Abstract
Context
Transsphenoidal surgery is standard care in the treatment of hormone-secreting pituitary adenomas. Current clinician-reported surgical outcome measures are one-dimensional, typically focusing primarily on complete or partial resection, and secondarily on complication rates. However, outcomes are best reflected by the delicate balance of efficacy and complications at patient level.
Objective
This study proposes a novel way to classify and report outcomes, integrating efficacy and safety at the patient level.
Methods
Retrospective chart review of all pure endoscopic transsphenoidal surgical procedures for acromegaly, Cushing’s disease, and prolactinoma between 2010 and 2018 in a single tertiary referral center. We present our results in a classic (remission and complications separate) and in a novel outcome square integrating both outcomes, focusing on intended and adverse effects (long-term complications). This resulted in 4 outcome groups, ranging from good to poor. We use this approach to present these outcomes for several subgroups.
Results
A total of 198 surgical procedures were included (44 reoperations). Remission was achieved in 127 operations (64%). Good outcome was observed after 121 (61%), and poor outcome after 6 (3%) operations. When intended effect of surgery was applied (instead of remission), good outcome as intended was achieved after 148 of 198 surgeries (75%) and poor outcome after 4 (2%).
Conclusion
Quality of a surgical intervention can be presented in 4 simple categories, integrating both efficacy and safety with flexibility to adapt to the individualized situation at patient, disease, and surgical strategy and to the outcome of interest.
Keywords: Pituitary adenoma, transsphenoidal surgery, outcomes, patient counselling
Outcomes of surgical procedures are often reported in literature in a clinician-centered fashion. Classic surgical outcome papers primarily report on measures of efficacy, like remission rates and gross total resection. Often, they report safety outcomes, such as complications, separately. However, as Porter advocates in his value-based healthcare (VBHC) model, a comprehensive set of clinician- and patient-reported outcomes measuring functional outcome is more meaningful to patients (1, 2). The VBHC approach was embraced in our high-volume tertiary referral practice for pituitary and skull base surgery. A care pathway is in place, wherein prospective collection of clinician- and patient-reported outcome measures (PROMs) and individualized preoperative counselling are implemented (3, 4). The comprehensive VBHC outcome set provides a more holistic view of outcomes. However, the results are not easily summarized or compared, which is particularly true in our heterogeneous population with different pituitary conditions and objectives for surgery.
For most pituitary adenomas, endoscopic transsphenoidal surgery remains the cornerstone of treatment. Functioning pituitary adenomas are benign adenomas producing excess hormones, resulting in heterogeneous syndromes, namely acromegaly, Cushing’s disease, and prolactinoma. Patients present with variable clinical manifestations, severity of hormone excess, tumor size and extension, and preoperative pituitary function. The ultimate goal of surgery is to achieve remission without pituitary gland injury. However, this aim is unrealistic in larger and invasive tumors. In those cases, surgical tumor debulking can also be used to achieve other objectives, such as decompression of the anterior optic pathway or to allow better medical management. The quality of a surgical procedure is best reflected by the balance between the best possible resection and minimal adverse effects: maximal safe resection. This is especially important in pituitary surgery, as loss of pituitary function is reported in 2% to 11.5% of operations for functioning tumors in meta-analyses (5-7). During the preparations of surgery, the odds of achieving the surgical goal should be weighed against the chances of a negative outcome of treatment. This should be accompanied by an assessment of the necessity to perform surgery or whether alternative treatment options provide a better option for the patient.
As proper randomized controlled trials and evidence-based medicine are lacking in this field of rare diseases, outcome registries and clinical benchmarking is vital for service evaluations and individualized clinical decision making. Therefore, we felt the need for a simple 2-dimensional outcome square integrating efficacy and safety at a patient level, resulting in 4 integrated outcome quadrants (IOQs). The proposed outcome squares may serve multiple goals and may facilitate in counselling of patients, global outcome evaluation for a heterogeneous patient population, meaningful data evaluation of relatively small subgroups, and further development of quality evaluations with great flexibility to adapt efficacy and safety parameters of interest. This approach is not limited to pituitary surgery and can be extrapolated to other treatment modalities to allow comparison between treatment options, and to other complex conditions.
Patients and Methods
A retrospective chart review of all patients that underwent fully endoscopic transsphenoidal pituitary surgery between January 1, 2010, and December 31, 2018, was performed. Only surgeries for acromegaly, Cushing’s disease, or prolactinomas were included. We included all operations for these tumors; no exclusion was made based on size, invasive growth, or reoperation. As a structured VBHC pituitary care pathway was implemented in 2016 (3), we compared the results of pituitary surgery before and after this time point. Data of patients not included in previous studies were obtained after a waiver of the medical ethical review was received from our institutional medical ethics review board (G19·011).
Preoperative Assessment
A standardized preoperative assessment including an magnetic resonance imaging (MRI) and computed tomography was performed in all 198 patients. When no MRI or computed tomography was available prior to admission, the preoperative imaging was performed on the day of admission (1 day preoperative). Tumors were defined as giant, micro-, or macroadenoma when maximal diameter was ≥40 mm, <10 mm, or between 10 and 40 mm, respectively. Cavernous sinus invasion was defined as a Knosp score of ≥3 (8). A full endocrine assessment was performed in all patients and the diagnosis of hormone excess and deficiency was reached based on published guidelines (9-12). In case of corticotroph or thyrotroph deficiency, preoperative hormone replacement therapy was initiated. An ophthalmological assessment was performed, including at least assessment of visual acuity and perimetry. All patients were reviewed at the combined endocrinology–neurosurgery outpatient clinic for a personalized consultation discussing surgery and alternative treatment options. A surgical plan detailing intended effects, expectations for success, and risks for adverse effects was set after thorough discussion with the patient. This plan could be either total resection or debulking only. Routinely, all patients were preoperatively discussed by the pituitary multidisciplinary team, including endocrinologists, neurosurgeons, ophthalmologists, radiation oncologists, and neuroradiologists, and when indicated pediatric endocrinologists, oncologists, and nuclear medicine specialists.
Operative Technique
All surgical procedures were performed by a combination of 2 experienced pituitary neurosurgeons (W.R.v.F., M.J.T.V., or P.J.S.) who used the “3-hand technique,” with 1 neurosurgeon handling the instruments and the other mainly handling the endoscope. Of the 198 procedures, in 25 (12.6%) the surgery was performed via an extended endoscopic endonasal approach (13).
Postoperative Assessments
All patients were closely monitored for the occurrence of complications according to our standard perioperative protocol. From 2016 onwards, patients had daily contact with a pituitary case manager after discharge until postoperative day 14 via telephone or email (3). Routine follow-up consisted of a visit to the combined outpatient clinic at 6 weeks and 6 months after surgery. Full hormonal panels and, if indicated, dynamic endocrinological tests were performed for a detailed assessment of pituitary function. Routinely, an MRI was performed at 6 months for the assessment of a tumor remnant as well as for a baseline measurement for future assessments regarding tumor growth. When indicated, this MRI was performed earlier during follow-up. After 6 months of follow-up visits were performed at yearly intervals, or more frequently when indicated.
Efficacy Parameters
The main efficacy parameter was biochemical remission according to published guidelines (9-12, 14-16). During the preoperative counselling, a patient is counselled according to intended effect of surgery. In our clinic there are 3 distinct counselling groups: (1) safe complete surgical tumor resection seems likely and biochemical remission is pursued, (2) complete resection might be possible, but is, however, dependent on the intraoperative situation, and potentially with an increased risk of complications. Intraoperative risk assessment is necessary to evaluate maximal safe resection, as in small Cushing adenomas and potential cavernous sinus invasion or abnormal tumor consistency, (3) remission is clearly not possible, but tumor debulking is performed to achieve clinical or biochemical improvement, such as restoration of visual function or biochemical control with a lower, tolerable dose of medication. For the current study, groups 1 and 2 were combined and the surgical objective for both was assessed as the achievement of remission. For group 3, the primary objective for debulking as specified in the conclusion of the combined outpatient clinic visit was extracted. If the objective of surgery was to restore visual function, the intended effect was defined as restoration of visual acuity or visual field defects to levels before chiasm compression. Normal postoperative visual field analysis was defined as a postoperative mean deviation of –4.0 or higher on perimetry analysis (17). If the objective was to achieve lower dosages of antitumor medication, the intended effect was achieved when the dose was indeed lowered 6 months postoperatively, irrespective of the decrease. In case of another objective, namely enabling targeted radiotherapy, biopsy for pathology, closure of a cerebrospinal fluid (CSF) leak, and alleviation of obstructive hydrocephalus, the intended effect achieved at 6 months postoperatively was taken as the efficacy outcome.
Complications
Complications were defined as any complication occurring during the first 30 days after the operation and necessitating medical intervention. If a patient had preoperative anterior panhypopituitarism (caused by the tumor or earlier surgery), postoperative endocrine deficiencies were not recorded as a complication. Diabetes insipidus (DI) was defined as polyuria (urine production >300 mL/hour for 3 consecutive hours) and urine specific gravity <1.005, in addition to at least 1 relative criterion: excessive thirst, serum osmolality >300 mosmol/kg, or serum sodium >145 mmol/L (18).
Adverse Effects
An adverse effect was defined as a long-term complication, in other words a duration of over 6 months, requiring ongoing medical management or with irreversible symptoms. Adverse effects included new-onset permanent anterior or posterior pituitary hormone deficiencies, neurological deficits, anosmia, and death.
Construction of Outcome Squares
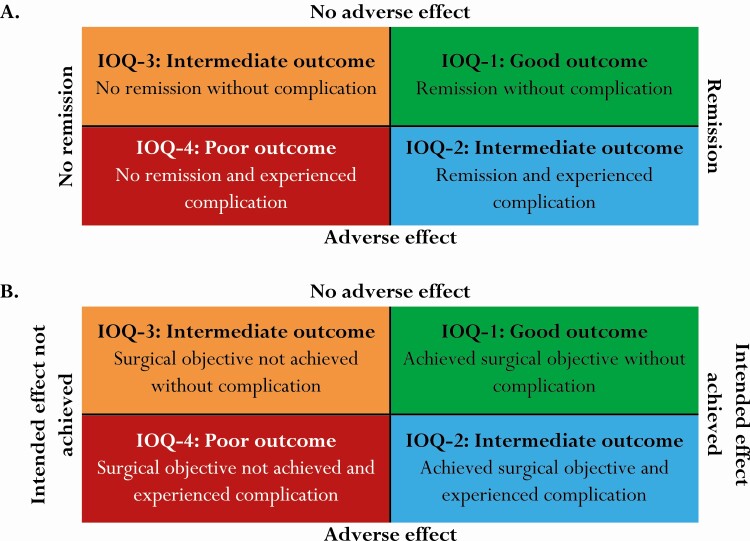
First, a “classical presentation” of surgical results was constructed reporting remission rates and complications separately. Second, outcome of surgery was presented in an outcome square, based on remission status and adverse effects (Fig. 1A). This resulted in the following categories: good outcome/IOQ1 (remission without adverse effect), poor outcome/IOQ4 (no remission with adverse effect), and 2 intermediate outcome categories: IOQ2 (remission with adverse effect) and IOQ3 (no remission without adverse effect). These outcome squares were constructed for different clinically relevant subgroups according to disease, tumor size, surgical status, year of operation, and tumor invasiveness. Finally, outcome squares were created integrating the achievement of the intended effect instead of remission and the occurrence of adverse effects. In these tables, categorization was based on achievement of the particular intended effect and the occurrence of adverse effects (Fig. 1B).
Figure 1.
Construction of the outcome square, a contingency table integrating efficacy with safety. Definitions of remission, intended effect and adverse effect are provided in the methods section. IOQ, integrated outcome quadrant (1-4).
Quality of Life Measurements
For a prognostic study on VBHC in pituitary surgery, questionnaires on quality of life (QoL) were filled in by 41 patients in this cohort. The analyzed questionnaires in this study are the Leiden Bother and Needs Questionnaire-Pituitary (LBNQ-P) and the Short Form 36 Health Survey (SF-36), which is divided in a mental component score (MCS) and a physical component score (PCS). The LBNQ-P is especially developed for pituitary patients, whereas the SF-36 is a more general QoL questionnaire. The occurrence of improvement, deterioration and no change per IOQ on these questionnaires is presented. Improvement and deterioration were defined as a change of at least 0.5 standard deviations from preoperatively to 6 months postoperatively.
Statistical Analysis
Statistical analysis was performed using IBM SPSS statistics 25 (IBM Corp. Armonk, NY, USA). Descriptive statistics were used for baseline characteristics. Continuous variables were reported as means ± SD and dichotomous variables as frequencies with percentage of the cohort. Differences between outcome groups were assessed with a 1-way analysis of variance. Statistical analyses of categorical variables were carried out using χ 2 or Fisher’s exact tests, as appropriate.
Results
Baseline Criteria
During the study period 198 operations were performed: 71 for patients with acromegaly (among 67 patients), 71 for Cushing’s disease (57 patients), and 56 for prolactinomas (54 patients). The mean age was 43.4 years at time of surgery and 60% of patients was female. The intended effect of surgery was to achieve remission in 159 operations (80.4%), and debulking in 39 (19.7%) (in order to restore visual function in 16 [8.1%], lower dose of medication in 17 [8.6%], to allow targeted radiotherapy in 1 [0.5%], biopsy for pathology in 2 [1.0%], closure of a CSF leak in 2 [1.0%], and alleviation of obstructive hydrocephalus in 1 patient [0.5%]). Of the 56 prolactinoma surgeries, primary indication for surgery was a contraindication to dopamine agonists in 3 patients (5.4%), moderate to severe intolerance to dopamine agonists 24 patients (42.9%), mild intolerance to dopamine agonists with a patient preference for surgical therapy in 4 (7.1%), a treatment refractory tumor in 11 (19.6%), compression of the anterior optic pathway in 6 (10.7%), apoplexy in 3 (5.4%), a CSF leak in 2 (3.6%), growth hormone cosecretion in 1 (1.8%), pathology in 1 (1.8%), and patient preference for primary surgical treatment in 1 (1.8%). One hundred and fifty-four operations were primary surgical treatment, and 44 patients underwent reoperations (34 a second, 8 a third, 1 a fourth, and 1 a sixth).
Detailed statistics can be found in Table 1 and elsewhere (Table S1 (19)).
Table 1.
Baseline criteria
| n (%) (n = 198) | |
|---|---|
| Female | 121 (61.1) |
| Age | 43.4 |
| Body mass index | 28.1 |
| Acromegaly | 71 (35.9) |
| Cushing’s disease | 71 (35.9) |
| Prolactinoma | 56 (28.4) |
| Microadenoma | 96 (48.5) |
| Macroadenoma | 97 (48.7) |
| Giant adenoma | 6 (3.0) |
| Visual field deficit | 24 (12.1) |
| Cavernous sinus invasion | 39 (19.6) |
| Apoplexy | 6 (3.0) |
| Reoperation | 44 (22.1) |
| Extended procedure | 5 (2.5) |
| No anterior pituitary deficiency | 135 (68.2) |
| One anterior pituitary deficiency | 34 (17.1) |
| Multiple anterior pituitary deficiency | 18 (9.0) |
| Anterior panhypopituitarism | 10 (5.0) |
| Unknown endocrine status | 2 (1.0) |
| Diabetes insipidus | 3 (1.5) |
| Surgical objective | |
| Remission | 159 (80.4) |
| Debulking | 39 (19.7) |
Remission Rates
Remission was achieved in 127 of 198 operations (64.1%). This was higher for microadenomas (78.1%) than for macroadenomas (54.2%), while no remission was achieved in giant adenomas. The remission rate between 2010 and 2015 was 57.4% (59/103), which was significantly higher after 2016 (68 out of 95 operations, 71.6%; P = .036).
Among patients with acromegaly, remission was achieved in 60.6% of operations (43/71 operations). The remission rate was 83.4% (15/18) in microadenomas, 57.1% (28/49) in macroadenomas, and 0% (0/4) in giant adenomas. The remission rate for patients undergoing primary surgery was 66.7% (36/54), in contrast to 41.2% (7/17) among patients that underwent reoperation. Recurrence occurred in 1 patient with a noninvasive macroadenoma. Remission rates did not significantly differ between those patients treated before 2016 (52.6%, 20/38), and those thereafter (69.7%, 23/33).
Among patients with Cushing’s disease, remission was achieved in 64.8% of surgeries (46/71). The remission rate was 71.2% (37/52) in microadenomas, 50.0% (9/18) in macroadenomas, and 0% (0/1) in giant adenomas. After primary surgery for a microadenoma the remission rate was 82.4% (28/34), and 50.0% (9/18) after reoperation. When inferior petrosal sinus sampling was necessary to confirm pituitary adrenocorticotropin overexcretion because the MRI did not show a clear adenoma, remission rates following sellar exploration were 55.2% (16/29). Recurrence occurred in 6 patients (3 microadenomas) (13%). The remission rate did not significantly differ between those treated before 2016 (24/39, 61.5%) and thereafter (22/32, 68.8%).
In prolactinomas, remission was achieved in 67.9% of surgeries (38/56). Remission rate was 88.5% (23/26) in microadenomas, 51.7% (15/29) in macroadenomas, and 0% (0/1) in giant adenomas. Recurrence occurred in 2 patients (1 microadenoma). Remission rates did not significantly differ between those patients treated before 2016 (57.7%, 15/26) and those thereafter (76.7%, 23/30).
Detailed statistics can be found in Table 2 and elsewhere (Table S2 (19)).
Table 2.
Remission and complication rates
| n (%) (n = 198) | |
|---|---|
| Remission at 6 months | 127 (64.1) |
| Recurrence | 8 (7.4) |
| Any complication | 81 (40.9) |
| Adverse effect (long-term complication | 12 (6.1) |
| Transient DI | 47 (23.6) |
| Permanent DI | 7 (3.5) |
| SIADH | 15 (7.6) |
| New anterior hypopituitarism | 6 (3.0) |
| Transient | 2 (1.0) |
| Permanent | 4 (2.0) |
| CSF-leak | 11 (5.6) |
| Needing reoperation | 5 (2.5) |
| Meningitis | 4 (2.0) |
| Epistaxis (needing coagulation) | 4 (2.0) |
| Cranial nerve palsy | 2 (1.0) |
| Permanent | 0 |
| Permanent anosmia | 2 (1.0) |
| Vascular complication | 2a (1.0) |
| Thrombo-embolism | 3 (1.5) |
| Readmission | 25 (12.6) |
| Mortality | 1 (0.5) |
Abbreviations: CSF, cerebrospinal fluid; DI, diabetes insipidus; SIADH, syndrome of inappropriate antidiuretic hormone secretion.
a 1 internal carotid artery injury and 1 bleeding in resection cavity.
Complication Rates
One or more complications occurred in 81 patients (40.7%) within 30 days after surgery (Table 2). The most common complication was transient DI (n = 47, 23.6%), followed by syndrome of inappropriate antidiuretic hormone secretion (n = 16, 8.0%). Adverse effects occurred in 12 (6.0%) patients. Thromboembolic events occurred in 2 patients with acromegaly, and in 1 patient with Cushing’s disease, all with giant adenomas. One bleeding in the resection cavity without permanent damage occurred after debulking of a large treatment-resistant macroprolactinoma, causing a transient cranial nerve palsy. One patient (0.5%) died within 30 days after surgery. This was caused by an intraoperative injury to the carotid artery, occurring during a cavernous sinus exploration in a patient previously irradiated for the treatment of severe recurrent Cushing’s disease. The bleeding was stopped using a balloon occlusion. However, during the occlusion the patient had a massive infarction caused by thrombosis of both anterior A1 arteries. The patient eventually died of the neurological consequences.
Between 2010 and 2015 a complication occurred following 45 out of 103 operations (43.7%). Adverse effects occurred after 8 (7.8%) operations. Between 2016 and 2018 a complication was observed in 36 out of 95 surgeries (37.9%), while adverse effects occurred in 4 (4.2%) operations. These decreases were nonsignificant.
Detailed statistics can be found in Table 2 and elsewhere (Table S2 (19)).
Outcome Squares Integrating Remission Status and Adverse Effect
When integrating remission and adverse effects, good outcome was achieved in 121 surgeries (61.1%). The good outcome group consisted of 50 operations for acromegaly (56.4%), 43 operations for Cushing’s disease (60.6%), and 38 operations for a prolactinoma (67.9%). The subgroup with the highest proportion of good outcome was the microprolactinomas (88.5%). Good outcome was present in 56 out of 103 surgeries (54.4%) before 2016, and in 65 out of 95 (68.4%) from 2016 onwards, a significant improvement (P = .043).
Intermediate outcome was observed in 71 patients (36%). Most of these patients (n = 65, 33%) did not achieve remission, but had no adverse effects either. Another 6 (3%) achieved remission, but had permanent loss of function postoperative. Intermediate outcome was most prevalent in acromegaly (40%) and least prevalent in prolactinomas (30%).
A poor outcome was seen after 6 operations (3.0%). The poor outcome group consisted of 3 acromegaly cases (4.2%), 2 Cushing patients (2.8%), and 1 prolactinoma patient (1.8%). The highest proportion of poor outcome was seen in patients with acromegaly with cavernous sinus invasion (13.4%). Poor outcome was present in 5 out of 103 surgeries (4.9%) before 2016, and in 1 out of 95 (1.1%) from 2016 onwards (ns).
Detailed statistics can be found in Table 3, section A, and elsewhere (Tables S3a, S4a, and S5a (19)).
Table 3.
Outcome squares with (A) remission versus adverse effect and (B) intended effect and adverse effect
| A | B | ||||||
|---|---|---|---|---|---|---|---|
| All | All | ||||||
| No. (%) | R– | R+ | No. (%) | IE– | IE+ | ||
| AE– | 65 (32.8) | 121 (61.1) | 186 (95.9) | AE– | 38 (19.2) | 148 (74.7) | 186 (93.9) |
| AE+ | 6 (3.0) | 6 (3.0) | 12 (6.1) | AE+ | 4 (2.0) | 8 (4.0) | 12 (6.1) |
| 71 (35.9) | 127 (64.1) | 198 | 42 (21.2) | 156 (78.8) | 198 | ||
| Disease | Disease | ||||||
| Acromegaly | R– | R+ | Acromegaly | IE– | IE+ | ||
| AE– | 25 (35.2) | 40 (56.4) | 65 (91.5) | AE– | 15 (21.1) | 50 (70.4) | 65 (91.5) |
| AE+ | 3 (4.2) | 3 (4.2) | 6 (8.5) | AE+ | 1 (1.4) | 5 (7.0) | 6 (8.5) |
| 28 (39.4) | 43 (60.6) | 71 | 16 (22.5) | 55 (77.5) | 71 | ||
| Cushing | R– | R+ | Cushing | IE– | IE+ | ||
| AE– | 23 (32.4) | 43 (60.6) | 66 (93.0) | AE– | 18 (25.4) | 48 (67.6) | 66 (93.0) |
| AE+ | 2 (2.8) | 3 (4.2) | 5 (7.0) | AE+ | 2 (2.8) | 3 (4.2) | 20 (28.2) |
| 25 (35.2) | 46 (64.8) | 71 | 20 (28.2) | 51 (71.8) | 71 | ||
| Prolactinoma | R– | R+ | Prolactinoma | IE– | IE+ | ||
| AE– | 17 (30.4) | 38 (67.9) | 38 (67.9) | AE– | 5 (8.9) | 50 (89.4) | 50 (89.4) |
| AE+ | 1 (1.8) | — | 1 (1.8) | AE+ | 1 (1.8) | — | 1 (1.8) |
| 18 (32.1) | 38 (67.9) | 56 | 6 (10.7) | 50 (89.4) | 56 | ||
| Tumor size | Tumor size | ||||||
| Microadenoma | R– | R+ | Microadenoma | IE– | IE+ | ||
| AE– | 19 (19.8) | 71 (74.0) | 90 (93.8) | AE– | 19 (19.8) | 71 (74.0) | 90 (93.8) |
| AE+ | 2 (2.1) | 4 (4.2) | 6 (6.4) | AE+ | 2 (2.1) | 4 (4.2) | 6 (6.4) |
| 21 (21.9) | 75 (78.1) | 96 | 21 (21.9) | 75 (78.1) | 96 | ||
| Macroadenoma | R– | R+ | Macroadenoma | IE– | IE+ | ||
| AE– | 40 (41.7) | 50 (52.1) | 90 (93.7) | AE– | 19 (19.8) | 71 (74.0) | 90 (93.8) |
| AE+ | 4 (4.2) | 2 (2.1) | 6 (6.4) | AE+ | 2 (2.1) | 4 (4.2) | 6 (6.4) |
| 44 (45.8) | 52 (54.2) | 96 | 21 (21.9) | 75 (78.1) | 96 | ||
| Giant adenoma | R– | R+ | Giant adenoma | IE– | IE+ | ||
| AE– | 6 (100) | — | 6 (100) | AE– | — | 6 (100) | 6 (100) |
| AE+ | — | — | — | AE+ | — | — | — |
| 6 (100) | — | 6 | — | 6 (100) | 6 | ||
| Surgical status | Surgical status | ||||||
| First operation | R– | R+ | First operation | IE– | IE+ | ||
| AE– | 42 (27.4) | 105 (68.2) | 147 (95.5) | AE– | 21 (13.6) | 126 (81.8) | 147 (95.5) |
| AE+ | 3 (1.9) | 4 (2.6) | 7 (4.5) | AE+ | 1 (0.6) | 6 (3.9) | 7 (4.5) |
| 45 (29.2) | 109 (70.8) | 154 | 22 (14.4) | 132 (85.7) | 154 | ||
| Reoperation | R– | R+ | Reoperation | IE– | IE+ | ||
| AE– | 23 (52.4) | 16 (36.4) | 39 (88.6) | AE– | 17 (38.6) | 22 (50.0) | 39 (88.6) |
| AE+ | 3 (6.8) | 18 (40.9) | 5 (11.4) | AE+ | 3 (6.8) | 2 (4.5) | 5 (11.4) |
| 26 (59.1) | 39 (88.6) | 44 | 20 (45.5) | 24 (54.5) | 44 | ||
| Year of operation | Year of operation | ||||||
| 2010-2015 | R– | R+ | 2010-2015 | IE– | IE+ | ||
| AE– | 39 (37.9) | 56 (54.4) | 95 (92.2) | AE– | 22 (21.4) | 73 (70.9) | 95 (92.2) |
| AE+ | 5 (4.9) | 3 (2.9) | 8 (7.8) | AE+ | 4 (3.9) | 4 (3.9) | 8 (7.8) |
| 44 (42.7) | 59 (57.4) | 103 | 26 (25.2) | 77 (74.8) | 103 | ||
| 2016-2018 | R– | R+ | 2016-2018 | IE– | IE+ | ||
| AE– | 26 (27.4) | 65 (68.4) | 91 (95.8) | AE– | 16 (16.8) | 75 (78.9) | 91 (95.8) |
| AE+ | 1 (1.1) | 3 (3.2) | 4 (4.2) | AE+ | — | 4 (4.2) | 4 (4.2) |
| 27 (28.4) | 68 (71.6) | 95 | 16 (16.8) | 79 (83.2) | 95 |
Definitions of remission, intended effect and adverse effect are provided in “Patients and Methods.”
Abbreviations: R+, remission achieved; R–, remission not achieved; AE+, adverse effect; AE– no adverse effect; IE+, intended effect achieved; IE–, intended effect not achieved.
Of the patients with filled in QoL questionnaires, 32 patients were in the good outcome group, of which 24 improved on the LBNQ-P (75.0%), 18 on the SF-36 MCS (56.3%), and 17 on the SF-36 PCS (53.1%). No patients showed a deterioration on the LBNQ-P, 7 on the SF-36 MCS (21.9%), and 9 on the SF-36 PCS (28.1%). Of the 9 patients with an intermediate outcome, 3 improved on the LBNQ-P (33.3%), 3 on the SF-36 MCS (33.3%), and 4 on the SF-36 PCS (44.4%). Deterioration occurred in 3 patients on the LBNQ-P (33.3%), 3 on the SF-36 MCS (33.3%), and 4 on the SF-36 PCS (44.4%). There were no patients with filled in questionnaires in the poor outcome group (Table 4, section A).
Table 4.
Change on quality of life questionnaires in the different IOQs; relevant change was defined as a change of at least 0.5 standard deviations. (A) Integrated outcome quadrants with remission versus adverse effect. (B) Integrated outcome quadrants with intended effect and adverse effect
| A | B | ||||||||
|---|---|---|---|---|---|---|---|---|---|
| LBNQ-P | IOQ-1 | IOQ-2 | IOQ-3 | IOQ-4 | LBNQ-P | IOQ-1 | IOQ-2 | IOQ-3 | IOQ-4 |
| Improved | 24 (75.0) | 0 | 3 (37.5) | — | Improved | 26 (74.3) | 0 | 1 (20.0) | — |
| No change | 8 (25.0) | 0 | 3 (37.5) | — | No change | 9 (25.7) | 0 | 2 (40.0) | — |
| Deteriorated | 0 | 1 (100) | 2 (25.0) | — | Deteriorated | 0 | 1 (100) | 2 (40.0) | — |
| SF-36 MCS | IOQ-1 | IOQ-2 | IOQ-3 | IOQ-4 | SF-36 MCS | IOQ-1 | IOQ-2 | IOQ-3 | IOQ-4 |
| Improved | 18 (56.3) | 1 (100) | 2 (25.0) | — | Improved | 19 (54.3) | 1 (100) | 1 (20.0) | — |
| No change | 7 (21.9) | 0 | 3 (37.5) | — | No change | 8 (22.9) | 0 | 2 (40.0) | — |
| Deteriorated | 7 (21.9) | 0 | 3 (37.5) | — | Deteriorated | 8 (22.9) | 0 | 2 (40.0) | — |
| SF-36 PCS | IOQ-1 | IOQ-2 | IOQ-3 | IOQ-4 | SF-36 PCS | IOQ-1 | IOQ-2 | IOQ-3 | IOQ-4 |
| Improved | 17 (53.1) | 0 | 4 (50.0) | — | Improved | 20 (57.1) | 0 | 1 (20.0) | — |
| No change | 6 (18.8) | 0 | 1 (12.5) | — | No change | 6 (17.1) | 0 | 1 (20.0) | — |
| Deteriorated | 9 (28.1) | 1 (100) | 3 (37.5) | — | Deteriorated | 9 (25.7) | 1 (100) | 3 (60.0) | — |
Abbreviations: IOQ, integrated outcome quadrant; IOQ1, intended effect achieved without adverse effect; IOQ2, intended effect achieved with adverse effect; IOQ3, intended effect not achieved without adverse effect; IOQ4, intended effect not achieved with adverse effect.
Outcome Squares Integrating Intended Effect and Adverse Effect
The intended effect (remission or specified alternative objective) was achieved in 156 of 198 operations (78.8%). Of these, 148 (74.7%) had a good, while 4 (2.0%) had a poor outcome. Good outcome was present in 73 out of 103 surgeries (70.9%) before 2016, and in 75 out of 95 (78.9%) from 2016 onwards (P = .052). Poor outcome was observed in 4 out of 103 surgeries (3.9%) before 2016, and in none of the cases from 2016 onwards (ns).
Intermediate outcome was observed in 46 patients (23%). In 38 (19%) the intended effect was not achieved but no permanent damage occurred either. In 8 the intended effect of surgery was achieved, however, adverse effects were also present. Intermediate outcome was most prevalent in Cushing’s disease (30%) and least prevalent in prolactinomas (9%).
When the objective of surgery was achieving remission, the goal was achieved in 118 of 159 operations (74.2%): 41/57 (71.9%) for acromegaly, 45/65 (69.2%) for Cushing’s disease, and 32/37 (86.5%) for prolactinomas. One hundred and twelve operations (70.4%) were classified as good, and 4 (2.5%) as poor outcome. Remission was not the primary intended effect of surgery in 39 operations. In these debulking operations, the intended effect was achieved in 38 (97.4%), and 36 (92.4%) had a good outcome. No patients had a poor outcome. Unexpectedly, in 7 operations intended as debulking, remission was achieved (17.9%).
Detailed statistics can be found in Table 3, section B, and elsewhere (Tables S3b, S4b, and S5b (19)).
Of the patients with filled in QoL-questionnaires, 35 patients were in the good outcome group, of which 26 improved on the LBNQ-P (74.3%), 19 on the SF-36 MCS (54.3%), and 20 on the SF-36 PCS (57.1%). No patients showed a deterioration on the LBNQ-P, 8 on the SF-36 MCS (22.9%), and 9 on the SF-36 PCS (25.7%). Of the 6 patients with an intermediate outcome, 1 improved on the LBNQ-P (16.7%), 2 on the SF-36 MCS (33.3%), and 1 on the SF-36 PCS (16.7%). Deterioration occurred in 3 patients on the LBNQ-P (50.0%), 2 on the SF-36 MCS (33.3%), and 4 on the SF-36 PCS (66.7%). There were no patients with filled in questionnaires in the poor outcome group (Table 4, section B).
Discussion
This study uses a novel way to classify and present patient relevant integrated outcomes of surgery, which provides an overall view of the important balance between the efficacy and safety of an intervention at a glance. This approach results in uniform reporting, even in a heterogeneous population with various tumors and several surgical objectives and facilitates more accurate information for individualized patient counselling. Additionally, these figures can be used to compare outcomes of different treatments of a disease, such as surgery and medication, and allow flexibility in definition of intended and adverse effects.
In the complex care for pituitary adenoma patients, personalized medicine has been emerging over the last decades, as there is a highly individual optimal balance between efficacy and safety in differing disease characteristics and treatment options (20). We recognize the value of different grading systems in various endocrine diseases aiming at providing personalized medicine. In particular in acromegaly, grading systems and treatment algorithms provide views on biomarkers, pathology, and clinical characteristics that are predictive of outcome of medical treatment or multimodality treatment outcome in acromegaly (21, 22). Outcome squares provide an integrated representation of efficacy and safety outcomes of a complete cohort of patients, or any case mix. Therefore, outcome squares can be used in combination with any preferred grading or predictor system. The proposed outcome squares system is flexible. The only requirement are a well-defined patient (sub)group, with a clear definition of the intended and adverse effect of the analyzed intervention.
The categories reported in this study are based on clinician-reported core data as the intended effect is based on tumor resection and endocrine remission or liaised objective achieved and adverse effects presented as surgical complications. With this categorization, improvement of QoL seems to occur more often in the good outcome group, especially on the LBNQ-P, although the sample size is still small. Ultimately the categories should be further evaluated by including also other patient-relevant outcomes, such as symptomatology, health-related QoL, disease burden, and functional outcomes into the intended/adverse effect definition. Changes in wellbeing and disease burden could, theoretically, be seen as the intended effect (when positive) or adverse effect (when negative) of an intervention. This approach can be used to show outcomes in all surgical fields, both endocrine and nonendocrine, where the delicate balance of efficacy and safety dictates the outcome of interventions.
The outcome square also uncovers 2 intermediate outcome groups of interest: a “no harm done” group (IOQ-3) and the “intended effect at a cost” group (IOQ-2). In the IOQ-3 group, an additional intervention may still result in achieving the intended effect without adverse. In milder cases of functioning tumors this may be a favorable outcome of surgery, leaving perspective of reoperation and alternative treatment options. In contrast, the IOQ-2 may be an acceptable scenario in severe disease cases where accepting pituitary function loss is preferred over ongoing hormone hypersecretion, particularly when this is not accompanied by significant loss of QOL. Outcome squares are a basis to start this discussion and to link the balance efficacy/safety to QoL.
With the presentation of results as illustrated in this study, a care provider can easily recognize patient groups at risk for good or adverse outcomes during treatment and modify treatment strategies accordingly. Patient groups with less favorable outcome pattern anticipated should be thoroughly counselled including critical appraisal of treatment alternatives. Alternatively, patients facing a good outcome pattern can be reassured about the anticipated chances to have a good outcome and the consequences of intermediate outcomes can be placed into the individual perspective. At a group level, patient groups with different outcome patterns can be compared to better understand performance of the team and assess why this group performs better than others and apply this knowledge in counselling and treatment decisions. In this process it is as important to analyze the patients with a poor outcome as all the patients in which a good outcome is achieved to assess which factors and processes lead to this outcome (23). For care providers, it is imminent to critically analyze the results of the care delivered at their center. A system of quality registration should, therefore, be in place. In the pituitary field, this registration is required for all Pituitary Tumor Centers of Excellence (24). For the comparability between centers and studies, it is of critical importance that centers use the same set of definitions for outcome parameters, and international discussion and consensus is, therefore, warranted.
The presentation of objectives/complications we present in this study is especially relevant in pituitary surgery. As a team we believe the balance between positive effects of the intervention and surgery-induced loss of function are of pivotal importance to the patient’s wellbeing; however, that has not been proven with data linking this balance and QoL (25). Functioning pituitary adenomas constitute a rare, heterogeneous, and complex disease. Groups of patients with a similar tumor type, growth pattern, and surgical objective are very small. To allow analysis of the effect of intervention, subgroups must be bundled to be able to have an adequate sample size. This presentation with IOQs allows the bundling of interventions with differing objectives, while still capturing what is important to the patient. Within the pituitary field further refinement can be made by analyzing larger groups of patients and, secondly, by obtaining international consensus by clinicians as well as with patient representatives on which outcomes are most relevant for decision-making.
Remission rates in our series are in agreement with recent meta-analyses (60.6% vs 59.8% for acromegaly, 64.8% vs 79.2% for Cushing’s disease, and 67.9% vs 67% for prolactinomas, with our results vs meta-analysis, respectively) (7, 26, 27). As can be appreciated, an important difference between our study and most other similar studies is that we included all surgeries, including those for giant adenomas, apoplexy, and reoperations. Outcomes of centers should always be interpreted with caution and in the context of the setting and case mix. The results in this study project the outcomes of a tertiary referral center, which, in general, may differ from those of regional centers with a lower surgical volume. Our case mix includes patients with surgically challenging tumors, patients with previous treatment that did not result in biochemical control, or intolerance for medication referred to us to consider alternative treatment options. The effect on outcome of these challenging tumors is counterbalanced by growing experience with these tumors. However, results in this study are also from an experienced, high-volume, multidisciplinary team with many diagnostic and therapeutic options.
This consecutive surgical series contains an overrepresentation of microprolactinomas comparison with other surgical cohorts. We want to stress that this article aims to provide a novel way to report outcomes, and is not a justification of our policy regarding this condition. To some degree this relative overrepresentation is caused by a lower threshold for considering surgery for prolactinomas than in other centers, but mainly it is the result of an increasing number of referrals of microprolactinoma patients during the study period, which was partly generated by an ongoing trial investigating medical and surgical management of microprolactinomas to elucidate the role of surgery (ClinicalTrials.gov Identifier: NCT04107480). As microadenomas in general have relatively favorable outcomes compared with other subgroups, the overrepresentation of microprolactinomas may have skewed the overall outcomes of our center. We have included baseline criteria and outcomes of many subgroups in the tables and elsewhere (19) to overcome this limitation. Readers are encouraged to carefully assess outcomes of the most relevant subgroups for their own daily practice.
Limitations of our study are the small sample size, unavoidable in rare diseases, and the heterogeneity in patient groups. Moreover, during the study period a few important innovations were implemented. Most notably, the volume of operations per year has increased more than 3-fold during the studied period. As clearly described in a review by Honegger and Grimm, the surgeon’s volume of transsphenoidal pituitary surgeries is pivotal for its outcomes (28). Honegger and Grimm take a cut-off point of 50 surgeries per year in defining high-volume centers. From 2015 onwards, this cut-off was consistently exceeded when counting endoscopic transsphenoidal surgeries for pituitary tumors only. When comparing outcomes before and after this time point, remission rates increase and complication rates decline (Fig. 2). This underlines the importance of the surgeon’s experience and volumes in this procedure. Another change was that we introduced a new protocol for our pituitary care pathway in 2016, including the introduction of our short-stay protocol and prospective outcomes registrations including PROMs (4). In this protocol, we decreased the minimal postoperative length of stay for select patients from 5 to 2 days and monitored patients remotely on a daily basis after discharge, and this initially resulted in a higher rate of readmissions.
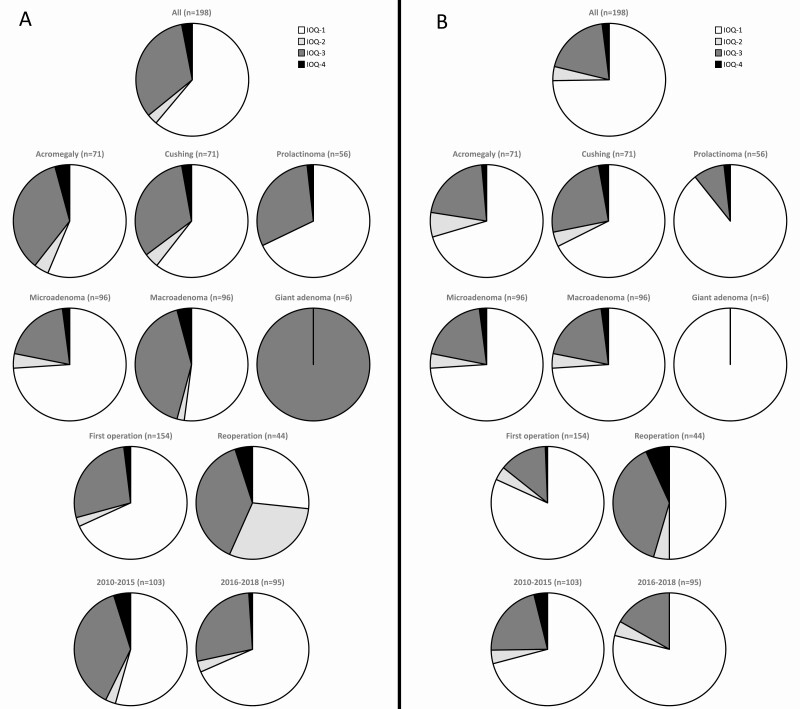
Figure 2.
Visual presentation of outcome squares presented in Table 3. Definitions of intended effect and adverse effect are provided in the methods section. (A) intended effect is always remission; (B) intended effect is as specified preoperative. IOQ1, intended effect achieved without adverse effect; IOQ2, intended effect achieved with adverse effect; IOQ3, intended effect not achieved without adverse effect; IOQ4, intended effect not achieved with adverse effect. Reporting of intended effect as specified preoperative provides a more reliable view of anticipated results. This is in particular true for subgroups in which debulking surgeries are more common (acromegaly, prolactinoma, macroadenomas, and giant adenomas). Irrespective of reporting remission or intended effect, we report improved outcomes from 2016 onwards with less patients with poor outcome (IOQ-4). The highest proportion IOQ-4 is observed in the reoperation subgroup, followed by the Cushing’s disease.
In conclusion, we present our results of endoscopic transsphenoidal surgery for pituitary tumors using a novel approach that provides a balanced view between efficacy, safety, and the surgical plan, which will assist physicians in the counselling of patients with a functioning tumor. It prompts physicians to critically analyze the risks and benefits of this complex procedure for each case individually and at a group level for quality of care purposes has potential for future refinement. The model shows that the majority of patients with a functioning pituitary adenoma can expect remission without the occurrence of adverse effects and that when taking into account the intended effect these percentages are even more favorable. With this integrated representation we are able to show improvement in our outcomes with higher volumes recent years, which were not significant when remission and adverse effect are reported separately. Pilot data suggest a better outcome in QoL data in the good outcome group; however, more data are needed to link PROMs with clinician-reported outcome data and further understand the consequences of intermediate outcomes and poor outcomes. The small subset of patients with a poor outcome (no remission and adverse effect) requires specific evaluation in the quality of full cycle of care evaluation (eg, decision-making, surgery, postoperative care) in order to minimize this group.
Acknowledgments
Financial Support: No specific funding was received for this research.
Glossary
Abbreviations
- CSF
cerebrospinal fluid
- DI
diabetes insipidus
- IOQ
integrated outcome quadrant
- MCS
mental component score
- MRI
magnetic resonance imaging
- PCS
physical component score
- PROM
patient-reported outcome measure
- QoL
quality of life
- VBHC
value-based healthcare
Additional Information
Disclosures: The authors have no relevant disclosures regarding this study.
Data Availability
Datasets generated during and/or analyzed during the current study are not publicly available but are available from the corresponding author on reasonable request.
References
- 1. Porter ME. Value-based health care delivery. Ann Surg. 2008;248(4):503-509. [DOI] [PubMed] [Google Scholar]
- 2. Porter ME, Teisberg EO. How physicians can change the future of health care. JAMA. 2007;297(10):1103-1111. [DOI] [PubMed] [Google Scholar]
- 3. Lobatto DJ, Vliet Vlieland TPM, van den Hout WB, et al. . Feasibility, safety and outcomes of a stratified fast-track care trajectory in pituitary surgery. Endocrine. 2020;69(1):175-187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Lobatto DJ, Zamanipoor Najafabadi AH, de Vries F, et al. . Towards value-based health care in pituitary surgery: application of a comprehensive outcome set in perioperative care. Eur J Endocrinol. 2019;181(4):375-387. [DOI] [PubMed] [Google Scholar]
- 5. Zamanipoor Najafabadi AH, Zandbergen IM, de Vries F, et al. . Surgery as a viable alternative first-line treatment for prolactinoma patients. a systematic review and meta-analysis. J Clin Endocrinol Metab. 2020;105(3):e32-e41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Broersen LHA, van Haalen FM, Biermasz NR, et al. . Microscopic versus endoscopic transsphenoidal surgery in the Leiden cohort treated for Cushing’s disease: surgical outcome, mortality, and complications. Orphanet J Rare Dis. 2019;14(1):64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Phan K, Xu J, Reddy R, Kalakoti P, Nanda A, Fairhall J. Endoscopic endonasal versus microsurgical transsphenoidal approach for growth hormone-secreting pituitary adenomas-systematic review and meta-analysis. World Neurosurg. 2017;97:398-406. [DOI] [PubMed] [Google Scholar]
- 8. Knosp E, Steiner E, Kitz K, Matula C. Pituitary adenomas with invasion of the cavernous sinus space: a magnetic resonance imaging classification compared with surgical findings. Neurosurgery. 1993;33(4):610-7; discussion 617. [DOI] [PubMed] [Google Scholar]
- 9. Casanueva FF, Molitch ME, Schlechte JA, et al. . Guidelines of the Pituitary Society for the diagnosis and management of prolactinomas. Clin Endocrinol (Oxf). 2006;65(2):265-273. [DOI] [PubMed] [Google Scholar]
- 10. Katznelson L, Laws ER Jr, Melmed S, et al. ; Endocrine Society . Acromegaly: an endocrine society clinical practice guideline. J Clin Endocrinol Metab. 2014;99(11):3933-3951. [DOI] [PubMed] [Google Scholar]
- 11. Nieman LK, Biller BM, Findling JW, et al. ; Endocrine Society . Treatment of Cushing’s syndrome: an endocrine society clinical practice guideline. J Clin Endocrinol Metab. 2015;100(8):2807-2831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Melmed S, Bronstein MD, Chanson P, et al. . A Consensus Statement on acromegaly therapeutic outcomes. Nat Rev Endocrinol. 2018;14(9):552-561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. van Furth WR, de Vries F, Lobatto DJ, et al. . Endoscopic surgery for pituitary tumors. Endocrinol Metab Clin North Am. 2020;49(3):487-503. [DOI] [PubMed] [Google Scholar]
- 14. Giustina A, Chanson P, Bronstein MD, et al. ; Acromegaly Consensus Group . A consensus on criteria for cure of acromegaly. J Clin Endocrinol Metab. 2010;95(7):3141-3148. [DOI] [PubMed] [Google Scholar]
- 15. Biller BM, Grossman AB, Stewart PM, et al. . Treatment of adrenocorticotropin-dependent Cushing’s syndrome: a consensus statement. J Clin Endocrinol Metab. 2008;93(7):2454-2462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Melmed S, Casanueva FF, Hoffman AR, et al. ; Endocrine Society . Diagnosis and treatment of hyperprolactinemia: an Endocrine Society clinical practice guideline. J Clin Endocrinol Metab. 2011;96(2):273-288. [DOI] [PubMed] [Google Scholar]
- 17. Pelsma ICM, Verstegen MJT, de Vries F, et al. . Quality of care evaluation in non-functioning pituitary adenoma with chiasm compression: visual outcomes and timing of intervention clinical recommendations based on a systematic literature review and cohort study. Pituitary. 2020;23(4):417-429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. de Vries F, Lobatto DJ, Verstegen MJT, van Furth WR, Pereira AM, Biermasz NR. Postoperative diabetes insipidus: how to define and grade this complication? Pituitary. 2021;24(2):284-291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Friso de Vries F, , LobattoDJ, , VerstegenMJT et al. Supplemental tables to Outcome squares integrating efficacy and safety, as applied to functioning pituitary adenoma surgery. Deposited November 14, 2020. ProMED-mail website. Figshare. https://figshare.com/articles/online_resource/Supplemental_tables_to_Outcome_Squares_integrating_efficacy_and_safety_as_applied_to_functioning_pituitary_adenoma_surgery/13134821.
- 20. Melmed S. Pituitary medicine from discovery to patient-focused outcomes. J Clin Endocrinol Metab. 2016;101(3):769-777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Cuevas-Ramos D, Carmichael JD, Cooper O, et al. . A structural and functional acromegaly classification. J Clin Endocrinol Metab. 2015;100(1):122-131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Kasuki L, Wildemberg LE, Gadelha MR. Management of endocrine disease: personalized medicine in the treatment of acromegaly. Eur J Endocrinol. 2018;178(3):R89-R100. [DOI] [PubMed] [Google Scholar]
- 23. Braithwaite J, Wears RL, Hollnagel E. Resilient health care: turning patient safety on its head. Int J Qual Health Care. 2015;27(5):418-420. [DOI] [PubMed] [Google Scholar]
- 24. Casanueva FF, Barkan AL, Buchfelder M, et al. ; Pituitary Society, Expert Group on Pituitary Tumors . Criteria for the definition of pituitary tumor centers of excellence (PTCOE): a Pituitary Society Statement. Pituitary. 2017;20(5):489-498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Webb SM, Santos A, Aulinas A, et al. . Patient-centred outcomes with pituitary and parasellar disease. Neuroendocrinology. 2020;110(9-10):882-888. [DOI] [PubMed] [Google Scholar]
- 26. Zamanipoor Najafabadi AH, Zandbergen IM, de Vries F, et al. . Surgery as a viable alternative first-line treatment for prolactinoma patients. A systematic review and meta-analysis. J Clin Endocrinol Metab. 2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Broersen LHA, Biermasz NR, van Furth WR, et al. . Endoscopic vs. microscopic transsphenoidal surgery for Cushing’s disease: a systematic review and meta-analysis. Pituitary. 2018;21(5):524-534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Honegger J, Grimm F. The experience with transsphenoidal surgery and its importance to outcomes. Pituitary. 2018;21(5):545-555. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Datasets generated during and/or analyzed during the current study are not publicly available but are available from the corresponding author on reasonable request.