Abstract
Context
The peptide neurotensin is implicated in insulin resistance, diabetes mellitus (DM), and cardiovascular disease.
Objective
We studied the association of neurotensin’s stable precursor, pro-neurotensin/neuromedin N (pro-NT/NMN) with incident metabolic syndrome (MetS) and DM.
Methods
We included 3772 participants from the REasons for Geographic and Racial Differences in Stroke (REGARDS) study who completed the baseline exam (2003-2007), the follow-up exam (2013-2016), and had pro-NT/NMN measured by immunoassay. Weighted logistic regression models were fitted to incident DM, incident MetS, and each MetS component, separately, incorporating demographics, metabolic risk factors, homeostasis model of insulin resistance (HOMA-IR), and diet scores. Incident MetS was defined by 3 or more harmonized criteria at follow-up in those with fewer than 3 at baseline. Incident DM was defined by use of hypoglycemic drugs/insulin, fasting glucose 126 mg/dL or greater, or random glucose 200 mg/dL or greater in those without these at baseline.
Results
Median (IQR) plasma pro-NT/NMN was 160 pmol/L (118-218 pmol/L). A total of 564 (of 2770 without baseline MetS) participants developed MetS, and 407 (of 3030 without baseline DM) developed DM. Per SD higher log-pro-NT/NMN, the demographic-adjusted odds ratio (OR) and 95% CI of incident MetS was 1.22 (1.11-1.35), 1.16 (1.00-1.35) for incident low high-density lipoprotein (HDL), and 1.25 (1.11-1.40) for incident dysglycemia. The association of pro-NT/NMN with MetS was attenuated in the model adding HOMA-IR (OR per SD log-pro-NT/NMN 1.14; 95% CI, 1.00-1.30). There was no association with incident DM (OR per SD log-pro-NT/NMN 1.06; 95% CI, 0.94-1.19).
Conclusion
Pro-NT/NMN was associated with MetS and 2 components, dysglycemia and low HDL, likely explained by insulin resistance.
Keywords: metabolic syndrome, diabetes mellitus, neurotensin, insulin resistance, biomarkers, prospective studies
In 2015, more than 24 million global deaths were jointly attributable to high fasting plasma glucose, abnormal cholesterol, high body mass index (BMI), and elevated systolic blood pressure (SBP) (1). Related through insulin resistance and obesity, these risk factors comprise metabolic syndrome (MetS), increasing risk for diabetes mellitus (DM), and cardiovascular disease (CVD) (2). The global prevalence of DM is predicted to soar to nearly 600 million by 2035 (3), with a parallel surge in the prevalence of MetS (4). Despite this, investigations examining the prospective risk of MetS or of its components are sparse.
Neurotensin is a tridecapeptide (5) released into circulation from specialized enteroendocrine cells in the small intestine (6) in response to a meal (7), found to upregulate glycogenolysis (8) and promote the absorption of dietary fatty acids in animal models (9-11). Neurotensin and its stable precursor, pro-neurotensin/neuromedin N (pro-NT/NMN) (12), have been implicated in obesity and insulin resistance in humans (13). Higher concentrations of pro-NT/NMN have been associated with risk of CVD (14-18) and DM (14, 16), but most of these studies were limited by a lack of participant geographic and racial diversity. Therefore, we examined the association of pro-NT/NMN with incident DM and MetS and assessed the factors underlying this association in a large, contemporary, and biracial cohort study.
Materials and Methods
Sample
The REasons for Geographic And Racial Differences in Stroke (REGARDS) cohort study enrolled 30 239 US White and Black participants age 45 years or older from 2003 to 2007 (19). Potential participants were randomly contacted from a public list and initial contact was made by telephone, during which verbal consent and medical history were obtained. Initial exclusion criteria included insufficient English proficiency, apparent cognitive impairment, anticipation of or actual residence in a nursing home, active malignancy, or any medical condition expected to preclude long-term follow-up. An initial in-home assessment was conducted for each participant to obtain written consent, biometrics, medication inventory, electrocardiogram, phlebotomy, and urine samples.
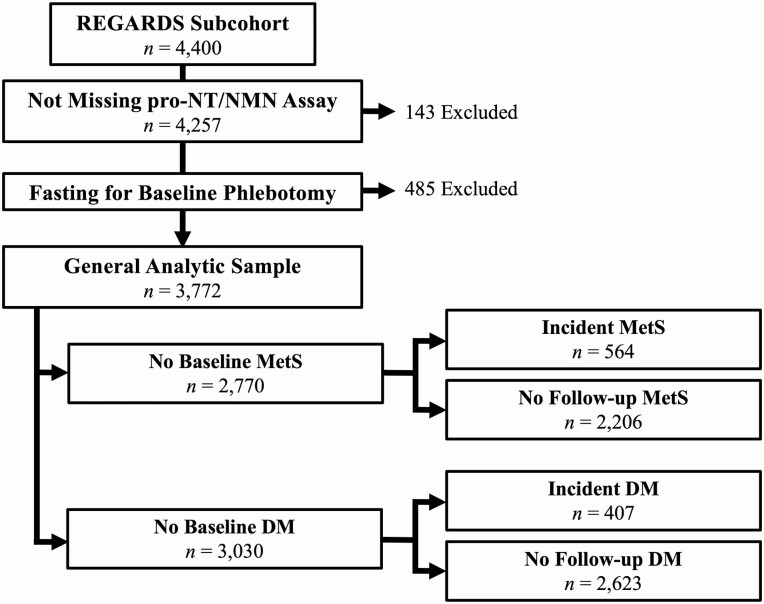
Participants were contacted by telephone semiannually for assessments and monitoring for outcomes. Additionally, a second in-home evaluation similar to the first was conducted from 2013 to 2016. Of the 13 912 eligible participants who completed the second in-home visit, a nested subcohort of 4400 participants was chosen by stratified random sampling and comprise the population included in this analysis. Participants were selected with equal allocation across sex-race groups, an approach that minimized bias in simulations. Of these participants, the analytic sample for cross-sectional analyses included participants fasting for phlebotomy and with a pro-NT/NMN measurement. For investigation of outcomes (MetS, MetS components, or DM), participants with the outcome at baseline were further excluded from the sample, as shown in Fig. 1.
Figure 1.
Participant inclusion diagram. DM, diabetes mellitus; MetS, metabolic syndrome; REGARDS, REasons for Geographic and Racial Differences in Stroke.
The methods of the REGARDS study and this investigation were approved by the institutional review boards of each involved institution. The REGARDS Publications Committee reviewed and approved the data analysis plan for this manuscript and reviewed and approved the final manuscript and its adherence to this plan.
Variables
Demographic variables included age (years), sex, and race. Lifestyle variables included tobacco use in pack-years and status (current, past, or never smoker) and current alcohol use (none, moderate [1-7 weekly drinks in women, 1-14 weekly drinks in men], or heavy [> 7 weekly drinks in women, > 14 weekly drinks in men]), or weekly exercise frequency (none, 1-3 times, or ≥ 4 times weekly). Biometric variables included waist circumference (in centimeters [cm]), SBP, and diastolic blood pressure (DBP; in millimeters of mercury [mmHg], mean of 2 resting measurements taken 5 minutes apart, via aneroid sphygmomanometer over the brachial artery) (20), and BMI (calculated as weight in kilograms divided by height in meters squared, using weight from a standard 300-lb (136-kg) calibrated scale and height obtained using an 8-foot (2.5-m) metal tape measure and square, both measured without shoes).
Participants’ regular dietary intake over the previous year was estimated with Block 98 Food Frequency Questionnaires (21) left with each participant after the baseline in-home visit to be completed and returned by mail. Exploratory and confirmatory factor analysis of the intake of 56 food groups was previously used to generalize dietary pattern scores in REGARDS (22). For the present analysis, 3 factor groups with relevance to dietary fat intake were considered. A pattern with heavy use of fried food, processed meats, added fats, egg products, and sweetened beverages similar to dietary patterns observed in the Southern United States was deemed the “Southern diet” pattern. Larger intake of a variety of dishes and fast foods was labeled the “convenience diet” pattern, and larger intake of miscellaneous sugars and desserts, sweetened breakfast food, chocolate, candy, solid fats, and oils was called the “sweets/fats” pattern. Diet factor scores were considered continuously in analyses, with a higher score indicating greater concordance with a specific dietary pattern.
Laboratory Variables
Fasting blood samples were drawn from each participant at both in-home visits, locally centrifuged for 10 minutes, then sent overnight on ice packs to the central laboratory at the University of Vermont, where they were centrifuged at 30 000g, aliquoted, and stored at –80 °C. Insulin was measured using a 2-step chemiluminescence immunoassay using the Roche Elecsys 2010 system (Roche Diagnostics). The homeostasis model of insulin resistance (HOMA-IR) score was calculated as the product of insulin (milli–international unit/liter; mIU/L) and glucose (milligram/deciliter; mg/dL), divided by 405 (23). At both visits, colorimetric reflectance spectrophotometry was used to measure glucose, total cholesterol, high-density lipoprotein (HDL), and triglycerides (TG) using the Ortho Vitros Clinical Chemistry System 950 IRC (Johnson & Johnson Clinical Diagnostics). Low-density lipoprotein (LDL) was estimated using the Friedewald equation (24).
Baseline pro-NT/NMN was measured in the subcohort by technicians blinded to clinical information at an independent facility (ASKA Biotech GmbH) using EDTA plasma on a 1-step sandwich immunoassay (SphingoTec GmbH), reported in picomoles per liter (pmol/L). This assay had a lower detection limit of 1.9 pmol/L, limit of quantification of 3 pmol/L, with a mean (SD) coefficient of variation of 3.7% (0.8%). Pro-NT/NMN was log-transformed for analyses in which it was considered continuously or dichotomized at the 50th percentile within each analytic sample for other analyses.
Study Outcomes
MetS was defined using the harmonized definition (2), requiring 3 or more of the following criteria for classification: increased waist circumference (≥ 102 cm in men, ≥ 88 cm in women), elevated TGs (≥150 mg/dL), decreased high-density lipoprotein (HDL < 40 mg/dL in men, < 50 mg/dL in women), elevated BP (SBP ≥ 130 mm Hg, DBP ≥ 85 mm Hg, or self-reported use of antihypertensive medications), or dysglycemia (fasting glucose ≥ 100 mg/dL or self-reported use of hypoglycemic medications or insulin). Incident MetS was identified in participants who were free of MetS at baseline (< 3 criteria) but were classified as having MetS (≥ 3 criteria) at the follow-up visit. DM was defined by self-reported use of hypoglycemic drugs or insulin, fasting glucose 126 mg/dL or greater, or random glucose 200 mg/dL or greater. Incident DM was identified in participants who were free of DM at the first in-home visit and were classified as having DM at the follow-up visit.
Statistical Analysis
Probability weighting of all analyses was used to account for sampling of the nested subcohort, stratified by sex and race. Participants missing values were excluded from the analyses for which data were absent. All tests were 2 sided, with α = .05 for main effects and interactions tested at α = .10, and performed with Stata version 16.1 (StataCorp). Four sequential and weighted logistic regression models were used to investigate the association of plasma pro-NT/NMN with each of incident MetS and DM, yielding odds ratios (OR) per SD log pro-NT/NMN or above vs below median pro-NT/NMN. Model 1 included age, race, and sex. Model 2 included model 1 variables plus waist circumference, weekly exercise frequency, alcohol use, tobacco use (pack-years), BMI (continuous), SBP, LDL, HDL, and TGs. Model 3 added HOMA-IR to model 2. Model 4 added convenience, Southern, and sweets/fats diet scores to model 3. Additionally, the association of pro-NT/NMN with development of each component of MetS was analyzed separately among participants not meeting the criterion at baseline in similar logistic regression models. Potential multicollinearity was excluded in each model with postestimation of variance inflation factors; all were fewer than 2 and thus not concerning for multicollinearity (25). The age-, race-, and sex-adjusted associations of log pro-NT/NMN with incident MetS, incident DM, and individual MetS criteria were displayed graphically using restricted cubic splines referencing the sample-specific median and using knots specified according to Harrell’s method (26). Kernel density plots were displayed contiguously.
A planned attenuation analysis was conducted to evaluate the confounding effect of individual risk factors (covariates in models 2-4) on a potential association of pro-NT/NMN with MetS or DM. Logistic regression model 1 was the base model, yielding ORwithout. Then, each individual covariate from models 2 to 4 was separately added to model 1, yielding an ORwith for each covariate. The degree of attenuation by each covariate of a model 1 association of pro-NT/NMN with the outcome was calculated by the following equation: %atten = (ORwithout – ORwith)/(ORwithout – 1) × 100 (27). A 95% CI on the degree of attenuation was estimated via percentiles from bootstrap procedures with 1000 replicate samples.
We conducted a sensitivity analysis to account for the impact of missing data for covariates used in regression models. Because diet scores were calculated based on paper mail-in surveys and HOMA-IR was not determined by design in those with baseline DM, these were not missing at random like other covariates, so multiple imputation was not used for these missing values (28). Participants missing diet score data were set as the median Southern, sweet/fats, and convenience diet score values for nonmissing participants classified as having a “poor” diet according to the American Heart Association’s “Life’s Simple 7.” (29) Since insulin level was not measured for those with DM at baseline, participants missing HOMA-IR were assigned the maximum value. For other covariates, chained regression equations were used to generate 20 multiple imputed datasets to account for missing data.
Results
Sample Characteristics
The general analytic sample included 3772 participants (see Fig. 1). Median (interquartile range; IQR) follow-up was 9.5 years (range, 8.7-10.0 years). Pro-NT/NMN ranged from 9 to 1431 pmol/L, with median (IQR) of 165 pmol/L (range, 119-227 pmol/L). Baseline characteristics of participants in the general analytic sample across pro-NT/NMN quartiles are provided in Table 1.
Table 1.
Baseline characteristics of the general analytic sample by pro-neurotensin/neuromedin N quartilea
| Characteristic (mean [95% CI] or %) | |||||
|---|---|---|---|---|---|
| Pro-NT/NMN quartiles | No. missing | Q1 (9-118 pmol/L) | Q2 (119-164 pmol/L) | Q3 (165-226 pmol/L) | Q4 (227-1430 pmol/L) |
| No. | 943 | 943 | 943 | 943 | |
| Age, y | 0 | 62.7 (62.1 to 63.3) | 63.4 (62.9 to 64.0) | 62.6 (62.1 to 63.1) | 63.3 (62.8 to 63.9) |
| Black race | 0 | 23.5 | 30.1 | 39.5 | 53.6 |
| Male sex | 0 | 48.5 | 45.2 | 44.1 | 39.9 |
| Tobacco use, pack-years | 83 | 10.6 (9.3 to 11.9) | 10.3 (9.0 to 11.5) | 9.5 (8.3 to 10.7) | 11.2 (9.7 to 12.6) |
| Current alcohol use | 58 | ||||
| None | 51.2 | 54.3 | 59.5 | 66.0 | |
| Moderate | 42.3 | 39.0 | 36.8 | 31.4 | |
| Heavy | 6.5 | 6.7 | 3.7 | 2.6 | |
| Weekly exercise frequency | 53 | ||||
| None | 26.5 | 25.8 | 30.9 | 32.3 | |
| 1-3 times | 38.0 | 40.4 | 39.0 | 38.9 | |
| ≥ 4 times | 35.6 | 33.8 | 30.1 | 28.9 | |
| Body mass index | 16 | 28.4 (28.0 to 28.7) | 28.8 (28.4 to 29.1) | 29.7 (29.3 to 30.1) | 30.5 (20.1 to 30.9) |
| Waist circumference, cm | 12 | 94 (92 to 95) | 94 (94 to 95) | 96 (95 to 97) | 98 (97 to 99) |
| Systolic blood pressure, mm Hg | 4 | 124 (123 to 125) | 125 (124 to 126) | 125 (124 to 126) | 127 (126 to 128) |
| Glucose, mg/dL | 0 | 167 (162 to 173) | 160 (154 to 166) | 152 (146 to 158) | 138 (132 to 145) |
| Insulin, mIU/L | 640b | 10.4 (9.8 to 10.9) | 11.3 (10.8 to 11.9) | 12.6 (11.7 to 13.6) | 15.8 (14.6 to 17.1) |
| HOMA-IR | 640b | 4.0 (3.7 to 4.2) | 4.1 (3.9 to 4.4) | 4.5 (4.1 to 4.8) | 5.0 (4.6 to 5.4) |
| Diabetes mellitus | 0 | 8.8 | 11.5 | 19.2 | 31.9 |
| Metabolic syndrome | 0 | 25.2 | 22.6 | 25.6 | 35.0 |
| Southern diet score | 886 | –0.24 (–0.30 to –0.18) | –0.15 (–0.22 to –0.08) | –0.07 (–0.14 to 0.01) | 0.08 (0.003 to 0.16) |
| Sweets/Fats diet score | 886 | 0.06 (–0.02 to 0.13) | 0.03 (–0.05 to 0.11) | 0.002 (–0.07 to 0.07) | –0.07 (–0.15 to 0.01) |
| Convenience diet score | 886 | 0.09 (0.03 to 0.16) | 0.13 (0.04 to 0.21) | 0.07 (–0.01 to 0.14) | –0.02 (–0.10 to 0.07) |
| Low-density lipoprotein, mg/dL | 59 | 118 (116 to 120) | 117 (115 to 119) | 114 (112 to 116) | 111 (109 to 113) |
| High-density lipoprotein, mg/dL | 17 | 52 (51 to 53) | 52 (51 to 54) | 53 (52 to 55) | 52 (51 to 53) |
| Triglycerides, mg/dL | 3 | 135 (129 to141) | 124 (119 to 129) | 120 (115 to 125) | 127 (122 to 132) |
Abbreviations: HOMA-IR, homeostasis model of insulin resistance; pro-NT/NMN, pro-neurotensin/neuromedin N; Q quartile.
a Weighted to parent cohort and excluding participants not fasting for phlebotomy and missing pro-NT/NMN measurement; n = 3772.
b Insulin was not measured in those with diabetes mellitus at baseline.
Association of Pro-Neurotensin/Neuromedin N With Incident Metabolic Syndrome
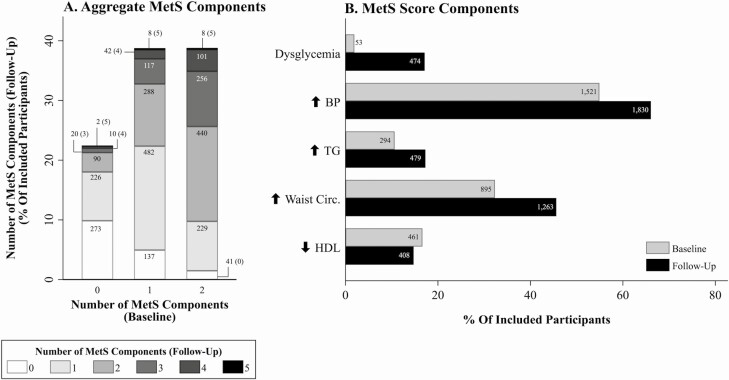
We identified 2770 individuals within the general analytic sample without MetS at baseline (see Fig. 1). Mean (SD) baseline BMI and waist circumference were 27.3 (5.3) and 91 cm (15 cm), respectively. In this subsample, pro-NT/NMN ranged from 14 to 1431 pmol/L, with median (IQR) of 160 pmol/L (range, 118-218 pmol/L). At baseline, 621 (22%) had 0; 1074 (39%) had 1; and 1075 (39%) had 2 MetS components. Incident MetS was observed in 564 (20%) participants. Changes in the number of components of MetS and each component between baseline and follow-up assessments are shown in Fig. 2.
Figure 2.
Bar graphs depicting number of A, metabolic syndrome (MetS) components and B, presence or absence of MetS at baseline and follow-up assessments in the MetS analytic sample. Value labels denote number of participants. BP, blood pressure; HDL, high-density lipoprotein; TG, triglycerides.
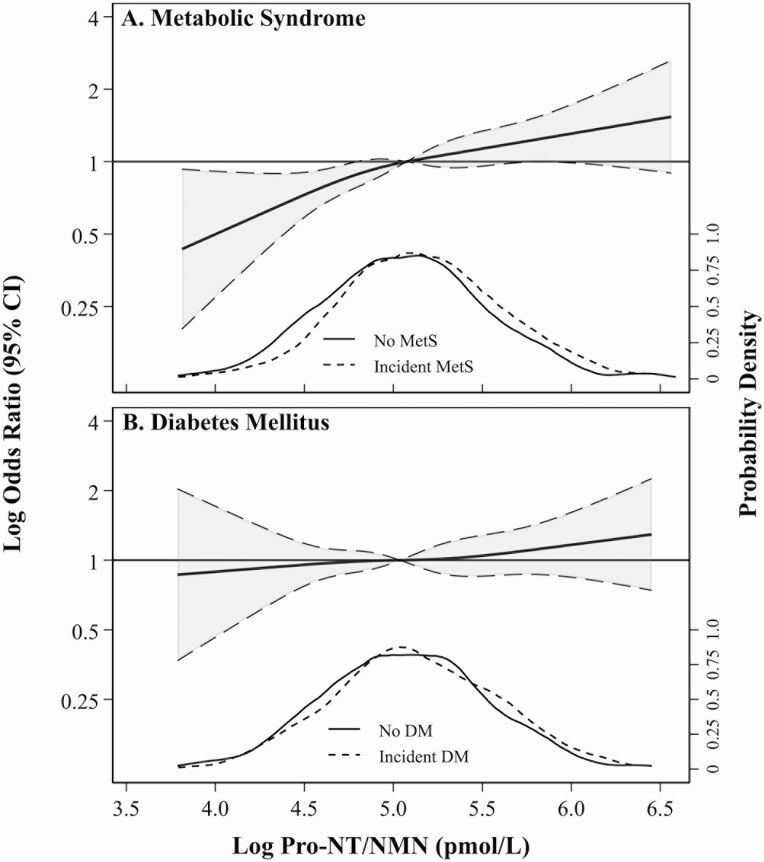
Table 2 and Fig. 3 show results of multivariable logistic regression models 1 to 4 of pro-NT/NMN with MetS. Pro-NT/NMN was associated with MetS overall in models 1 and 2; the association was attenuated and became statistically insignificant in models 3 and 4. The association of pro-NT/NMN with MetS did not significantly differ by sex (model 4 interaction P = .72), BMI (model 4 interaction P = .94), or race (model 4 interaction P = .53), so stratified analyses were not pursued.
Table 2.
Odds ratios (95% CI) for incident metabolic syndrome and diabetes mellitusa
| MetS (564 incident MetS/2770 at risk) | DM (407 incident DM/3030 at risk) | |||
|---|---|---|---|---|
| Per SD log pro-NT/NMN | Above vs below median pro-NT/NMN | Per SD log pro-NT/NMN | Above vs below median pro-NT/NMN | |
| Model 1 | 1.22 (1.11-1.35) | 1.30 (1.07-1.59) | 1.06 (0.94-1.19) | 1.12 (0.90-1.41) |
| Model 2 | 1.20 (1.07-1.34) | 1.25 (1.00-1.57) | 1.10 (0.97-1.24) | 1.15 (0.90-1.47) |
| Model 3 | 1.14 (1.00-1.30) | 1.08 (0.84-1.40) | 1.03 (0.91-1.17) | 1.04 (0.81-1.34) |
| Model 4 | 1.11 (0.96-1.28) | 1.11 (0.96-1.28) | 1.04 (0.90-1.20) | 1.12 (0.84-1.49) |
Median Pro-NT/NMN values: 160 pmol/L (for MetS analyses), 154 pmol/L (for DM analyses).
Covariates in weighted multivariable models: model 1: age, race, sex; model 2: model 1 + waist circumference, smoking (pack-years), weekly exercise frequency, alcohol use, systolic blood pressure, body mass index, low-density lipoprotein, high-density lipoprotein, and triglycerides; model 3: model 2 + HOMA-IR; and model 4: model 3 + Southern, sweets/fats, and convenience diet scores.
Abbreviations: DM, diabetes mellitus; MetS, metabolic syndrome; OR, odds ratio; pro-NT/NMN, pro-neurotensin/neuromedin N.
a Weighted to parent cohort.
Figure 3.
Restricted cubic splines presenting odds ratios (OR) and 95% CI (shaded) of incident A, metabolic syndrome (MetS) and B, diabetes mellitus (DM) across log pro-neurotensin/neuromedin N (pro-NT/NMN) considering age, race, and sex as covariates (model 1). Each spline references the subgroup-specific median and uses knots specified by Harrell’s method, set at 4.3, 4.9, 5.3, and 5.9 pmol/L (MetS); and 4.3, 4.9, 5.2, and 5.8 pmol/L (DM). Spline functions were cut at the subsample-specific 0.5th and 99.5th percentiles of log pro-NT/NMN. Kernel density plots show distributions of pro-NT/NMN within each subgroup, stratified by participants without (solid) and with (dashed) incident MetS or DM.
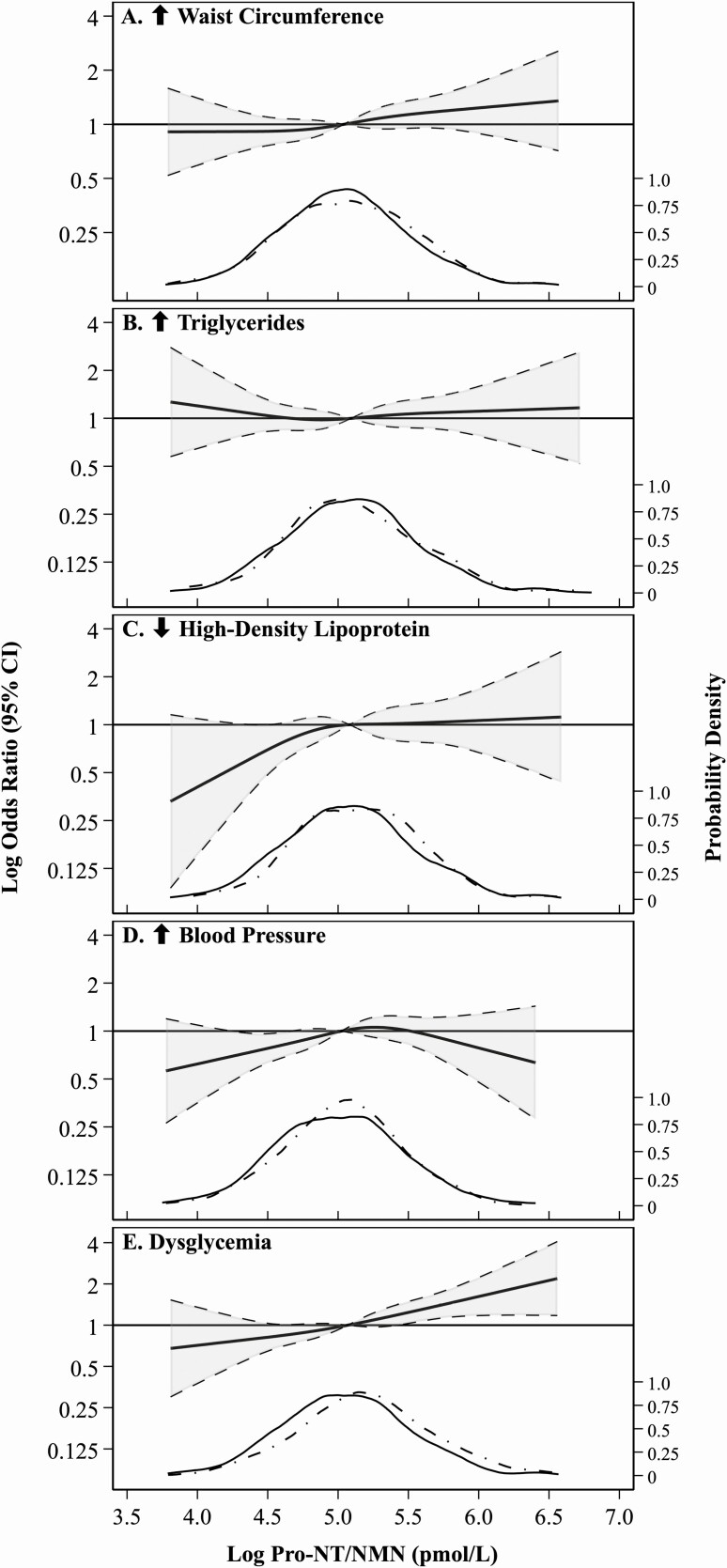
The associations of log pro-NT/NMN with incident MetS criteria are shown in Table 3 and Fig. 4. Along with excluding those with baseline MetS, participants meeting each given criterion at baseline were further excluded for each component-specific analysis. Pro-NT/NMN was significantly associated with incident low HDL in model 1 only (188 cases), and dysglycemia in models 1 and 2 (436 cases). Pro-NT/NMN was not associated with incidence of elevated waist circumference (528 cases), elevated BP (494 cases) or hypertriglyceridemia (344 cases) in any model.
Table 3.
Odds ratios for incident individual metabolic syndrome componentsa
| No. incident MetS/No. at risk | Odds ratio (95% CI) per SD log pro-NT/NMN | ||||
|---|---|---|---|---|---|
| Model 1 | Model 2 | Model 3 | Model 4 | ||
| ↑ Waist circumference | 528/1875 | 1.10 (0.98-1.22) | 1.02 (0.90-1.16) | 0.98 (0.85-1.12) | 0.94 (0.81-1.10) |
| ↑ Triglycerides | 344/2476 | 1.00 (0.88-1.15) | 1.04 (0.90-1.20) | 0.98 (0.83-1.15) | 0.97 (0.81-1.16) |
| ↓ HDL | 188/2309 | 1.16 (1.001-1.35) | 1.15 (0.98-1.36) | 1.14 (0.96-1.37) | 1.06 (0.87-1.29) |
| ↑ Blood pressure | 494/1249 | 1.09 (0.96-1.24) | 1.05 (0.92-1.20) | 1.09 (0.95-1.26) | 1.09 (0.93-1.28) |
| Dysglycemia | 436/2717 | 1.25 (1.11-1.40) | 1.21 (1.06-1.37) | 1.01 (0.85-1.20) | 1.07 (0.88-1.31) |
a Analyses include participants without MetS and without the given criterion at baseline; sample-weighted.
Abbreviations: HOMA-IR, homeostasis model of insulin resistance; MetS, metabolic syndrome; pro-NT/NMN, pro-neurotensin/neuromedin N.
MetS component definitions: ↑ waist circumference: ≥ 102 cm in men or ≥ 88 cm in women; ↑ triglycerides: ≥150 mg/dL; ↓ HDL < 40 mg/dL in men, < 50 mg/dL in women; ↑ blood pressure: systolic blood pressure ≥ 130 mm Hg, diastolic blood pressure ≥ 85 mm Hg, or self-reported use of antihypertensive medications; dysglycemia: fasting glucose ≥ 100 mg/dL or self-reported use of hypoglycemic medications or insulin.
Covariates in weighted multivariable models: model 1: age, race, sex; model 2: model 1 + waist circumference, smoking (pack-years), exercise level, alcohol use, systolic blood pressure, body mass index, low-density lipoprotein, high-density lipoprotein, and triglycerides; model 3: model 2 + HOMA-IR; model 4: model 3 + Southern, sweets/fats, and convenience diet scores.
Figure 4.
Restricted cubic splines presenting risk of each criterion of metabolic syndrome (MetS) in the MetS analytic sample across log pro-neurotensin/neuromedin N (pro-NT/NMN), considering model 1 covariates and referencing the subsample-specific median. Knots were specified by Harrell’s method, set at 4.3, 4.9, 5.2, and 5.9 pmol/L (increased waist circumference); 4.4, 4.9, 5.3, and 5.9 pmol/L (elevated triglycerides); 4.4, 4.9, 5.3, and 5.9 pmol/L (decreased high-density lipoprotein); 4.3, 4.9, 5.2, and 5.8 pmol/L (elevated blood pressure); and 4.3, 4.9, 5.3, and 5.9 pmol/L (dysglycemia). Spline functions were cut at the subgroup-specific 0.5th and 99.5th percentiles of log pro-NT/NMN. Kernel density plots within each subgroup show distributions of pro-NT/NMN, stratified by participants without (solid) and with (dashed) incidence of the specific MetS criterion.
Table 4 shows estimated attenuation of the overall model 1 association of log pro-NT/NMN with incident MetS by each model 2 to 4 covariate. Baseline HOMA-IR had the largest estimated attenuation; LDL also statistically significantly attenuated the association. Other covariates did not significantly attenuate the association of log pro-NT/NMN with incident MetS.
Table 4.
Covariate attenuation of association of log pro-neurotensin/neuromedin N with incident metabolic syndromea
| Modelb | OR per SD (95% CI) log pro-NT/NMN | % Attenuation (95% CI)c | |
|---|---|---|---|
| Model 1, with age, race, and sex | 1.22 (1.11 to 1.35) | ||
| Waist circumference | 1.19 (1.08 to 1.32) | 13.3% | (–3.9% to 30.6%) |
| Smoking, pack-years | 1.23 (1.12 to 1.35) | –3.5% | (–14.9% to 7.9%) |
| Weekly exercise frequency | 1.22 (1.11 to 1.35) | -0.8% | (–9.6% to 8.0%) |
| Alcohol use | 1.21 (1.09 to 1.33) | 6.0% | (–3.3% to 15.4%) |
| Systolic blood pressure | 1.22 (1.11 to 1.35) | 0.8% | (–5.9% to 7.5%) |
| Body mass index | 1.17 (1.06 to 1.30) | 21.1% | (–0.4% to 42.6%) |
| Low-density lipoprotein | 1.20 (1.09 to 1.32) | 10.9% | (0.3% to 21.5%) |
| High-density lipoprotein | 1.24 (1.13 to 1.37) | –10.4% | (–27.3% to 6.6%) |
| Triglycerides | 1.24 (1.12 to 1.37) | –7.3% | (–15.9% to 1.3%) |
| HOMA-IR | 1.12 (1.00 to 1.25) | 47.9% | (1.7% to 94.1%) |
| Southern diet score | 1.20 (1.07 to 1.34) | 9.2% | (–23.1% to 41.5%) |
| Sweets/Fats diet score | 1.20 (1.08 to 1.35) | 7.9% | (–24.1% to 39.8%) |
| Convenience diet score | 1.20 (1.07 to 1.34) | 10.1% | (–22.4% to 42.7%) |
n = 2770
Abbreviations: HOMA-IR, homeostatic model assessment of insulin resistance; OR, odds ratio; pro-NT/NMN, pro-neurotensin/neuromedin N.
a Weighted to analytic cohort.
b Each covariate was individually added to model 1.
c Estimates obtained using bootstrapping with 1000 replicate samples.
Association of Pro-Neurotensin/Neuromedin N With Incident Diabetes Mellitus
There were 3030 individuals within the general analytic sample without DM at baseline (see Fig. 1). In this subsample, pro-NT/NMN ranged from 19 to 921 pmol/L, with median (IQR) of 154 pmol/L (range, 114-210 pmol/L). Incident DM was observed in 407 (13%) participants. Table 2 and Fig. 3 show results of multivariable logistic regression models 1 to 4 of pro-NT/NMN with DM. Pro-NT/NMN was not associated with DM overall in any model.
BMI, race, and sex did not significantly interact with log pro-NT/NMN in any model (model 4 race interaction P = .80; model 4 sex interaction P = .63; model 4 BMI interaction P = .65), so stratification was not pursued.
Interpretation of results of the sensitivity analyses accounting for missing data did not differ substantially from the primary analysis (data not shown).
Discussion
In the REGARDS study, a biracial and contemporary prospective cohort, plasma pro-NT/NMN was associated with incident MetS, but this association was attenuated and not statistically significant after multivariable adjustment. Specifically, each SD higher pro-NT/NMN increased the odds of incident MetS by 22% when considering demographics and other cardiometabolic risk factors. Controlling for HOMA-IR attenuated nearly half the association of pro-NT/NMN with incident MetS, suggesting that much of the association is explained by baseline insulin resistance or its associated physiology. Furthermore, each SD higher pro-NT/NMN was associated with 25% increased odds of developing dysglycemia and 16% increased odds of developing low HDL (according to the MetS definition) at follow-up. Plasma pro-NT/NMN was not associated with incident DM.
This investigation is the first to report a prospective association of a neurotensin-related peptide with the risk of MetS. This finding was not surprising, given the importance of insulin resistance in MetS (4) and implications of neurotensin in the pathogenesis of insulin resistance and its sequelae (30). Neurotensin appears to drive insulin resistance both at the tissue level and through facilitation of digestion and absorption of dietary lipids. Neurotensin likely increases fat emulsification and digestion through changes in small bowel motility (31), enhanced bile acid release (11), and increased pancreatic exocrine output (32). Augmentation of fatty acid absorption by intestinal mucosal cells appears to be mediated through neurotensin receptor-driven inhibition of adenosine 5′-monophosphate–activated protein kinase (13).
The influence of neurotensin on systemic glycemic homeostasis also appears multifaceted. Circulating neurotensin causes glucagon-independent, glycogenolysis-mediated hyperglycemia (8) and also inhibits insulin release from isolated pancreatic islets when plasma glucose is high (33). Neurotensin receptor 3, also known as sortilin, regulates translocation of the insulin-sensitive glucose transporter 4 in adipocytes and myocytes, offering a potential mechanism of tissue-level insulin insensitivity (34, 35). Systemic (36) and central nervous system (37) neurotensin-related peptides have been associated with the anorexigenic peptide leptin, offering a potential mechanism for long-term regulation of energy balance. Lastly, circulating neurotensin may penetrate the central nervous system to influence hypothalamic and pituitary neurotensin content (38, 39), driving interactions with other endocrine systems that could promote weight gain and insulin resistance, such as inhibition of thyrotropin-releasing hormone (40) or tonic stimulation of corticotropin-releasing hormone secretion (41, 42).
Interestingly, pro-NT/NMN was associated with the development of only 2 of 5 MetS criteria in the overall group. Associations of pro-NT/NMN with increased odds of incident dysglycemia and low HDL may be attributable to the aforementioned effects of neurotensin on insulin resistance. However, neurotensin may also decrease HDL through neurotensin receptor 2–mediated inhibition of serum lipolytic activity (43), paralleling similar effects of insulin resistance (44). That we observed no association of pro-NT/NMN with development of elevated plasma TGs over follow-up was unanticipated, given that insulin resistance increases TGs (44), neurotensin appears to independently increase plasma TGs in the short term (43), and a cross-sectional correlation of pro-NT/NMN with TG concentration was previously reported in one study of individuals with morbid obesity (45). Based on our findings and the literature, further study of the influence of neurotensin-related peptides on lipid metabolism and lipoproteins is important. That pro-NT/NMN was not associated with development of elevated BP is consistent with our recent report (C.D. Nicoli, unpublished data; manuscript submitted). Finding no association of pro-NT/NMN with incident increased waist circumference contrasts with a small positive association of pro-NT/NMN with waist circumference reported by Li et al (13), who excluded obese participants at baseline (13), whereas average BMI and waist circumference at baseline were high in the present study.
In human studies, neurotensin-related peptides are implicated in sequelae of insulin resistance, including new-onset obesity (13), nonalcoholic fatty liver disease (46), incident cardiovascular events (14-16, 18), and all-cause and cardiovascular mortality (14, 17). In obese individuals, higher plasma pro-NT/NMN is associated with vascular adipose tissue inflammation, suggesting a role for neurotensin in the pathogenesis of metabolic dysfunction in obesity (45). Interestingly, variation at the SORT1 locus (1p13), encoding sortilin, is a major risk factor for coronary heart disease (47). It is therefore necessary that subsequent studies explore MetS as a mediating factor in associations of pro-NT/NMN with these outcomes. We were surprised to find no significant association of pro-NT/NMN with incident DM, given several previous studies reporting prospective (14, 16) and cross-sectional (17, 46, 48) associations. These different results may be due to a lack of diversity of these prior prospective studies (49, 50). That pro-NT/NMN was otherwise associated with incident dysglycemia of lower threshold for the MetS definition would suggest that neurotensin drives a lower-grade insulin resistance than is necessary to meet the clinical threshold of DM, or that undetermined compensatory mechanisms counteract its physiologic effects.
This study has several limitations. While REGARDS improves greatly on the racial homogeneity of previous cohorts, results from our investigations may be less generalizable to race groups other than Black or White. Because plasma pro-NT/NMN concentrations increase markedly following a meal (51), variation between participants in the magnitude of this postprandial increase could have an effect on the outcome. Our exclusion of participants who were not fasting for phlebotomy and controlling for dietary patterns with high fat intake attempted to minimize this. Because DM and the MetS criteria are treated as dichotomies both clinically and in the present analysis, individuals with values closer to a given cutoff at baseline may be at higher risk to have a suprathreshold value in follow-up. We attempted to minimize the impact of this by controlling for continuous baseline values of the criteria in multivariable modeling. There were missing data for a number of covariates, but sensitivity analysis accounting for this confirmed the main results. Lastly, that the time interval between participants’ phlebotomy dates and measurement of pro-NT/NMN varied by participant raises concern for breakdown of pro-NT/NMN over time, but we have shown that peptides stored at –80 °C or colder are generally stable (52).
Strengths of this analysis include the high retention, inclusion of Black or White adults from a broad geographic representation in the United States, and long-term follow-up of REGARDS participants. Sex- and race-specific sampling weights reduced possible sampling bias. This subcohort has high power to observe associations of biomarkers with outcomes of interest. Furthermore, the use of in-person assessments and biological sampling of participants allowed us to determine the presence or absence of biometric and laboratory characteristics not otherwise self-reported by participants. Lastly, we were able to consider a laboratory proxy for pancreatic β-cell function, HOMA-IR, in multivariable modeling.
In conclusion, among participants in the REGARDS cohort, concentrations of the neurotensin precursor pro-NT/NMN were associated with incident MetS and its dysglycemia and low HDL components. A component of this association was attributable to baseline insulin resistance. Despite this and in contrast to prior studies, pro-NT/NMN was not associated with incident DM. Further investigation of neurotensin’s molecular interactions with lipid metabolism, long-term glycemic homeostasis, and other hormonal regulators of metabolism is needed.
Acknowledgments
The authors thank the other investigators, the staff, and the participants of the REGARDS study for their valuable contributions. A full list of participating REGARDS investigators and institutions can be found at https://www.uab.edu/soph/regardsstudy/.
Financial Support: This work was supported by the National Institute of Neurological Disorders and Stroke (NINDS) and the National Institute on Aging (NIA), National Institutes of Health, Department of Health and Human Service (cooperative agreement No. U01 NS041588). The content is solely the responsibility of the authors and does not necessarily represent the official views of the NINDS or the NIA. Representatives of the NINDS were involved in the review of the manuscript but were not directly involved in the collection, management, analysis, or interpretation of the data.
Glossary
Abbreviations
- BMI
body mass index
- CVD
cardiovascular disease
- DBP
diastolic blood pressure
- DM
diabetes mellitus
- HDL
high-density lipoprotein
- HOMA-IR
homeostasis model of insulin resistance
- LDL
low-density lipoprotein
- MetS
metabolic syndrome
- OR
odds ratio
- pro-NT/NMN
pro-neurotensin/neuromedin N
- REGARDS
REasons for Geographic and Racial Differences in Stroke
- SBP
systolic blood pressure
- TGs
triglycerides
Additional Information
Disclosures: A.P.C. has received investigator-initiated research funding from Amgen, Inc for unrelated work. J.S. is employed by SphingoTec GmbH, which developed and markets the pro-NT/NMN assay. The other authors have nothing to disclose. The authors report no other potential conflicts of interest, financial or otherwise.
Data Availability
Restrictions apply to the availability of data generated or analyzed during this study to preserve patient confidentiality or because they were used under license. The corresponding author will on request detail the restrictions and any conditions under which access to some data may be provided.
References
- 1. GBD 2015 Risk Factors Collaborators. Global, regional, and national comparative risk assessment of 79 behavioural, environmental and occupational, and metabolic risks or clusters of risks, 1990-2015: a systematic analysis for the Global Burden of Disease Study 2015. Lancet. 2016;388(10053):1659-1724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Alberti KG, Eckel RH, Grundy SM, et al. ; International Diabetes Federation Task Force on Epidemiology and Prevention; National Heart, Lung, and Blood Institute; American Heart Association; World Heart Federation; International Atherosclerosis Society; International Association for the Study of Obesity . Harmonizing the metabolic syndrome: a joint interim statement of the International Diabetes Federation Task Force on Epidemiology and Prevention; National Heart, Lung, and Blood Institute; American Heart Association; World Heart Federation; International Atherosclerosis Society; and International Association for the Study of Obesity. Circulation. 2009;120(16):1640-1645. [DOI] [PubMed] [Google Scholar]
- 3. Guariguata L, Whiting DR, Hambleton I, Beagley J, Linnenkamp U, Shaw JE. Global estimates of diabetes prevalence for 2013 and projections for 2035. Diabetes Res Clin Pract. 2014;103(2):137-149. [DOI] [PubMed] [Google Scholar]
- 4. Saklayen MG. The global epidemic of the metabolic syndrome. Curr Hypertens Rep. 2018;20(2):12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Carraway R, Leeman SE. The isolation of a new hypotensive peptide, neurotensin, from bovine hypothalami. J Biol Chem. 1973;248(19):6854-6861. [PubMed] [Google Scholar]
- 6. Polak JM, Sullivan SN, Bloom SR, et al. Specific localisation of neurotensin to the N cell in human intestine by radioimmunoassay and immunocytochemistry. Nature. 1977;270(5633):183-184. [DOI] [PubMed] [Google Scholar]
- 7. Go VLW, Demol P. Role of nutrients in the gastrointestinal release of immunoreactive neurotensin. Peptides. 1981;2(Suppl 2):267-269. [DOI] [PubMed] [Google Scholar]
- 8. Carraway RE, Demers LM, Leeman SE. Hyperglycemic effect of neurotensin, a hypothalamic peptide. Endocrinology. 1976;99(6):1452-1462. [DOI] [PubMed] [Google Scholar]
- 9. Armstrong MJ, Parker MC, Ferris CF, Leeman SE. Neurotensin stimulates [3H]oleic acid translocation across rat small intestine. Am J Physiol. 1986;251(6 Pt 1):G823-G829. [DOI] [PubMed] [Google Scholar]
- 10. Ferris CF, Hammer RA, Leeman SE. Elevation of plasma neurotensin during lipid perfusion of rat small intestine. Peptides. 1981;2(Suppl 2):263-266. [DOI] [PubMed] [Google Scholar]
- 11. Gui X, Dobner PR, Carraway RE. Endogenous neurotensin facilitates enterohepatic bile acid circulation by enhancing intestinal uptake in rats. Am J Physiol Gastrointest Liver Physiol. 2001;281(6):G1413-G1422. [DOI] [PubMed] [Google Scholar]
- 12. Friry C, Feliciangeli S, Richard F, Kitabgi P, Rovere C. Production of recombinant large proneurotensin/neuromedin N-derived peptides and characterization of their binding and biological activity. Biochem Biophys Res Commun. 2002;290(4):1161-1168. [DOI] [PubMed] [Google Scholar]
- 13. Li J, Song J, Zaytseva YY, et al. An obligatory role for neurotensin in high-fat-diet-induced obesity. Nature. 2016;533(7603):411-415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Melander O, Maisel AS, Almgren P, et al. Plasma proneurotensin and incidence of diabetes, cardiovascular disease, breast cancer, and mortality. JAMA. 2012;308(14):1469-1475. [DOI] [PubMed] [Google Scholar]
- 15. Januzzi JL Jr, Lyass A, Liu Y, et al. Circulating proneurotensin concentrations and cardiovascular disease events in the community: the Framingham Heart Study. Arterioscler Thromb Vasc Biol. 2016;36(8):1692-1697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Fawad A, Bergmann A, Struck J, Nilsson PM, Orho-Melander M, Melander O. Proneurotensin predicts cardiovascular disease in an elderly population. J Clin Endocrinol Metab. 2018;103(5):1940-1947. [DOI] [PubMed] [Google Scholar]
- 17. Wettersten N, Cushman M, Howard VJ, et al. Usefulness of proneurotensin to predict cardiovascular and all-cause mortality in a United States population (from the Reasons for Geographic and Racial Differences in Stroke study). Am J Cardiol. 2018;122(1):26-32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Nicoli CD, Wettersten N, Judd SE, et al. Pro-neurotensin/neuromedin N and risk of ischemic stroke: the REasons for Geographic and Racial Differences in Stroke (REGARDS) study. Vasc Med. 2020;25(6):534-540. [DOI] [PubMed] [Google Scholar]
- 19. Howard VJ, Cushman M, Pulley L, et al. The Reasons for Geographic and Racial Differences in Stroke study: objectives and design. Neuroepidemiology. 2005;25(3):135-143. [DOI] [PubMed] [Google Scholar]
- 20. Howard VJ, Woolson RF, Egan BM, et al. Prevalence of hypertension by duration and age at exposure to the stroke belt. J Am Soc Hypertens. 2010;4(1):32-41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Block G, Hartman AM, Dresser CM, Carroll MD, Gannon J, Gardner L. A data-based approach to diet questionnaire design and testing. Am J Epidemiol. 1986;124(3):453-469. [DOI] [PubMed] [Google Scholar]
- 22. Judd SE, Letter AJ, Shikany JM, Roth DL, Newby P. Dietary patterns derived using exploratory and confirmatory factor analysis are stable and generalizable across race, region, and gender subgroups in the REGARDS study. Front Nutr. 2015;1:29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Matthews DR, Hosker JP, Rudenski AS, Naylor BA, Treacher DF, Turner RC. Homeostasis model assessment: insulin resistance and beta-cell function from fasting plasma glucose and insulin concentrations in man. Diabetologia. 1985;28(7):412-419. [DOI] [PubMed] [Google Scholar]
- 24. Friedewald WT, Levy RI, Fredrickson DS. Estimation of the concentration of low-density lipoprotein cholesterol in plasma, without use of the preparative ultracentrifuge. Clin Chem. 1972;18(6):499-502. [PubMed] [Google Scholar]
- 25. Kock N, Lynn G. Lateral collinearity and misleading results in variance-based SEM: an illustration and recommendations. J Assoc Inf Syst. 2012;13(7):546-580. [Google Scholar]
- 26. Harrell FE Jr. Ordinal logistic regression. In: Regression Modeling Strategies: With Applications to Linear Models, Logistic and Ordinal Regression, and Survival Analysis. 2nd ed. Springer International Publishing; 2015:311-325. [Google Scholar]
- 27. VanderWeele TJ. Explanation in Causal Inference: Methods for Mediation and Interaction. Oxford University Press; 2015. [Google Scholar]
- 28. White IR, Royston P, Wood AM. Multiple imputation using chained equations: issues and guidance for practice. Stat Med. 2011;30(4):377-399. [DOI] [PubMed] [Google Scholar]
- 29. Kulshreshtha A, Vaccarino V, Judd SE, et al. Life’s Simple 7 and risk of incident stroke: the Reasons for Geographic and Racial Differences in Stroke study. Stroke. 2013;44(7):1909-1914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Blondeau N, Béraud-Dufour S, Lebrun P, Hivelin C, Coppola T. Sortilin in glucose homeostasis: from accessory protein to key player? Front Pharmacol. 2018;9:1561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Pellissier S, Eribon O, Chabert J, Gully D, Roche M. Peripheral neurotensin participates in the modulation of pre- and postprandial intestinal motility in rats. Neuropeptides. 1996;30(5):412-419. [DOI] [PubMed] [Google Scholar]
- 32. Wood JG, Hoang HD, Bussjaeger LJ, Solomon TE. Effect of neurotensin on pancreatic and gastric secretion and growth in rats. Pancreas. 1988;3(3):332-339. [DOI] [PubMed] [Google Scholar]
- 33. Dolais-Kitabgi J, Kitabgi P, Brazeau P, Freychet P. Effect of neurotensin on insulin, glucagon, and somatostatin release from isolated pancreatic islets. Endocrinology. 1979;105(1):256-260. [DOI] [PubMed] [Google Scholar]
- 34. Morris NJ, Ross SA, Lane WS, et al. Sortilin is the major 110-kDa protein in GLUT4 vesicles from adipocytes. J Biol Chem. 1998;273(6):3582-3587. [DOI] [PubMed] [Google Scholar]
- 35. Shi J, Kandror KV. Sortilin is essential and sufficient for the formation of Glut4 storage vesicles in 3T3-L1 adipocytes. Dev Cell. 2005;9(1):99-108. [DOI] [PubMed] [Google Scholar]
- 36. Barchetta I, Ciccarelli G, Cimini FA, et al. Association between systemic leptin and neurotensin concentration in adult individuals with and without type 2 diabetes mellitus. J Endocrinol Invest. 2018;41(10):1159-1163. [DOI] [PubMed] [Google Scholar]
- 37. Sahu A, Carraway RE, Wang YP. Evidence that neurotensin mediates the central effect of leptin on food intake in rat. Brain Res. 2001;888(2):343-347. [DOI] [PubMed] [Google Scholar]
- 38. Dorsa DM, de Kloet ER, Mezey E, de Wied D. Pituitary-brain transport of neurotensin: functional significance of retrograde transport. Endocrinology. 1979;104(6):1663-1666. [DOI] [PubMed] [Google Scholar]
- 39. von Euler G, Meister B, Hökfelt T, Eneroth P, Fuxe K. Intraventricular injection of neurotensin reduces dopamine D2 agonist binding in rat forebrain and intermediate lobe of the pituitary gland. Relationship to serum hormone levels and nerve terminal coexistence. Brain Res. 1990;531(1-2):253-262. [DOI] [PubMed] [Google Scholar]
- 40. Stolakis V, Kalafatakis K, Botis J, Zarros A, Liapi C. The regulatory role of neurotensin on the hypothalamic-anterior pituitary axons: emphasis on the control of thyroid-related functions. Neuropeptides. 2010;44(1):1-7. [DOI] [PubMed] [Google Scholar]
- 41. Nicot A, Rowe WB, De Kloet ER, et al. Endogenous neurotensin regulates hypothalamic-pituitary-adrenal axis activity and peptidergic neurons in the rat hypothalamic paraventricular nucleus. J Neuroendocrinol. 1997;9(4):263-269. [DOI] [PubMed] [Google Scholar]
- 42. Nussdorfer GG, Malendowicz LK, Meneghelli V, Mazzocchi G. Neurotensin enhances plasma adrenocorticotropin concentration by stimulating corticotropin-releasing hormone secretion. Life Sci. 1992;50(9):639-643. [DOI] [PubMed] [Google Scholar]
- 43. Piatek J, Witmanowski H, Paluszak J, Krauss H, Krawczyk J. The effects of neurotensin on selected parameters of lipid metabolism in rats. Peptides. 2005;26(5):837-843. [DOI] [PubMed] [Google Scholar]
- 44. Bjornstad P, Eckel RH. Pathogenesis of lipid disorders in insulin resistance: a brief review. Curr Diab Rep. 2018;18(12):127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Barchetta I, Cimini FA, Capoccia D, et al. Neurotensin is a lipid-induced gastrointestinal peptide associated with visceral adipose tissue inflammation in obesity. Nutrients. 2018;10(4):526:1-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Barchetta I, Cimini FA, Leonetti F, et al. Increased plasma proneurotensin levels identify NAFLD in adults with and without type 2 diabetes. J Clin Endocrinol Metab. 2018;103(6):2253-2260. [DOI] [PubMed] [Google Scholar]
- 47. Musunuru K, Strong A, Frank-Kamenetsky M, et al. From noncoding variant to phenotype via SORT1 at the 1p13 cholesterol locus. Nature. 2010;466(7307):714-719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Fawad A, Nilsson PM, Struck J, Bergmann A, Melander O, Bennet L. The association between plasma proneurotensin and glucose regulation is modified by country of birth. Sci Rep. 2019;9(1):13640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Lahmann PH, Lissner L, Gullberg B, Berglund G. Differences in body fat and central adiposity between Swedes and European immigrants: the Malmö Diet and Cancer Study. Obes Res. 2000;8(9):620-631. [DOI] [PubMed] [Google Scholar]
- 50. Berglund G, Nilsson P, Eriksson KF, et al. Long-term outcome of the Malmö Preventive Project: mortality and cardiovascular morbidity. J Intern Med. 2000;247(1):19-29. [DOI] [PubMed] [Google Scholar]
- 51. Ernst A, Hellmich S, Bergmann A. Proneurotensin 1-117, a stable neurotensin precursor fragment identified in human circulation. Peptides. 2006;27(7):1787-1793. [DOI] [PubMed] [Google Scholar]
- 52. Lewis MR, Callas PW, Jenny NS, Tracy RP. Longitudinal stability of coagulation, fibrinolysis, and inflammation factors in stored plasma samples. Thromb Haemost. 2001;86(6):1495-1500. [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Restrictions apply to the availability of data generated or analyzed during this study to preserve patient confidentiality or because they were used under license. The corresponding author will on request detail the restrictions and any conditions under which access to some data may be provided.