Abstract
Context
Pseudohypoparathyroidism type 1B (PHP1B), also referred to as inactivating PTH/PTHrP signaling disorder (iPPSD), is characterized by proximal renal tubular resistance to parathyroid hormone (PTH) leading to hypocalcemia, hyperphosphatemia, and elevated PTH values. Autosomal dominant PHP1B (AD-PHP1B) with loss of methylation at the maternal GNAS A/B:TSS-DMR (transcription start site-differentially methylated region) alone can be caused by maternal deletions involving STX16.
Objective
Characterize a previously not reported AD-PHP1B family with loss of methylation at GNAS A/B:TSS-DMR, but without evidence for a STX16 deletion on the maternal allele and assess GNAS-AS2:TSS-DMR methylation.
Methods
DNA from 24 patients and 10 controls were investigated. AD-PHP1B patients without STX16 deletion from a single family (n = 5), AD-PHP1B patients with STX16 deletion (n = 9), sporPHP1B (n = 10), unaffected controls (n = 10), patUPD20 (n = 1), and matUPD20 (n = 1). Methylation and copy number analyses were performed by pyrosequencing, methylation-sensitive multiplex ligation-dependent probe amplification, and multiplex ligation-dependent probe amplification.
Results
Molecular cloning of polymerase chain reaction–amplified, bisulfite-treated genomic DNA from healthy controls revealed evidence for 2 distinct GNAS-AS2:TSS-DMR subdomains, named AS2-1 and AS2-2, which showed 16.0 ± 2.3% and 31.0 ± 2.2% methylation, respectively. DNA from affected members of a previously not reported AD-PHP1B family without the known genetic defects revealed incomplete loss of methylation at GNAS A/B:TSS-DMR, normal methylation at the 3 well-established maternal and paternal DMRs, and, surprisingly, increased methylation at AS2-1 (32.9 ± 3.5%), but not at AS2-2 (30.5 ± 2.9%).
Conclusion
The distinct methylation changes at the novel GNAS-AS2:TSS-DMR will help characterize further different PHP1B/iPPSD3 variants and will guide the search for underlying genetic defects, which may provide novel insights into the mechanisms underlying GNAS methylation.
Keywords: pseudohypoparathyroidism, iPPSD, GNAS, DMR, imprinting
Pseudohypoparathyroidism (PHP) (also known as inactivating PTH/PTHrP signaling disorder [iPPSD]) (1) was described first by Fuller Albright in 1942 as a rare developmental disease with characteristic clinical features referred to as Albright hereditary osteodystrophy (AHO) that occur in combination with hypocalcemia and hyperphosphatemia due to renal proximal tubular resistance to parathyroid hormone (PTH), frequently resistance to other hormones such as thyroid-stimulating hormone (TSH), as well as resistance to other hormones in some patients (2-4). These hormones all mediate their actions through G protein–coupled receptors that require the alpha-subunit of the stimulatory G protein (Gsα) for cAMP (3′,5′-cyclic adenosine monophosphate) generation by adenylyl cyclase (5).
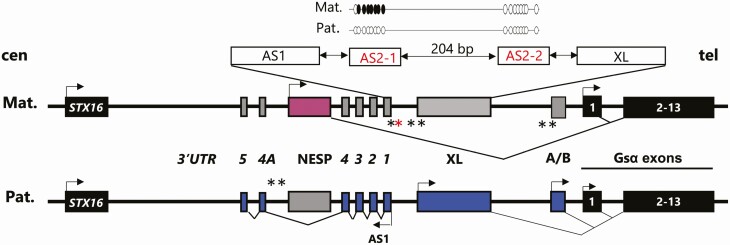
Gsα is encoded by exons 1-13 of GNAS, a complex imprinted locus on chromosome 20q13.3 (6), which gives rise to at least 4 additional transcripts using promoters that undergo parent-specific methylation (Fig. 1). These include NESP (NeuroEndocrine Secretory Protein) that is derived from the nonmethylated maternal allele, as well as XLαs (the eXtra Large variant of Gsα), the A/B transcript (referred to as 1A in rodents), and an antisense transcript of unknown function that are transcribed from the nonmethylated paternal GNAS allele. While Gsα is derived in most tissues from both parental alleles, its expression from the paternal GNAS allele can be reduced or abolished through as yet unknown mechanisms in some tissues such as renal proximal tubules, brown adipose tissue, pituitary, gonads, thyroid, and hypothalamus (7, 8). Loss-of-function mutations involving GNAS exons 1-13 cause iPPSD type 2; the patients’ phenotype differs when the mutation lies on the maternal GNAS allele, namely, PHP1A, or on the paternal GNAS allele, namely, PseudoPHP (9, 10).
Figure 1.
The GNAS locus (not to genomic scale). Mat, maternal chromosome; Pat, paternal chromosome; cen, centromere; tel, telomere; arrow, direction of transcription; pink rectangle, maternal transcript (NESP55); blue rectangles, paternal transcripts (A/B, XL and the GNAS-AS1 antisense); number in italic exons and 3′ untranslated region (UTR) of GNAS-AS1; gray rectangles, transcripts not expressed; black rectangles, transcripts expressed from both alleles; black stars, GNAS DMRs; GNAS A/B:TSS-DMR, GNAS-XL:EX1-DMR; GNAS-AS1:TSS-DMR (chr20:57,425,649-57,428,033; hg19); and GNAS-NESP:TSS-DMR; red star, GNAS-AS2:TSS-DMR (AS2-1: chr20: 57,427,685-57,427,748 and AS2-2: chr20:57,427,933-57,427,996; hg19); broken lines, splicing patterns; STX16 is a gene located approximately 220 kb upstream of the GNAS locus. A deletion of STX16-5-418nt and ST16-6-244nt probes by MS-MPLA is consistent with LOM at GNAS A/B:TSS-DMR for AD-PHP1B patients. A more detailed view of the GNAS-AS2:TSS-DMR: white circles CpG not methylated, black circles CpG methylated.
Methylation defects at 1 or more GNAS promoters cause PHP type 1B (PHP1B/iPPSD3). PTH resistance is the main laboratory manifestation in patients with PHP1B/iPPSD3, but moderate resistance to TSH is also common. Approximately 10% of PHP1B/iPPSD3 patients have clinical signs of AHO such as brachydactyly, but these are usually less severe (11-13). Approximately 10% of PHP1B/iPPSD3 patients are affected by an autosomal dominant form of the disorder (termed AD-PHP1B) that is characterized by loss of methylation (LOM) restricted to the maternal GNAS A/B:TSS-DMR (14, 15). The majority of these patients have a recurrent maternal 3-kb microdeletion within the genomic region of STX16, the gene encoding syntaxin 16, located approximately 220 kb upstream of the GNAS locus (14). Other maternal mutations leading to LOM at GNAS A/B:TSS-DMR alone include additional STX16 deletions (15-17) a large deletion involving exon NESP and the region centromeric thereof (18), several different duplications/triplications of portions of the GNAS locus (19, 20), and a large inversion involving exon A/B and all exons encoding Gsα (21). Furthermore, several different deletions within GNAS have been described that cause AD-PHP1B with LOM at all 3 maternal DMRs due to deletions involving exons NESP and AS3-4, or exons AS3-4 alone (22-24).
However, approximately 90% of all PHP1B/iPPSD3 patients have a sporadic form of the disorder (sporPHP1B) that is characterized by complete LOM at the maternal GNAS A/B:TSS-DMR, often incomplete or no methylation changes at the maternal GNAS-XL:EX1-DMR and GNAS-AS1:TSS-DMR, and increased methylation at the paternal GNAS-NESP:TSS-DMR (25, 26). The mechanism(s) underlying these parent-specific methylation abnormalities for sporPHP1B cases are not known, with the exception of approximately 10% of these patients, who have paternal uniparental isodisomy (or rarely heterodisomy) of chromosome 20q [iUPD(20q)]pat (27-30); DNA from these latter patients show complete LOM at GNAS A/B:TSS-DMR, GNAS-XL:EX1-DMR, and GNAS-AS1:TSS-DMR, and complete gain of methylation (GOM) at GNAS-NESP:TSS-DMR.
Recently, Rochtus et al. identified, through a methylome-wide analysis using the Human Methylation 450K chip, a previously not recognized DMR that is located telomeric of GNAS-AS1:TSS-DMR, named GNAS-AS2:TSS-DMR (31). DNA from unaffected controls showed approximately 30% methylation; due to the 3-kb STX16 deletion, sporPHP1B and AD-PHP1B patients exhibited an almost complete LOM at this DMR. In addition to the methylation differences at the 4 well-established DMRs, the genome-wide methylome analysis provided evidence for DNA methylation abnormalities at other imprinted loci for several sporPHP1B cases and 1 AD-PHP1B patient (31).
We investigated this novel GNAS-AS2:TSS-DMR in several PHP1B/iPPSD3 patients with genetically defined mutations other than the 3-kb STX16 deletion, including a small kindred with AD-PHP1B in which the affected patients show LOM limited to GNAS A/B:TSS-DMR, but no evidence for any of the known mutations associated with this epigenetic abnormality.
Materials and Methods
Patient Samples
In total, 22 patients diagnosed with PHP1B/iPPSD3 and 10 controls were included in this study. The diagnosis of PHP1B/iPPSD3 was based on elevated PTH, with or without low calcium and elevated phosphate levels, and in some cases elevated TSH levels. These biochemical changes were associated with GNAS methylation abnormalities involving the GNAS A/B:TSS-DMR alone or abnormalities at all 4 GNAS DMRs. DNA from 1 [iUPD(20q)]pat and 1 [iUPD(20q)]mat patient were also included.
Informed written consent was obtained from the patients and controls.
DNA Extraction
Genomic DNA (gDNA) was extracted from whole blood using Gentra Kit extraction (QIAGEN, Hilden, Germany) according to the manufacturer’s protocol or by the proteinase K/phenol/chloroform method, as previously described (21, 32).
MLPA amplification of STX16 and GNAS
Multiplex ligation-dependent probe amplification (MLPA) and methylation-sensitive MLPA (MS-MLPA) were performed using the SALSA MLPA kit ME031 GNAS (MRC-Holland, Amsterdam, The Netherlands) following the manufacturer’s instructions (https://www.mlpa.com/). Polymerase chain reaction (PCR) products were examined using the ABI3730xl Genetic Analyzer at the service de Génétique, Pharmacogénétique et Hormonologie of the Bicêtre Hospital.
Search for STX16 Deletion
Long-range PCR and genomic quantitative PCR (qPCR) were performed. qPCR was performed on a light cycler 480 (Roche, Basel, Switzerland) using primers that flank STX16 exon 5 that comprises the several microdeletions involving the STX16 gene (14, 15, 33). The gene encoding albumin (ALB) was used as internal amplification control. The Ct values for amplification of STX16 (CtStx16) were subtracted from the Ct value for ALB (CtAlb); a difference of –1 revealed the presence of a monoallelic deletion of exon 5 of STX16.
GNAS Methylation Analysis
Epigenetic GNAS changes were assessed by MS-MLPA, as described (21). Quantification of methylation at 3 maternal GNAS DMRs (GNAS AB:TSS-DMR, GNAS-AS1:TSS-DMR and GNAS-AS2:TSS-DMR) was performed by pyrosequencing, as described (34). In brief, 600 ng of DNA was bisulfite converted (EZ DNA Methylation-Gold Kit; Zymo Research, CA, USA) according to the manufacturer’s protocol. One microliter of bisulfite-converted DNA was then amplified with specific primers for each DMR. Pyrosequencing reactions were carried out on a PyroMark Q96 ID (QIAGEN, Hilden, Germany) using either 1 sequencing primer for 1 bisulfited PCR product (GNAS AB:TSS-DMR and GNAS-AS1:TSS-DMR) or 2 different sequencing primers for 1 bisulfited PCR product (GNAS-AS2:TSS-DMR). Primers sequences are presented in elsewhere (Table 1 (33)). Peak heights were determined using the provided software (PyroMark Q24 v2.0.6.20). Results are the mean ± SD of methylation measured at each cytosine for each DMR (GNAS AB:TSS-DMR and GNAS-AS1:TSS-DMR: 6 cytosines; GNAS-AS2:TSS-DMR: 16 cytosines). Two DNAs from healthy controls were analyzed in parallel during each run and served as internal standards to ensure reproducibility; these included unmethylated DNA (whole-genome amplified control DNA generated using the REPLI-g Mini Kit; QIAGEN, Hilden, Germany) and fully methylated DNA (unmethylated DNA that had been treated with SSI DNA methyltransferase; New England Biolabs, Beverly, MA).
Allelic Cloning of GNAS-AS2:TSS-DMR
DNA was bisulfite converted as previously described (34). One microliter of bisulfite-converted DNA was then amplified using GNAS-AS2:TSS-DMR primers (Table 1 (33)). PCR products were checked on QIAxcel advanced system (Qiagen, Hilden, Germany) for quality control and absence of primers dimers before ligation into the TOPO® TA Cloning® vector and subsequent transformation of competent bacteria (E.coli and TOPO® TA Cloning® Kit, Life Technologies, Carlsbad, CA) according to the manufacturer’s protocol. Ten white colonies were picked and cultured overnight at 37°C in Luria-Bertani (LB) containing 100 μg/μL ampicillin for extraction of plasmid DNA. Inserts were PCR-amplified using a vector-specific primer and a biotinylated primer that hybridizes to the GNAS-AS2:TSS-DMR (AS2-biotR). Pyrosequencing reactions were carried out on a PyroMark Q96 ID (Qiagen Hilden, Germany) as previously described using GNAS-AS2-seq1F and GNAS-AS2-seq2F as sequencing primers (34).
Analysis of Microsatellite Markers
Microsatellite markers (listed centromeric to telomeric) D20S100, DS20S86, 907-rep2, 261P9-CA, 806M20-CA, 543J19-TTA, D20S171, D20S196, D20S173, and D20S93 were analyzed at the Center for Human Genetic Research of the Massachusetts General Hospital, as described (24).
Whole Genome Sequencing
Whole genome sequencing was performed at the Broad Institute, Cambridge, MA, as described (21). DNA sequences were analyzed in IGV (https://software.broadinstitute.org/software/igv/).
Statistics
Methylation indices (MIs) at each DMR and dispersion of MI were compared by 1-way analysis of variance followed by Dunnett’s multiple comparison test. P < .05 was considered statistically significant. To calculate the dispersion of MI, the mean of DMR MI for each group was calculated and the deviation of each sample from the mean was calculated. Statistical analyses were performed using Prism 6.0 software.
Results
Patient Characteristics
In order to better understand the elements that control methylation at the different GNAS promoters and expression of transcripts derived from this locus, we analyzed the affected members of an AD-PHP1B family (family 208) who presented with PTH-resistant hypocalcemia and LOM at GNAS A/B:TSS-DMR; we excluded the known genetic defects in affected individuals of this family (referred to as AD–) (Fig. 2). We furthermore investigated genomic DNA from several patients with PTH-resistance due to LOM restricted to the GNAS A/B:TSS-DMR that is caused by 3-kb STX16 deletion (referred to as AD+; n = 9), 1 patient with the previously reported large inversion of exon A/B and all exons encoding Gsα (21), sporPHP1B cases (n = 10) (35), and patients affected by [iUPD(20q)]pat and [iUPD(20q)]mat, as well as unaffected controls (n = 9); the biochemical findings for the 21 PHP1B patients are provided elsewhere (Table 2 (33)).
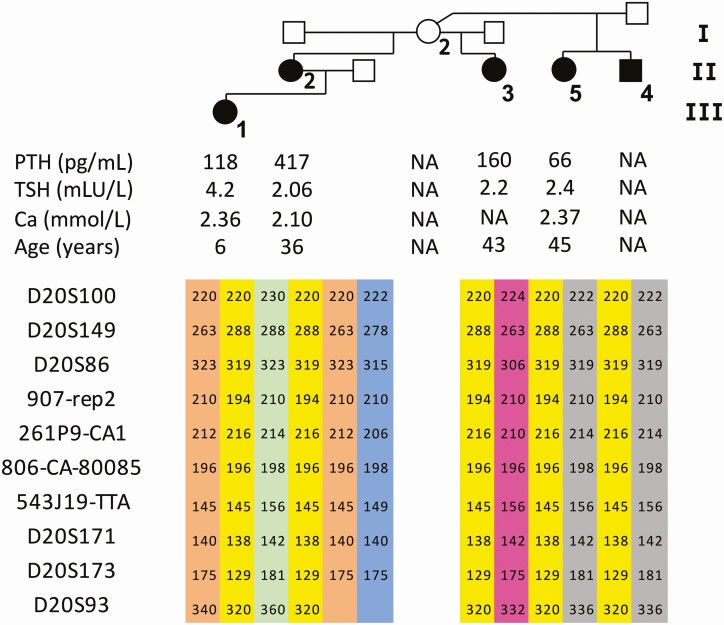
Figure 2.
Pedigree, laboratory results at the time of establishing the diagnosis and microsatellite markers of the iPPSD3/AD-PHP1B family (AD–), in which the recurrent 3-kb deletion of STX16 was excluded.
The unaffected female 208/I-2 has 4 affected children with 3 different partners, her son 208/II-4 and her 3 daughters: 208/II-2, 208/II-3, and 208/II-5; the female patient 208/II-2 has an affected daughter (208/III-1). Patient 208/II-2 (index case) presented with symptoms of hypocalcemia during her adolescence, yet the diagnosis of AD-PHP1B was not made until the age of 25 years when she came to medical attention due to cramps and weariness, after her daughter 208/III-1 was born. She had low blood calcium levels and elevated PTH and phosphate levels, as well as an elevated TSH level and reduced urinary calcium excretion (Fig. 2). The affected patients 208/II-3 and 208/II-4 were diagnosed in early adulthood because of an elevated PTH; they presented with only minor symptoms. Analysis of the 4 GNAS DMRs by pyrosequencing for 3 patients (208/II-2, 208/II-3, and 208/III-1) showed a reduction of methylation at GNAS A/B:TSS-DMR to 10.4 ± 8.4% (Fig. 3A), but no evidence for abnormal methylation at GNAS-XL:EX1-DMR, GNAS-AS1:TSS-DMR, and GNAS-NESP:TSS-DMR (data not shown). Haplotype analysis of the affected individuals using several microsatellite markers revealed that all 5 patients share the same allele at the GNAS locus; each color represent a different haplotype (Fig. 2).
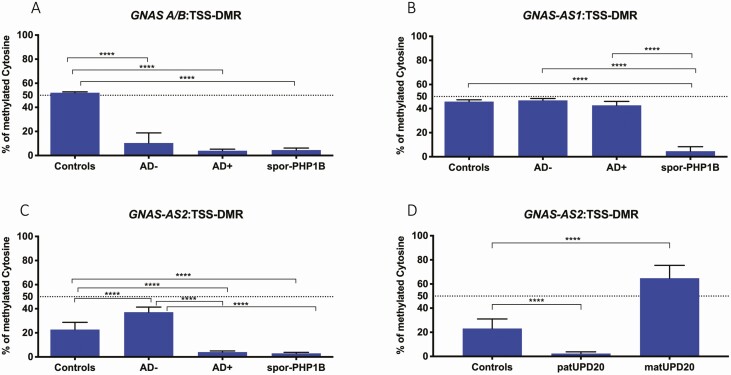
Figure 3.
Average methylation indexes of GNAS-DMRs. (A) GNAS AB:TSS-DMR, (B) GNAS-AS1:TSS-DMR, (C) GNAS-AS2:TSS-DMR, and (D) GNAS-AS2:TSS-DMR: [iUPD(20q)]pat and [iUPD(20q)]mat patients. AD–, PHP1B patients without STX16 deletion (pyrosequencing for 3 patients [208/II-2, 208/II-3, and 208/III-1]); AD+, PHP1B patients with STX16 deletion (n = 9), sporPHP1B (n = 10), controls (n = 10), patUPD20 (n = 1), matUPD20 (n = 1).
Analysis of Methylation at GNAS-AB:TSS-DMR, GNAS-AS1:TSS-DMR, and GNAS-AS2:TSS-DMR by Pyrosequencing for Different PHP1B Variants
We initially characterized the GNAS DMRs using genomic DNA extracted from peripheral blood lymphocytes of patients affected by sporPHP1B, AD+, or AD–, and from 10 healthy controls. Compared with DNA from healthy individuals, who showed 52 ± 0.8% methylation at the GNAS A/B:TSS-DMR, AD+ and sporPHP1B patients showed only 4 ± 1.3% and 4.5 ± 1.7% methylation, respectively (Fig. 3A).
SporPHP1B patients revealed a reduction in methylation at the GNAS AS1:TSS-DMR to 4.6 ± 3.8%, while AD– and AD+ patients showed methylation at this DMR that was indistinguishable from controls (Fig. 3B). At the GNAS AS2:TSS-DMR, DNA from healthy controls showed 22.7 ± 6% methylation, while DNA from patients affected by AD+ and sporPHP1B revealed a reduction at this DMR to 4.0 ± 0.9% and to 2.9 ± 0.8%. Surprisingly, GNAS AS2:TSS-DMR methylation was increased to 37.1 ± 4.2% for the 3 AD– patients (Fig. 3C).
At the GNAS AS2:TSS-DMR, DNA from a patient with [iUPD(20q)]pat showed a reduction to 2.3 ± 1.3%, while DNA from a patient with [iUPD(20q)]mat revealed an increase to 64.7 ± 10% (Fig. 3D). Methylation indexes for the GNAS-XL:EX1-DMR and for the GNAS-NESP:TSS-DMR were similar to the results obtained with DNA from healthy controls and from AD + and AD– patients (data not shown).
Allelic Methylation at the GNAS-AS2:TSS-DMR in Patients With Maternal or Paternal UPD20q
Compared with controls, DNA from fibroblasts of a [iUPD(20q)]pat patient exhibited a complete LOM at GNAS-AS2:TSS-DMR while a [iUPD(20q)]mat patient showed a gain of methylation at GNAS-AS2:TSS-DMR (Fig. 3D), which is consistent with methylation of the 2 maternal alleles.
No Deletions in the STX16/GNAS Region in AD– Patients of Family 208
Genomic DNA analysis of AD– patients by MLPA revealed no evidence for a deletion within GNAS and STX16 by MS-MLPA (Figure 1 (33)). Analysis of DNA from AD– patients by long-range PCR to cover STX16 exons 3-6 confirmed absence of a STX16 deletion (data not shown). Furthermore, whole genome sequence analysis of patients 208/II-3 and 208/III-1 revealed no evidence for a deletion, duplication, or inversion within the STX16-GNAS region.
Further Molecular Characterization of the GNAS-AS2:TSS-DMR
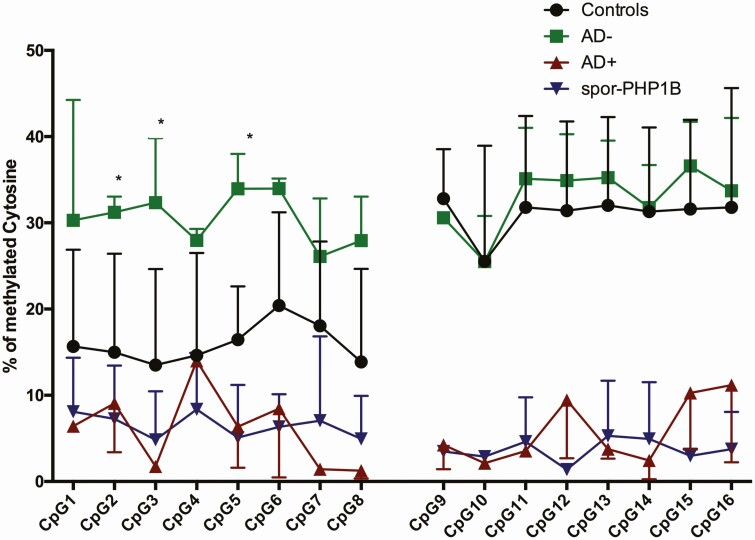
We selected 3 AD– patients, 2 AD+ patients, 3 sporPHP1B patients, and 3 controls to further characterize the GNAS-AS2:TSS-DMR by cloning PCR products amplified from bisulfite-treated genomic DNA for analysis of 2 CG-rich subdomains of the GNAS AS2:TSS-DMR, AS2-1 and AS2-2, that are separated by 184 bp and contain 8 CpGs each (Fig. 4 and Figure 2 (33)).
Figure 4.
GNAS-AS2:TSS-DMR methylation indexes determined by pyrosequencing after subcloning. black circles, controls; green squares, AD– patients; red triangles, AD+ patients; and blue triangles, sporPHP1B patients.
DNA from healthy controls revealed 13.5% to 20.4% methylation for AS2-1 and 25.5% to 32.8% methylation for AS2-2. AD+ and sporPHP1B patients showed much reduced methylation at both subdomains (AD+: 1.2–14.0% for AS2-1 and 2.1-11.1% for AS2-2; sporPHP1B: 4.8-8.3% for AS2-1 and 1.3-5.3% for AS2-2). In contrast, AD– patients revealed increased methylation at the first subdomain of GNAS-AS2:TSS-DMR: 26.1% to 34.0% methylation, but not at AS2-2: 25.5% to 36.5% (Fig. 4).
In addition, cloning of PCR amplicons allowed us to determine 3 distinct methylation profiles for DNA from healthy controls and from iPPSD3/PHP1B patients with defined mutations (Figure 2 (33)). Healthy controls showed 16.0 ± 2.3% methylation at AS2-1 and 31.0 ± 2.2% methylation at AS2-2; AD+ and sporPHP1B patients had 6.0 ± 4.4%, and 6.5 ± 1.4% at AS2-1 and 5.8 ± 3.7% and 3.6 ± 1.2% at AS2-2. In contrast, AD– patients showed 4 distinct methylation profiles with methylation of 30.4 ± 2.9% at AS2-1 and 32.9 ± 3.5% at AS2-2.
Discussion
The notion that most patients affected by AD+ show loss of methylation restricted to GNAS A/B:TSS-DMR and that only sporPHP1B patients have broader methylation defects has been challenged by the discovery of the GNAS-AS2:TSS-DMR. In fact, as for the GNAS A/B:TSS-DMR, the GNAS-AS2:TSS-DMR shows reduced methylation when using genomic DNA from AD+ patients (31), which is indistinguishable from the epigenetic changes observed in patients affected by sporPHP1B and patUPD20q. Furthermore, an increase in methylation at this novel GNAS-AS2:TSS-DMR was observed when analyzing DNA from an affected member of a family with AD-PHP1B, for whom LOM at GNAS A/B:TSS-DMR is caused by a large inversion involving exon A/B as well as all exons encoding Gsα (21). In addition, we have now shown for a patient affected by matUPD20q that methylation at this DMR occurs on the maternal allele and that it is almost 3 times higher than in controls, but does not approach complete methylation as observed for the other maternal DMRs. In contrast to the concordant findings at GNAS-AS2:TSS-DMR for sporPHP1B and AD+ patients, methylation at GNAS-AS1:TSS-DMR was reduced only in sporPHP1B indicating that different mechanisms contribute to remethylation at the 2 GNAS-AS2 subdomains that presumably occurs during oogenesis (36). It remains unknown, however, whether a shared regulatory genomic DNA element controls methylation of GNAS A/B:TSS-DMR and GNAS-AS2:TSS-DMR in AD+ patients.
We have now characterized both GNAS-AS2 subdomains in an AD-PHP1B family (AD–), in which affected individuals revealed none of the known mutations that result in LOM at GNAS A/B:TSS-DMR alone. These patients showed laboratory abnormalities that are indistinguishable from those observed in other familial or sporPHP1B cases. The analysis of several microsatellite markers for the GNAS region indicated that the 5 affected individuals all share the same allele. Thus, although the family is not large enough to conclusively link their AD-PHP1B to chr.20q13.3, the GNAS methylation studies as well as the microsatellite data are consistent with the conclusion that the disease-causing genetic defect is located in that region of the genome. Analysis of methylation at the GNAS-AS1:TSS-DMR did not differ from healthy controls for patients affected by AD– and AD+. In contrast, the GNAS-AS2:TSS-DMR showed for affected individuals of our AD– family almost twice the methylation observed in controls, but less than that detected in the patient with matUPD20q, which is distinctly different from the findings in controls and patients affected by AD+ and sporPHP1B. The increase in GNAS-AS2:TSS-DMR methylation most likely occurred on the maternal allele because patients 208/II-2 and 208/III-1 revealed equivalent changes. As for the matUPD20q patient, the gain in methylation was incomplete. It could indicate that a methyltransferase or a connecting protein does not fully interact with that portion of the GNAS locus thus preventing a more complete epigenetic change at the maternal GNAS-AS2:TSS-DMR.
In conclusion, we characterized the novel DMR GNAS-AS2:TSS-DMR through the analysis of DNA from different, genetically defined patients and discovered a previously not described AD-PHP1B family, in which the affected members show LOM at GNAS A/B:TSS-DMR alone, yet GOM at the novel GNAS-AS2:TSS-DMR. The identified distinct epigenetic findings will help characterize further the different PHP1B variants and will help guide the search for underlying genetic defects, which may provide novel insights into the mechanisms underlying GNAS methylation.
Acknowledgments
Financial Support: This work was supported by the AP-HP recurrent funds from the rare disease plan, a grant from the French Society of Pediatric Endocrinology and Diabetology (SFEDP) (to P.H.), a 2019 fellowship grant from ESPE (to P.H.), a Sandoz grant (to A.L.), a grant from the French patient’s advocacy group (to P.H.), the 2019 research unit grant from ESPE (to A.L.), and NIH grant DK046718 (to H.J.).
Glossary
Abbreviations
- AD
autosomal dominant
- AHO
Albright hereditary osteodystrophy
- iPPSD
inactivating PTH/PTHrP signaling disorder
- LOM
loss of methylation
- MI
methylation index
- MLPA
multiplex ligation-dependent probe amplification
- MS
methylation sensitive
- NESP
NeuroEndocrine Secretory Protein
- PHP
pseudohypoparathyroidism
- PHP1B
pseudohypoparathyroidism type 1B
- PTH
parathyroid hormone
- TSH
thyroid-stimulating hormone
- TSS-DMR
transcription start site-differentially methylated region
Additional Information
Disclosures: The authors have nothing to disclose in relation to the study.
Data Availability
Some or all data generated or analyzed during this study are included in this published article or in the data repositories listed in References.
References
- 1. Thiele S, Mantovani G, Barlier A, et al. From Pseudohypoparathyroidism to inactivating PTH/PTHrP Signalling Disorder (iPPSD), a novel classification proposed by the European EuroPHP network. Eur J Endocrinol. 2016;175(6):P1-P17. 10.1530/EJE-16-0107 [DOI] [PubMed] [Google Scholar]
- 2. Mantovani G. Clinical review: pseudohypoparathyroidism: diagnosis and treatment. J Clin Endocrinol Metab. 2011;96(10):3020-3030. [DOI] [PubMed] [Google Scholar]
- 3. Turan S, Bastepe M. The GNAS complex locus and human diseases associated with loss-of-function mutations or epimutations within this imprinted gene. Horm Res Paediatr. 2013;80(4):229-241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Weinstein LS, Yu S, Warner DR, Liu J. Endocrine manifestations of stimulatory G protein alpha-subunit mutations and the role of genomic imprinting. Endocr Rev. 2001;22(5):675-705. [DOI] [PubMed] [Google Scholar]
- 5. Bastepe M, Jüppner H. Pseudohypoparathyroidism. New insights into an old disease. Endocrinol Metab Clin North Am. 2000;29(3):569-589. [DOI] [PubMed] [Google Scholar]
- 6. Kozasa T, Itoh H, Tsukamoto T, Kaziro Y. Isolation and characterization of the human Gs alpha gene. Proc Natl Acad Sci U S A. 1988;85(7):2081-2085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Hayward BE, Barlier A, Korbonits M, et al. Imprinting of the G(s)alpha gene GNAS1 in the pathogenesis of acromegaly. J Clin Invest. 2001;107(6):R31-R36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Yu S, Yu D, Lee E, et al. Variable and tissue-specific hormone resistance in heterotrimeric Gs protein alpha-subunit (Gsalpha) knockout mice is due to tissue-specific imprinting of the gsalpha gene. Proc Natl Acad Sci U S A. 1998;95(15):8715-8720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Shore EM, Ahn J, Jan de Beur S, et al. Paternally inherited inactivating mutations of the GNAS1 gene in progressive osseous heteroplasia. N Engl J Med. 2002;346(2):99-106. [DOI] [PubMed] [Google Scholar]
- 10. Weinstein LS, Gejman PV, Friedman E, et al. Mutations of the Gs alpha-subunit gene in Albright hereditary osteodystrophy detected by denaturing gradient gel electrophoresis. Proc Natl Acad Sci U S A. 1990;87(21):8287-8290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Mantovani G, Ferrante E, Giavoli C, et al. Recombinant human GH replacement therapy in children with pseudohypoparathyroidism type Ia: first study on the effect on growth. J Clin Endocrinol Metab. 2010;95(11):5011-5017. [DOI] [PubMed] [Google Scholar]
- 12. Maupetit-Méhouas S, Mariot V, Reynès C, et al. Quantification of the methylation at the GNAS locus identifies subtypes of sporadic pseudohypoparathyroidism type Ib. J Med Genet. 2011;48(1):55-63. [DOI] [PubMed] [Google Scholar]
- 13. de Nanclares GP, Fernández-Rebollo E, Santin I, et al. Epigenetic defects of GNAS in patients with pseudohypoparathyroidism and mild features of Albright’s hereditary osteodystrophy. J Clin Endocrinol Metab. 2007;92(6):2370-2373. [DOI] [PubMed] [Google Scholar]
- 14. Bastepe M, Fröhlich LF, Hendy GN, et al. Autosomal dominant pseudohypoparathyroidism type Ib is associated with a heterozygous microdeletion that likely disrupts a putative imprinting control element of GNAS. J Clin Invest. 2003;112(8):1255-1263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Linglart A, Gensure RC, Olney RC, Jüppner H, Bastepe M. A novel STX16 deletion in autosomal dominant pseudohypoparathyroidism type Ib redefines the boundaries of a cis-acting imprinting control element of GNAS. Am J Hum Genet. 2005;76(5):804-814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Mantovani G, Bondioni S, Linglart A, et al. Genetic analysis and evaluation of resistance to thyrotropin and growth hormone-releasing hormone in pseudohypoparathyroidism type Ib. J Clin Endocrinol Metab. 2007;92(9):3738-3742. [DOI] [PubMed] [Google Scholar]
- 17. Yang Y, Chu X, Nie M, et al. A novel long-range deletion spanning STX16 and NPEPL1 causing imprinting defects of the GNAS locus discovered in a patient with autosomal-dominant pseudohypoparathyroidism type 1B. Endocrine. 2020;69(1):212-219. [DOI] [PubMed] [Google Scholar]
- 18. Richard N, Abeguilé G, Coudray N, et al. A new deletion ablating NESP55 causes loss of maternal imprint of A/B GNAS and autosomal dominant pseudohypoparathyroidism type Ib. J Clin Endocrinol Metab. 2012;97(5):E863-E867. [DOI] [PubMed] [Google Scholar]
- 19. Perez-Nanclares G, Velayos T, Vela A, Muñoz-Torres M, Castaño L. Pseudohypoparathyroidism type Ib associated with novel duplications in the GNAS locus. PLoS One. 2015;10(2):e0117691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Nakamura A, Hamaguchi E, Horikawa R, et al. complex genomic rearrangement within the GNAS region associated with familial pseudohypoparathyroidism type 1b. J Clin Endocrinol Metab. 2016;101(7):2623-2627. [DOI] [PubMed] [Google Scholar]
- 21. Grigelioniene G, Nevalainen PI, Reyes M, et al. A large inversion involving GNAS exon A/B and all exons encoding Gsα is associated with autosomal dominant pseudohypoparathyroidism type Ib (PHP1B). J Bone Miner Res Off J Am Soc Bone Miner Res. 2017;32(4):776-783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Bastepe M, Fröhlich LF, Linglart A, et al. Deletion of the NESP55 differentially methylated region causes loss of maternal GNAS imprints and pseudohypoparathyroidism type Ib. Nat Genet. 2005;37(1):25-27. [DOI] [PubMed] [Google Scholar]
- 23. Chillambhi S, Turan S, Hwang DY, Chen HC, Jüppner H, Bastepe M. Deletion of the noncoding GNAS antisense transcript causes pseudohypoparathyroidism type Ib and biparental defects of GNAS methylation in cis. J Clin Endocrinol Metab. 2010;95(8):3993-4002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Takatani R, Molinaro A, Grigelioniene G, et al. Analysis of multiple families with single individuals affected by pseudohypoparathyroidism type Ib (PHP1B) reveals only one novel maternally inherited GNAS deletion. J Bone Miner Res. 2016;31(4):796-805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Elli FM, Linglart A, Garin I, et al. The prevalence of GNAS deficiency-related diseases in a large cohort of patients characterized by the EuroPHP network. J Clin Endocrinol Metab. 2016;101(10):3657-3668. [DOI] [PubMed] [Google Scholar]
- 26. Takatani R, Molinaro A, Grigelioniene G, et al. Analysis of multiple families with single individuals affected by pseudohypoparathyroidism type Ib (PHP1B) reveals only one novel maternally inherited GNAS deletion. J Bone Miner Res. 2016;31(4):796-805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Bastepe M, Lane AH, Jüppner H. Paternal uniparental isodisomy of chromosome 20q–and the resulting changes in GNAS1 methylation–as a plausible cause of pseudohypoparathyroidism. Am J Hum Genet. 2001;68(5):1283-1289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Dixit A, Chandler KE, Lever M, et al. Pseudohypoparathyroidism type 1b due to paternal uniparental disomy of chromosome 20q. J Clin Endocrinol Metab. 2013;98(1):E103-E108. [DOI] [PubMed] [Google Scholar]
- 29. Fernández-Rebollo E, Lecumberri B, Garin I, et al. ; Spanish PHP Group . New mechanisms involved in paternal 20q disomy associated with pseudohypoparathyroidism. Eur J Endocrinol. 2010;163(6):953-962. [DOI] [PubMed] [Google Scholar]
- 30. Takatani R, Minagawa M, Molinaro A, et al. Similar frequency of paternal uniparental disomy involving chromosome 20q (patUPD20q) in Japanese and Caucasian patients affected by sporadic pseudohypoparathyroidism type Ib (sporPHP1B). Bone. 2015;79:15-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Rochtus A, Martin-Trujillo A, Izzi B, et al. Genome-wide DNA methylation analysis of pseudohypoparathyroidism patients with GNAS imprinting defects. Clin Epigenetics. 2016;8:10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Grybek V, Aubry L, Maupetit-Méhouas S, et al. Methylation and transcripts expression at the imprinted GNAS locus in human embryonic and induced pluripotent stem cells and their derivatives. Stem Cell Reports. 2014;3(3):432-443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Hanna P, Francou B, Delemer B, et al. GNAS-AS2 supplemental materials. figshare. Figure. Deposited December 10, 2020. 10.6084/m9.figshare.13363097.v1 [DOI] [Google Scholar]
- 34. Maupetit-Méhouas S, Azzi S, Steunou V, et al. Simultaneous hyper- and hypomethylation at imprinted loci in a subset of patients with GNAS epimutations underlies a complex and different mechanism of multilocus methylation defect in pseudohypoparathyroidism type 1b. Hum Mutat. 2013;34(8):1172-1180. [DOI] [PubMed] [Google Scholar]
- 35. Hanna P, Grybek V, de Nanclares GP, et al. Genetic and epigenetic defects at the GNAS locus lead to distinct patterns of skeletal growth but similar early-onset obesity. J Bone Miner Res Off J Am Soc Bone Miner Res. 2018;33(8):1480-1488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Smallwood SA, Kelsey G. De novo DNA methylation: a germ cell perspective. Trends Genet. 2012;28(1):33-42. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Some or all data generated or analyzed during this study are included in this published article or in the data repositories listed in References.