Abstract
Aims:
Metastasis to the thyroid gland is a rare occurrence that may pose a diagnostic challenge. In this study, we aimed to report the clinicopathologic features, immunoprofile, molecular alterations, and outcome of 30 patients treated at our center from 2003 to 2019.
Results:
The most common site of the primary tumor was the kidney, followed by the lung, lower gastrointestinal tract, and breast. In seven (23%) patients, the thyroid metastases were resected prior to the diagnosis of the primary tumors. Six patients (20%) had thyroid as the sole metastatic site. Three (10%) patients harbored tumor-to-tumor metastasis. 71% had unilateral unifocal thyroid mass, which might be mistaken for primary thyroid tumors. Among the 13 cases that were initially diagnosed at an outside hospital, 4 (31%) were misinterpreted as a thyroid primary. An immunohistochemical panel of thyroid follicular cell markers was most useful to differentiate primary thyroid tumors from metastasis. Molecularly, the metastasis showed alterations characteristic of the primary tumor, which may be helpful in establishing diagnosis and primary site. Although the prognosis was poor with a 5-year disease specific survival of 58%, a long-term cure was possible in cases with oligometastasis treated successfully with surgery.
Conclusions:
Metastasis to the thyroid gland is an uncommon phenomenon with an incidence of 0.36% in all thyroid malignancies. It may present as a solitary thyroid mass before the discovery of the primary tumor, posing a diagnostic challenge. Although the overall prognosis is poor, a subset of patients with oligometastasis can be managed surgically.
Keywords: Thyroid, metastasis, renal cell carcinoma, tumor-to-tumor metastasis
Introduction
Metastasis to the thyroid gland is an uncommon occurrence with a reported frequency of up to 2.1% among all thyroid malignancies diagnosed in surgical specimens 1-3. The most common primary site is the lung in autopsy series and kidney in surgical specimens 4. Given its rarity, metastasis to the thyroid may pose a diagnostic challenge especially when the primary tumor is undiagnosed at the time of thyroid sampling, resulting in misinterpretation of metastasis as a primary thyroid carcinoma 5-7.
In recent years, immunohistochemistry (IHC) of various thyroid-specific markers (e.g. PAX8, TTF-1 and thyroglobulin) 8-12 as well as molecular diagnostic tools 13 became readily available in routine clinical practice, assisting pathologists in reaching a correct diagnosis in challenging cases. However, publications on thyroid metastasis, especially those including comprehensive immunophenotypic and molecular profiles, are relatively sparse.
In this study, we performed a detailed clinical, pathologic, immunophenotypic, and molecular analysis of 30 cases of metastasis to the thyroid gland in patients seen at the Memorial Sloan Kettering Cancer Center (MSKCC, New York, NY, US) spanning a period of 16 years. Additionally, a literature review on the contemporary studies of metastasis to the thyroid gland in surgical pathology within the past 4 decades was conducted, aiming to further our understanding of the diagnosis and management of this rare entity.
Materials and Methods
The study was approved by the Institutional Review Board of MSKCC. The pathology database was searched and reviewed for all surgical thyroid specimens from 2003 to 2019 with a diagnosis of malignancy (including carcinoma, lymphoma, and sarcoma) or noninvasive follicular thyroid neoplasm with papillary-like nuclear features (NIFTP). Thyroid specimens with a pathologic diagnosis of non-neoplastic conditions or adenoma were excluded. A total of 8410 cases diagnosed with malignancy involving the thyroid gland were identified. Among them, 30 patients harbored hematogenous metastasis to the thyroid gland. Cases with secondary involvement of the thyroid gland by direct extension of carcinoma or sarcoma from adjacent organs (e.g. larynx, trachea, and parathyroid gland) were excluded.
All available slides and clinical charts of the 30 cases of thyroid metastases were reviewed by an endocrine pathologist (BX). Pathologic features gathered included specimen type (biopsy vs. resection), diagnosis of the metastasis, size, focality, and laterality of the metastasis, co-existing thyroid neoplasms, and their relationship with the metastasis, vascular invasion, immunohistochemical profile, and molecular alterations. Clinical characteristics included were age, sex, site and pathologic diagnosis of the primary tumor and its temporal relationship to the thyroid metastasis, other sites of distant metastasis, and clinical follow up. The 3-year, 5-year, and 10-year disease specific survival was calculated from the diagnosis of thyroid metastasis using SPSS software 24.0 (IBM corporation, Westchester, NY, USA).
IHC was performed at the discretion of the pathologist at the time of diagnosis. Antibodies utilized in this study included PAX8 (Proteintech, polyclonal, dilution 1:100), TTF-1 (Ventana, 8G7G3/1, monoclonal, ready to use RTU), thyroglobulin (Cell Marque, 2H11+6E1, RTU), Napsin-A (Leica, IP64, monoclonal, dilution 1:100), CD10 (Leica, 56C6, monoclonal, RTU), carbonic anhydrase IX (CAIX, Novus, polyclonal, dilution: 1:2000), synaptophysin (Biogenex, SNP88, monoclonal, dilution 1: 2000), chromogranin (Ventana, LK2H10, monoclonal, RTU), CDX2 (Biogenex, CDX2.88, monoclonal, dilution 1:100), GATA3 (Biocare, L50.823, monoclonal, dilution 1:200), estrogen receptor (ER, Leica, 6F11, monoclonal, RTU), progesterone receptor (PR, Leica, 16, monoclonal, RTU) , Histone H3-G34W (HH3-G34W, RevMab Bioscience, RM263, monoclonal, RTU), smooth muscle actin (SMA, Cell Marque, 1A4, Monoclonal, dilution 1:200), smooth muscle myosin (SMM, Cell Marque, SMMS-1, monoclonal, RTU), CKIT (DAKO, polyclonal, dilution 1:150), CK7 (DAKO, OV-TL-12/30, monoclonal, dilution 1: 800), and anaplastic lymphoma (ALK, Cell Signaling, D5F3, Monoclonal, dilution 1:200).
Molecular profile, when available, was collected from the patient’s chart. Five pulmonary carcinomas were subjected to Sequenom MassARRAY system (Sequenom, San Diego, CA) targeting 8 oncogenes: EGFR, KRAS, BRAF, PIK3CA, NRAS, AKT1, ERBB2, and MAP2K1/MEK as previously described 14, 15. Additionally, 6 tumors, including a pulmonary adenocarcinoma, a pulmonary non-small cell carcinoma, a clear cell renal cell carcinoma, a malignant giant cell tumor, a polymorphous adenocarcinoma of minor salivary gland origin, and an undifferentiated spindle sarcoma, were tested using MSK-IMPACT™ (Memorial Sloan Kettering-Integrated Mutation Profiling of Actionable Cancer Targets) platform, which is a Food and Drug Administration (FDA)-approved deep-coverage, targeted next-generation sequencing assay detecting single nucleotide variants (SNVs), small insertions/deletion (indels), copy number variants (CNVs) and fusion/structural variants in 468 oncogenes, using custom DNA probes designed for targeted sequencing of all exons and selected introns, including canonical and selected non-canonical transcripts 16, 17.
Results
Among the 8410 cases of thyroid biopsies and resections with a diagnosis of malignancy or NIFTP, 30 patients (0.36%) were diagnosed with metastasis to the thyroid gland. The detailed clinical, pathologic, immunophenotypic, and molecular features of the study cohort are shown in Table 1.
Table 1.
Clinicopathologic features and outcomes of metastases to the thyroid gland.
| N | Age/ gender |
Primary site |
Diagnosis | Time interval from primary tumor diagnosis to thyroid metastasis (months ) (a) |
Other sites of metastasis |
Thyroid as the only site of metastasis |
Thyroid as first site of metastasis |
Status at last follow up |
Follow up period after diagnosis of thyroid metastasis (months ) |
Specimen type |
Laterality and focality of metastasis |
Tumor greatest dimension (cm) |
Thyroid neoplasms and geographic relationship with the metastasis |
Discrepant initial diagnosis |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Kidney primary (n=9) | ||||||||||||||
| 1 | 65/F | Kidney | Clear cell RCC | −2 | Lung | No | Yes | AWD | 9 | Resection | Unilateral, unifocal | 1.1 | NIFTP (tumor-to-tumor metastasis) | No |
| 2 | 59/F | Kidney | Clear cell RCC | −1.5 | None | Yes | Yes | NED | 108.9 | Resection | Bilateral, multifocal | 1.8 | PMC (not related) | NA |
| 3 | 62/F | Kidney | Clear cell RCC | 92.8 | None | Yes | Yes | AWD | 45.9 | Resection | Unilateral, unifocal | 2.5 | PMC (not related) | No |
| 4 | 83/M | Kidney | Clear cell RCC | 6.3 | Liver | No | Yes | DOD | 35.4 | Resection | Unilateral, unifocal | 1.3 | PMC (not related) | NA |
| 5 | 63/M | Kidney | Clear cell RCC | 97.7 | skin, bone, brain, lung, spine | No | No | DOD | 33.4 | Resection | Bilateral, multifocal | 5.5 | Absent | NA |
| 6 | 69/M | Kidney | Clear cell RCC | 129.5 | None | Yes | Yes | NED | 61.3 | Resection | Bilateral, multifocal | 2.9 | Absent | No |
| 7 | 72/M | Kidney | Clear cell RCC | 71.7 | None | Yes | Yes | NED | 35.4 | Resection | Unilateral, unifocal | 2.4 | Absent | NA |
| 8 | 60/M | Kidney | Clear cell RCC | 61.1 | Pancreas, lung | No | No | DOD | 124.5 | Resection | Unilateral, unifocal | 2.5 | Absent | NA |
| 9 | 71/F | Kidney | Chromophobe RCC | 55.7 | Liver | No | Yes | AWD | 3.4 | Resection | Unilateral, unifocal | 1.8 | PTC, FV (adjacent) | PTC, FV |
| Lung primary (n=7) | ||||||||||||||
| 10 | 83/F | Lung | Adenocarcinoma | 36.8 | Bone, spine | No | No | AWD | 21.2 | Resection | Unilateral, unifocal | 1.4 | Absent | NA |
| 11 | 44/F | Lung | Adenocarcinoma | 1.1 | Bone, liver | No | Yes | DOD | 16.4 | Resection | Bilateral, multifocal | 2.5 | Absent | No |
| 12 | 63/M | Lung | Adenocarcinoma | NA | Brain, intrapulmonary, cervical lymph nodes | No | Yes | AWD | 14 | Resection | Unilateral, unifocal | 3 | PMC (adjacent) | ATC |
| 13 | 28/M | Lung | Adenocarcinoma | NA | Bone, gastrointestinal tract, peritoneum | No | Yes | DOD | 6.3 | Resection | Unilateral, unifocal | 6.2 | Absent | NA |
| 14 | 77/M | Lung | Non-small cell carcinoma | NA | Brain, bone, intrapulmonary, peritoneal | No | Yes | AWD | 18.2 | Resection | Unilateral, unifocal | 6 | Absent | ATC |
| 15 | 65/M | Lung | LCNEC | 8.7 | Intrapulmonary | No | No | AWD | 63.9 | Resection | Unilateral, unifocal | 7.2 | Absent | NA |
| 16 | 54/M | Lung | Carcinoid tumor | 34.7 | Liver, intrapulmonary | No | No | DOD | 88.2 | Resection | Unilateral, unifocal | 0.5 | PMC (not related) | NA |
| Colorectal primary (n=4) | ||||||||||||||
| 17 | 66/F | Colorectal | Adenocarcinoma | 145 | Ovary, lung, duodenum | No | No | AWD | 52.1 | Resection | Unilateral, unifocal | 1.2 | Classic PTC (tumor-to-tumor metastasis) | NA |
| 18 | 49/F | Colorectal | Adenocarcinoma | 67.8 | Liver | No | No | NED | 49.4 | Resection | Bilateral, multifocal | 0.8 | PMC (not related) | No |
| 19 | 63/M | Colorectal | Adenocarcinoma | 23.1 | Liver, omentum, abdominal wall | No | No | DOD | 55.4 | Resection | Unilateral, unifocal | 2.2 | Absent | NA |
| 20 | 56/M | Colorectal | Adenocarcinoma | 124.3 | Brain, lung | No | No | DOD | 106.2 | Resection | Unilateral, unifocal | 4 | Absent | NA |
| 21 | 56/F | Colorectal | Adenocarcinoma | 71 | Lung, liver | No | No | DOD | 1 | Biopsy | NA | NA | NA | NA |
| Other primary sites (n=9) | ||||||||||||||
| 22 | 59/F | Breast | Mammary carcinoma | 161.5 | Bone | No | No | AWD | 11.2 | Resection | Bilateral, multifocal | 1.5 | PTC, TCV (adjacent) | No |
| 23 | 58/F | Breast | Mammary carcinoma | 76.1 | Cervical lymph nodes | No | Yes | NED | 9.6 | Resection | Bilateral, multifocal | 1 | Absent | No |
| 24 | 51/F | Salivary gland (minor, buccal) | PAC | 257.4 | Mediastinum, brain, spine | No | Yes | AWD | 43.4 | Resection | Unilateral, unifocal | 2 | Absent | No |
| 25 | 78/F | Salivary gland (submandibular) | Adenocarcinoma, NOS | 24.6 | Lung, bone | No | Yes | DOD | 29.1 | Resection | Unilateral, unifocal | 2 | Absent | No |
| 26 | 72/F | Esophagus | Adenocarcinoma | 2.2 | None | Yes | Yes | DOUC/NED | 123.9 | Resection | Unilateral, unifocal | 3.4 | FA (tumor-to-tumor metastasis) | NA |
| 27 | 51/F | Bone (femur) | Malignant giant cell tumor | NA | Bone, spine, lung | No | No | DOD | 3 | Biopsy | NA | NA | NA | NA |
| 28 | 22/M | Bone (femur) | Osteosarcoma | 78.3 | Lung, liver | No | No | DOD | 12.4 | Resection | Unilateral, unifocal | 3.9 | Absent | NA |
| 29 | 47/M | Soft tissue (peralaryngeal) | Undifferentiated spindle sarcoma | 46.2 | Skin, neck soft tissue, spine, lung, pleura | No | No | DOD | 45.8 | Resection | Unilateral, unifocal | 5 | Absent | NA |
| 30 | 81/M | Soft tissue (retroperitonum) | Leiomyosarcoma | NA | None | Yes | Yes | AWD | 13.4 | Resection | Bilateral, multifocal | 6.5 | Absent | ATC |
F: female, M: male, PAC: polymorphous adenocarcinoma, RCC: renal cell carcinoma, LCNEC: large cell neuroendocrine carcinoma, NOS: not otherwise specified, AWD: alive with disease, NED: no evidence of disease, DOD: dead of disease, DOUC: dead of unknown cause, NED: no evidence of disease, PTC: papillary thyroid carcinoma, TCV: tall cell variant, FV: follicular variant, PMC: papillary microcarcinoma, NIFTP: noninvasive follicular thyroid neoplasm with papillary-like nuclear features, FA: follicular adenoma.
A negative value indicates that the thyroid metastasis is diagnosed prior to primary tumor. NA: not applicable in four cases in which the primary tumor was not sampled for pathologic diagnosis.
Clinical characteristics
The median age at the diagnosis of thyroid metastasis was 63 years (range: 23 to 83). The male to female ratio was 1:1.
In 23 (77%) cases, the primary tumors were diagnosed prior to the presentation of thyroid metastasis. The median time interval from the pathologic diagnosis of the primary tumor to thyroid metastasis was 64.5 months (range: 1 to 257 months). Late metastasis (10 years or more) to the thyroid gland was observed in 5 patients, including 2 with colorectal adenocarcinoma, 1 with clear cell renal cell carcinoma, 1 with mammary carcinoma, and 1 with salivary gland carcinoma. In 2 cases, the primary tumor was resected to provide a definite pathologic diagnosis approximately 2 months after the diagnosis of thyroid metastasis, whereas in the remaining 5 patients the primary tumors were never sampled.
Among these 5 patients, the site and diagnosis of primary tumors were determined using a combination of clinical, radiologic, immunophenotypic, and molecular findings. Three patients (cases 12, 13, and 14) presented with large pulmonary mass and widely metastatic disease with the thyroid metastasis demonstrated typical immunophenotypic (TTF-1 positive/napsin-A positive/PAX8 negative) and/or molecular features (ALK fusion or KRAS mutation) of lung carcinoma; one patient (case 27) had large femoral mass with multifocal metastasis to lung and other bones showing diagnostic H3F3A mutation of giant cell tumor, and one (case 30) showed 8-cm unresectable stable retroperitoneal mass and multiple bilateral thyroid nodules that were diffusely and strongly positive for myoid marker smooth muscle actin and myosin.
Six patients (20%) had thyroid as the sole metastatic site, including 4 patients with clear cell renal cell carcinoma, 1 with retroperitoneal leiomyosarcoma, and 1 with esophageal adenocarcinoma. The remaining 24 patients had widely metastatic disease involving other organs, most common being lung (n=13), bone (n=8), liver (n=8), and brain (n=5). Among these 24 patients, the thyroid gland was present as the first site of metastasis in 10 patients.
The histologic features
Twenty-five cases had a diagnosis of carcinoma; one case was diagnosed as metastatic carcinoid tumor; whereas the remaining four were mesenchymal neoplasms. Two cases were biopsies, while the remaining 28 cases were resection specimens. The most common type and site of the primary tumor that metastasized to the thyroid gland were clear cell renal cell carcinoma (n=8, 27%) and the kidney (n=9, 30%) respectively. The sites of primary tumors in a descending order were kidney (n=9), lung (n=7), colon/rectum (n=5), breast (n=2), femur (n=2), buccal minor salivary gland (n=1), submandibular gland (n=1), esophagus (n=1), neck soft tissue (n=1), and retroperitoneum (n=1).
The histotypes of the metastasis were clear cell renal cell carcinoma (n=8), chromophobe renal cell carcinoma (n=1), pulmonary adenocarcinoma (n=4), pulmonary non-small cell carcinoma (n=1), pulmonary large cell neuroendocrine carcinoma (n=1), pulmonary carcinoid tumor (n=1), colorectal adenocarcinoma (n=5), mammary carcinoma (n=2), polymorphous adenocarcinoma of salivary gland (n=1), adenocarcinoma not otherwise specified of salivary gland (n=1), esophageal adenocarcinoma (n=1), malignant giant cell tumor (n=1), osteosarcoma (n=1), leiomyosarcoma (n=1), and undifferentiated spindle sarcoma (n=1).
Among the 28 resected metastases, 8 (29%) contained bilateral and multifocal disease in the thyroid gland, while the remaining 20 (71%) were present as a unilateral and unifocal thyroid mass. Vascular invasion was identified in 5 (18%), including 2 with extensive vascular invasion. The median size of the metastatic focus was 2.5 cm (range: 0.5 to 7.2 cm).
Eleven (39%) thyroid resection specimens also contained neoplasms of thyroid follicular cell origin, including papillary microcarcinoma (n=6), NIFTP (n=1), papillary carcinoma follicular variant (n=1), noninvasive encapsulated classic papillary carcinoma with predominant follicular pattern (n=1), papillary carcinoma tall cell variant (n=1), and follicular adenoma (n=1). Among them, 3 showed tumor-to-tumor metastasis (Figure 1), including an esophageal adenocarcinoma to follicular adenoma, a colorectal adenocarcinoma to classic papillary carcinoma, and a clear cell renal cell carcinoma to NIFTP. Two cases had coincidental papillary carcinoma immediately adjacent to the metastasis, whereas in the remaining cases, the thyroid neoplasms and the metastases showed no spatial relationship.
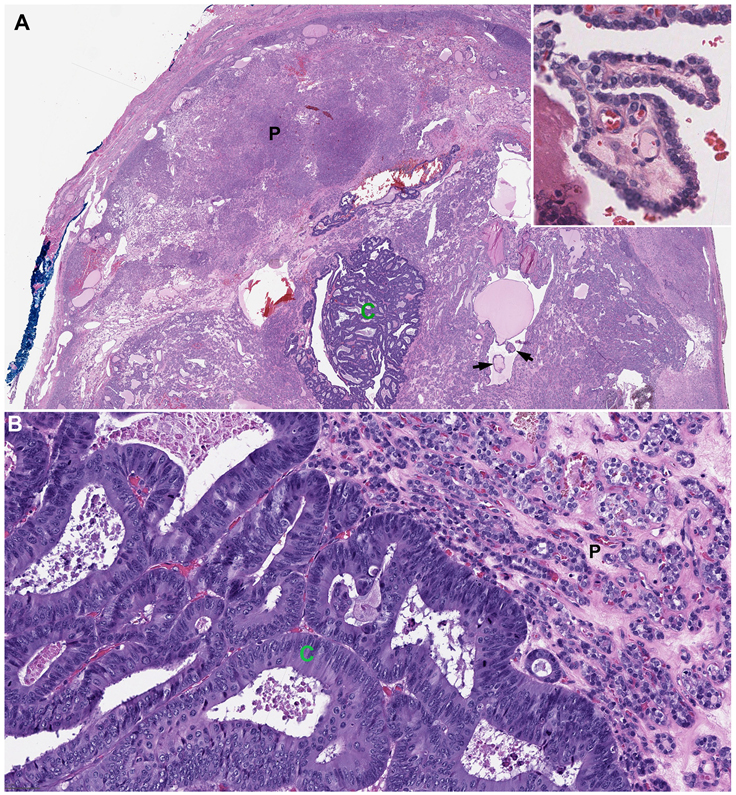
Figure 1. Tumor-to-tumor metastasis.
A colorectal adenocarcinoma (C) metastasizes to an encapsulated classic papillary thyroid carcinoma with predominant follicular growth pattern (P, patient #17). The metastatic colorectal adenocarcinoma contains pseudostratified nuclei, luminal necrosis, and frequent mitotic activity. Note the presence of scattered well-formed papillae within the background thyroid lesion (arrows and insert). Magnification: top panel 20X; bottom panel 200X.
IHC and molecular profile
Among the 22 cases tested for TTF-1 IHC, 5 (23%) were positive for TTF-1, all of which were of pulmonary origin. PAX8 IHC using polyclonal antibody was positive in 4 of 13 (31%) cases, including 2/2 (100%) clear cell renal cell carcinoma, and 2/6 (33%) pulmonary tumors. Thyroglobulin IHC was universally negative in all metastases tested.
Molecular testing was performed in 11 cases, which demonstrated alterations characteristic of the primary tumors in 5 cases, including VHL mutation in clear cell renal cell carcinoma (n=1), KRAS mutations (n=2) or EML4-ALK fusion (n=1) in pulmonary adenocarcinoma, and H3F3A G34W mutation in malignant giant cell tumor.
Diagnostic discrepancy and challenges
Thirteen cases were consultation cases that were initially diagnosed at an outside institute. Among them, 4 cases were misinterpreted as primary thyroid carcinomas. The discrepant diagnoses were as follows (initial – revised): anaplastic thyroid carcinoma (ATC) – metastatic leiomyosarcoma; ATC – metastatic non-small cell lung carcinoma; ATC – metastatic EML4-ALK translocated pulmonary adenocarcinoma; and papillary thyroid carcinoma follicular variant – metastatic chromophobe renal cell carcinoma. The primary tumors were not diagnosed at the time of thyroid metastasis in the first three cases, while the resection of chromophobe renal cell carcinoma was performed 5 years prior to the diagnosis of thyroid metastasis. The two cases of pulmonary metastasis showed an immunoprofile of TTF-1 positivity, napsin-A positivity, and PAX8 negativity, which was incompatible with a diagnosis of ATC (Figure 2). The metastatic chromophobe renal cell carcinoma was positive for PAX8, CK7, and CKIT, whereas negative for TTF-1 and thyroglobulin, which did not support the diagnosis of a well-differentiated thyroid carcinoma (Figure 3).
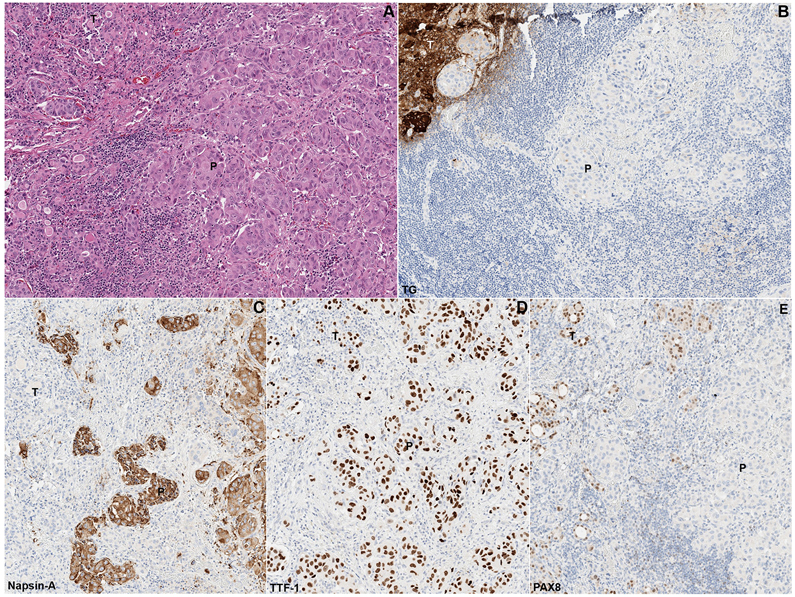
Figure 2. Metastatic pulmonary adenocarcinoma (P) to the thyroid gland (T),
hematoxylin and eosin (H&E, A) and immunohistochemistry for thyroglobulin (TG, B), napsin-A (C), TTF-1 (D), and PAX8 (E, Case #12). The metastatic pulmonary adenocarcinoma is positive for napsin-A and TTF-1, whereas negative for TG and PAX8. The background thyroid gland is positive for PAX8, TTF-1, and TG, and is negative for napsin-A.
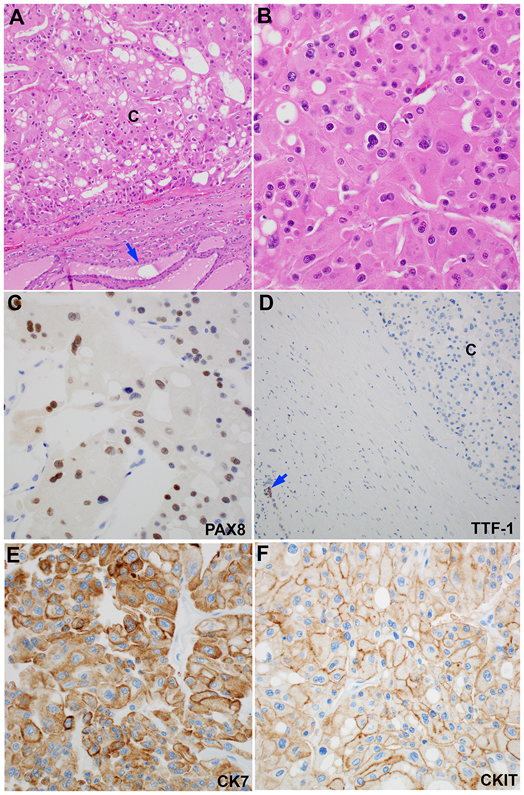
Figure 3. Metastatic chromophobe renal cell carcinoma to the thyroid gland.
(A) At low power (100X), the tumor is separated from the background thyroid (blue arrow) by a fibrous capsule. (B) At high power (400X), the tumor cells have wrinkled nuclei, binucleation, perinuclear halo, and abundant eosinophilic cytoplasm. (C-F) The tumor shows a PAX8-positive, TTF-1-negative, CK7-positive, and CKIT-negative immunoprofile. Note that the background thyroid is TTF-1 positive (blue arrow in panel D).
Clinical outcome
The median follow up of the study cohort after the diagnosis of thyroid metastasis was 35 months (range: 1 to 200 months). The median disease-specific survival from the diagnosis of thyroid metastasis was 94 months. The 3-year, 5-year, and 10-year disease specific survival after metastasis was 70%, 58%, and 39% respectively.
Six cases (20%) were considered as no evidence of disease (NED) given that both the primary tumors and metastases were completely resected surgically; 13 suffered disease specific death; whereas the remaining cases were alive with disease at the time of the last follow up. Among the 6 patients with a status of NED at the last follow up, four patients had metastasis limited to the thyroid gland (3 with clear cell renal cell carcinoma and 1 with esophageal adenocarcinoma), while the remaining two patients had resectable oligometastasis to the liver or cervical lymph node beside the thyroid gland (one with mammary carcinoma, the other with colorectal adenocarcinoma). The 3-year, 5-year, and 10-year disease specific survival was 100%, 100%, and 100% respectively for the subgroup with isolated metastasis limited to the thyroid gland, and 56%, 41%, and 14% for patients with multi-site/widely-spread metastatic disease.
Discussion
A detailed literature review summarizing studies of metastasis to the thyroid gland in surgical pathology from 1985 to present is provided in supplementary table 1. Autopsy series 18-20, fine needle aspiration reports 21, 22, and surgical studies conducted before 1985 5, 7, 18, 23-25 were excluded to generate a contemporary dataset with supportive diagnostic ancillary studies used in current surgical pathology practice. Two series included lymphoma 26 and leukemia 27 as metastasis: such cases were considered as systemic involvement and were excluded from the literature review. Overall, metastasis to the thyroid gland was exceedingly rare, accounting for 0.15% of all thyroid resections 1, 2, 28, and 0.63% of all thyroid malignancies 1, 2. Of note, the frequency of metastasis among all thyroid malignancies was lower in the present study, being 0.36%, compared with 1.1% to 2.1% as reported in the literature 1-3. One explanation for the discrepancy could be the selection bias of patients treated at our center with high frequency of malignancy diagnosis in all thyroid pathology specimens and a high rate of surgical resection in patients with metastasis to the thyroid gland. Thyroidectomy is often reserved for those with oligometastasis as reflected in the higher number of patients with metastatic tumors limited to the thyroid (20%) in our study compared to other surgical series 29, 30.
In our cohort, renal cell carcinoma was the most common histotype of thyroid metastasis, followed by pulmonary tumors, colorectal adenocarcinoma, and mesenchymal tumors. Similarly, the literature review showed that the kidney was the most common primary site, accounting for 42% of all cases 1, 2, 28-39. Other common sites of primary tumors in descending order of frequency were lung (16%), head and neck (10%), breast (9%), lower gastrointestinal tract (7%), upper gastrointestinal tract (5%), melanoma (2%), and sarcoma (2%). However, the rate of head and neck carcinoma metastasis to the thyroid gland needs to be interpreted with caution, as several studies included thyroid involvement from carcinomas of the adjacent head and neck organs, such as trachea, larynx, and parathyroid gland 2, 31, 33, 39. It is questionable whether these cases represent a direct extension of the primary tumor into the thyroid gland given the anatomic proximity.
To date, all renal cell carcinomas metastasized to the thyroid gland reported in the literature were described as of clear cell type 1, 12, 35, 40. In this study, we reported the first case of hematogenous spread of chromophobe renal cell carcinoma to the thyroid gland, widening the differential diagnoses in this specific setting.
Contrary to the common belief that thyroid metastases frequently present as multifocal deposits 41, we found that the majority (71%) manifested as a unifocal and unilateral distinct nodule within the thyroid gland. Additionally, 39% of resection specimens contained primary thyroid neoplasms as well, including scenarios of tumor-to-tumor metastasis or adjacent primary and metastatic tumors. Furthermore, in two cases, the primary tumor was discovered after the thyroid resection. Together, these factors resulted in the possibility of a metastasis masquerading as a primary thyroid tumor, which presented diagnostic challenges to the practicing pathologists. Indeed, in our study, 36% (4/11) of the consult cases were initially misinterpreted as primary thyroid carcinomas, in particular ATC and less frequently papillary thyroid carcinoma. In this setting, a high clinical suspicion and a combined IHC panel of thyroid-specific markers (in particular TTF-1, thyroglobulin, and PAX8), as well as primary site/tumor-specific markers, might lead to the correct diagnosis 8-11. Among the thyroid-specific markers, PAX8 is particularly useful in the setting of ATC as a large proportion of it is positive for PAX8 and entirely negative for TTF-1 and thyroglobulin 8, 11, while pulmonary carcinomas are in their vast majority typically PAX8 negative. However, as shown in this study and others, lung carcinomas can express PAX8 if a polyclonal antibody is used 42. Some authors found that 55 (8%) of 687 lung carcinomas are PAX8 positive using a polyclonal antibody while no immunopositivity was seen in these lung carcinomas stained with monoclonal PAX8 42. We therefore recommend the use of monoclonal PAX8 when metastasis is suspected especially a lung metastasis. At the other end of the spectrum, thyroglobulin IHC is the most specific marker and is universally expressed in differentiated thyroid carcinoma 10, 43. We however encountered in our practice non-thyroid carcinomas focally positive for thyroglobulin because of cross-reactivity (especially with Müllerian primaries) or passive diffusion from normal thyroid follicles, a well-known phenomenon 44. The confusion of a metastatic tumor to the thyroid with a primary thyroid carcinoma can have significant clinical impacts on the patient leading to morbidities. In our opinion, the best approach to avoid such a mistake is to use a combination of immunohistochemical markers along with a careful clinical-pathologic correlation.
Among all pathologic features studied, tumor-to-tumor metastasis deserves a special mention. It is a rare phenomenon defined as metastasis into another tumor 45-48. Thyroid tumors are one of the most common “recipient” tumors 47, and the most common histotypes reported in the literature are follicular adenoma and follicular variant of papillary thyroid carcinoma 45-48. In this study, we reported three cases of tumor-to-tumor metastasis with the “recipient” tumors being a follicular adenoma, a noninvasive encapsulated classic papillary carcinoma with predominant follicular growth pattern, and a NIFTP. Coincidentally, all three tumors are indolent neoplasms with a predominant or exclusive follicular growth pattern.
Molecular testing, when performed, might show signature alterations of the primary tumors, which might be useful in identifying the primary site in an appropriate clinical setting, e.g. widely metastatic disease without a clear-cut primary. The characteristic molecular alterations reported in our cohort included VHL somatic mutation in clear cell renal cell carcinoma 49, KRAS mutations, or EML4-ALK translocation in pulmonary adenocarcinoma 50, and H3F3A G34W mutation and immunopositivity of the mutation product HH3-G34W in giant cell tumor 51, 52.
Literature varied regarding the outcome of thyroid metastasis. The reported median overall survival ranged from 12 to 34 months 2, 29, 37, 5-year overall survival ranged from 31% to 51% 28, 35, 36, and the rate of disease-specific mortality ranged from 33% to 73% 1, 28, 35, 36, 38, 39. We herein reported a median disease specific survival of 94 months, 5-year overall survival of 58%, and disease-specific mortality of 43%. The prolonged survival in our cohort might in part be attributed to the finding of a small but significant amount of cases (20%) with thyroid being the only metastatic site. Indeed as mentioned above, the proportion of patients with metastasis restricted to the thyroid was much lower in other series 29, 30. A subgroup of patients (6/30, 20%) was considered clinically as disease-free following a curative surgical attempt to remove oligometastasis. Similarly, a recent meta-analysis has found surgical removal of the metastasis to the thyroid gland was associated with improved survival compared to a conservative approach in all metastasis as well as in subsite analysis focusing specifically on metastases from kidney, colon, or pulmonary origin 30. Together, these data suggest that surgical approach with curative intent may be considered in the setting of oligometastasis to the thyroid gland, which may result in improved survival and even long-term cure.
In conclusion, we herein reported the comprehensive clinical, pathologic, immunophenotypic, and molecular features of 30 cases of metastasis to the thyroid gland, a rare entity accounting for less than 1% of all thyroid malignancies. Thyroid metastasis typically is presented as a unifocal thyroid mass and might occur prior to the discovery of the primary tumor leading to its misinterpretation as a thyroid primary in a significant number of cases. This error has drastic consequences for the patient. Immunostains for TTF-1, thyroglobulin, and PAX8, as well as molecular testing are useful diagnostic tools in the appropriate clinical setting that can prevent this serious misdiagnosis. Although the outcome was poor overall with a 5-year disease-specific survival of 58%, long-term remission might be achieved with surgery for patients with oligometastasis.
Supplementary Material
Acknowledgments
Research reported in this publication was supported in part by the Cancer Center Support Grant of the National Institutes of Health/National Cancer Institute under award number P30CA008748. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
Footnotes
Conflict of interest statement: No competing financial interests exist for all contributory authors. No competing financial interests exist for all contributory authors.
REFERENCES
- 1.Cichoń S, Anielski R, Konturek A, Barczyński M, Cichoń W. Metastases to the thyroid gland: Seventeen cases operated on in a single clinical center. Langenbeck's archives of surgery / Deutsche Gesellschaft fur Chirurgie 2006;391;581–587. [DOI] [PubMed] [Google Scholar]
- 2.Papi G, Fadda G, Corsello SM et al. Metastases to the thyroid gland: Prevalence, clinicopathological aspects and prognosis: A 10-year experience. Clin Endocrinol (Oxf) 2007;66;565–571. [DOI] [PubMed] [Google Scholar]
- 3.Wood K, Vini L, Harmer C. Metastases to the thyroid gland: The royal marsden experience. European journal of surgical oncology : the journal of the European Society of Surgical Oncology and the British Association of Surgical Oncology 2004;30;583–588. [DOI] [PubMed] [Google Scholar]
- 4.Nixon IJ, Coca-Pelaz A, Kaleva AI et al. Metastasis to the thyroid gland: A critical review. Annals of surgical oncology 2017;24;1533–1539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Elliott RH Jr., Frantz VK. Metastatic carcinoma masquerading as primary thyroid cancer: A report of authors' 14 cases. Annals of surgery 1960;151;551–561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Matias-Guiu X, LaGuette J, Puras-Gil AM, Rosai J. Metastatic neuroendocrine tumors to the thyroid gland mimicking medullary carcinoma: A pathologic and immunohistochemical study of six cases. The American journal of surgical pathology 1997;21;754–762. [DOI] [PubMed] [Google Scholar]
- 7.Ivy HK. Cancer metastatic to the thyroid: A diagnostic problem. Mayo Clinic proceedings 1984;59;856–859. [DOI] [PubMed] [Google Scholar]
- 8.Xu B, Fuchs T, Dogan S et al. Dissecting anaplastic thyroid carcinoma: A comprehensive clinical, histologic, immunophenotypic, and molecular study of 360 cases. Thyroid : official journal of the American Thyroid Association 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Cimino-Mathews A, Sharma R, Netto GJ. Diagnostic use of pax8, caix, ttf-1, and tgb in metastatic renal cell carcinoma of the thyroid. The American journal of surgical pathology 2011;35;757–761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Harach HR, Franssila KO. Thyroglobulin immunostaining in follicular thyroid carcinoma: Relationship to the degree of differentiation and cell type. Histopathology 1988;13;43–54. [DOI] [PubMed] [Google Scholar]
- 11.Bishop JA, Sharma R, Westra WH. Pax8 immunostaining of anaplastic thyroid carcinoma: A reliable means of discerning thyroid origin for undifferentiated tumors of the head and neck. Human pathology 2011;42;1873–1877. [DOI] [PubMed] [Google Scholar]
- 12.Queipo FJ, Panizo A, Yagüe A et al. Metastatic clear cell renal cell carcinoma to the thyroid gland: A clinicopathological and immunohistochemical study of 8 cases and review of the literature. Revista espanola de patologia : publicacion oficial de la Sociedad Espanola de Anatomia Patologica y de la Sociedad Espanola de Citologia 2019;52;81–86. [DOI] [PubMed] [Google Scholar]
- 13.Afrogheh AH, Meserve E, Sadow PM et al. Molecular characterization of an endometrial endometrioid adenocarcinoma metastatic to a thyroid hürthle cell adenoma showing cancerization of follicles. Endocrine pathology 2016;27;213–219. [DOI] [PubMed] [Google Scholar]
- 14.Rekhtman N, Paik PK, Arcila ME et al. Clarifying the spectrum of driver oncogene mutations in biomarker-verified squamous carcinoma of lung: Lack of egfr/kras and presence of pik3ca/akt1 mutations. Clin Cancer Res 2012;18;1167–1176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kadota K, Sima CS, Arcila ME et al. Kras mutation is a significant prognostic factor in early-stage lung adenocarcinoma. The American journal of surgical pathology 2016;40;1579–1590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Cheng DT, Mitchell TN, Zehir A et al. Memorial sloan kettering-integrated mutation profiling of actionable cancer targets (msk-impact): A hybridization capture-based next-generation sequencing clinical assay for solid tumor molecular oncology. The Journal of molecular diagnostics : JMD 2015;17;251–264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Morris LG, Chandramohan R, West L et al. The molecular landscape of recurrent and metastatic head and neck cancers: Insights from a precision oncology sequencing platform. JAMA oncology 2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Silverberg SG, Vidone RA. Carcinoma of the thyroid in surgical and postmortem material. Analysis of 300 cases at autopsy and literature review. Annals of surgery 1966;164;291–299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Berge T, Lundberg S. Cancer in malmö 1958-1969. An autopsy study. Acta pathologica et microbiologica Scandinavica. Supplement1977;1–235. [PubMed] [Google Scholar]
- 20.Lam KY, Lo CY. Metastatic tumors of the thyroid gland: A study of 79 cases in chinese patients. Archives of pathology & laboratory medicine 1998;122;37–41. [PubMed] [Google Scholar]
- 21.HooKim K, Gaitor J, Lin O, Reid MD. Secondary tumors involving the thyroid gland: A multi-institutional analysis of 28 cases diagnosed on fine-needle aspiration. Diagnostic cytopathology 2015;43;904–911. [DOI] [PubMed] [Google Scholar]
- 22.Michelow PM, Leiman G. Metastases to the thyroid gland: Diagnosis by aspiration cytology. Diagnostic cytopathology 1995;13;209–213. [DOI] [PubMed] [Google Scholar]
- 23.Wychulis AR, Beahrs OH, Woolner LB. Metastasis of carcinoma to the thyroid gland. Annals of surgery 1964;160;169–177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Czech JM, Lichtor TR, Carney JA, van Heerden JA. Neoplasms metastatic to the thyroid gland. Surg Gynecol Obstet 1982;155;503–505. [PubMed] [Google Scholar]
- 25.Shimaoka K, Sokal JE, Pickren JW. Metastatic neoplasms in the thyroid gland. Pathological and clinical findings. Cancer 1962;15;557–565. [DOI] [PubMed] [Google Scholar]
- 26.Pusztaszeri M, Wang H, Cibas ES et al. Fine-needle aspiration biopsy of secondary neoplasms of the thyroid gland: A multi-institutional study of 62 cases. Cancer Cytopathol 2015;123;19–29. [DOI] [PubMed] [Google Scholar]
- 27.Mangussi-Gomes J, Danelon-Leonhardt F, Moussalem GF, Ahumada NG, Oliveira CL, Hojaij FC. Thyroid gland invasion in advanced squamous cell carcinoma of the larynx and hypopharynx. Brazilian journal of otorhinolaryngology 2017;83;269–275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Calzolari F, Sartori PV, Talarico C et al. Surgical treatment of intrathyroid metastases: Preliminary results of a multicentric study. Anticancer research 2008;28;2885–2888. [PubMed] [Google Scholar]
- 29.Lièvre A, Leboulleux S, Boige V et al. Thyroid metastases from colorectal cancer: The institut gustave roussy experience. European journal of cancer (Oxford, England : 1990) 2006;42;1756–1759. [DOI] [PubMed] [Google Scholar]
- 30.Russell JO, Yan K, Burkey B, Scharpf J. Nonthyroid metastasis to the thyroid gland: Case series and review with observations by primary pathology. Otolaryngology--head and neck surgery : official journal of American Academy of Otolaryngology-Head and Neck Surgery 2016;155;961–968. [DOI] [PubMed] [Google Scholar]
- 31.McCabe DP, Farrar WB, Petkov TM, Finkelmeier W, O'Dwyer P, James A. Clinical and pathologic correlations in disease metastatic to the thyroid gland. American journal of surgery 1985;150;519–523. [DOI] [PubMed] [Google Scholar]
- 32.Green LK, Ro JY, Mackay B, Ayala AG, Luna MA. Renal cell carcinoma metastatic to the thyroid. Cancer 1989;63;1810–1815. [DOI] [PubMed] [Google Scholar]
- 33.Nakhjavani MK, Gharib H, Goellner JR, van Heerden JA. Metastasis to the thyroid gland. A report of 43 cases. Cancer 1997;79;574–578. [DOI] [PubMed] [Google Scholar]
- 34.Chen H, Nicol TL, Udelsman R. Clinically significant, isolated metastatic disease to the thyroid gland. World journal of surgery 1999;23;177–180; discussion 181. [DOI] [PubMed] [Google Scholar]
- 35.Heffess CS, Wenig BM, Thompson LD. Metastatic renal cell carcinoma to the thyroid gland: A clinicopathologic study of 36 cases. Cancer 2002;95;1869–1878. [DOI] [PubMed] [Google Scholar]
- 36.Iesalnieks I, Winter H, Bareck E et al. Thyroid metastases of renal cell carcinoma: Clinical course in 45 patients undergoing surgery. Assessment of factors affecting patients' survival. Thyroid : official journal of the American Thyroid Association 2008;18;615–624. [DOI] [PubMed] [Google Scholar]
- 37.Romero Arenas MA, Ryu H, Lee S et al. The role of thyroidectomy in metastatic disease to the thyroid gland. Annals of surgical oncology 2014;21;434–439. [DOI] [PubMed] [Google Scholar]
- 38.Saito Y, Sugitani I, Toda K, Yamada K, Fujimoto Y. Metastatic thyroid tumors: Ultrasonographic features, prognostic factors and outcomes in 29 cases. Surg Today 2014;44;55–61. [DOI] [PubMed] [Google Scholar]
- 39.Zhang L, Liu Y, Li X, Gao W, Zheng C. Metastases to the thyroid gland: A report of 32 cases in pumch. Medicine (Baltimore) 2017;96;e7927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Medas F, Calò PG, Lai ML, Tuveri M, Pisano G, Nicolosi A. Renal cell carcinoma metastasis to thyroid tumor: A case report and review of the literature. Journal of medical case reports 2013;7;265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Rosai J, DeLellis RA, Carcangiu ML, Frable WJ, Tallini G. Tumor of the thyroid and parathyroid gland (afip atlas of tumor pathology series 4). Silver Spring, MD: American Registry of Pathology Press, 2015;606. [Google Scholar]
- 42.Toriyama A, Mori T, Sekine S, Yoshida A, Hino O, Tsuta K. Utility of pax8 mouse monoclonal antibody in the diagnosis of thyroid, thymic, pleural and lung tumours: A comparison with polyclonal pax8 antibody. Histopathology 2014;65;465–472. [DOI] [PubMed] [Google Scholar]
- 43.Kavishwar VS, Phatak AM, Rege JD. Immunohistochemistry of thyroid carcinoma--an experience with thyroglobulin and calcitonin. Indian journal of pathology & microbiology 1998;41;163–167. [PubMed] [Google Scholar]
- 44.Rosai J, Kuhn E, Carcangiu ML. Pitfalls in thyroid tumour pathology. Histopathology 2006;49;107–120. [DOI] [PubMed] [Google Scholar]
- 45.Gowda KK, Bal A, Agrawal P, Verma R, Das A. Tumor-to-tumor metastasis: Small cell carcinoma lung metastasising into a follicular adenoma of the thyroid. Indian journal of pathology & microbiology 2017;60;133–135. [DOI] [PubMed] [Google Scholar]
- 46.Wey SL, Chang KM. Tumor-to-tumor metastasis: Lung carcinoma metastasizing to thyroid neoplasms. Case reports in pathology 2015;2015;153932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Matsukuma S, Kono T, Takeo H, Hamakawa Y, Sato K. Tumor-to-tumor metastasis from lung cancer: A clinicopathological postmortem study. Virchows Archiv : an international journal of pathology 2013;463;525–534. [DOI] [PubMed] [Google Scholar]
- 48.Baloch ZW, LiVolsi VA. Tumor-to-tumor metastasis to follicular variant of papillary carcinoma of thyroid. Archives of pathology & laboratory medicine 1999;123;703–706. [DOI] [PubMed] [Google Scholar]
- 49.Ricketts CJ, De Cubas AA, Fan H et al. The cancer genome atlas comprehensive molecular characterization of renal cell carcinoma. Cell reports 2018;23;3698. [DOI] [PubMed] [Google Scholar]
- 50.Comprehensive molecular profiling of lung adenocarcinoma. Nature 2014;511;543–550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Yamamoto H, Iwasaki T, Yamada Y et al. Diagnostic utility of histone h3.3 g34w, g34r, and g34v mutant-specific antibodies for giant cell tumors of bone. Human pathology 2018;73;41–50. [DOI] [PubMed] [Google Scholar]
- 52.Cleven AH, Höcker S, Briaire-de Bruijn I, Szuhai K, Cleton-Jansen AM, Bovée JV. Mutation analysis of h3f3a and h3f3b as a diagnostic tool for giant cell tumor of bone and chondroblastoma. The American journal of surgical pathology 2015;39;1576–1583. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.