Abstract
Purpose
To determine whether periodontal disease is positively associated with incident diabetes across the continuum of body mass levels (BMI) and test the hypothesis that the periodontal risk for incident diabetes is modified by BMI.
Methods
We included 5569 diabetes-free participants from Visit 4 (1996-1998) of the Atherosclerosis Risk in Communities study and followed them until 2018. Periodontal disease status was classified by periodontal profile class (PPC)-Stages , and incident diabetes was based on participant report of physician diagnosis. We estimated the hazard ratios (HR) for diabetes using a competing risk model for each PPC-Stage. We assessed multiplicative interactions between periodontal disease and BMI (as a continuous variable) on risk of diabetes.
Results
During a median time of 19.4 years of follow-up, 1348 incident diabetes cases and 1529 deaths occurred. Compared to the “Health/Incidental Disease” stage, participants with PPC “Severe Periodontal Disease” or “Severe Tooth Loss” stage and lower BMI had elevated risk for diabetes adjusting for demographic, smoking, education, and biological variables when accounting for death as a competing risk with HRs of 1.76 (95% CI 1.10-2.80) and 2.11 (95% CI 1.46-3.04), respectively. The interaction between PPC-Stages and BMI was significant (P = 0.01). No significant associations of PPC-Stages with incident diabetes were present when BMI was above 31 kg/m2.
Conclusion
Periodontal disease was associated with incident diabetes, especially in nonobese participants. Dentists should be aware that periodontal disease is associated with incident diabetes but the association may be modified for patient’s at higher BMI levels.
Keywords: periodontal-systemic disease interaction, diabetes, body mass index, periodontal disease
Periodontal disease, obesity, and diabetes are strongly linked (1). Based on a recent meta-analysis, a nonsurgical oral plaque elimination treatment of periodontitis patients with diabetes resulted in a significant overall 0.56% A1c reduction (2). Studies have generally shown that individuals with normal glucose tolerance and periodontitis are more likely to develop diabetes than subjects with shallow periodontal probing depths (3,4). However, specific studies reporting risks of subgroups of periodontal disease for diabetes have generated inconsistent results. The heterogeneity of periodontal disease definitions, which were traditionally observation-driven and expert opinion-based, used in those studies may partially account for the inconsistent findings that periodontal disease is associated with incident diabetes (5,6).
Body mass index (BMI) and inflammation are well-defined risk factors for diabetes (7,8). Studies indicate that BMI may modify the associations between inflammatory markers and incident diabetes. For example, the association of periodontal disease with C-reactive protein, a systemic inflammatory marker, in the Atherosclerosis Risk In Communities (ARIC) cohort was BMI-dependent: as BMI increased, the association became weaker (9). In addition, clinical, epidemiological and basic science research have demonstrated a clear quantifiable inflammation-mediated association between obesity and periodontal disease (1). Some prospective studies demonstrated that high BMI is a risk factor for periodontal disease (10,11). According to several meta-analyses, overweight and obesity were significantly associated with periodontitis (12). The risk-modifying effect of BMI on periodontal disease for developing diabetes was also suggested in several clinical intervention studies reporting that the nonsurgical periodontal therapy for periodontitis patients with diabetes achieved better diabetic control, as measured by A1c reduction, in patients with lower BMI than higher BMI (13-15).
The goal of this report is to assess whether periodontal disease, defined by Periodontal Profile Class (PPC)-Stages, is associated with prospective self-reported diabetes risk in ARIC (16,17). Since periodontal disease can manifest into a systemic inflammatory condition and that obesity can modify inflammatory biomarkers, an interaction of obesity and periodontal disease may exist in determining diabetes development. Therefore, we hypothesize that BMI modifies the association between periodontal disease as defined by the data-driven PPC-Stages and incident diabetes.
Materials and Methods
Study Sample
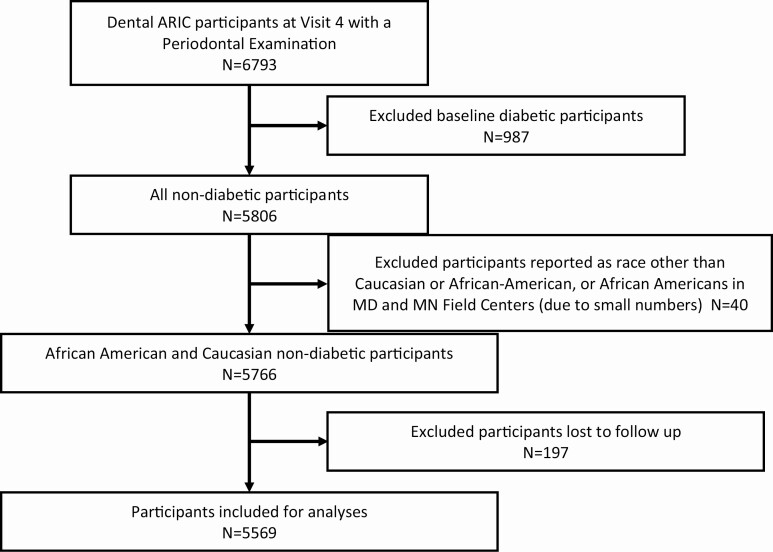
This report is based on a subset of the ARIC study participants who had a periodontal examination record. Details of the ARIC study and the dental component of ARIC study have been described in previous publications (16-19). The institutional review board at each study site approved the protocol, and participants provided written informed consent at each clinic visit. Of the 6793 ARIC participants who had complete periodontal examinations at Visit 4 (1996-1998; baseline of our study), 987 participants with prevalent diabetes, which was defined as the fasting glucose level above 140 mg/dL, nonfasting glucose level above 200 mg/dL, or taking diabetes medications, were excluded. Among the remaining 5806 nondiabetic participants, those who self-reported race other than white or African American were excluded (N = 40). We additionally excluded 197 participants who were lost to follow-up after baseline. The remaining 5569 African American and Caucasian nondiabetic participants were included in our study (Fig. 1). The data collected for this study were from January 1996 to December 2018.
Figure 1.
Screening and inclusion process of ARIC cohort Participants for Incident Diabetes. Of the 6793 ARIC participants who had complete periodontal examinations, 987 participants with existing diabetes at the fourth clinic visit (Visit 4, baseline) were excluded. Among the remaining 5806 nondiabetic participants, those who self-reported race other than Caucasian or African American and African Americans from the Minnesota or Maryland field centers were excluded (N = 40). Since we plan to do a competing risk analysis using the ARIC annual phone follow-up, we excluded 197 participants who did not respond to the phone call. The remaining 5569 African American and Caucasian nondiabetic participants were used in the competing risk analysis.
Measurement of Exposures
A dental component was added to the ARIC Visit 4. Full-mouth examinations on all teeth present were performed by trained and calibrated examiners (20). Periodontal disease status was defined using the PPC-Stages, which was based on a latent class analysis (LCA) applied to the data from 7 periodontal indices, all of which are routinely collected in a dental clinic. LCA created 7 mutually exclusive homogenous classes that were based on clinical disease phenotypes of ARIC study participants (16). The 7 tooth parameters, which were dichotomized, include (1) ≥1 site with interproximal attachment level ≥ 3 mm; (2) ≥1 site with probing depth ≥ 4 mm; (3) extent of bleeding on probing (dichotomized at 50% or ≥3 sites per tooth); (4) gingival inflammation index (GI; dichotomized as GI = 0 vs GI ≥ 1); (5) plaque index (PI; dichotomized as PI = 0 vs Pl ≥ 1); (6) the presence/absence of full prosthetic crowns for each tooth; (7) tooth status (present vs absent) (16,17). The 7 PPC-Stages have very high probability (most of them >95%) of being correctly assigned to their group (16). PPC-Stages was a simple modification of the original PPC system, the details of which are described in several other publications (16,17,19). Briefly, PPC-Health became “PPC-Stage I” and was labeled as “PPC-Health/Incidental disease” (abbreviated as “Healthy/Incidental”). PPC-Stage I serves as the reference group in this study. PPC-Mild disease became “PPC-Stage II” and was labeled as “Mild Periodontal Disease” (abbreviated as “Mild Perio”); PPC-Posterior disease became “PPC-Stage III” labeled as “Moderate Periodontal Disease” (abbreviated as “Mod Perio”); PPC-Severe disease became “PPC-Stage IV” and was labeled as “Severe Periodontal Disease” (abbreviated as “Severe Perio”); PPC-High GI became “PPC-Stage V” and was labeled as “Mild Tooth Loss and High Gingival Inflammation” (abbreviated as “Mild TL/High GI”); PPC-Tooth Loss became “PPC-Stage VI,” labeled as “Moderate Tooth Loss and Reduced Periodontium” (abbreviated as “Mod TL/Reduced Perio”); and PPC-Severe Tooth Loss became “PPC-Stage VII” and was labeled as “PPC-Severe Tooth Loss” (abbreviated as “Severe TL”). Even though PPC-Stages are categorical variables and nonhierarchical, we attempted to label them in a manner similar to other periodontal indices based on disease severity. However, some ordinality is seen for Stages V to VII, which have increasing numbers of missing teeth in patients (16).
The ARIC cohort was a longitudinal observational survey and primarily designed to investigate cardiovascular disease risks. Records related to dental clinic visits and treatment during the surveillance period were not included in the study design; therefore, that information was not collected.
Measurement of Incident Diabetes
ARIC participants were called annually through 2011 and semi-annually starting in 2012. We classified incident diabetes mellitus using a self-report from the annual telephone follow-up that their physician had stated they had diabetes. That question was “Since we last contacted you, has a doctor said you have diabetes or sugar in the blood?” and the follow-up question was “What was the date and year the doctor told you that you had diabetes?”
Diabetes mellitus was not classified as type 1 diabetes mellitus or type 2 diabetes mellitus because diabetes condition was reported by the participants rather than a physician’s diagnosis. However, given the age of the participants, most of the new diabetes cases were presumably type 2. For the competing risk of death, all-cause deaths were classified based on linkage to the National Death Index using death certificates (21). Time-to-event was calculated from ARIC Visit 4 examination date to the date the participant was told they had diabetes, the date of death, loss to follow-up, or December 31, 2018, whichever came first.
Other Variables of Interest
Age, sex, race/center, BMI and lipid profile were measured according to published methods (18). Education was self-reported during ARIC Visit 1 and was categorized as basic (≤11 years), intermediate (12-16 years), and advanced (17-21 years). A 3-level smoking status was used in this report including never smoker, former smoker, and current smoker at Visit 4 via interview. BMI was interpreted using the Centers of Disease Control and Prevention standard weight status categories for adults as follows: BMI < 18.5 is underweight, 18.5 ≤ 25 is normal weight, 25 ≤ 30 is overweight, and ≥30 is obese (22). Obesity is frequently subdivided into 3 classes: Class I is 30 to <35, Class II is 35 to <40, and Class III is 40 or higher (22,23). Hypertension was defined as the average of 2 blood pressure readings at the visit (with systolic blood pressure having a cutoff point of 140 mmHg or higher and diastolic blood pressure having a cutoff point of 90 mmHg or higher) or the use of hypertension medication. Level of high-density lipoprotein (HDL) and triglycerides was dichotomized at 40 mg/dL and 200 mg/dL, respectively, to indicate low or high lipid profiles. Whether participants took cholesterol-lowering medications (ie, statins) or not was also recorded.
Statistical Analysis
We analyzed participant’s demographics, lipid profiles, mean BMI, smoking status, and education level by PPC-Stages. Incident diabetes and mortality were also examined by PPC-Stages. Descriptive statistics were computed using chi-squared for categorical variables and general linear models for continuous variables.
To determine the association of PPC-Stage with incident diabetes while accounting for the competing risk of death due to the high mortality risk (27%) during follow-up, we calculated subdistribution hazard models using the Fine and Gray method (24) and adjusted for covariates in a series of models (PROC PHREG, SAS Version 9.4, SAS Institute Inc., Cary, NC, USA). Participants who developed diabetes before death would be identified as a case in the competing risk model. We presented the risk in a nested subdistribution model where Model 1 (M1) was a crude unadjusted model, Model 2 was M1 adjusting BMI, and Model 3 was the fully adjusted model including the following variables: race/center, age, sex, BMI, smoking (3 levels), HDL, triglycerides, cholesterol-lowering medications, hypertension, and education (3 levels).
To test whether the effect of periodontal disease reflected by PPC-Stages on incident diabetes is modified by BMI, we used a similar competing risk model including an interaction term of BMI * PPC-Stage to test the overall interaction effect for PPC-Stage and *BMI on incident diabetes. We then tested the significance of interaction between PPC-Stages (categorical variable) and BMI (continuous variable) for incident diabetes in the competing risk model. We used centering for the BMI groups, which is a statistical method of exploring an interaction of a categorical (PPC-Stages) and continuous variable (BMI). This categorical-continuous interaction tests the effect of PPC-Stage at the y-intercept, which would normally be when BMI = 0. To test the effect of PPC-Stage at different BMI levels, we first subtract a constant from BMI (eg, BMI = BMI − 30.0) for each of the BMI levels. This allowed us to test the effect of PPC-Stages at specific BMI values for incident diabetes. We used the BMI values at the lower and higher boundaries rather than mid-point for each weight status category to provide a full range of hazard ratios across each weight classification within each PPC-Stage category. However, none of statistical analysis was performed to show differences among weight groups, which appear simply to designate the boundaries of the weight categories.
Results
Demographics and Periodontal Disease Categories of ARIC Participants
The mean age (SD) of ARIC participants was 62.4 (±5.6) at the baseline visit (ARIC Visit 4). Females accounted for 55.1% of the participants and 16.4% of the participants were African American (Table 1). During a median of 19.4 years of follow-up there were 1348 (24.2%) cases of incident diabetes and 1529 (27.5%) deaths. During the study period, the observed cumulative rates of incident diabetes were 21.0%, 23.2%, 20.9%, 29.0%, 25.3%, 25.2%, and 32.9% for PPC-Stages I to VII, respectively.
Table 1.
Percentage or mean demographics, biological markers, smoking, and education according to PPC-Stage in ARIC participants (1996-2018)
| Study variables | Study population (n = 5569) | PPC-Stage I health/incidental (n = 1612) | PPC-Stage II mild (n = 887) | PPC-Stage III moderate (n = 863) | PPC-Stage IV severe (n = 366) | PPC-Stage V high GI/mild TL (n = 513) | PPC-Stage VI reduced periodontium moderate TL (n = 648) | PPC-Stage VII severe TL (n = 680) | P-value |
|---|---|---|---|---|---|---|---|---|---|
| Age (years), mean (SD) | 62.4 (5.6) | 61.7 (5.5) | 62.3 (5.9) | 62.8 (5.6) | 61.7 (5.8) | 61.7 (5.6) | 63.8 (5.6) | 63.0 (5.5) | <0.0001 |
| Male (%) | 44.9 | 32.0 | 52.1 | 54.5 | 65.9 | 46.6 | 43.8 | 42.5 | <0.0001 |
| Mississippi (African American) (%) | 14.7 | 2.1 | 1.5 | 0.6 | 38.3 | 70.2 | 12.2 | 27.7 | |
| North Carolina (Caucasian) (%) | 24.5 | 35.7 | 31.0 | 13.2 | 15.9 | 7.2 | 29.8 | 16.3 | |
| North Carolina (African American) (%) | 1.7 | 0.7 | 1.0 | 0.6 | 3.6 | 1.8 | 3.9 | 2.8 | |
| Maryland (Caucasian) (%) | 25.8 | 15.4 | 39.2 | 26.3 | 33.6 | 11.3 | 31.3 | 33.5 | |
| Minnesota (Caucasian) (%) | 33.4 | 46.0 | 27.3 | 59.3 | 8.5 | 9.6 | 22.8 | 19.7 | <0.0001 |
| BMI, mean (SD) | 28.1 (5.1) | 27.1 (4.6) | 28.0 (4.7) | 27.8 (4.7) | 29.0 (6.0) | 29.3 (5.5) | 28.4 (5.0) | 29.0 (6.0) | <0.0001 |
| Weight | |||||||||
| Underweight (BMI < 18.5) | 0.8 | 0.6 | 0.8 | 0.4 | 1.4 | 1.0 | 0.9 | 1.0 | |
| Normal (BMI 18.5 ≤ 25.0) | 27.8 | 34.7 | 26.9 | 29.7 | 24.9 | 18.0 | 24.3 | 22.7 | |
| Overweight (BMI 25 ≤ 30) | 42.1 | 43.0 | 44.4 | 40.8 | 36.4 | 42.6 | 41.6 | 42.2 | |
| Obese Class I (BMI 30 ≤ 35) | 20.4 | 15.8 | 19.8 | 23.8 | 23.0 | 23.8 | 23.1 | 20.9 | |
| Obese Class II and above (BMI ≥ 35) | 8.9 | 6.0 | 8.1 | 5.5 | 14.3 | 14.7 | 10.1 | 13.2 | <0.0001 |
| Hypertension (%) | 30.7 | 24.6 | 29.2 | 26.1 | 39.5 | 41.5 | 36.3 | 35.1 | <0.0001 |
| Low HDL (<40 mg/dL) (%) | 24.9 | 18.1 | 29.0 | 27.6 | 29.0 | 21.8 | 29.6 | 28.2 | <0.0001 |
| High triglycerides (>200 mg/dL) | 15.3 | 17.0 | 17.8 | 15.9 | 7.4 | 7.8 | 16.8 | 15.3 | <0.0001 |
| Cholesterol-lowering medications (%) | 12.2 | 12.7 | 13.1 | 12.8 | 9.6 | 8.6 | 13.0 | 12.7 | 0.12 |
| Smoking | |||||||||
| Current smoker (%) | 12.3 | 6.8 | 6.2 | 16.6 | 17.0 | 11.8 | 16.0 | 22.3 | <0.0001 |
| Former smoker (%) | 38.8 | 37.5 | 37.1 | 45.6 | 35.1 | 33.6 | 41.0 | 39.3 | |
| Never smoker (%) | 48.9 | 55.7 | 56.7 | 37.8 | 48.0 | 54.6 | 43.0 | 38.5 | |
| Education level | <0.0001 | ||||||||
| Basic (<12 Years) (%) | 12.2 | 5.1 | 7.9 | 5.7 | 22.1 | 22.1 | 15.6 | 26.8 | |
| Intermediate (12-16 years) (%) | 43.5 | 42.3 | 44.2 | 44.7 | 39.1 | 32.8 | 48.8 | 49.4 | |
| Advanced (17-21 years) (%) | 44.3 | 52.6 | 47.9 | 49.7 | 38.8 | 45.1 | 35.7 | 23.8 | |
| Outcomes | <0.0001 | ||||||||
| Alive (%) | 48.3 | 57.8 | 50.7 | 49.5 | 38.5 | 46.2 | 41.1 | 35.2 | |
| Incident diabetes (%) | 24.2 | 21.0 | 23.2 | 20.9 | 29.0 | 25.3 | 25.2 | 32.9 | |
| All-cause mortality (%) | 27.5 | 21.2 | 26.0 | 29.7 | 32.5 | 28.5 | 33.8 | 31.9 |
Abbreviations: BMI, body mass index; GI, gingival inflammation index; HDL, high-density lipoprotein; PPC, periodontal profile class; TL, tooth loss.
All demographic and general health variables in Table 1 were statistically different across PPC-Stages except cholesterol-lowering medications. The percentage of males increased in all PPC-Stages other than Stage I (“Health/Incidental Disease.” Participants in the more severe PPC-Stages (Stage IV-VII) had a slightly higher BMI, were less educated, were more likely to be hypertensive, and were more likely to be current smokers compared to those in the less severe stages.
Diabetes Incidence According to BMI and PPC-Stages
Table 2 presents the incidence rate for diabetes and 3 nested models for BMI and PPC-Stages. The diabetes incidence rate (95% CI) was lowest for PPC-Stage I [1.22 (CI 1.09-1.34)]; was [1.99 (CI 1.62-2.37)] for PPC-Stage IV, and was highest for participants with PPC-Stage VII [2.30 (CI 2.00-2.59)]. The unadjusted M1 shows the risk of incident diabetes [HR (95% CI)] for PPC-Stages IV through VII were significantly higher compared to PPC-Stage I (periodontal health/incident disease). Partially adjusted M2 resulted in somewhat diminished, but significant HRs for PPC-Stage IV [1.39 (1.11-1.73)] as well as PPC-Stage VI [1.23 (1.02-1.48)] and PPC-Stage VII [1.59 (1.34-1.89)]. Fully adjusted M3 resulted in PPC-Stage VII remaining significantly elevated as compared to PPC-Stage I control [1.25 (1.03-1.51)]. The PPC-Stage Type III P-values for all 3 models were significant, as shown in Table 2.
Table 2.
Multivariable hazard ratios (95% CI) of incident diabetes by PPC-Stages in ARIC (January 1996-December 2018)
| PPC-Stage I health/incidental | PPC-Stage II mild | PPC-Stage III moderate | PPC-Stage IV severe | PPC-Stage V high GI/mild TL | PPC-Stage VI reduced periodontium/Mod TL | PPC-Stage VII severe TL | |
|---|---|---|---|---|---|---|---|
| N | 1612 | 887 | 863 | 366 | 513 | 648 | 680 |
| Diabetes (n) | 339 | 206 | 180 | 106 | 130 | 163 | 224 |
| Person-years | 27 856 | 14 560 | 14 016 | 5324 | 8041 | 9902 | 9757 |
| Incidence rate/100 years (95% CI) | 1.22 (1.09-1.34) | 1.41 (1.22-1.61) | 1.28 (1.10-1.47) | 1.99 (1.62-2.37) | 1.62 (1.34-1.89) | 1.65 (1.40-1.90) | 2.30 (2.00-2.59) |
| M1a | Ref | 1.18 (0.99-1.40) | 1.06 (0.89-1.27) | 1.70 (1.37-2.11) | 1.34 (1.09-1.64) | 1.37 (1.14-1.65) | 1.91 (1.61-2.26) |
| M2b | Ref | 1.09 (0.92-1.29) | 1.00 (0.83-1.20) | 1.39 (1.11-1.73) | 1.12 (0.92-1.38) | 1.23 (1.02-1.48) | 1.59 (1.34-1.89) |
| M3c | Ref | 0.96 (0.80-1.14) | 0.93 (0.77-1.12) | 1.10 (0.86-1.40) | 0.88 (0.69-1.12) | 1.02 (0.84-1.24) | 1.25 (1.03-1.51) |
Bold type indicates a statistically significant HR.
Abbreviations: GI, gingival inflammation index; PPC, periodontal profile class; Ref, reference; TL, tooth loss.
a Model 1: unadjusted subdistribution model (PPC-Stage Type III; P-value < 0.0001).
b Model 2: adjusted for BMI (PPC-Stage Type III; P-value < 0.0001).
c Model 3: fully adjusted model for all demographic parameters, health parameter, and education level. (PPC-Stage Type III; P = 0.03 with 6 degree of freedom)
BMI-Modified Risk of Periodontal Disease PPC-Stages for Incident Diabetes
The interaction of PPC and BMI on diabetes risk was significant (P = 0.01, 6-degree freedom), indicating the risk of diabetes from periodontal disease was modified by BMI (Table 3). While the interaction model was calculated on BMI as a continuous variable, it is possible to calculate HRs for points along the continuum.
Table 3.
Competing risk HRs (95% CI) for incident diabetes by PPC-Stages interaction with boundary BMIs for each BMI weight status category
| HR 95% CI | Incident diabetes | ||||||
|---|---|---|---|---|---|---|---|
| Weight categories with BMI group (km/m2) | PPC-Stage I health/incidental | PPC-Stage II mild | PPC-Stage III moderate | PPC-Stage IV severe | PPC-Stage V high GI/mild TL | PPC-Stage VI reduced period/moderate TL | PPC-Stage VII severe TL |
| Underweight (N = 42) | |||||||
| L 14.0 | Ref | 1.55 (0.92-2.60) | 1.31 (0.77-2.23) | 2.11 (1.16-3.86) | 1.08 (0.58-2.01) | 0.95 (0.53-1.71) | 2.59 (1.60-4.19) |
| H 18.5 | 1.35 (0.91-1.98) | 1.19 (0.80-1.77) | 1.76 (1.10-2.80) | 1.01 (0.62-1.64) | 0.96 (0.61-1.50) | 2.11 (1.46-3.04) | |
| Normal weight (N = 1547) | |||||||
| L 18.5 | Ref | 1.35 (0.91-1.98) | 1.19 (0.80-1.77) | 1.76 (1.10-2.80) | 1.01 (0.62-1.64) | 0.96 (0.61-1.50) | 2.11 (1.46-3.04) |
| H 25.0 | 1.10 (0.88-1.38) | 1.03 (0.81-1.30) | 1.34 (1.00-1.82) | 0.97 (0.67-1.26) | 0.97 (0.75-1.27) | 1.56 (1.24-1.97) | |
| Overweight (N = 2344) | |||||||
| L 25.0 | Ref | 1.10 (0.88-1.38) | 1.03 (0.81-1.30) | 1.34 (1.00-1.82) | 0.97 (0.67-1.26) | 0.97 (0.75-1.27) | 1.56 (1.24-1.97) |
| H 30.0 | 0.94 (0.79-1.13) | 0.92 (0.76-1.11) | 1.09 (0.86-1.39) | 0.85 (0.67-1.09) | 0.98 (0.81-1.20) | 1.24 (1.03-1.50) | |
| Obese Class I (N = 1132) | |||||||
| L 30.0 | Ref | 0.94 (0.79-1.13) | 0.92 (0.76-1.11) | 1.09 (0.86-1.39) | 0.85 (0.67-1.09) | 0.98 (0.81-1.20) | 1.24 (1.03-1.50) |
| H 35.0 | 0.80 (0.62-1.04) | 0.82 (0.63-1.06) | 0.89 (0.67-1.19) | 0.79 (0.60-1.06) | 1.00 (0.77-1.29) | 0.99 (0.78-1.25) |
While the analysis of HRs was calculated on BMI as a continuous variable, it is possible to calculate HRs for any point along the continuum. The first column contains weight categories and for each weight category, the second column has an L, which is the lowest BMI for that group or lower boundary for that group and an H, which is the highest BMI for that group or higher boundary for that group. For example, the lowest BMI in the normal weight group is 18.5 and the highest BMI is 25.0. This allows the reader to track changes in HRs within weight groups. BMI weight status categories are adjusted for race/center, age, sex, hypertension, HDL, triglycerides, cholesterol lowering meds, smoking and education. Type III P-value for PPC-Stage and BMI interaction is 0.01. Bold type indicates a statistically significant HR.
Abbreviations: BMI, body mass index; GI, gingival inflammation index; HDL, high-density lipoprotein; HR, hazard ratio; PPC, periodontal profile class; Ref, reference; TL, tooth loss.
Table 3 shows that after adjusting for race/center, sex, age, smoking, education, hypertension, low HDL, high triglycerides, and cholesterol-lowering medications, PPC-Stages II, III, V, and VI did not present excess risk for diabetes in any weight group. However, PPC-Stages IV and VII did present excess risk for diabetes in some weight groups. For example, if a participant has periodontal disease classified as PPC-Stage IV and is underweight, the lower boundary of the underweight category has an HR of 2.11, 95% CI (1.16-3.86), but if a participant has almost normal BMI (upper boundary of the underweight category) the HR decreases to 1.76 (1.10-2.80). The closer a participant is to being obese, the further the HRs decrease. For PPC-Stage IV, the lower confidence level goes below 1.0 at a BMI of 25 (but was significant at a BMI of 24), indicating that for individuals who are overweight and beyond, periodontitis no longer represents excess risk for incident diabetes. We did not extend BMI beyond 35 as all higher levels continue to be not significant.
PPC-Stage VII had a pattern of excess risk due to periodontitis, similar to Stage IV, but became nonsignificant around a higher BMI of 31 (lower boundary of obese group) than BMI for PPC-Stage IV. If a participant had PPC-Stage VII disease and is underweight, the left boundary of the underweight category had an HR of 2.59 [95% CI (1.60-4.19)], but if a participant was almost normal weight (highest boundary of the underweight category) the HR is 2.11 (1.46-3.04). For both PPC-Stages IV and VII, the 95% CIs are widest for the underweight group, likely due to the small sample size that is likely even smaller at the lowest BMI levels.
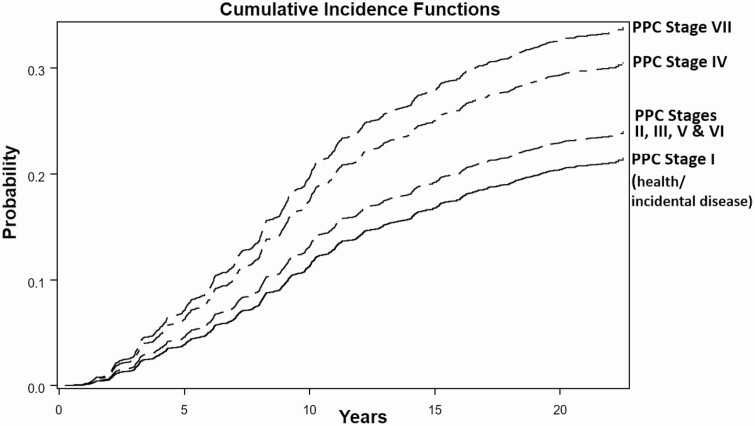
Figure 2 presents the cumulative incidence functions for developing diabetes for PPC-Stages during the follow up period. To reduce the number of functions plotted, we grouped PPC-Stages II, III, V, and VI, which were not related to incident diabetes. Compared to the reference group, PPC-Stage VII (“Severe TL”) had the highest probability to develop diabetes. The stage with the next highest probability was PPC-Stage IV (“Severe Perio”) category.
Figure 2.
Unadjusted cumulative incidence functions for PPC-Stages and incident diabetes. The cumulative probability to develop incident diabetes for different PPC-Stages during the follow-up period is shown. Compared to PPC-Stage I, PPC-Stage VII (severe TL) had the highest probability to develop diabetes followed by PPC-Stage IV (severe perio).
Discussion
We followed a well-characterized community-based cohort of initially nondiabetic adults for a median of 19.4 years and found that 2 phenotypes of periodontitis as defined by PPC-Stages showed an increased risk of diabetes. This indicates that only individuals classified as having specific stages, PPC-Stage IV (severe periodontal disease) and VII (severe tooth loss) of periodontal disease appear to be at excess risk for incident diabetes and in those stages the risks were modified by BMI levels. In PPC-Stage IV category, incident diabetes was almost exclusively present in underweight and in normal weight individuals (BMI < 25), while PPC-Stage VII incident diabetes risk extended to overweight group and included some obese participants with BMI ≤ 31.
One mechanism linking both obesity and periodontal disease to diabetes appears to be inflammation. The inflammation underlying the relationship of periodontal disease and diabetes is mainly due to the individual or host’s response to a chronic oral infection and subsequent shift of oral flora to a dysbiotic microbial community. Through 16s rDNA sequencing, Matsha et al has found a markedly increased abundance of Bacteroidetes in diabetic or prediabetic patients with bleeding upon probing, a clinical sign of oral inflammation as compared to diabetic or prediabetic subjects without bleeding (25). Functional analysis has shown that bacterial metabolites including creatine, several monoacylglycerols, and urate significantly increased the risk for incident diabetes by at least 24% (26). Therefore, the hypothesis that biomarkers, such as signature oral bacterial metabolites or plaque bacteria-associated inflammatory response, mediates the periodontal risk for incident diabetes is attractive and warrants ongoing investigation with a longitudinal study design. Alternatively, the observed risk for incident diabetes from the underweight participants in this study could also be attributed from unidentified genetic susceptibility that does not appear to associate with periodontal inflammation (27). However, this mechanism is less likely because genetic risks are usually revealed at an earlier age than the participants from ARIC.
Our data suggest that nonobese study participants with severe forms of periodontal inflammation, such as PPC-Stage IV (severe periodontal disease) and PPC-Stage VII (severe tooth loss), had significantly higher risk for incident diabetes than individuals with lower levels of periodontal disease (Table 3). However, Table 3 indicates that the relationship between PPC-Stages is modified by BMI so that the HRs in the competing risk models for Stages IV and VII remain significant but decrease as BMI increases. When BMI scores reached 25, PPC-Stage IV did not appear to present a significant risk for incidence of diabetes and for PPC-Stage VII, a BMI above 31 had the same effect. This absence of excess risk due to periodontal disease, even from the more severe forms of periodontal disease at higher BMI levels, may mean that the inflammation contributed by periodontal disease in obese individuals does not contribute to the overall burden of systemic inflammation. Alternatively, periodontal disease may contribute to the risk of diabetes, but the level of inflammation inherent in obesity overshadows the contribution from periodontal disease—that is, the excess risk due to periodontal disease is only apparent at lower BMI levels. Thus, BMI-modified risk from periodontal disease can be explained by causal redundancy in that the moderate risk from different periodontal phenotypes for ongoing diabetes may be difficult to assess in the presence of strong risk factors such as obesity.
The quantifiable risk of periodontal disease for diabetes is sometimes blurred by the heterogeneity of the most commonly applied periodontal definitions. Patients within the same moderate or severe periodontitis diagnosis using the 1999 American Association of Periodontology definition do not necessarily share the same risk for incident diabetes (28). For example, 1 study reported that participants with the most advanced periodontitis at baseline had a 5-fold increase in glycosylated hemoglobin (hemoglobin A1c) over 5 years compared to participants with mild to no periodontitis (29). However, in another prospective study neither moderate nor severe periodontitis was significantly associated with increased risk of diabetes (6). It appears a more refined and consistent periodontal classification system may reconcile the discrepancy of the periodontitis-associated risk assessment for incident diabetes. PPC-Stages are based on the homogeneity of clinical phenotypes through an unbiased LCA approach (16). We found that PPC-Stages have higher heritability than other current indices, indicating a biological basis (30). In addition, PPC-Stages have demonstrated utility in predicting future tooth and attachment loss and is more sensitive in detecting associations with systemic conditions than the widely used American Association of Periodontology definition (16-18,31).
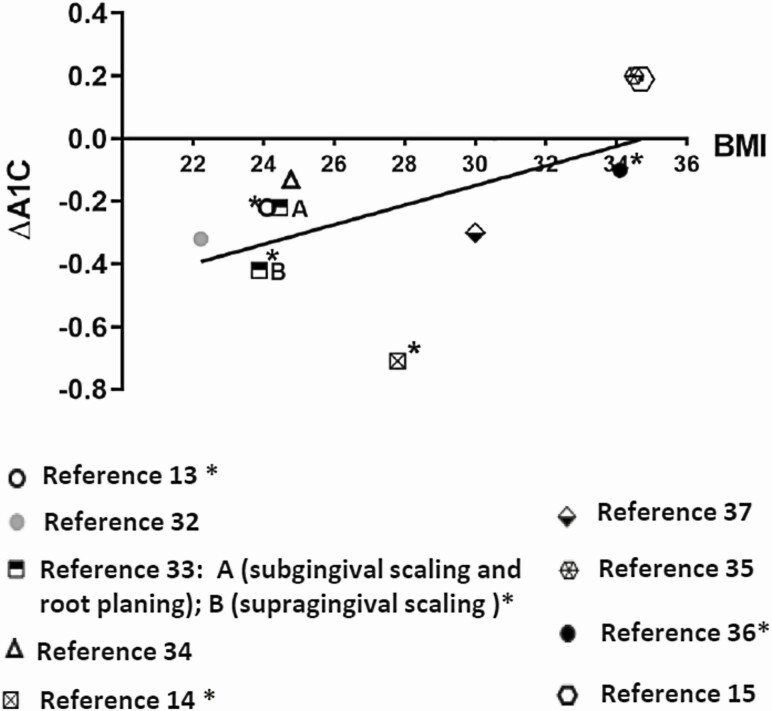
The results show that the relationship between periodontal disease and incident diabetes is strongest in people with lower BMI, indicating that periodontal treatment may be more effective in individuals with lower BMI scores to prevent or control diabetes. Therefore, it is reasonable to suggest that future study designs that involve periodontal therapy to impact diabetes may be more successful if limited to groups of people with lower BMI. Appropriately designed studies are needed to determine the actual relationships. We examined the literature to explore the relationship between baseline BMI levels and glycemic control improvement as reflected by reduction in A1c after nonsurgical periodontal treatment by analyzing several clinical trials (13-15,32-37) (Fig. 3). It appears that baseline BMI in diabetic subjects with periodontitis is inversely related to the A1c reduction at the follow-up visits after periodontal therapy. This means that individuals with diabetes and higher BMI tended to have less reduction of A1c levels following periodontal therapy than diabetics with lower BMIs. For example, in Katagiri’s study, diabetic patients with a mean BMI less than 25 significantly reduced their A1c 1 month after active periodontal treatment (13). Koromantzos and colleagues reported that in a diabetic population with a mean BMI of 27.8 (overweight), nonsurgical periodontal therapy significantly reduced A1c level at 1 month after treatment and the reduced A1c level persisted through 3 and 6 months follow-ups (14). In contrast, published data from a multicentered random clinical trial by Engebretson and colleagues demonstrate periodontal treatment failed to reduce A1c value at the 6-month follow-up visit in an obese diabetic population with an average BMI greater than 34 (15). A clinical trial conducted by Geisinger and colleagues reported that diabetic participants with a mean BMI score of 34.5 who received scaling and root planning periodontal therapy exhibited an 0.2 increase of A1c at the 6-month follow-up visit (35).
Figure 3.
Illustration of HbA1c changes (ΔA1c) in relation to BMI level after the nonsurgical periodontal therapy from several clinical studies. The BMI level of participants was either reported or inferred from different clinical trials. Each symbol represents a clinical study indicated in the reference. A trend line indicates that an increase in baseline BMI was associated with a diminished HbA1c improvement after the periodontal therapy. Abbreviation: HbA1c, hemoglobin A1c. *HbA1c was significantly lower after than before the periodontal treatment. The follow up period of those studies was ranging from 1 month to 1 year.
PPC-Stages may allow clinicians to identify specific intervention measures for subgroups of people who could benefit more than others and, due to the homogeneity of the people in each group, could reduce the phenomenon of nonresponders commonly observed with one-size-fits-all treatments. Both outcomes support a move toward precision dentistry/medicine.
Study Strengths and Limitations
Study strengths include a large sample size from a community cohort with multiple biomarkers, medical history, rigorous methodology, and a full-mouth periodontal examination that measured all teeth present at 6 sites per tooth.
Participants in this ARIC cohort came from an older population with an average age of 62.4 at baseline. Not surprisingly, a high percentage of (27.5%) participants died during the long follow-up period, which is about 20 years. Death is a competing risk event that precludes incident diabetes. The strength of using a competing risk model in this study is to address the cumulative incident diabetes while taking competing risk, which in this study is death, into account (24). In comparison to a Cox proportional hazards model, this competing risk model produces conservative associations and is particularly suitable to apply in the event of a high competing risk occurring during the observation period (24).
The first limitation of the study is that ARIC participants have an average age of about 63 years and represent an older group. However, national data indicate that the average age for people with periodontal disease is 54; thus, ARIC participants are about 9 years older (38). Another limitation is that study participants were randomly selected to be representative of their communities. Over time, however, they have become less representative due to mortality, loss to follow-up, and changes in the communities. Thus, they may remain representative of others who had the same life experiences but are no longer considered to be representative of their communities. Another limitation is the long monitoring period from baseline to the incident event. Periodontal disease was only measured at Visit 4, which may introduce a systematic bias in the interpretation of the magnitude of the effect of periodontal disease on diabetes because periodontal disease tends to get worse over time. This could result in an underestimation of periodontal disease and the interactive effect with BMI on incident diabetes. The established association of periodontal disease status subcategories with incident diabetes in ARIC cohort needs to be further validated by clinical interventional studies or clinical trials. BMI is strongly associated with incident diabetes, and it is possible that increased inflammation due to weight gain from baseline may be enough to cause the onset of diabetes, and periodontal disease is not a risk for incident diabetes. The ARIC study did not record participant weight during the annual telephone follow-ups, so we calculated the change in weight from Visit 4 to Visit 5, which occurred 15 years later and created 3 groups based on their change in weight status. The data in Table 4 are from the subset of ARIC participants who participated in Visit 5 and shows that 33% of the 689 study participants who gained 10 or more pounds over the 15 years developed diabetes while 25% of PPC-Stage I participants, who are periodontally healthy, developed diabetes. Of those classified as having severe disease (Stages IV and VII), 46% developed diabetes. The patterns were similar for study participants who gained less weight or who lost weight. It appears that periodontitis may convey excess risk for diabetes in individuals who have gained weight or lost weight during the follow-up period of this study. However, this subgroup may not be representative of our study sample and validation of these findings in additional studies is needed. Another limitation of the study is the lack of dental treatment records from the surveyed participants. It is known that periodontal therapy improves glycemic control and A1c in diabetic patients (2,39,40). It is likely that regular dental prophylaxis or periodontal maintenance may delay the onset of diabetes by reducing episodical systemic spreads of inflammatory molecules and plaque bacteria from periodontal pockets. However, we are not able to dissect the impact of dental treatment, especially periodontal therapy, on the BMI-modified risk of PPC-Stages for incident diabetes.
Table 4:
Percentage of Participants with Incident Diabetes over 15 years (Visits 4 to 5) by Weight Change and PPC-Stages IV & VII
| 15-Year Weight Change (N) | Incident Diabetes, n (%) |
|---|---|
| Lost >10 pounds (N=1132) | 328 (29) |
| Stage 1 | 86 (25) |
| Stage IV and Stage VII | 76 (36) |
| Negligible (-10 to 10 pounds) (N=1676) | 385 (23) |
| Stage I | 120 (21) |
| Stage IV and Stage VII | 83 (34) |
| Gained > 10 pounds (N=689) | 227 (33) |
| Stage I | 53 (25) |
| Stage IV and Stage VII | 62 (46) |
These results were obtained only for the participants of the ARIC cohort that attended Visit 5 and are not representative of the ARIC cohort participants at baseline.
In summary, by following a well-characterized community-dwelling US sample for a median of 19.4 years, we report that 2 periodontal disease stages in the PPC-Stages system have modest, but significant, excess risk for incident diabetes. This periodontal disease-associated risk was modified by BMI and only associated with incident diabetes in study participants with lower levels of BMI. For researchers, these results may indicate that not all periodontitis may have systemic effects and even severe periodontitis may only have systemic effects under certain circumstances, which implies that future treatment studies to determine reductions in systemic markers and diseases may not want to include obese study participants. Our data were supported by intervention studies suggesting that periodontal treatment achieved a better A1c reduction of diabetic patients with lower BMI than patients with high BMI scores (Fig. 1). Dentists should be aware that periodontal disease is associated with incident diabetes, but the association may be modified for patient’s at higher BMI levels.
Acknowledgments
The authors want to recognize Dr. Steven Offenbacher, a pioneer in the area of periodontal medicine, whose leadership and energy contributed to early drafts of this paper. The authors also thank the staff and participants of the ARIC study for their important contributions.
Author Contributions: SZ, RTD, ES, and JDB were responsible for conception. SZ, KM, HA, ES, RTD, and JDB contributed to the overall study design. SZ, KHP, HA, KM, IZM, and JDB were responsible for manuscript drafting. KM performed data analysis. DW, HA, ES, RTD, and FN provided statistical expertise. KHP performed literature search. All authors participated in data interpretation. All authors participated in critical revision of the manuscript for important intellectual content and gave final approval of the manuscript.
Financial support: The Atherosclerosis Risk in Communities study has been funded in whole or in part with federal funds from the National Heart, Lung, and Blood Institute, National Institutes of Health, Department of Health and Human Services, under contract nos. HHSN268201700001I, HHSN268201700002I, HHSN268201700003I, HHSN268201700005I, and HHSN268201700004I. This study was also partially supported by an National Institute of Dental and Craniofacial Research (NIDCR) R00 grant (4R00DE027086) awarded to SZ.
Additional Information
Disclosures: JDB and KM report that the University of North Carolina has submitted a patent for the PPC technology, and computational algorithms. Currently, no financial arrangements exist. The other authors report no potential conflicts of interest relevant to this article.
Data Availability
Restrictions apply to some or all the availability of data generated or analyzed during this study to preserve patient confidentiality or because they were used under license. The corresponding author will on request detail the restrictions and any conditions under which access to some data may be provided.
References
- 1. Arboleda S, Vargas M, Losada S, Pinto A. Review of obesity and periodontitis: an epidemiological view. Br Dent J. 2019;227(3):235-239. [DOI] [PubMed] [Google Scholar]
- 2. Baeza M, Morales A, Cisterna C, et al. Effect of periodontal treatment in patients with periodontitis and diabetes: systematic review and meta-analysis. J Appl Oral Sci. 2020;28:e20190248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Saito T, Shimazaki Y, Kiyohara Y, et al. The severity of periodontal disease is associated with the development of glucose intolerance in non-diabetics: the Hisayama study. J Dent Res. 2004;83(6):485-490. [DOI] [PubMed] [Google Scholar]
- 4. Demmer RT, Jacobs DR Jr, Desvarieux M. Periodontal disease and incident type 2 diabetes: results from the First National Health and Nutrition Examination Survey and its epidemiologic follow-up study. Diabetes Care. 2008;31(7):1373-1379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Taylor GW, Burt BA, Becker MP, et al. Severe periodontitis and risk for poor glycemic control in patients with non-insulin-dependent diabetes mellitus. J Periodontol. 1996;67(Suppl 10S):1085-1093. [DOI] [PubMed] [Google Scholar]
- 6. Ide R, Hoshuyama T, Wilson D, Takahashi K, Higashi T. Periodontal disease and incident diabetes: a seven-year study. J Dent Res. 2011;90(1):41-46. [DOI] [PubMed] [Google Scholar]
- 7. Vazquez G, Duval S, Jacobs DR Jr, Silventoinen K. Comparison of body mass index, waist circumference, and waist/hip ratio in predicting incident diabetes: a meta-analysis. Epidemiol Rev. 2007;29:115-128. [DOI] [PubMed] [Google Scholar]
- 8. Liu C, Feng X, Li Q, Wang Y, Li Q, Hua M. Adiponectin, TNF-α and inflammatory cytokines and risk of type 2 diabetes: a systematic review and meta-analysis. Cytokine. 2016;86:100-109. [DOI] [PubMed] [Google Scholar]
- 9. Slade GD, Ghezzi EM, Heiss G, Beck JD, Riche E, Offenbacher S. Relationship between periodontal disease and C-reactive protein among adults in the Atherosclerosis Risk in Communities study. Arch Intern Med. 2003;163(10):1172-1179. [DOI] [PubMed] [Google Scholar]
- 10. Morita I, Okamoto Y, Yoshii S, et al. Five-year incidence of periodontal disease is related to body mass index. J Dent Res. 2011;90(2):199-202. [DOI] [PubMed] [Google Scholar]
- 11. Jimenez M, Hu FB, Marino M, Li Y, Joshipura KJ. Prospective associations between measures of adiposity and periodontal disease. Obesity (Silver Spring). 2012;20(8):1718-1725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Nascimento GG, Leite FR, Do LG, et al. Is weight gain associated with the incidence of periodontitis? A systematic review and meta-analysis. J Clin Periodontol. 2015;42(6):495-505. [DOI] [PubMed] [Google Scholar]
- 13. Katagiri S, Nitta H, Nagasawa T, et al. Multi-center intervention study on glycohemoglobin (HbA1c) and serum, high-sensitivity CRP (hs-CRP) after local anti-infectious periodontal treatment in type 2 diabetic patients with periodontal disease. Diabetes Res Clin Pract. 2009;83(3):308-315. [DOI] [PubMed] [Google Scholar]
- 14. Koromantzos PA, Makrilakis K, Dereka X, Katsilambros N, Vrotsos IA, Madianos PN. A randomized, controlled trial on the effect of non-surgical periodontal therapy in patients with type 2 diabetes. Part I: effect on periodontal status and glycaemic control. J Clin Periodontol. 2011;38(2):142-147. [DOI] [PubMed] [Google Scholar]
- 15. Engebretson SP, Hyman LG, Michalowicz BS, et al. The effect of nonsurgical periodontal therapy on hemoglobin A1c levels in persons with type 2 diabetes and chronic periodontitis: a randomized clinical trial. Jama. 2013;310(23):2523-2532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Morelli T, Moss KL, Beck J, et al. Derivation and validation of the periodontal and tooth profile classification system for patient stratification. J Periodontol. 2017;88(2):153-165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Morelli T, Moss KL, Preisser JS, et al. Periodontal profile classes predict periodontal disease progression and tooth loss. J Periodontol. 2018;89(2):148-156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Beck JD, Moss KL, Morelli T, Offenbacher S. Periodontal profile class is associated with prevalent diabetes, coronary heart disease, stroke, and systemic markers of C-reactive protein and interleukin-6. J Periodontol. 2018;89(2):157-165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Beck JD, Moss KL, Morelli T, Offenbacher S. In search of appropriate measures of periodontal status: the periodontal profile phenotype (P3) system. J Periodontol. 2018;89(2):166-175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Beck JD, Elter JR, Heiss G, Couper D, Mauriello SM, Offenbacher S. Relationship of periodontal disease to carotid artery intima-media wall thickness: the atherosclerosis risk in communities (ARIC) study. Arterioscler Thromb Vasc Biol. 2001;21(11):1816-1822. [DOI] [PubMed] [Google Scholar]
- 21. ARIC Investigators. The Atherosclerosis Risk in Communities (ARIC) study: design and objectives. Am J Epidemiol. 1989;129(4):687-702. [PubMed] [Google Scholar]
- 22. Centers for Disease Control and Prevention. Defining adult overweight and obesity. Published May 13, 2021. https://www.cdc.gov/obesity/adult/defining.html.
- 23. Jensen MD, Ryan DH, Apovian CM, et al. ; American College of Cardiology/American Heart Association Task Force on Practice Guidelines; Obesity Society . 2013 AHA/ACC/TOS guideline for the management of overweight and obesity in adults: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines and the Obesity Society. J Am Coll Cardiol. 2014;63(25 Pt B):2985-3023. [DOI] [PubMed] [Google Scholar]
- 24. Fine JP, Gray RJ. A proportional hazards model for the subdistribution of a competing risk. J Am Stat Assoc. 1999;94(446):496-509. [Google Scholar]
- 25. Matsha TE, Prince Y, Davids S, et al. Oral microbiome signatures in diabetes mellitus and periodontal disease. J Dent Res. 2020;99(6):658-665. [DOI] [PubMed] [Google Scholar]
- 26. Vangipurapu J, Fernandes Silva L, Kuulasmaa T, Smith U, Laakso M. Microbiota-related metabolites and the risk of type 2 diabetes. Diabetes Care. 2020;43(6):1319-1325. [DOI] [PubMed] [Google Scholar]
- 27. Dorajoo R, Liu J, Boehm BO. Genetics of type 2 diabetes and clinical utility. Genes (Basel). 2015;6(2):372-384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Page RC, Eke PI. Case definitions for use in population-based surveillance of periodontitis. J Periodontol. 2007;78(7 Suppl):1387-1399. [DOI] [PubMed] [Google Scholar]
- 29. Demmer RT, Desvarieux M, Holtfreter B, et al. Periodontal status and A1C change: longitudinal results from the Study of Health in Pomerania (SHIP). Diabetes Care. 2010;33(5):1037-1043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Agler CS, Moss K, Philips KH, et al. Biologically defined or biologically informed traits are more heritable than clinically defined ones: the case of oral and dental phenotypes. Adv Exp Med Biol. 2019;1197:179-189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Sen S, Giamberardino LD, Moss K, et al. Periodontal disease, regular dental care use, and incident ischemic stroke. Stroke. 2018;49(2):355-362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Wu Y, Chen L, Wei B, Luo K, Yan F. Effect of non-surgical periodontal treatment on visfatin concentrations in serum and gingival crevicular fluid of patients with chronic periodontitis and type 2 diabetes mellitus. J Periodontol. 2015;86(6):795-800. [DOI] [PubMed] [Google Scholar]
- 33. Chen L, Luo G, Xuan D, et al. Effects of non-surgical periodontal treatment on clinical response, serum inflammatory parameters, and metabolic control in patients with type 2 diabetes: a randomized study. J Periodontol. 2012;83(4):435-443. [DOI] [PubMed] [Google Scholar]
- 34. Goel K, Pradhan S, Bhattarai MD. Effects of nonsurgical periodontal therapy in patients with moderately controlled type 2 diabetes mellitus and chronic periodontitis in Nepalese population. Clin Cosmet Investig Dent. 2017;9:73-80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Geisinger ML, Michalowicz BS, Hou W, et al. Systemic inflammatory biomarkers and their association with periodontal and diabetes-related factors in the diabetes and periodontal therapy trial, a randomized controlled trial. J Periodontol. 2016;87(8):900-913. [DOI] [PubMed] [Google Scholar]
- 36. Taşdemir Z, Özsarı Taşdemir F, Koçyiğit İ, Yazıcı C, Gürgan CA. The clinical and systemic effects of periodontal treatment in diabetic and non-diabetic obese patients. J Oral Sci. 2016;58(4):523-531. [DOI] [PubMed] [Google Scholar]
- 37. D’Aiuto F, Gkranias N, Bhowruth D, et al. ; TASTE Group . Systemic effects of periodontitis treatment in patients with type 2 diabetes: a 12 month, single-centre, investigator-masked, randomised trial. Lancet Diabetes Endocrinol. 2018;6(12):954-965. [DOI] [PubMed] [Google Scholar]
- 38. Eke PI, Thornton-Evans GO, Wei L, Borgnakke WS, Dye BA, Genco RJ. Periodontitis in US adults: national health and nutrition examination survey 2009-2014. J Am Dent Assoc (1939). 2018;149(7):576-588.e576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Mauri-Obradors E, Merlos A, Estrugo-Devesa A, Jané-Salas E, López-López J, Viñas M. Benefits of non-surgical periodontal treatment in patients with type 2 diabetes mellitus and chronic periodontitis: a randomized controlled trial. J Clin Periodontol. 2018;45(3):345-353. [DOI] [PubMed] [Google Scholar]
- 40. Teeuw WJ, Gerdes VE, Loos BG. Effect of periodontal treatment on glycemic control of diabetic patients: a systematic review and meta-analysis. Diabetes Care. 2010;33(2):421-427. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Restrictions apply to some or all the availability of data generated or analyzed during this study to preserve patient confidentiality or because they were used under license. The corresponding author will on request detail the restrictions and any conditions under which access to some data may be provided.