Abstract
Autism Spectrum Disorder (ASD) is a neurodevelopmental disorder with a behavioral phenotype characterized by impaired development of social-communicative skills as well as excessive repetitive and stereotyped behaviors. Despite high phenotypic heterogeneity in ASD, a meaningful subpopulation of children with ASD (~90%) show significant general motor impairment.. More focused studies on the nature of motor impairment in ASD reveal that children with ASD are particularly impaired on tasks such as ball catching and motor imitation that require efficient visual-motor integration (VMI). Motor computational approaches also provide evidence for VMI-impairment showing that children with ASD form internal sensorimotor representations that bias proprioceptive over visual-feedback. Impaired integration of visual information to form internal representations of others’ and the external world may explain observed impairments on VMI-tasks and motor imitation of others. Motor imitation is crucial for acquiring both social and motor skills and impaired imitation skill may contribute to the observed core behavioral phenotype of ASD. The current review examines evidence supporting VMI-impairment as a core feature of ASD that may contribute to both impaired motor imitation and social-communicative skill development. We propose that understanding the neurobiological mechanisms underlying VMI-impairment in ASD may be key to discovery of therapeutics to address disability in children and adults with ASD.
Keywords: autism, visual-motor integration, internal-representations, motor imitation
INTRODUCTION
Autism Spectrum Disorder (ASD) is a prevalent neurodevelopmental disorder, affecting 1 in 54 children in the US [1], and is characterized by social-communication impairments and restricted and repetitive behaviors and interests [2]. In addition to the core behavioral phenotype of ASD, motor impairment is very common among children with ASD (~90%) [3] and understanding the specificity of autism-associated motor impairments may lead to the discovery of neural circuits and processes contributing to the emergence of the core ASD behavioral phenotype.
In addition to general motor impairments observed in individuals with ASD [3–5], motor imitation impairments are consistently observed [6,7] with the degree of imitation impairment predictive of core ASD symptoms [8,9]. Motor imitation is crucial for social-communication and forming social bonds [10] and may contribute to the emergence of core ASD symptoms. Neural processes involved in motor imitation are complex and findings from our group and others suggest that some individuals with ASD may have a specific impairment in efficient use of visual information to form internal representations of the self and of the external world for predictive motor control [11–15]. This proposed visual-motor integration (VMI) impairment hypothesis in autism suggests that transformation of visual representations of the self and the external world into internal perceptual-motor representations for action is a crucial neural process important to the development of social-communicative behavior. Deficient VMI, with poor ability to imitate others’ actions, could thereby explain core autism impairments in development of both social skills (as efferent representations of these internal action models) and social perception/awareness (as afferent representations of these internal action models).
Motor computational approaches have provided evidence in support of the VMI-impairment hypothesis showing anomalous patterns in how individuals with ASD execute and acquire novel motor skills. Methods which weighted visual vs. proprioceptive sensory integration during motor learning show that children with ASD tend bias proprioceptive feedback (from their own internal body space) and to discount visual feedback (from the external world) [11–13,15]. Additional studies examining movement in response to varying visual dynamics and feedback have helped specify the nature of VMI impairment in ASD, as compared with both typically developing (TD) controls as well as with individuals with other neurodevelopmental disorders (e.g., ADHD) [14,16–19].
In the current review article, we synthesize research that has been performed by our group and others exploring the VMI-impairment hypothesis in ASD. First, we review research showing that individuals with ASD form anomalous internal representations that bias proprioception (“somatosensation”) over vision (“sight”). Second, we review studies that show individuals with ASD show difficulty integrating dynamic (less predictable) visual information into motor commands. Finally, we discuss specific neural processes that may contribute to motor imitation impairment in ASD and examine the potential for therapeutics to mitigate VMI-impairment allowing for improved acquisition of social-communicative skills and awareness.
INDIVIDUALS WITH ASD BIAS PROPRIOCEPTIVE OVER VISUAL FEEDBACK DURING SELF-GENERATED GOAL DIRECTED MOVEMENTS
In one of the earliest investigations of how children with ASD build internal representations, Masterton and Biederman (1983) examined whether children (7–15 years) with ASD prefer proximal (proprioception) as compared to distal (visual) sensory information when learning a novel coin-placement task [20]. In their study, children with ASD, children with intellectual disability, and TD children reached into a transparent box to place a coin into a slot indicated by an LED. In subsequent trials, a prism was inserted into the box and the children adapted to a prism-induced lateral displacement of their observed hand producing approximately 60mm coin placement error. Such unilateral prism-induced visual field shifts cause adaptation specific to the adapted arm viewed through the prism, with no inter-manual transfer to the non-adapted arm [21]. Post-adaptation aftereffects were examined with the prism removed and coin-placement trials were performed for each hand. While TD controls and children with intellectual disability (ID) showed virtually no aftereffects on the non-adapted hand, children with ASD showed a large degree of adaptation between hands, such that post-adaptation accuracy decreased on both the non-adapted and adapted hand. Based on the findings, the authors concluded that although children with ASD adapted typically to the prism-induced visuomotor transformation, they showed bias for relying on proprioception-feedback over visual-feedback. This conclusion, while preliminary, has been supported since by findings from several motor computational studies, as detailed below.
Motor Computational Approaches Reveal Proprioceptive-Bias Forming Internal Representations in Children with ASD
Initial findings suggested that children with ASD have the capacity to learn novel motor tasks and update internal representations of action [22,23]; however, these studies did not examine the underlying sensori-motor processes involved in motor learning. In both Haswell et al. (2009) and Izawa et al. (2012), children performed a series of reaches to a target with a robotic manipulandum while a velocity-dependent force field “pushed” their hand perpendicular to the movement direction. After the child adapted their reaches to counteract the force-field perturbation, the arm configuration was rotated and reaches were performed in error-clamp trials, such that a virtual channel wall fixed the manipulandum to move in one direction producing no error (no lateral deviation from target). Error-clamp trials were used to assess the generalization of learning to both visual coordinates (same visual trajectory to target but with different arm joint configuration) and proprioceptive coordinates (same arm joint configuration but with different visual trajectory). In the initial Haswell et al (2009) study, we discovered that, as compared with TD children, children with ASD showed a much greater degree of generalization in proprioceptive space, with less generalization in visual coordinate space. The findings suggested that, when forming novel movement patterns, children with ASD show a bias towards relying on proprioceptive feedback, with a tendency to discount visual feedback. Further, the degree of proprioceptive-bias was associated with both impaired motor imitation performance as well as clinical measures of social-communication impairment in children with ASD [10]. In a follow up study by Izawa et al. (2012), it was shown that this bias towards relying on proprioceptive (vs. visual) feedback appears specific to autism: Children with ASD showed an abnormal bias in comparison to both TD children as well as children with Attention Deficit/Hyperactivity Disorder (ADHD), with those in the ADHD group showing a learning pattern that was similar to the TD children [11].
Haswell et al. (2009) and Izawa et al. (2012) demonstrated that the formed internal representation of the learned motor task biased proprioception in children with ASD, such that “somatosensation” drove learning as compared to “sight”. However, the channel-specific sensitivity to visual and proprioceptive perturbations during task learning was not examined. Using the same 2D ballistic force-field reaching paradigm as [11,12], Marko et al. (2015) perturbed visual and proprioception errors during task acquisition itself, so as to more directly examine whether children with ASD were more sensitive to proprioceptive error as compared to visual error while learning a novel movement pattern. Proprioceptive error sensitivity was measured from conditions where a force field “pushed” the hand perpendicular to the reach direction, at various magnitudes, while the visual error was fixed at zero. Visual error sensitivity was examined by holding the force field “push” constant while the visual error (gain) was magnified. The findings revealed that while TD children show significantly more sensitivity to and learning from visually-sensed errors as compared to proprioceptively-sensed errors; children with ASD showed the exact opposite.. Collectively, the findings presented in Haswell et al. (2009), Izawa et al. (2012), and Marko et al. (2015) tell a compelling story that when forming internal sensori-motor representation of action, children with ASD tend to rely more on “somatosensation” as compared to “sight.” However, these studies do not elucidate the underlying developmental factors leading to proprioceptive-bias. For example, it is unknown whether VMI is intact early in development but disrupted by a later emerging proprioceptive-bias, or whether proprioceptive-bias emerges as a consequence of disrupted VMI early in development. This question needs to be resolved through careful longitudinal study of infant/childhood development in populations of children at risk for ASD.
Proprioceptive Sensory-Bias Revealed using Naturalistic Target Reaching Tasks
In the studies examined in the previous section, reaches were constrained to 2D and proprioception was augmented using novel force fields. It follows that examining proprioception bias in the internal representation of a ‘naturalistic’ 3D reach to target would be crucial to understanding “real-world” implications. In a sample of adults with ASD and neurotypical controls, Glazebrook et al. (2009) examined eye and arm reaching movements in 3D space to a target both with visual-feedback and without visual-feedback during the reach [15]. In contrast to neurotypical adults, for the adults with ASD, when visual-feedback was removed immediately after reach initiation, reach duration and spatial variability were significantly reduced as compared to reaching trials with visual-feedback. Furthermore, on reaching trials with visual-feedback, endpoint error was higher in the ASD group compared to the TD group, a finding that was not observed in reaches without visual-feedback. The findings suggest that the internal representation of the arm in individuals with ASD may be biased towards proprioception (“somatosensation”) as online visual feedback was disruptive to reaching performance and its removal normalized reaching performance to neurotypical levels.
Similar to Glazebrook et al. (2009), Zheng et al. (2019) used a 3D reach aiming task to a visual target but included reach trials were the reach degrees of freedom were constrained to 2D and 1D [24]. In the 2D-reaching trials, the participants (TD and ASD) reached to a target while sliding their index finger along a low friction sheet of Plexiglas, whereas in the 1D-reaching trials a groove (track) was cut in the sheet that provided tactile error feedback to the participant and guided the finger towards the target. While 3D-reaches are dominated by visual feedback for online correction, 2D and 1D-reaches provide additional tactile and proprioceptive information to the participants. Zheng et al. (2019) revealed a group-by-reach type interaction such that the ASD group spent more time executing the 3D reaches as compared to the TD group. This finding supports several studies that also show prolonged 3D reach movement times in children and adults with ASD as compared to TD controls [25]. Further, within each group, only individuals with ASD showed significantly longer 3D reach movement times as compared to 2D and 1D reaches. Therefore, by providing individuals with ASD additional proprioceptive and tactile sensory feedback, reliance on visual feedback was decreased and movement times were normalized to neurotypical levels.
Proprioceptive-Bias in Postural Control
In the previous section, findings in manual reaching paradigms revealed that individuals with ASD may have difficulty using visual information to build internal representations of novel tool dynamics [11–13] and their own limbs [15,24], such that “somatosensation” is more central to skill execution and learning than is “sight”. Evidence of anomalous internal representations have also been observed using postural maintenance tasks where the brain must compare the internal state of the body relative to the external world [26,27]. Minshew et al. (2004) examined postural sway responses from sensory perturbations to visual, proprioceptive, and vestibular systems in a large sample of individuals with ASD (5–52 years; N=79) [26]. In conditions where the platform was sway-referenced, such that proprioceptive feedback was inaccurate, individuals with ASD showed the greatest disruptions to postural control. Further, the developmental trajectories of proprioceptive-induced disruptions to postural sway in individuals with ASD did not normalize to neurotypical levels with increasing age. Using a different experimental paradigm, Morris et al. (2015) examined automatic postural adjustments resulting from a proprioceptive posterior neck muscle vibration illusion during conditions where vision was available or occluded [27]. Through posterior neck muscle vibration, an illusion of backward trunk movement is produced causing a forward shift in the center of pressure (COP). When vision was available, TD adults were able to counter the vibration-induced postural illusion by increasing reliance on vision for postural control, whereas in the same condition (vision + vibration), adults with ASD showed forward shift in COP that suggests inaccurate proprioceptive information was “trusted” over the accurate visual feedback. Collectively, the findings from both manual and postural studies suggest that individuals with ASD build an internal representation of actions that is more strongly reliant upon proprioception as compared to TD individuals, and that the observed proprioceptive bias persists across development.
INDIVIDUALS WITH ASD SHOW HYPO-RESPONSIVENESS TO DYNAMIC VISUAL FEEDBACK AND ENVIRONMENTAL STIMULI
In the previous section, we examined studies that show that individuals with ASD tend to “trust” proprioceptive feedback more strongly than normal when forming internal representations. In the current section, we will review studies that examine how integration of visual information for motor control and learning is specifically altered in individuals with ASD.
Children with ASD Show Decreased Motor Anticipatory Responses and Performance During Ball Catching
Our group, has found that among items from a standardized developmental motor assessment (the Movement Assessment Battery for Children, mABC), ball catching was the feature that most reliably distinguished children with ASD from TD children as well as those with ADHD [28]. These findings have been replicated by other groups [29,30] and ball catching performance has been shown to distinguish children with ASD (with intellectual disability) from TD children as well as those with intellectual disability (without ASD) [30]. Impaired ball catching performance in children with ASD, may result from difficulty forming internal representations of visually sensed trajectories (i.e., gravity) [31]. Infants (6 months) at high-familial risk for ASD also show evidence of impaired trajectory prediction during ball interception as they show a decreased motor anticipatory response when a ball rolled towards them as compared to infants at low familial risk for ASD [32]. These findings suggest that VMI-impairment in infants at high-risk for ASD may be present early in development impacting how these children interact with others’ and their environment.
Individuals with ASD Show Hypo-Responsiveness to Increased Visual Scene Dynamics
Gepner & Mestre (2002) examined postural reactivity to dynamic visual perturbations in a small number of children with ASD and TD controls. The dynamic visual perturbation was an oscillating tunnel expanding and contracting in depth at a frequency of 0.2 Hz, such that it induced anterior-posterior sway. In TD controls, as compared to children with ASD, the frequency of the visual stimulus (0.2 Hz) showed greater entrainment in the postural sway dynamics measured by the force platform. The findings suggest hypo-reactivity to dynamic visual stimuli in children with ASD, as compared to TD controls, since manipulating the visual dynamics of the world in the children with ASD did not change the internal representation of the self as relative to the world. In a larger group of individuals with ASD and TD controls, Greffou et al. (2012) used a similar virtual tunnel paradigm as Gepner & Mestre (2002) to induce anterior-posterior sway oscillations. As compared to their respective TD control groups, the children with ASD, but not adults with ASD, showed postural hypo-reactivity to the oscillating tunnel visual perturbation. Together, the findings from Gepner & Mestre (2002) and Greffou et al., (2012) suggest hypo-reactivity to dynamic visual stimuli in children, but not adults, with ASD. The findings may result from an internal representation of the self that relies more strongly upon proprioceptive “somatosensation” than upon “sight” to maintain a given posture.
Individuals with ASD Show Hypo-Responsivity to Increasing Visual Feedback Dynamics During Manual Motor Performance
Visuomotor grip-force tracking paradigms have been used in combination with fMRI to study visuomotor circuitry [33,34] and to determine the integrity of visuomotor circuitry to integrate visual feedback with the motor system [16,35–37]. During visuomotor grip-force tracking tasks, the visual-feedback can be manipulated by increasing the dynamics of either the force-feedback or the target force. While visuomotor grip-force tracking seems to predominately rely on visual feedback to update motor commands, predicted internal multisensory representations of course also include proprioceptive and tactile feedback, as these feedback systems are always present (except in rare cases of de-afferented individuals).
Mosconi et al. (2015) examined force output during a visuomotor grip force tracking task in children and adults with ASD and TD controls (5–35 years) [16]. The task required individuals to use a squeeze a pair of load cells to align a cursor with a static force target at various visual gains. For the TD group, as the visual-feedback gain was increased, the complexity (i.e., irregularity) of force output also increased up to the highest visual gain levels. In contrast, the ASD group showed a diminished response to progressive increases in visual feedback magnitude, such that the force output complexity plateaued before the TD group at which point they were no longer able to use the amplified visual error feedback to update motor commands.
Using a similar experimental paradigm as Mosconi et al. (2015), Lidstone et al. (2020) examined whether modifying the dynamics of the target force would also reveal VMI-impairment in children ASD [37]. To examine the diagnosis-specificity of VMI-impairment, Lidstone et al. (2020) recruited children (7–17 years) with ASD, ADHD, Fetal Alcohol Spectrum Disorder (FASD), and TD controls. The children performed two grip force tracking tasks: 1) A static task, similar to that used in Mosconi et al. (2015), during which children squeezed a load cell using a precision-grip (index and thumb) to match a static target force and, 2) A dynamic task during which children adjusted their grip force to track a constant-velocity dynamic target. The dynamic task thereby had increased VMI demands as compared to the static task, as children had to form and adjust an internal representation such that grip forces scaled in synchrony with the dynamics of the visual target. The findings revealed that for the static task, all groups showed similar force tracking accuracy. In contrast, for the dynamic task the children in the ASD group showed a particular difficulty tracking the target as compared to the TD group, whereas tracking accuracy differences between the TD and ADHD and FASD groups were not as prominent. These findings suggest that for children with ASD there may be a particular deficit in forming internal representations of dynamic targets for predictive motor control. As noted above, the findings may help to explain observed deficits in object control and ball interception skills in children with ASD as compared to children with ADHD and TD controls [28,29,32,38,39].
MOTOR IMITATION IMPAIRMENT IN ASD: BRIDGING THE GAP BETWEEN VISUAL-MOTOR INTEGRATION AND SOCIAL-SKILL DEVELOPMENT
In the previous sections we have reviewed evidence from motor computational literature supporting the VMI-impairment hypothesis in ASD. How might VMI-impairment affect the emergence of and development of skills crucial for efficient social-communication? We posit that motor imitation, a skill reliant on efficient VMI, may bridge the gap between VMI and social-communication impairment in ASD. Motor imitation, which is highly dependent on efficient translation of visually-observed actions into internal action representations, has been consistently shown to be impaired in both children and adults with ASD [6,19,40–44]. Learning new skills often involves observing, and then imitating others’ actions. Impaired motor imitation may thereby lead to anomalous development of a wide range of skilled behaviors, including both motor and social [7]. In terms of social development, imitation has been shown to be crucial for the development of language, play, and joint attention for sharing experiences with others [10].
Visually-guided motor control and learning is central to the acquisition of a wide range of skilled behaviors, including those crucial to motor, social and communicative development. Indeed, our group and others have repeatedly and consistently found children with ASD to show impaired performance of skilled manual gestures (i.e., praxis), both in comparison to TD children as well as children with ADHD [42,45,46], with degree of dyspraxia among children with ASD correlated with measures of core social-communicative autism impairment [42] as well as proprioceptive bias during motor learning [12,47]. Furthermore, children with ASD not only show impaired performance of skilled motor gestures but also impaired ability to recognize these gestures as performed by others [46]. The findings thereby suggest that anomalous formation of internal action models in autism contributes to anomalous development of both afferent (inferior parietal lobule, IPL) and efferent (dorsal/ventral premotor, PMd/PMv) representations within the imitation network.
A Motor Computational Approach to Objectively Quantify Motor Imitation Skill
Motor computational approaches are widely used to assess VMI, however, motor imitation is traditionally scored using Human Observation Coding (HOC). The HOC scoring method has potential for experimenter bias [48], is time intensive, and has limited power to stratify interindividual differences in performance [44]. Recently, Tunçgenç et al. (2020) developed and validated a Computational Assessment of Motor Imitation (CAMI) in children with ASD to automatically score motor imitation by comparing the spatial and temporal similarity between the movements of the imitating child and a performer [44]. CAMI demonstrated validity with traditional human observation coded (HOC) imitation scores and outperformed HOC at classifying children with ASD from TD children using a machine learning classifier. Further, CAMI outperformed HOC at predicting the severity of social-communication impairment. Importantly, the task used was brief (one minute) and incorporated a highly-engaging video game format, so that it can be readily scalable for use in clinic and home settings, with strong potential as a tractable phenotypic biomarker for diagnosis and targeted intervention.
Neural Mechanisms Underlying VMI, Impaired Motor Imitation, and Core ASD Symptoms
Motor imitation involves action perception (higher-order visual processing), forming an internal multisensory representation of the observed action (embodied representation of other) [49,50], and selecting a motor sequence that best matches the configuration of the internal representations of the self and the embodied other [51]. Key nodes of the imitation network include superior temporal sulcus (STS), rostral inferior parietal lobule (IPL), primary somatosensory cortex (S1), dorsal/ventral premotor cortices (PMd, PMv), supplementary motor area (SMA), and posterolateral cerebellum [49,52–56]. Of the key imitation nodes, structural MRI findings in children with ASD show consistent anomalies in the left IPL [57,58] and right posterolateral cerebellum [59–64]. The IPL and posterolateral cerebellum are functionally connected nodes of the imitation network [59,65–68]. IPL disruptions may impair the function of the imitation network to form internal multisensory representations from biological motion [69], whereas cerebellar processing deficits may disrupt forward modelling processes to predict the sensory consequences of motor commands [70–73] and to detect errors between predicted and actual sensory feedback to update internal representations [74,75]. While internal multisensory representations of the self and others are suggested to be mapped in the IPL [76,77] in a common reference frame [69], evidence suggests the posterolateral cerebellum forms predictions of an internal representation of the arm to guide manual movements during a visuomotor tracking task [78] and predicts an internal representation of object dynamics in the world (i.e., a moving target) [79]. Further, evidence suggests that, during voluntary action the right cerebellum compares predicted internal multisensory representations to actual sensory states to update beliefs and subsequent motor commands [80]. Therefore, disrupted communication between the cerebellum and IPL may influence how children use sensory information to form and update internal representations of the self, others, and object dynamics in the external world. We show a schematic (Figure 1) summarizing hypothesized common neural processes involved in both motor imitation and ball catching – both tasks requiring efficient VMI. Interestingly, object interception and motor imitation networks both involve visual cortex, rostral parietal, premotor, and the cerebellar brain regions [31,50,81,82]. Shared impairment using visual information to form internal representations for action may explain observation of impaired motor imitation and ball catching that are both associated with the severity of core ASD symptoms [28,44].
Figure 1:
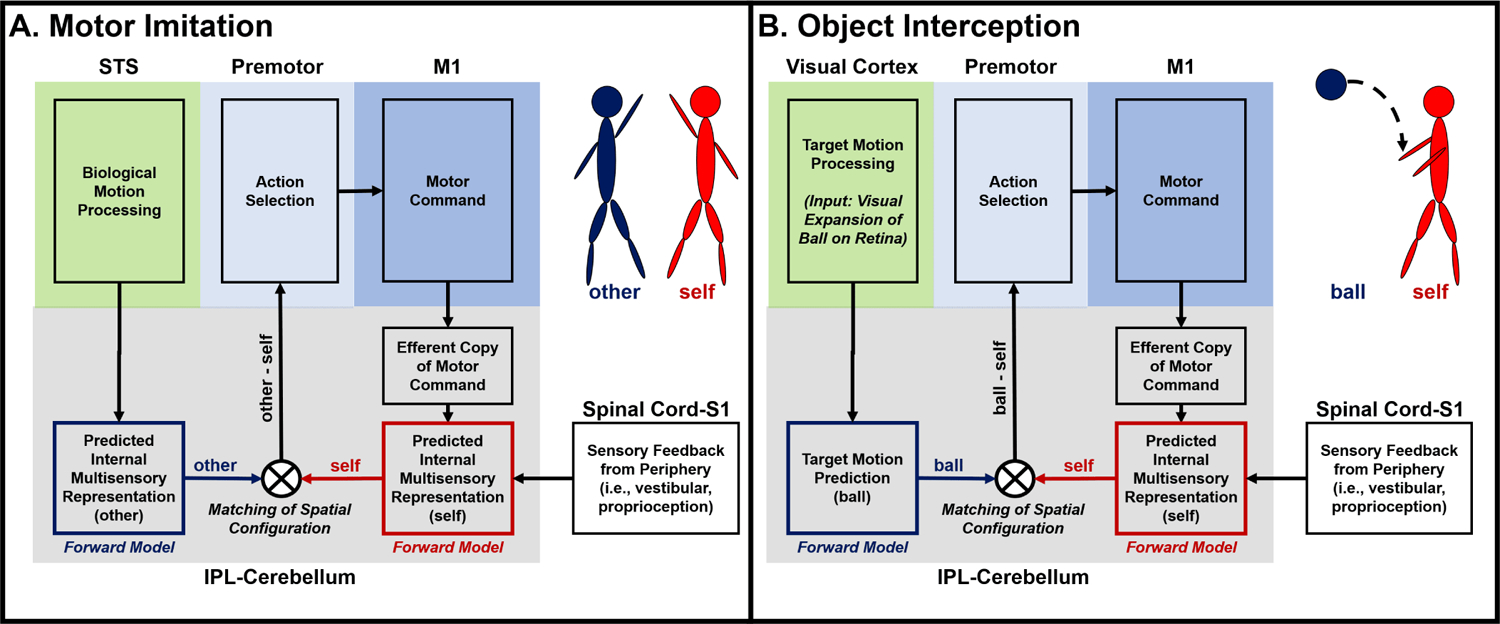
A proposed internal model of (A) motor imitation and (B) ball catching adapted from concepts described in [83–85]. (A) Motor Imitation [49,52–56]: Biological motion processing occurs within posterior STS that generates an embodied representation of the performer (other) in the IPL. A forward model in the cerebellum (tightly integrated with the IPL) uses an efferent copy of the outgoing motor command and actual (current) sensory feedback to predict the current sensory state of the imitator (internal representation of the self). The predicted internal representation of the self, generated by the cerebellar forward model, is compared to embodied internal representation of the performer (predicted by action observation) that drives corrections to the motor command or the selection of a new motor sequences from the supplementary motor area (SMA). (B) Object Interception (i.e., ball catching) [31]: The visual expansion of the projectile on the retina is processed in the visual cortex and used to by the cerebellum to predict an internal representation of the target motion (IPL-cerebellum). Peripheral sensory information is integrated to form an internal representation of the self and motor commands are selected based upon a matching process between the internal representations of the projectile and the self.
Anomalous Functional Connectivity Between Key Nodes of the Imitation Network
The integration of visual information (biological or inanimate) is crucial for efficient VMI and motor imitation. Nebel et al. (2016) used a data-driven resting-state functional connectivity approach to examine connectivity strength between the visual network and the somatosensory network in a large-sample (N=100; ASD: n=50; TD: n=50) of children with ASD and TD children (8–12 years). Findings revealed that, as compared to TD children, children with ASD showed more asynchronous connectivity between the visual and somatosensory networks and that asynchronous visual-motor connectivity was associated with the severity of social-communication impairment in the children with ASD [86]. Findings of disrupted connectivity between visual association and somatosensory networks has also been observed in a separate large-scale study by Oldehinkel et al. (2019) [87], with decreased visual-motor connectivity associated with more severe social-communication impairment in the ASD group [93] Collectively, the findings presented here suggest disrupted connectivity between visual and somatosensory networks in individuals with ASD that is associated with the severity core ASD symptoms [86,87].
The cerebellum and IPL are crucial to forming and updating internal multisensory representations and therefore represent key nodes of the imitation network that may be disrupted in individuals with ASD. Stoodley et al. (2017), showed disrupted functional connectivity between the right posterolateral cerebellum and the left IPL in children with ASD as compared to TD controls [88]. Recently, using a data-driven approach and a large sample of children (N=405; ASD: n=104; TD: n=301), we observed significant group differences in functional connectivity between the right posterolateral cerebellum subnetwork and motor system subnetworks corresponding to bilateral dorsolateral premotor cortex, dorsomedial M1, left rostral IPL, and SMA – all key imitation network nodes. Further, right posterolateral connectivity with the left rostral IPL was found to be significantly associated with the severity of core ASD symptoms [88]. Lastly, recent findings from Wymbs et al. (2020) show that disrupted IPL connectivity with the cerebellum and dorsal premotor cortex in children with ASD is associated with both impaired imitation/praxis and social skills [89].
Proposed Therapeutic Approaches to Address Potential VMI-Impairment in ASD
This review has provided evidence supporting the VMI-impairment hypothesis. We suggest that, in individuals with ASD, general VMI-impairment may lead to impaired formation of embodied representations of others’, contributing to observed impairments with motor imitation and ultimately disrupted learning of both motor and social skills. We propose two potential interventions to address VMI impairment in individuals with ASD (Figure 2). In the “Work Through” approach, we propose that decreasing the speed of the visual information in a systematic way may help individuals with ASD visually track and integrate biological motion information, providing a foundation for repeated practice and strengthening of visual-motor connections necessary to imitation and subsequent development of social skills and cognition. Supporting this approach, Laine et al. (2011) reported improved imitation in individuals with ASD when slowing the speed of observed biological motion [19]. The “Work Around” approach seeks to bypasses the visual system and either using “top-down” approaches in which skills are taught using more explicit instruction (e.g., social skills groups) that sometimes includes reward-based behavior modification or by enriching the sensory experiences of individuals with ASD using haptic technology (i.e., vibrotactile stimulators, force-feedback). For the latter, if individuals with ASD build internal representations of the world that bias “somatosensation” over “sight”, then representing the visual world through “somatosensation” may help individuals with ASD build more accurate internal representations and ultimately improve predictions from dynamic visual information. Vibrotactile sensors are one example of a haptic technology that is widely available and embedded in video game controllers, tablets, and smartphones and could be used to encode visual stimuli (“sight”) into a somatosensory representation (“somatosensation”). Future studies should investigate whether vibrotactile-encoded visual information can improve visual-based predictive control in individuals with ASD. Such evidence may support the idea that internally sensing the external world via haptic technology may help children build more accurate internal representations of the world and improve prediction of sensory consequences from visually-sensed information. Future research is needed to examine the therapeutic utility of haptic technology to assist children with ASD acquire both social and motor skills.
Figure 2:
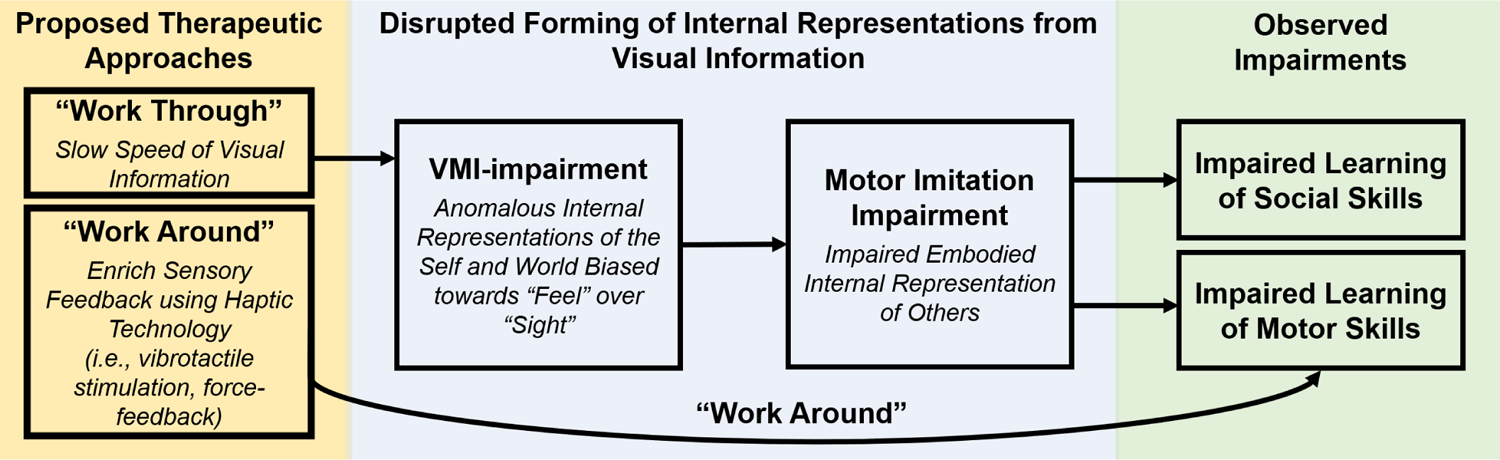
Proposed interventions to address VMI-impairment in individuals with ASD. The “Work Through” approach involves manipulating the speed of visual information to promote learning of motor and social skills from observation. The “Work Around” approach involves providing an enriched sensory experience using haptic technology (e.g., vibrotactile stimulation) in a way that promotes learning of both motor and social skills, thereby decreasing reliance on vision-only for acquiring novel skills.
CONCLUSION
In summary, there is substantial evidence that suggests that while individuals with ASD are able to form internal sensorimotor representations of learned tasks [22,23], the representations are biased towards “somatosensation” (i.e., proprioceptive-feedback) as compared to “sight” (i.e., visual-feedback) [11–13,15,24,27]. Individuals with ASD appear to have particular difficulty incorporating increasing amounts of visual information to update internal motor representations [90] and forming internal representations of dynamic visual stimuli for predictive motor control [28,29,37]. Impaired forming of internal representations using visual feedback may also impair embodied representations of others that is crucial for motor imitation. The evidence presented in this review shows copious evidence that motor imitation is impaired in individuals with ASD and associated with anomalous social-communicative skill development [6,19,40–42,44,46]. Brain regions for predicting and updating internal representations include the cerebellum and IPL, and are both brain regions that show anomalous structure and connectivity in individuals with ASD [57–64]. Future studies are needed to examine the neurobiological mechanisms underlying VMI-impairment, motor imitation, and core ASD symptoms in individuals with ASD to inform the development of targeted therapeutics to decrease disability in individuals with ASD.
ACKNOWLEDGEMENTS:
Ken Swaiman’s vision and his determination to see this vision through were exemplary. These qualities have benefited the field of Pediatric Neurology and Dr. Mostofsky personally. Ken’s foresight in recognizing the importance of understanding how the developing brain contributes to behavioral and cognitive development and how this deeper understanding can be applied to improving the lives of children and their families was foundational. Dr. Mostofsky was fortunate to have been trained in a program, established and fostered by Ken, where a strong working relationship between neurologists and neuropsychologists was the rule. This was crucial to his education as a clinician-scientist and has served as a working model throughout my career.
FUNDING SOURCES:
The authors are supported in part by the National Institutes of Health [grant numbers R01 MH106564–05, R01 MH113652–04] and the Simons Foundation for Autism Research.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Declaration of interests
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
REFERENCES
- [1].Maenner MJ, Shaw KA, Baio J, Washington A, Patrick M, DiRienzo M, et al. Prevalence of Autism Spectrum Disorder Among Children Aged 8 Years — Autism and Developmental Disabilities Monitoring Network, 11 Sites, United States, 2016. MMWR Surveill Summ 2020;69:1–12. 10.15585/mmwr.ss6904a1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [2].American Psychological Association. Diagnostic and statistical manual of mental disorders: DSM-IV-TR. 4th ed. Washington: American Psychiatric Association; 2000. [Google Scholar]
- [3].Bhat AN. Motor Impairment Increases in Children With Autism Spectrum Disorder as a Function of Social Communication, Cognitive and Functional Impairment, Repetitive Behavior Severity, and Comorbid Diagnoses: A SPARK Study Report. Autism Res 2021;14:202–19. 10.1002/aur.2453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].Fournier KA, Hass CJ, Naik SK, Lodha N, Cauraugh JH. Motor coordination in autism spectrum disorders: A synthesis and meta-analysis. J Autism Dev Disord 2010;40:1227–40. 10.1007/s10803-010-0981-3. [DOI] [PubMed] [Google Scholar]
- [5].Green D, Charman T, Pickles A, Chandler S, Loucas T, Simonoff E, et al. Impairment in movement skills of children with autistic spectrum disorders. Dev Med Child Neurol 2009;51:311–6. 10.1111/j.1469-8749.2008.03242.x. [DOI] [PubMed] [Google Scholar]
- [6].Edwards LA. A Meta-Analysis of Imitation Abilities in Individuals With Autism Spectrum Disorders. Autism Res 2014;7:363–80. 10.1002/aur.1379. [DOI] [PubMed] [Google Scholar]
- [7].Mostofsky SH, Ewen JB. Altered Connectivity and Action Model Formation in Autism Is Autism. Neurosci 2011;17:437–48. 10.1177/1073858410392381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Tunçgenç B, Pacheco C, Rochowiak R, Nicholas R, Rengarajan S, Zou E, et al. Computerised Assessment of Motor Imitation (CAMI) as a scalable method for distinguishing children with autism. Biol Psychiatry Cogn Neurosci Neuroimaging 2020. 10.1016/j.bpsc.2020.09.001. [DOI] [PMC free article] [PubMed]
- [9].Toth K, Munson J, Meltzoff AN, Dawson G. Early predictors of communication development in young children with autism spectrum disorder: Joint attention, imitation, and toy play. J Autism Dev Disord 2006;36:993–1005. 10.1007/s10803-006-0137-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Rogers SJ, Pennington BF. A theoretical approach to the deficits in infantile autism. Dev Psychopathol 1991;3:137–62. 10.1017/S0954579400000043. [DOI] [Google Scholar]
- [11].Haswell CC, Izawa J, Dowell LR, Mostofsky SH, Shadmehr R. Representation of internal models of action in the autistic brain. Nat Neurosci 2009;12:970–2. 10.1038/nn.2356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Izawa J, Pekny SE, Marko MK, Haswell CC, Shadmehr R, Mostofsky SH. Motor learning relies on integrated sensory inputs in ADHD, but over-selectively on proprioception in autism spectrum conditions. Autism Res 2012;5:124–36. 10.1002/aur.1222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Marko MK, Crocetti D, Hulst T, Donchin O, Shadmehr R, Mostofsky SH. Behavioural and neural basis of anomalous motor learning in children with autism. Brain 2015;138:784–97. 10.1093/brain/awu394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Lidstone DE, Miah FZ, Poston B, Beasley JF, Mostofsky SH, Dufek JS. Children with Autism Spectrum Disorder Show Impairments During Dynamic Versus Static Grip-force Tracking. Autism Res 2020;13:2177–89. 10.1002/aur.2370. [DOI] [PubMed] [Google Scholar]
- [15].Glazebrook CM, Gonzalez D, Hansen S, Elliott D. The role of vision for online control of manual aiming movements in persons with autism spectrum disorders. Autism 2009;13:411–33. 10.1177/1362361309105659. [DOI] [PubMed] [Google Scholar]
- [16].Mosconi MW, Mohanty S, Greene RK, Cook EH, Vaillancourt DE, Sweeney JA, et al. Feedforward and Feedback Motor Control Abnormalities Implicate Cerebellar Dysfunctions in Autism Spectrum Disorder. J Neurosci 2015;35:2015–25. 10.1523/JNEUROSCI.2731-14.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].Greffou S, Bertone A, Hahler EM, Hanssens JM, Mottron L, Faubert J. Postural hypo-reactivity in autism is contingent on development and visual environment: A fully immersive virtual reality study. J Autism Dev Disord 2012;42:961–70. 10.1007/s10803-011-1326-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].Gepner B, Mestre DR. Brief Report: Postural Reactivity to Fast Visual Motion Differentiates Autistic from Children with Asperger Syndrome. J Autism Dev Disord 2002;32:231–8. 10.1023/A:1015410015859. [DOI] [PubMed] [Google Scholar]
- [19].Lainé F, Rauzy S, Tardif C, Gepner B. Slowing down the presentation of facial and body movements enhances imitation performance in children with severe autism. J Autism Dev Disord 2011;41:983–96. 10.1007/s10803-010-1123-7. [DOI] [PubMed] [Google Scholar]
- [20].Masterton BA, Biederman GB. Proprioceptive versus visual control in autistic children. J Autism Dev Disord 1983;13:141–52. 10.1007/BF01531815. [DOI] [PubMed] [Google Scholar]
- [21].Hamilton CR. Intermanual Transfer of Adaptation to Prisms. Am J Psychol 1964;77:457–62. 10.2307/1421017. [DOI] [PubMed] [Google Scholar]
- [22].Gidley Larson JC, Bastian AJ, Donchin O, Shadmehr R, Mostofsky SH. Acquisition of internal models of motor tasks in children with autism. Brain 2008;131:2894–903. 10.1093/brain/awn226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [23].Mostofsky SH, Bunoski R, Morton SM, Goldberg MC, Bastian AJ. Children with autism adapt normally during a catching task requiring the cerebellum. Neurocase 2004;10:60–4. 10.1080/13554790490960503. [DOI] [PubMed] [Google Scholar]
- [24].Zheng R, Skultety J, Lyons J, Naiman ID, Passmore SR, Glazebrook CM. The impact of different movement types on motor planning and execution in individuals with autism spectrum disorder. Motor Control 2019;23:398–417. 10.1123/mc.2017-0084. [DOI] [PubMed] [Google Scholar]
- [25].Sacrey L-AR, Germani T, Bryson SE, Zwaigenbaum L. Reaching and grasping in autism spectrum disorder: a review of recent literature. Front Neurol 2014;5:Article 6. 10.3389/fneur.2014.00006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [26].Minshew NJ, Sung K, Jones BL, Furman JM. Underdevelopment of the postural control. Neurology 2004;63:2056–61. 10.1212/01.WNL.0000145771.98657.62. [DOI] [PubMed] [Google Scholar]
- [27].Morris SL, Foster CJ, Parsons R, Falkmer M, Falkmer T, Rosalie SM. Differences in the use of vision and proprioception for postural control in autism spectrum disorder. Neuroscience 2015;307:273–80. 10.1016/j.neuroscience.2015.08.040. [DOI] [PubMed] [Google Scholar]
- [28].Ament K, Mejia A, Buhlman R, Erklin S, Caffo B, Mostofsky S, et al. Evidence for Specificity of Motor Impairments in Catching and Balance in Children with Autism. J Autism Dev Disord 2015;45:742–51. 10.1007/s10803-014-2229-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [29].Whyatt C, Craig CM. Interceptive skills in children aged 9–11 years, diagnosed with Autism Spectrum Disorder. Res Autism Spectr Disord 2013;7:613–23. 10.1016/j.rasd.2013.01.003. [DOI] [Google Scholar]
- [30].Craig F, Lorenzo A, Lucarelli E, Russo L, Fanizza I, Trabacca A. Motor competency and social communication skills in preschool children with autism spectrum disorder. Autism Res 2018;11:893–902. 10.1002/aur.1939. [DOI] [PubMed] [Google Scholar]
- [31].Zago M, McIntyre J, Senot P, Lacquaniti F. Visuo-motor coordination and internal models for object interception. Exp Brain Res 2009;192:571–604. 10.1007/s00221-008-1691-3. [DOI] [PubMed] [Google Scholar]
- [32].Landa RJ, Haworth JL, Nebel MB. Ready, set, go! Low anticipatory response during a dyadic task in infants at high familial risk for autism. Front Psychol 2016;7:1–12. 10.3389/fpsyg.2016.00721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [33].Vaillancourt DE, Thulborn KR, Corcos DM. Neural Basis for the Processes That Underlie Visually Guided and Internally Guided Force Control in Humans. J Neurophysiol 2006;90:3330–40. 10.1152/jn.00394.2003. [DOI] [PubMed] [Google Scholar]
- [34].Vaillancourt DE, Mayka MA, Corcos DM. Intermittent Visuomotor Processing in the Human Cerebellum, Parietal Cortex, and Premotor Cortex. J Neurophysiol 2005;95:922–31. 10.1152/jn.00718.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [35].Baweja HS, Patel BK, Martinkewiz JD, Vu J, Christou EA. Removal of visual feedback alters muscle activity and reduces force variability during constant isometric contractions. Exp Brain Res 2009;197:35–47. 10.1007/s00221-009-1883-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [36].Baweja HS, Kennedy DM, Vu J, Vaillancourt DE, Christou EA. Greater amount of visual feedback decreases force variability by reducing force oscillations from 0–1 and 3–7 Hz. Eur J Appl Physiol 2010;108:935–43. 10.1007/s00421-009-1301-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [37].Lidstone DE, Miah FZ, Poston B, Beasley JF, Mostofsky SH, Dufek JS. Children with Autism Spectrum Disorder Show Impairments During Dynamic Versus Static Grip-force Tracking. Autism Res 2020;13:2177–89. 10.1002/aur.2370. [DOI] [PubMed] [Google Scholar]
- [38].Pan C-Y, Tsai C-L, Chu C-H. Fundamental Movement Skills in Children Diagnosed with Autism Spectrum Disorders and Attention Deficit Hyperactivity Disorder. J Autism Dev Disord 2009;39:1694–705. 10.1007/s10803-009-0813-5. [DOI] [PubMed] [Google Scholar]
- [39].Whyatt CP, Craig CM. Motor Skills in Children Aged 7 – 10 Years, Diagnosed with Autism Spectrum Disorder 2012:1799–809. 10.1007/s10803-011-1421-8. [DOI] [PubMed]
- [40].McAuliffe D, Pillai AS, Tiedemann A, Mostofsky SH, Ewen JB. Dyspraxia in ASD: Impaired coordination of movement elements. Autism Res 2017;10:648–52. 10.1002/aur.1693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [41].DeMyer MK, Alpern GD, Barton S, DeMyer WE, Churchill DW, Hingtgen JN, et al. Imitation in autistic, early schizophrenic, and non-psychotic subnormal children. J Autism Child Schizophr 1972;2:264–87. 10.1007/BF01537618. [DOI] [PubMed] [Google Scholar]
- [42].Dziuk MA, Larson JCG, Apostu A, Mahone EM, Denckla MB, Mostofsky SH. Dyspraxia in autism: Association with motor, social, and communicative deficits. Dev Med Child Neurol 2007;49:734–9. 10.1111/j.1469-8749.2007.00734.x. [DOI] [PubMed] [Google Scholar]
- [43].Gizzonio V, Avanzini P, Campi C, Orivoli S, Piccolo B, Cantalupo G, et al. Failure in Pantomime Action Execution Correlates with the Severity of Social Behavior Deficits in Children with Autism: A Praxis Study. J Autism Dev Disord 2015;45:3085–97. 10.1007/s10803-015-2461-2. [DOI] [PubMed] [Google Scholar]
- [44].Tunçgenç B, Pacheco C, Rochowiak R, Nicholas R, Rengarajan S, Zou E, et al. Computerized Assessment of Motor Imitation as a Scalable Method for Distinguishing Children With Autism. Biol Psychiatry Cogn Neurosci Neuroimaging 2020. 10.1016/j.bpsc.2020.09.001. [DOI] [PMC free article] [PubMed]
- [45].Mostofsky SH, Dubey P, Jerath VK, Jansiewicz EM, Goldberg MC, Denckla MB. Developmental dyspraxia is not limited to imitation in children with autism spectrum disorders. J Int Neuropsychol Soc 2006;12:314–26. 10.1017/S1355617706060437. [DOI] [PubMed] [Google Scholar]
- [46].Dowell LR, Mahone EM, Mostofsky SH. Associations of Postural Knowledge and Basic Motor Skill with Dyspraxia in Autism: Implication for Abnormalities in Distributed Connectivity and Motor Learning. Neuropsychology 2009;23:563–70. 10.1037/a0015640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [47].Haswell CC, Izawa J, R Dowell L, H Mostofsky S, Shadmehr R. Representation of internal models of action in the autistic brain. Nat Neurosci 2009;12:970–2. 10.1038/nn.2356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [48].Davis J, Redshaw J, Suddendorf T, Nielsen M, Kennedy-Costantini S, Oostenbroek J, et al. Does Neonatal Imitation Exist? Insights From a Meta-Analysis of 336 Effect Sizes. Perspect Psychol Sci 2021:174569162095983. 10.1177/1745691620959834. [DOI] [PubMed]
- [49].Keysers C, Paracampo R, Gazzola V. What neuromodulation and lesion studies tell us about the function of the mirror neuron system and embodied cognition 2018. 10.1016/j.copsyc.2018.04.001. [DOI] [PMC free article] [PubMed]
- [50].Iacoboni M. Neurobiology of imitation. Curr Opin Neurobiol 2009;19:661–5. 10.1016/j.conb.2009.09.008. [DOI] [PubMed] [Google Scholar]
- [51].Schaal S, Ijspeert A, Billard A. Computational approaches to motor learning by imitation. Philos Trans R Soc B Biol Sci 2003;358:537–47. 10.1098/rstb.2002.1258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [52].Rizzolatti G, Sinigaglia C. The mirror mechanism: A basic principle of brain function. Nat Rev Neurosci 2016;17:757–65. 10.1038/nrn.2016.135. [DOI] [PubMed] [Google Scholar]
- [53].Iacoboni M Neural mechanisms of imitation. Curr Opin Neurobiol 2005;15:632–7. 10.1016/j.conb.2005.10.010. [DOI] [PubMed] [Google Scholar]
- [54].Jack A, Englander ZA, Morris JP. Subcortical contributions to effective connectivity in brain networks supporting imitation. Neuropsychologia 2011;49:3689–98. 10.1016/j.neuropsychologia.2011.09.024. [DOI] [PubMed] [Google Scholar]
- [55].Jack A, Morris JP. Neocerebellar contributions to social perception in adolescents with autism spectrum disorder. Dev Cogn Neurosci 2014;10:77–92. 10.1016/j.dcn.2014.08.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [56].Vogt S, Buccino G, Wohlschläger AM, Canessa N, Shah NJ, Zilles K, et al. Prefrontal involvement in imitation learning of hand actions: Effects of practice and expertise. Neuroimage 2007;37:1371–83. 10.1016/j.neuroimage.2007.07.005. [DOI] [PubMed] [Google Scholar]
- [57].Mahajan R, Dirlikov B, Crocetti D, Mostofsky SH. Motor Circuit Anatomy in Children with Autism Spectrum Disorder With or Without Attention Deficit Hyperactivity Disorder. Autism Res 2016;9:67–81. 10.1002/aur.1497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [58].Yang Q, Huang P, Li C, Fang P, Zhao N, Nan J, et al. Mapping alterations of gray matter volume and white matter integrity in children with autism spectrum disorder. Neuroreport 2018:1. 10.1097/WNR.0000000000001094. [DOI] [PubMed]
- [59].Stoodley CJ, D’Mello AM, Ellegood J, Jakkamsetti V, Liu P, Nebel MB, et al. Author Correction: Altered cerebellar connectivity in autism and cerebellar-mediated rescue of autism-related behaviors in mice (Nature Neuroscience DOI: 10.1038/s41593-017-0004-1). Nat Neurosci 2018;21:1016. . [DOI] [PubMed] [Google Scholar]
- [60].D’Mello AM, Crocetti D, Mostofsky SH, Stoodley CJ. Cerebellar gray matter and lobular volumes correlate with core autism symptoms. NeuroImage Clin 2015;7:631–9. 10.1016/j.nicl.2015.02.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [61].D’Mello AM, Moore DM, Crocetti D, Mostofsky SH, Stoodley CJ. Cerebellar gray matter differentiates children with early language delay in autism. Autism Res 2016;9:1191–204. 10.1002/aur.1622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [62].Stoodley CJ. Distinct regions of the cerebellum show gray matter decreases in autism, ADHD, and developmental dyslexia. Front Syst Neurosci 2014;8:1–17. 10.3389/fnsys.2014.00092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [63].Wilson LB, Tregellas JR, Hagerman RJ, Rogers SJ, Rojas DC. A voxel-based morphometry comparison of regional gray matter between fragile X syndrome and autism. Psychiatry Res - Neuroimaging 2009;174:138–45. 10.1016/j.pscychresns.2009.04.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [64].Yang X, Si T, Gong Q, Qiu L, Jia Z, Zhou M, et al. Brain gray matter alterations and associated demographic profiles in adults with autism spectrum disorder: A meta-analysis of voxel-based morphometry studies. Aust N Z J Psychiatry 2016;50:741–53. 10.1177/0004867415623858. [DOI] [PubMed] [Google Scholar]
- [65].Clower DM, West RA, Lynch JC, Strick PL. The inferior parietal lobule is the target of output from the superior colliculus, hippocampus, and cerebellum. J Neurosci 2001;21:6283–91. https://doi.org/21/16/6283 [pii]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [66].Glickstein M. How are visual areas of the brain connected to motor areas for the sensory guidance of movement? Trends Neurosci 2000;23:613–7. 10.1016/S0166-2236(00)01681-7. [DOI] [PubMed] [Google Scholar]
- [67].Moulton E, Galléa C, Kemlin C, Valabregue R, Maier MA, Lindberg P, et al. Cerebello-Cortical Differences in Effective Connectivity of the Dominant and Non-dominant Hand during a Visuomotor Paradigm of Grip Force Control. Front Hum Neurosci 2017;11:1–12. 10.3389/fnhum.2017.00511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [68].Blakemore SJ, Sirigu A. Action prediction in the cerebellum and in the parietal lobe. Exp Brain Res 2003;153:239–45. 10.1007/s00221-003-1597-z. [DOI] [PubMed] [Google Scholar]
- [69].Cohen YE, Andersen RA. A common reference frame for movement plans in the posterior parietal cortex. Nat Rev Neurosci 2002;3:553–62. 10.1038/nrn873. [DOI] [PubMed] [Google Scholar]
- [70].Coltz JD, Johnson MT, Ebner TJ. Cerebellar Purkinje cell simple spike discharge encodes movement velocity in primates during visuomotor arm tracking. J Neurosci 1999;19:1782–803. 10.1523/JNEUROSCI.19-05-01782.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [71].Liu X, Robertson E, Miall RC. Neuronal activity related to the visual representation of arm movements in the lateral cerebellar cortex. J Neurophysiol 2003;89:1223–37. 10.1152/jn.00817.2002. [DOI] [PubMed] [Google Scholar]
- [72].Pasalar S, Roitman A V., Durfee WK, Ebner TJ. Force field effects on cerebellar Purkinje cell discharge with implications for internal models. Nat Neurosci 2006;9:1404–11. 10.1038/nn1783. [DOI] [PubMed] [Google Scholar]
- [73].Roitman A V Position, Direction of Movement, and Speed Tuning of Cerebellar Purkinje Cells during Circular Manual Tracking in Monkey. J Neurosci 2005;25:9244–57. 10.1523/JNEUROSCI.1886-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [74].Rondi-Reig L, Paradis A-L, Lefort JM, Babayan BM, Tobin C. How the cerebellum may monitor sensory information for spatial representation. Front Syst Neurosci 2014;8:1–13. 10.3389/fnsys.2014.00205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [75].Tseng Y -w., Diedrichsen J, Krakauer JW, Shadmehr R, Bastian AJ. Sensory Prediction Errors Drive Cerebellum-Dependent Adaptation of Reaching. J Neurophysiol 2007;98:54–62. 10.1152/jn.00266.2007. [DOI] [PubMed] [Google Scholar]
- [76].Wolpert DM, Goodbody SJ, Husain M. Maintaining internal representations: The role of the human superior parietal lobe. Nat Neurosci 1998;1:529–33. 10.1038/2245. [DOI] [PubMed] [Google Scholar]
- [77].Ticini LF, Dolk T, Waszak F, Schütz-Bosbach S. IPL-M1 interaction shapes pre-reflective social differentiation in the human action system: new insights from TBS and TMS combined. Sci Rep 2018;8:1–10. 10.1038/s41598-018-30480-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [78].Liu X. Neuronal Activity Related to the Visual Representation of Arm Movements in the Lateral Cerebellar Cortex. J Neurophysiol 2002;89:1223–37. 10.1152/jn.00817.2002. [DOI] [PubMed] [Google Scholar]
- [79].Cerminara NL, Apps R, Marple-Horvat DE. An internal model of a moving visual target in the lateral cerebellum. J Physiol 2009;587:429–42. 10.1113/jphysiol.2008.163337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [80].Van Kemenade BM, Arikan BE, Podranski K, Steinsträter O, Kircher T, Straube B. Distinct Roles for the Cerebellum, Angular Gyrus, and Middle Temporal Gyrus in Action-Feedback Monitoring. Cereb Cortex 2019;29:1520–31. 10.1093/cercor/bhy048. [DOI] [PubMed] [Google Scholar]
- [81].Iacoboni M. Visuo-motor integration and control in the human posterior parietal cortex: Evidence from TMS and fMRI. Neuropsychologia 2006;44:2691–9. 10.1016/j.neuropsychologia.2006.04.029. [DOI] [PubMed] [Google Scholar]
- [82].Caspers S, Zilles K, Laird AR, Eickhoff SB. ALE meta-analysis of action observation and imitation in the human brain. Neuroimage 2010;50:1148–67. 10.1016/j.neuroimage.2009.12.112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [83].Desmurget M, Grafton S. Forward modeling allows feedback control for fast reaching movements. Trends Cogn Sci 2000;4:423–31. [DOI] [PubMed] [Google Scholar]
- [84].Ishikawa T, Tomatsu S, Izawa J, Kakei S. The cerebro-cerebellum: Could it be loci of forward models? Neurosci Res 2016;104:72–9. 10.1016/j.neures.2015.12.003. [DOI] [PubMed] [Google Scholar]
- [85].Scott SH. Optimal feedback control and the neural basis of volitional motor control. Nat Rev Neurosci 2004;5:532–44. 10.1038/nrn1427. [DOI] [PubMed] [Google Scholar]
- [86].Nebel MB, Eloyan A, Nettles CA, Sweeney KL, Ament K, Ward RE, et al. Intrinsic visual-motor synchrony correlates with social deficits in autism. Biol Psychiatry 2016;79:633–41. 10.1016/j.biopsych.2015.08.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [87].Oldehinkel M, Mennes M, Marquand A, Charman T, Tillmann J, Ecker C, et al. Altered Connectivity Between Cerebellum, Visual, and Sensory-Motor Networks in Autism Spectrum Disorder: Results from the EU-AIMS Longitudinal European Autism Project. Biol Psychiatry Cogn Neurosci Neuroimaging 2019;4:260–70. 10.1016/j.bpsc.2018.11.010. [DOI] [PubMed] [Google Scholar]
- [88].Stoodley CJ, D’Mello AM, Ellegood J, Jakkamsetti V, Liu P, Nebel MB, et al. Altered cerebellar connectivity in autism and cerebellar-mediated rescue of autism-related behaviors in mice. Nat Neurosci 2017;20:1744–51. 10.1038/s41593-017-0004-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [89].Wymbs NF, Nebel MB, Ewen JB, Mostofsky SH. Altered Inferior Parietal Functional Connectivity is Correlated with Praxis and Social Skill Performance in Children with Autism Spectrum Disorder. Cereb Cortex 2020. 10.1093/cercor/bhaa380. [DOI] [PMC free article] [PubMed]
- [90].Mosconi MW, Mohanty S, Greene RK, Cook EH, Vaillancourt DE, Sweeney JA. Feedforward and Feedback Motor Control Abnormalities Implicate Cerebellar Dysfunctions in Autism Spectrum Disorder. J Neurosci 2015;35:2015–25. 10.1523/JNEUROSCI.2731-14.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]


