Abstract
Prospective memory involves setting an intention to act that is maintained over time and executed when appropriate. Slow wave sleep (SWS) has been implicated in maintaining prospective memories, although which SWS oscillations most benefit this memory type remains unclear. Here, we investigated SWS spectral power correlates of prospective memory. Healthy young adult participants completed three ongoing tasks in the morning or evening. They were then given the prospective memory instruction to remember to press “Q” when viewing the words “horse” or “table” when repeating the ongoing task after a 12-h delay including overnight, polysomnographically recorded sleep or continued daytime wakefulness. Spectral power analysis was performed on recorded sleep EEG. Two additional groups were tested in the morning or evening only, serving as time-of-day controls. Participants who slept demonstrated superior prospective memory compared with those who remained awake, an effect not attributable to time-of-day of testing. Contrary to prior work, prospective memory was negatively associated with SWS. Furthermore, significant increases in spectral power in the delta-theta frequency range (1.56 Hz–6.84 Hz) during SWS was observed in participants who failed to execute the prospective memory instructions. Although sleep benefits prospective memory maintenance, this benefit may be compromised if SWS is enriched with delta–theta activity.
Prospective memory refers to the maintenance, retrieval, and execution of a previously formed intention (Einstein and McDaniel 1990). Successful prospective memory is essential for a large number of tasks in daily life, such as remembering to attend a doctor's appointment, to pick up a prescribed medication after that appointment, and to also pick up other needed items (e.g., groceries) while at the drugstore. The above described hypothetical sequence of events integrates previously studied prospective memory variants including time-based (i.e., maintaining a memory to complete an intention at a prespecified time; e.g., Esposito et al. 2015; Occhionero et al. 2017), activity-based (i.e., maintaining a memory to perform an intention before or after a particular activity; e.g., Occhionero et al. 2020), and cue-based (i.e., relying on external cues to prompt a maintained memory for a set intention; e.g., Scullin and McDaniel 2010; Leong et al. 2019b; Scullin et al. 2019).
When it is required that memories be maintained across longer periods of time, prospective memory may become less reliable unless sleep occurs (Scullin and McDaniel 2010; Diekelmann et al. 2013a,b; Grundgeiger et al. 2014; Leong et al. 2019a,b; Scullin et al. 2019). Sleep appears to most strongly aid spontaneous retrieval of cue-based prospective memories (Leong et al. 2019a). Several reports have found that slow wave sleep (SWS) supports spontaneous retrieval of cue-based prospective memory intentions (e.g., Diekelmann et al. 2013a; Leong et al. 2019b), although at least one study found an association with rapid eye movement (REM) sleep instead (Scullin et al. 2019). Cue-based prospective memory is hypothesized to be a type of associative memory that binds prospective components (the prospective memory cue) and retrospective components (maintenance of the memory for the prospective memory intention when presented with the cue; Diekelmann et al. 2013b; Leong et al. 2019a).
Rodent and human literature, implementing a variety of invasive and noninvasive brain imaging techniques, show that cortical slow oscillations (SOs; <1 Hz) and fast thalamocortical sleep spindles during SWS facilitate associative memory retention (Niknazar et al. 2015; Latchoumane et al. 2017; Helfrich et al. 2018; Mikutta et al. 2019; Muehlroth et al. 2019), whereas faster oscillations, such as those in the theta frequency band (∼4–7 Hz), may inhibit declarative associative memory (Marshall et al. 2011). We therefore hypothesize that prospective memory performance, like other studied associative memory variants, should benefit from oscillations during SWS (Klinzing et al. 2019). However, it remains unknown which SWS microarchitectural features may facilitate or inhibit prospective memory performance.
Here, we aimed to first replicate prior findings that prospective memories are better maintained across a 12-h interval including sleep compared with an equivalent interval of wakefulness (e.g., Scullin and McDaniel 2010). We next explored whether sleep-associated memory maintenance might be linked to SWS microarchitectural features. To our knowledge, this is the first experiment to examine whether SWS oscillations differentiate successful from unsuccessful prospective memory performance. Given the role of hippocampal engagement in both associative memory binding (e.g., Yonelinas et al. 2019) and oscillatory coupling during SWS that supports associative memory (Niknazar et al. 2015; Latchoumane et al. 2017; Helfrich et al. 2018; Mikutta et al. 2019; Muehlroth et al. 2019), we hypothesized that prospective memory performance would be supported by SWS and specifically SOs and sleep spindle activity.
Results
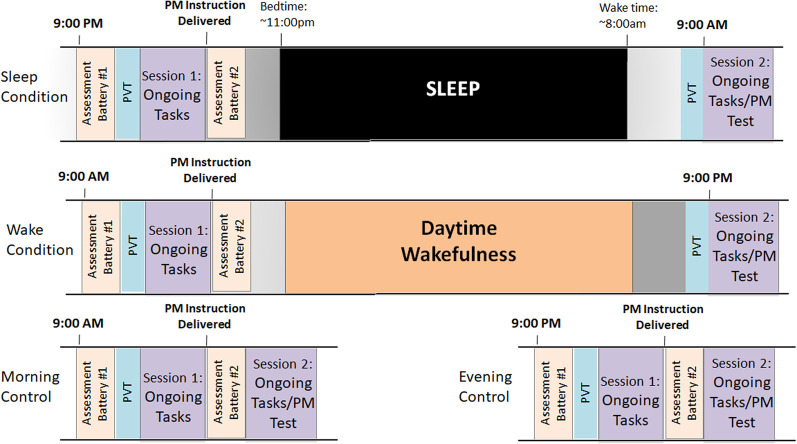
A schematic of the study timeline is presented in Figure 1. Ninety-five participants (age range 18–30, mean = 19.9 ± 1.9, 61 female) (see Table 1 for demographic characteristics) completed the study protocol. Briefly, participants arrived at the lab at 9:00 a.m. (Wake condition; n = 33) or 9:00 p.m. (Sleep condition, n = 30) and completed a psychomotor vigilance task (PVT) (Doran et al. 2001) and a battery of assessments, including the Positive and Negative Affect Schedule (PANAS) (Watson et al. 1988), Beck Anxiety Inventory (BAI) (Beck et al. 1988), Beck Depression Inventory (BDI-II) (Beck et al. 1996), Pittsburgh Sleep Quality Index (PSQI) (Buysse et al. 1989), and the State-Trait Anxiety Inventory (state version; STAI) (Spielberger 1983). See the Materials and Methods for additional details.
Figure 1.
Schematic of study timeline for each experimental condition.
Table 1.
Participant demographics
Participants then completed Session 1 of the prospective memory paradigm involving three computer-based ongoing tasks in the following order: (1) Living/Nonliving Decision, (2) Lexical Decision, and (3) Semantic Categorization. After the final ongoing task, participants were given the prospective memory instruction to press the letter “Q” any time the words “horse” or “table” were presented during the second session (see the Materials and Methods for more details). Participants then completed a second battery of assessments to prevent rehearsal, which included the Morningness-Eveningness Questionnaire (MEQ) (Horne and Östberg 1976), the Iowa Sleep Disturbance Inventory—Expanded Version (ISDI-E) (Koffel 2011), and reassessment of the PANAS and STAI. Overnight sleep was monitored in the Sleep Condition with polysomnography (PSG) (see the Materials and Methods for more details).
For Session 2, participants returned to the lab 12 h later at 9:00 p.m. (Wake condition) or 9:00 a.m. (Sleep condition). After a second PVT, participants then completed the same three ongoing tasks with the prospective memory cues (“horse” and “table”) presented twice per task and were tested on their memory to press the letter “Q” in response to these prompts (without a reminder). To control for time-of-day effects on task performance, 32 additional participants were tested with only a 20-min delay of questionnaire completion between Sessions 1 and 2 at 9:00 a.m. (Morning control, n = 16) or 9:00 p.m. (Evening control, n = 16). Participants were identified as being “Successful” on the prospective memory task if they remembered to respond correctly to a prospective memory cue at least once during Session 2. We chose this as a binary threshold to determine those who had (1) some or complete ability to retrieve the prospective memory intention compared with (2) those who did not have this ability. Given our interest in memory consolidation, we felt this was a straightforward way to categorize those who consolidated the prospective memory intention versus those who did not. We see this >1 cutoff as a conservative, bare-minimum cutoff for successful memory retention. Participants who did not meet this threshold were classified as “Unsuccessful.” Additionally, the proportion of successful prospective memory intention executions was calculated for each participant as the number of times the participant successfully responded to the prospective memory prompt divided by the total number of prospective memory prompts during the ongoing tasks at Session 2.
Participant demographics, self-report questionnaires, and PVT performance
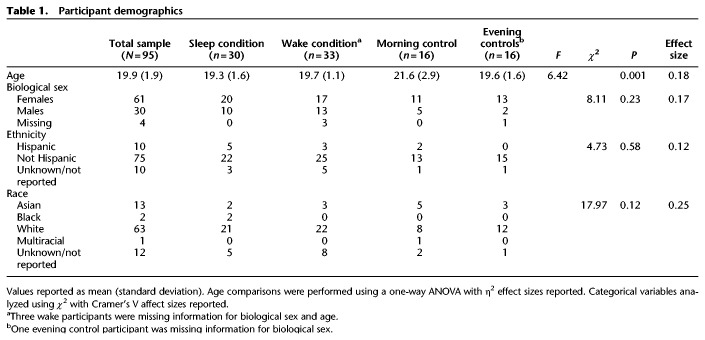
Participant demographics
χ2 tests revealed no significant differences between conditions in terms of biological sex, race, and ethnicity, but there was a significant difference on the one-way ANOVA analysis of age (see Table 1). Follow up t-tests revealed that this was due to the evening control condition being slightly, yet significantly, older than the other conditions (Sleep condition comparison: t(44) = 3.5, P = 0.001; Wake condition comparison: t(44) = 3.2, P = 0.003; Morning control comparison: t(29) = 2.3, P = 0.03). There were no differences in age between the other conditions (most notably no difference between the Sleep and Wake conditions).
Self‐report questionnaires
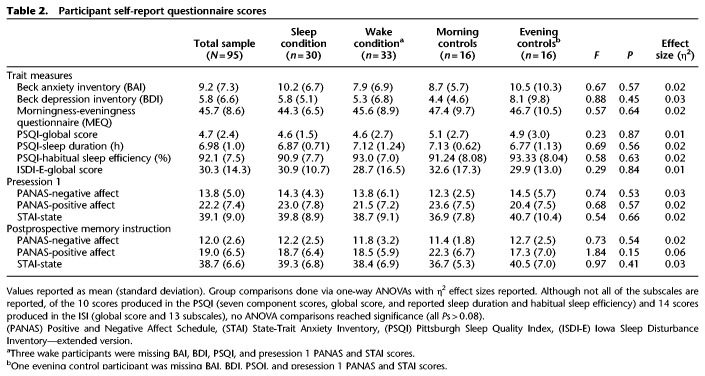
One-way ANOVAs revealed that conditions did not differ on self-report questionnaires including the MEQ, BAI, BDI, global PSQI, and global ISDI-E scores (all Ps > 0.45) (see Table 2). Furthermore, there were no condition effects on any ISDI-E subscale, PSQI component score, PSQI-reported sleep duration, or PSQI-reported habitual sleep efficiency (all Ps > 0.08). There was also no difference in either the initial or reassessment of positive affect, negative affect, or state anxiety as measured by the PANAS and STAI-state measures (all Ps > 0.05) (see the Materials and Methods for questionnaire details).
Table 2.
Participant self-report questionnaire scores
PVT performance
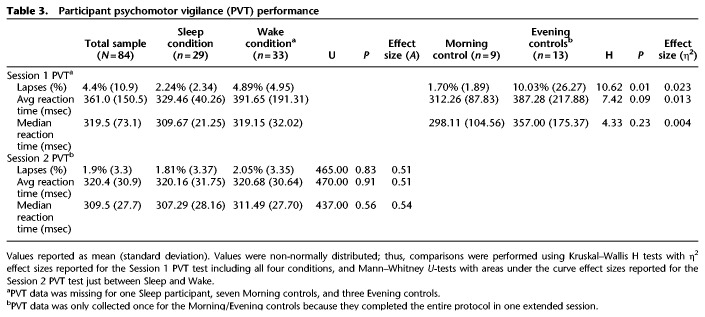
On the PVT, using Kruskal–Wallis H comparisons for nonparametric testing, the four conditions did not differ in median or average reaction time at Session 1 (see Table 3). There was, however, a significant difference in lapse rate at Session 1. Follow-up Mann–Whitney U comparisons for nonparametric testing revealed that the Wake condition had a significantly higher lapse rate (4.9%) than the Sleep condition (2.2%, U = 289.0 P = 0.007) and the Morning control condition (1.7%, U = 69.5, P = 0.015), but not the Evening control condition. There were no other lapse rate differences between the conditions at Session 1. At Session 2, Mann–Whitney U comparisons between the Sleep and Wake conditions revealed no differences in lapse rates, average reaction time, or median reaction time.
Table 3.
Participant psychomotor vigilance (PVT) performance
Prospective memory performance
We first conducted a 3 (Ongoing Task) × 2 (Delay) × 2 (Encoding Time-of-Day) mixed analysis of variance (ANOVA) including a within-subjects factor Task (living/nonliving decision, lexical decision, or semantic categorization) and between subject factors of Delay (20 min or 12 h) and Encoding Time-of-Day (morning or evening). We observed a significant main effect of Delay (20 min > 12 h), F(1,91) = 12.29, P = 0.001, ηp2 = 0.12, and a significant Delay × Encoding Time-of-Day interaction, F(1,91) = 4.85, P = 0.03, ηp2 = 0.05. Results remained significant when controlling for PVT lapse rates (Delay: F(1,79) = 12.35, P = 0.001, ηp2 = 0.14; Delay × Encoding Time-of-Day: F(1,79) = 4.50, P = 0.03, ηp2 = 0.054). We observed no main effects or interactions with Task (all Ps > 0.15), nor a main effect of Encoding Time-of-Day, F(1,91) = .07, P = 0.79, ηp2 = 0.001, which remained nonsignificant when controlling for PVT. Post-hoc t-tests revealed that the 12-h delay group that encoded in the evening (i.e., the Sleep condition) demonstrated a higher proportion of successful prospective memory intention executions compared with the 12-h delay group that encoded in the morning (i.e., the Wake condition), t(61) = 2.038, P = 0.046, d = 0.513 (see Fig. 2). Seventeen out of 30 (65.4%) Sleep condition participants successfully completed the prospective memory intention at least once, whereas only nine out of 33 (34.6%) did so in the wake condition. χ2 analysis between the sleep and wake conditions revealed a significant difference in the number of prospective memory Successful/Unsuccessful participants within each condition, χ2 (1, N = 63) = 5.6, P = 0.018. Importantly, no statistically significant differences were observed between the 20-min delay conditions (i.e., Time-of-Day Control Conditions), t(30) = 1.32, P = 0.20, d = 0.47, χ2 (1, N = 32) = 0.24, P = 0.63 (see Fig. 2). These findings suggest that sleep facilitates the retrieval of prospective memory intentions to a greater extent than continued wakefulness and that these effects likely are not driven by time-of-day effects.
Figure 2.
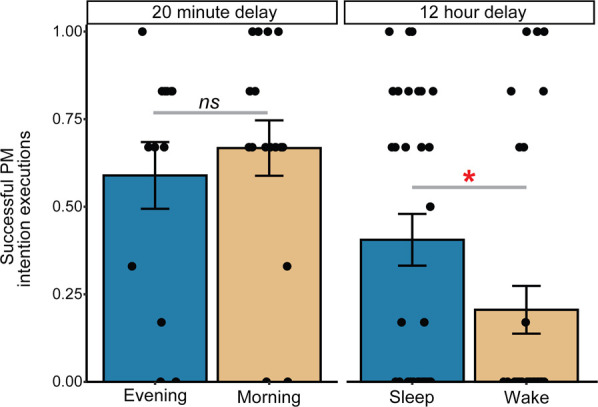
Behavioral data. Proportion of successful prospective memory intention executions (i.e., the number of times the participant successfully responded to the prospective memory prompt divided by the total number of prospective memory prompts) in each condition. Error bars indicate the standard error. (*) P < 0.05, (ns) nonsignificant.
Correlation between prospective memory performance and sleep physiology
Based on our a priori hypothesis, we were primarily interested in whether SWS sleep was associated with the proportion of successfully executed prospective memory intentions. Contrary to our hypothesis, we observed a significant negative correlation between SWS% and proportion of successfully executed prospective memory intentions (ρ = −0.38, P = 0.039). No other sleep stage correlations were significant. Exploratory analyses in other sleep metrics did not reach statistical significance (all Ps > 0.26) (see Supplemental Table S1 in the Supplemental Material).
Sleep and prospective memory performance success
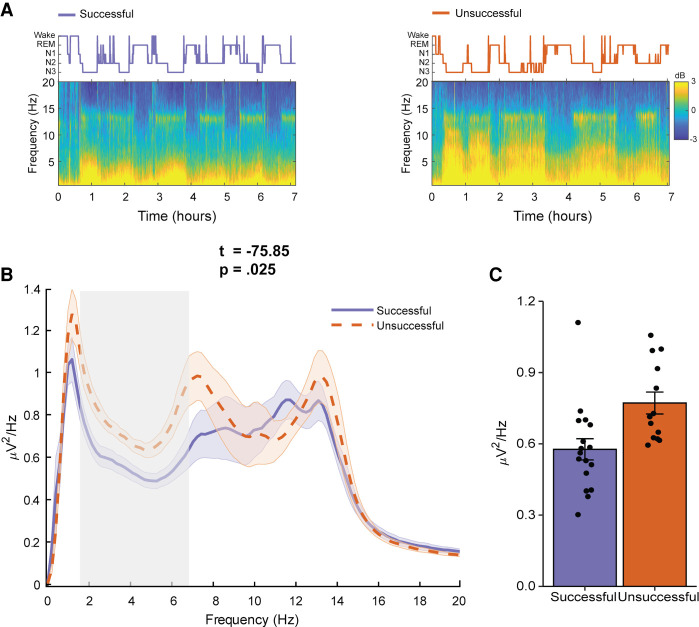
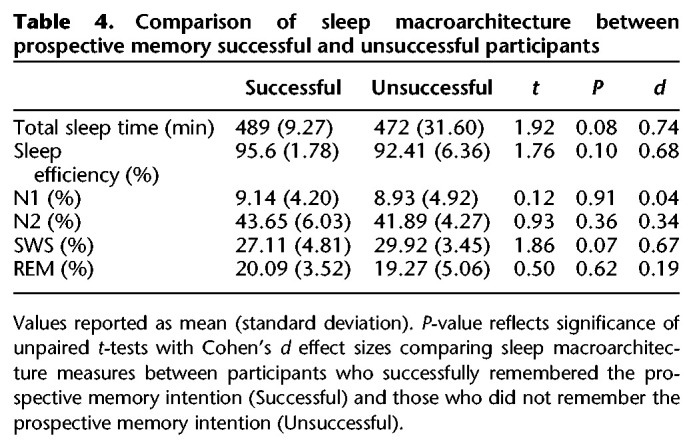
We next determined whether consolidation night sleep characteristics differentiated sleep participants who were successful at completing the prospective memory intention at least once (“Successful”) compared with those who never completed the prospective memory intention (“Unsuccessful”). We first compared the power spectral density of SWS in Successful and Unsuccessful participants. A cluster-based permutation test revealed a significant cluster between 1.56Hz and 6.84Hz (cluster tsum = 75.85, P = 0.025, d = 1.10), suggesting significantly higher spectral power in Unsuccessful participants relative to Successful participants, in a band encompassing delta and theta activity in SWS (see Fig. 3). Given prior work that activity in the delta–theta range increases as a function of prior sleep loss (Cajochen et al. 1999; Åkerstedt et al. 2009), we compared Successful and Unsuccessful participants’ habitual sleep in the month prior to the study with the PSQI. No statistically significant differences were observed, t(28) = 1.40, P = 0.17, d = 0.51. Finally, Successful and Unsuccessful sleep participants showed no significant differences in sleep macroarchitecture nor SWS slow oscillation or spindle characteristics (see Table 4; Supplemental Table S2 in the Supplemental Material). Further comparison of sleep Successful and Unsuccessful participant demographics, psychometrics, PVT, and ongoing task analyses are presented in the Supplemental Material.
Figure 3.
Spectral power analysis. (A) Hypnogram (top) and full night spectrogram (bottom) at electrode C4 of a single participant who did successfully remember the prospective memory intention (left) and a single participant who did not successfully remember the prospective memory intention (right). (B) Group-level SWS power spectrum (averaged power across frontal and central electrodes). Power spectral density (PSD) was normalized within each participant by dividing power at each frequency bin by the average PSD in the 0–20 Hz range. The gray box highlights frequencies in which there were significant differences between participants who remembered the prospective memory intention (solid purple line) and participants who did not remember the prospective memory intention (dashed orange line). Cluster statistic displayed above plot. Shaded areas indicate the standard error. (C) PSD averaged across frequencies in the significant cluster (B) for successful and unsuccessful participants. Error bars indicate the standard error.
Table 4.
Comparison of sleep macroarchitecture between prospective memory successful and unsuccessful participants
Ongoing task performance
Changes in ongoing task performance from Session 1 to Session 2 may be indicative of prospective memory recall strategy. For example, if participants remember to look for the prospective memory cues before the ongoing tasks begin at Session 2 (i.e., “strategic monitoring”), ongoing task performance may show immediate slowing compared with Session 1 (e.g., Scullin McDaniel and Shelton 2013). Conversely, participants may only remember to execute the prospective memory intention upon seeing the cue (i.e., “spontaneous retrieval”), resulting in a delayed or targeted slowing. Prior work suggests that sleep may most benefit spontaneous retrieval because of strengthening of cue-intention associative memory (e.g., Diekelmann et al. 2013a; Leong et al. 2019a). To explore this possibility, we analyzed session and condition differences for ongoing task success (i.e., percent correct) and decision reaction times (see the Supplemental Material for a full description of these analyses and expanded results). Most relevant to the present investigation, we found a significant main effect of ongoing task for both percent correct and reaction time (Ps < 0.001), replicating results from Scullin McDaniel and Shelton (2013). Intriguingly, we found that participants who successfully responded to at least one prospective memory prompt (i.e., Successful) demonstrated significantly greater accuracy (i.e., greater percent correct) to the ongoing tasks across both sessions, t(90) = 2.36, P = 0.02, but also showed significant slowing in response times from Session 1 to Session 2, t(90) = 7.40, P < 0.001, compared with participants that never successfully responded to a prospective memory prompt (i.e., Unsuccessful). This slowing of response time to the ongoing tasks could suggest that participants were utilizing a strategic monitoring technique to respond to the prospective memory prompts (see the Supplemental Material for additional exploratory analysis). However, we did not explicitly aim to answer this question with the current task design, and thus our conclusion remains only speculative. Last, we observed no Condition × Prospection Success interactions, suggesting that sleep did not alter the strategy used during prospective memory retrieval.
Discussion
Here, we replicated prior work by demonstrating that a 12-h interval including sleep results in superior prospective memory performance compared with an equivalent interval of daytime wakefulness. Importantly, we confirmed that this sleep-dependent effect was not simply the result of time-of-day effects and did not seem to determine prospective memory performance strategy. Contrary to Scullin and McDaniel (2010), prospective memory performance was similar across all ongoing tasks, rather than limited to Semantic Categorization. Furthermore, our findings support a deleterious, rather than beneficial, role of SWS on prospective memory performance. When we investigated SWS oscillatory correlates of prospective memory performance, we observed that delta/theta spectral power was elevated in participants who were unsuccessful at maintaining memory for the prospective memory intention across the 12-h, sleep-filled delay. Although it is not immediately clear why such an association between delta/theta spectral power was associated with poorer prospective memory performance, evidence in rodents suggests that slow EEG activity may be dissociable, with SOs facilitating and delta activity inhibiting memory (e.g., Kim et al. 2019). The presence of uncharacteristic rhythms during SWS (e.g., theta activity) has also been associated with memory performance impairments. For example, Zhang et al. (2020) demonstrated that administration of zolpidem during the night following learning improved associative memory in parallel with increases in sigma and decreases in theta and delta power (though theta power was still positively correlated with memory performance). Theta-frequency transcranial direct current stimulation during NREM sleep produces a global decrease in SO activity along with local reductions in frontal slow EEG spindle power (8–12 Hz) and a decrement in declarative memory consolidation (Marshall et al. 2011). Theta bursts during NREM sleep also appear to precede the SO downstate (Gonzalez et al. 2018). Although we did not specifically explore temporal dynamics of theta rhythm during NREM sleep, one possibility is that increased theta power resulted in improperly timed theta bursts, impairing the typical memory-promoting function of SOs, although this remains highly speculative. Evidence in waking EEG suggests that theta rhythms produce cognitive interference for episodic memories (Hanslmayr et al. 2010; Staudigl et al. 2010). Memories are theorized to be repeatedly reactivated in the hippocampus during SWS and consolidation occurs by enhanced hippocampal–neocortical dialog (Zhang et al. 2018; Klinzing et al. 2019). Aberrant theta rhythms during sleep may thus increase memory interference resulting in impaired prospective memory cue-intention consolidation. This interpretation is only speculative at this point, but could be tested through causal manipulation of sleep rhythms using techniques such as acoustic stimulation (Ong et al. 2016; Choi et al. 2018; Simor et al. 2018) or noninvasive electrical brain stimulation protocols (e.g., Marshall et al. 2011).
Last, both delta and theta rhythm activity increase during recovery sleep following sleep deprivation (Cajochen et al. 1999; Åkerstedt et al. 2009). Sleep is commonly curtailed in young, college-aged samples, like the sample used here. Therefore, increased broadband delta–theta activity may serve as a proxy for those who slept more poorly in the nights leading up to the study. However, our findings do not support this hypothesis as self-reported habitual sleep quality prior to study participation was similar between participants who were both successful and unsuccessful at completing the prospective memory task. This outstanding question may be resolved in future studies by implementing objective baseline assessments of sleep quality using, for example, actigraphy or PSG.
Limitations
We recognize some limitations to the current study. As noted in the Materials and Methods, following completion of the second session we conducted a retrospective memory test for the prospective memory prompts (“horse” and “table”) and response key (“Q”). Unfortunately, a majority of these responses were lost during a laboratory transition. Without this data, we are currently unable to determine whether prospective memory unsuccessful participants forgot the correct cues or the intention they were required to execute. We did, however, ensure that participants correctly encoded the prospective memory intention and were clear about what actions to perform when presented with the cue words (see the Materials and Methods for more details). We further speculate that enhanced delta–theta activity observed here might benefit other forms of cognition or memory not tested here, which should be a focus of future work. Moreover, a greater number and variety of prospective memory intentions, like those implemented in Diekelmann et al. (2013b) and Leong et al. (2019b), may have provided better resolution to explore the association between sleep oscillatory correlates of prospective memory.
Conclusion
Sleep promotes cue-based prospective memory performance to a greater extent than continued wake. However, and surprisingly, we observed a deleterious effect of both the amount of SWS and delta–theta band activity during SWS, on prospective memory performance. We hypothesize that these middle frequencies may intrude on typical slow (i.e., SOs) and fast (i.e., spindles) SWS oscillatory rhythms, resulting in impaired prospective memory cue-intention consolidation, although additional work is needed to substantiate this interpretation.
Materials and Methods
Participants
Ninety-five English-speaking young adult participants (age range 18–30, mean = 19.9 ± 1.9, 61 female) with normal or corrected-to-normal vision (see Table 1 for demographic characteristics) were recruited from the University of Notre Dame student population. Participants reported no history of neurological, psychiatric, major medical, or sleep disorders or use of psychoactive or sleep-altering medications. All participants were instructed to refrain from tobacco, caffeine, alcohol, and recreational drugs for 24 h prior to and during study participation. All procedures were approved by the University of Notre Dame Institutional Review Board (IRB), and participants were provided with written informed consent prior to study participation. Initial intake data (including demographic information) for four participants was lost either because of technical difficulties (3) or experimenter error (1).
Prospective memory task
The prospective memory task involved two sessions. During Session 1, participants completed three computer-based ongoing tasks in the following order: Living/Nonliving Decision (deciding if a word presented represented a living [e.g., dog] or nonliving [e.g., kite] object), lexical decision (deciding if a string of letters formed a word [e.g., ship] or nonword [e.g., ihsp]), and semantic categorization (determine if a word presented in lowercase belonged to the category represented by a word simultaneously presented in uppercase [e.g., couch FURNITURE]). In an attempt to replicate findings from Scullin and McDaniel (2010), the ongoing task order was not counterbalanced. After completing the tasks, the prospective memory instruction was introduced by instructing participants that the “next time you do these tasks, any time you see the words ‘horse’ or ‘table,’ press the letter ‘Q’ instead of whatever you would normally press.” The prospective memory instruction was presented via E-Prime immediately following completion of the ongoing tasks. After receiving the instruction, the participants were then given a sheet of paper and were asked to write down the two words they were asked to respond to at the next session (“horse” and “table”) and the key that they were to press in response (“Q”). If they did not respond correctly, they were verbally reminded of the PM instruction by the experimenter and were given another sheet of paper until they responded correctly. They were also verbally instructed by the experimenter that they would not be reminded of the PM instruction at the next session.
During Session 2, participants completed the same three ongoing tasks outlined above containing two prospective memory cues per task. As previously established (Scullin et al. 2010, 2011), to allow for inhibition errors while still successfully responding to the prospective memory prompt, successful execution of a prospective memory intention was defined as remembering to press “Q” in close temporal proximity to “horse” or “table” trials (i.e., either on the prospective memory probe trial or n + 1). Across all participants, no prospective memory responses (i.e., letter “Q” entries) were given outside of either the prospective memory probe or the following trial. At the end of Session 2, a retrospective memory check was conducted. Participants were asked to report the prospective memory prompts and response key to determine if there were condition differences in entirely forgetting the prompts and responses. Unfortunately, approximately half of these sheets were lost during a laboratory move. As such, we were unable to entirely confirm if condition differences were driven by forgetting of the intention or by forgetting of the cues, which is a limitation of this study.
Procedures
Participants arrived at the lab at 9:00 a.m. (Wake condition; n = 33) or 9:00 p.m. (Sleep condition, n = 30) and completed an initial battery of intake questionnaires and a psychomotor vigilance task (PVT). As clinical state has been demonstrated to impact prospective memory performance (Bowman et al. 2019), intake questionnaires included the Positive and Negative Affect Schedule (PANAS; Watson et al. 1988), Beck Anxiety Inventory (BAI; Beck et al. 1988), Beck Depression Inventory (BDI-II; A. Beck et al. 1996), Pittsburgh Sleep Quality Index (PSQI; Buysse et al. 1989), and the State-Trait Anxiety Inventory (state version; STAI; (Spielberger 1983). The PVT is an objective measurement of reaction time and sustained attention and has been shown to be sensitive to sleep loss and circadian variation in performance, allowing for the determination of differences in attention between conditions prior to cognitive testing (Doran et al. 2001). Participants viewed a black screen with dimly lit numbers (0000) in the center. Participants were asked to press the space bar using their preferred hand whenever they saw the numbers light up and begin to count upward. When the participants responded to the stimulus the numbers would stop. Participants were informed that the numbers in the display represented their reaction time—the smaller the number, the faster they responded. The interstimulus interval varied randomly (delay ranging from 2 to 10 sec) and the task duration was 5 min.
Participants then completed Session 1 of the prospective memory paradigm described above. Participants practiced the three ongoing tasks and, after the final ongoing task, were given the prospective memory instruction to press the letter “Q” any time the words “horse” or “table” were presented during the second session. Participants were told that they would not be reminded about the prospective memory instruction. After the prospective memory instruction was delivered, participants completed a second battery of questionnaires for ∼20 min to prevent rehearsal. The questionnaires during the delay period included the Morningness-Eveningness Questionnaire (MEQ; Horne and Östberg 1976) and reassessment of the PANAS and STAI. Overnight sleep was monitored in the Sleep condition with polysomnography (PSG).
For Session 2, participants returned to the lab 12 h later at 9:00 p.m. (Wake Condition) or 9:00 a.m. (Sleep Condition). Session 2 again began with the PVT. Participants then completed the same three ongoing tasks with the prospective memory cues (“horse” and “table”) presented twice per task. Following completion of the prospective memory task, participants were debriefed and dismissed.
To control for time-of-day effects on task performance, 32 additional participants were tested with only a 20-min delay of questionnaire completion between Sessions 1 and 2 at 9:00 a.m. (Morning control, n = 16) or 9:00 p.m. (Evening control, n = 16).
Self-report questionnaires
Demographics questionnaire
Participants completed a demographics questionnaire that included age, biological sex, race, and ethnicity.
Beck Depression Inventory (BDI‐II)
The BDI-II (Beck et al. 1996) is a 21-item self-report measure of depression that has been validated in adolescents and adults. Respondents rate their experience of each symptom (e.g., “It's hard to get interested in anything”) over the past 2 wk using a four-point intensity scale (from 0–3, with unique descriptors for each question). Scores 0–13 indicate minimal depression, 14–19 indicate mild depression, 20–28 indicate moderate clinical depression, and >29 indicate severe clinical depression.
Beck Anxiety Inventory (BAI)
The BAI (Beck et al. 1988) is a 21-item self-report measure of anxiety that focuses on somatic content to minimize the overlap with depression. Respondents rate their experience of each symptom (e.g., “unable to relax”) over the past month using a four-point intensity scale (0 = “Not at all,” 3 = “Severely, it bothered me a lot”). Scores between 0–21 are interpreted as indicating low anxiety, 22–35 as indicating moderate anxiety, and 36 and above as indicating severe anxiety.
State‐Trait Anxiety Inventory (STAI)
The STAI (Spielberger 1983) assesses current levels of anxiety (i.e., state anxiety) and how anxious participants tend to feel in general (i.e., trait anxiety). Only the state anxiety subscale was utilized for this study, which consists of 20 items (e.g., “I feel anxious”). Respondents rate their current experience of each symptom using a four-point intensity scale (1 = “not at all,” 4 = “very much so”), and higher scores indicate greater state anxiety.
Positive and Negative Affect Schedule (PANAS)
The PANAS (Watson et al. 1988) is a 20-item self-report measure of affect comprising two scales: Positive Affect (PA) and Negative Affect (NA). The NA scale contains 10 negative affect terms (e.g., “ashamed”), whereas the PA scale contains 10 positive affect terms (e.g., “excited”). This study used state instructions for the PANAS, such that participants were asked to rate the extent they were experiencing each affect “right now” using a five-point scale (1 = “not at all,” 5 = “extremely”). The PA and NA scales are independent of each other and both show strong internal consistency under present-moment time instructions (Watson et al. 1988).
Pittsburgh Sleep Quality Index (PSQI)
The PSQI (Buysse et al. 1989) differentiates between “poor-” and “good-”quality sleepers by measuring seven areas over the course of the last month: subjective sleep duration, sleep quality, sleep efficiency, sleep latency, use of sleep medications, sleep disturbances, and daytime dysfunction. The survey is a mix of free response and multiple choice, with all scores transformed into a 0–3 Likert scale, in which a score of 3 reflects the negative extreme. A global score of 5 or less is indicative of a good-quality sleeper, whereas a score of >5 is indicative of a poor-quality sleeper. The internal consistency of the PSQI, estimated by Cronbach's alpha, is 0.73. In addition to running analyses on the global PSQI score, at the request of a reviewer exploratory analyses were conducted on the habitual hours of total sleep duration and habitual sleep efficiency (total sleep time/time in bed) reported by the participants on the PSQI, as well as the seven PSQI component scores: daytime dysfunction, use of sleep medications, sleep disturbances, sleep efficiency, sleep latency, sleep duration, and subjective sleep quality.
Morningness‐Eveningness Questionnaire (MEQ)
The MEQ (Horne and Östberg 1976) is used to distinguish between chronotypes (an endogenous characteristic describing one's preference for either morning or evening patterns of activity). Scores range from 16 to 86, corresponding to extreme eveningness (lower numbers) to extreme morningness (higher numbers), whereas intermediate chronotypes fall within the extremes. Questions target individual preferences for sleep and wake times and preferred times for daytime activities, such as: “What time would you go to bed if you were entirely free to plan your evening?” Each question is given its own set of responses with various scores associated with each response, which are then summed for the final MEQ score. Internal consistency for the MEQ, estimated by Cronbach's alpha, is 0.86.
Iowa Sleep Disturbance Inventory—Extended version (ISDI‐E)
The ISDI-E (Koffel 2011) comprises 95 true–false questions assessing sleep difficulties. Each query has a short statement, and participants are instructed to check “true” if it sounds like them and “false” if it does not sound like them. The time frame of symptom presentation is not explicitly stated. In addition to a global score, the ISDI-E contains 13 separate subscales: initial insomnia, nightmares, fatigue, fragmented sleep, nonrestorative sleep, light sleep, anxiety at night, movement at night, sensations at night, irregular sleep, excessive sleep, sleep paralysis, and sleep hallucinations. The global score ranges from 0 to 95 and is measured by summing all “true” responses. The subscales are similarly calculated by summing the relevant items (with some items reverse-scored). The items were ultimately selected from a pool of >3000 items following an exploratory factor analysis to narrow down the final items and cluster them into subgroups (Koffel and Watson 2010). Psychometric properties were found to be acceptable in both healthy and clinical cohorts.
Three Wake condition participants and one Evening control participant were missing demographic information, BDI, BAI, PSQI, and presession PANAS, and STAI scores.
Sleep monitoring and staging
Sleep was monitored with PSG, which included six-channel electroencephalography (EEG; F3, F4, C3, C4, O1, O2) referenced to contralateral mastoid electrodes (M1, M2), two-channel electrooculogram, and two-channel chin electromyography. Sleep EEG was staged in 30-sec epochs using standard American Academy of Sleep Medicine criteria (Iber et al. 2007). Our main PSG sleep outcome measures included total sleep time (TST), sleep latency (minutes), REM latency (minutes), wake after sleep onset (WASO), sleep efficiency (percentage), and percentage and minutes spent in Stage 1 (N1), Stage 2 (N2), slow-wave sleep (SWS), and rapid-eye movement (REM) sleep.
Sleep EEG analysis
The power spectrum was determined separately at each EEG channel (F3, F4, C3, C4) for all artifact-free SWS data. Power spectral density (PSD) was estimated using Welch's method with 5-sec windows and 50% overlap (using the pwelch function in Matlab). To minimize the typical 1/f scaling of the power spectrum, estimates were obtained from the temporal derivative of the EEG time series (Cox et al. 2017). We then normalized each electrode's power spectrum by dividing the spectrum by that electrode's average power. PSD estimates from the four channels were then averaged together for use in further analysis. Sleep spindles, SOs, and their coupling were detected using previously validated methods (see the Supplemental Material for additional details).
Statistical analysis
The primary analyses of interest included comparing prospective memory performance between sleep and wake conditions. We conducted exploratory analyses comparing sleep macroarchitecture (i.e., stage percentage) and microarchitecture (i.e., spectral power density and spindle/SO density and spindle and SO coupling) between participants who successfully remembered the prospective memory probe (Successful) and those who did not (Unsuccessful). Differences in SWS spectral power between Successful and Unsuccessful participants were assessed using a cluster-based permutation approach implemented in the FieldTrip toolbox for Matlab, using the ft_statfun_indepsamplesT function (Oostenveld et al. 2011). The following parameters were used: 10,000 iterations, a cluster alpha of 0.05 with the default maxsum method to determine cluster significance, and a significance threshold of 0.05. PSD in the 0- to 20-Hz range was included in the analysis. We focused on this range because it encompassed the frequency bands typically associated with memory processes during SWS. Condition differences in memory performance were assessed with analysis of variance (ANOVA) and post-hoc, unpaired t-tests. As this study was designed as a replication of Scullin and McDaniel (2010), we maintained the same ANOVA analysis plan: 3 (ongoing task) × 2 (delay) × 2 (encoding time-of-day) mixed ANOVAs including a within-subjects factor of Task (living/nonliving decision, lexical decision, or semantic categorization) and a between subject factors of Delay (20 min or 12 h) and Encoding Time-of-Day (morning or evening). Notably, a reviewer suggested utilizing the Recognition Time-of-Day as opposed to the Encoding Time-of-Day for the ANOVAs. Although we wanted to maintain consistency with previous work but also recognized this analysis as an intriguing consideration, we have included the Recognition Time-of-Day analysis and a discussion of its merits and limitations in the Supplemental Material. Furthermore, we observed condition differences in PVT performance during the first experimental session in the 12-h delay groups; thus, we conducted an additional confirmatory memory performance analysis of covariance (ANCOVA), covarying PVT during the first session. Analysis of performance differences on the various tasks and differences in sleep macroarchitecture and demographics were done using one-way ANOVAs, Kruskal–Wallis H tests, t-tests, χ2 tests, and Mann–Whitney U-tests where appropriate. Correlations were conducted using Spearman's rho. Analyses were conducted in SPSS version 12 unless otherwise noted.
Data Availability
The data for this project has been made available for open access at the following link: https://osf.io/va8ft.
Supplementary Material
Acknowledgments
We thank Dr. Michael Scullin for sharing the Prospective Memory Task materials. Funding for this project was provided by the Institute for Scholarship in the Liberal Arts Founders’ and Directors’ 30th Anniversary Research Award from the University of Notre Dame. R.B. and T.J.C. thank their National Institutes of Health (NIH) T32 funding sources for supporting their work and ongoing training. R.B. and T.J.C. are currently funded by the Research Training Program in Sleep, Circadian, and Respiratory Neurobiology (NIH T32 HL007901) through the Division of Sleep Medicine at Harvard Medical School and Brigham and Women's Hospital.
Footnotes
[Supplemental material is available for this article.]
Article is online at http://www.learnmem.org/cgi/doi/10.1101/lm.053412.121.
References
- Åkerstedt T, Kecklund G, Ingre M, Lekander M, Axelsson J. 2009. Sleep homeostasis during repeated sleep restriction and recovery: support from EEG dynamics. Sleep 32: 217–222. 10.1093/sleep/32.2.217 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beck AT, Epstein N, Brown G, Steer RA. 1988. An inventory for measuring clinical anxiety: psychometric properties. J Consult Clin Psychol 56: 893–897. 10.1037/0022-006X.56.6.893 [DOI] [PubMed] [Google Scholar]
- Beck A, Steer R, Brown G. 1996. Beck depression inventory-II. The Psychological Corporation, San Antonio, TX. [Google Scholar]
- Bowman MA, Cunningham TJ, Levin-Aspenson HF, O'Rear AE, Pauszek JR, Ellickson-Larew S, Martinez BS, Payne JD. 2019. Anxious, but not depressive, symptoms are associated with poorer prospective memory performance in healthy college students: preliminary evidence using the tripartite model of anxiety and depression. J Clin Exp Neuropsychol 41: 694–703. 10.1080/13803395.2019.1611741 [DOI] [PubMed] [Google Scholar]
- Buysse DJ, Reynolds CF, Monk TH, Berman SR, Kupfer DJ. 1989. The Pittsburgh sleep quality index: a new instrument for psychiatric practice and research. Psychiatr Res 28: 193–213. 10.1016/0165-1781(89)90047-4 [DOI] [PubMed] [Google Scholar]
- Cajochen C, Foy R, Dijk D-J. 1999. Frontal predominance of a relative increase in sleep delta and theta EEG activity after sleep loss in humans. Sleep Res Online 2: 65–69. [PubMed] [Google Scholar]
- Choi J, Han S, Won K, Jun SC. 2018. The neurophysiological effect of acoustic stimulation with real-time sleep spindle detection. 2018 40th Annual International Conference of the IEEE Engineering in Medicine and Biology Society (EMBC), pp 470–473. [DOI] [PubMed] [Google Scholar]
- Cox R, Schapiro AC, Manoach DS, Stickgold R. 2017. Individual differences in frequency and topography of slow and fast sleep spindles. Front Hum Neurosci 11: 433. 10.3389/fnhum.2017.00433 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Diekelmann S, Wilhelm I, Wagner U, Born J. 2013a. Sleep to implement an intention. Sleep 36: 149–153. 10.5665/sleep.2322 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Diekelmann S, Wilhelm I, Wagner U, Born J. 2013b. Sleep improves prospective remembering by facilitating spontaneous-associative retrieval processes. PLoS ONE 8: e77621. 10.1371/journal.pone.0077621 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Doran SM, Van Dongen HP, Dinges DF. 2001. Sustained attention performance during sleep deprivation: evidence of state instability. Arch Ital Biol 139: 253–267. [PubMed] [Google Scholar]
- Einstein GO, McDaniel MA. 1990. Normal aging and prospective memory. J Exp Psychol Learn Mem Cogn 16: 717–726. 10.1037/0278-7393.16.4.717 [DOI] [PubMed] [Google Scholar]
- Esposito MJ, Occhionero M, Cicogna P. 2015. Sleep deprivation and time-based prospective memory. Sleep 38: 1823–1826. 10.5665/sleep.5172 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gonzalez CE, Mak-McCully RA, Rosen BQ, Cash SS, Chauvel PY, Bastuji H, Rey M, Halgren E. 2018. theta bursts precede, and spindles follow, cortical and thalamic downstates in human NREM sleep. J Neurosci 38: 9989–10001. 10.1523/JNEUROSCI.0476-18.2018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grundgeiger T, Bayen UJ, Horn SS. 2014. Effects of sleep deprivation on prospective memory. Memory 22: 679–686. 10.1080/09658211.2013.812220 [DOI] [PubMed] [Google Scholar]
- Hanslmayr S, Staudigl T, Aslan A, Bäuml K-H. 2010. Theta oscillations predict the detrimental effects of memory retrieval. Cogn Affect Behav Neurosci 10: 329–338. 10.3758/CABN.10.3.329 [DOI] [PubMed] [Google Scholar]
- Helfrich RF, Mander BA, Jagust WJ, Knight RT, Walker MP. 2018. Old brains come uncoupled in sleep: slow wave-spindle synchrony, brain atrophy, and forgetting. Neuron 97: 221–230.e4. 10.1016/j.neuron.2017.11.020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Horne JA, Östberg O. 1976. A self-assessment questionnaire to determine morningness-eveningness in human circadian rhythms. Int J Chronobiol 4: 97–110. [PubMed] [Google Scholar]
- Iber C, Ancoli-Israel S, Chesson AL, Quan SF. 2007. The AASM manual for the scoring of sleep and associated events: rules, terminology and technical specifications (vol. 1). American Academy of Sleep Medicine, Westchester, IL. [Google Scholar]
- Kim J, Gulati T, Ganguly K. 2019. Competing roles of slow oscillations and delta waves in memory consolidation versus forgetting. Cell 179: 514–526.e13. 10.1016/j.cell.2019.08.040 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klinzing JG, Niethard N, Born J. 2019. Mechanisms of systems memory consolidation during sleep. Nat Neurosci 22: 1598–1610. 10.1038/s41593-019-0467-3 [DOI] [PubMed] [Google Scholar]
- Koffel E. 2011. Further validation of the Iowa sleep disturbances inventory. Psychol Assess 23: 587. 10.1037/a0022818 [DOI] [PubMed] [Google Scholar]
- Koffel E, Watson D. 2010. Development and initial validation of the Iowa sleep disturbances inventory. Assessment 17: 423–439. 10.1177/1073191110362864 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Latchoumane C-FV, Ngo H-VV, Born J, Shin H-S. 2017. Thalamic spindles promote memory formation during sleep through triple phase-locking of cortical, thalamic, and hippocampal rhythms. Neuron 95: 424–435.e6. 10.1016/j.neuron.2017.06.025 [DOI] [PubMed] [Google Scholar]
- Leong RLF, Cheng GH-L, Chee MWL, Lo JC. 2019a. The effects of sleep on prospective memory: a systematic review and meta-analysis. Sleep Med Rev 47: 18–27. 10.1016/j.smrv.2019.05.006 [DOI] [PubMed] [Google Scholar]
- Leong RLF, Koh SYJ, Chee MWL, Lo JC. 2019b. Slow wave sleep facilitates spontaneous retrieval in prospective memory. Sleep 42: zsz003. 10.1093/sleep/zsz003 [DOI] [PubMed] [Google Scholar]
- Marshall L, Kirov R, Brade J, Mölle M, Born J. 2011. Transcranial electrical currents to probe EEG brain rhythms and memory consolidation during sleep in humans. PLoS One 6: e16905. 10.1371/journal.pone.0016905 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mikutta C, Feige B, Maier JG, Hertenstein E, Holz J, Riemann D, Nissen C. 2019. Phase-amplitude coupling of sleep slow oscillatory and spindle activity correlates with overnight memory consolidation. J Sleep Res 28: e12835. 10.1111/jsr.12835 [DOI] [PubMed] [Google Scholar]
- Muehlroth BE, Sander MC, Fandakova Y, Grandy TH, Rasch B, Shing YL, Werkle-Bergner M. 2019. Precise slow oscillation–spindle coupling promotes memory consolidation in younger and older adults. Sci Rep 9: 1940. 10.1038/s41598-018-36557-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- Niknazar M, Krishnan GP, Bazhenov M, Mednick SC. 2015. Coupling of thalamocortical sleep oscillations are important for memory consolidation in humans. PLoS One 10: e0144720. 10.1371/journal.pone.0144720 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Occhionero M, Cicogna P, Esposito MJ. 2017. The effect of sleep loss on dual time-based prospective memory tasks. Am J Psychol 130: 93–103. 10.5406/amerjpsyc.130.1.0093 [DOI] [PubMed] [Google Scholar]
- Occhionero M, Tonetti L, Fabbri M, Boreggiani M, Martoni M, Giovagnoli S, Natale V. 2020. Prospective memory, sleep, and age. Brain Sci 10: 422. 10.3390/brainsci10070422 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ong JL, Lo JC, Chee NIYN, Santostasi G, Paller KA, Zee PC, Chee MWL. 2016. Effects of phase-locked acoustic stimulation during a nap on EEG spectra and declarative memory consolidation. Sleep Med 20: 88–97. 10.1016/j.sleep.2015.10.016 [DOI] [PubMed] [Google Scholar]
- Oostenveld R, Fries P, Maris E, Schoffelen J-M. 2011. FieldTrip: open source software for advanced analysis of MEG, EEG, and invasive electrophysiological data. Comput Intell Neurosci 2011: 156869. 10.1155/2011/156869 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scullin MK, McDaniel MA. 2010. Remembering to execute a goal: sleep on it! Psychol Sci 21: 1028–1035. 10.1177/0956797610373373 [DOI] [PubMed] [Google Scholar]
- Scullin MK, McDaniel MA, Einstein GO. 2010. Control of cost in prospective memory: evidence for spontaneous retrieval processes. J Exp Psychol Learn Mem Cogn 36: 190–203. 10.1037/a0017732 [DOI] [PubMed] [Google Scholar]
- Scullin MK, Bugg JM, McDaniel MA, Einstein GO. 2011. Prospective memory and aging: preserved spontaneous retrieval, but impaired deactivation, in older adults. Mem Cognit 39: 1232–1240. 10.3758/s13421-011-0106-z [DOI] [PubMed] [Google Scholar]
- Scullin MK, McDaniel MA, Shelton JT. 2013. The dynamic multiprocess framework: evidence from prospective memory with contextual variability. Cogn Psychol 67: 55–71. 10.1016/j.cogpsych.2013.07.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scullin MK, Gao C, Fillmore P, Roberts RL, Pruett N, Bliwise DL. 2019. Rapid eye movement sleep mediates age-related decline in prospective memory consolidation. Sleep 42: zsz055. 10.1093/sleep/zsz055 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simor P, Steinbach E, Nagy T, Gilson M, Farthouat J, Schmitz R, Gombos F, Ujma PP, Pamula M, Bódizs R, et al. 2018. Lateralized rhythmic acoustic stimulation during daytime NREM sleep enhances slow waves. Sleep 41: zsy176. 10.1093/sleep/zsy176 [DOI] [PubMed] [Google Scholar]
- Spielberger CD. 1983. Manual for the State-Trait Anxiety Inventory; Palo Alto, CA, Ed. Consulting Psychologists Press, Inc., Columbia, MO. [Google Scholar]
- Staudigl T, Hanslmayr S, Bäuml K-HT. 2010. Theta oscillations reflect the dynamics of interference in episodic memory retrieval. J Neurosci 30: 11356–11362. 10.1523/JNEUROSCI.0637-10.2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watson D, Clark LA, Tellegen A. 1988. Development and validation of brief measures of positive and negative affect: the PANAS scales. J Pers Soc Psychol 54: 1063–1070. 10.1037/0022-3514.54.6.1063 [DOI] [PubMed] [Google Scholar]
- Yonelinas AP, Ranganath C, Ekstrom AD, Wiltgen BJ. 2019. A contextual binding theory of episodic memory: systems consolidation reconsidered. Nat Rev Neurosci 20: 364–375. 10.1038/s41583-019-0150-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang H, Fell J, Axmacher N. 2018. Electrophysiological mechanisms of human memory consolidation. Nat Commun 9: 4103. 10.1038/s41467-018-06553-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang J, Yetton B, Whitehurst LN, Naji M, Mednick SC. 2020. The effect of zolpidem on memory consolidation over a night of sleep. Sleep 43: zsaa084. 10.1093/sleep/zsaa084 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The data for this project has been made available for open access at the following link: https://osf.io/va8ft.