Abstract
Context
Observational studies suggest that low vitamin D status may be a risk factor for cancer.
Objective
In a population with prediabetes and overweight/obesity that is at higher risk of cancer than the general population, we sought to determine if vitamin D supplementation lowers the risk of cancer and precancers.
Methods
The Vitamin D and type 2 diabetes (D2d) cancer outcomes study (D2dCA) is an ancillary study to the D2d study, which was conducted at 22 academic medical centers in the United States. Participants had prediabetes and overweight/obesity and were free of cancer for the previous 5 years. Participants were randomized to receive vitamin D3 4000 IU daily or placebo. At scheduled study visits (4 times/year), cancer and precancer events were identified by questionnaires. Clinical data were collected and adjudicated for all reported events. Cox proportional hazard models compared the hazard ratio (HR) of incident cancers and precancers between groups.
Results
Over a median follow-up period of 2.9 years, among 2385 participants (mean age 60 years and 25-hydroxyvitamin D 28 ng/mL), there were 89 cases of cancer. The HR of incident cancer for vitamin D vs placebo was 1.07 (95% CI 0.70, 1.62). Of 241 participants with incident precancers, 239 had colorectal adenomatous polyps. The HR for colorectal polyps for vitamin D vs placebo was 0.83 (95% CI 0.64, 1.07).
Conclusion
In the D2d population of participants with prediabetes and overweight/obesity, not selected for vitamin D insufficiency, vitamin D supplementation did not have a significant effect on risk of incident cancer or colorectal polyps.
Keywords: vitamin D, cancer, clinical trial, prediabetes, colorectal polyps
Evidence from different study designs, including basic science experiments, epidemiologic studies, and clinical trials, lends credence to the potential benefit of vitamin D supplementation on overall cancer risk, although results have been inconsistent (1-7). Large-scale clinical trials recently completed have tested the hypothesis that vitamin D supplementation lowers risk of cancer. One US-based trial of postmenopausal women reported a nearly statistically significant lower risk of cancer among women randomized to a vitamin D/calcium combination (hazard ratio [HR] 0.70; 95% CI 0.47, 1.02) compared with placebo (6). Other studies, including a post hoc analysis of the New Zealand Vitamin D Assessment (ViDA) study (7) and the US-based Vitamin D and Omega-3 Trial (VITAL) trial, reported no significant effect of vitamin D supplementation on cancer risk (8).
Certain health conditions can increase risk of cancer, and people with these health conditions would be particularly suited for testing interventions for cancer prevention, such as vitamin D supplementation. The conditions of overweight/obesity, prediabetes, and diabetes, which are all associated with insulin resistance/hyperinsulinemia, hyperglycemia, and inflammation, are linked to higher cancer risk than the general population. Many biological mechanisms have been proposed to explain these associations (9-11). There are no data on whether vitamin D supplementation reduces cancer risk in such higher-risk populations.
The Vitamin D and type 2 diabetes (D2d) study is a randomized clinical trial of US adults with prediabetes and overweight/obesity, designed to test the effect of vitamin D3 supplementation versus placebo on diabetes risk (12, 13). The D2d cancer outcomes ancillary study (D2dCA) tested for an effect of vitamin D3 supplementation vs placebo on risk of cancer and precancers in D2d participants, who are at higher risk for developing these conditions than the general population.
Methods
Overview of Trial Design and Oversight
D2d is a randomized, double-blind, placebo-controlled, multisite clinical trial designed to evaluate the safety and efficacy of vitamin D supplementation for diabetes prevention in adults with prediabetes (clinicaltrials.gov NCT01942694). The methods of D2d and the results of the primary outcome have been reported (12, 13). The institutional review board at each clinical site approved the protocol, and all participants provided written informed consent. A sponsor-appointed data and safety monitoring board approved the protocol and provided independent monitoring. A single interim analysis was conducted for the primary outcome of diabetes, but no interim analysis was planned for or conducted for the outcome of cancer.
D2dCA is a prespecified ancillary study of D2d to test the hypothesis that, compared to placebo, high-dose daily oral vitamin D supplementation reduces risk of incident cancer and precancerous lesions in a cohort of overweight/obese adults with prediabetes. Methods for D2dCA have been previously described (14).
Participants
Participants had to meet at least 2 of 3 glycemic criteria for prediabetes as defined by the 2010 American Diabetes Association (ADA) guidelines, without exceeding any (12, 13). The other inclusion criteria were age ≥30 years (≥25 years for American Indians, Alaska Natives, Native Hawaiians, and other Pacific Islanders) and body mass index (BMI) of 24 to 42 kg/m2 (22.5-42 kg/m2 for Asians). Key exclusion criteria included hyperparathyroidism, nephrolithiasis, use of supplements containing vitamin D or calcium over study limitation (1000 IU/day or 600 mg/day respectively), use of medications or conditions that could interfere with absorption or metabolism of vitamin D, and history of cancer within the past 5 years. History of precancers was not ascertained. Blood 25-hydroxyvitamin D [25(OH)D] concentration was not an inclusion criterion. The recruitment process has been described previously (12, 15).
Procedures
Participants were randomized to take a once-daily soft-gel that contained either 4000 IU of vitamin D3 (cholecalciferol) or matching placebo, with stratification by site, BMI (<30 or ≥30 kg/m2), and race (white or nonwhite). To maximize the study’s ability to observe a treatment effect, participants were asked to limit the use of outside-of-study vitamin D to 1000 IU per day from all supplements, including multivitamins. To optimize safety, participants were asked to limit calcium supplements to 600 mg per day (12).
Participants had study visits at month 3, month 6, and twice per year thereafter. Midway between each study visit, beginning at month 9, an interim encounter (by phone or email) took place (12, 14). At scheduled study encounters (4 times/year), cancer and precancer outcomes were assessed by asking the participant specific questions related to cancer screening (ie, endoscopic examinations and prostate, breast, or skin biopsies) and any diagnoses of cancer or precancer that had occurred during that time period. For all reported events (cancer diagnoses, possible cancerous or precancerous lesions, all endoscopic colorectal examinations, and prostate, breast, and skin biopsies), the site collected supporting clinical documentation related to the event (pathology and histology reports, radiology reports, laboratory results, etc); the site submitted the de-identified records to the Coordinating Center; and the Coordinating Center forwarded event files to the Cancer Clinical Events adjudicator, a board-certified oncologist unaware of treatment assignment, for adjudication. To ensure that all cancers were captured, the Coordinating Center reviewed all adverse events reported by participants for any events that were reported as cancer or that may “harbor cancer” (eg, pancreatic mass) and asked sites to submit such events and supporting documentation for adjudication (14).
Outcome
The cancer outcome was defined as first diagnosis during D2d (ie, since randomization) of any type of invasive cancer (excluding nonmelanoma skin cancers). In analyses, each participant with incident cancer was counted only once. Because history of cancer within the past 5 years was a study exclusion criterion, a diagnosis of cancer was considered “new” unless the adjudicator had sufficient or unequivocal evidence to consider otherwise. Clinical data on deaths were obtained from medical records and death certificates, and cause of death was determined by the Cancer Clinical Events adjudicator. If a participant did not return for a visit during the closeout period, site coordinators were instructed to determine the vital status of the participant through different methods (eg, next of kin contact, local electronic health record, obituary records).
The precancer outcome was defined as first diagnosis during D2d (ie, since randomization) for each type of breast, prostate, or colorectal precancerous lesion. For colorectal adenomatous polyps, the indication for the colonoscopy (screening, diagnostic, surveillance, undetermined) was adjudicated and recorded. Given the predominance of colorectal precancerous lesions, our analyses for precancers included the outcome of colorectal adenomatous polyps only.
Information on potential confounders or effect modifiers, including demographics, family history of cancer, use of aspirin, physical activity, smoking history, and dietary supplement intake, was collected by report from participants, and anthropometric measurements were taken in-person at study visits (14). Stored serum samples from the baseline, month 12, 24, 36, and 48 visits were used to measure 25(OH)D by liquid chromatography–tandem mass spectrometry with calibrators that are traceable to NIST (from the Bureau of Standards), validated by the quarterly proficiency testing program administered by the Vitamin D External Quality Assessment Scheme (DEQAS, United Kingdom) (16, 17).
Sample Size Calculations
D2d was conducted as an event-driven trial for the primary outcome of diabetes. End-of-study procedures were initiated when the minimum number of required diabetes events was reached. Power for D2dCA was estimated a priori based on potential effect of vitamin D supplementation on cancer risk and is described in the methods paper (14).
Based on guidance from the CONSORT statement, a post hoc power calculation is not presented (18).
Statistical Analyses
Descriptive data included means and standard deviations, medians and interquartile ranges, or percentages. Intention-to-treat analyses compared groups defined by the randomization procedure and included all participants and events observed during the study, irrespective of adherence to assigned treatment. Follow-up time was calculated as time from randomization until the occurrence of cancer or precancer, death free of cancer, withdrawal or last follow-up encounter, or determination of vital status if the last scheduled encounter was missed.
All analyses described below were prespecified, except for those described as exploratory, which were decided post hoc. Cox proportional hazard models were used to compare the hazard rates of incident cancer and precancer between vitamin D and placebo groups (19). Each model included randomization assignment as its main predictor variable. The stratification variables (trial site, BMI, and race) were also included in the baseline hazard function. The P value from the primary analysis was based on the chi-square statistic from a likelihood ratio test obtained from proportional hazards models with and without the term for intervention arm. All randomized participants of D2dCA were included in the analysis. Two analyses for incident cancer were conducted: one analysis includes the entire cohort; another analysis was conducted within each sex (men, women), as cancers and the effect of vitamin D on cancer risk may differ between sexes (20, 21).
Sensitivity analyses were conducted after excluding cancers that were diagnosed within the first 6 months or within the first 12 months (exploratory) since randomization, as these cancers may have been preexisting and previously unrecognized. There is some evidence that vitamin D supplementation may increase the risk of prostate cancer (22, 23); therefore, a sensitivity analysis in the entire cohort was done where the primary outcome was defined as above (any type of cancer, excluding nonmelanoma skin cancers) but excluding cases of prostate cancer.
Additionally, we conducted per protocol analyses for both the outcomes of cancer and precancer that censored follow-up at the time of permanent study pill discontinuation for any reason or the first time a participant started taking out-of-trial vitamin D supplements above the trial limit of 1000 IU per day.
We compared key clinical characteristics between the subgroup of participants who had a colonoscopy and the entire D2dCA. In exploratory analyses, we used Fisher’s Exact test to compare the proportion of participants in each treatment group who had a colonoscopy (0, 1, ≥2 colonoscopies during the study); and we compared the proportions of participants who had colonoscopies for different indications. In an exploratory sensitivity analysis, we evaluated the outcome of colorectal polyps among participants who had a screening colonoscopy to minimize any potential bias from having a history of colorectal polyps.
Two-sided P values less than 0.05 were considered statistically significant. Statistical analyses were conducted using SAS software, Version 9.4 (SAS Institute Inc., Cary, NC).
Subgroup Analyses
Variability of response to vitamin D supplementation was assessed in subgroups defined by key baseline variables as described previously (14). No adjustments were made for multiple comparisons; therefore, only point estimates and 95% CIs are presented without P values.
Results
Of the 2423 participants in the D2d study, 2385 consented to the D2dCA study and were included in the intention-to-treat analyses for the cancer/precancer outcomes, with 1194 randomized to vitamin D and 1191 randomized to placebo (Fig. 1). The mean age of participants was 60 years (SD 10), mean BMI was 32 kg/m2 (SD 5), and mean serum 25(OH)D was 28.0 ng/mL (SD 10.1); 44.5% of participants were women and 25.2% were Black or African American (Table 1). Study medication was well-tolerated with few adverse events overall and with no significant differences between groups in the protocol-specified adverse events, including hypercalcemia and nephrolithiasis (13). Safety results will be presented in detail elsewhere (manuscript in preparation).
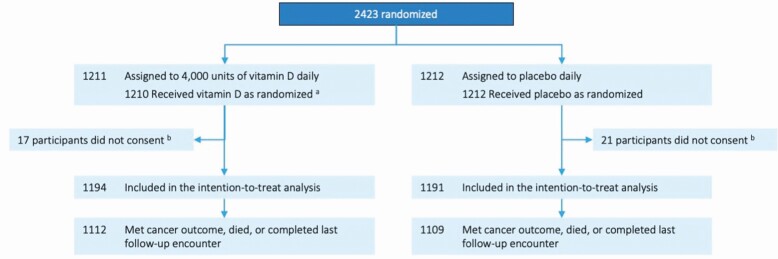
Figure 1.
Flow of participants through the D2dCA study. a One participant did not receive study pills because the participant was randomized with Urine Albumin Creatinine Ratio above the eligibility safety threshold. b Participants did not consent to the D2d cancer ancillary study because the cancer protocol was not approved at two sites.
Table 1.
Characteristics of D2dCA participants
| Overall D2dCA cohort (n = 2385) | Vitamin D (N = 1194) | Placebo (N = 1191) | |
|---|---|---|---|
| Baseline characteristics | |||
| Age, years | 60.0 ± 9.9 | 59.6 ± 9.8 | 60.4 ± 10.0 |
| Women, no. (%) | 1062 (44.5) | 531 (44.5) | 531 (44.6) |
| Race, no. (%) a | |||
| Asian | 129 (5.4) | 66 (5.5) | 63 (5.3) |
| Black or African American | 601 (25.2) | 293 (24.5) | 308 (25.9) |
| White | 1595 (66.9) | 802 (67.2) | 793 (66.6) |
| Other | 60 (2.5) | 33 (2.8) | 27 (2.3) |
| Hispanic or Latino ethnicity, no. (%) a | 222 (9.3) | 120 (10.1) | 102 (8.6) |
| Personal history of cancer, no. (%) | 135 (5.7) | 63 (5.3) | 72 (6.0) |
| Family history of cancer (first degree relative), no. (%) b | 1325 (59.7) | 675 (60.6) | 650 (58.7) |
| Smoking, no. (%) | |||
| Never | 1385 (58.1) | 696 (58.3) | 689 (57.9) |
| Former | 827 (34.7) | 414 (34.7) | 413 (34.7) |
| Current | 153 (6.4) | 74 (6.2) | 79 (6.6) |
| Unknown or not reported | 20 (0.8) | 10 (0.8) | 10 (0.8) |
| Dietary supplement use c | |||
| Vitamin D | |||
| Participants taking vitamin D supplements, no. (%) | 1022 (42.9) | 504 (42.2) | 518 (43.5) |
| Vitamin D intake among all participants, IU/day d | 314 ± 399 | 313 ± 402 | 314 ± 395 |
| Vitamin D intake among participants using supplements, IU/day | 732 ± 255 | 741 ± 256 | 722 ± 253 |
| Calcium | |||
| Participants taking calcium supplements, no. (%) | 791 (33.2) | 381 (31.9) | 410 (34.4) |
| Calcium intake among all participants, mg/day d | 104 ± 176 | 101 ± 175 | 106 ± 176 |
| Calcium intake among participants using supplements, mg/day | 313 ± 167 | 316 ± 168 | 309 ± 166 |
| Physical activity, total MET hour/week e | 109.7 ± 158.6 | 110.3 ± 157.7 | 109.1 ± 159.5 |
| Body mass index, kg/m2 | 32.0 ± 4.5 | 32.0 ± 4.5 | 32.1 ± 4.4 |
| Body mass index <30 kg/m2, no. (%) | 1532 (64.2) | 762 (63.8) | 770 (64.7) |
| Aspirin use, no. (%) | 701 (29.4) | 338 (28.3) | 363 (30.5) |
| HMG coA reductase inhibitors (statins) use, no. (%) f | 1012 (42.4) | 511 (42.8) | 501 (42.1) |
| Laboratory-based baseline characteristics | |||
| Impaired glucose tolerance (IGT), no. (%) g | 1224 (51.3) | 597 (50.0) | 627 (52.6) |
| Prediabetes categories, no. (%) g | |||
| Met all 3 glycemic criteria (IGT + iA1c + IFG) | 846 (35.5) | 423 (35.4) | 423 (35.5) |
| Met 2 glycemic criteria only | |||
| IGT + IFG | 150 (6.3) | 72 (6.0) | 78 (6.5) |
| IGT + iA1c | 228 (9.6) | 102 (8.5) | 126 (10.6) |
| IFG + iA1c | 1161 (48.7) | 597 (50.0) | 564 (47.4) |
| Serum 25-hydroxyvitamin D, ng/mL | 28.0 ± 10.1 | 27.8 ± 10.2 | 28.2 ± 10.1 |
| Serum 25-hydroxyvitamin D categories, no. (%) h | |||
| <12 ng/mL | 100 (4.2) | 57 (4.8) | 43 (3.6) |
| 12–19 ng/mL | 412 (17.3) | 212 (17.8) | 200 (16.8) |
| 20–29 ng/mL | 868 (36.4) | 450 (37.7) | 418 (35.1) |
| ≥ 30 ng/mL | 1004 (42.1) | 475 (39.8) | 529 (44.5) |
Plus-minus values are means ± SD. Percentages may not add up to 100 because of rounding. To convert 25-hydroxyvitamin D from ng/mL to nmol/L, multiply by 2.496. To convert glucose from mg/dL to mmol/L, multiply by 0.055. To convert vitamin D intake from IU to mcg, divide by 40. There were no statistically significant differences between the 2 groups in mean (ie, all P values higher than 0.05).
a Race and ethnicity were reported by the participant. The category “other” includes Asian, American Indian or Alaska Native, Native Hawaiian or other Pacific Islander, or other race. Ethnicity includes any race.
b Data available in 2161 participants because 224 participants did not complete the Cancer Screening/Family History Questionnaire
c Data on vitamin D and calcium intake are derived from a question about dietary supplements, including multivitamins and high-dose prescribed doses. Participants were allowed to take, from supplements, up to 1000 IU/day of vitamin D and 600 mg/day of calcium. Dietary intake of vitamin D and calcium was not limited.
d Value shown is among all participants regardless of whether they reported use of supplements or not.
e Based on International Physical Activity Questionnaire
f HMG coA reductase inhibitors (statins): atorvastatin, fluvastatin, lovastatin, pitavastatin, pravastatin, rosuvastatin, simvastatin
g IFG, impaired fasting glucose defined as fasting plasma glucose 100 to 125 mg/dL (5.6-6.9 mmol per liter); IGT, impaired glucose tolerance defined as 2-hour post-load plasma glucose after a 75-gram glucose load 140 to 199 mg/dL (7.8-11.0 mmol per liter); iA1c, impaired A1c defined as HbA1c 5.7% to 6.4% (39-47 mmol/mol).
h Categories based on 2010 Dietary Reference Intakes for Calcium and Vitamin D (32)
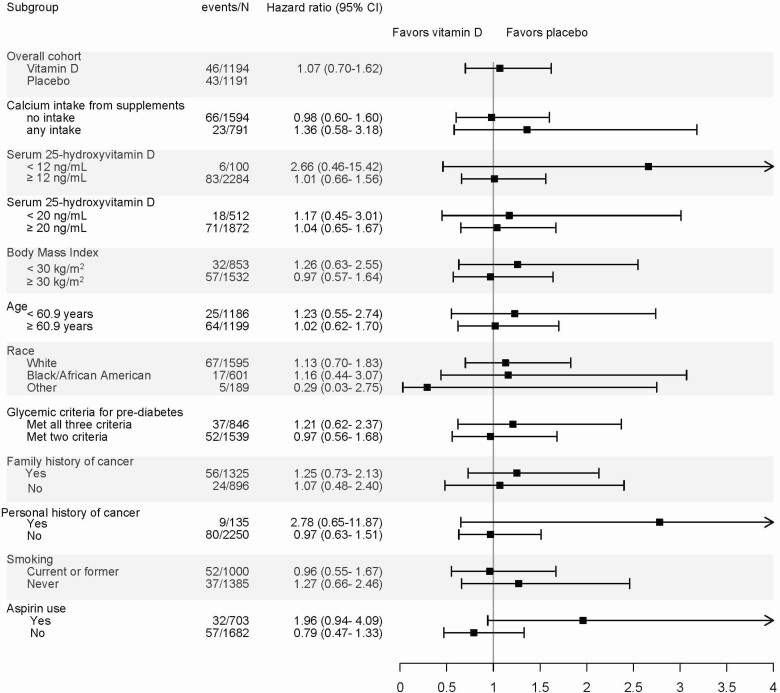
Over a median follow-up time of 2.9 years (interquartile range 2.0, 3.5), 89 participants were diagnosed with cancer: 46 in the vitamin D group and 43 in the placebo group. Compared with placebo, participants in the vitamin D group had a hazard ratio for incident cancer of 1.07 (95% CI 0.70, 1.62) (Table 2). There were no significant differences in incidence of invasive cancer by treatment group in either women or men separately, after excluding incident cancer occurring within the first 6 months or within the first 12 months, after excluding cases of prostate cancer, or in the per protocol analysis (Table 2). In an exploratory analysis, we examined the incidence of prostate cancer separately given prior associations of increased risk with vitamin D. Among men, the hazard ratio for vitamin D vs placebo for prostate cancer was 1.46 (95% CI 0.64, 3.44). In prespecified subgroup analyses, there were no significant differences in risk of cancer between vitamin D and placebo groups in any of the subgroups (Fig. 2).
Table 2.
Summary of results for risk of incident cancer among D2dCA participants
| Overall (N = 2385) | Vitamin D (N = 1194) | Placebo (N = 1191) | Hazard ratio with vitamin D, (95% CI)a | |
|---|---|---|---|---|
| Incident cancer | No. events (cancers per 100 person-years) | |||
| Any type of cancer, intention-to-treat | 89 (1.33) | 46 (1.37) | 43 (1.29) | 1.07 (0.70, 1.62) |
| Any type of cancer, women, intention-to-treat | 33 (1.1) | 18 (1.18) | 15 (1.01) | 1.38 (0.67, 2.85) |
| Any type of cancer, men, intention-to-treat | 56 (1.52) | 28 (1.52) | 28 (1.51) | 1.18 (0.68, 2.03) |
| Any type of cancer, excluding cancers detected in first 6 months, intention-to-treat | 73 (1.09) | 38 (1.13) | 35 (1.05) | 1.09 (0.68, 1.74) |
| Any type of cancer, excluding cancers detected in first 12 months, intention-to-treat | 56 (0.84) | 29 (0.86) | 27 (0.81) | 1.06 (0.62, 1.79) |
| Any type of cancer, excluding prostate cancers, intention-to-treat | 67 (1) | 33 (0.98) | 34 (1.02) | 0.97 (0.60, 1.57) |
| Any type of cancer, per protocol analysis | 83 (1.33) | 44 (1.40) | 39 (1.26) | 1.11 (0.72, 1.71) |
a All hazard ratios are adjusted for stratification variables (site, BMI [<30 or ≥30] and race [white or nonwhite])
Figure 2.
Subgroup analyses: incidence of cancer. All hazard ratios are adjusted for stratification variables [site, BMI (<30 or ≥30) and race (white or non-white)] P > .05 for the interaction terms for all subgroups.
During follow-up, 241 participants were diagnosed with precancers: 239 participants had colorectal adenomatous polyps and 2 participants developed precancers of the breast (one developed atypical ductal hyperplasia and another developed atypical lobular hyperplasia). Key participant characteristics among those who underwent colonoscopy did not differ compared with the entire D2dCA cohort (mean age 63 vs 60 years; BMI 31.5 vs 32.0; 42% vs 45% women). Of participants diagnosed with colorectal adenomatous polyps, 109 were in the vitamin D group and 130 were in the placebo group. Compared with the placebo group, participants in the vitamin D group had a hazard ratio for adenomatous colorectal polyps of 0.83 (95% CI 0.64, 1.07). In the per protocol analysis, the hazard ratio for adenomatous polyps with vitamin D compared to placebo was 0.88 (95% CI 0.68, 1.14) (Table 3).
Table 3.
Summary of results for risk of colorectal adenomatous polyps among D2dCA participants
| Overall (N = 2385) | Vitamin D (N = 1194) | Placebo (N = 1191) | Hazard Ratio with vitamin D, (95% CI)a | |
|---|---|---|---|---|
| No. events (# of participants with polyps per 100 person-years) | ||||
| Any colorectal adenomatous polyp, intention-to-treat | 239 (3.69) | 109 (3.33) | 130 (4.05) | 0.83 (0.64, 1.07) |
| Any colorectal adenomatous polyp excluding polyps detected in first 6 months, intention-to-treat | 204 (3.10) | 93 (2.80) | 111 (3.40) | 0.83 (0.63, 1.10) |
| Any colorectal adenomatous polyp excluding polyps detected in first 12 months, intention-to-treat | 165 (2.48) | 75 (2.34) | 90 (2.72) | 0.83 (0.61, 1.13) |
| Any colorectal adenomatous polyp, per protocol | 227 (3.77) | 107 (3.51) | 120 (4.05) | 0.88 (0.68, 1.14) |
| Among participants who had a screening colonoscopy (n = 259) | ||||
| Overall (N = 259) | Vitamin D (N = 117) | Placebo (N = 142) | Hazard Ratio with vitamin D, (95% confidence interval) a | |
| No. events (# of participants with polyps per 100 person-years) | ||||
| Any colorectal adenomatous polyp | 105 (15.9) | 44 (14.1) | 61 (17.6) | 0.76 (0.49, 1.17) |
| Any colorectal adenomatous polyp excluding polyps detected in first 6 months | 83 (11.6) | 35 (10.5) | 48 (12.6) | 0.77 (0.47, 1.26) |
| Any colorectal adenomatous polyp excluding polyps detected in first 12 months | 67 (9.0) | 29 (8.4) | 38 (9.4) | 0.79 (0.46, 1.35) |
a All hazard ratios are adjusted for stratification variables (site, BMI [<30 or ≥30] and race [white or nonwhite])
Among the entire D2dCA cohort, 559 participants reported having at least one colonoscopy during follow-up, either for screening, diagnostic, or surveillance purposes. Of these 559 participants, 531 had 1 colonoscopy while 28 had ≥2 colonoscopies. A greater proportion of participants in the vitamin D group had no colonoscopy during follow-up compared with those in the placebo group (78.1% vs 75%, respectively; P = 0.03). There were no significant differences in the indications for colonoscopic examinations between the groups. Among the 259 participants who had a screening colonoscopy, the hazard ratio for adenomatous polyps for vitamin D group vs placebo group was 0.76 (95% CI 0.49, 1.17) (Table 3).
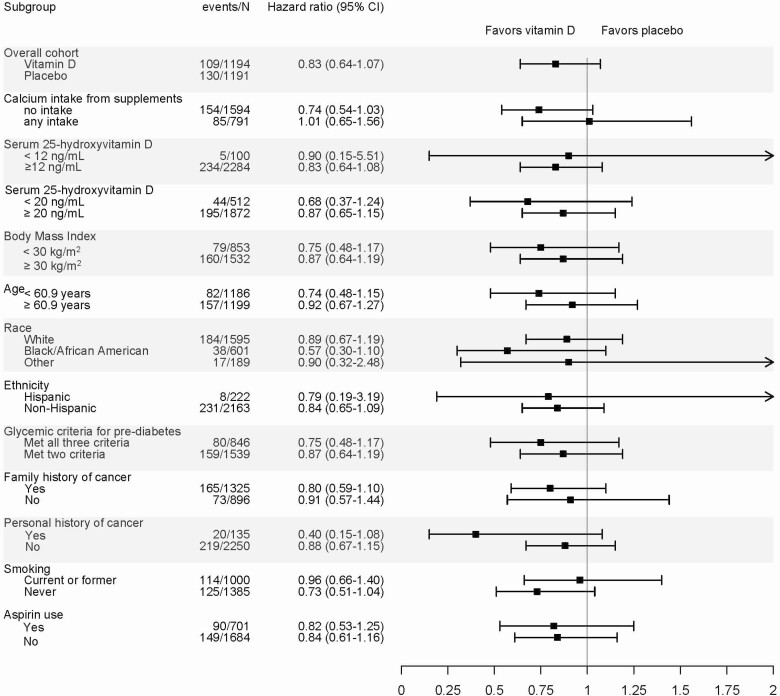
Among the entire D2dCA cohort, in prespecified subgroup analyses related to the outcome of precancers, there were no significant differences in risk of colorectal adenomatous polyps between vitamin D and placebo groups (Fig. 3).
Figure 3.
Subgroup analyses: risk of colorectal adenomatous polyps. All hazard ratios are adjusted for stratification variables [site, BMI (<30 or ≥30) and race (white or non-white)] P > .05 for the interaction terms for all subgroups.
During follow-up, 11 deaths were captured. Six deaths were determined to be related to cancer; 5 participants were assigned to placebo and one participant was assigned to vitamin D.
Discussion
The D2dCA study, an ancillary study of D2d, evaluated the effect of vitamin D supplementation on incident cancers and precancers among participants with prediabetes and overweight/obesity who are at increased risk for cancer as compared to the general population. Over a median follow-up period of 2.9 years, we observed 89 cases of incident cancer with an annual incidence rate of 1.33% per year. This incidence rate of 1.33% in the D2dCA cohort is slightly higher than the incidence observed in the general population with similar demographics, age 60 to 64 years and 45% women (incidence rate of 1.1%) based on national data (24). There was no significant difference in risk of incident cancer or colorectal adenomatous precancers between the participants assigned to vitamin D3 compared to placebo.
Findings from this study are consistent with findings from recent clinical trials that have reported on the effect of vitamin D supplementation on cancer, either as a primary or secondary outcome, and which have reported no statistically significant effects of vitamin D supplementation on cancer risk (6-8). In particular, results from the large, long-term VITAL study did not show a reduced risk of incident cancer among those assigned to 2000 IU/day of vitamin D3 compared to placebo (8). An ongoing trial in Australia (D-Health) is testing the effect of vitamin D3 supplementation at a dose of 60 000 IU/month compared to placebo in adults aged 65 to 84 on multiple health outcomes, including cancer (25).
Recently conducted or ongoing trials examining the effect of vitamin D supplementation on cancer risk have included participants from the general population who were not selected for being at high risk for cancer, other than due to age. The D2dCA population differs from other trial cohorts because D2d participants had prediabetes and overweight/obesity, both of which have been associated with increased risk of cancer (9-11, 26, 27). Similar to other clinical trials of vitamin D, D2dCA participants were not selected based on baseline vitamin D status, and the majority of participants had vitamin D levels that would be considered sufficient: the mean baseline serum 25(OH)D level in D2dCA was 28 ng/mL; in VITAL, 31 ng/mL; in ViDA, 26 ng/mL; and in the trial by Lappe et al, 33 ng/mL (6-8). Based on our knowledge of how vitamins work, the potential benefits of vitamin D supplementation on cancer and other health risks may only be evident in people with lower vitamin D status, but the number of D2dCA participants in the subgroup with vitamin D deficiency [25(OH)D < 12 ng/mL] or the subgroup with vitamin D insufficiency [25(OH)D < 20 ng/mL] and the number of participants with incident cancer in these subgroups were too small to test this hypothesis with any reliability.
Prior studies have examined the associations between serum 25(OH)D levels and risk of colorectal adenomatous polyps and the effects of vitamin D intake on risk of colon polyp recurrence. Meta-analyses of observational studies report significant inverse associations between blood 25(OH)D levels and incidence, but not recurrence, of colorectal adenomas (28, 29). One clinical trial tested the effects of calcium and vitamin D supplementation on risk of recurrence of colorectal polyps. In that trial, 2259 participants with a history of colorectal adenomas resected within 3 months of enrollment were randomized to vitamin D3 1000 IU/day, calcium carbonate 1200 mg/day, both, or neither, in a 2 × 2 factorial design (30). The trial found no significant effect of vitamin D, calcium, or the combination on recurrent colorectal adenomas over 3 to 5 years (30). During long-term follow-up, without study treatment, a higher risk of sessile serrated adenomas or polyps was found among participants taking a combination of calcium and vitamin D or calcium alone which was not found among those taking vitamin D supplementation alone (31). In D2dCA, the risk of colorectal adenomatous polyps was lower in the vitamin D group (HR 0.83), but the result was not statistically significant. When we examined only the cohort that underwent a screening colonoscopy, the risk of colorectal adenomatous polyps was even lower (HR 0.76), but the result remained statistically nonsignificant. Although we did not collect information on colonoscopies and history of colorectal polyps prior to enrollment, colonoscopy reports during the trial provided information about whether the polyps detected during follow-up were incident or recurrent. Not all participants had a colonoscopy during follow-up, but key baseline characteristics among those who had a colonoscopy were similar to the entire D2dCA cohort. Regarding the incidence of colon cancer, in the D2dCA there were 4 cases of incident colorectal cancer, 3 in the placebo group and 1 in the intervention group. This observed incidence rate of colorectal cancer is within the range of expected incidence for the age range of this cohort (24). Because the number of colorectal cancers was small, we did not attempt to draw any conclusions about generalizability.
While the present study provides additional insight into the effects of vitamin D supplementation in a specific at-risk population with prediabetes and overweight/obesity, the D2d study was designed and powered to test for an effect of vitamin D for prevention of diabetes, not for cancer or precancer. Ultimately, the D2dCA study was not powered to detect small effects on cancer risk, and follow-up was too short to fully test the hypothesis that vitamin D supplementation reduces risk of cancer or precancer. However, our results can be combined with results from other trials with cohorts or subcohorts of similar risk (eg, participants with dysglycemia or overweight/obesity) to better define the role of vitamin D in cancer/precancer prevention in these populations.
D2dCA has several strengths. Participants were queried about the outcomes of cancer/precancers more often than in other studies (4 times a year) to improve recall and capture of outcomes and procedures relevant to cancer/precancer outcomes. Records were reviewed and adjudicated by a board-certified oncologist blinded to the assignment. Finally, D2dCA is also unique in the administered dose of vitamin D. Daily vitamin D3 at 4000 IU was chosen to balance safety (by staying within the tolerable upper limit set by the Institute of Medicine) (32) and efficacy in terms of obtaining a large difference in blood 25(OH)D concentration between the treatment and control groups, which was achieved (13).
In conclusion, vitamin D3 supplementation at a dose of 4000 IU/day in participants with prediabetes and overweight/obesity who had sufficient levels of vitamin D at baseline did not have a significant effect on the risk of incident cancer or colorectal adenomatous polyps.
Acknowledgments
Dr. John Erban, hematology-oncologist at Tufts Medical Center, served as the cancer events adjudicator for D2dCA and contributed to the initial drafts of this manuscript. We are saddened by his death and we wish to recognize and appreciate his vital contributions to this study and to this manuscript.
The authors thank the D2d investigators, staff, and trial participants for their outstanding dedication and commitment to the study. Dr. Pittas was supported in part by generous donations to the Tupper Research Fund at Tufts Medical Center.
Financial Support: The planning phase of D2d was funded by the National Institute of Diabetes and Digestive and Kidney Diseases (NIDDK) through a multicenter clinical study implementation planning grant (U34DK091958; principal investigator A.G.P.) to Tufts Medical Center in Boston, MA. Planning was also supported in part by the Intramural Research Program of the NIDDK. The conduct of D2d is primarily supported by NIDDK and the Office of Dietary Supplements of the National Institutes of Health through the multicenter clinical study cooperative agreement (U01DK098245; principal investigator A.G.P.) to Tufts Medical Center where the D2d Coordinating Center is based. The U01 grant mechanism establishes the NIDDK project scientist as a member of the D2d Research Group. The study also received secondary funding from the American Diabetes Association (1-14-D2d- 01).
Clinical Trial Information : D2d ClinicalTrials.gov no. NCT01942694
Author Contributions: R.C., P.F., E.M.V., E.S.L., P.R.S., M.R.L., R.J.D., K.C.J., S.R.K., J.N., and A.G.P. designed and conducted research, analyzed data, wrote the paper, and had primary responsibility for final content. All authors read and approved the final manuscript.
Glossary
Abbreviations
- 25(OH)D
25-hydroxyvitamin D
- BMI
body mass index
- D2d
Vitamin D and type 2 diabetes study
- D2dCA
D2d cancer outcomes ancillary study
- HR
hazard ratio
Contributor Information
D2d Research Group:
Anastassios G Pittas, Irwin Brodsky, Lisa Ceglia, Chhavi Chadha, Ranee Chatterjee, Bess Dawson-Hughes, Cyrus Desouza, Rowena Dolor, John Foreyt, Adline Ghazi, Daniel S Hsia, Karen C Johnson, Sangeeta R Kashyap, Sun Kim, Erin S LeBlanc, Michael R Lewis, Emilia Liao, Saul Malozowski, Lisa M Neff, Patrick O’Neil, Jean Park, Anne Peters, Lawrence S Phillips, Richard Pratley, Philip Raskin, Neda Rasouli, David Robbins, Clifford Rosen, Dave Reboussin, Vanita R Aroda, James H Ware, Patricia Sheehan,, Myrlene A Staten, and William C Knowler
D2d Research Group Collaborators
Steering Committee
Anastassios G. Pittas, MD MS, Tufts Medical Center, Boston, MA (Chair)
Irwin Brodsky, MD, Maine Medical Center Research Institute, Scarborough, ME
Lisa Ceglia, MD MS, Tufts Medical Center, Boston, MA
Chhavi Chadha, MD, HealthPartners Research Foundation, Minneapolis, MN
Ranee Chatterjee, MD MPH, Duke University Medical Center, Durham, NC
Bess Dawson-Hughes, MD, Jean Mayer USDA Human Nutrition Research Center on Aging at Tufts University, Boston, MA
Cyrus Desouza, MBBS, Omaha VA Medical Center, University of Nebraska Medical Center, Omaha, NE
Rowena Dolor, MD MHS, Duke University Medical Center, Durham, NC
John Foreyt, PhD, Baylor College of Medicine, Houston, TX
Adline Ghazi, MD, MedStar Good Samaritan Hospital, Baltimore, MD
Daniel S. Hsia, MD, Pennington Biomedical Research Center, Baton Rouge, LA
Karen C. Johnson, MD MPH, University of Tennessee Health Science Center, Memphis, TN
Sangeeta R. Kashyap, MD, Cleveland Clinic, Cleveland, OH
Sun Kim, MD, Stanford University Medical Center, Stanford, CA
Erin S. LeBlanc, MD MPH, Kaiser Permanente Center for Health Research NW, Portland, OR
Michael R. Lewis, MD MBA, University of Vermont–Central Laboratory, Burlington, VT
Emilia Liao, MD, Northwell Health Lenox Hill Hospital, New York, NY
Saul Malozowski, MD PhD, National Institute of Diabetes and Digestive and Kidney Diseases, Bethesda, MD (NIDDK Project Scientist)
Lisa M. Neff, MD, Northwestern University, Chicago, IL
Patrick O’Neil, PhD, Medical University of South Carolina, Charleston, SC
Jean Park, MD, MedStar Health Research Institute, Hyattsville, MD
Anne Peters, MD, Keck School of Medicine of the University of Southern California, Los Angeles, CA
Lawrence S. Phillips, MD, Atlanta VA Medical Center, Decatur, GA and Emory University School of Medicine, Atlanta, GA
Richard Pratley, MD, AdventHealth Translational Research Institute for Metabolism and Diabetes, Orlando, FL
Philip Raskin, MD, University of Texas Southwestern Medical Center, Dallas, TX
Neda Rasouli, MD, University of Colorado, School of Medicine and VA Eastern Colorado Health Care System, Aurora, CO
David Robbins, MD, University of Kansas Medical Center, Kansas City, KS
Clifford Rosen, MD, Maine Medical Center Research Institute, Scarborough, ME
Lead Biostatistician
Dave Reboussin, PhD, Wake Forest School of Medicine, Winston-Salem, NC
Past Steering Committee Members
Vanita R. Aroda, MD, Brigham and Women’s Hospital, Boston, MA
James H. Ware, PhD, Harvard T.H. Chan School of Public Health, Boston, MA (deceased)
Patricia Sheehan, RN MPH MS, Spaulding Rehabilitation Network, Boston, MA
Myrlene A. Staten, MD, National Institute of Diabetes and Digestive and Kidney Diseases, Bethesda, MD (NIDDK Project Scientist)
Advisor
William C. Knowler, MD DrPH, National Institute of Diabetes and Digestive and Kidney Diseases, Phoenix, AZ
Additional Information
Disclosure Summary: R.C., P.F., E.M.V., E.S.L., P.R.S., M.R.L., R.J.D., K.C.J., S.R.K., J.N., and A.G.P. have no potential conflict of interest.
Data Availability
Some or all datasets generated during and/or analyzed during the current study are not publicly available but are available from the corresponding author on reasonable request.
References
- 1. Krishnan AV, Feldman D. Mechanisms of the anti-cancer and anti-inflammatory actions of vitamin D. Annu Rev Pharmacol Toxicol. 2011;51:311-336. [DOI] [PubMed] [Google Scholar]
- 2. Leyssens C, Verlinden L, Verstuyf A. Antineoplastic effects of 1,25(OH)2D3 and its analogs in breast, prostate and colorectal cancer. Endocr Relat Cancer. 2013;20(2):R31-R47. [DOI] [PubMed] [Google Scholar]
- 3. Welsh J. Cellular and molecular effects of vitamin D on carcinogenesis. Arch Biochem Biophys. 2012;523(1):107-114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Gandini S, Boniol M, Haukka J, et al. . Meta-analysis of observational studies of serum 25-hydroxyvitamin D levels and colorectal, breast and prostate cancer and colorectal adenoma. Int J Cancer. 2011;128(6):1414-1424. [DOI] [PubMed] [Google Scholar]
- 5. Bjelakovic G, Gluud LL, Nikolova D, Whitfield K, Krstic G, Wetterslev J, -Gluud C. Vitamin D supplementation for prevention of cancer in adults. Cochrane Database Syst Rev. 2014(6):CD007469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Lappe J, Watson P, Travers-Gustafson D, et al. . Effect of vitamin D and calcium supplementation on cancer incidence in older women: a randomized clinical trial. JAMA. 2017;317(12):1234-1243. [DOI] [PubMed] [Google Scholar]
- 7. Scragg R, Khaw KT, Toop L, et al. . Monthly high-dose vitamin D supplementation and cancer risk: a post hoc analysis of the vitamin D assessment randomized clinical trial. JAMA Oncol. 2018;4(11):e182178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Manson JE, Cook NR, Lee IM, et al. ; VITAL Research Group . Vitamin D supplements and prevention of cancer and cardiovascular disease. N Engl J Med. 2019;380(1):33-44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Huang Y, Cai X, Qiu M, et al. . Prediabetes and the risk of cancer: a meta-analysis. Diabetologia. 2014;57(11):2261-2269. [DOI] [PubMed] [Google Scholar]
- 10. Giovannucci E, Harlan DM, Archer MC, et al. . Diabetes and cancer: a consensus report. Diabetes Care. 2010;33(7):1674-1685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Shlomai G, Neel B, LeRoith D, Gallagher EJ. Type 2 diabetes mellitus and cancer: the role of pharmacotherapy. J Clin Oncol. 2016;34(35):4261-4269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Pittas AG, Dawson-Hughes B, Sheehan PR, et al. ; D2d Research Group . Rationale and design of the vitamin D and Type 2 diabetes (D2d) study: a diabetes prevention trial. Diabetes Care. 2014;37(12):3227-3234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Pittas AG, Dawson-Hughes B, Sheehan P, et al. ; D2d research group. Vitamin D supplementation and prevention of type 2 diabetes. N Engl J Med. 2019;381(6):520-530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Chatterjee R, Erban JK, Fuss P, et al. ; D2d Research Group . Vitamin D supplementation for prevention of cancer: the D2d cancer outcomes (D2dCA) study. Contemp Clin Trials. 2019;81:62-70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Aroda VR, Sheehan PR, Vickery EM, et al. ; D2d Research Group . Establishing an electronic health record-supported approach for outreach to and recruitment of persons at high risk of type 2 diabetes in clinical trials: the vitamin D and type 2 diabetes (D2d) study experience. Clin Trials. 2019;16(3):306-315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Bedner M, Lippa KA, Tai SS. An assessment of 25-hydroxyvitamin D measurements in comparability studies conducted by the Vitamin D metabolites quality assurance program. Clin Chim Acta. 2013;426:6-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. DEQAS Vitamin D External Quality Assessment Scheme. Accessed February 1, 2018.http://www.deqas.org
- 18. Moher D, Hopewell S, Schulz KF, et al. . CONSORT 2010 explanation and elaboration: updated guidelines for reporting parallel group randomised trials. BMJ. 2010;340:c869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Cox D. Regression models and life tables (with Discussion). J RStatist Soc. 1972;Series B(34):187-220. [Google Scholar]
- 20. Bansal D, Bhansali A, Kapil G, Undela K, Tiwari P. Type 2 diabetes and risk of prostate cancer: a meta-analysis of observational studies. Prostate Cancer Prostatic Dis. 2013;16(2):151-8, S1. [DOI] [PubMed] [Google Scholar]
- 21. Ohkuma T, Peters SAE, Woodward M. Sex differences in the association between diabetes and cancer: a systematic review and meta-analysis of 121 cohorts including 20 million individuals and one million events. Diabetologia. 2018;61(10):2140-2154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Xu Y, Shao X, Yao Y, et al. . Positive association between circulating 25-hydroxyvitamin D levels and prostate cancer risk: new findings from an updated meta-analysis. J Cancer Res Clin Oncol. 2014;140(9):1465-1477. [DOI] [PubMed] [Google Scholar]
- 23. Batai K, Murphy AB, Ruden M, et al. . Race and BMI modify associations of calcium and vitamin D intake with prostate cancer. BMC Cancer. 2017;17(1):64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Howlader N, Noone AM, Krapcho M, et al. (eds). SEER Cancer Statistics Review 1975–2017. Based on November 2019 SEER data submission, posted to the SEER web site, April 2020. https://seer.cancer.gov/csr/1975_2017. Accessed April 2, 2020. [Google Scholar]
- 25. Neale RE, Armstrong BK, Baxter C, et al. . The D-Health trial: a randomized trial of vitamin D for prevention of mortality and cancer. Contemp Clin Trials. 2016;48:83-90. [DOI] [PubMed] [Google Scholar]
- 26. Kyrgiou M, Kalliala I, Markozannes G, et al. . Adiposity and cancer at major anatomical sites: umbrella review of the literature. BMJ. 2017;356:j477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Freisling H, Arnold M, Soerjomataram I, et al. . Comparison of general obesity and measures of body fat distribution in older adults in relation to cancer risk: meta-analysis of individual participant data of seven prospective cohorts in Europe. Br J Cancer. 2017;116(11):1486-1497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Yin L, Grandi N, Raum E, Haug U, Arndt V, Brenner H. Meta-analysis: Serum vitamin D and colorectal adenoma risk. Prev Med. 2011;53(1-2):10-16. [DOI] [PubMed] [Google Scholar]
- 29. Wei MY, Garland CF, Gorham ED, Mohr SB, Giovannucci E. Vitamin D and prevention of colorectal adenoma: a meta-analysis. Cancer Epidemiol Biomarkers Prev. 2008;17(11):2958-2969. [DOI] [PubMed] [Google Scholar]
- 30. Baron JA, Barry EL, Mott LA, et al. . A Trial of calcium and vitamin D for the prevention of colorectal adenomas. N Engl J Med. 2015;373:1519-1530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Crockett SD, Barry EL, Mott LA, et al. . Calcium and vitamin D supplementation and increased risk of serrated polyps: results from a randomised clinical trial. Gut. 2019;68(3):475-486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Ross AC, Taylor CL, Yaktine AL, Del Valle HB. Institute of medicine dietary reference intakes for calcium and vitamin D. Washington, DC:The National Academies Press; 2011. [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Some or all datasets generated during and/or analyzed during the current study are not publicly available but are available from the corresponding author on reasonable request.