Abstract
Context
Suboptimal endometrial thickening is associated with lower pregnancy rates and occurs in some infertile women treated with clomiphene.
Objective
To examine cellular and molecular differences in the endometrium of women with suboptimal vs optimal endometrial thickening following clomiphene.
Methods
Translational prospective cohort study from 2018 to 2020 at a university-affiliated clinic. Reproductive age women with unexplained infertility treated with 100 mg of clomiphene on cycle days 3 to 7 who developed optimal (≥8mm; n = 6, controls) or suboptimal (<6mm; n = 7, subjects) endometrial thickness
underwent preovulatory blood and endometrial sampling.
The main outcome measures were endometrial tissue architecture, abundance and location of specific proteins, RNA expression, and estrogen receptor (ER) α binding.
Results
The endometrium of suboptimal subjects compared with optimal controls was characterized by a reduced volume of glandular epithelium (16% vs 24%, P = .01), decreased immunostaining of markers of proliferation (PCNA, ki67) and angiogenesis (PECAM-1), increased immunostaining of pan-leukocyte marker CD45 and ERβ, but decreased ERα immunostaining (all P < .05). RNA-seq identified 398 differentially expressed genes between groups. Pathway analysis of differentially expressed genes indicated reduced proliferation (Z-score = –2.2, P < .01), decreased angiogenesis (Z-score = –2.87, P < .001), increased inflammation (Z-score = +2.2, P < .01), and ERβ activation (Z-score = +1.6, P < .001) in suboptimal subjects. ChIP-seq identified 6 genes bound by ERα that were differentially expressed between groups (P < .01), some of which may play a role in implantation.
Conclusion
Women with suboptimal endometrial thickness after clomiphene exhibit aberrant ER expression patterns, architectural changes, and altered gene and protein expression suggesting reduced proliferation and angiogenesis in the setting of increased inflammation.
Keywords: Endometrium, infertility, clomiphene, proliferation, estrogen receptor, ChIP-seq
Adequate endometrial estrogen action during the follicular phase of the cycle is necessary to establish normal endometrial receptivity to embryo implantation (1). The essential action of estrogen offers a logical foundation for the strong, positive association between measurements of endometrial thickness and pregnancy rates during infertility treatment, including in vitro fertilization (2-4), unstimulated cycles (5), and insemination cycles (6). Clomiphene is a selective estrogen receptor modulator that acts centrally as an estrogen antagonist to induce pituitary gonadotropin synthesis and release. For more than 50 years, clomiphene has been the medication used most commonly for the treatment of infertility worldwide (7). However, clomiphene also has antiestrogenic effects within the female reproductive tract (8-10), which may impair endometrial growth and embryo implantation, thereby decreasing cycle fecundity (11-13).
Whereas most women receiving clomiphene treatment exhibit normal endometrial proliferation, a significant minority do not (14-19). Poor endometrial growth during treatment has been widely assumed to result from increased endometrial sensitivity to peripheral clomiphene estrogen antagonism, although the mechanism(s) responsible have not been investigated in detail (8-11, 20, 21).
Unexplained infertility is a diagnosis that generally includes unrecognized endometriosis, implantation defects, and, in theory, luteal phase defects or dysfunction (LPD). Whereas the importance and prevalence of LPD is unknown because there is no validated method for diagnosis (22, 23), decreased endometrial receptivity (24) and altered expression of receptivity markers (25-28) have been observed among women with unexplained infertility.
In recent years, clinicians have used letrozole in place of clomiphene. Compared with the central estrogen antagonistic effects and slow metabolism of clomiphene, letrozole decreases ovarian estrogen production directly by inhibiting aromatase activity and has a shorter half-life (29). Consequently, letrozole is not thought to have a direct effect on endometrium and thus should be less likely to adversely affect estrogen-stimulated endometrial growth than clomiphene (30). However, limited clinical studies observed no significant difference in average peak endometrial thickness among infertile women receiving clomiphene or letrozole (14, 30, 31). These observations suggest that the endometrium of women who exhibit poor endometrial thickening following clomiphene may be intrinsically different in a way that is revealed by such treatment (5). They also raise the intriguing possibility that an altered response to estradiol and clomiphene may contribute to otherwise unexplained infertility.
We hypothesized that poor endometrial proliferation is a consequence of inadequate or aberrant estrogen actions that may result from altered estrogen receptor expression and/or postreceptor responses. We recruited women with unexplained infertility receiving a fixed regimen of clomiphene treatment and identified those with robustly normal endometrial growth (endometrial thickness ≥8 mm) and others with clearly suboptimal growth (endometrial thickness <6 mm) (5), and compared serum sex hormone concentrations, tissue morphometrics, global gene expression, mRNA and protein expression, and genome-wide analysis of estrogen receptor binding in the 2 groups.
Materials and Methods
Human Subjects and Ethics Approval
Poor Endometrial Proliferation after Clomiphene (PEPC) was a translational prospective cohort study conducted at the University of North Carolina at Chapel Hill from 2018 to 2020. All infertile women aged 18-45 years receiving clomiphene citrate treatment (100 mg cycle days 3-7) for a diagnosis of unexplained infertility were screened for eligibility. All couples were English speaking and had a diagnosis of unexplained infertility in accordance with published best practices guidelines (32, 33), including objective evidence of normal ovulatory function (in natural cycles or after ovulation induction), a normal uterine cavity and bilateral tubal patency (documented by hysterosalpingogram), and a partner with normal semen quality (ejaculate containing ≥10 million total motile sperm). Women with any known endometriosis, adenomyosis, intrauterine lesion or congenital uterine malformation, recurrent pregnancy loss, undiagnosed vaginal bleeding or abnormal uterine bleeding, history of uterine surgery (operative hysteroscopy, myomectomy, or Cesarean delivery) or pelvic infection, any uncontrolled intercurrent illness, a body mass index (BMI) >38 kg/m2, or current tobacco use were excluded. Women who took clomiphene in the preceding month were not excluded due to the short half-life of the biologically active isomer (29), but women who had endometrial biopsy or diagnostic hysteroscopy within 2 months of enrollment were excluded due to potential functional effects related to tissue injury (34, 35).
This study was carried out in accordance with federal regulations governing human subjects research. All procedures were reviewed and approved by the Committee for the Protection of Human Subjects at the University of North Carolina at Chapel Hill under file #17-3106 prior to study initiation. Informed consent was obtained from all subjects before their participation in the study. Separate consents were obtained for consent to undergoing the endometrial biopsy procedure and for the storage of specimens.
Group Assignment
At the time of a clinically scheduled transvaginal ultrasound examination performed between stimulation cycle days 12 to 14 that demonstrated 1 or more mature ovarian follicles (mean diameter ≥19 mm2), women were assigned to 1 of 2 exposure groups based on their observed endometrial response, defined as “optimal” controls (endometrial thickness ≥8 mm) or “suboptimal” subjects (endometrial thickness <6 mm). Those women exhibiting an endometrial thickness measuring from 6.0 to 7.99 mm were excluded. Endometrial thickness measurements were performed in the midsagittal plane, in accordance with standard procedure at our facility (36). Assessment of endometrial grade was visually determined by LHB and confirmed by an additional experienced observer (S.L.Y.).
Intervention
Investigations of the relationship between endometrial thickness and pregnancy rates suggest that endometrial thickness has greatest predictive value when measured in the late proliferative phase just before ovulation (37-39). We collected serum and endometrium in the late proliferative phase, defined as the day on which at least 1 mature ovarian follicle was observed. Continued participation required that subjects have a serum estradiol concentration >200 pg/mL and a serum progesterone concentration <1.5 ng/mL, as measured in our clinical assay (Siemens Immulite 1000 Analyzer, Siemens Medical Solution, Malvern, PA; estradiol 17β: solid-phase, 2-site chemiluminescent immunometric assay (40); progesterone: solid-phase, ligand-labeled, competitive chemiluminescent immunoassay (41)).
Endometrial samples were obtained using an aspiration sampling device (M0015 Preferred Curette SureFlex Endo Sampler, Bioteque America, San Jose, CA). Tissue specimens were divided into 2 portions; 1 was fixed in formalin for histopathologic examination and the second was snap frozen in liquid nitrogen and stored at −80°C for later RNA and cistrome analysis.
Sample Size Justification
Previous research from our group suggested that a sample size of 6 patients per group would yield useful results for analysis of both endometrial protein and gene expression (42). Although no relevant empiric data on this subject were available, we hypothesized that if inadequate endometrial proliferation after clomiphene treatment was associated with a change in endometrial receptivity sufficient to adversely affect pregnancy rates, the difference should be detectable even with this small sample size.
Histopathology
Sections of human endometrial tissue stained with hematoxylin and eosin (H&E) were scanned on an Aperio ScanScope XT (Leica Biosystems). Images were uploaded to eSlide Manager and visualized with ImageScope 12.4 (Leica Biosystems) to permit whole slide imaging analysis (43-45). Analysis was performed with Genie pattern recognition software (Aperio Technologies) as previously described (46-48). Briefly, the Genie algorithm was trained to segment tissue into different regions of interest (ROIs): glandular epithelium, luminal epithelium, stroma, artifact, and glass. Each scanned image was annotated manually for analysis by a third party (B.R.M.) to confirm appropriate ROIs and exclude areas of glass or poor sample quality. The final Genie classifier then calculated the total tissue area and the area percentages for each of the ROIs (49).
Immunohistochemistry and Multiplex Immunofluorescence
Immunohistochemical staining was performed using established protocols at the Translational Pathology Laboratory at UNC. Tissue slides were analyzed using proliferating cell nuclear antigen (PCNA; Leica Microsystems Inc, Wetzlar, Germany, PA0042, 1:4800 dilution), Ki67 (Biocare Medical, Pacheco, CA, monoclonal CRM325A, 1:100 dilution), platelet endothelial cell adhesion molecule (PECAM-1; Leica Microsystems Inc, Wetzlar, Germany, monoclonal PA0250, RTU), CD45 (Leica Microsystems Inc, Wetzlar, Germany, monoclonal X16/99, RTU), CD138 (Leica Microsystems Inc, Wetzlar, Germany, monoclonal MI15, RTU), ESR1 antibody (Cell Marque/Sigma Aldrich, Rocklin, CA, monoclonal 249R-16-ASR, 1:200 dilution), ESR2 antibody (BioGenex, San Roman, CA, polyclonal AR385-5R, 1:2 dilution), and a second ESR2 antibody (Invitrogen, Monoclonal IgG2b Clone # PPZ0506 (50), 1:100 dilution).
To ensure specificity of binding, samples were stained with Rabbit or Mouse IgG, as appropriate, as controls for nonspecific secondary antibody binding. Acknowledging known challenges with anti-estrogen receptor (ER) β antibodies (51), we used both a polyclonal antibody and the most specific and well-performing ERβ antibody (50).
Multiplex immunofluorescence was performed with ERα and ERβ antibodies to permit comparison in precisely the same tissue section. For immunofluorescence, we used ERα antibody (Cell Marque/Sigma Aldrich, Rocklin, CA, monoclonal 249R-16-ASR, 1:100 dilution) and ERβ antibody (Invitrogen, Monoclonal Mouse IgG2b Clone # PPZ0506 (50), 1:100 dilution).
For analysis, genie pattern recognition software was trialed but algorithm accuracy appeared poor at tissue edges in some samples and decision was made to proceed with histologic scoring. Previous work demonstrated low intra- and interobserver variability of the HSCORE system applied to immunostaining of endometrium (52). A single experienced observer (L.H.B.) blinded to the identity of the slides by a third party (J.L.P.) used the semiquantitative digital histologic scoring (dSCORE) system and Fiji (ImageJ) software (53), which has previously been validated for image analysis in endometrial assessment (54, 55).
Data Analysis
Statistical analysis software (Stata 15.1, SAS Institute Inc., Cary, NC) was used for all data analysis. Dot plots were generated using GraphPad Prism software (Version 8.1.2, GraphPad Software, La Jolla, CA). Assumption of non-normal distribution was planned to minimize risk of Type I error in the setting of a small sample size and use of human tissue samples, which introduce more intrinsic variability (56-58). Clinical and demographic variables were interrogated using the Wilcoxon rank sum test for continuous variables and the Mantel–Haenszel test for dichotomous and count variables where appropriate. The Wilcoxon rank sum test generated P values comparing semiquantitative dSCOREs between groups.
RNA Extraction
RNA was extracted using Trizol and RNEasy® (Qiagen) and reverse transcribed using a Transcriptor First Strand cDNA Synthesis Kit (Roche; 04379012001) as previously described by our group (59).
RNA Sequencing Analysis
RNA sequencing was performed as we have previously described (59). Six optimal and 5 suboptimal endometrial specimens were subjected to the RNA-seq assay. The libraries prepared from endometrial specimens were sequenced with approximately 200 millions of 75 bp paired-end reads per sample. The raw reads were processed by filtering with average quality scores greater than 20. The reads passing the initial processing were aligned to the human reference genome (hg19; Genome Reference Consortium Mouse Build 37) using TopHat version 2.0.4 (60). Expression values of RNA-seq were expressed as FPKM (fragments per kilobase of exon per million fragments) values. Sequencing data are deposited in the National Center for Biotechnology Information Gene Expression Omnibus with accession number GSE164768 (https://www.ncbi.nlm.nih.gov/geo/query/acc.cgi?acc=GSE164768). Differential expression was calculated using Cufflink version 2.2.1 (61). Differentially expressed genes (DEGs) were defined as absolute fold change ≥1.2, FPKM > 1 in at least 1 of the samples, and P < .05 (62, 63). As suggested by Jeffrey and colleagues as best practice, substantive conclusions were restricted to DEGs with P < .001 (64).
DEGs generated from RNA sequencing data were analyzed using several software programs to study their biological functions (65-67). Ingenuity Pathway Analysis software (IPA, www.ingenuity.com) and the Database for Annotation, Visualization, and Integrated Discovery (DAVID) v6.8. were used to predict molecular activity in upstream regulator pathways (65, 66). Hierarchical clustering and heat maps of DEGs were generated by the Partek© Genomics Suite software version 6.6. Heat maps were generated from statistically significant upstream regulator pathways denoted by IPA. FPKM values were log2 transformed, shifted to a mean of 0, and rescaled to a standard deviation of 1 for display on heat maps.
ERα Chromatin Immunoprecipitation Sequencing
Frozen endometrial biopsy tissue specimens were analyzed by the Active Motif company (Carlsbad, CA) for chromatin immunoprecipitation sequencing (ChIP-seq) analysis using the ERα antibody (Millipore/Merck, Darmstadt, Germany, rabbit polyclonal #06935) from a subset of patient samples (total n = 4; optimal controls n = 2, suboptimal subjects n = 2) as described by our research group (68). Limited human endometrial tissue, particularly in the suboptimal group whose endometrium was <6 mm in thickness at the time of biopsy, as well as cost limitations restricted our use to 4 samples. Peak calling was performed with MACS2 by the Active Motif data analysis service (69). Area-proportional Venn diagrams were generated comparing ERα occupancy intervals and DEGs bound by ERα in suboptimal and optimal groups (70). Sequencing data is deposited in the National Center for Biotechnology Information Gene Expression Omnibus with accession number GSE164764 (https://www.ncbi.nlm.nih.gov/geo/query/acc.cgi?acc=GSE164764).
Validation of RNA Sequencing Data by Reverse Transcription Polymerase Chain Reaction
Quantitative real-time reverse transcription polymerase chain reaction (RT-PCR) was performed on total RNA, isolated as above, in technical duplicate, using probe–primer sets specific for genes of interest to provide further confirmation of RNA-seq data. As previously described by our group (59), DNA was generated using the Transcriptor First Strand cDNA Synthesis Kit (Roche Life Science, Penzberg, Germany) in accordance with the manufacturer’s manual. PCR was conducted using SsoAdvanced Universal SYBR Green Supermix (1725270, Bio-Rad, Hercules, CA) or SsoAdvanced Universal Probes Supermix (1725280, Bio-Rad, Hercules, CA) on the CFX Connect Real-Time PCR Detection System (1855201, Bio-Rad, Hercules, CA) per manufacturer’s instructions.
Probe–primer sets cross introns and, therefore, provide a specific signal from mRNA rather than genomic DNA. Primers for HMOX1, PRSS35, and ADAMT5 were designed using ProbeFinder version 2.53 for Humans (Roche Diagnostics). The primer sequences used in this study were as follows (from 5′ to 3′, F = forward and R = reverse): HMOX1—TGAACTCCCTGGAGATGACTC (F) and CCTGCAACTCCTCAAAGAGC (R); PRSS35— GTTCAAGCGATCCTGCTGA (F) and TCAACCAAAG CAGCATATTTTC (R); ADAMTS5- TGGCAGCACC AACACAAC (F) and AATGATGCCCACATAAATCCTC (R). Gene expression was normalized to 18s rRNA by the ΔΔCT method. The 2-tailed Student’s t-test with equal variance was used for the quantitative qPCR assays.
Results
Thirteen whole endometrial tissue samples from the late proliferative phase, after clomiphene exposure, were collected for examination of molecular differences between endometrium of optimal (n = 6) and suboptimal, (n = 7) thickness. Specimens from 2 women who were found to have serum progesterone levels >1.5 ng/mL at the time of endometrial biopsy were excluded from subsequent analysis, leaving a total of 11 samples (optimal: n = 6, suboptimal: n = 5) analyzed. The minimum criterion was met by a Shapiro–Wilkes test of normalcy (P = .06) but nonparametric analyses were performed as planned in accordance with expert consensus (56-58).
Serum estradiol was not different between groups (P = .56), nor were cycle day or number of mature follicles measured on the day of tissue sampling (Table 1). Clinical and demographic variables were similar between groups with the exception of dysmenorrhea, which was more commonly reported among suboptimal subjects (40% vs 0%, P = .10), and body mass index (kg/m2), which was higher among suboptimal subjects (32 vs 23, P = .01, Table 1). Normal, trilaminar endometrial architecture was similarly frequent in both groups (P = .36). In subsequent cycles, a higher proportion of suboptimal subjects underwent a letrozole treatment cycle than optimal controls (P = .045). Notably, among women who subsequently underwent a letrozole treatment cycle, endometrial thickness following letrozole was similar between groups (P = .48, Table 1).
Table 1.
Clinical and demographic variables
| Variable | Optimal (n = 6) | Suboptimal (n = 5) | P |
|---|---|---|---|
| Age, years | 31 (29-36) | 33 (27-36) | 0.46 |
| BMI, kg/m2 | 23 (21-25) | 32 (29-34) | 0.01* |
| Gravidity | 0 (0-1) | 1 (0-1) | 0.23 |
| Parity | 0 (0-1) | 0 (0-0) | 0.64 |
| AMH (ng/mL) | 3.8 (2.7-6.3) | 1.9 (0.8-2.3) | 0.05 |
| PCOS | 0 (0%) | 1 (20%) | 0.27 |
| LPD | 1 (14%) | 0 (0%) | 0.68 |
| Dysmenorrhea | 0 (0%) | 2 (40%) | 0.10 |
| Cycle day | 13 (12-13) | 13 (13-13) | 0.61 |
| Estradiol (pg/mL) | 678 (317-1138) | 792 (679-875) | 0.58 |
| Progesterone (ng/mL) | 0.7 (0.5-0.7) | 0.5 (0.5-0.6) | 0.14 |
| No. of eggs ≥14 mm | 2 (1-3) | 2 (1-3) | 0.79 |
| ES (mm) at time of biopsy | 9.7 (8.9-10.4) | 5.5 (5-5.6) | 0.006* |
| Trilaminar endometrial grade at biopsy | 5 (83%) | 5 (100%) | 0.36 |
| Letrozole usea | 1 (16.7) | 4 (80.0) | 0.045* |
| ES (mm) on Letrozolea | 9.4 (9.4) | 8.1 (6.9-9.3) | 0.48 |
| Prior clomiphene use 1 month before biopsy | 2 (33%) | 2 (40%) | 0.12 |
| Prior clomiphene use ever | 3 (50%) | 3 (60%) | 0.76 |
Presented as N (%) or median (interquartile range: quartile 1-quartile 3).
Mann–Whitney used for continuous variables, M-H odds used for categorical or count variables. *Statistical significance (P < .05).
Abbreviations: BMI, body mass index; AMH, anti-Mullerian hormone; PCOS, polycystic ovarian syndrome; LPD, luteal phase defect; ES, endometrial stripe thickness.
a One optimal control and 4 suboptimal subjects used letrozole.
Tissue Level Differences
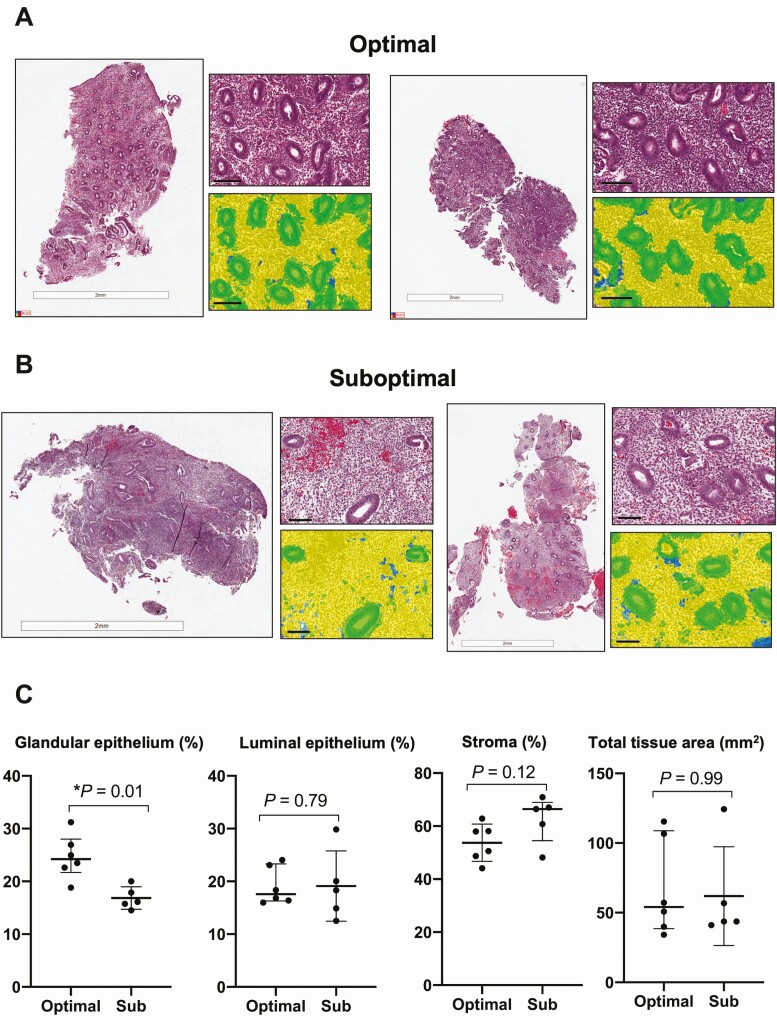
H&E staining of sections was performed using Aperio ImageScope with Genie automated software analysis to determine cell type composition as described above. Results demonstrated a lower percentage of glandular epithelium in suboptimal subjects than in optimal controls (16.1% vs. 24.2%, P = 0.01, Fig. 1). The proportion of tissue occupied by stromal cells and luminal epithelium as well as the total tissue area were similar between the groups (P > .05 for all). No H&E-stained sections demonstrated increased plasma cell numbers that would support a histologic diagnosis of chronic endometritis.
Figure 1.
Endometrial tissue level changes by histopathology examination of infertile women with optimal and suboptimal endometrial thickening following clomiphene citrate treatment. (A) Hematoxylin and eosin staining of whole slide tissue image and filter from optimal controls and (B) suboptimal subjects (filter: yellow = stromal cells, green = glandular epithelium, blue = luminal epithelium). Scale = 100µm. (C) Dot-plots depicting differences in tissue composition area (mm2) and percentages (%) in glandular epithelium, luminal epithelium, stroma, and total tissue area. Wilcoxon rank-sum generated P values.
Comparative Gene Expression
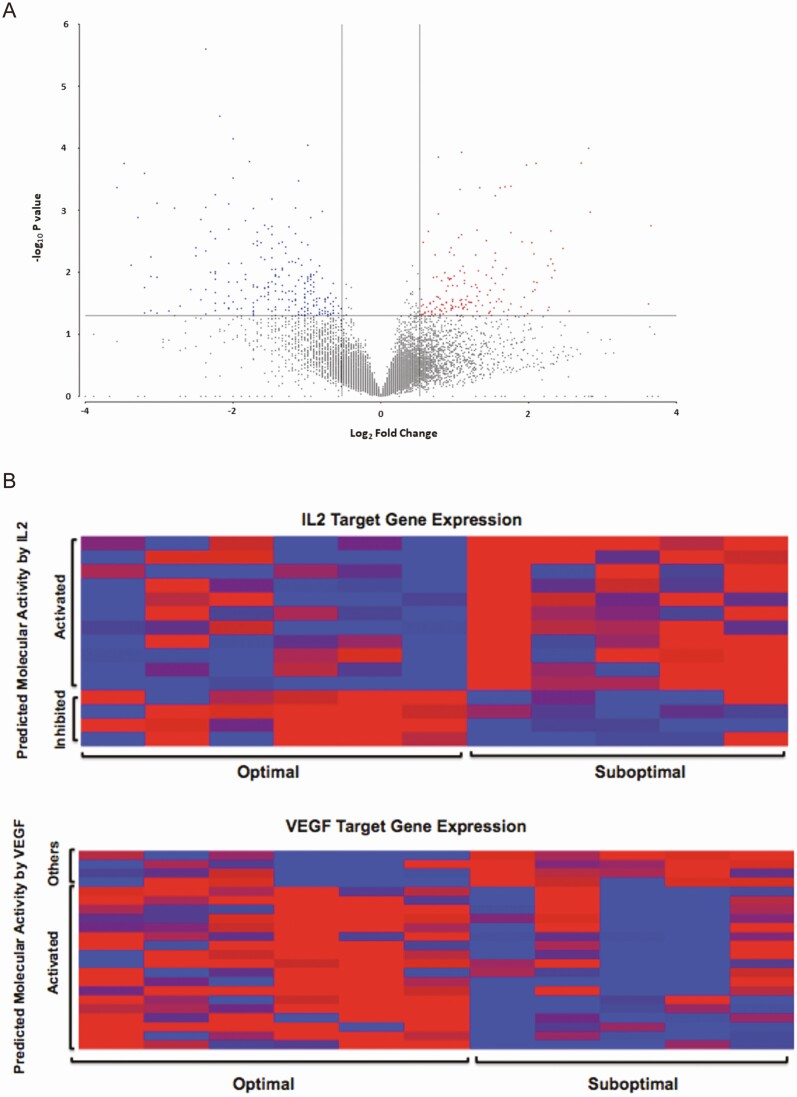
Whole endometrial RNA Sequencing analysis identified over 20 000 genes that had least 1 sample with FPKM > 1. Comparison of endometrium between optimal and suboptimal groups identified 398 DEGs (P < .05) (Fig. 2A).
Figure 2.
Comparative gene expression between infertile women with optimal and suboptimal endometrial thickening following clomiphene citrate treatment. (A) Volcano plot of 398 differentially regulated genes identified by RNA-seq whereby |fold change| > 1.2, P < .05 and genes had at least 1 sample with FPKM > 1. Red denotes increased expression and blue denotes decreased expression in suboptimal vs optimal controls. (B) Heat map of IL2 and VEGF target gene expression in infertile women exposed to clomiphene citrate who developed an optimal vs. suboptimal endometrial thickness (x-axis) by predicted molecular activity (y-axis). Red denotes increased expression and blue denotes decreased expression in suboptimal subjects vs optimal controls.
Upstream regulator pathway analysis of DEGs via IPA identified significant differences between groups for predicted molecular activity in proliferation, angiogenesis, and inflammation pathways (Table 2). Compared with optimal controls, suboptimal subjects had reduced predicted molecular activity in cellular proliferation (FOXM1 Z-score = –2.2, P < .01) and angiogenesis (VEGF Z-score = –2.87, P < .0001) pathways and increased predicted molecular activity in inflammatory pathways (interleukin [IL]-2 Z-score = +2.2, P < .01, Table 2 and Fig. 2B). Suboptimal subjects also demonstrated a decrease in predicted molecular activity of the anti-inflammatory drug dexamethasone pathway (Z-score = –3.6, P < .0001). Heat map representation of gene expression levels determined by RNA-seq identified alterations in pathways related to inflammation and angiogenesis (Fig. 2B). Individual genes are presented elsewhere (Table 1 (71)).
Table 2.
Upstream regulators identified using ingenuity pathway analysis
| Upstream regulator | Molecule type | Activation Z-score | P of overlap |
|---|---|---|---|
| FOXM1 | Transcription regulator | –2.23 | 1.71 × 10–3 |
| VEGF | Group | –2.87 | 3.46 × 10–5 |
| IL2 | Cytokine | 2.22 | 2.53 × 10–3 |
| ESR1 | Ligand-dependent nuclear receptor | –0.57 | 5.08 × 10–5 |
| ESR2 | Ligand-dependent nuclear receptor | 1.60 | 3.0 × 10–5 |
| Beta-estradiol | Hormone | 0.63 | 3.20 × 10–3 |
| Dexamethasone | Chemical drug | –3.58 | 1.06 × 10–10 |
| SMAD7 | Transcriptional regulator | 3.10 | 9.22 × 10–5 |
| ERBB3 | Kinase | 2.50 | 6.0 × 10–4 |
| ERBB4 | Kinase | 2.06 | 8.87 × 10–5 |
| TGFB1 | Growth Factor | –3.48 | 4.22 × 10–13 |
+Z-score implies activation of downstream molecular pathway in the suboptimal group
Immunohistochemical Staining
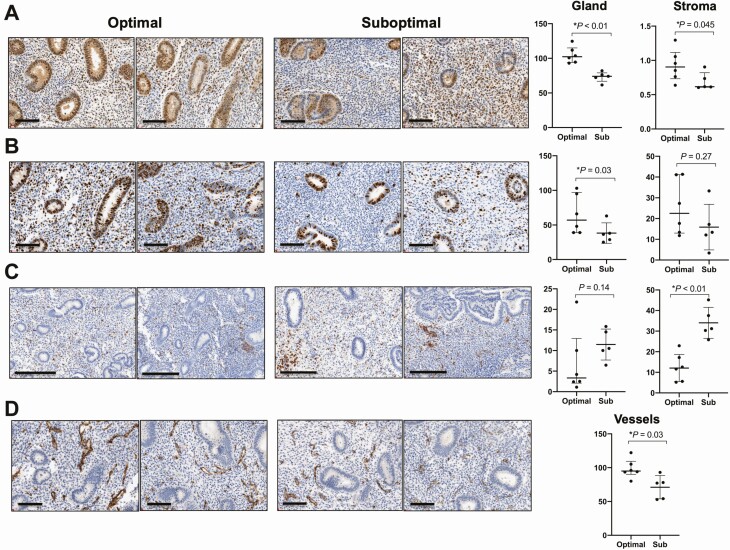
Immunohistochemical staining was performed to evaluate differences in proliferation, angiogenesis and inflammation at the protein level. Suboptimal subjects demonstrated evidence of reduced proliferation by decreased glandular and stromal PCNA immunostaining (dSCORE = 74 vs 101, P < 0.01; 27 vs 82, P <0.05, respectively, Fig. 3A) and decreased glandular ki67 immunostaining (dSCORE = 38.3 vs 65.0, P = .03, Fig. 3B). Evidence of inflammation was assessed by immunostaining for bone marrow–derived immune marker CD45 (cluster of differentiation 45) and showed increased stromal staining in suboptimal compared with optimal subjects (dSCORE = 31 vs 12, P < .01, Fig. 3C). Immunostaining for the plasma cell marker CD138 (syndecan-1) was similar between groups in both glandular (P = .93) and stromal (P = .13) compartments. Immunohistochemical staining for an angiogenic marker, PECAM-1 (CD31), was reduced among suboptimal compared to optimal subjects (dSCORE = 77 vs 95, P = .03, Fig. 3D).
Figure 3.
Protein level changes by immunohistochemical staining in late proliferative endometrium from women with optimal compared to suboptimal endometrial thickening following follicular phase exposure to clomiphene citrate. (A) Tissue staining in representative samples and dot-plots depicting semiquantitative analyses of digital H-score (dSCORE) from all samples stained for PCNA (scale = 100µm), (B) ki67, (scale = 100μm) (C) pan-leukocyte inflammatory marker CD45 (scale = 200µm), and (D) angiogenesis marker PECAM-1 (scale = 100µm). Dot-plots represent medians and interquartile ranges (Q1-Q3). Wilcoxon rank-sum generated P values.
Estrogen Signaling
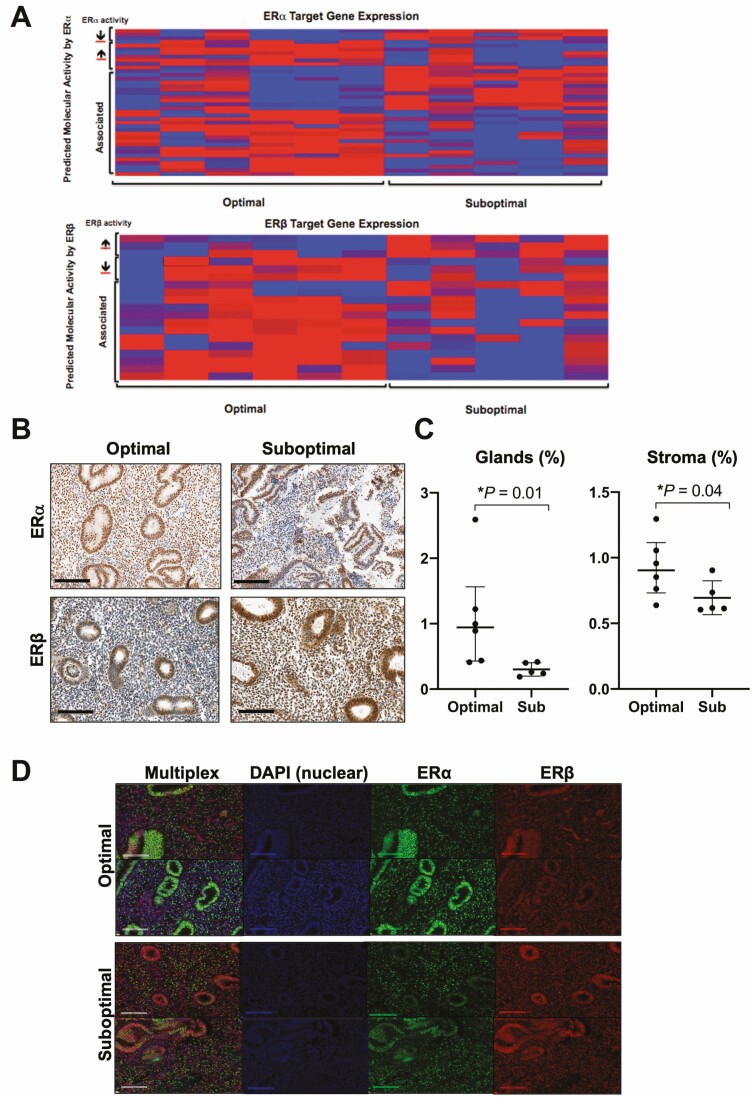
As estrogen is known to promote endometrial proliferation and enhance both angiogenesis and inflammation, initial attention was focused on evaluating for differences in estrogen signaling between groups. Pathway analysis, using IPA software, demonstrated altered ER subtype activity between groups with suboptimal subjects demonstrating increased ERβ expression (Z-score = +1.6, P < .0001) compared with optimal controls and slightly decreased predicted ERα activity (Z-score = –0.57, P < .0001). Heat map representation of gene expression levels demonstrated visually distinct profiles between groups for predicted molecular activity of ERα and ERβ downstream pathways (Fig. 4A).
Figure 4.
Examination of estrogen signaling and estrogen action between infertile women with optimal compared to suboptimal endometrial thickening after clomiphene citrate. (A) Heat map of differentially expressed ERα and ERβ target gene expression in infertile women exposed to clomiphene citrate who developed optimal vs suboptimal endometrial thickening (x-axis) by predicted molecular activity (y-axis). Red denotes increased expression and blue denotes decreased expression in suboptimal subjects vs optimal controls. (B) Immunohistochemical staining of ERα and ERβ showing altered estrogen receptor subtype abundance between suboptimal and optimal controls. (C) Dot-plots depicting semiquantitative analysis of digital H-score (dSCORE) from all samples. (D) Variation in ER subtype abundance by multiplex immunofluorescence analysis. Scale = 100µm.
To further examine the potential role of altered estrogen signaling, we examined the localization and intensity of ERα and ERβ immunostaining. Subjects with a suboptimal endometrial thickness demonstrated decreased immunostaining of ERα and increased immunostaining of ERβ compared with optimal controls in both glands (P = .01) and stroma (P = .04, Fig. 4C). Experiments were repeated with a highly specific ERβ antibody (Monoclonal Mouse IgG2b Clone # PPZ0506) (50) and yielded highly similar results (data not presented due to lack of antibody validation in the application of immunohistochemical staining (50)).
Since analysis of tissue composition (vide supra) demonstrated a higher proportion of glandular epithelium in optimal subjects, multiplex immunofluorescence was performed to permit visualization of ERα and ERβ immunostaining in the same tissue section. Results demonstrated colocalization of ERα and ERβ in the glandular nuclei (Fig. 4D).
ERα ChIPSeq
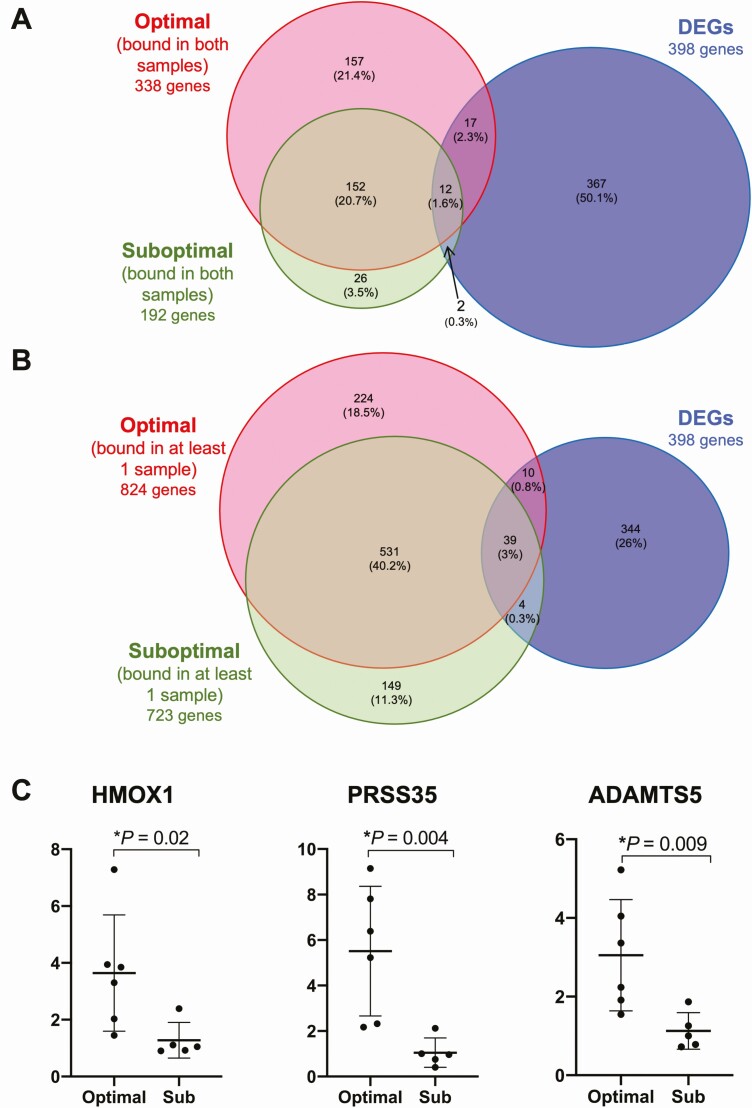
To further evaluate altered estrogen receptor function between groups, we examined genome-wide alterations in ERα binding using ERα ChIP-seq in a subset of samples (total n = 4; optimal controls n = 2, suboptimal subjects n = 2). The majority of ESR1 target genes bound by ERα in suboptimal samples were also bound by ERα in optimal controls, whether comparing target genes bound in both endometrial samples per group (Fig. 5A) or at least 1 sample per group (Fig. 5B).
Figure 5.
ERα ChIP-seq results and confirmation of differentially expressed genes identified by RNA-seq. (A) Venn Diagram representation of ChIP-Seq results overlapped with differentially expressed genes (DEGs) identified by RNA-seq comparing genes bound by ERα in both samples in the optimal and suboptimal groups. (B) Venn diagram depicting DEGs bound by ERα in at least 1 sample per group. (C) Dot-plots depicting evaluation of gene expression by RT-PCR in 3 DEGs identified on RNA-seq: HMOX1, PRSS35, and ADAMTS5.
A total of 53 target genes were bound by ERα on ChIPSeq and were also DEGs using our RNA sequencing data (Table 3). Of the 53 differentially expressed ERα target genes, 6 genes were found to have an absolute fold change of >1.5, P < .001 (64), and were bound by ERα on ChIPSeq in at least 1 sample per group: ADAM metallopeptidase with thrombin type motif 5 (ADAMTS5; fold change from suboptimal to optimal of –1.77, P < .0001), Laminin subunit alpha 4 (LAMA4; fold change –1.72, P < .0001), Myotilin (MYOT; fold change +1.587, P < .0001), Ribosomal Protein S6 Kinase Like 1 (RPS6KL1; fold change +1.61, P < 0.001), Sphingomyelin phosphodiesterase 3 (SMPD3; fold change +1.71, P < .001), and Thrombospondin 2 (THSB2; fold change –1.68, P < 0.001) (Table 1 (71)).
Table 3.
Differentially expressed genes (RNA-Seq) that are bound by ERα (ChIP-seq)
| Gene symbol | Fold change** | P | Gene description |
|---|---|---|---|
| ABCA3 | 1.338 | 3.4 × 10–02 | ATP binding cassette subfamily A member 3 |
| *ADAMTS5 | –1.773 | 3.1 × 10–05 | ADAM metallopeptidase with thrombospondin type 1 motif 5 |
| ADAMTS8 | –1.361 | 3.6 × 10–02 | ADAM metallopeptidase with thrombospondin type 1 motif 8) |
| ADAMTSL5 | 1.41 | .000117 | ADAMTS-Like Protein 5 |
| AFAP1L2 | –1.522 | .00175 | Actin filament associated protein 1 like 2 |
| AKAP1 | 1.49 | .000435 | A-Kinase Anchoring Protein 1 |
| ALOX5 | –1.3 | .0078 | arachidonate 5-lipoxygenase |
| ANXA5 | –1.351 | .001 | Thromboplastin inhibitor |
| AOC3 | 0.6 | 3.0 × 10–04 | Amine Oxidase Copper Containing 3 |
| ARHGAP26 | 1.567 | .0004 | Rho GTPase activating protein 26 |
| BCL6 | –1.325 | .016 | B cell CLL/lymphoma 6 |
| C1orf198 | –1.381 | .003 | chromosome 1 open reading frame 198 |
| C1QTNF7 | –1.41 | .028 | C1q and TNF related 7 |
| C8orf48 | –1.299 | .0282 | chromosome 8 open reading frame 48 |
| CCBE1 | –1.385 | .0273 | collagen and calcium binding EGF domains 1 |
| CDH2 | –1.302 | .0468 | cadherin 2 |
| CISH | –1.222 | .046 | Cytokine Inducible SH2 Containing Protein |
| COL18A1 | –1.272 | .0396 | collagen type XVIII alpha 1 chain |
| COL21A1 | –1.422 | .0245 | collagen type XXI alpha 1 chain |
| DAB2 | –1.355 | 3.6 × 10–03 | Differentially Expressed Protein 2 |
| EDN2 | –1.511 | .0105 | endothelin 2 |
| FAT4 | –1.451 | .0212 | FAT atypical cadherin 4 |
| FMO1 | –1.488 | .0109 | flavin containing monooxygenase 1 |
| GABRR2 | 1.281 | .0287 | gamma-aminobutyric acid type A receptor rho2 subunit |
| GRK5 | –1.259 | .0299 | G protein–coupled receptor kinase 5 |
| HAPLN2 | –1.387 | .0419 | hyaluronan and proteoglycan link protein 2 |
| HSD17B2 | –1.439 | .0176 | hydroxysteroid 17-beta dehydrogenase 2 |
| ITGB5 | –1.253 | .0371 | integrin subunit beta 5 |
| ITPK1 | 1.255 | .0365 | inositol-tetrakisphosphate 1-kinase |
| KDM4B | 1.2 | .03 | Lysine Demethylase 4B |
| KLK2 | 1.31 | .03 | Kallikrein Related Peptidase 2) |
| *LAMA4 | –1.715 | .0000705 | laminin subunit alpha 4 |
| LOC389895 | –1.335 | .0454 | chromosome 16 open reading frame 72-like |
| MN1 | 1.345 | .0166 | MN1 proto-oncogene, transcriptional regulator |
| MYB | 1.379 | .0102 | MYB proto-oncogene, transcription factor |
| *MYOT | 1.587 | .00042 | myotilin |
| NXNL2 | –1.348 | .03 | Nucleoredoxin-Like Protein 2 |
| PKDCC | –1.289 | .0338 | protein kinase domain containing, cytoplasmic |
| PLCE1 | –1.316 | .0145 | phospholipase C epsilon 1 |
| PLCG2 | 1.369 | .0151 | phospholipase C gamma 2 |
| PXK | –1.41 | .00239 | PX domain containing serine/threonine kinase like |
| RBM47 | 1.275 | .047 | RNA binding motif protein 47 |
| *RPS6KL1 | 1.609 | .000411 | ribosomal protein S6 kinase like 1 |
| RSPO1 | –1.447 | .0174 | R-spondin 1 |
| RUNX1 | 1.327 | .0127 | runt related transcription factor 1 |
| RXRA | 1.245 | .0295 | retinoid X receptor alpha |
| SLC7A5 | 1.37 | .03 | Solute Carrier Family 7 Member 5) |
| *SMPD3 | 1.708 | .000176 | sphingomyelin phosphodiesterase 3 |
| SPARCL1 | –1.389 | .027 | Proliferation Inducing Protein 33 |
| *THBS2 | –1.684 | .000563 | thrombospondin 2 |
| TSPAN5 | –1.33 | .0115 | tetraspanin 5 |
| USP31 | 1.29 | .038 | Ubiquitin Specific Peptidase 31 |
| WWC1 | 1.319 | .0132 | WW and C2 domain containing 1 |
*Absolute fold change >1.2, P < .05, and bound by ERα in at least 1 sample per group. **Positive fold change denotes increased expression in the Suboptimal group.
ERα genome occupancy profiles were also compared and found to be similar between groups, with 86% of occupancy intervals bound by ERα in suboptimal subjects also bound by ERα in optimal specimens (Fig. 1A (71)). ERα-occupied regions exhibited similar contexts between groups, with similar proportions of occupied intervals occurring in exons, intergenic, and promoter regions in optimal (Fig. 1B (71)) and suboptimal (Fig. 1C (71)) groups.
To estimate which endometrial ERα-binding intervals occur in the stromal compartment, we compared ERα-binding intervals with a recently published ESR1-binding profile in stroma (72) (Fig. 1A (71)). A total of 36 stromal ESR1 occupancy sites were shared between groups and 14 are associated with known genes (Table 3 (71)), although none were differentially expressed between groups with P < .001.
RT-PCR Confirmation of DEGs
Differences in RNA abundance in specific genes involved in proliferation (HMOX1 (73, 74)), angiogenesis (ADAMTS5 (75), HMOX1 (76)), and inflammatory pathways (PRSS35 (77)) were validated via RT-PCR. The expression of HMOX1, ADAMTS5, PRSS35 was +3.6, +3.1, and +6.6-fold higher, respectively, in optimal vs suboptimal samples, consistent with results from our RNA-seq analysis (Fig. 5B). These findings further support the concept of reduced proliferation and angiogenesis combined with increased inflammation in subjects exhibiting poor endometrial thickening after clomiphene.
Discussion
Poor endometrial growth correlates with worse treatment outcomes (3-5). However, the molecular biology defining the mechanisms causing poor endometrial growth among some women receiving clomiphene treatment has not been investigated. We hypothesized that women with poor endometrial growth after clomiphene treatment have aberrant endometrial ER signaling. We performed a set of complementary experiments to identify unique tissue and molecular differences between women with optimal or suboptimal endometrial thickening after clomiphene treatment, including morphologic examination, immunostaining, mRNA expression patterns, and the ERα cistrome. Key findings included reduced biomarkers of proliferation and angiogenesis, increased markers of inflammation, and aberrant estrogen receptor expression in patients with poor endometrial thickening.
Hypothesized Downstream Pathways of Altered ER Subtype Abundance and Activity
We found that most women with suboptimal endometrial thickness following clomiphene went on to develop normal midcycle endometrial thickness following letrozole treatment, suggesting that alterations in cellular pathways rather than intrinsic endometrial abnormality drives an abnormal response to clomiphene in these women. A higher proportion of women in the suboptimal group subsequently used letrozole, which is consistent with most patients’ preference to trial letrozole before proceeding with in vitro fertilization, which is not covered by insurance in our nonmandated state, after clomiphene treatment resulted in the development of a suboptimal endometrial thickness. A similar incidence of trilaminar endometrial architecture in both groups after clomiphene treatment implies that differences in endometrial thickening are not driven by differences in endometrial grade (2).
Proliferation, angiogenesis, and inflammation pathways differed by endometrial thickness after clomiphene treatment and appeared to reflect differences in estrogen receptor expression. Women with a suboptimal endometrial thickness demonstrated a lower proportion of glandular epithelium on H&E staining despite a similar overall area of tissue in the sections. Recognition that glandular hyperplasia characteristic of the late proliferative phase is an estrogen-dependent process (1, 78-81) offers mechanistic insight into how the development of a suboptimal endometrial thickness following clomiphene might represent a suboptimal response to clomiphene’s selective agonism and antagonism of the estrogen receptor. Evidence of reduced proliferation in suboptimal subjects also was demonstrated by decreased immunostaining of proliferation markers PCNA and ki67. Results from RNA-seq and pathway analysis corroborated this finding with reduced FOXM1 pathway activity in subjects with a suboptimal growth response to clomiphene. The decreased proliferation observed in some women after clomiphene treatment may be a downstream effect of alterations in estrogen signaling, as endometrial proliferation is largely driven by estradiol in a process characterized by an increase in the number and size of endometrial glands (2, 4, 5). Endometrial glands are hypothesized to play an important role in normal uterine receptivity to implantation (82) and, thus, the reduced abundance of glandular cells might herald altered endometrial function.
Angiogenesis normally occurs during the proliferative phase, is promoted by estrogen, and is critical to endometrial receptivity and fetal survival (83, 84). Specifically, VEGF has been identified as an essential protein for implantation (85). Our findings of decreased immunostaining of the blood vessel marker PECAM-1, reduced predicted molecular activity of the VEGF pathway, and lower levels of HMOX-1 transcript in RT-PCR in patients with poor endometrial proliferation suggest a defect in endometrial angiogenesis at the protein and mRNA transcript levels. Angiogenesis itself might also drive endometrial epithelial proliferation (86), implying that alterations in angiogenesis may be fundamental to endometrial dysfunction.
Activation of IL-2 signaling mediators and reduction in anti-inflammatory signaling via the dexamethasone pathway along with findings of increased CD45 immunostaining suggest an association between a proinflammatory environment and poor endometrial growth after clomiphene. IL-2 signaling can affect the differentiation and survival of both effector and memory T cells (87, 88) as well as Th17 regulatory cells, both of which are thought to be important to fetal survival (89). Endometrial inflammation is evident in women with endometriosis by evaluation of eutopic endometrium (90, 91) and, as such, our findings and a higher prevalence of dysmenorrhea in the suboptimal group could suggest undiagnosed endometriosis or other causes of inflammation among suboptimal subjects. Though angiogenesis and inflammation are typically mutually dependent processes (92), defects in angiogenesis and decidualization occur in the setting of increased inflammation in the eutopic endometrium of women with endometriosis (90) and additionally suggest that development of a suboptimally thin endometrium after clomiphene may identify early or clinically unrecognized endometriosis.
In the present study, women with poor endometrial proliferation had a significantly higher BMI than women with optimal endometrial thickness. Although small sample size precludes reliably meaningful correlations with demographic variables, the documented association of obesity with increased endometrial inflammation provides a biologically plausible explanation (93-95). Although women in our study were neither clinically hyperandrogenic nor anovulatory (96), it is possible that women with a higher BMI experience subclinical hyperandrogenism (97) that impacts their endometrium. This speculation may be supported by elegant experiments in rhesus monkeys showing that chronic hyperandrogenism and high-calorie, high-fat diets led to the development of a thinner endometrium (98), infertility (99), and placental dysfunction (100).
Differences in ER Subtype Abundance
Women who exhibited poor endometrial growth after clomiphene demonstrated decreased ERα and increased ERβ gene expression at the transcript and protein levels. Women with endometriosis demonstrate increased ERβ gene expression in endometriotic lesions (101, 102) and mechanistic investigations suggest that increased ERβ gene expression drives a decrease in ERα gene expression (103). ERβ has been shown to inhibit ERα-induced cell proliferation through formation of ERα/ERβ heterodimers with different molecular activity (104), and other nongenomic mechanisms (105).
ERα Cistrome Results
Key findings from ChIPSeq analysis included significant overlap between groups. ERα occupancy profiles were largely similar and the majority of genes bound by ERα in subjects with poor endometrial growth were also bound by ERα in normal controls. In addition, most DEGs identified by RNA sequencing were not differentially bound between groups.
Our observations have numerous potential explanations. Downstream modifications including post-transcriptional or post-translational alterations may affect differences in ER subtype abundance and localization, which would be consistent with our findings on immunohistochemical staining. It is also possible that the activity of coactivators or corepressors, rather than ERα binding itself, drives differential ER effects on gene expression, or that nongenomic functions of ER vary between groups (106). The relative affinity of ERα binding to target genes may not be captured by ChIPSeq, such that while the threshold for binding was met in both groups, differential affinity for binding might exist above the ChiPSeq detection threshold. Lastly, our observations may be driven by differences in ERβ gene expression, which has been shown to alter ERα activity and expression (102, 104, 105).
ChIP-seq identified 6 genes bound by ERα that were also identified by RNA-seq as differentially regulated between groups: ADAMTS5, LAMA4, THSB2, SMPD3, MYOT, RPS6KL1. ADAMTS5 expression is negatively regulated by cytokines in human decidual stromal cells (107) and in our study was lower in suboptimal subjects, consistent with higher levels of inflammation in this group. Suboptimal subjects had lower levels of LAMA4, an extracellular matrix glycoprotein that has been associated with pre-eclampsia (108, 109) and decreased VEGF gene expression (110), which may relate to altered angiogenesis in these women. THBS2, a glycoprotein that mediates cell-to-cell and cell-to-matrix interactions, is associated with angiogenesis in a receptor-dependent fashion and was lower in suboptimal subjects (111, 112). SMPDE3 was increased in suboptimal compared with optimal subjects and is a potential biomarker for endometriosis that is upregulated in the eutopic endometrium of women with endometriosis (113). Expression of myotilin, a protein necessary for microfilament assembly (114), was increased in suboptimal subjects, as was a protein kinase RPS6KL1 (115). No specific role for either myotilin or RPS6KL1 in endometrial function or pathology has been previously reported.
To date, 1 previous investigation has assessed the ERα cistrome in the stromal endometrium of healthy women undergoing hysterectomy (72). These women were not known to be infertile and endometrial tissue was not collected at a specific time in the menstrual cycle. Yet, as the only existing investigation of ERα occupancy intervals in endometrium, we compared our data directly with these. We did not expect overlap due to baseline differences and did not identify significant differences.
Strengths/Limitations
Our study is a novel, mechanistic investigation of a commonly encountered clinical observation of poor endometrial proliferation after clomiphene treatment. Rigorous inclusion criteria were used to identify subjects with the diagnosis of unexplained infertility without history of uterine procedure or pathology. Study subjects had the same diagnosis, were subjected to the same treatment dose and duration and underwent tissue sampling in the same (late proliferative) phase of the endometrial cycle. Serum sex hormone measurement confirmed typical and similar late follicular phase estradiol concentrations in both groups and demonstrated that neither ovulation nor premature luteinization had occurred. Transvaginal ultrasound permitted assessment of follicle sizes, measurement of endometrial thickness, and assessment of endometrial grade.
To the best of our knowledge, there has not been a previously reported mechanistic investigation of endometrial tissue in women who exhibit poor endometrial proliferation after clomiphene exposure or in any other context. ERα ChIP-seq has not been performed on human endometrial tissue from women with infertility. ERα ChIP-seq in murine models (116) may not be informative in humans due to species conservation in mouse–human comparison of just 6% of predicted estrogen response elements (117).
The greatest insight into whether the extent of endometrial growth after clomiphene treatment impacts implantation mechanisms might be gained by study of tissues obtained during the secretory phase, but endometrial tissue sampling after ovulation may disrupt implantation or early pregnancy and thus is not acceptable in women actively seeking pregnancy. Previous investigations suggest that the late proliferative phase is the next-most ideal time to identify key differences in endometrial gene expression in patients with unexplained infertility (118-120). Intra- and interobserver variability from the recording of measurements by multiple providers may have introduced bias, although research demonstrates excellent reproducibility of measurements within 1 mm and specifically when using a cutoff value of 6 mm (36, 121). To mitigate potential bias, we excluded women with endometrial thickness measurements from 6.0 to 7.99 mm in efforts to ensure enrollment of only those women with either clearly suboptimal (<6 mm) or clearly optimal (>8 mm) endometrial thickening following clomiphene.
Our results are limited by small sample size. To account for this, conservative statistical methods, including use of nonparametric tests, were employed to mitigate the risk of Type I error. The limited amount of available human tissue and cost constraints restricted evaluation of ChIP-seq to 4 samples, which limits our insight into causal genomic mechanisms and generalizability of findings. At present, no ERβ antibody has been validated for ChIP-seq and this complicated our ability to more thoroughly evaluate the impact of altered ER subtype expression on gene expression. Considering controversy over specificity and sensitivity of ERβ antibodies, we used 2 different antibodies for immunostaining, 1 of which has previously been shown to be highly specific for ERβ in endometrium (50). While we focused our immunohistochemical analysis on protein targets with known roles in endometrial proliferation, Immunohistochemical analysis of those transcripts identified as differentially regulated by RNA-seq may provide additional insight.
Conclusion
We investigated mechanisms that might explain the common clinical observation of poor endometrial thickening after clomiphene treatment in some infertile women. Women with inadequate endometrial growth demonstrated reduced proliferation, increased inflammation and altered estrogen signaling. Altered ER subtype abundance and activity suggest an intrinsic endometrial mechanism for the altered action of estrogen after clomiphene in some women. We speculate that these endometrial abnormalities may underlie a distinct cause of infertility among patients currently deemed unexplained.
These foundational data allow for an improved understanding of why a sizable minority of women with infertility exhibit inadequate endometrial proliferation. Ongoing efforts to obtain endometrium from the same patient groups in natural (unmedicated) cycles, organoid culture and, when feasible, assessing the ERβ cistrome would provide additional foundational information on the molecular regulation of poor endometrial proliferation after clomiphene treatment.
Acknowledgments
We thank Bentley R. Midkoff and the UNC Translational Pathology Laboratory (TPL) for expert technical assistance. The UNC TPL is supported in part, by grants from the NCI (5P30CA016086-42), NIH (U54-CA156733), NIEHS (5 P30 ES010126-17), UCRF, and NCBT (2015-IDG-1007). This work was supported by grant R01 HD100329 (SLY) from the Eunice Shriver Kennedy NICHD, by an Intramural Research Program of the National Institute of Environmental Health Sciences, National Institutes of Health (NIH) Z1AES103311 (F.J.D.) and Z99-ES999999 (S.P.W.). S.C.K. is supported by the NIEHS Scholars Connect Program. We also thank research coordinator Jana Phillips, whose hard work was critical to accomplishing this research.
Financial Support: This work was supported by the Intramural Research Program of the National Institute of Health: Project Z1AES103311 (F.J.D.) and the Eunice Kennedy Shriver National Institute of Child Health and Human Development: Projects 5R01HD067721-05 (S.L.Y.) and the Nova Carda Fund, UNC Department of Obstetrics & Gynecology (M.A.F.).
Glossary
Abbreviations
- BMI
body mass index
- CD
cluster of differentiation
- ChIP-seq
chromatin immunoprecipitation sequencing
- DEG
differentially expressed gene
- ER
estrogen receptor
- FPKM
fragments per kilobase of transcript per million mapped reads
- H&E
hematoxylin and eosin
- IL
interleukin
- IPA
Ingenuity Pathway Analysis
- LPD
luteal phase defects or dysfunction
- PCNA
proliferating cell nuclear antigen
- ROI
region of interest
- RT-PCR
reverse transcription polymerase chain reaction
Additional Information
Disclosures: L.H.B. is a shareholder in 2minutemedicine®. All other authors have nothing to disclose.
Data Availability
All data generated or analyzed during this study are included in this published article or in the data repositories listed in References.
Supplemental tables and figures can be found at: https://doi.org/10.5061/dryad.9kd51c5gk
Gene expression data can be found at: https://www.ncbi.nlm.nih.gov/geo/query/acc.cgi?acc=GSE164764
https://www.ncbi.nlm.nih.gov/geo/query/acc.cgi?acc=GSE164768
References
- 1. Lessey BA, Killam AP, Metzger DA, Haney AF, Greene GL, McCarty KS Jr. Immunohistochemical analysis of human uterine estrogen and progesterone receptors throughout the menstrual cycle. J Clin Endocrinol Metab. 1988;67(2):334-340. [DOI] [PubMed] [Google Scholar]
- 2. Gingold JA, Lee JA, Rodriguez-Purata J, et al. Endometrial pattern, but not endometrial thickness, affects implantation rates in euploid embryo transfers. Fertil Steril. 2015;104(3):620-628.e5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. El-Toukhy T, Coomarasamy A, Khairy M, et al. The relationship between endometrial thickness and outcome of medicated frozen embryo replacement cycles. Fertil Steril. 2008;89(4):832-839. [DOI] [PubMed] [Google Scholar]
- 4. Glujovsky D, Pesce R, Fiszbajn G, Sueldo C, Hart RJ, Ciapponi A. Endometrial preparation for women undergoing embryo transfer with frozen embryos or embryos derived from donor oocytes. Cochrane Database Syst Rev. 2010;( 1):CD006359. [DOI] [PubMed] [Google Scholar]
- 5. von Wolff M, Fäh M, Roumet M, et al. Thin endometrium is also associated with lower clinical pregnancy rate in unstimulated menstrual cycles: a study based on natural cycle IVF. Front Endocrinol (Lausanne). 2018;9:776. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Esmailzadeh S, Faramarzi M. Endometrial thickness and pregnancy outcome after intrauterine insemination. Fertil Steril. 2007;88(2):432-437. [DOI] [PubMed] [Google Scholar]
- 7. Cudmore DW, Tupper WR. Induction of ovulation with clomiphene citrate. A double-blind study. Fertil Steril. 1966;17(3):363-373. [DOI] [PubMed] [Google Scholar]
- 8. Van Campenhout JC, Simard R, Leduc B. Antiestrogenic effect of clomiphene in the human being. Fertil Steril. 1968;19(5):700-706. [DOI] [PubMed] [Google Scholar]
- 9. Massai MR, de Ziegler D, Lesobre V, Bergeron C, Frydman R, Bouchard P. Clomiphene citrate affects cervical mucus and endometrial morphology independently of the changes in plasma hormonal levels induced by multiple follicular recruitment. Fertil Steril. 1993;59(6):1179-1186. [DOI] [PubMed] [Google Scholar]
- 10. Palopoli FP, Feil VJ, Allen RE, Holtkamp DE, Richardson A Jr. Substituted aminoalkoxytriarylhaloethylenes. J Med Chem. 1967;10(1):84-86. [DOI] [PubMed] [Google Scholar]
- 11. Wysowski DK. Use of fertility drugs in the United States, 1973 through 1991. Fertil Steril. 1993;60(6):1096-1098. [DOI] [PubMed] [Google Scholar]
- 12. Bhattacharya S, Harrild K, Mollison J, et al. Clomifene citrate or unstimulated intrauterine insemination compared with expectant management for unexplained infertility: pragmatic randomised controlled trial. BMJ. 2008;337:a716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Medicine PCftASfR. Evidence-based treatments for couples with unexplained infertility: a guideline. Fertil Steril. 2020;113(2):305- 322. [DOI] [PubMed] [Google Scholar]
- 14. Diamond MP, Legro RS, Coutifaris C, et al. ; NICHD Reproductive Medicine Network . Letrozole, gonadotropin, or clomiphene for unexplained infertility. N Engl J Med. 2015;373(13):1230-1240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Takasaki A, Tamura H, Taketani T, Shimamura K, Morioka H, Sugino N. A pilot study to prevent a thin endometrium in patients undergoing clomiphene citrate treatment. J Ovarian Res. 2013;6(1):94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Dehbashi S, Parsanezhad ME, Alborzi S, Zarei A. Effect of clomiphene citrate on endometrium thickness and echogenic patterns. Int J Gynaecol Obstet. 2003;80(1):49-53. [DOI] [PubMed] [Google Scholar]
- 17. Haritha S, Rajagopalan G. Follicular growth, endometrial thickness, and serum estradiol levels in spontaneous and clomiphene citrate-induced cycles. Int J Gynaecol Obstet. 2003;81(3):287-292. [DOI] [PubMed] [Google Scholar]
- 18. Nakamura Y, Ono M, Yoshida Y, Sugino N, Ueda K, Kato H. Effects of clomiphene citrate on the endometrial thickness and echogenic pattern of the endometrium. Fertil Steril. 1997;67(2):256-260. [DOI] [PubMed] [Google Scholar]
- 19. Quaas AM, Gavrizi SZ, Peck JD, et al. ; Eunice Kennedy Shriver National Institute of Child Health and Human Development Reproductive Medicine Network . Endometrial thickness after ovarian stimulation with gonadotropin, clomiphene, or letrozole for unexplained infertility, and association with treatment outcomes. Fertil Steril. 2021;115(1):213-220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Weiss NS, van Vliet MN, Limpens J, et al. Endometrial thickness in women undergoing IUI with ovarian stimulation. How thick is too thin? A systematic review and meta-analysis. Hum Reprod. 2017;32(5):1009-1018. [DOI] [PubMed] [Google Scholar]
- 21. Gadalla MA, Huang S, Wang R, et al. The effect of clomiphene citrate on endometrial thickness, ovulation, pregnancy and live birth in anovulatory women: a systematic review and meta-analysis. Ultrasound Obstet Gynecol. 2018;51(1):64-76. [DOI] [PubMed] [Google Scholar]
- 22. Coutifaris C, Myers ER, Guzick DS, et al. ; NICHD National Cooperative Reproductive Medicine Network . Histological dating of timed endometrial biopsy tissue is not related to fertility status. Fertil Steril. 2004;82(5):1264-1272. [DOI] [PubMed] [Google Scholar]
- 23. Murray MJ, Meyer WR, Zaino RJ, et al. A critical analysis of the accuracy, reproducibility, and clinical utility of histologic endometrial dating in fertile women. Fertil Steril. 2004;81(5):1333-1343. [DOI] [PubMed] [Google Scholar]
- 24. Strowitzki T, Germeyer A, Popovici R, von Wolff M. The human endometrium as a fertility-determining factor. Hum Reprod Update. 2006;12(5):617-630. [DOI] [PubMed] [Google Scholar]
- 25. Lessey BA, Castelbaum AJ, Sawin SW, Sun J. Integrins as markers of uterine receptivity in women with primary unexplained infertility. Fertil Steril. 1995;63(3):535-542. [PubMed] [Google Scholar]
- 26. Foulk RA, Zdravkovic T, Genbacev O, Prakobphol A. Expression of L-selectin ligand MECA-79 as a predictive marker of human uterine receptivity. J Assist Reprod Genet. 2007;24(7):316-321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Laird SM, Tuckerman EM, Dalton CF, Dunphy BC, Li TC, Zhang X. The production of leukaemia inhibitory factor by human endometrium: presence in uterine flushings and production by cells in culture. Hum Reprod. 1997;12(3):569-574. [DOI] [PubMed] [Google Scholar]
- 28. Hambartsoumian E. Endometrial leukemia inhibitory factor (LIF) as a possible cause of unexplained infertility and multiple failures of implantation. Am J Reprod Immunol. 1998;39(2):137-143. [DOI] [PubMed] [Google Scholar]
- 29. Young SL, Opsahl MS, Fritz MA. Serum concentrations of enclomiphene and zuclomiphene across consecutive cycles of clomiphene citrate therapy in anovulatory infertile women. Fertil Steril. 1999;71(4):639-644. [DOI] [PubMed] [Google Scholar]
- 30. Ganesh A, Chauhan N, Das S, Chakravarty B, Chaudhury K. Endometrial receptivity markers in infertile women stimulated with letrozole compared with clomiphene citrate and natural cycles. Syst Biol Reprod Med. 2014;60(2):105-111. [DOI] [PubMed] [Google Scholar]
- 31. Al-Fozan H, Al-Khadouri M, Tan SL, Tulandi T. A randomized trial of letrozole versus clomiphene citrate in women undergoing superovulation. Fertil Steril. 2004;82(6):1561-1563. [DOI] [PubMed] [Google Scholar]
- 32. Gianaroli L, Racowsky C, Geraedts J, Cedars M, Makrigiannakis A, Lobo R. Best practices of ASRM and ESHRE: a journey through reproductive medicine. Hum Reprod. 2012;27(12):3365-3379. [DOI] [PubMed] [Google Scholar]
- 33. Marcoux S, Maheux R, Bérubé S. Laparoscopic surgery in infertile women with minimal or mild endometriosis. Canadian Collaborative Group on Endometriosis. N Engl J Med. 1997;337(4):217-222. [DOI] [PubMed] [Google Scholar]
- 34. Nastri CO, Lensen SF, Gibreel A, et al. Endometrial injury in women undergoing assisted reproductive techniques. Cochrane Database Syst Rev. 2015;( 3):CD009517. [DOI] [PubMed] [Google Scholar]
- 35. Nastri CO, Lensen S, Polanski L, Raine-Fenning N, Farquhar CM, Martins WP. Endometrial injury and reproductive outcomes: there’s more to this story than meets the horse’s blind eye. Hum Reprod. 2015;30(3):749. [DOI] [PubMed] [Google Scholar]
- 36. Delisle MF, Villeneuve M, Boulvain M. Measurement of endometrial thickness with transvaginal ultrasonography: is it reproducible? J Ultrasound Med. 1998;17(8):481-4; quiz 485. [DOI] [PubMed] [Google Scholar]
- 37. Haas J, Smith R, Zilberberg E, et al. Endometrial compaction (decreased thickness) in response to progesterone results in optimal pregnancy outcome in frozen-thawed embryo transfers. Fertil Steril. 2019;112(3):503-509.e1. [DOI] [PubMed] [Google Scholar]
- 38. Ohno Y, Fujimoto Y. Endometrial oestrogen and progesterone receptors and their relationship to sonographic appearance of the endometrium. Hum Reprod Update. 1998;4(5):560-564. [DOI] [PubMed] [Google Scholar]
- 39. Talbi S, Hamilton AE, Vo KC, et al. Molecular phenotyping of human endometrium distinguishes menstrual cycle phases and underlying biological processes in normo-ovulatory women. Endocrinology. 2006;147(3):1097-1121. [DOI] [PubMed] [Google Scholar]
- 40. Dancoine F, Couplet G, Buvat J, Guittard C, Marcolin G, Fourlinnie JC. Analytical and clinical evaluation of the Immulite estradiol assay in serum from patients undergoing in vitro fertilization: estradiol increase in mature follicles. Clin Chem. 1997;43(7):1165-1171. [PubMed] [Google Scholar]
- 41. De Boever J, Kohen F, Vandekerckhove D, Van Maele G. Solid-phase chemiluminescence immunoassay for progesterone in unextracted serum. Clin Chem. 1984;30(10):1637-1641. [PubMed] [Google Scholar]
- 42. Young SL, Savaris RF, Lessey BA, et al. Effect of randomized serum progesterone concentration on secretory endometrial histologic development and gene expression. Hum Reprod. 2017;32(9):1903-1914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Hanna MG, Reuter VE, Hameed MR, et al. Whole slide imaging equivalency and efficiency study: experience at a large academic center. Mod Pathol. 2019;32(7):916-928. [DOI] [PubMed] [Google Scholar]
- 44. Cornish TC, Swapp RE, Kaplan KJ. Whole-slide imaging: routine pathologic diagnosis. Adv Anat Pathol. 2012;19(3):152-159. [DOI] [PubMed] [Google Scholar]
- 45. Fónyad L, Krenács T, Nagy P, et al. Validation of diagnostic accuracy using digital slides in routine histopathology. Diagn Pathol. 2012;7:35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Webster JD, Dunstan RW. Whole-slide imaging and automated image analysis: considerations and opportunities in the practice of pathology. Vet Pathol. 2014;51(1):211-223. [DOI] [PubMed] [Google Scholar]
- 47. Angell HK, Gray N, Womack C, Pritchard DI, Wilkinson RW, Cumberbatch M. Digital pattern recognition-based image analysis quantifies immune infiltrates in distinct tissue regions of colorectal cancer and identifies a metastatic phenotype. Br J Cancer. 2013;109(6):1618-1624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Laurinavicius A, Plancoulaine B, Laurinaviciene A, et al. A methodology to ensure and improve accuracy of Ki67 labelling index estimation by automated digital image analysis in breast cancer tissue. Breast Cancer Res. 2014;16(2):R35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Taylor NJ, Nikolaishvili-Feinberg N, Midkiff BR, Conway K, Millikan RC, Geradts J. Rational manual and automated scoring thresholds for the immunohistochemical detection of TP53 missense mutations in human breast carcinomas. Appl Immunohistochem Mol Morphol. 2016;24(6):398-404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Andersson S, Sundberg M, Pristovsek N, et al. Insufficient antibody validation challenges oestrogen receptor beta research. Nat Commun. 2017;8:15840. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Nelson AW, Groen AJ, Miller JL, et al. Comprehensive assessment of estrogen receptor beta antibodies in cancer cell line models and tissue reveals critical limitations in reagent specificity. Mol Cell Endocrinol. 2017;440:138-150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Budwit-Novotny DA, McCarty KS, Cox EB, et al. Immunohistochemical analyses of estrogen receptor in endometrial adenocarcinoma using a monoclonal antibody. Cancer Res. 1986;46(10):5419-5425. [PubMed] [Google Scholar]
- 53. Schindelin J, Arganda-Carreras I, Frise E, et al. Fiji: an open-source platform for biological-image analysis. Nat Methods. 2012;9(7):676-682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Fuhrich DG, Lessey BA, Savaris RF. Comparison of HSCORE assessment of endometrial beta3 integrin subunit expression with digital HSCORE using computerized image analysis (ImageJ). Anal Quant Cytopathol Histpathol. 2013;35(4):210-216. [PMC free article] [PubMed] [Google Scholar]
- 55. Schneider CA, Rasband WS, Eliceiri KW. NIH Image to ImageJ: 25 years of image analysis. Nat Methods. 2012;9(7):671-675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Divine G, Norton HJ, Hunt R, Dienemann J. Statistical grand rounds: a review of analysis and sample size calculation considerations for Wilcoxon tests. Anesth Analg. 2013;117(3):699-710. [DOI] [PubMed] [Google Scholar]
- 57. Dwivedi AK, Mallawaarachchi I, Alvarado LA. Analysis of small sample size studies using nonparametric bootstrap test with pooled resampling method. Stat Med. 2017;36(14):2187-2205. [DOI] [PubMed] [Google Scholar]
- 58. Ghasemi A, Zahediasl S. Normality tests for statistical analysis: a guide for non-statisticians. Int J Endocrinol Metab. 2012;10(2):486-489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Wu SP, Anderson ML, Wang T, et al. Dynamic transcriptome, accessible genome, and PGR cistrome profiles in the human myometrium. FASEB J. 2020;34(2):2252-2268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Trapnell C, Pachter L, Salzberg SL. TopHat: discovering splice junctions with RNA-Seq. Bioinformatics. 2009;25(9):1105-1111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Trapnell C, Williams BA, Pertea G, et al. Transcript assembly and quantification by RNA-Seq reveals unannotated transcripts and isoform switching during cell differentiation. Nat Biotechnol. 2010;28(5):511-515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Miller RA, Galecki A, Shmookler-Reis RJ. Interpretation, design, and analysis of gene array expression experiments. J Gerontol A Biol Sci Med Sci. 2001;56(2):B52-B57. [DOI] [PubMed] [Google Scholar]
- 63. Witten DMTR. A Comparison of Fold-Change and the T-Statistic for Microarray Data Analysis. Stanford University; 2007:1-13. [Google Scholar]
- 64. Jeffery IB, Higgins DG, Culhane AC. Comparison and evaluation of methods for generating differentially expressed gene lists from microarray data. BMC Bioinformatics. 2006;7:359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Huang da W, Sherman BT, Lempicki RA. Systematic and integrative analysis of large gene lists using DAVID bioinformatics resources. Nat Protoc. 2009;4(1):44-57. [DOI] [PubMed] [Google Scholar]
- 66. da Huang W, Sherman BT, Lempicki RA. Bioinformatics enrichment tools: paths toward the comprehensive functional analysis of large gene lists. Nucleic Acids Res. 2009;37(1):1-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. Krämer A, Green J, Pollard J Jr, Tugendreich S. Causal analysis approaches in Ingenuity Pathway Analysis. Bioinformatics. 2014;30(4):523-530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68. Chi RA, Wang T, Adams N, et al. Human endometrial transcriptome and progesterone receptor cistrome reveal important pathways and epithelial regulators. J Clin Endocrinol Metab. 2020;105(4):e1419-e1439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69. Zhang Y, Liu T, Meyer CA, et al. Model-based analysis of ChIP-Seq (MACS). Genome Biol. 2008;9(9):R137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70. Hulsen T, de Vlieg J, Alkema W. BioVenn – a web application for the comparison and visualization of biological lists using area-proportional Venn diagrams. BMC Genomics. 2008;9:488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71. Hawkins Bressler LFM, Wu S, Yuan L, et al. 2021. Data from: Poor endometrial proliferation after clomiphene is associated with altered estrogen action.Dryad. Deposited February 5, 2021. doi: 10.5061/dryad.9kd51c5gk. [DOI] [PMC free article] [PubMed]
- 72. Yilmaz BD, Sison CAM, Yildiz S, et al. Genome-wide estrogen receptor-a binding and action in human endometrial stromal cells. Fertil Steril Dialogue. 2020;1(1):59-66. [DOI] [PubMed] [Google Scholar]
- 73. Yoshiki N, Kubota T, Aso T. Identification of heme oxygenase in human endometrium. J Clin Endocrinol Metab. 2001;86(10):5033-5038. [DOI] [PubMed] [Google Scholar]
- 74. Dassen H, Kamps R, Punyadeera C, et al. Haemoglobin expression in human endometrium. Hum Reprod. 2008;23(3):635-641. [DOI] [PubMed] [Google Scholar]
- 75. McMahon M, Ye S, Izzard L, et al. ADAMTS5 is a critical regulator of virus-specific T cell immunity. PLoS Biol. 2016;14(11):e1002580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76. Zenclussen ML, Linzke N, Schumacher A, et al. Heme oxygenase-1 is critically involved in placentation, spiral artery remodeling, and blood pressure regulation during murine pregnancy. Front Pharmacol. 2015;5:291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77. Ichii O, Kimura J, Okamura T, et al. IL-36α regulates tubulointerstitial inflammation in the mouse kidney. Front Immunol. 2017;8:1346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78. Okulicz WC, Ace CI, Scarrell R. Zonal changes in proliferation in the rhesus endometrium during the late secretory phase and menses. Proc Soc Exp Biol Med. 1997;214(2):132-138. [DOI] [PubMed] [Google Scholar]
- 79. Okulicz WC, Balsamo M. A double immunofluorescent method for simultaneous analysis of progesterone-dependent changes in proliferation and the oestrogen receptor in endometrium of rhesus monkeys. J Reprod Fertil. 1993;99(2):545-549. [DOI] [PubMed] [Google Scholar]
- 80. Okulicz WC, Balsamo M, Tast J. Progesterone regulation of endometrial estrogen receptor and cell proliferation during the late proliferative and secretory phase in artificial menstrual cycles in the rhesus monkey. Biol Reprod. 1993;49(1):24-32. [DOI] [PubMed] [Google Scholar]
- 81. Slayden OD, Brenner RM. Hormonal regulation and localization of estrogen, progestin and androgen receptors in the endometrium of nonhuman primates: effects of progesterone receptor antagonists. Arch Histol Cytol. 2004;67(5):393-409. [DOI] [PubMed] [Google Scholar]
- 82. Spencer TE. Biological roles of uterine glands in pregnancy. Semin Reprod Med. 2014;32(5):346-357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83. Karizbodagh MP, Rashidi B, Sahebkar A, Masoudifar A, Mirzaei H. Implantation window and angiogenesis. J Cell Biochem. 2017;118(12):4141-4151. [DOI] [PubMed] [Google Scholar]
- 84. Zhang Y, Lin X, Dai Y, et al. Endometrial stem cells repair injured endometrium and induce angiogenesis via AKT and ERK pathways. Reproduction. 2016;152(5):389-402. [DOI] [PubMed] [Google Scholar]
- 85. Billhaq DH, Lee SH, Lee S. The potential function of endometrial-secreted factors for endometrium remodeling during the estrous cycle. Anim Sci J. 2020;91(1):e13333. [DOI] [PubMed] [Google Scholar]
- 86. Koos RD. Minireview: Putting physiology back into estrogens’ mechanism of action. Endocrinology. 2011;152(12):4481-4488. [DOI] [PubMed] [Google Scholar]
- 87. Zelante T, Fric J, Wong AY, Ricciardi-Castagnoli P. Interleukin-2 production by dendritic cells and its immuno-regulatory functions. Front Immunol. 2012;3:161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88. Malek TR. The biology of interleukin-2. Annu Rev Immunol. 2008;26:453-479. [DOI] [PubMed] [Google Scholar]
- 89. Hyde KJ, Schust DJ. Immunologic challenges of human reproduction: an evolving story. Fertil Steril. 2016;106(3):499-510. [DOI] [PubMed] [Google Scholar]
- 90. Lessey BA, Kim JJ. Endometrial receptivity in the eutopic endometrium of women with endometriosis: it is affected, and let me show you why. Fertil Steril. 2017;108(1):19-27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91. Ahn SH, Khalaj K, Young SL, Lessey BA, Koti M, Tayade C. Immune-inflammation gene signatures in endometriosis patients. Fertil Steril. 2016;106(6):1420-1431.e7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92. Ribatti D, Crivellato E. Immune cells and angiogenesis. J Cell Mol Med. 2009;13(9A):2822-2833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93. Borghi C, Indraccolo U, Scutiero G, et al. Biomolecular basis related to inflammation in the pathogenesis of endometrial cancer. Eur Rev Med Pharmacol Sci. 2018;22(19):6294-6299. [DOI] [PubMed] [Google Scholar]
- 94. Snider AP, Wood JR. Obesity induces ovarian inflammation and reduces oocyte quality. Reproduction. 2019;158(3):R79-R90. [DOI] [PubMed] [Google Scholar]
- 95. Saltiel AR, Olefsky JM. Inflammatory mechanisms linking obesity and metabolic disease. J Clin Invest. 2017;127(1):1-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96. Legro RS. Obesity and PCOS: implications for diagnosis and treatment. Semin Reprod Med. 2012;30(6):496-506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97. Pasquali R. Obesity and androgens: facts and perspectives. Fertil Steril. 2006;85(5):1319-1340. [DOI] [PubMed] [Google Scholar]
- 98. Bishop CV, Mishler EC, Takahashi DL, et al. Chronic hyperandrogenemia in the presence and absence of a western-style diet impairs ovarian and uterine structure/function in young adult rhesus monkeys. Hum Reprod. 2018;33(1):128-139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99. True CA, Takahashi DL, Burns SE, et al. Chronic combined hyperandrogenemia and western-style diet in young female rhesus macaques causes greater metabolic impairments compared to either treatment alone. Hum Reprod. 2017;32(9):1880-1891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100. Kuo K, Roberts VHJ, Gaffney J, et al. Maternal high-fat diet consumption and chronic hyperandrogenemia are associated with placental dysfunction in female rhesus macaques. Endocrinology. 2019;160(8):1937-1949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101. Bulun SE, Monsavais D, Pavone ME, et al. Role of estrogen receptor-β in endometriosis. Semin Reprod Med. 2012;30(1):39-45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102. Trukhacheva E, Lin Z, Reierstad S, Cheng YH, Milad M, Bulun SE. Estrogen receptor (ER) beta regulates ERalpha expression in stromal cells derived from ovarian endometriosis. J Clin Endocrinol Metab. 2009;94(2):615-622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103. Bulun SE, Cheng YH, Pavone ME, et al. Estrogen receptor-beta, estrogen receptor-alpha, and progesterone resistance in endometriosis. Semin Reprod Med. 2010;28(1):36-43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104. Pace P, Taylor J, Suntharalingam S, Coombes RC, Ali S. Human estrogen receptor beta binds DNA in a manner similar to and dimerizes with estrogen receptor alpha. J Biol Chem. 1997;272(41):25832-25838. [DOI] [PubMed] [Google Scholar]
- 105. Simmen RC, Kelley AS. Reversal of fortune: estrogen receptor-β in endometriosis. J Mol Endocrinol. 2016;57(2):F23-F27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106. Fuentes N, Silveyra P. Estrogen receptor signaling mechanisms. Adv Protein Chem Struct Biol. 2019;116:135-170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107. Zhu H, Leung PC, MacCalman CD. Expression of ADAMTS-5/implantin in human decidual stromal cells: regulatory effects of cytokines. Hum Reprod. 2007;22(1):63-74. [DOI] [PubMed] [Google Scholar]
- 108. Shan N, Zhang X, Xiao X, et al. The role of laminin α4 in human umbilical vein endothelial cells and pathological mechanism of preeclampsia. Reprod Sci. 2015;22(8):969-979. [DOI] [PubMed] [Google Scholar]
- 109. Shan N, Zhang X, Xiao X, et al. Laminin α4 (LAMA4) expression promotes trophoblast cell invasion, migration, and angiogenesis, and is lowered in preeclamptic placentas. Placenta. 2015;36(8):809-820. [DOI] [PubMed] [Google Scholar]
- 110. Ji Y, Zhou L, Wang G, Qiao Y, Tian Y, Feng Y. Role of LAMA4 gene in regulating extravillous trophoblasts in pathogenesis of preeclampsia. Med Sci Monit. 2019;25:9630-9636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111. Streit M, Riccardi L, Velasco P, et al. Thrombospondin-2: a potent endogenous inhibitor of tumor growth and angiogenesis. Proc Natl Acad Sci U S A. 1999;96(26):14888-14893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112. Seki N, Kodama J, Hashimoto I, Hongo A, Yoshinouchi M, Kudo T. Thrombospondin-1 and -2 messenger RNA expression in normal and neoplastic endometrial tissues: correlation with angiogenesis and prognosis. Int J Oncol. 2001;19(2):305-310. [DOI] [PubMed] [Google Scholar]
- 113. Lee YH, Tan CW, Venkatratnam A, et al. Dysregulated sphingolipid metabolism in endometriosis. J Clin Endocrinol Metab. 2014;99(10):E1913-E1921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114. Schessl J, Bach E, Rost S, et al. Novel recessive myotilin mutation causes severe myofibrillar myopathy. Neurogenetics. 2014;15(3):151-156. [DOI] [PubMed] [Google Scholar]
- 115. Ghosh S, Kobayashi N, Nagashima S, et al. Molecular characterization of the VP1, VP2, VP4, VP6, NSP1 and NSP2 genes of bovine group B rotaviruses: identification of a novel VP4 genotype. Arch Virol. 2010;155(2):159-167. [DOI] [PubMed] [Google Scholar]
- 116. Hewitt SC, Winuthayanon W, Lierz SL, et al. Role of ERα in mediating female uterine transcriptional responses to IGF1. Endocrinology. 2017;158(8):2427-2435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117. Lin CY, Ström A, Vega VB, et al. Discovery of estrogen receptor alpha target genes and response elements in breast tumor cells. Genome Biol. 2004;5(9):R66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118. Petracco RG, Kong A, Grechukhina O, Krikun G, Taylor HS. Global gene expression profiling of proliferative phase endometrium reveals distinct functional subdivisions. Reprod Sci. 2012;19(10):1138-1145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119. Burney RO, Talbi S, Hamilton AE, et al. Gene expression analysis of endometrium reveals progesterone resistance and candidate susceptibility genes in women with endometriosis. Endocrinology. 2007;148(8):3814-3826. [DOI] [PubMed] [Google Scholar]
- 120. Cho S, Mutlu L, Grechukhina O, Taylor HS. Circulating microRNAs as potential biomarkers for endometriosis. Fertil Steril. 2015;103(5):1252-60.e1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121. Spandorfer SD, Arrendondo-Soberon F, Loret de Mola JR, Feinberg RF. Reliability of intraobserver and interobserver sonographic endometrial stripe thickness measurements. Fertil Steril. 1998;70(1):152-154. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
All data generated or analyzed during this study are included in this published article or in the data repositories listed in References.
Supplemental tables and figures can be found at: https://doi.org/10.5061/dryad.9kd51c5gk
Gene expression data can be found at: https://www.ncbi.nlm.nih.gov/geo/query/acc.cgi?acc=GSE164764
https://www.ncbi.nlm.nih.gov/geo/query/acc.cgi?acc=GSE164768