Abstract
A visible light-induced palladium-catalyzed oxidative C–H alkylation of oximes has been developed. This mild protocol allows for an efficient atom economical C–C bond construction of alkyl-substituted oximes. A broad range of primary, secondary, and tertiary alkyl bromides and iodides, as well as a range of different formaldoximes, can efficiently undergo this transformation. The method features visible light-induced generation of nucleophilic hybrid alkyl Pd radical intermediates, which upon radical addition at the imine moiety and a subsequent β-hydrogen elimination deliver substituted imines.
Keywords: oxime, alkylation, Heck reaction, palladium, light-induced, radical
Graphical Abstract

Addition of alkyl radicals to imines is an established strategy toward synthesis of multisubstituted amines.1 Most often, these reactions rely on employment of stochiometric radical mediators in combination with a catalytic Lewis acid, which is required for activation of an imine moiety toward addition of a nucleophilic radical (Scheme 1a).2 With recent development of photoredox catalysis,3 as well as metal-catalyzed hydrogen atom transfer techniques,4 a number of catalytic versions of such transformations have appeared in the literature. Nevertheless, these transformations remain overall reductive, which limits potential for further functionalization. In order to retain a synthetically attractive imine group upon radical alkylations, an addition/elimination strategy has been developed (Scheme 1b).5 In 1996, Kim and co-workers introduced oxime 1′ bearing sulfonate as a suitable radical leaving group.5a Major problems with such approach are the lengthy synthesis of starting materials (1′) and the poor atom economy, as the high-molecular-weight byproduct is produced. In addition, with a few exceptions,6 these methods rely on employment of stoichiometric tin reagents. Despite these synthetic drawbacks, the discussed protocols remain in demand because of the vast synthetic7,8 and biological9 relevance of oxime-containing molecules. In this light, the development of catalytic oxidative C–H functionalization of oximes 1 is warranted.10,11 Herein, we report mild and general visible light-induced Pd-catalyzed method for synthesis of substituted oximes 3 operating via a direct C–H Heck-type alkylation protocol (Scheme 1c).
Scheme 1.

Radical Reactions of Imines
Scheme 3.
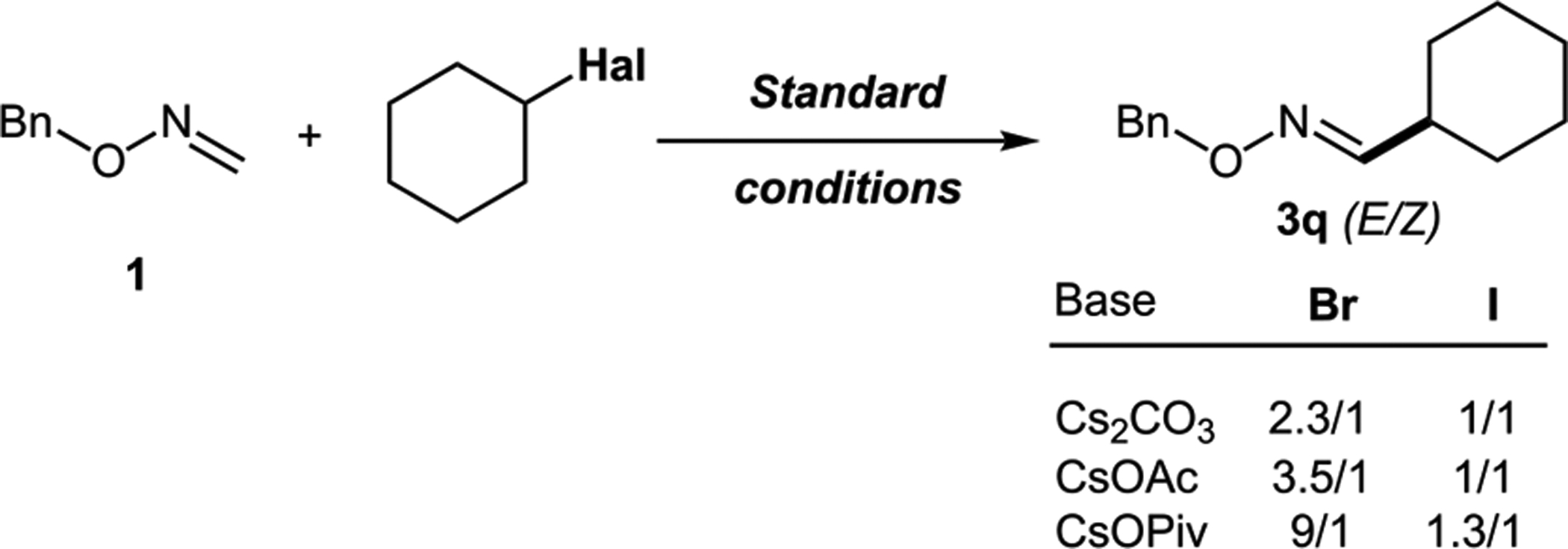
Impact of Base and Halide on E/Z Selectivity
In recent years, the visible light-induced photoexcited chemistry of palladium has become an emerging field of study.12 Particularly, we13 and others14 have demonstrated the feasibility of the visible light-induced palladium-catalyzed alkyl-Heck reaction of a broad range of alkyl electrophiles proceeding via hybrid palladium C(sp3)-centered radical species. Encouraged by this novel mild protocol for generation of alkyl radicals13,14 and motivated by the need of new synthetic methods toward substituted oximes,7 we aimed at the development of alkyl imino-Heck reaction. The feasibility of this transformation was supported by the effectiveness for generation of nucleophilic alkyl radicals under light-induced Pd-catalyzed conditions;12 the affinity of oximes toward nucleophilic alkyl radicals;15 and the propensity of palladium catalysis for the oxidative end-game.16
Toward this end, we investigated the reaction between formaldoxime (1) and iodocyclohexane (2) under our standard palladium(II) acetate/Xantphos catalytic system13 in the presence of indium(III) chloride additive. Gratifyingly, it was found that under these conditions, the desired coupling product 3 was formed, albeit in moderate yield (Table 1, entry 1). The combination of monodentate and bidentate phosphine ligands17 resulted in a significant improvement of the reaction efficiency (entry 2). Further optimization revealed indium(III) acetate to be a superior additive with high reproducibility (entry 3). Control experiments demonstrated that thermal reaction (entry 4) is much less efficient and Pd catalyst is essential for this transformation (entry 5). Notably, this reaction can proceed without Lewis acid additive, however with lower reproducibility (entry 6).
Table 1.
Reaction Optimization
| |||
|---|---|---|---|
| entry | L1, L2 | additive | yield, %a |
| 1 | Xantphos | InCl3 | 37 |
| 2 | Xantphos, PPh3 | InCl3 | 68 |
| 3 | Xantphos, PPh 3 | In(OAc) 3 | 99 b |
| 4c | Xantphos, PPh3 | In(OAc)3 | 27 |
| 5d | Xantphos, PPh3 | In(OAc)3 | 0 |
| 6 | Xantphos, PPh3 | none | 90 |
GC-MS yield.
E/Z 1/1.
80 °C, no light.
No Pd(OAc)2.
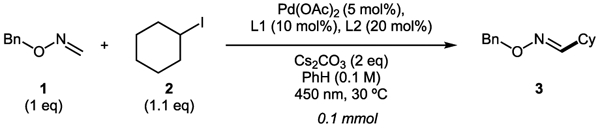
With the optimized conditions in hand, the scope of alkyl halides was examined first (Scheme 4). It was found that all linear alkyl halides tested, including those having distant functional groups, such as phenyl (3b), ester (3c), alkyl and aryl ethers (3d, 3e), terminal alkene (3g), chloride (3f), cyano- (3h), and ketone (3i), have proven to be capable substrates for this coupling reaction. Interestingly, compound 3j having silicon atom beta to imine could also be synthesized via this protocol. This is a particularly surprising finding, considering silyl methyl radicals are more electrophilic compared with simple alkyl radicals.18 Notably, β-D-ribofuranoside derivative 3k, as well as dipeptide derivative 3l, could be accessed via this approach, thus indicating the applicability of this method to functionalization reactions in a more complex setting. Secondary alkyl halides were found to be the most effective coupling partners. Thus, oximes possessing acyclic substituents (3m, 3n), could be obtained in good yields. Oximes having varied size carbocycles (3o–3s) could also be efficiently synthesized. Oxime, possessing secondary adamantyl derivative (3t), was obtained in nearly quantitative yield. Saturated heterocyclic derivatives, such as oxetane (3u), tetrahydropyran (3v), and piperidine (3w), were also successfully obtained. Intriguingly, this reaction exhibited a notable halide effect (3p, 3q). While efficiencies of processes for alkyl bromides and iodides were comparable, the use of iodides resulted in products with substantially higher Z isomer content. This counterion effect was observed throughout the entire scope of the reaction, where alkyl iodides in general resulted in products with higher amount of Z oxime. Tertiary alkyl halides 3x–3ab are also capable coupling partners. In the reaction with tert-adamantyl iodide, however, a substantial amount of nonseparable Friedel–Crafts arylation side product was observed. Accordingly, the product in reduced form (3ac) was isolated. A simple tert-butyl derivative 3x was obtained in good yield from both respective iodide and bromide. As in the cases of primary and secondary alkyl halides, the reaction gave a mixture of E/Z isomers. In this case, however, it is particularly surprising, as Z isomer containing bulky tertiary substituent is thermodynamically much less favorable. On the other hand, cyclobutene-containing product 3ab formed with almost exclusive E selectivity.
Scheme 4. Scope of Alkyl Heck-Type Coupling of Oximesa.
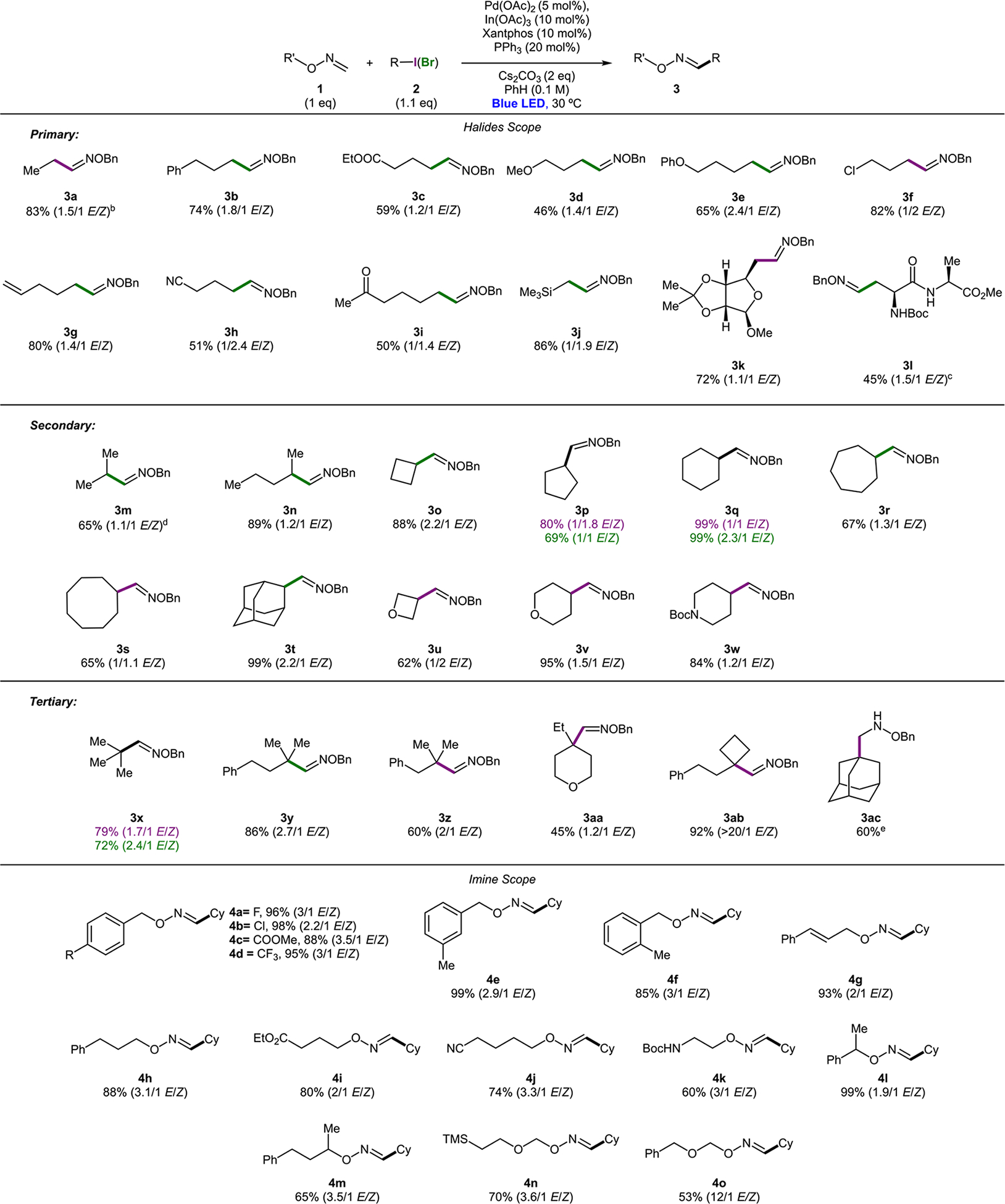
a0.3 mmol scale, isolated yields. b3 equiv of EtI. c3 equiv of oxime. d0.5 mmol scale, 3 equiv of i-PrBr. eYield after reduction with NaBH3CN.
Next, the scope of oxime in reaction with cyclohexyl bromide was tested. First, benzylic formaldoximes possessing different functional groups at the aryl group were examined. Overall, the process demonstrated outstanding efficiencies for these substrates (4a–4f). Notably, styrene derivative 4g could be synthesized in an excellent yield, despite a potential side addition of the alkyl radical at the double bond leading to a highly stabilized benzylic radical.13,14 Linear molecules possessing various distant functionalities, such as phenyl (4h), ester (4i), cyano- (4j), and protected primary amine (4k), all worked well. Synthesis of formaldoximes bearing secondary alkoxy groups posed no problem either (4l, 4m). Gratifyingly, imines 4n and 4o having easily removable trimethylsilylethoxymethyl (SEM) and benzyloxymethyl (BOM) groups were synthesized with moderate to good yields, which opens access to O-unprotected oximes. At this point, attempts to perform this imine-Heck reaction on aldoximes resulted in reductive radical additions only. Obviously, the presence of additional substituent hampers the reoxidation of amine into imine.
As discussed in the introduction, oximes enjoy vast synthetic applications.7,8 There is a number of reported protocols highlighting transformations of oximes 3 obtained via our alkyl Heck-type alkylation protocol and its derivatives (Scheme 2). Primarily, these feature the construction of different nitrogen containing heterocycles 8–10.19 Diverse additions to electrophilic carbon of oxime are also well-established (11, 12).20 We have also found that 3 could undergo partial (6) or exhaustive (7) dehydrogenation upon exposure to different amounts of N-bromosuccinimide.
Scheme 2.
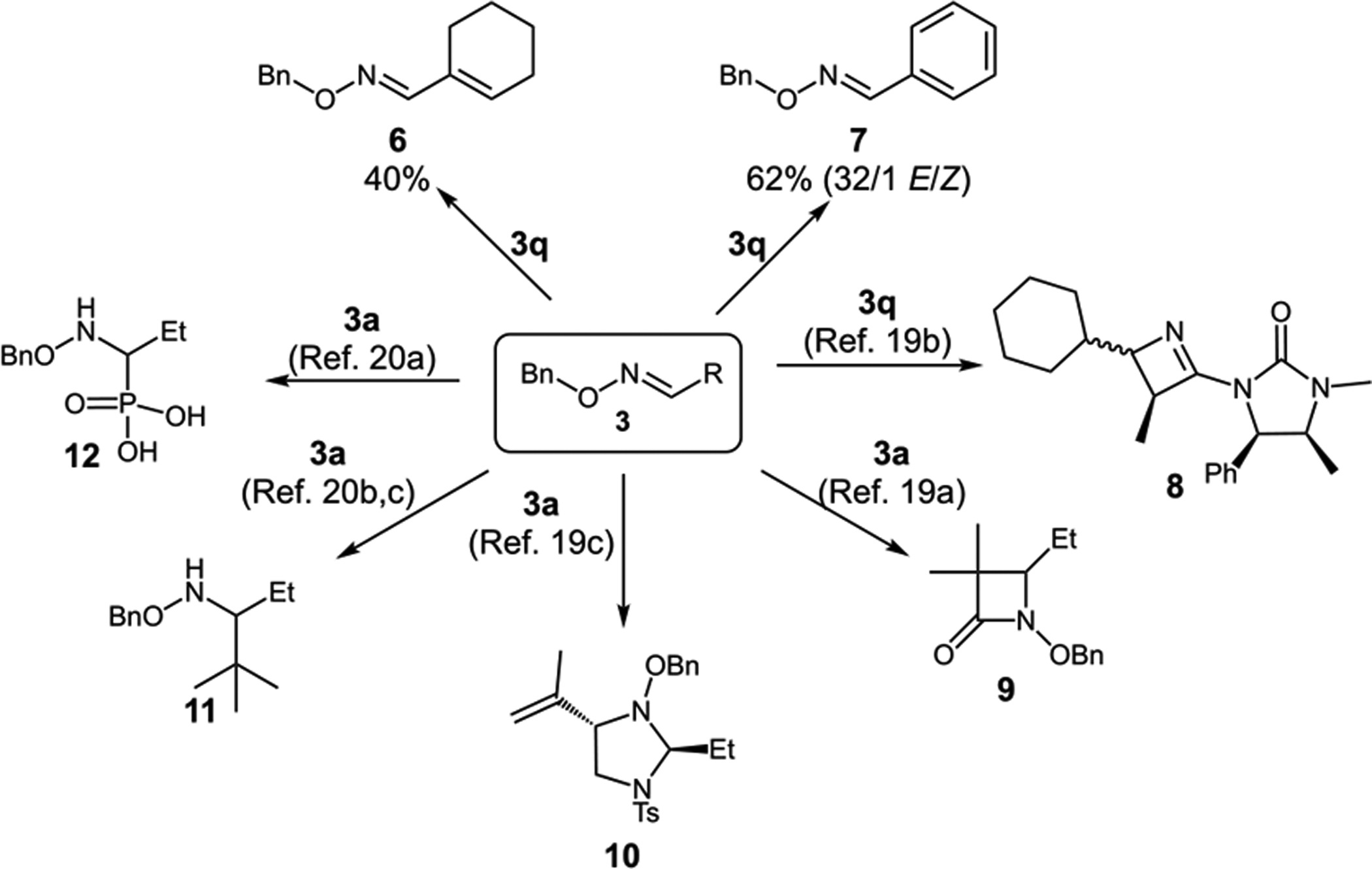
Synthetic Utility of Obtained Products
Notably, in contrast to the thermal21 and visible light-induced13,14 alkyl-Heck protocols, which exclusively or pre-dominantly deliver the thermodynamically more stable E isomers, the alkyl-Heck reaction of oximes shows broadly varied stereoselectivity: from nearly exclusive E (3ab) to preferentially Z (3u). As alluded above, the stereochemistry depended on the halide used. In addition, it was found to be base-dependent (Scheme 3).22 It seems apparent that, in the case of alkyl bromides, the size of the base (cf. CsOAc vs CsOPiv) rather than the basicity governs the stereochemical outcome of the reaction. This may imply that the classical β-hydride elimination process, where the base is only required for scavenging acid from HPdX for the catalyst regeneration,23 is an unlikely end-game scenario in the alkylation of imines with alkyl bromides. Possibly, a base-assisted elimination takes place, which is responsible for the observed base-dependent selectivity. In contrast, this effect is muted for alkyl iodides, suggesting a potential switch of the mechanism.
On the basis of the relevance to alkyl-Heck reaction of alkenes13,14 and above-mentioned observations, the following plausible mechanism is proposed for the novel alkyl-Heck reaction of imines (Scheme 5). First, a hybrid alkyl Pd-radical species A is formed either via a direct SET from the generated in situ photoexcited Pd(0) complex or via oxidative addition of alkyl halide with Pd(0) complex, followed by homolysis of the C–Pd bond. The radical nature of this transformation was supported by radical clock, radical trapping, and spin-trapping experiments.24 Next, the well-established addition of alkyl radical to oxime takes place,15 resulting in the formation of the hybrid nitrogen centered radical B. A direct hydrogen atom transfer (HAT) by palladium would result in the formation of the reaction product 3 (path a). In an alternative scenario (path b), the radical recombination would produce Pd(II) complex C, which could be in equilibrium with alkoxyamine D and PdX2 salt. Pd(II) complex C may either undergo a classical β-hydride-(path b-1) or a base-assisted elimination process via a classical bimolecular (E2) or a concerted ligand-assisted elimination path (path b-2).25
Scheme 5.
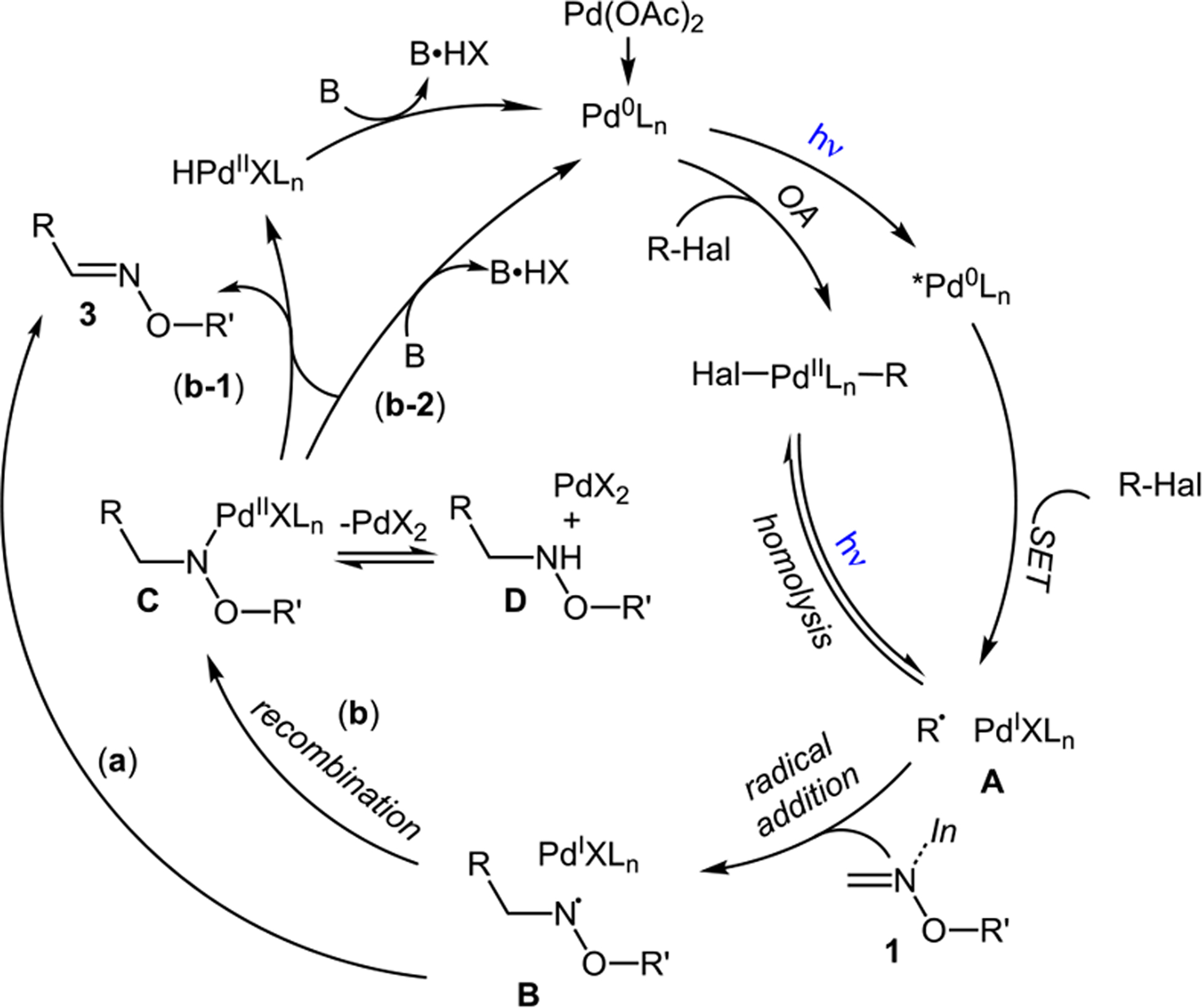
Proposed Mechanism
The feasibility of path b was supported by the observation of small amounts of intermediate D by early stage GC/MS analysis of the reaction mixtures. This was further validated by the test experiment, where benzyloxyamine 13 produced substantial amounts of oxime derivative 3q under our standard alkyl-Heck reaction of oximes (1 → 3p, eq 1). At this point, none of the proposed pathways can be reliably ruled out. It is likely that the reaction path is governed by the nature of counterion X at Pd, contingent on the choice of halide and base used. Obviously, more detailed studies are required to establish a concise mechanism for this novel alkyl imino-Heck reaction.26
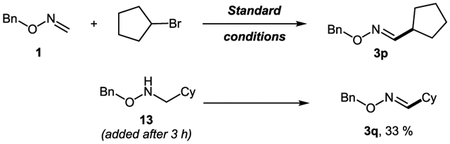 |
(1) |
In conclusion, the first example of visible light-induced palladium-catalyzed Heck-type alkylation of oximes has been developed. In this transformation, the affinity of oximes to nucleophilic carbon-centered radicals, combined with the oxidative nature of palladium catalysts, allowed for a new C–H functionalization protocol. It is anticipated that this mild visible light-induced method will find application in synthesis and will stimulate development of new C–H functionalization methods.
Supplementary Material
ACKNOWLEDGMENTS
This paper is dedicated to the memory of a great Person, outstanding Scientist, and the Cardinal, Professor Victor Snieckus (1937-2020). We thank the National Institute of Health (GM120281), National Science Foundation (CHE-1955663), and Welch Foundation (Chair, AT-0041) for financial support.
Footnotes
Supporting Information
The Supporting Information is available free of charge at https://pubs.acs.org/doi/10.1021/acscatal.1c00267.
Experimental procedures; analytical data for all new compounds (PDF)
Complete contact information is available at: https://pubs.acs.org/10.1021/acscatal.1c00267
The authors declare no competing financial interest.
REFERENCES
- (1).(a) Friestad GK Addition of carbon-centered radicals to imines and related compounds. Tetrahedron 2001, 57, 5461–5496. [Google Scholar]; (b) Miyabe H; Ueda M; Naito T Carbon-Carbon Bond Construction Based on Radical Addition to C=N Bond. Synlett 2004, 7, 1140–1157. [Google Scholar]
- (2).(a) Halland N; Anker Jørgensen K Intermolecular addition of alkyl radicals to imines in the absence and in the presence of a Lewis acid. J. Chem. Soc., Perkin Trans 12001, 1290–1295. [Google Scholar]; (b) Yamada K-I; Fujihara H; Yamamoto Y; Miwa Y; Taga T; Tomioka K Radical Addition of Ethers to Imines Initiated by Dimethylzinc. Org. Lett 2002, 4, 3509–3511. [DOI] [PubMed] [Google Scholar]; (c) Lee S; Kim S Enantioselective radical addition reactions to imines using binaphthol-derived chiral N-triflyl phosphor-amides. Tetrahedron Lett. 2009, 50, 3345–3348. [Google Scholar]
- (3).Garrido-Castro AF; Maestro MC; Alemán J α-Functionalization of Imines via Visible Light Photoredox Catalysis. Catalysts 2020, 10, 562. [Google Scholar]
- (4).Matos JLM; Vásquez-Céspedes S; Gu J; Oguma T; Shenvi RA Branch-Selective Addition of Unactivated Olefins into Imines and Aldehydes. J. Am. Chem. Soc 2018, 140, 16976–16981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (5).(a) Kim S; Lee IY; Yoon J-Y; Oh DH Novel Radical Reaction of Phenylsulfonyl Oxime Ethers. A Free Radical Acylation Approach. J. Am. Chem. Soc 1996, 118, 5138–5139. [Google Scholar]; (b) Kim S; Song H-J; Choi T-L; Yoon J-Y Tin-Free Radical Acylation Reactions with Methanesulfonyl Oxime Ether. Angew. Chem., Int. Ed 2001, 40, 2524–2526. [DOI] [PubMed] [Google Scholar]; (c) Rouquet G; Robert F; Méreau R; Castet F; Landais Y Allylsilanes in “Tin-free” Oximation, Alkenylation, and Allylation of Alkyl Halides. Chem. - Eur. J 2011, 17, 13904–13911. [DOI] [PubMed] [Google Scholar]; (d) Kamijo S; Takao G; Kamijo K; Hirota M; Tao K; Murafuji T Photo-induced Substitutive Introduction of the Aldoxime Functional Group to Carbon Chains: A Formal Formylation of Non-Acidic C(sp3)-H Bonds. Angew. Chem., Int. Ed 2016, 55, 9695–9699. [DOI] [PubMed] [Google Scholar]
- (6).Gaspar B; Carreira EM Cobalt Catalyzed Functionalization of Unactivated Alkenes: Regioselective Reductive C-C Bond Forming Reactions. J. Am. Chem. Soc 2009, 131, 13214–13215. [DOI] [PubMed] [Google Scholar]
- (7).(a) Mikhaleva AI; Zaitsev AB; Trofimov BA Oximes as reagents. Russ. Chem. Rev 2006, 75, 797–823. [Google Scholar]; (b) Mirjafary Z; Abdoli M; Saeidian H; Boroon S; Kakanejadifard A Oxime ethers as versatile precursors in organic synthesis: a review. RCS Adv. 2015, 5, 79361–79384. [Google Scholar]; (c) Bolotin DS; Bokach NA; Demakova MY; Kukushkin VY Metal-Involving Synthesis and Reactions of Oximes. Chem. Rev 2017, 117, 13039–13122. [DOI] [PubMed] [Google Scholar]
- (8).Hui C; Xu J Palladium-catalyzed sp3 C-H oxidation using oxime as directing group—applications in total synthesis. Tetrahedron Lett. 2016, 57, 2692–2696. [Google Scholar]
- (9).(a) Abele E; Lukevics E Furan and Thiophene Oximes: Synthesis, Reactions, and Biological Activity. (Review). Chem. Heterocycl. Compd 2001, 37, 141–169. [Google Scholar]; (b) Abele E; Abele R; Dzenitis O; Lukevics E Indole and Isatin Oximes: Synthesis, Reactions, and Biological Activity. (Review). Chem. Heterocycl. Compd 2003, 39, 3–35. [Google Scholar]; (c) Abele E; Abele R; Lukevics E Pyrrole Oximes: Synthesis, Reactions, and Biological Activity. (Review).Chem. Heterocycl. Compd 2004, 40, 1–15. [Google Scholar]; (d) Abele E; Abele R; Rubina K; Lukevics E Quinoline Oximes: Synthesis, Reactions, and Biological Activity. (Review).Chem. Heterocycl. Compd 2005, 41, 137–162. [Google Scholar]; (e) Faiz Norrrahim MN; Idayu Abdul Razak MA; Ahmad Shah NA; Kasim H; Wan Yusoff WY; Halim NA; Mohd Nor SA; Jamal SH; Ong KK; Zin Wan Yunus WM; Knight VF; Mohd Kasim NA Recent developments on oximes to improve the blood brain barrier penetration for the treatment of organophosphorus poisoning: a review. RCS Adv. 2020, 10, 4465–4489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (10).Ohno H; Aso A; Kadoh Y; Fujii N; Tanaka T Heck-Type Cyclization of Oxime Ethers: Stereoselective Carbon-Carbon Bond Formation with Aryl Halides To Produce Heterocyclic Oximes. Angew. Chem., Int. Ed 2007, 46, 6325–6328. [DOI] [PubMed] [Google Scholar]
- (11).Gou Q; Deng B; Qin J Palladium-Catalyzed Arylation of (Di)azinyl Aldoxime Ethers by Aryl Iodides: Stereoselective Synthesis of Unsymmetrical (E)-(Di)azinylaryl Ketoxime Ethers. Chem. - Eur. J 2015, 21, 12586–12591. [DOI] [PubMed] [Google Scholar]
- (12).(a) Chuentragool P; Kurandina D; Gevorgyan V Catalysis with Palladium Complexes Photoexcited by Visible Light. Angew. Chem., Int. Ed 2019, 58, 11586–11598. [DOI] [PMC free article] [PubMed] [Google Scholar]; (b) Kancherla R; Muralirajan K; Sagadevan A; Rueping M Visible Light-Induced Excited-State Transition-Metal Catalysis. Trends in Chemistry 2019, 1, 510–523. [Google Scholar]; (c) Zhou W-J; Cao G-M; Zhang Z-P; Yu D-G Visible Light-induced Palladium-catalysis in Organic Synthesis. Chem. Lett 2019, 48, 181–191. [Google Scholar]
- (13).(a) Kurandina D; Parasram M; Gevorgyan V Visible Light-Induced Room-Temperature Heck Reaction of Functionalized Alkyl Halides with Vinyl Arenes/Heteroarenes. Angew. Chem., Int. Ed 2017, 56, 14212–14216. [DOI] [PMC free article] [PubMed] [Google Scholar]; (b) Kurandina D; Rivas M; Radzhabov M; Gevorgyan V Heck Reaction of Electronically Diverse Tertiary Alkyl Halides. Org. Lett 2018, 20, 357–360. [DOI] [PMC free article] [PubMed] [Google Scholar]; (c) Chuentragool P; Yadagiri D; Morita T; Sarkar S; Parasram M; Wang Y; Gevorgyan V Aliphatic Radical Relay Heck Reaction at Unactivated C(sp3)-H Sites of Alcohols. Angew. Chem., Int. Ed 2019, 58, 1794–1798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (14).(a) Wang G-Z; Shang R; Cheng W-M; Fu Y Irradiation-Induced Heck Reaction of Unactivated Alkyl Halides at Room Temperature. J. Am. Chem. Soc 2017, 139, 18307–18312. [DOI] [PubMed] [Google Scholar]; (b) Koy M; Sandfort F; Tlahuext-Aca A; Quach L; Daniliuc CG; Glorius F Palladium-Catalyzed Decarboxylative Heck-Type Coupling of Activated Aliphatic Carboxylic Acids Enabled by Visible Light. Chem. - Eur. J 2018, 24, 4552–4555. [DOI] [PubMed] [Google Scholar]; (c) Wang G-Z; Shang R; Fu Y Irradiation-Induced Palladium-Catalyzed Decarboxylative Heck Reaction of Aliphatic N-(Acyloxy)phthalimides at Room Temperature. Org. Lett 2018, 20, 888–891. [DOI] [PubMed] [Google Scholar]
- (15).(a) Miyabe H; Fujii K; Naito T Highly Diastereoselective Radical Addition to Oxime Ethers: Asymmetric Synthesis of β-Amino Acids. Org. Lett 1999, 1, 569–572. [Google Scholar]; (b) Miyabe H; Fujii K; Naito T Radical addition to oxime ethers for asymmetric synthesis of β-amino acid derivatives. Org. Biomol. Chem 2003, 1, 381–390. [DOI] [PubMed] [Google Scholar]; (c) Miyabe H; Ueda M; Fujii K; Nishimura A; Naito T Tandem Carbon-Carbon Bond-Forming Radical Addition-Cyclization Reaction of Oxime Ether and Hydrazone. J. Org. Chem 2003, 68, 5618–5626. [DOI] [PubMed] [Google Scholar]; (d) Kim J-G; Mishra MK; Jang DO Synthesis of sterically hindered ketones from aldehydes via O-silyl oximes. Tetrahedron Lett. 2012, 53, 3527–3529. [Google Scholar]
- (16).Wang D; Weinstein AB; White PB; Stahl SS Ligand-Promoted Palladium-Catalyzed Aerobic Oxidation Reactions. Chem. Rev 2018, 118, 2636–2679. [DOI] [PubMed] [Google Scholar]
- (17).(a) Cheng W-M; Shang R; Fu Y Irradiation-induced palladium-catalyzed decarboxylative desaturation enabled by a dual ligand system. Nat. Commun 2018, 9, 5215. [DOI] [PMC free article] [PubMed] [Google Scholar]; (b) Zhao B; Shang R; Wang G-Z; Wang S; Chen H; Fu Y Palladium-Catalyzed Dual Ligand-Enabled Alkylation of Silyl Enol Ether and Enamide under Irradiation: Scope, Mechanism, and Theoretical Elucidation of Hybrid Alkyl Pd(I)-Radical Species. ACS Catal. 2020, 10, 1334–1343. [Google Scholar]
- (18).Kurandina D; Yadagiri D; Rivas M; Kavun A; Chuentragool P; Hayama K; Gevorgyan V Transition-Metal- and Light-Free Directed Amination of Remote Unactivated C(sp3)-H Bonds of Alcohols. J. Am. Chem. Soc 2019, 141, 8104–8109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (19).(a) Ikeda K; Achiwa K; Sekiya M A Convenient Synthesis of N-Benzyloxy-β-lactams via N-Benzyloxyimines. Chem. Pharm. Bull 1989, 37, 1179–1184. [Google Scholar]; (b) MacNevin CJ; Moore RL; Liotta DC Stereoselective Synthesis of Quaternary Center Bearing Azetines and Their β-Amino Acid Derivatives. J. Org. Chem 2008, 73, 1264–1269. [DOI] [PubMed] [Google Scholar]; (c) Lin T-Y; Wu H-H; Feng J-J; Zhang J Chirality Transfer in Rhodium(I)-Catalyzed [3 + 2]-Cycloaddition of Vinyl Aziridines and Oxime Ethers: Atom-Economical Synthesis of Chiral Imidazolidines. Org. Lett 2018, 20, 3587–3590. [DOI] [PubMed] [Google Scholar]
- (20).(a) Elhaddadi M; Jacquier R; Petrus F; Petrus C A New and Convenient Synthesis of 1-Benzyloxyaminoalkyl Phosphonic and Phosphinic Acids from Oximes. Phosphorus, Sulfur Silicon Relat. Elem 1989, 45, 161–164. [Google Scholar]; (b) Miyabe H; Shibata R; Sangawa M; Ushiro C; Naito T Intermolecular alkyl radical addition to the carbon-nitrogen double bond of oxime ethers and hydrazones. Tetrahedron 1998, 54, 11431–11444. [Google Scholar]; (c) Miyabe H; Shibata R; Ushiro C; Naito T Carbon-carbon bond formation via intermolecular carbon radical addition to aldoxime ethers. Tetrahedron Lett. 1998, 39, 631–634. [Google Scholar]
- (21).(a) McMahon CM; Alexanian EJ Palladium-Catalyzed Heck-Type Cross-Couplings of Unactivated Alkyl Iodides. Angew. Chem., Int. Ed 2014, 53, 5974–5977. [DOI] [PubMed] [Google Scholar]; (b) Zou Y; Zhou J Palladium-catalyzed intermolecular Heck reaction of alkyl halides. Chem. Commun 2014, 50, 3725–3728. [DOI] [PubMed] [Google Scholar]
- (22).The test experiments showed no change of E/Z ratios of the products in time.
- (23).(a) Beletskaya IP; Cheprakov AV The Heck Reaction as a Sharpening Stone of Palladium Catalysis. Chem. Rev 2000, 100, 3009–3066. [DOI] [PubMed] [Google Scholar]; (b) Le Bras J; Muzart J Intermolecular Dehydrogenative Heck Reactions. Chem. Rev 2011, 111, 1170–1214. [DOI] [PubMed] [Google Scholar]
- (24).See Supporting Information for details.
- (25).The possibility of the chain reaction was not supported by low quantum yield, as well as by BDE calculations.24
- (26).(a) McAtee JR; Martin SES; Ahneman DT; Johnson KA; Watson DA Preparation of Allyl and Vinyl Silanes by the Palladium-Catalyzed Silylation of Terminal Olefins: A Silyl-Heck Reaction. Angew. Chem., Int. Ed 2012, 51, 3663–3667. [DOI] [PMC free article] [PubMed] [Google Scholar]; (b) Martin SES; Watson DA Preparation of Vinyl Silyl Ethers and Disiloxanes via the Silyl-Heck Reaction of Silyl Ditriflates. J. Am. Chem. Soc 2013, 135, 13330–13333. [DOI] [PMC free article] [PubMed] [Google Scholar]; (c) Reid WB; Spillane JJ; Krause SB; Watson DA Direct Synthesis of Alkenyl Boronic Esters from Unfunctionalized Alkenes: A Boryl-Heck Reaction. J. Am. Chem. Soc 2016, 138, 5539–5542. [DOI] [PMC free article] [PubMed] [Google Scholar]; (d) Reid WB; Watson DA Synthesis of Trisubstituted Alkenyl Boronic Esters from Alkenes Using the Boryl-Heck Reaction. Org. Lett 2018, 20, 6832–6835. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


