myotonic dystrophy in transgenic mice expressing an expanded CUG repeat. Science. 2000;289:1769–1773. doi: 10.1126/science.289.5485.1769. [DOI] [PubMed] [Google Scholar]
- 65.Mahadevan M.S. Reversible model of RNA toxicity and cardiac conduction defects in myotonic dystrophy. Nat. Genet. 2006;38:1066–1070. doi: 10.1038/ng1857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Gomes-Pereira M. CTG trinucleotide repeat ‘big jumps’: large expansions, small mice. PLoS Genet. 2005;3 doi: 10.1371/journal.pgen.0030052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Vignaud A. Progressive skeletal muscle weakness in transgenic mice expressing CTG expansions is associated with the activation of the ubiquitin-proteasome pathway. Neuromuscul. Disord. 2010;20:319–325. doi: 10.1016/j.nmd.2010.03.006. [DOI] [PubMed] [Google Scholar]
- 68.Huguet A. Molecular, physiological, and motor performance defects in DMSXL mice carrying> 1,000 CTG repeats from the human DM1 locus. PLoS Genet. 2012;8 doi: 10.1371/journal.pgen.1003043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Sicot G. Downregulation of the glial GLT1 glutamate transporter and Purkinje cell dysfunction in a mouse model of myotonic dystrophy. Cell Reports. 2017;19:2718–2729. doi: 10.1016/j.celrep.2017.06.006. [DOI] [PMC free article] [PubMed] [Google Scholar]